Submitted:
16 August 2024
Posted:
19 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
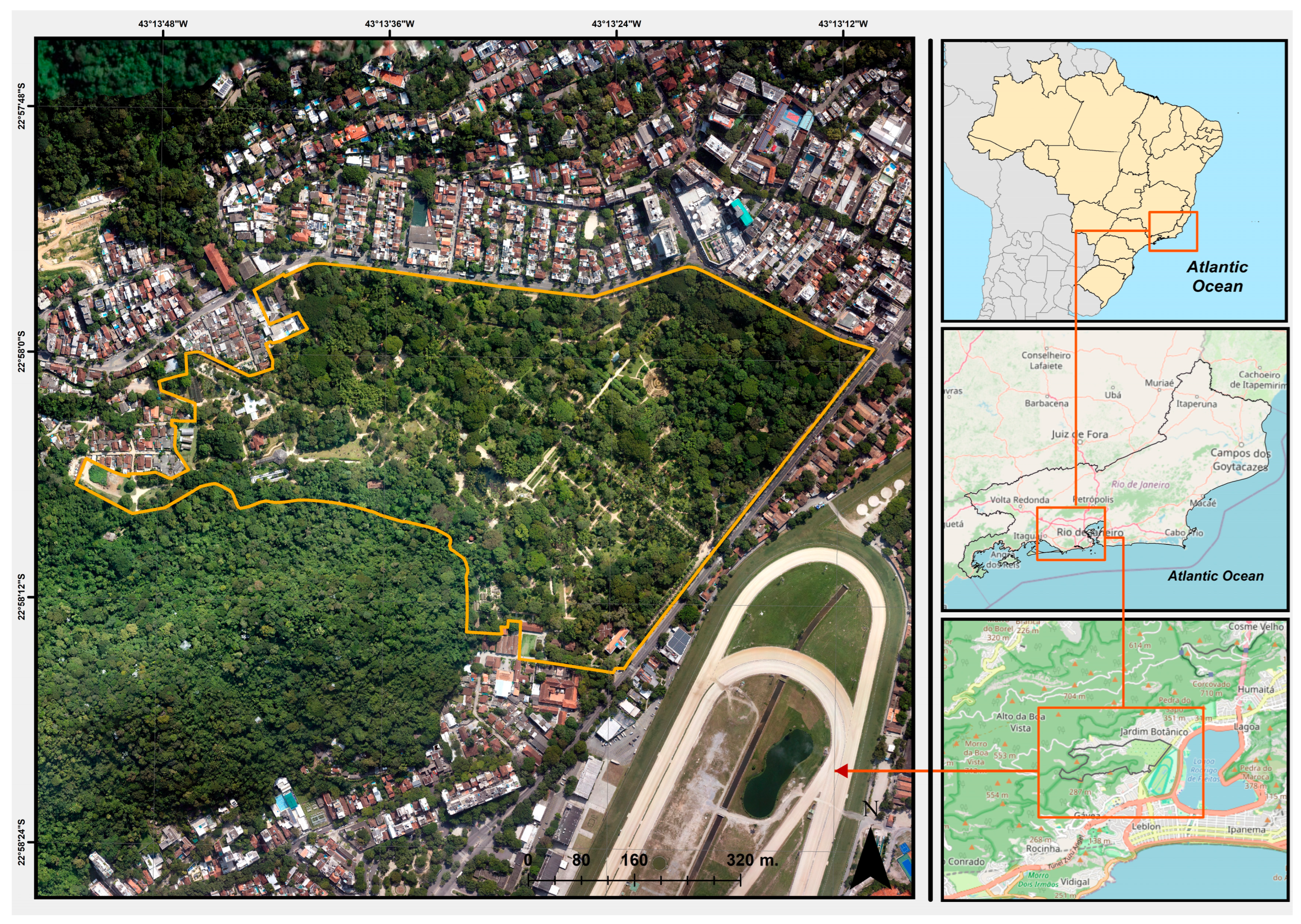
2. Materials and Methods
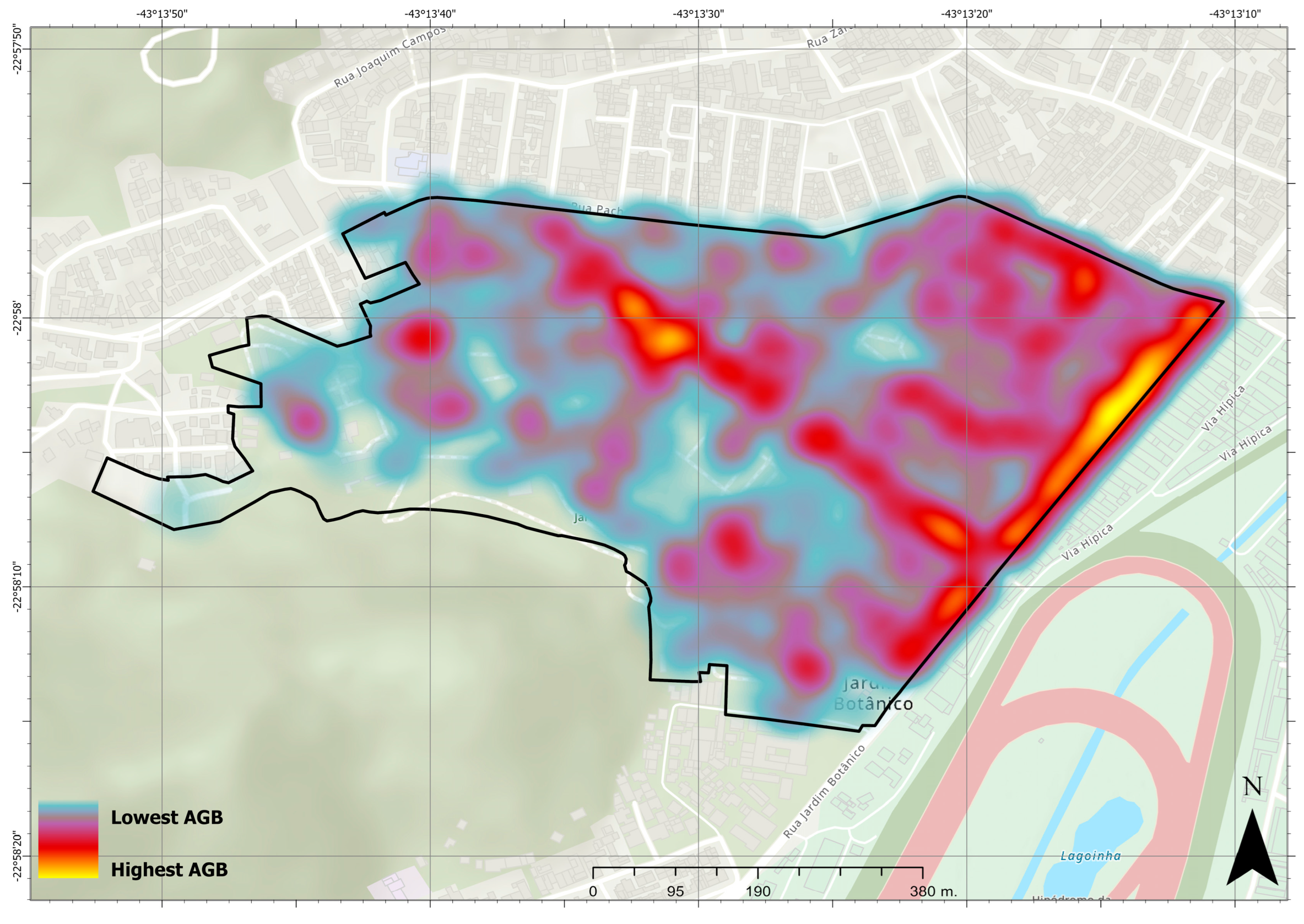
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC—Intergovernmental Panel on Climate Change. Summary for policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, United Kingdom and New York, NY, USA, 2021; pp. 3−32. [CrossRef]
- IPCC—Intergovernmental Panel on Climate Change. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- United Nations. Paris Agreement. Available online: chromeextension://efaidnbmnnnibpcajpcglclefindmkaj/https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 6 August 2024).
