Submitted:
16 August 2024
Posted:
19 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
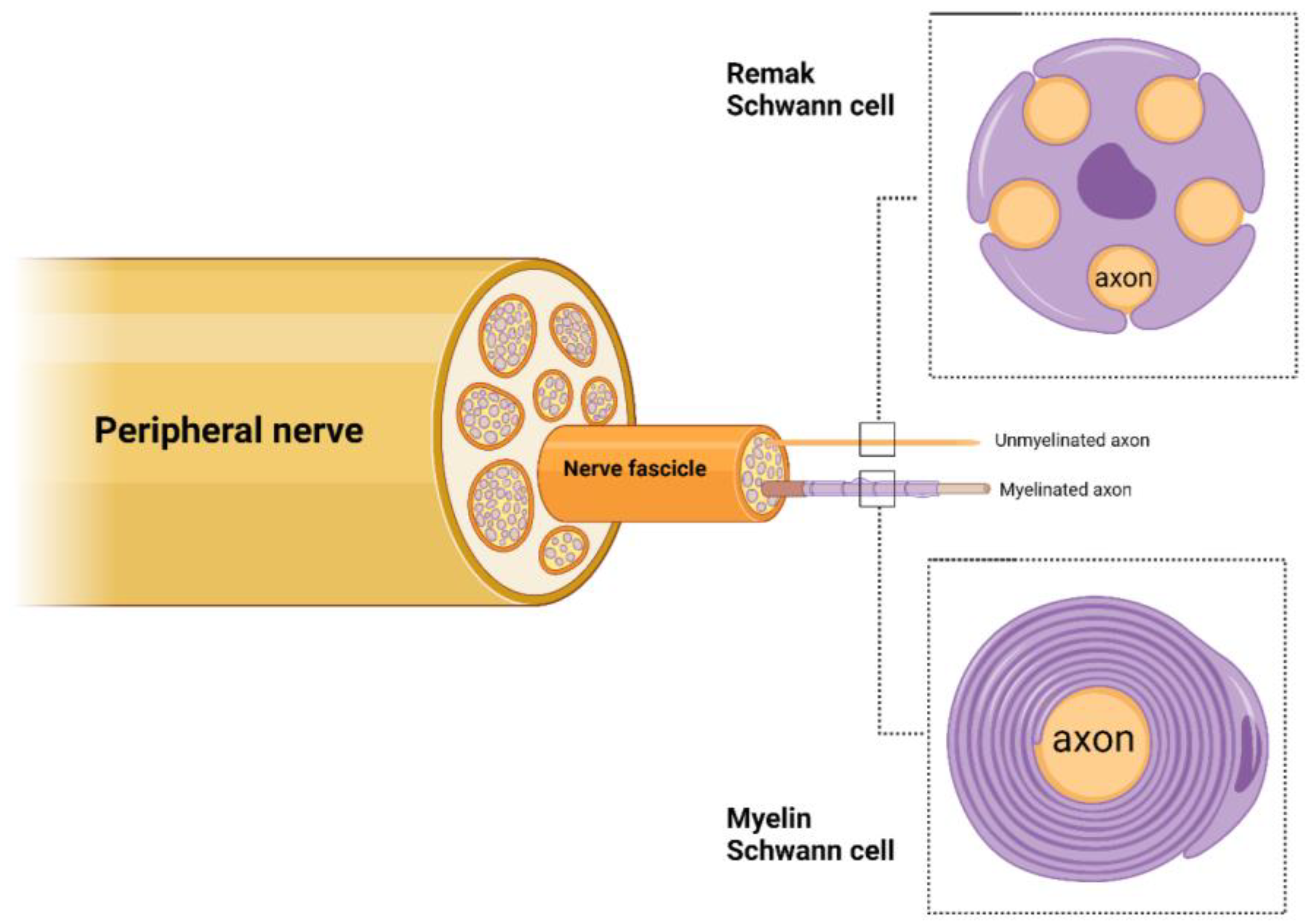
2. Schwann Cell Roles in Peripheral Nerves
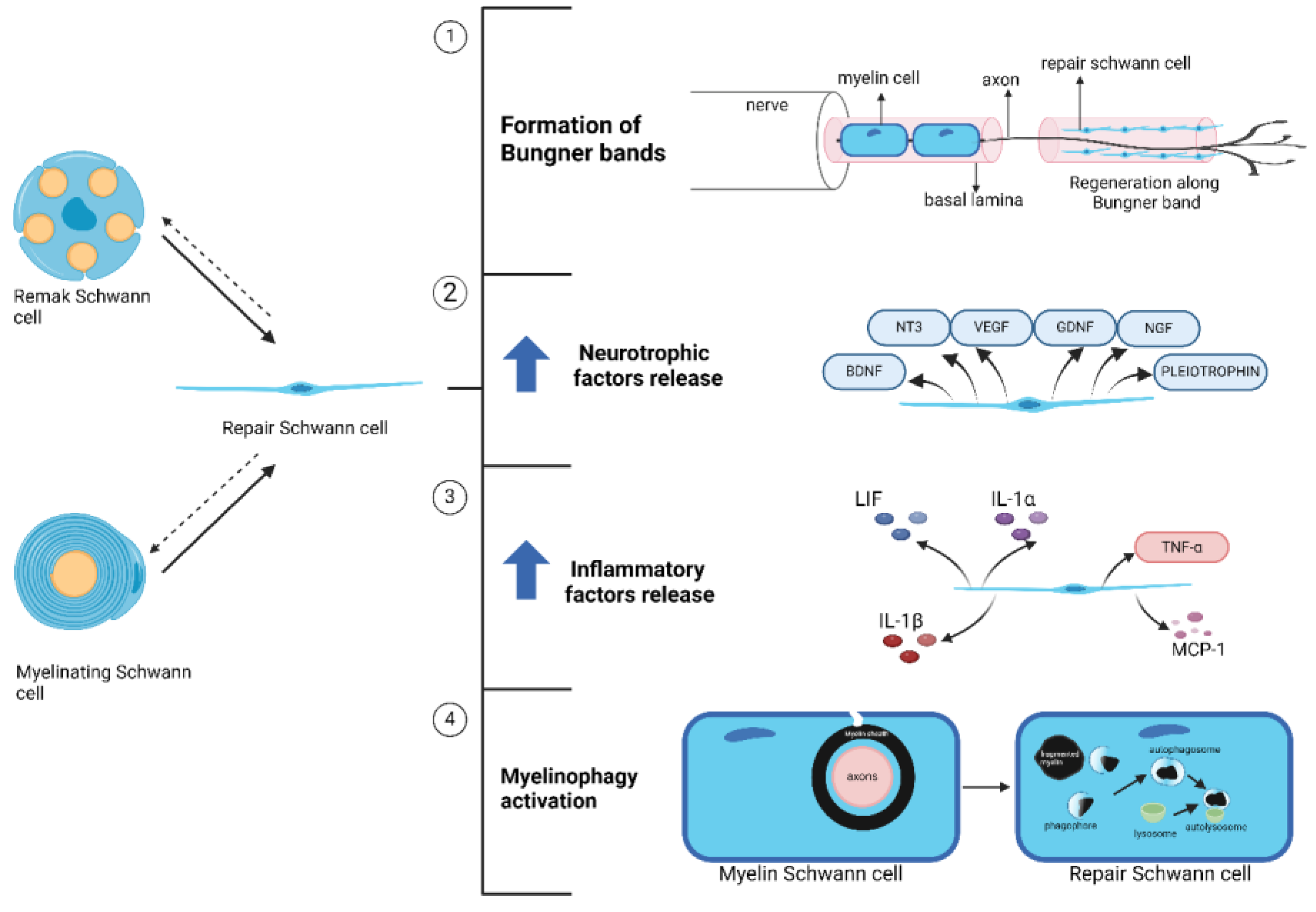
3. Schwann Cell Plasticity during Tissue Regeneration
4. Pathological Changes in Schwann Cell under Diabetic Conditions
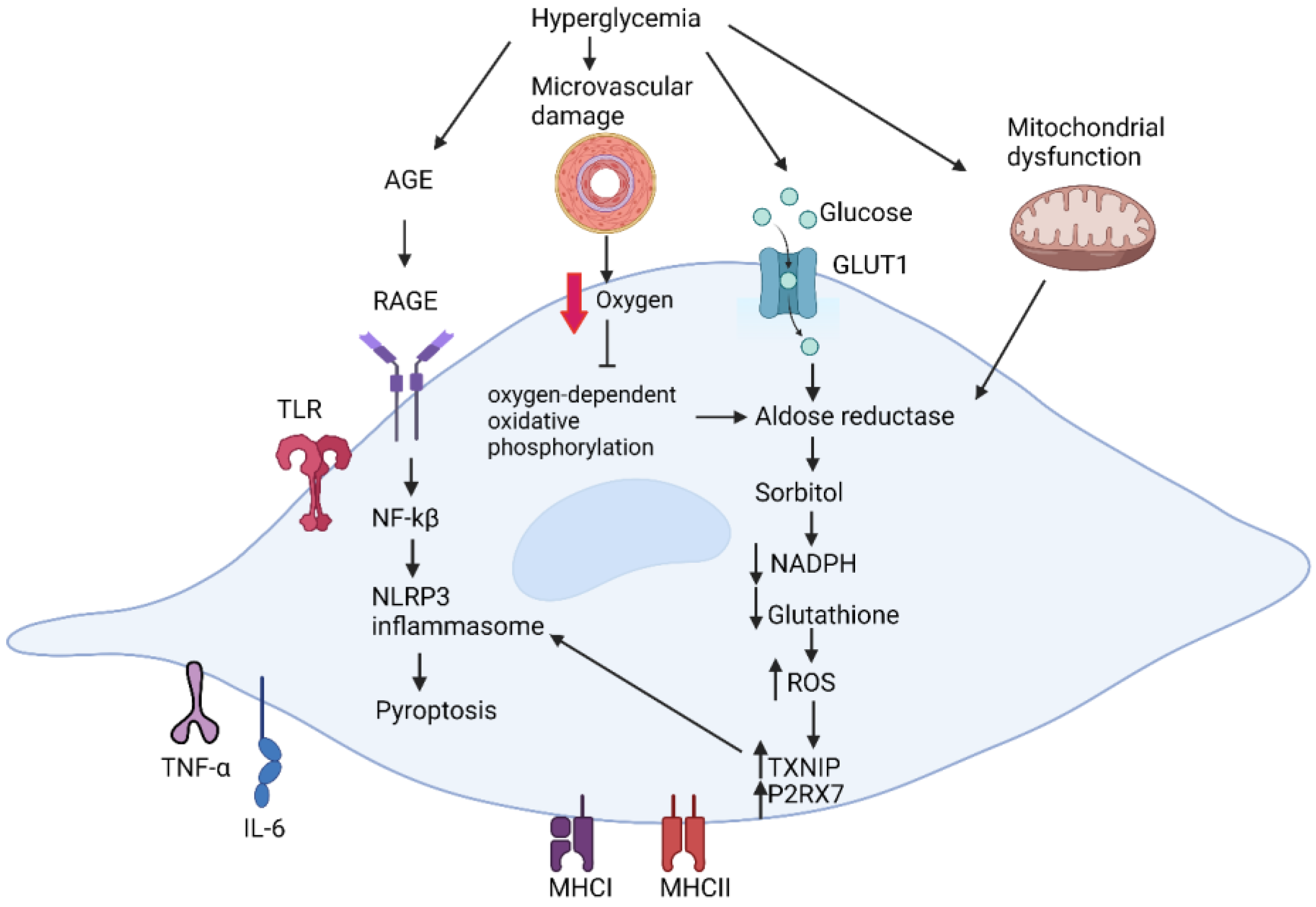
4.1. Metabolic Dysfunction in Diabetic Schwann Cells
4.2. Effect of Microvascular Damage on Schwann Cells
4.3. Inflammatory Dyregulation in Diabetic Schwann Cells
5. Schwann Cell Dysfunctions Compromise the Functional Integrity of Peripheral Nerve in Diabetic Neuropathy
6. Dysfunctional Schwann Cell Plasticity in Diabetic Neuropathy: Diabetes-Induced Schwann Cell Reprogramming into Repair Phenotypes
6.1. Phenotypic Alteration in Diabetic Schwann Cells Resembles in Nerve Injury Response
6.2. Diabetes Elevates the Activity of Repair Regulatory Pathways in Schwann Cells
7. Failure of Repair Schwann Cells to Rescue Tissue Damage in Diabetic Neuropathy
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbott, C.A.; Malik, R.A.; Van Ross, E.R.E.; Kulkarni, J.; Boulton, A.J.M. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011, 34, 2220–2224. [Google Scholar] [CrossRef]
- Lu, Y.; Xing, P.; Cai, X.; Luo, D.; Li, R.; Lloyd, C.; et al. Prevalence and Risk Factors for Diabetic Peripheral Neuropathy in Type 2 Diabetic Patients From 14 Countries: Estimates of the INTERPRET-DD Study. Front Public Health 2020, 8, 534372. [Google Scholar] [CrossRef] [PubMed]
- Truini, A.; Biasiotta, A.; Di Stefano, G.; La Cesa, S.; Leone, C.; Cartoni, C.; et al. Peripheral nociceptor sensitization mediates allodynia in patients with distal symmetric polyneuropathy. J Neurol 2013, 260, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Nave, K.-A.; Jensen, T.S.; Bennett, D.L.H. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 2017, 93, 1296–1313. [Google Scholar]
- Callaghan, B.C.; Cheng, H.T.; Stables, C.L.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 2012, 11, 521–34. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Azmi, S.; Yadav, R.; Ferdousi, M.; Kumar, M.; Cuthbertson, D.J.; et al. Diabetic Peripheral Neuropathy: Epidemiology, Diagnosis, and Pharmacotherapy. Clin Ther 2018, 40, 828–849. [Google Scholar] [PubMed]
- Schreiber, A.K.; Nones, C.F.; Reis, R.C.; Chichorro, J.G.; Cunha, J.M. Diabetic neuropathic pain: Physiopathology and treatment. World J Diabetes 2015, 6, 432–44. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Prezioso, G.; Scattoni, R.; Chiarelli, F. Autonomic neuropathy in diabetes mellitus. Front Endocrinol (Lausanne) 2014, 5, 205. [Google Scholar] [CrossRef]
- Zenker, J.; Ziegler, D.; Chrast, R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci 2013, 36, 439–449. [Google Scholar] [PubMed]
