Submitted:
23 August 2024
Posted:
26 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Materials Harvest
2.2. Preparation of the Immature Carob Juice
2.3. Quantification of Total Sugar Content
2.4. Quantification of Total Phenolic Content
2.5. Quantification of Flavonoid Content
2.6. Quantification of Total Condensed Tannins
2.7. Antioxidant Activity Evaluation Assays
2.7.1. 2,2-Diphényl 1-Picrylhydrazyle Assay
2.7.2. ABTS Scavenging Assay
2.7.3. Ferric Reducing Antioxidant Power Assay
2.8. Identification of Unripe Carob Pulp Juice Phytochemical Compounds
2.8.1. Solubilization of Polyphenols of Unripe Carob Pulp Juice
2.8.2. HPLC- UV-MS/MS Analysis
2.9. Animals
2.10. Acute Toxicity Test
2.11. Evaluation of the Inhibitory Effect of Unrip Carob Pulp on Pancreatic α-Amylase Activity
2.11.1. In Vitro Assay
2.11.2. In Vivo Assay
2.12. Assessment of the Inhibitory Effect of Unrip Carob Pulp on Intestinal α-Glucosidase Activity
2.12.1. In Vitro Assay
2.12.2. In Vivo Assay
2.13. Molecular Docking Analysis
2.13.1. Ligand Preparation
2.13.2. Protein Preparation
2.14. ADME Studies
2.15. Statistical Analysis
3. Results
3.1. Total Sugar Content
3.2. Total Phenolics, Flavonoids, and Condensed Tannins Contents
3.3. Antioxidant Activities
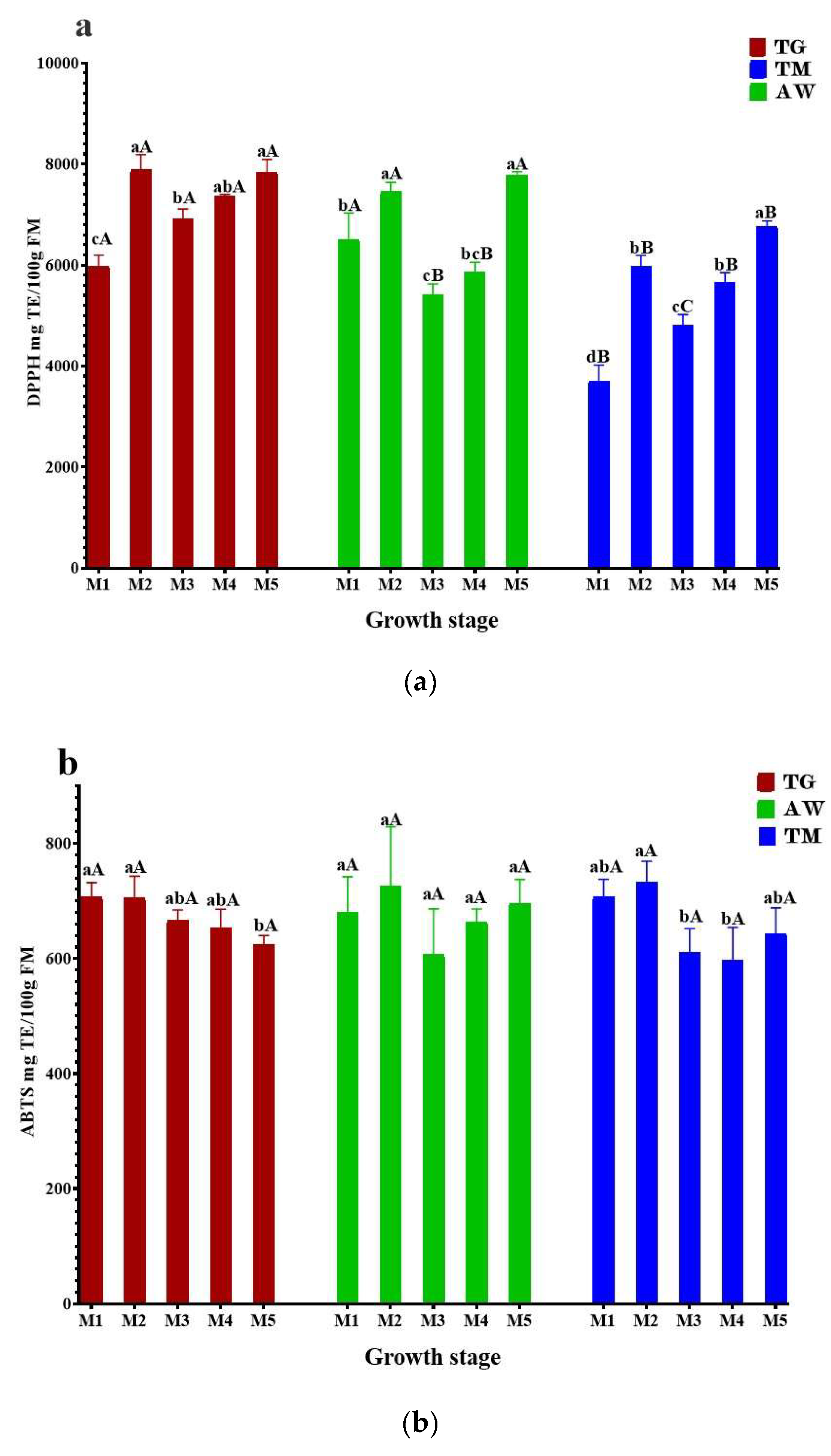
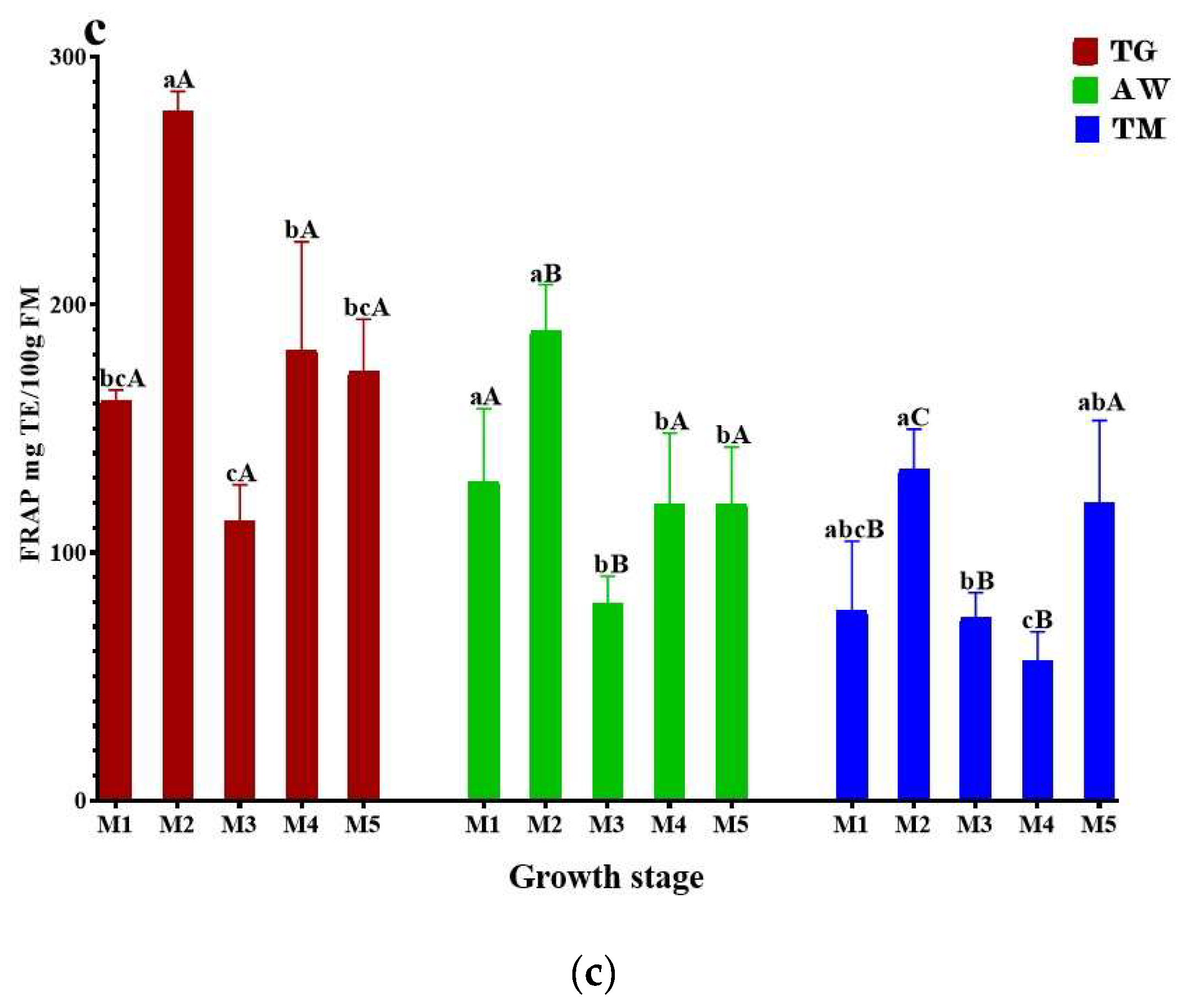
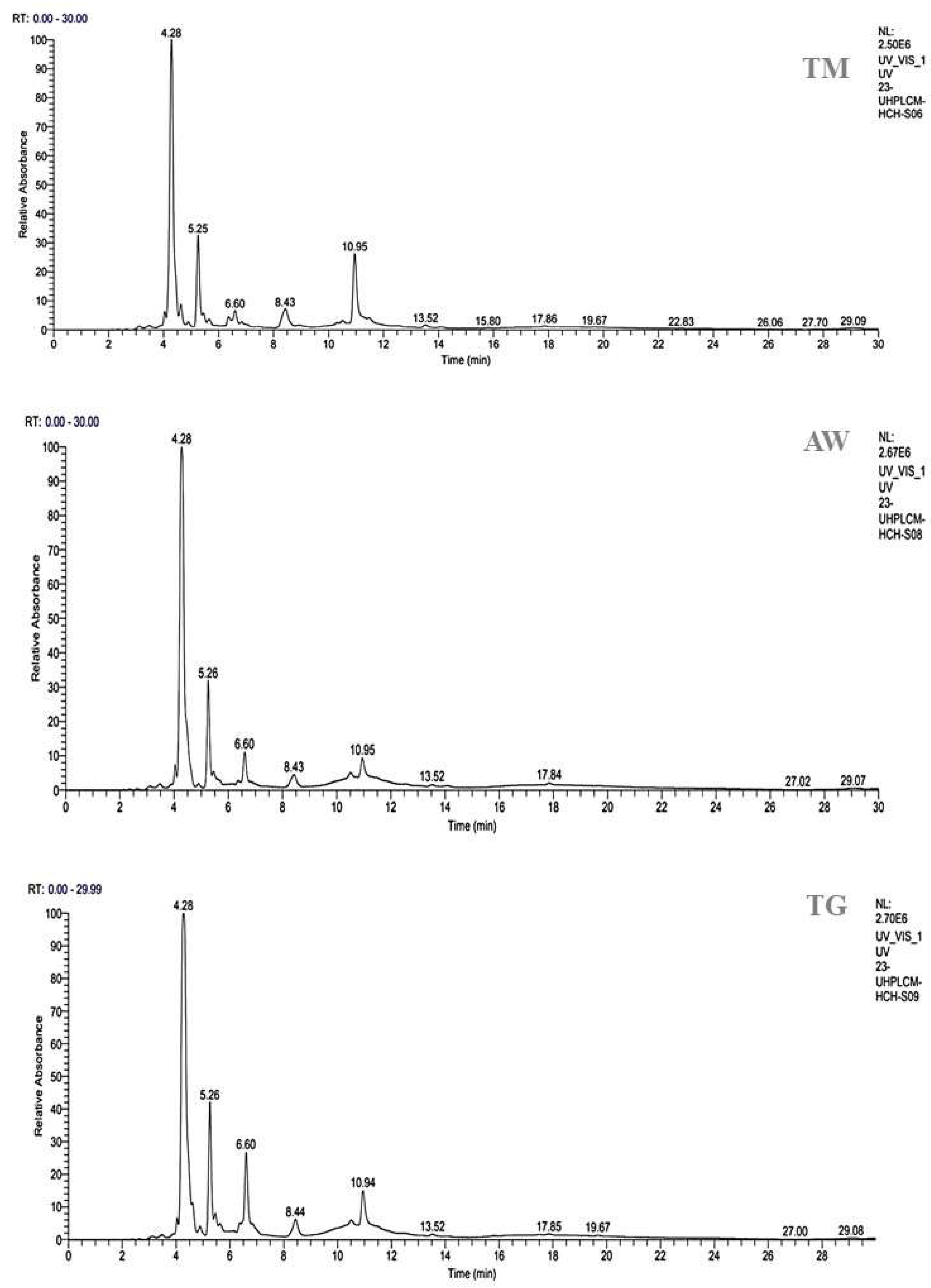
3.4. HPLC- UV-MS/MS Analysis
3.5. Acute Safety
3.6. In Vitro, Inhibitory Effect of the Plant Extract on the Pancreatic α-Amylase and Intestinal α-Glucosidase Activities
3.6.1. Pancreatic α-Amylase
