Submitted:
22 August 2024
Posted:
23 August 2024
You are already at the latest version
Abstract
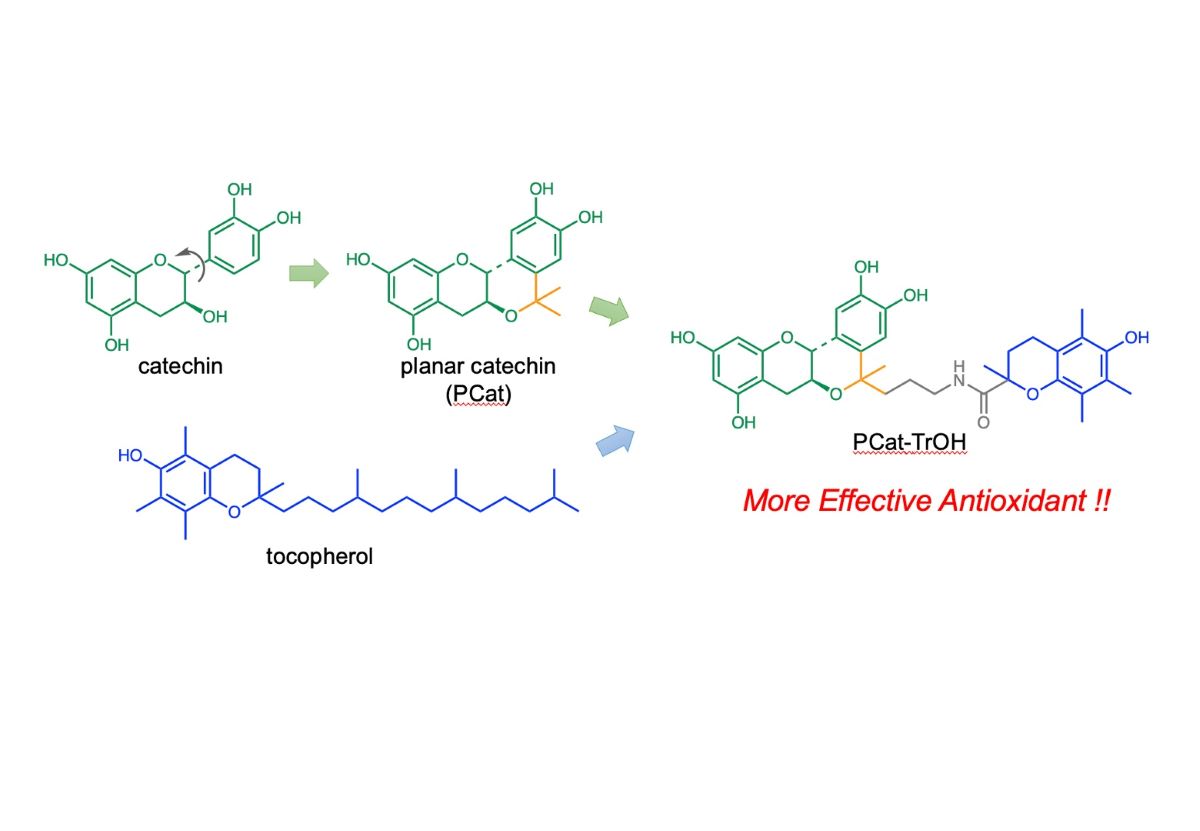
Keywords:
1. Introduction
2. Materials and Methods
2.1. General Methods
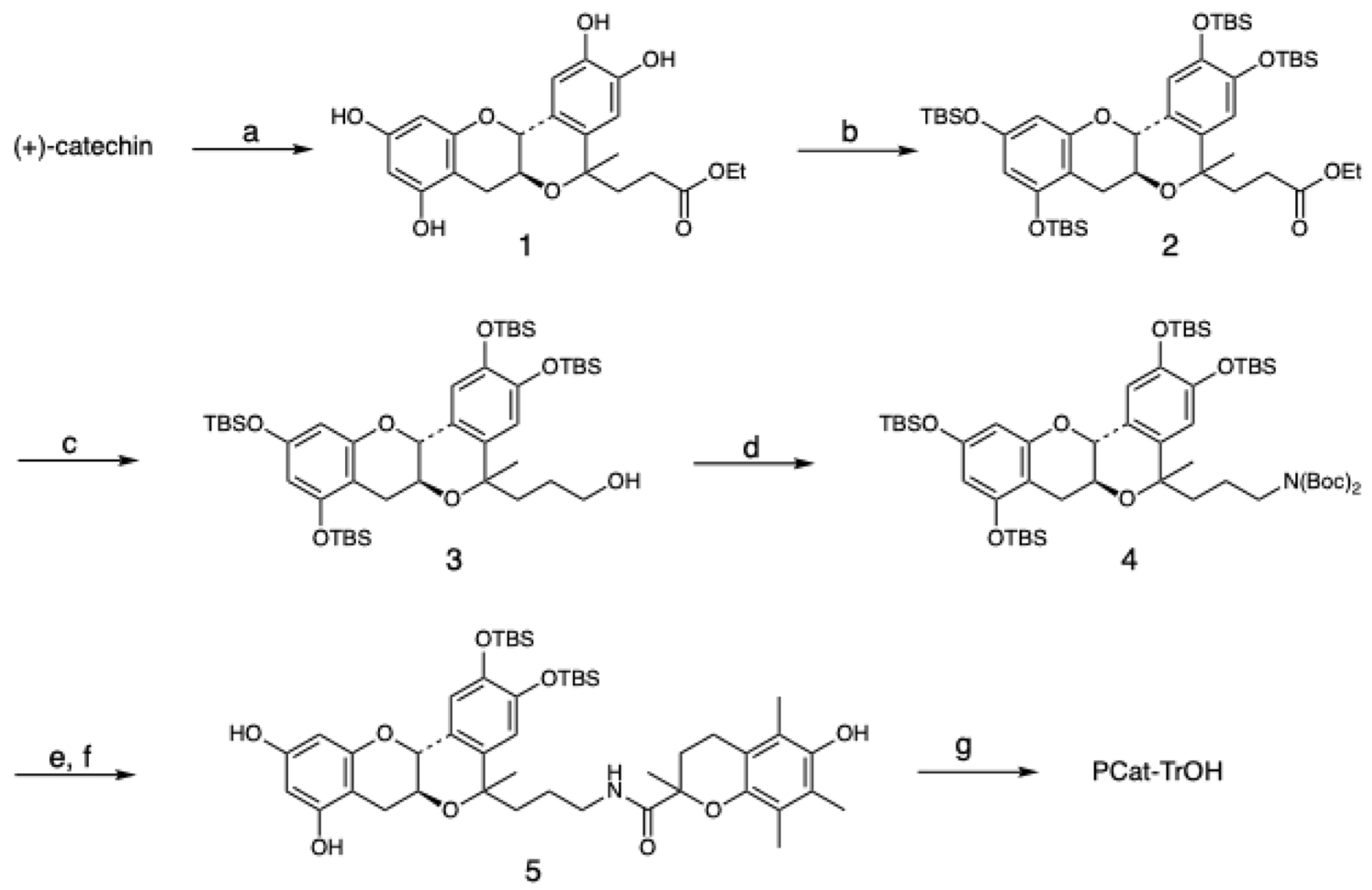
2.2. Synthesis of PCat-TrOH
2.2.1. Ethyl 3-((6aS,12aR)-2,3,8,10-tetrahydroxy-5-methyl-5,6a,7,12a-tetrahydroisochromeno [4,3-b]chromen-5-yl)propanoate (1)
2.2.2. Ethyl 3-((6aS,12aR)-2,3,8,10-tetrakis((tert-butyldimethylsilyl)oxy)-5-methyl-5,6a,7,12a-tetrahydroisochromeno[4,3-b]chromen-5-yl)propanoate (2)
2.2.3. 3-((6aS,12aR)-2,3,8,10-tetrakis((tert-butyldimethylsilyl)oxy)-5-methyl-5,6a,7,12a-tetrahydroisochromeno[4,3-b]chromen-5-yl)propan-1-ol (3)
2.2.4. tert-butyl (tert-butoxycarbonyl)(3-((6aS,12aR)-2,3,8,10-tetrakis((tert-butyldimethylsilyl)oxy)-5-methyl-5,6a,7,12a-tetrahydroisochromeno[4,3-b]chromen-5-yl)propyl)carbamate (4)