- Sima, A.A.F.; Zhang, W. Mechanisms of diabetic neuropathy: axon dysfunction. Handb Clin Neurol 2014, 126, 429–442. [Google Scholar] [PubMed]
- Kobayashi, M.; Zochodne, D.W. Diabetic neuropathy and the sensory neuron: New aspects of pathogenesis and their treatment implications. J Diabetes Investig 2018, 9, 1239–1254. [Google Scholar]
- Gonçalves, N.P.; Vægter, C.B.; Andersen, H.; Østergaard, L.; Calcutt, N.A.; Jensen, T.S. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol 2017, 13, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cellular and Molecular Life Sciences 2020, 77, 3977–3989. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Aguayo, A.J. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 1981, 214, 931–3. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.M.; McGuinness, U.M.; Aguayo, A.J. Axons from CNS neurones regenerate into PNS grafts. Nature 1980, 284, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front Cell Neurosci 2019, 13, 33. [Google Scholar] [CrossRef]
- Menorca, R.M.G.; Fussell, T.S.; Elfar, J.C. Peripheral Nerve Trauma: Mechanisms of Injury and Recovery. Hand Clin 2013, 29, 317. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Brennan, A.; Liu, N.; Yarden, Y.; Lefkowitz, G.; Mirsky, R.; et al. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat schwann cell precursors. Neuron 1995, 15, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Brennan, A.; Morgan, L.; Mirsky, R.; Kent, A.; Hashimoto, Y.; et al. The Schwann cell precursor and its fate: A study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron 1994, 12, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Feltri, M.L.; Poitelon, Y.; Previtali, S.C. How Schwann Cells Sort Axons: New concepts. Neuroscientist 2016, 22, 252–265. [Google Scholar] [CrossRef]
- Poliak, S.; Peles, E. The local differentiation of myelinated axons at nodes of ranvier. Nat Rev Neurosci 2003, 4, 968–980. [Google Scholar] [PubMed]
- Simons, M.; Trotter, J. Wrapping it up: the cell biology of myelination. Curr Opin Neurobiol 2007, 17, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Poliak, S.; Peles, E. The local differentiation of myelinated axons at nodes of ranvier. Nat Rev Neurosci 2003, 4, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Morell, P.; Quarles, R.H. The Myelin Sheath. 1999. [Google Scholar]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 2005, 6, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Harty, B.L.; Monk, K.R. Unwrapping the unappreciated: recent progress in Remak Schwann cell biology. Curr Opin Neurobiol 2017, 47, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.T.; Yanick, C.; Wein, N.; Gomez Limia, C.E. Neuron-Schwann cell interactions in peripheral nervous system homeostasis, disease, and preclinical treatment. Front Cell Neurosci 2023, 17, 1248922. [Google Scholar] [CrossRef] [PubMed]