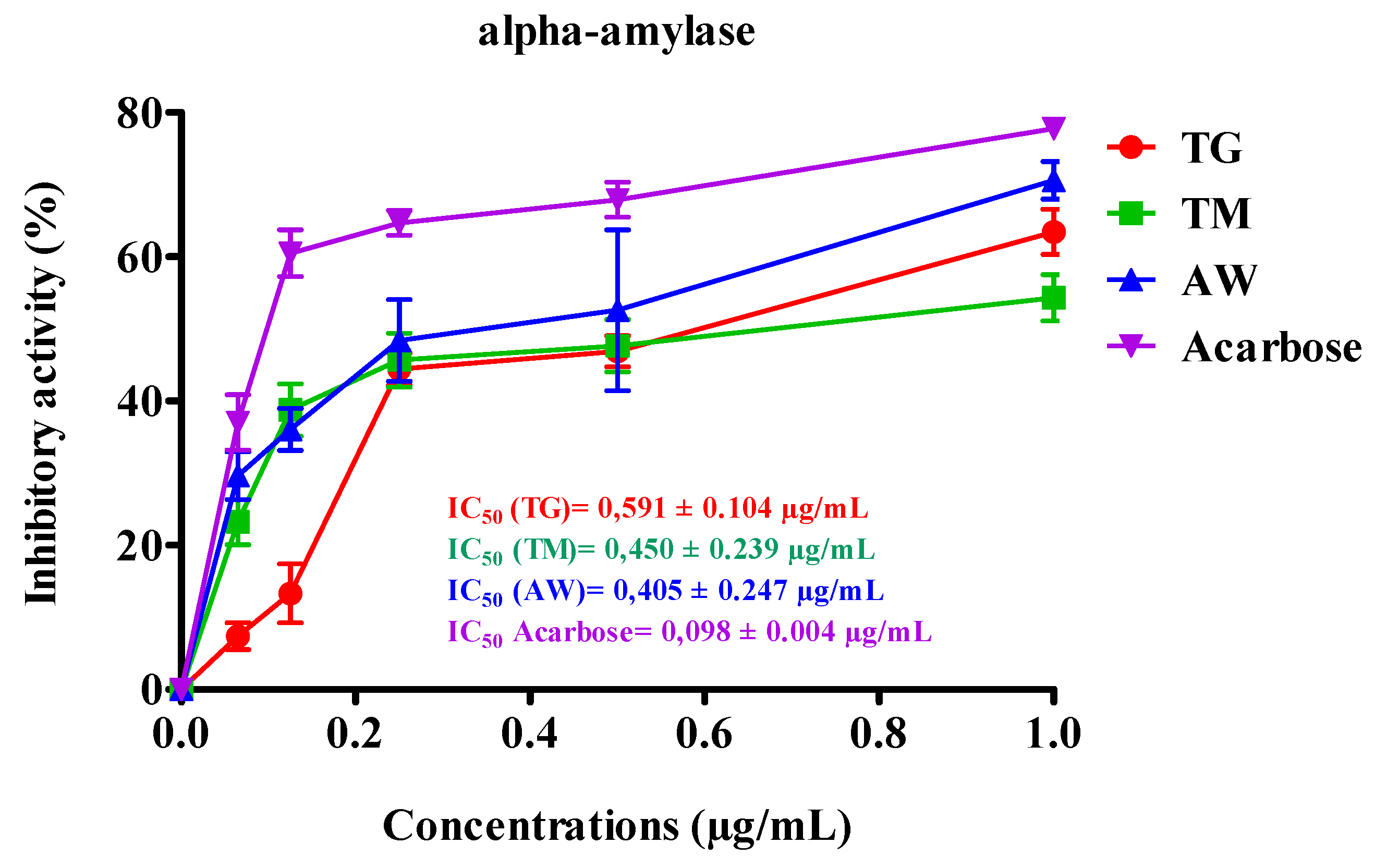
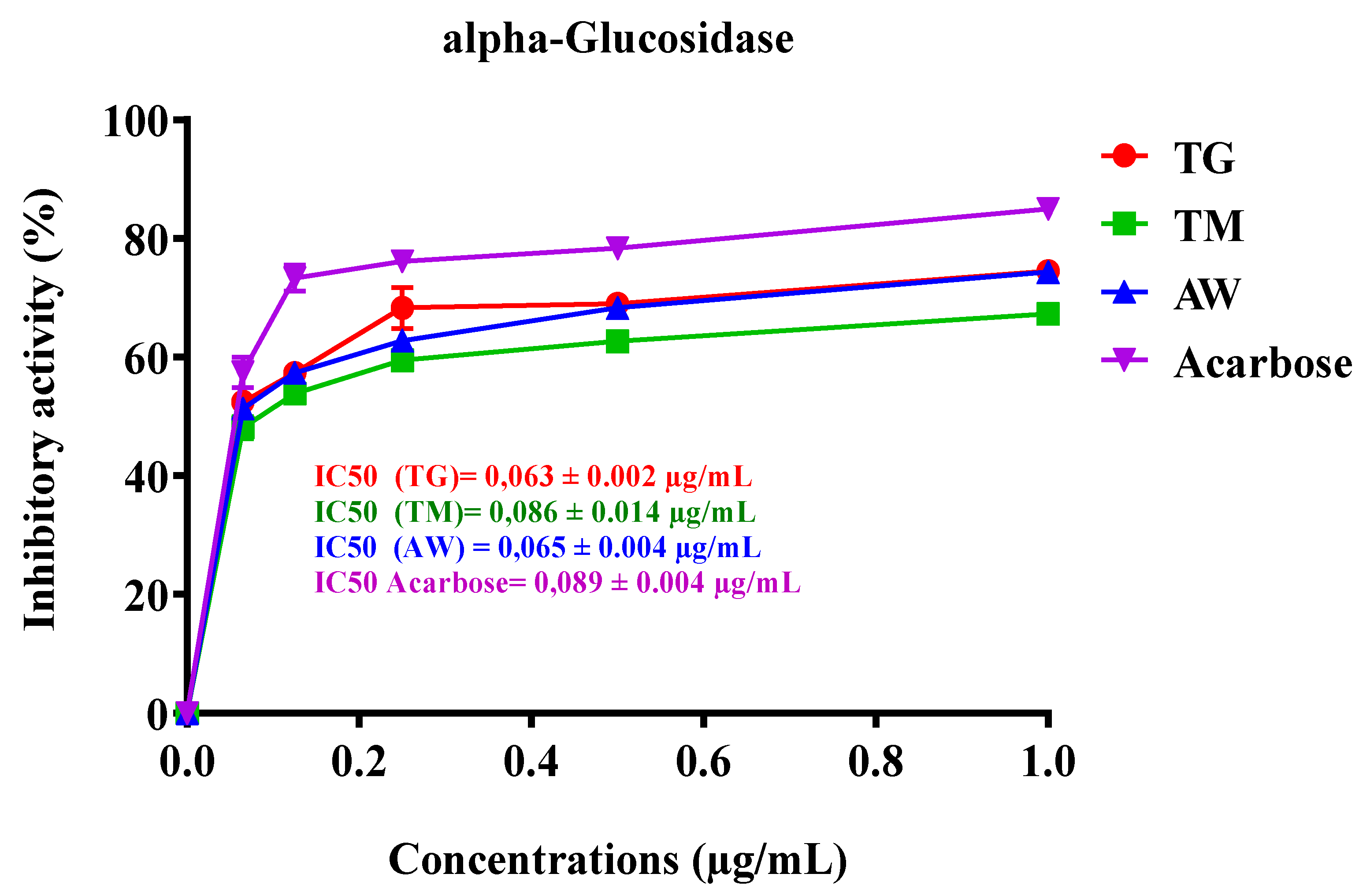
3.6.2. Intestinal α-Glucosidase
3.7. In Vivo, the Plant Extract’s Effect on Inhibiting Pancreatic α-Amylase and Intestinal α-Glucosidase Activities
3.7.1. Pancreatic α-Amylase

3.7.2. Intestinal α-Glucosidase
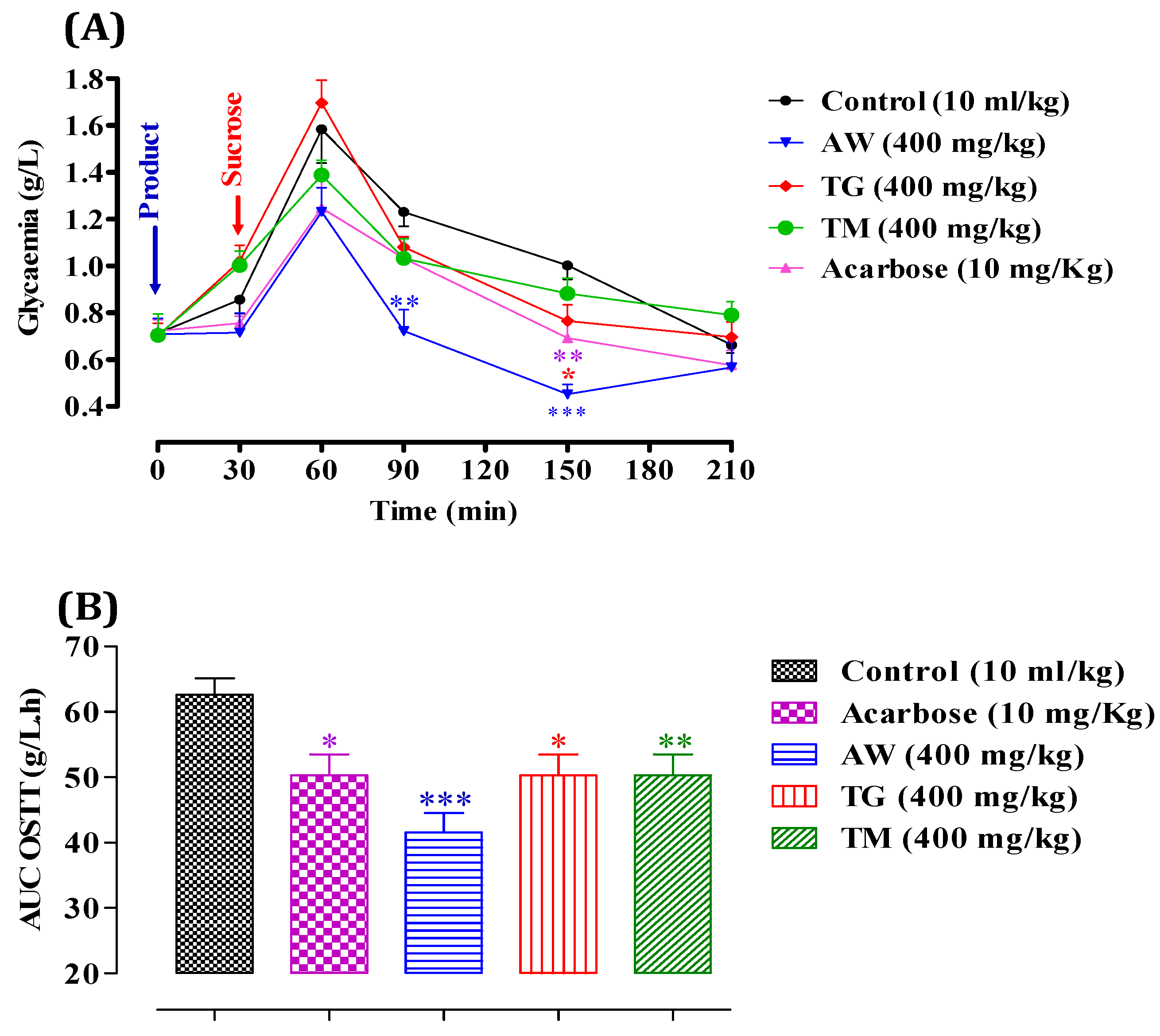
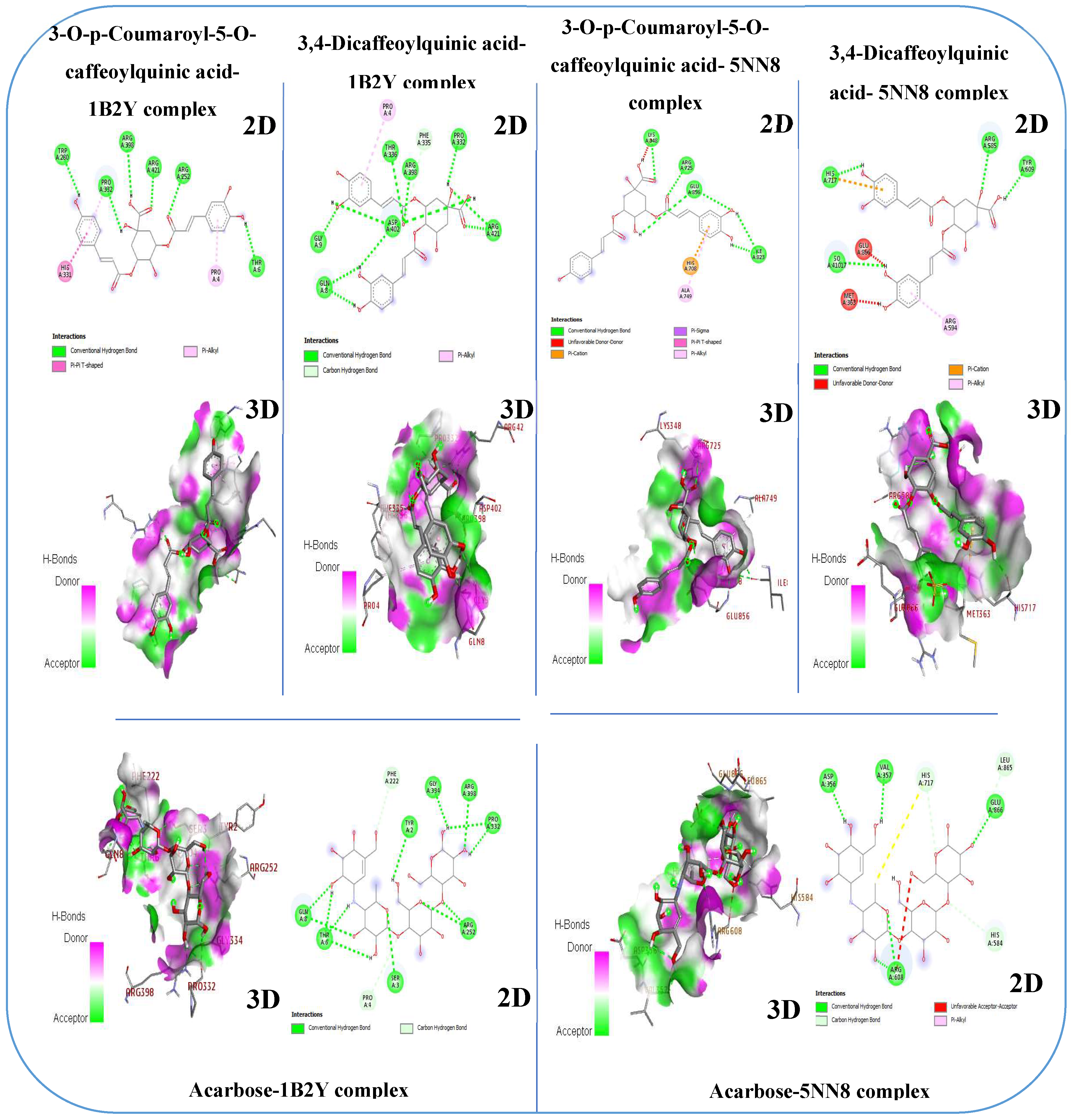
3.8. Molecular Docking: Targeting α-Glucosidase and α-Amylase
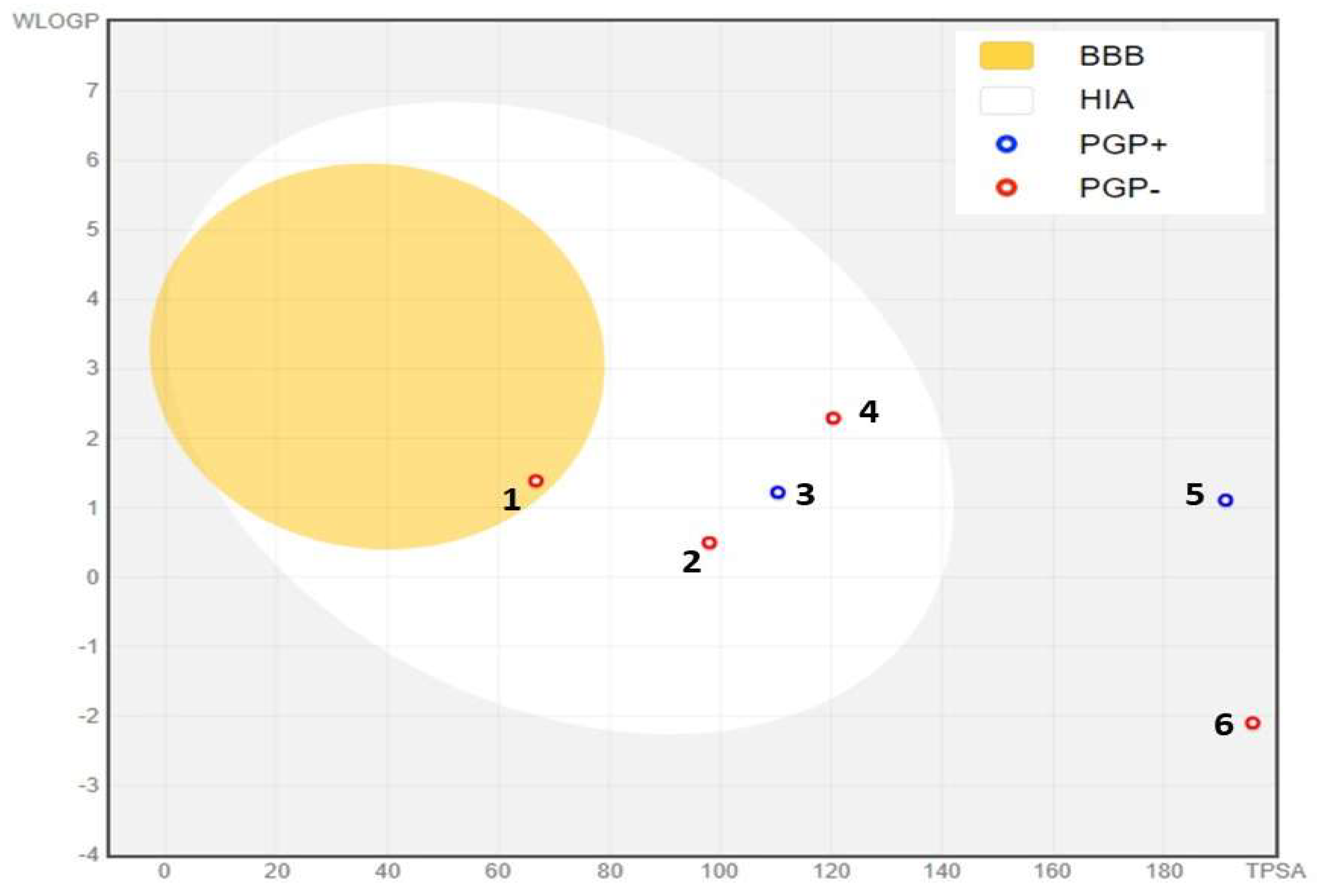
3.9. ADME Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kassout J, Hmimsa Y, Fatehi S El, Kadaoui K, Houssni M, Chakkour S, et al. Aridity Gradients Shape Intraspecific Variability of Morphological Traits in Native Ceratonia siliqua L. of Morocco. Plants 2023;12. [CrossRef]
- Frühbauerová M, Červenka L, Hájek T, Pouzar M, Palarčík J. Bioaccessibility of phenolics from carob (Ceratonia siliqua L.) pod powder prepared by cryogenic and vibratory grinding. Food Chem 2022;377. [CrossRef]
- Papaefstathiou E, Agapiou A, Giannopoulos S, Kokkinofta R. Nutritional characterization of carobs and traditional carob products. Food Sci Nutr 2018;6:2151–61. [CrossRef]
- Roseiro LB, Tavares CS, Roseiro JC, Rauter AP. Antioxidants from aqueous decoction of carob pods biomass (Ceretonia siliqua L.): Optimisation using response surface methodology and phenolic profile by capillary electrophoresis. Ind Crops Prod 2013;44:119–26. [CrossRef]
- Kyriacou MC, Antoniou C, Rouphael Y, Graziani G, Kyratzis A. Mapping the primary and secondary metabolomes of carob (Ceratonia siliqua l.) fruit and its postharvest antioxidant potential at critical stages of ripening. Antioxidants 2021;10:1–21. [CrossRef]