- IPCC—Intergovernmental Panel on Climate Change. Summary for policymakers. In Global Warming of 1.5°C. An IPCC Special Report on the Impacts of Global Warming of 1.5°C above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, United Kingdom and New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The structure, distribution, and biomass of the world’s forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Global Change Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Nogueira, E.M.; Fearnside, P.M.; Nelson, B.W.; Barbosa, R.I.; Keizer, E.W.H. Estimates of forest biomass in the Brazilian Amazon: New allometric equations and adjustments to biomass from wood-volume inventories. For. Ecol. Manag. 2008, 256, 1853–1867. [Google Scholar] [CrossRef]
- Vieira, S.A.; Alves, L.F.; Duarte-Neto, P.J.; Martins, S.C.; Veiga, L.G.; Scaranello, M.A.; Picollo, M.C.; Camargo, P.B.; Carmo, J.B.; Neto, E.S.; et al. Stocks of carbon and nitrogen and partitioning between above- and belowground pools in the Brazilian coastal Atlantic Forest elevation range. Ecol. Evol. 2011, 1, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Mitchard, E.T.A.; Feldpausch, T.R.; Brienen, R.J.W.; Lopez-Gonzalez, G.; Monteagudo, A.; Baker, T.R.; Lewis, S.L.; Lloyd, J.; Quesada, C.A.; Gloor, M.; et al. Markedly divergent estimates of Amazon forest carbon density from ground plots and satellites. Global Ecol. Biogeogr. 2014, 23, 935–946. [Google Scholar] [CrossRef]
- Araujo, E.C.G.; Sanquetta, C.R.; Dalla Corte, A.P.; Pelissari, A.L.; Orso, G.A.; Silva, T.C. Global review and state-of-the-art of biomass and carbon stock in the Amazon. J. Environ. Manage. 2023, 331, 117251. [Google Scholar] [CrossRef]
- Gomes, V.H.F.; Vieira, I.C.G.; Salomão, R.P.; ter Steege, H. Amazonian tree species threatened by deforestation and climate change. Nat. Clim. Change 2019, 9, 547–553. [Google Scholar] [CrossRef]
- Gatti, L.V.; Basso, L.S.; Miller, J.B.; Gloor, M.; Gatti Domingues, L.; Cassol, H.L.G.; Tejada, G.; Aragão, L.E.O.C.; Nobre, C.; Peters, W.; et al. Amazonia as a carbon source linked to deforestation and climate change. Nature 2021, 595, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Velasco, E.; Chen, K.W. Carbon storage estimation of tropical urban trees by an improved allometric model for aboveground biomass based on terrestrial laser scanning. Urban For. Urban Greening 2019, 44, 126387. [Google Scholar] [CrossRef]
- Roy, S.; Byrne, J.; Pickering, C. A systematic quantitative review of urban tree benefits, costs, and assessment methods across cities in different climatic zones. Urban For. Urban Greening 2012, 11, 351–363. [Google Scholar] [CrossRef]
- Nowak, D.J.; Greenfield, E.J.; Hoehn, R.E.; Lapoint, E. Carbon storage and sequestration by trees in urban and community areas of the United States. Environ. Pollut. 2013, 178, 229–236. [Google Scholar] [CrossRef]
- Ngo, K.M.; Lum, S. Aboveground biomass estimation of tropical street trees. J. Urban Ecol. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Zhao, D.; Cai, J.; Xu, Y.; Liu, Y.; Yao, M. Carbon sinks in urban public green spaces under carbon neutrality: A bibliometric analysis and systematic literature review. Urban For. Urban Greening 2023, 86, 128037. [Google Scholar] [CrossRef]
- Helen; Jarzebski, M. P.; Gasparatos, A. Land use change, carbon stocks and tree species diversity in green spaces of a secondary city in Myanmar, Pyin Oo Lwin. PLoS One 2019, 14, e0225331. [Google Scholar] [CrossRef]