- Keswani, S.C.; Buldanlioglu, U.; Fischer, A.; Reed, N.; Polley, M.; Liang, H.; et al. A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann Neurol 2004, 56, 815–826. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, B.; Gu, G.; Zhang, W.; Li, J.; Wang, H.; et al. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death & Disease, 2023; 14, 1–17. [Google Scholar]
- Hyung, S.; Kim, J.Y.; Yu, C.J.; Jung, H.S.; Hong, J.W. Neuroprotective Effect of Glial Cell-Derived Exosomes on Neurons. Immunotherapy: Open Access, 2019; 5, 1–8. [Google Scholar]
- Court, F.A.; Hendriks, W.T.J.; MacGillavry, H.D.; Alvarez, J.; Van Minnen, J. Schwann Cell to Axon Transfer of Ribosomes: Toward a Novel Understanding of the Role of Glia in the Nervous System. Journal of Neuroscience 2008, 28, 11024–11029. [Google Scholar] [CrossRef] [PubMed]
- Mietto, B.S.; Jhelum, P.; Schulz, K.; David, S. Schwann Cells Provide Iron to Axonal Mitochondria and Its Role in Nerve Regeneration. Journal of Neuroscience 2021, 41, 7300–7313. [Google Scholar] [CrossRef]
- Deck, M.; Van Hameren, G.; Campbell, G.; Bernard-Marissal, N.; Devaux, J.; Berthelot, J.; et al. Physiology of PNS axons relies on glycolytic metabolism in myelinating Schwann cells. PLoS One 2022, 17, e0272097. [Google Scholar] [CrossRef] [PubMed]
- Viader, A.; Golden, J.P.; Baloh, R.H.; Schmidt, R.E.; Hunter, D.A.; Milbrandt, J. Schwann cell mitochondrial metabolism supports long-term axonal survival and peripheral nerve function. Journal of Neuroscience 2011, 31, 10128–10140. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.A.A.; Chen, Y.; Dao, D.Q.; Mayoral, S.R.; Wu, L.; Meijer, D.; et al. Phosphorylation of LKB1/Par-4 establishes Schwann cell polarity to initiate and control myelin extent. Nat Commun 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Beirowski, B.; Babetto, E.; Golden, J.P.; Chen, Y.J.; Yang, K.; Gross, R.W.; et al. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat Neurosci 2014, 17, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Pooya, S.; Liu, X.; Kumar, V.B.S.; Anderson, J.; Imai, F.; Zhang, W.; et al. The tumour suppressor LKB1 regulates myelination through mitochondrial metabolism. Nat Commun 2014, 5. [Google Scholar]
- Rigby, M.J.; Gomez, T.M.; Puglielli, L. Glial Cell-Axonal Growth Cone Interactions in Neurodevelopment and Regeneration. Front Neurosci 2020, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Stierli, S.; Napoli, I.; White, I.J.; Cattin, A.L.; Cabrejos, A.M.; Calavia, N.G.; et al. The regulation of the homeostasis and regeneration of peripheral nerve is distinct from the CNS and independent of a stem cell population. Development (Cambridge) 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Yu, W.M.; Strickland, S. Peripheral regeneration. Annu Rev Neurosci 2007, 30, 209–233. [Google Scholar] [CrossRef] [PubMed]