- Owen RW, Haubner R, Hull WE, Erben G, Spiegelhalder B, Bartsch H, et al. Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food and Chemical Toxicology 2003;41:1727–38. [CrossRef]
- Ortega N, Macià A, Romero MP, Trullols E, Morello JR, Anglès N, et al. Rapid determination of phenolic compounds and alkaloids of carob flour by improved liquid chromatography tandem mass spectrometry. J Agric Food Chem 2009;57:7239–44. [CrossRef]
- Bengoechea C, Romero A, Villanueva A, Moreno G, B MA, Milla F, et al. Composition and structure of carob (Ceratonia siliqua L.) germ proteins. Food Chem 2008;107:675–83. [CrossRef]
- Achchoub M, Azzouzi H, Elhajji L, Benbati M, Elfazazi K, Salmaoui S. Evaluation of Physicochemical, Functional and Sensory Properties of Carob Pulp Beverage (Ceratonia Siliqua L). Biosci Biotechnol Res Asia 2021;18:611–8. [CrossRef]
- Elfazazi K, Harrak H, Achchoub M, Benbati M. Physicochemical criteria, bioactive compounds and sensory quality of Moroccan traditional carob drink. Mater Today Proc 2020;27:3249–53. [CrossRef]
- Goulas V, Stylos E, Chatziathanasiadou M V., Mavromoustakos T, Tzakos AG. Functional Components of Carob Fruit: Linking the Chemical and Biological Space. International Journal of Molecular Sciences 2016, Vol 17, Page 1875 2016;17:1875. [CrossRef]
- Maier H, Anderson M, Karl C, Magnuson K, Whistler RL. Guar, Locust Bean, Tara, and Fenugreek Gums. Industrial Gums: Polysaccharides and Their Derivatives: Third Edition 2013:181–226. [CrossRef]