2.2.5. N-(3-((6aS,12aR)-2,3-bis((tert-butyldimethylsilyl)oxy)-8,10-dihydroxy-5-methyl-5,6a,7,12a-tetrahydroisochromeno[4,3-b]chromen-5-yl)propyl)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxamide (5)
2.2.6. 6-hydroxy-2,5,7,8-tetramethyl-N-(3-((6aS,12aR)-2,3,8,10-tetrahydroxy-5-methyl-5,6a,7,12a-tetrahydroisochromeno[4,3-b]chromen-5-yl)propyl)chromane-2-carboxamide (PCat–TrOH)
2.3. Antioxidant activity measurements
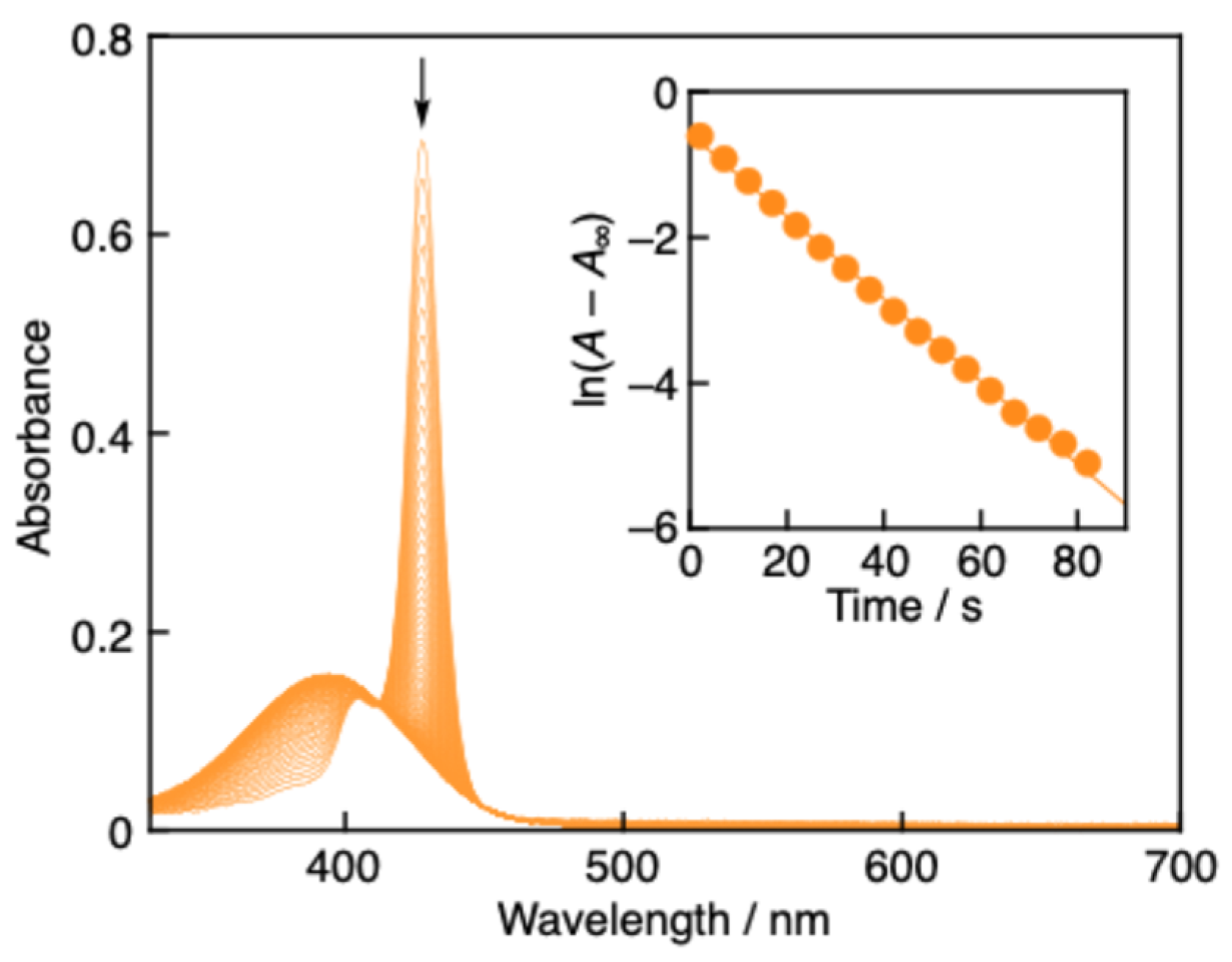
2.4. Spectral titrations
3. Results
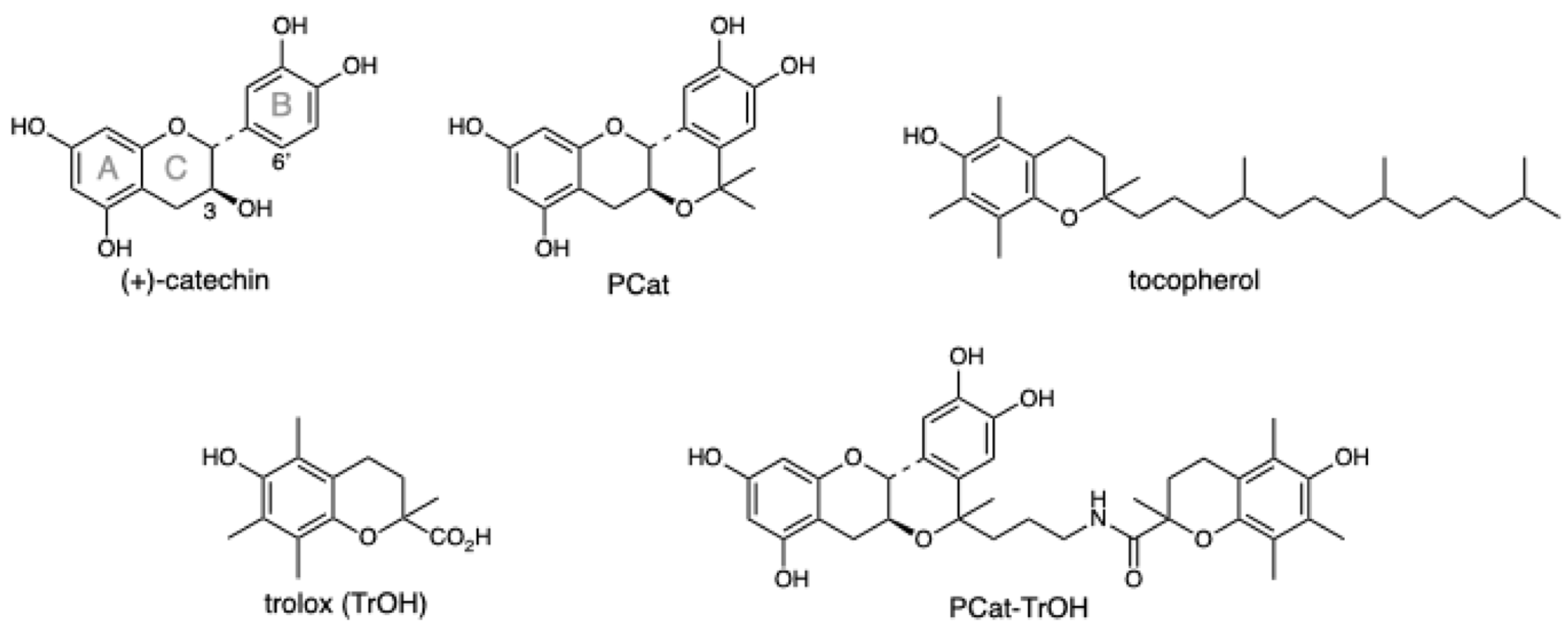
3.1. Chemistry
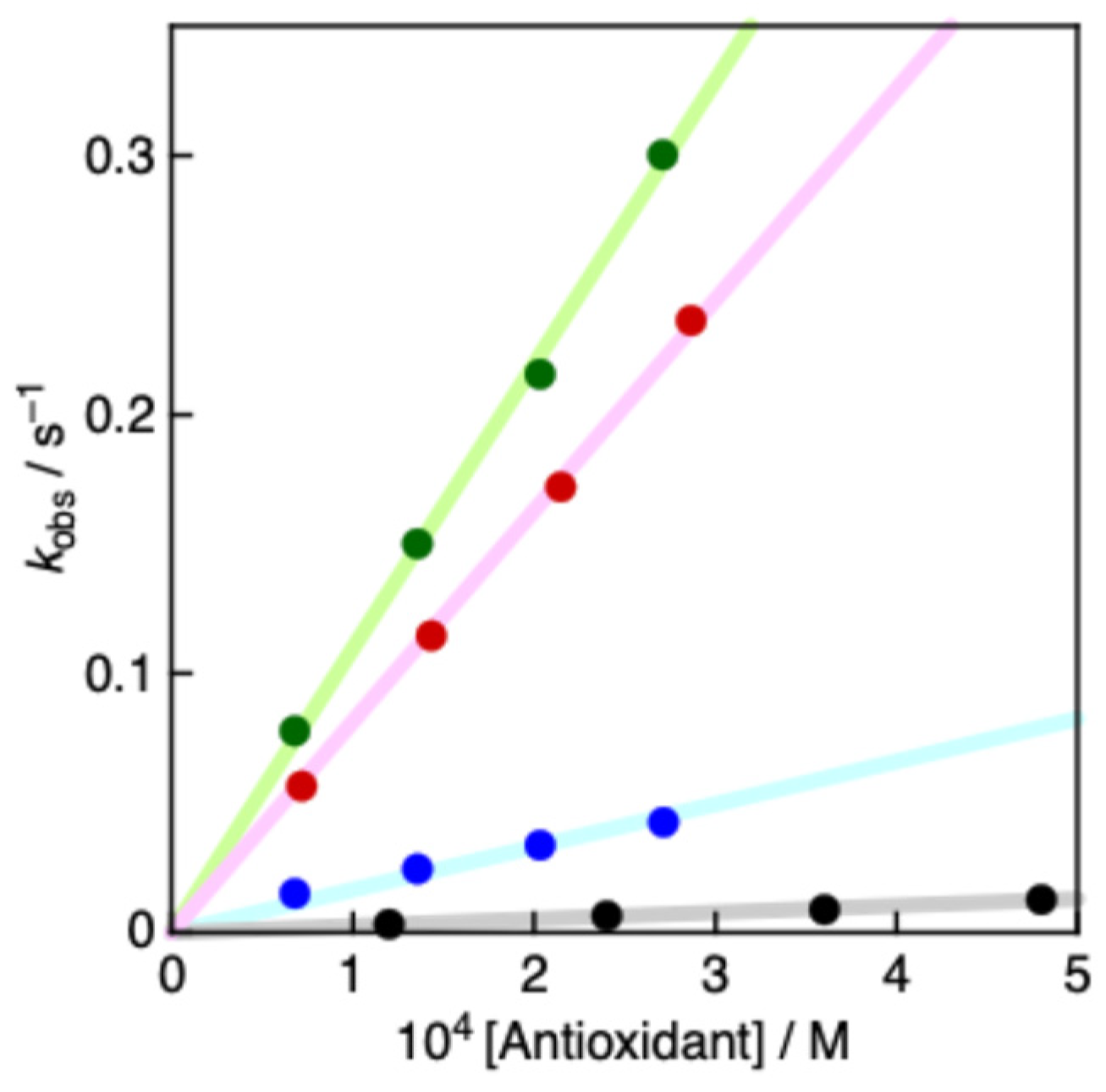
2.4. Radical Scavenging Activity
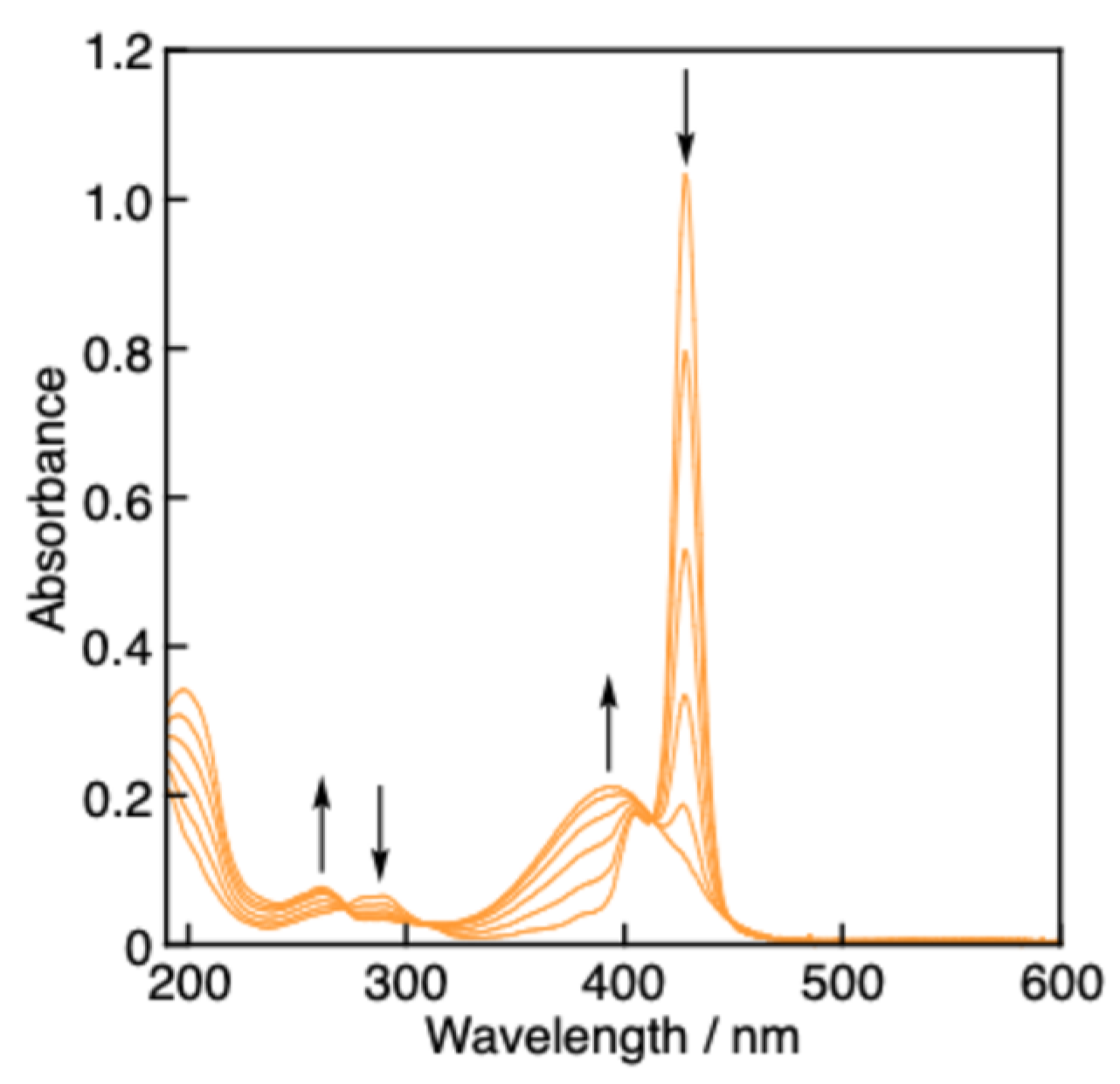
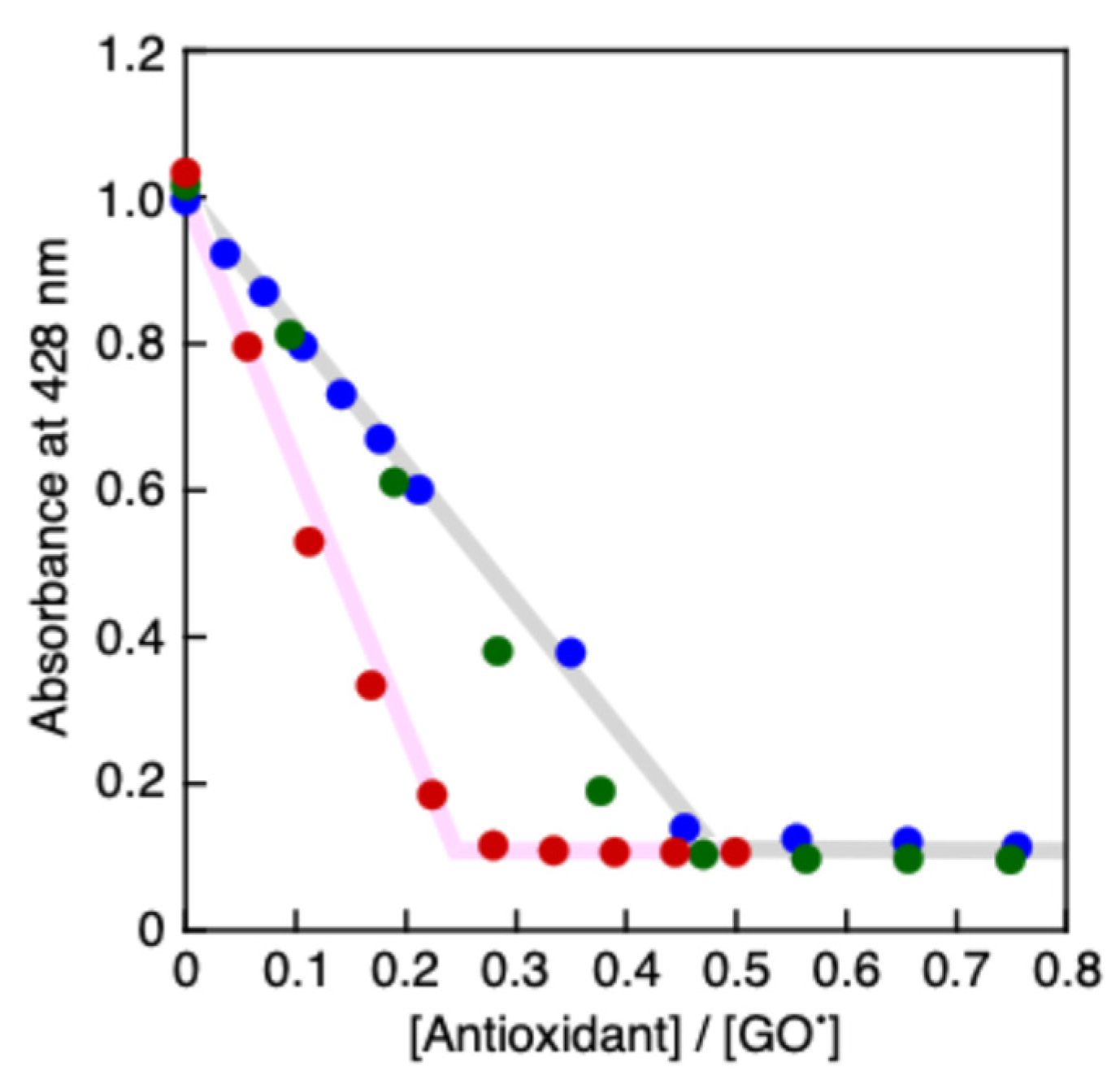
2.4. Spectral titrations
3. Discussions
3. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feitosa, C. M.; da Silva Oliveira, G. L.; do Nascimento Cavalcante, A.; Morais