- Stoll G.; Müller H.W. Nerve injury, axonal degeneration and neural regeneration: Basic insights, In: Brain Pathology, International Society of Neuropathology, 1999, 313–325.
- Gomez-Sanchez, J.A.; Pilch, K.S.; Van Der Lans, M.; Fazal S., V; Benito, C.; Wagstaff, L.J.; et al. Development/Plasticity/Repair After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J Neurosci 2017, 37, 9086–9099. [Google Scholar] [CrossRef]
- Scheib, J.; Höke, A. Advances in peripheral nerve regeneration. Nat Rev Neurol 2013, 9, 668–676. [Google Scholar] [PubMed]
- Grothe, C.; Haastert, K.; Jungnickel, J. Physiological function and putative therapeutic impact of the FGF-2 system in peripheral nerve regeneration-Lessons from in vivo studies in mice and rats. Brain Res Rev 2006, 51, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M.; Aspalter, M.; Griffin, J.W.; Redett, R.; Hameed, H.; Zhou, C.; et al. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Exp Neurol 2013, 247, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Mackinnon, S.E. Pathways regulating modality-specific axonal regeneration in peripheral nerve. Exp Neurol 2015, 265, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Fontana, X.; Hristova, M.; Da Costa, C.; Patodia, S.; Thei, L.; Makwana, M.; et al. C-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. Journal of Cell Biology 2012, 198, 127–141. [Google Scholar] [PubMed]
- Rotshenker, S. Wallerian degeneration: The innate-immune response to traumatic nerve injury. J Neuroinflammation 2011, 8. [Google Scholar] [CrossRef]
- Boivin, A.; Pineau, I.; Barrette, B.; Filali, M.; Vallières, N.; Rivest, S.; et al. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. Journal of Neuroscience 2007, 27, 12565–12576. [Google Scholar] [CrossRef]
- Perrin, F.E.; Lacroix, S.; Avilés-Trieueros, M.; David, S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1α and interleukin-1β Wallerian degeneration. Brain 2005, 128, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Subang, M.C.; Richardson, P.M. Influence of injury and cytokines on synthesis of monocyte chemoattractant protein-1 MRNA in peripheral nervous tissue. European Journal of Neuroscience 2001, 13, 521–528. [Google Scholar] [CrossRef]
- Brück, W.; Huitinga, I.; Dykstra, C.D. Liposome-mediated monocyte depletion during Wallerian degeneration defines the role of hematogenous phagocytes in myelin removal. J Neurosci Res 1996, 46, 477–484. [Google Scholar] [CrossRef]
- Carroll S.L.; Worley S.H. Wallerian degeneration, In: The Curated Reference Collection in Neuroscience and Biobehavioral Psychology, Elsevier Science Ltd., 2016, 485–491.