- Patmore J V., Goff HD, Fernandes S. Cryo-gelation of galactomannans in ice cream model systems. Food Hydrocoll 2003;17:161–9. [CrossRef]
- Laaraj S, Hussain A, Mouhaddach A, Noutfia Y, Gorsi FI, Yaqub S, et al. Nutritional Benefits and Antihyperglycemic Potential of Carob Fruit (Ceratonia siliqua L.): An Overview. Ecological Engineering and Environmental Technology 2024;25:124–32. [CrossRef]
- Laaraj S, Salmaoui S, Addi M, El-Rhouttais C, Tikent A, Elbouzidi A, et al. Carob (Ceratonia siliqua L.) Seed Constituents: A Comprehensive Review of Composition, Chemical Profile, and Diverse Applications. J Food Qual 2023;2023. [CrossRef]
- Palipoch S, Punsawad C, Suwannalert P. Thunbergia laurifolia , a new choice of natural antioxidant to prevent oxidative stress-related pathology : A review 2013;7:698–701. [CrossRef]
- Slavin J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013;5:1417–35. [CrossRef]
- Raninen K, Lappi J, Mykkänen H, Poutanen K. Dietary fiber type reflects physiological functionality: comparison of grain fiber, inulin, and polydextrose. Nutr Rev 2011;69:9–21. [CrossRef]
- de Bock M, Derraik JGB, Cutfield WS. Polyphenols and glucose homeostasis in humans. J Acad Nutr Diet 2012;112:808–15. [CrossRef]
- Dewanjee S, Das AK, Sahu R, Gangopadhyay M. Antidiabetic activity of Diospyros peregrina fruit: Effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food and Chemical Toxicology 2009;47:2679–85. [CrossRef]