Chaves, S. K.; Rai, M. , Determination of Parameters of Oxidative Stress in vitro Models of Neurodegenerative Diseases-A Review. Curr Clin Pharmacol 2018, (2), 100–109. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D. M.; Niculescu, A. G. ; Lungu, II; Radu, C. I.; Vladacenco, O.; Roza, E.; Costachescu, B.; Grumezescu, A. M.; Teleanu, R. I., An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int J Mol Sci, 2022; 23. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M. R.; Yu, E. , The Role of Oxidative Stress in Atherosclerosis. Cells, 2022; 11. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Zeng, Y.; Mo, X.; Hong, S.; He, H.; Li, J.; Fatima, S.; Liu, Q. , Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci Rep 2023, (1), 15515. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Osko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczynska, K. , Mitochondrial Oxidative Stress-A Causative Factor and Therapeutic Target in Many Diseases. Int J Mol Sci, 2021; 22. [Google Scholar] [CrossRef]
- Bhatti, J. S.; Bhatti, G. K.; Reddy, P. H. , Mitochondrial dysfunction and oxidative stress in metabolic disorders - A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Forman, H. J.; Zhang, H. , Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S. V.; Simic, M. G. , Antioxidants in nutrition. Ann N Y Acad Sci 2000, 899, 326–34. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Curcio, M.; Vittorio, O.; Iemma, F.; Restuccia, D.; Spizzirri, U. G.; Puoci, F.; Picci, N. , Polyphenol Conjugates and Human Health: A Perspective Review. Crit Rev Food Sci Nutr 2016, 56, 326–37. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Narvaez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Minno, G. D.; Ritieni, A. , Red Wine Consumption and Cardiovascular Health. Molecules, 2019; 24. [Google Scholar] [CrossRef]