- David, S.; Lacroix, S. Molecular approaches to spinal cord repair. Annu Rev Neurosci 2003, 26, 411–440. [Google Scholar] [CrossRef]
- George, E.B.; Glass, J.D.; Griffin, J.W. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. Journal of Neuroscience 1995, 15, 6445–6452. [Google Scholar] [CrossRef]
- Beuche, W.; Friede, R.L. The role of non-resident cells in Wallerian degeneration. J Neurocytol 1984, 13, 767–796. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, J.A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M.; Griffith, M.; et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. Journal of Cell Biology 2015, 210, 153–168. [Google Scholar] [PubMed]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann cells: Development and role in nerve repair. Cold Spring Harb Perspect Biol 2015, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, T.; Bitsch, A.; Stadelmann, C.; Siebert, H.; Brück, W. Macrophages are eliminated from the injured peripheral nerve via local apoptosis and circulation to regional lymph nodes and the spleen. Journal of Neuroscience 2001, 21, 3401–3408. [Google Scholar] [CrossRef]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J 2012, 12, 556–569. [Google Scholar] [CrossRef]
- Yabe-Nishimura, C. Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Undefined 1998. [Google Scholar]
- El-Kabbani, O.; Ruiz, F.; Darmanin, C.; Chung, R.P.T. Aldose reductase structures: Implications for mechanism and inhibition. Cellular and Molecular Life Sciences 2004, 61, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Calcutt, N.A.; Rames, K.M.; Mizisin, A.P. Novel sites of aldose reductase immunolocalization in normal and streptozotocin-diabetic rats. Journal of the Peripheral Nervous System 2006, 11, 274–285. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ramana K., V. Aldose reductase: New insights for an old enzyme. Biomol Concepts 2011, 2, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Obrosova, I.G.; Fathallah, L.; Lang, H.J. Interaction between osmotic and oxidative stress in diabetic precataractous lens: Studies with a sorbitol dehydrogenase inhibitor. Biochem Pharmacol 1999, 58, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.J.; Lattimer, S.A.; Kamijo, M.; Van Huysen, C.; Sima, A.A.F.; Greene, D.A. Osmotically-induced nerve taurine depletion and the compatible osmolyte hypothesis in experimental diabetic neuropathy in the rat. Diabetologia 1993, 36, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Askwith, T.; Zeng, W.; Eggo, M.C.; Stevens, M.J. Taurine reduces nitrosative stress and nitric oxide synthase expression in high glucose-exposed human Schwann cells. Exp Neurol 2012, 233, 154–162. [Google Scholar] [PubMed]
- Askwith, T.; Zeng, W.; Eggo, M.C.; Stevens, M.J. Oxidative stress and dysregulation of the taurine transporter in high-glucose-exposed human Schwann cells: Implications for pathogenesis of diabetic neuropathy. Am J Physiol Endocrinol Metab 2009, 297, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; et al. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab 2018, 28, 337–352. [Google Scholar] [PubMed]
- Vincent, A.M.; Perrone, L.; Sullivan, K.A.; Backus, C.; Sastry, A.M.; Lastoskie, C.; et al. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology 2007, 148, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, K.M.; Neundörfer, B.; Schlötzer-Schrehardt, U.; Bierhaus, A.; Schleicher, E.; Pauli, E.; et al. Activation of the RAGE pathway: A general mechanism in the pathogenesis of polyneuropathies? Neurol Res 2007, 29, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Sekido, H.; Suzuki, T.; Jomori, T.; Takeuchi, M.; Yabe-Nishimura, C.; Yagihashi, S. Reduced cell replication and induction of apoptosis by advanced glycation end products in rat Schwann cells. Biochem Biophys Res Commun 2004, 320, 241–248. [Google Scholar] [CrossRef]
- Freeman, O.J.; Unwin, R.D.; Dowsey, A.W.; Begley, P.; Ali, S.; Hollywood, K.A.; et al. Metabolic dysfunction is restricted to the sciatic nerve in experimental diabetic neuropathy. Diabetes 2016, 65, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, C.; Vasquez, F.E.; Galeva, N.; Onyango, I.; Swerdlow, R.H.; et al. Hyperglycemia alters the Schwann cell mitochondrial proteome and decreases coupled respiration in the absence of superoxide production. J Proteome Res 2010, 9, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Isoda, F.; Kurland, I.; Mobbs C., V. Glucose-induced metabolic memory in Schwann cells: Prevention by PPAR agonists. Endocrinology 2013, 154, 3054–3066. [Google Scholar] [CrossRef] [PubMed]
- Thrainsdottir, S.; Malik, R.A.; Dahlin, L.B.; Wiksell, P.; Eriksson, K.F.; Rosén, I.; et al. Endoneurial capillary abnormalities presage deterioration of glucose tolerance and accompany peripheral neuropathy in man. Diabetes 2003, 52, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Dyck, P.J. Basement membrane reduplication and pericyte degeneration precede development of diabetic polyneuropathy and are associated with its severity. Ann Neurol 1995, 37, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, L.; Finnerup, N.B.; Terkelsen, A.J.; Olesen, R.A.; Drasbek, K.R.; Knudsen, L.; et al. The effects of capillary dysfunction on oxygen and glucose extraction in diabetic neuropathy. Diabetologia 2015, 58, 666–677. [Google Scholar] [PubMed]