- Kumar S, Kumar V, Prakash O, Kumar V. Antidiabetic, hypolipidemic and histopathological analysis of Dillenia indica (L.) leaves extract on alloxan induced diabetic rats Asian Pacific Journal of Tropical Medicine Antidiabetic Dillenia indica Hypolipidemic Rat Serum cholesterol. 2011.
- Rtibi K, Selmi S, Grami D, Sebai H, Marzouki L. Cronicon In Vitro α-Amylase/α-Glucosidase Inhibitory Activities and In Vivo Improving Glucose Tolerance and Hypoglycemic Effect of Ceratonia siliqua Leaves Aqueous Extract *Corresponding. vol. 13. 2018.
- Benchikh Y, Louaileche H, George B, Merlin A. Changes in bioactive phytochemical content and in vitro antioxidant activity of carob (Ceratonia siliqua L.) as influenced by fruit ripening. Ind Crops Prod 2014;60:298–303. [CrossRef]
- Benchikh Y, Paris C, Louaileche H, Charbonnel C, Ghoul M, Chebil L. Comparative characterization of green and ripe carob (Ceratonia siliqua L.): physicochemical attributes and phenolic profile. 2016.
- Benchikh Y, Louailèche H. Effects of extraction conditions on the recovery of phenolic compounds and in vitro antioxidant activity of carob (Ceratonia siliqua L.) pulp. Acta Botanica Gallica 2014;161:175–81. [CrossRef]
- Farag MA, El-Kersh DM, Ehrlich A, Choucry MA, El-Seedi H, Frolov A, et al. Variation in Ceratonia siliqua pod metabolome in context of its different geographical origin, ripening stage and roasting process. Food Chem 2019;283:675–87. [CrossRef]
- Ydjedd S, Chaalal M, Richard G, Kati DE, López-Nicolás R, Fauconnier ML, et al. Assessment of antioxidant potential of phenolic compounds fractions of Algerian Ceratonia siliqua L. pods during ripening stages. Int Food Res J 2017;24:2041–9.