- Ferreira, M.P.; Martins, G.B.; Almeida, T.M.H.; Ribeiro, R.S.; Veiga Júnior, V.F.; Paz, I.S.R.; Siqueira, M.F.; Kurtz, B.C. Estimating aboveground biomass of tropical urban forests with UAV-borne hyperspectral and LiDAR data. Urban For. Urban Greening 2024, 96, 128362. [Google Scholar] [CrossRef]
- McHale, M.R.; Burke, I.C.; Lefsky, M.A.; Peper, P.J.; McPherson, E.G. Urban forest biomass estimates: Is it important to use allometric relationships developed specifically for urban trees? Urban Ecosyst. 2009, 12, 95–113. [Google Scholar] [CrossRef]
- Heywood, V.H. The future of plant conservation and the role of botanic gardens. Plant Divers. 2017, 39, 309–313. [Google Scholar] [CrossRef]
- Westwood, M.; Cavender, N.; Meyer, A.; Smith, P. Botanic garden solutions to the plant extinction crisis. Plants People Planet 2021, 3, 22–32. [Google Scholar] [CrossRef]
- Catahan, N.; Hopwood, M.; Suraweera, P. Botanic garden tourism, social value, health, and well-being. J. Zool. Bot. Gard. 2024, 5, 187–199. [Google Scholar] [CrossRef]
- Gratani, L.; Catoni, R.; Tarquini, F. Carbon dioxide sequestration capability of the Botanical Garden of Rome: Environmental and economic benefits. Am. J. Plant Sci. 2019, 10, 1249–1260. [Google Scholar] [CrossRef]
- BGCI—Botanic Gardens Conservation International. The only global database of the world’s botanic gardens, with information on 3.571 institutions worldwide. Available online: https://gardensearch.bgci.org/ (accessed on 27 June 2024).
- Borelli, S.; Conigliaro, M.; Di Cagno, F. Urban Forests: A Global Perspective; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Bediaga, B.; Guedes-Bruni, R.R. Jardim Botânico do Rio de Janeiro: Dois séculos de história. In Guia de Árvores Notáveis: 200 Anos do Jardim Botânico do Rio de Janeiro; Ormindo, P., Ed.; Andrea Jakobsson Estúdio Editorial: Rio de Janeiro, Brazil, 2008; pp. 16–23. [Google Scholar]
- Coelho, M.A.N. O inventário da coleção. In Guia de Árvores Notáveis: 200 Anos do Jardim Botânico do Rio de Janeiro; Ormindo, P., Ed.; Andrea Jakobsson Estúdio Editorial: Rio de Janeiro, Brazil, 2008; pp. 24–31. [Google Scholar]
- Almeida, T.M.H.; Coelho, M.A.N.; Peixoto, A.L. Rio de Janeiro Botanical Garden: Biodiversity conservation in a tropical arboretum. J. Zool. Bot. Gard. 2024, 5, 378–394. [Google Scholar] [CrossRef]
- PPBio—Programa de Pesquisa em Biodiversidade. Instructions for surveying woody plants in RAPELD grids and modules. Available online: https://ppbio.inpa.gov.br/sites/default/files/Protocol_for_the_survey_vascular_plants_RAPELD_grids_modules_2.pdf (accessed on 7 August 2024).
- Goodman, R.C.; Phillips, O.L.; Torres, D.C.; Freitas, L.; Cortese, S.T.; Monteagudo, A.; Baker, T.R. Amazon palm biomass and allometry. For. Ecol. Manage. 2013, 310, 994–1004. [Google Scholar] [CrossRef]
- Randrianasolo, Z.H.; Razafimahatratra, A.R.; Razafinarivo, R.N.G.; Randrianary, T.; Rakotovololonalimanana, H.; Rajemison, A.H.; Mamitiana, A.; Andriamanalina, R.L.; Rakotosoa, A.; Ramananantoandro, T. Which allometric models are the most appropriate for estimating aboveground biomass in secondary forests of Madagascar with Ravenala madagascariensis? Sci. Afr. 2019, 6, e00147. [Google Scholar] [CrossRef]
- Aguaron, E.; McPherson, E.G. Comparison of methods for estimating carbon dioxide storage by Sacramento’s urban forest. In Carbon Sequestration in Urban Ecosystems; Lal, R., Augustin, B., Eds.; Springer: Dordrecht, Netherlands, 2012; pp. 43–71. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Global wood density database. Dryad, 2009. Available online: http://hdl.handle.net/10255/dryad.235 (accessed on 8 January 2023).