- Jespersen, S.N.; Østergaard, L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. Journal of Cerebral Blood Flow and Metabolism 2012, 32, 264–277. [Google Scholar]
- Tilton, R.G.; Chang, K.; Nyengaard, J.R.; Enden M.V., d.; Ido, Y.; Williamson, J.R. Inhibition of Sorbitol Dehydrogenase: Effects on Vascular and Neural Dysfunction in Streptozocin-Induced Diabetic Rats. Diabetes 1995, 44, 234–242. [Google Scholar] [CrossRef]
- Theriault, M.; Dort, J.; Sutherland, G.; Zochodne, D.W. Local human sural nerve blood flow in diabetic and other polyneuropathies. Brain 1997, 120, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Sullivan, K.A.; Pande, M.; Hong, Y.; Sima, A.A.F.; Jagadish H., V.; et al. The identification of gene expression profiles associated with progression of human diabetic neuropathy. Brain 2011, 134, 3222. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Lankisch, M.; Ziegler, D.; Rathmann, W.; Koenig, W.; Illig, T.; et al. Subclinical inflammation and diabetic polyneuropathy. Diabetes Care 2009, 32, 680–682. [Google Scholar] [CrossRef]
- Hussain, G.; Rizvi, S.A.A.; Singhal, S.; Zubair, M.; Ahmad, J. Serum levels of TNF-α in peripheral neuropathy patients and its correlation with nerve conduction velocity in type 2 diabetes mellitus. Diabetes Metab Syndr 2013, 7, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.P.; Wang, Y.G.; Li, C.Q.; Lv, W.S.; Wang, B.; Jing, Z.H.; et al. Association Between Tumor Necrosis Factor-α and Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes: a Meta-Analysis. Mol Neurobiol 2017, 54, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Rizvi, S.A.A.; Singhal, S.; Zubair, M.; Ahmad, J. Serum levels of TGF-β1 in patients of diabetic peripheral neuropathy and its correlation with nerve conduction velocity in type 2 diabetes mellitus. Diabetes and Metabolic Syndrome: Clinical Research and Reviews, 2016; 10, S135–S139. [Google Scholar]
- Conti, G.; Scarpini, E.; Baron, P.; Livraghi, S.; Tiriticco, M.; Bianchi, R.; et al. Macrophage infiltration and death in the nerve during the early phases of experimental diabetic neuropathy: A process concomitant with endoneurial induction of IL-1β and p75NTR. J Neurol Sci 2002, 195, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Newton, V.L.; Guck, J.D.; Cotter, M.A.; Cameron, N.E.; Gardiner, N.J. Neutrophils Infiltrate the Spinal Cord Parenchyma of Rats with Experimental Diabetic Neuropathy. J Diabetes Res 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Martini, R.; Fischer, S.; López-Vales, R.; David, S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia 2008, 56, 1566–1577. [Google Scholar] [CrossRef]
- Shamash, S.; Reichert, F.; Rotshenker, S. The Cytokine Network of Wallerian Degeneration: Tumor Necrosis Factor-α, Interleukin-1α, and Interleukin-1β. Journal of Neuroscience 2002, 22, 3052–3060. [Google Scholar] [CrossRef]
- Lee, H.; Jo, E.K.; Choi, S.Y.; Oh, S.B.; Park, K.; Soo Kim, J.; et al. Necrotic neuronal cells induce inflammatory Schwann cell activation via TLR2 and TLR3: Implication in Wallerian degeneration. Biochem Biophys Res Commun 2006, 350, 742–747. [Google Scholar] [PubMed]
- Ydens, E.; Lornet, G.; Smits, V.; Goethals, S.; Timmerman, V.; Janssens, S. The neuroinflammatory role of Schwann cells in disease. Neurobiol Dis 2013, 55, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Goethals, S.; Ydens, E.; Timmerman, V.; Janssens, S. Toll-like receptor expression in the peripheral nerve. Glia 2010, 58, 1701–1709. [Google Scholar] [PubMed]
- Ydens, E.; Demon, D.; Lornet, G.; De Winter, V.; Timmerman, V.; Lamkanfi, M.; et al. Nlrp6 promotes recovery after peripheral nerve injury independently of inflammasomes. J Neuroinflammation 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.M.; Yan S., Du; Fernandas, R.; Guimarães, A.; Stern, D.; Saraiva, M.J. Familial amyloid polyneuropathy: receptor for advanced glycation end products-dependent triggering of neuronal inflammatory and apoptotic pathways. J Neurosci 2001, 21, 7576–7586. [Google Scholar] [CrossRef] [PubMed]
- Baetas-Da-Cruz, W.; Alves, L.; Pessolani M.C., V.; Barbosa, H.S.; Régnier-Vigouroux, A.; Corte-Real, S.; et al. Schwann cells express the macrophage mannose receptor and MHC class II. Do they have a role in antigen presentation? J Peripher Nerv Syst 2009, 14, 84–92. [Google Scholar] [CrossRef]
- Weis, W.I.; Taylor, M.E.; Drickamer, K. The C-type lectin superfamily in the immune system. Immunol Rev 1998, 163, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Campana, W.M.; Li, X.; Dragojlovic, N.; Janes, J.; Gaultier, A.; Gonias, S.L. The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells: possible implications in peripheral nerve injury. J Neurosci 2006, 26, 11197–11207. [Google Scholar] [CrossRef]