- Ben Othmen K, Elfalleh W, Lachiheb B, Haddad M. Evolution of phytochemical and antioxidant activity of Tunisian carob (Ceratonia siliqua L.) pods during maturation. Eurobiotech Journal 2019;3:135–42. [CrossRef]
- Rtibi K, Selmi S, Grami D, Saidani K, Sebai H, Amri M, et al. Ceratonia siliqua L. (immature carob bean) inhibits intestinal glucose absorption, improves glucose tolerance and protects against alloxan-induced diabetes in rat. J Sci Food Agric 2017;97:2664–70. [CrossRef]
- Tikent A, Laaraj S, Marhri A, Taibi M, Elbouzidi A, Khalid I, et al. The Antioxidant and Antimicrobial Activities of Two Sun-Dried Fig Varieties (Ficus carica L.) Produced in Eastern Morocco and the Investigation of Pomological, Colorimetric, and Phytochemical Characteristics for Improved Valorization. International Journal of Plant Biology 2023;14:845–63. [CrossRef]
- Vekiari AS, Ouzounidou G, Gork G, Ozturk M, Asfi M. Compositional changes of major chemical compounds in Greek carob pods during development. Bull Chem Soc Ethiop 2012;26:343–51. [CrossRef]
- Prasanna V, Prabha TN, Tharanathan RN. Fruit Ripening Phenomena–An Overview. Crit Rev Food Sci Nutr 2007;47:1–19. [CrossRef]
- Song S, Abubaker MA, Akhtar M, Elimam AM, Zhu X, Zhang J. Chemical Characterization Analysis, Antioxidants, and Anti-Diabetic Activity of Two Novel Acidic Water-Soluble Polysaccharides Isolated from Baobab Fruits. Foods 2024;13. [CrossRef]
- Moumou M, Mokhtari I, Tayebi A, Milenkovic D, Amrani S, Harnafi H. Immature carob pods extract and its fractions prevent lipid metabolism disorders and lipoprotein-rich plasma oxidation in mice: A phytochemical and pharmacological study. J Ethnopharmacol 2023:117557. [CrossRef]
- Ouzounidou G, Vekiari S, Asfi M, Gork MG, Sakcali MS, Ozturk M. PHOTOSYNTHETIC CHARACTERISTICS OF CAROB TREE (CERATONIA SILIQUA L.) AND CHEMICAL COMPOSITION OF ITS FRUIT ON DIURNAL AND SEASONAL BASIS. Pak J Bot 2012;44:1689–95.
- Sebai H, Souli A, Chehimi L, Rtibi K, Amri M, El-Benna J, et al. In vitro and in vivo antioxidant properties of Tunisian carob (Ceratonia siliqua L.). Journal of Medicinal Plants Research 2013;7:85–90. [CrossRef]
- Gull J, Sultana B, Anwar F, Naseer R, Ashraf M, Ashrafuzzaman M. Variation in Antioxidant Attributes at Three Ripening Stages of Guava (Psidium guajava L.) Fruit from Different Geographical Regions of Pakistan. Molecules 2012, Vol 17, Pages 3165-3180 2012;17:3165–80. [CrossRef]
- Ben Othmen K, Elfalleh W, Lachiheb B, Haddad M. Evolution of phytochemical and antioxidant activity of Tunisian carob ( Ceratonia siliqua L.) pods during maturation . Eurobiotech J 2019;3:135–42. [CrossRef]
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry 2000;55:481–504. [CrossRef]
- Saci F, Bachir bey M, Louaileche H, Gali L, Bensouici C. Changes in anticholinesterase, antioxidant activities and related bioactive compounds of carob pulp (Ceratonia siliqua L.) during ripening stages. Journal of Food Measurement and Characterization 2020;14:937–45. [CrossRef]
- Saci F, Bachir bey M, Louaileche H, Gali L, Bensouici C. Changes in anticholinesterase, antioxidant activities and related bioactive compounds of carob pulp (Ceratonia siliqua L.) during ripening stages. Journal of Food Measurement and Characterization 2020;14:937–45. [CrossRef]
- Kim I, Lee J. Variations in anthocyanin profiles and antioxidant activity of 12 genotypes of mulberry (Morus spp.) fruits and their changes during processing. Antioxidants 2020;9. [CrossRef]
- Saensouk S, Senavongse R, Papayrata C, Chumroenphat T. Evaluation of Color, Phytochemical Compounds and Antioxidant Activities of Mulberry Fruit (Morus alba L.) during Ripening. Horticulturae 2022;8. [CrossRef]
- Dahmani W, Elaouni N, Abousalim A, Akissi ZLE, Legssyer A, Ziyyat A, et al. Exploring Carob (Ceratonia siliqua L.): A Comprehensive Assessment of Its Characteristics, Ethnomedicinal Uses, Phytochemical Aspects, and Pharmacological Activities. Plants 2023, Vol 12, Page 3303 2023;12:3303. [CrossRef]
- Jafri SAA, Khalid ZM, Khan MZ, Jogezai NU. Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan. Open Chem 2022;20:1337–56. [CrossRef]
- Ferreira-Santos P, Nobre C, Rodrigues RM, Genisheva Z, Botelho C, Teixeira JA. Extraction of phenolic compounds from grape pomace using ohmic heating: Chemical composition, bioactivity and bioaccessibility. Food Chem 2024;436:137780. [CrossRef]
- Inga M, Betalleluz-Pallardel I, Puma-Isuiza G, Cumpa-Arias L, Osorio C, Valdez-Arana J-D-C, et al. Chemical analysis and bioactive compounds from agrifood by-products of peruvian crops. Front Sustain Food Syst 2024;8:1341895. [CrossRef]
- Can-Cauich CA, Sauri-Duch E, Betancur-Ancona D, Chel-Guerrero L, González-Aguilar GA, Cuevas-Glory LF, et al. Tropical fruit peel powders as functional ingredients: Evaluation of their bioactive compounds and antioxidant activity. J Funct Foods 2017;37:501–6. [CrossRef]
- Rubio S, Quintana J, Eiroa JL, Triana J, Estévez F. Acetyl derivative of quercetin 3-methyl ether-induced cell death in human leukemia cells is amplified by the inhibition of ERK. Carcinogenesis 2007;28:2105–13. [CrossRef]
- Lee EH, Kim HJ, Song YS, Jin C, Lee KT, Cho J, et al. Constituents of the stems and fruits ofOpuntia ficus-indica var.saboten. Arch Pharm Res 2003;26:1018–23. [CrossRef]
- Takeara R, Albuquerque S, Lopes NP, Callegari Lopes JL. Trypanocidal activity of Lychnophora staavioides Mart. (Vernonieae, Asteraceae). Phytomedicine 2003;10:490–3. [CrossRef]
- Wei BL, Lu CM, Tsao LT, Wang JP, Lin CN. In vitro anti-inflammatory effects of quercetin 3-O-methyl ether and other constituents from Rhamnus species. Planta Med 2001;67:745–7. [CrossRef]
- Kim SH, Naveen Kumar C, Kim HJ, Kim DH, Cho J, Jin C, et al. Glucose-containing flavones—their synthesis and antioxidant and neuroprotective activities. Bioorg Med Chem Lett 2009;19:6009–13. [CrossRef]
- Li J, Mottamal M, Li H, Liu K, Zhu F, Cho Y-Y, et al. Quercetin-3-methyl ether suppresses proliferation of mouse epidermal JB6 P1 cells by targeting ERKs. Carcinogenesis 2012;33:459–65. [CrossRef]
- Motta EVS, Lemos M, Costa JC, Banderó-Filho VC, Sasse A, Sheridan H, et al. Galloylquinic acid derivatives from Copaifera langsdorffii leaves display gastroprotective activity. Chem Biol Interact 2017;261:145–55. [CrossRef]
- Hassani S, Ghanbari F, Lotfi M, Alam W, Aschner M, Popović-Djordjević J, et al. How gallic acid regulates molecular signaling: role in cancer drug resistance. Medical Oncology 2023 40:11 2023;40:1–18. [CrossRef]
- Bhuia MS, Rahaman MM, Islam T, Bappi MH, Sikder MI, Hossain KN, et al. Neurobiological effects of gallic acid: current perspectives. Chinese Medicine 2023 18:1 2023;18:1–19. [CrossRef]
- Bouaouda K, Elagdi C, El Hachlafi N, Mohtadi K, Hsaine M, Kettani A, et al. HPLC-UV-MS/MS Profiling of Phenolics from Euphorbia nicaeensis (All.) Leaf and Stem and Its Antioxidant and Anti-Protein Denaturation Activities. Progress In Microbes & Molecular Biology 2023;6. [CrossRef]
- Agarwal P, Gupta R. Alpha-amylase inhibition can treat diabetes mellitus. Research and Reviews Journal of Medical and Health Sciences RRJMHS 2016;5.