- Réjou-Méchain, M.; Tanguy, A.; Piponiot, C.; Chave, J.; Hérault, B. Biomass: an R package for estimating above-ground biomass and its uncertainty in tropical forests. Methods Ecol. Evol. 2017, 8, 1163–1167. [Google Scholar] [CrossRef]
- SEEG—Sistema de Estimativas de Emissões e Remoções de Gases de Efeito Estufa. Municípios 2022: Estatísticas. Observatório do Clima. Available online: https://public.tableau.com/app/profile/seeg2472/viz/SEEGMUNICPIOS2022Estatsticas/RANKINGGERAL (accessed on 15 July 2024).
- Fonsêca, N.C.; Cunha, J.S.A.; Albuquerque, E.R.G.M.; Lins-e-Silva, A.C.B. Carbon stock in aboveground biomass and necromass in the Atlantic Forest: An analysis of data published between 2000 and 2021. An. Acad. Bras. Cienc. 2024, 96, e20220761. [Google Scholar] [CrossRef] [PubMed]
- Lindén, L.; Riikonen, A.; Setälä, H.; Yli-Pelkonen, V. Quantifying carbon stocks in urban parks under cold climate conditions. Urban For. Urban Greening 2020, 49, 126633. [Google Scholar] [CrossRef]
- Davies, Z.G.; Edmondson, J.L.; Heinemeyer, A.; Leake, J.R.; Gaston, K.J. Mapping an urban ecosystem service: Quantifying above-ground carbon storage at a city-wide scale. J. Appl. Ecol. 2011, 48, 1125–1134. [Google Scholar] [CrossRef]
- Schreyer, J.; Tigges, J.; Lakes, T.; Churkina, G. Using airborne LiDAR and QuickBird data for modelling urban tree carbon storage and its distribution—a case study of Berlin. Remote Sens. 2014, 6, 10636–10655. [Google Scholar] [CrossRef]
- Malage, L. Bosque Urbano Reinhard Maack: Contribuição no Enfrentamento da Mudança Climática por Florestas Urbanas. Graduation Monograph, Universidade Federal do Paraná, Curitiba, 2023.
- Lowe, W.A.M.; Silva, G.L.L.P.; Pushpakumara, D.K.N.G. Homegardens as a modern carbon storage: Assessment of tree diversity and above-ground biomass of homegardens in Matale district, Sri Lanka. Urban For. Urban Greening 2022, 74, 127671. [Google Scholar] [CrossRef]
- Padmakumar, B.; Sreekanth, N.P.; Shanthiprabha, V.; Paul, J.; Sreedharan, K.; Augustine, T.; Jayasooryan, K.K.; Rameshan, M.; Arunbabu, V.; Mohan, M.; et al. Unveiling tree diversity and carbon density of homegarden in the Thodupuzha urban region of Kerala, India: A contribution towards urban sustainability. Trop. Ecol. 2021, 62, 508–524. [Google Scholar] [CrossRef]
- Pedreira, L.O.L. (Secretaria Municipal de Meio Ambiente, Rio de Janeiro, RJ, Brazil). Personal communication, 2024.
- SMAC—Secretaria Municipal de Meio Ambiente. Inventário da Cobertura Arbórea da Cidade do Rio de Janeiro; Prefeitura da Cidade do Rio de Janeiro: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- PCRJ—Prefeitura da Cidade do Rio de Janeiro. Cobertura vegetal e uso da terra 2018. Available online: https://www.data.rio/datasets/c32974e0db954842b7af9a4816d7a821/explore (accessed on 4 January 2024).