- Hartlehnert, M.; Derksen, A.; Hagenacker, T.; Kindermann, D.; Schäfers, M.; Pawlak, M.; et al. Schwann cells promote post-traumatic nerve inflammation and neuropathic pain through MHC class II. Scientific Reports, 2017; 7, 1–10. [Google Scholar]
- Meyer zu Hörste, G.; Mausberg, A.K.; Müller, J.I.; Lehmann, H.C.; Löber, S.; Gmeiner, P.; et al. Quinpramine Ameliorates Rat Experimental Autoimmune Neuritis and Redistributes MHC Class II Molecules. PLoS One 2011, 6, e21223. [Google Scholar] [CrossRef] [PubMed]
- Tsuyuki, Y.; Fujimaki, H.; Hikawa, N.; Fujita, K.; Nagata, T.; Minami, M. IFN-gamma induces coordinate expression of MHC class I-mediated antigen presentation machinery molecules in adult mouse Schwann cells. Neuroreport 1998, 9, 2071–2075. [Google Scholar] [CrossRef] [PubMed]
- Spierings, E.; Boer T., de; Wieles, B.; Adams, L.B.; Marani, E.; Ottenhoff, T.H.M. Mycobacterium leprae-Specific, HLA Class II-Restricted Killing of Human Schwann Cells by CD4+ Th1 Cells: A Novel Immunopathogenic Mechanism of Nerve Damage in Leprosy. The Journal of Immunology 2001, 166, 5883–5888. [Google Scholar] [CrossRef]
- Spierings, E.; Boer T., de; Wieles, B.; Adams, L.B.; Marani, E.; Ottenhoff, T.H.M. Mycobacterium leprae-Specific, HLA Class II-Restricted Killing of Human Schwann Cells by CD4+ Th1 Cells: A Novel Immunopathogenic Mechanism of Nerve Damage in Leprosy. The Journal of Immunology 2001, 166, 5883–5888. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, I.; Van Den Berg, L.H.; Bosboom, W.M.J.; Otten, H.G.; Logtenberg, T. Expression of accessory molecules for T-cell activation in peripheral nerve of patients with CIDP and vasculitic neuropathy. Brain 2000, 123 Pt 10, 2020–2029. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Chu, L.W.; Chen, J.Y.; Hsieh, S.L.; Chang, Y.C.; Dai, Z.K.; et al. Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation. Cells 2020, Vol. 9, Page 1948 2020, 9, 1948. [Google Scholar] [CrossRef] [PubMed]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front Aging Neurosci 2022, 14, 879021. [Google Scholar] [PubMed]
- Padilla, A.; Descorbeth, M.; Almeyda, A.L.; Payne, K.; De Leon, M. Hyperglycemia magnifies Schwann cell dysfunction and cell death triggered by PA-induced lipotoxicity. Brain Res 2011, 1370, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Tatsunami, R.; Yama, K.; Murao, Y.; Tampo, Y. Glycolaldehyde induces endoplasmic reticulum stress and apoptosis in Schwann cells. Toxicol Rep 2015, 2, 1454. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, S.; Yuan, Q.; Zhu, L.; Li, F.; Wang, H.; et al. TXNIP, a novel key factor to cause Schwann cell dysfunction in diabetic peripheral neuropathy, under the regulation of PI3K/Akt pathway inhibition-induced DNMT1 and DNMT3a overexpression. Cell Death & Disease, 2021; 12, 1–13. [Google Scholar]
- Song, X.M.; Xu, X.H.; Zhu, J.; Guo, Z.; Li, J.; He, C.; et al. Up-regulation of P2X7 receptors mediating proliferation of Schwann cells after sciatic nerve injury. Purinergic Signal 2015, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, H.; Gao, H.; Zhang, H.; Zhang, H.; Wang, Q.; et al. P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci 2020, 10, 1–9. [Google Scholar]
- Cheng, Y.C.; Chu, L.W.; Chen, J.Y.; Hsieh, S.L.; Chang, Y.C.; Dai, Z.K.; et al. Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation. Cells 2020, 9, 1948. [Google Scholar] [CrossRef] [PubMed]
- Dunnigan, S.K.; Ebadi, H.; Breiner, A.; Katzberg, H.D.; Lovblom, L.E.; Perkins, B.A.; et al. Conduction slowing in diabetic sensorimotor polyneuropathy. Diabetes Care 2013, 36, 3684–3690. [Google Scholar] [CrossRef] [PubMed]
- Hamid, W.S.; Ahmed, H.S.; Osman, M.A.; Babiker, R. Nerve conduction and its correlations with duration of diabetes mellitus and glycosylated haemoglobin in type 2 diabetes mellitus (T2DM). Journal of Endocrinology, Metabolism and Diabetes of South Africa, 2021; 26, 46–51. [Google Scholar]
- Guo, J.; Guo, Z.; Huang, Y.; Ma, S.; Yan, B.; Pan, C.; et al. Blockage of MLKL prevents myelin damage in experimental diabetic neuropathy. Proc Natl Acad Sci U S A 2022, 119, e2121552119. [Google Scholar] [CrossRef]
- Ying, Z.; Pan, C.; Shao, T.; Liu, L.; Li, L.; Guo, D.; et al. Mixed Lineage Kinase Domain-like Protein MLKL Breaks Down Myelin following Nerve Injury. Mol Cell, 2018; 72, 457–468.e5. [Google Scholar]
- Zhu, L.; Hao, J.; Cheng, M.; Zhang, C.; Huo, C.; Liu, Y.; et al. Hyperglycemia-induced Bcl-2/Bax-mediated apoptosis of Schwann cells via mTORC1/S6K1 inhibition in diabetic peripheral neuropathy. Exp Cell Res 2018, 367, 186–195. [Google Scholar] [CrossRef]
- Du, W.; Wang, N.; Li, F.; Jia, K.; An, J.; Liu, Y.; et al. STAT3 phosphorylation mediates high glucose—impaired cell autophagy in an HDAC1-dependent and -independent manner in Schwann cells of diabetic peripheral neuropathy. The FASEB Journal 2019, 33, 8008–8021. [Google Scholar] [CrossRef]