- Liberale, L.; Bonaventura, A.; Montecucco, F.; Dallegri, F.; Carbone, F. , Impact of Red Wine Consumption on Cardiovascular Health. Curr Med Chem 2019, 26, 3542–3566. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D. M. , The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules, 2018; 23. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S. J.; Khan, J.; Dukhyil, A. B.; Ansari, M. A.; Alomary, M. N.; Alshabrmi, F. M.; Palai, S.; Deb, P. K.; Devi, R. , Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front Pharmacol 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, K.; Nakanishi, I.; Kansui, H.; Sugiyama, E.; Kimura, M.; Shimada, T.; Urano, S.; Yamaguchi, K.; Miyata, N. , Enhanced radical-scavenging activity of a planar catechin analogue. J Am Chem Soc 2002, 124, 5952–3. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Mori, K.; Misawa, T.; Takaki, T.; Demizu, Y.; Shibanuma, M.; Fukuhara, K. , Inhibition of beta-amyloid-induced neurotoxicity by planar analogues of procyanidin B3. Bioorg Med Chem Lett 2019, 29, 2659–2663. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Mori, K.; Tsuchiya, K.; Takaki, T.; Misawa, T.; Demizu, Y.; Shibanuma, M.; Fukuhara, K. , Design, Synthesis, and Biological Activity of Conformationally Restricted Analogues of Silibinin. ACS Omega 2020, 5, 23164–23174. [Google Scholar] [CrossRef]
- Mustacich, D. J.; Bruno, R. S.; Traber, M. G. , Vitamin E. Vitam Horm 2007, 76, 1–21. [Google Scholar] [PubMed]
- Niki, E. , Interaction of ascorbate and alpha-tocopherol. Ann N Y Acad Sci 1987, 498, 186–99. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Stahl, W.; Sundquist, A. R. , Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann N Y Acad Sci, 1992; 669, 7–20. [Google Scholar]