- Pedreira, L.O.L.; Andrade, F.N.; Fico, B.V. Índices de Áreas Verdes do Município do Rio de Janeiro. Nota Técnica – No 37; Prefeitura da Cidade do Rio de Janeiro: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Liu, S.; Brandt, M.; Nord-Larsen, T.; Chave, J.; Reiner, F.; Lang, N.; Tong, X.; Ciais, P.; Igel, C.; Pascual, A.; et al. (2023). The overlooked contribution of trees outside forests to tree cover and woody biomass across Europe. Sci. Adv. 2023, 9, eadh4097. [Google Scholar] [CrossRef] [PubMed]

| Taxonomic component | Allometric model | Reference |
|---|---|---|
| Gymnosperms (excluding Cycadaceae and Zamiaceae), magnoliids, and eudicots | AGB = 0.1054(HpD2)0.9417 | [13] |
| Palms, arborescent monocotyledons, Cycadaceae and Zamiaceae | AGB0.25 = 0.55512(dmfD2Hstem)0.25 | [31] |
| Ravenala madagascariensis | AGB = EXP(-4.996+5.654ln(H)-0.772(ln(H))2) | [32] |
| Family/species | H | D | p | AGB |
|---|---|---|---|---|
| LEGUMINOSAE Samanea saman (Jacq.) Merr. | 27.73 | 224.54 | 0.59 | 39.32 |
| MELIACEAE Khaya senegalensis A.Juss. | 44.35 | 150.56 | 0.63 | 30.47 |
| MELIACEAE Khaya senegalensis A.Juss. | 38.35 | 155.97 | 0.63 | 28.40 |
| MALVACEAE Ceiba pentandra (L.) Gaertn. | 39.63 | 219.00 | 0.31 | 28.21 |
| MYRTACEAE Eucalyptus globulus Labill. | 33.69 | 150.62 | 0.72 | 26.90 |
| MYRTACEAE Corymbia citriodora (Hook.) K.D.Hill. & L.A.S.Johnson | 36.37 | 125.10 | 0.80 | 22.57 |
| MALVACEAE Sterculia apetala (Jacq.) H.Karst. | 30.51 | 194.81 | 0.39 | 22.39 |
| LEGUMINOSAE Swartzia langsdorffii Raddi | 24.64 | 144.51 | 0.85 | 21.59 |
| City, country | Estimation extent | Vegetation component | Carbon density | Reference |
|---|---|---|---|---|
| Rio de Janeiro, Brazil | Rio de Janeiro Botanical Garden arboretum | AGC; trees (DBH ≥ 10 cm) | 103.7 | This study |
| Rio de Janeiro, Brazil | Rio de Janeiro Botanical Garden arboretum | AGC + BGC; trees (DBH ≥ 10 cm) | 130.7 | This study |
| Leicester, England | Urban area | AGC; herbs, shrubs, and trees | 31.6 | [40] |
| Leicester, England | Areas of tree cover on publicly owned/managed sites | AGC; herbs, shrubs, and trees | 288.6 | [40] |
| Berlin, Germany | Urban area (urban forests not included) | AGC; trees | 11.5 | [41] |
| Jersey City, USA | Urban area | AGC + BGC; trees (DBH ≥ 2.54 cm) | 5 | [15] |
| Morgantown, USA | Urban area | AGC + BGC; trees (DBH ≥ 2.54 cm) | 37.7 | [15] |
| Helsinki, Finland | Urban constructed parks | AGC; trees (DBH ≥ 2.5 cm) | 22 - 28 | [39] |
| Singapore | Telok Kurau neighborhood | AGC + BGC; woody trees | 7.3 | [13] |
| Pyin Oo Lwin, Myanmar | National Botanical Gardens | AGC + BGC; herbs, shrubs, and trees | 177.1 | [18] |
| Pyin Oo Lwin, Myanmar | Other urban green spaces | AGC + BGC; herbs, shrubs, and trees | 71.9 - 225.5 | [18] |
| Curitiba, Brazil | Urban forest | AGC + BGC; trees (DBH > 5 cm) | 102.3 | [42] |
| Matale District, Sri Lanka | Urban to rural homegardens | AGC; trees (DBH > 5 cm) | 0.8 - 139.4 | [43] |
| Thodupuza, India | Urban homegardens | AGC + BGC; trees (DBH ≥ 3 cm) | 28.2 - 39.5 | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