- Dyck, P.J.; Sherman, W.R.; Hallcher, L.M.; John Service, F.; O’Brien, P.C.; Grina, L.A.; et al. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol 1980, 8, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.A.; Tesfaye, S.; Newrick, P.G.; Walker, D.; Rajbhandari, S.M.; Siddique, I.; et al. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia 2005, 48, 578–585. [Google Scholar] [CrossRef]
- Mizisin, A.P. Mechanisms of diabetic neuropathy: Schwann cells. Handb Clin Neurol 2014, 126, 401–428. [Google Scholar] [PubMed]
- Mizisin, A.P.; Shelton, G.D.; Wagner, S.; Rusbridge, C.; Powell, H.C. Myelin splitting, Schwann cell injury and demyelination in feline diabetic neuropathy. Acta Neuropathol 1998, 95, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Yagihashi, S.; Matsunaga, M. Ultrastructural Pathology of Peripheral Nerves in Patients with Diabetic Neuropathy. Tohoku J Exp Med 1979, 129, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Nukada, H.; McMorran, P.D.; Baba, M.; Ogasawara, S.; Yagihashi, S. Increased susceptibility to ischemia and macrophage activation in STZ-diabetic rat nerve. Brain Res 2011, 1373, 172–182. [Google Scholar]
- Tang, W.; Lv, Q.; Chen, X.; Zou, J.; Liu, Z.; Shi, Y. CD8+ T Cell-Mediated Cytotoxicity toward Schwann Cells Promotes Diabetic Peripheral Neuropathy. Cellular Physiology and Biochemistry 2013, 32, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Tashiro, S.; Hasegawa, T.; Sato, Y.; Kobayashi, T.; Tando, T.; et al. Hyperglycemia Promotes Schwann Cell De-differentiation and De-myelination via Sorbitol Accumulation and Igf1 Protein Down-regulation. J Biol Chem 2015, 290, 17106–15. [Google Scholar] [CrossRef]
- Cermenati, G.; Abbiati, F.; Cermenati, S.; Brioschi, E.; Volonterio, A.; Cavaletti, G.; et al. Diabetes-induced myelin abnormalities are associated with an altered lipid pattern: protective effects of LXR activation. J Lipid Res 2012, 53, 300–10. [Google Scholar]
- Yung, J.H.M.; Giacca, A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9. [Google Scholar] [CrossRef]
- Yung, J.H.M.; Giacca, A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. Journal of Physiology 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, D.B.; Bhaskaran, A.; Arthur-Farraj, P.; Noon, L.A.; Woodhoo, A.; Lloyd, A.C.; et al. c-Jun is a negative regulator of myelination. Journal of Cell Biology 2008, 181, 625–637. [Google Scholar] [CrossRef]
- Parkinson, D.B.; Bhaskaran, A.; Droggiti, A.; Dickinson, S.; D’Antonio, M.; Mirsky, R.; et al. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. Journal of Cell Biology 2004, 164, 385–394. [Google Scholar] [CrossRef]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; et al. c-Jun Reprograms Schwann Cells of Injured Nerves to Generate a Repair Cell Essential for Regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Tricaud, N.; Gautier, B.; Berthelot, J.; Gonzalez, S.; Van Hameren, G. Traumatic and Diabetic Schwann Cell Demyelination Is Triggered by a Transient Mitochondrial Calcium Release through Voltage Dependent Anion Channel 1. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liang, X.; Zhang, H. Effects of High Glucose on Cell Viability and Differentiation in Primary Cultured Schwann Cells: Potential Role of ERK Signaling Pathway. Neurochem Res 2016, 41, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Napoli, I.; Noon, L.A.; Ribeiro, S.; Kerai, A.P.; Parrinello, S.; Rosenberg, L.H.; et al. A Central Role for the ERK-Signaling Pathway in Controlling Schwann Cell Plasticity and Peripheral Nerve Regeneration In Vivo. Neuron 2012, 73, 729–742. [Google Scholar] [CrossRef]
- Boerboom, A.; Dion, V.; Chariot, A.; Franzen, R. Molecular mechanisms involved in schwann cell plasticity. Front Mol Neurosci 2017, 17, 38. [Google Scholar]
- Pan, P.; Dobrowsky, R.T. Differential expression of neuregulin-1 isoforms and downregulation of erbin are associated with Erb B2 receptor activation in diabetic peripheral neuropathy. Acta Neuropathol Commun 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Taveggia, C.; Zanazzi, G.; Petrylak, A.; Yano, H.; Rosenbluth, J.; Einheber, S.; et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 2005, 47, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Fledrich, R.; Akkermann, D.; Schütza, V.; Abdelaal, T.A.; Hermes, D.; Schäffner, E.; et al. NRG1 type I dependent autoparacrine stimulation of Schwann cells in onion bulbs of peripheral neuropathies. Nat Commun 2019, 10. [Google Scholar]
- Syed, N.; Reddy, K.; Yang, D.P.; Taveggia, C.; Salzer, J.L.; Maurel, P.; et al. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. Journal of Neuroscience 2010, 30, 6122–6131. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wang, N.; Li, F.; Jia, K.; An, J.; Liu, Y.; et al. STAT3 phosphorylation mediates high glucose—impaired cell autophagy in an HDAC1-dependent and -independent manner in Schwann cells of diabetic peripheral neuropathy. FASEB Journal 2019, 33, 8008–8021. [Google Scholar] [CrossRef] [PubMed]
- McKerracher, L.; Rosen, K. MAG, myelin and overcoming growth inhibition in the CNS. Frontiers in Molecular Neuroscience 2015, 8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