- Salvagno, M.; Sterchele, E. D.; Zaccarelli, M.; Mrakic-Sposta, S.; Welsby, I. J.; Balestra, C.; Taccone, F. S. , Oxidative Stress and Cerebral Vascular Tone: The Role of Reactive Oxygen and Nitrogen Species. Int J Mol Sci, 2024; 25. [Google Scholar] [CrossRef]
- La Torre, M. E.; Villano, I.; Monda, M.; Messina, A.; Cibelli, G.; Valenzano, A.; Pisanelli, D.; Panaro, M. A.; Tartaglia, N.; Ambrosi, A.; Carotenuto, M.; Monda, V.; Messina, G.; Porro, C. , Role of Vitamin E and the Orexin System in Neuroprotection. Brain Sci, 2021; 11. [Google Scholar]
- Engin, K. N. , Alpha-tocopherol: looking beyond an antioxidant. Mol Vis 2009, 15, 855–60. [Google Scholar] [PubMed]
- Mesa, T.; Munne-Bosch, S. , alpha-Tocopherol in chloroplasts: Nothing more than an antioxidant? Curr Opin Plant Biol 2023, 74, 102400. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, I.; Fukuhara, K.; Shimada, T.; Ohkubo, K.; Iizuka, Y.; Inami, K.; Mochizuki, M.; Urano, S.; Miyata, N.; Fukuzumi, S. , Effect of magnesiumu ion on kinetic stability and spin distribution of phenoxyl radical derived from a vitamin E analogue: mechanistic insight into antioxidative hydrogen-transfer reaction of vitamin E. J, Chem. Soc., Perkin Trans. 2002; 2, 1520–1524. [Google Scholar]
- Smirnoff, N. , Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic Biol Med 2018, 122, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, I.; Fukuhara, K.; Ohkubo, K.; Shimada, T.; Kansui, H.; Kurihara, M.; Urano, S.; Fukuzumi, S.; Miyata, N. , Superoxide anion generation via electron-transfer oxidation of catechin dianion by molecular oxygen in an aprotic medium. Chemistry Letters 2001, 1152–1153. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