- Custódio L, Patarra J, Alberício F, Neng NR, Nogueira JMF, Romano A. In vitro antioxidant and inhibitory activity of water decoctions of carob tree (Ceratonia siliqua L.) on cholinesterases, α-amylase and α-glucosidase. Nat Prod Res 2015;29:2155–9. [CrossRef]
- Darwish WS, Khadr AES, Kamel MAEN, Abd Eldaim MA, El Sayed IET, Abdel-Bary HM, et al. Phytochemical Characterization and Evaluation of Biological Activities of Egyptian Carob Pods (Ceratonia siliqua L.) Aqueous Extract: In Vitro Study. Plants 2021, Vol 10, Page 2626 2021;10:2626. [CrossRef]
- Kamtekar S, Keer V, Patil V. Estimation of Phenolic content, Flavonoid content, Antioxidant and Alpha amylase Inhibitory Activity of Marketed Polyherbal Formulation. J Appl Pharm Sci 2014;4;061–5. [CrossRef]
- Hanefeld M. The Role of Acarbose in the Treatment of Non–Insulin-Dependent Diabetes Mellitus. J Diabetes Complications 1998;12:228–37. [CrossRef]
- Subramanian R, Asmawi MZ, Sadikun A. In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. 2008.
- Reddy NVLS, Anarthe SJ, Raghavendra NM. In Vitro Antioxidant and Antidiabetic activity of Asystasia gangetica (Chinese Violet) Linn. (Acanthaceae). International Journal of Research in Pharmaceutical and Biomedical Sciences 2010;1.
- Picariello G, Sciammaro L, Siano F, Volpe MG, Puppo MC, Mamone G. Comparative analysis of C-glycosidic flavonoids from Prosopis spp. and Ceratonia siliqua seed germ flour. Food Research International 2017;99:730–8. [CrossRef]
- Qasem MA, Noordin MI, Arya A, Alsalahi A, Jayash SN. Evaluation of the glycemic effect of Ceratonia siliqua pods (Carob) on a streptozotocin-nicotinamide induced diabetic rat model. PeerJ 2018;2018:e4788. [CrossRef]
- Zheng Y, Tian J, Yang W, Chen S, Liu D, Fang H, et al. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem 2020;317:126346. [CrossRef]
- Singh A, Singh K, Sharma A, Kaur K, Kaur K, Chadha R, et al. Recent developments in synthetic α-glucosidase inhibitors: A comprehensive review with structural and molecular insight. J Mol Struct 2023;1281. [CrossRef]
- Ferreira LLG, Andricopulo AD. ADMET modeling approaches in drug discovery. Drug Discov Today 2019;24:1157–65. [CrossRef]
- Srivastava V, Yadav A, Sarkar P. Molecular docking and ADMET study of bioactive compounds of Glycyrrhiza glabra against main protease of SARS-CoV2. Mater Today Proc 2022;49:2999–3007. [CrossRef]
- Farihi A, Bouhrim M, Chigr F, Elbouzidi A, Bencheikh N, Zrouri H, et al. Exploring Medicinal Herbs’ Therapeutic Potential and Molecular Docking Analysis for Compounds as Potential Inhibitors of Human Acetylcholinesterase in Alzheimer’s Disease Treatment. Medicina (Lithuania) 2023;59. [CrossRef]
- Purushothaman K, Sivasankar E, Krishnamoorthy M, Karunakaran K, Muniyan R. Computational identification of potential tau tubulin kinase 1 (TTBK1) inhibitors: a structural analog approach. In Silico Pharmacology 2024 12:2 2024;12:1–11. [CrossRef]
- Stillhart C, Vučićević K, Augustijns P, Basit AW, Batchelor H, Flanagan TR, et al. Impact of gastrointestinal physiology on drug absorption in special populations––An UNGAP review. European Journal of Pharmaceutical Sciences 2020;147:105280. [CrossRef]
- Taşçıoğlu N, Saatçi Ç, Emekli R, Tuncel G, Eşel E, Dundar M. Investigation of cytochrome p450 CYP1A2, CYP2D6, CYP2E1 and CYP3A4 gene expressions and polymorphisms in alcohol withdrawal. Klinik Psikiyatri Dergisi 2021;24:298–306. [CrossRef]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric Method for Determination of Sugars and Related Substances. Anal Chem 1956;28:350–6. [CrossRef]
- Tavarini S, Degl’Innocenti E, Remorini D, Massai R, Guidi L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem 2008;107:282–8. [CrossRef]
- Lamaison JL, Petitjen-Freytet C, Carnat A. Teneurs en acide rosmarinique, en dérivés hydroxycinnamiques totaux et activité antioxydante chez les apiacées, les borraginacées et les laminacées médicinales. Ann Pharm Fr, vol. 48, 1990, p. 103–8.
- Broadhurst RB, Jones WT. Analysis of condensed tannins using acidified vanillin. J Sci Food Agric 1978;29:788–94. [CrossRef]
- Szabo K, Zorit a D, Adriana-Florinela Catoi, Vodnar DC. Screening of Ten Tomato Varieties Processing Waste for Bioactive Components and Their Related Antioxidant and Antimicrobial Activities. Antioxidants 2019;8(8), 292.
- Uysal S, Zengin G, Aktumsek A, Karatas S. Chemical and biological approaches on nine fruit tree leaves collected from the Mediterranean region of Turkey. J Funct Foods 2016;22:518–32. [CrossRef]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 1996;239:70–6. [CrossRef]
- Ma T, Tian C, Luo J, Zhou R, Sun X, Ma J. Influence of technical processing units on polyphenols and antioxidant capacity of carrot (Daucus carrot L.) juice. Food Chem 2013;141:1637–44. [CrossRef]
- Guide for the care and use of laboratory animals. National Academies Press; 2011.
- Elrherabi A, Bouhrim M, Abdnim R, Berraaouan A, Ziyyat A, Mekhfi H, et al. Antihyperglycemic potential of the Lavandula stoechas aqueous extract via inhibition of digestive enzymes and reduction of intestinal glucose absorption. J Ayurveda Integr Med 2023;14:100795. [CrossRef]
- Srivastava V, Yadav A, Sarkar P. Molecular docking and ADMET study of bioactive compounds of Glycyrrhiza glabra against main protease of SARS-CoV2. Mater Today Proc 2022;49:2999. [CrossRef]
- Zrouri H, Elbouzidi A, Bouhrim M, Bencheikh N, Kharchoufa L, Ouahhoud S, et al. Phytochemical analysis, antioxidant activity, and nephroprotective effect of the Raphanus sativus aqueous extract. Mediterranean Journal of Chemistry 2021;11:84. [CrossRef]
- Siddique MH, Ashraf A, Hayat S, Aslam B, Fakhar-e-Alam M, Muzammil S, et al. Antidiabetic and antioxidant potentials of Abelmoschus esculentus: In vitro combined with molecular docking approach. Journal of Saudi Chemical Society 2022;26:101418. [CrossRef]
- Mendie LE, Hemalatha S. Molecular Docking of Phytochemicals Targeting GFRs as Therapeutic Sites for Cancer: an In Silico Study. Appl Biochem Biotechnol 2022;194:215–31. [CrossRef]
- Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 2017;7. [CrossRef]
- van de Waterbeemd H, Gifford E. ADMET in silico modelling: towards prediction paradise? Nat Rev Drug Discov 2003;2:192–204. [CrossRef]
| Total sugar mg GE/100 g FM | |||
|---|---|---|---|
| TG | TM | AW | |
| M1 | 1720±76.22dA | 1709±72.63cA | 1889 ± 124.8bA |
| M2 | 1814±84.36cdA | 1721±91.97bcA | 1636 ± 36.63cA |
| M3 | 1880±7.31bcB | 1987±71.89aA | 1982 ±55.23abA |
| M4 | 2032±22.35bA | 1880±25.34abA | 1940±27.93abA |
| M5 | 2134±56.23aA | 1904 ± 20.8aA | 2098 ±34.57aA |
| TPC mg GAE/100g FM | TFC mg QE/100g FM | TCT mg CE/100g FM | |||||||
| TG | TN | AW | TG | TN | AW | TG | TN | AW | |
| M1 | 2813 ± 58.02bA |
3014 ± 59.13bA |
2734 ± 99.24aA |
826.2 ± 40.77bB |
298.7 ± 20.1cC |
1423 ± 78.47aA |
613.9 ± 74.18bA |
231.2 ± 69.86cB |
567.9 ± 21.35bA |
| M2 | 3475 ± 189.6aB |
3819 ± 226.4aA |
2765 ± 144.3aC |
925.4 ± 13.27aAB |
1034 ± 57.08aA |
830.1 ± 87.62bB |
848.1 ± 18.91aB |
1472 ± 28.46aA |
756.1 ± 92.54 aC |
| M3 | 2750 ± 98.37bcA |
2368 ± 43.12cB |
2209 ± 86.94bC |
900.6 ± 65.7abA |
827.5 ± 49.6bA |
848.4 ± 52.7bA |
628.5 ± 93.07bA |
633.7 ± 61.17bA |
539.6 ± 35.26bB |
| M4 | 2532 ± 48.3cA |
2120 ± 150.9cB |
1947 ± 55.37cB |
173.2 ± 11.25dA |
196.2 ± 10.96dA |
161.2 ± 13.73cA |
724.7 ± 22.69abA |
706.9 ± 37.82bAB |
565.8 ± 18.38bB |
| M5 | 2744 ± 39.47bcA |
2391 ± 76.58cC |
2595 ± 91.21aB |
294 ± 12.06cA |
249.6 ± 16.76cdA |
305.6 ± 11.95cA |
75.71 ± 5.52cA |
71.42 ± 3.32dA |
77.17 ± 6.11cA |
| N° | Compounds | Chemical formula | RT (min) | %AIR | Ref | ||
|---|---|---|---|---|---|---|---|
| TM | AW | TG | |||||
| 1 | 3-O-p-coumaroyl 5-O-caffeoylquinic acid | C25H24O11 | 4.28 | 53.40 | 63.62 | 55.84 | N |
| 2 | Quercetin 3-methyl ether | C16H12O7 | 5.26 | 13.87 | 12.91 | 13.83 | N |
| 3 | Gallic acid | C7H6O5 | 6.60 | 11.24 | 5.17 | 2.35 | S |
| 4 | 3,4-Dicaffeoylquinic acid | C25H24O12 | 8.43 | 3.04 | 2.85 | 5.06 | N |
| 5 | Galloylquinic acid | C14H16O10 | 10.95 | 12.55 | n.d | n.d | (1) |
| 6 | Ferulic acid | C10H10O4 | 13.52 | 0.34 | 0.37 | 0.60 | S |
| 7 | Catechine | C15H14O6 | 17.84 | n.d | 0.37 | n.d | S |
| Doses Symptoms |
2 g/kg | 1 g/kg | 0.5 g/kg |
|---|---|---|---|
| Locomotion and mobility | Normal | Normal | Normal |
| Hair loss or erection | - | - | - |
| Diarrhea | - | - | - |
| Abnormal agitation | - | - | - |
| Anorexia | - | - | - |
| Spontaneous startle | - | - | - |
| Isolation in the corner | - | - | - |
| Mortality | - | - | - |
| Body weight gain | Normal | Normal | Normal |
| Extraits | IC50 µg/mL |
|---|---|
| TG | 0,591 ± 0.104a |
| TM | 0,450 ± 0.239 ab |
| AW | 0,405 ± 0.247 ab |
| Acarbose (control) | 0,098 ± 0.004 b |
| Extraits | IC50 µg/mL |
| TG | 0,063 ± 0.002 b |
| TM | 0,086 ± 0.014 a |
| AW | 0,065 ± 0.004 b |
| Acarbose (control) | 0,089 ± 0.004 a |
|
α-amylase (PDB:1B2Y) |
α-glucosidase (PDB: 5NN8) |
|||
|
Compound name |
Affinity (Kcal/mol) |
Interaction Site |
Affinity (Kcal/mol) |
Interaction Site |
| Acarbose (Standard inhibitor) |
-8.2 | Tyr 2, Ser 3, Pro 4, Thr 6, Gln 8, Phe 222, Arg 252, Pro 332, Gly 334, Arg 398. | -7.2 | Asp 356, Val 357, Arg 608, His 584, His 717, Leu 865, Glu 866. |
| 3-O-p-Coumaroyl-5-O-caffeoylquinic acid | -9.2 | Pro 4, Tyr 6, Arg 252, Trp 280, His 331, Pro 332, Arg 398, Arg 421 | -8.2 | Lys 348, His 708, Arg 725, Ala 749, Ile 823 Glu 856 |
| Quercetin 3-methyl ether | -8.2 | Arg 267, Arg 303, Thr 314, Arg 346, Asp 356 | -7.1 | Met 363, Arg 585, Arg 594 |
| Gallic acid | -5.9 | Val 129, Gly 181, Tyr 182 | -5.8 | His 708, Gln 715, Glu 748, Ala 749, Ile 823, Glu 856. |
| 3,4-Dicaffeoylquinic acid | -8.2 | Pro4, Gln 8, Gly 9, Pro 332, Phe 335, Thy 336, Arg 398, Asp 402, Arg 421 | -7.9 | Met 363, Arg 585, Arg 594, Tyr 609, His 717, Glu 866, |
| Galloylquinic acid | -7.2 | Arg 10, Arg 252, Ser 289, Gly 334, Asp 402, Gly 403 | -6.4 | Leu 195, Arg 585, Tyr 609 |
| Ferulic acid | -6.3 | Arg 552, Ser 289, Pro 332, Phe 335, Arg 424 | -6.6 | His 708, Glu 721, Arg 725, Tyr 822, Gly 855, Glu 856 |
| Catechin | -7.8 | Asn 301, Ile 312, Thr 314, Asp 317, Arg 346, Asn 352 | -6.8 | Pro 194, Leu 195, Phe 490, Thr 491, Leu 577, Ile 581, Tyr 609 |
| Physicochemical Properties | Lipophilicity | Druglikeness | Pharmacokinetics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | MW g/mol | HBA | HBD | TPSA Ų | ROTB | MlogP | WlogP | Lipinski’s | Verber’s | GI absorption | BBB Permea- tion |
CYP1A2 Inhibitor |
| 3-O-p-Coumaroyl-5-O-caffeoylquinic acid | 500.4 | 11 | 6 | 191.0 | 9 | 0.1 | 1.1 | 3 | 1 | Low | No | No |
| Quercetin 3-methyl ether | 316.2 | 7 | 4 | 120.3 | 2 | -0.3 | 2.2 | 0 | 0 | High | No | No |
| Gallic acid | 170.1 | 5 | 4 | 97.9 | 1 | -0.1 | 0.5 | 0 | 0 | High | No | No |
| 3,4-Dicaffeoylquinic acid | 516.4 | 12 | 7 | 211.2 | 9 | -0.3 | 0.8 | 3 | 1 | Low | No | No |
| Galloylquinic acid | 344.2 | 10 | 8 | 195.8 | 3 | -2.7 | -2.1 | 1 | 1 | Low | No | No |
| Ferulic acid | 194.1 | 4 | 2 | 66.7 | 3 | 1.0 | 1.3 | 0 | 0 | High | Yes | No |
| Catechin | 290.2 | 6 | 5 | 110.3 | 1 | 0.2 | 1.2 | 0 | 0 | High | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





