Submitted:
13 August 2024
Posted:
15 August 2024
You are already at the latest version
Abstract
Keywords:
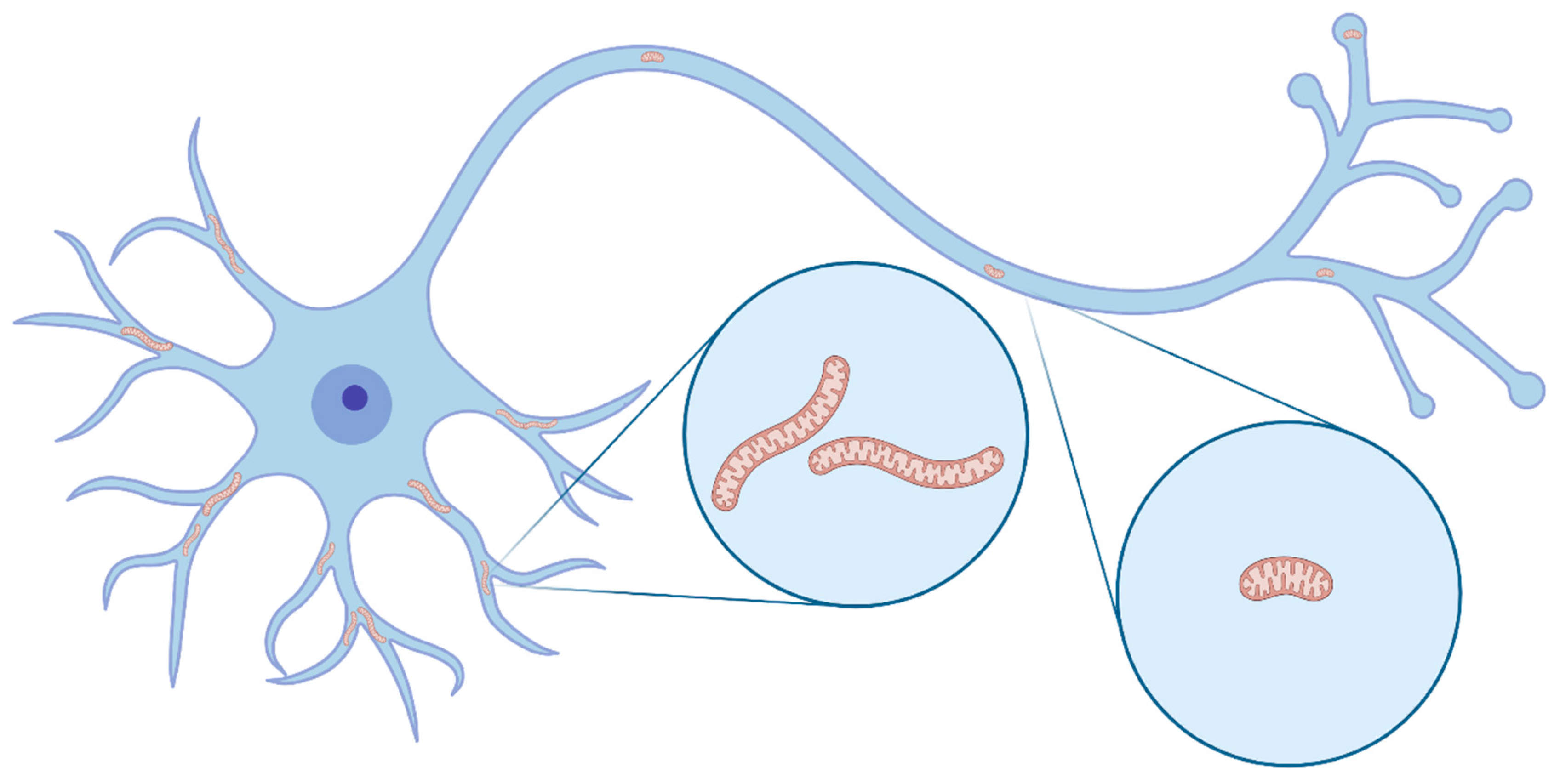
1. Intro: The Role of Mitochondria in Neurons
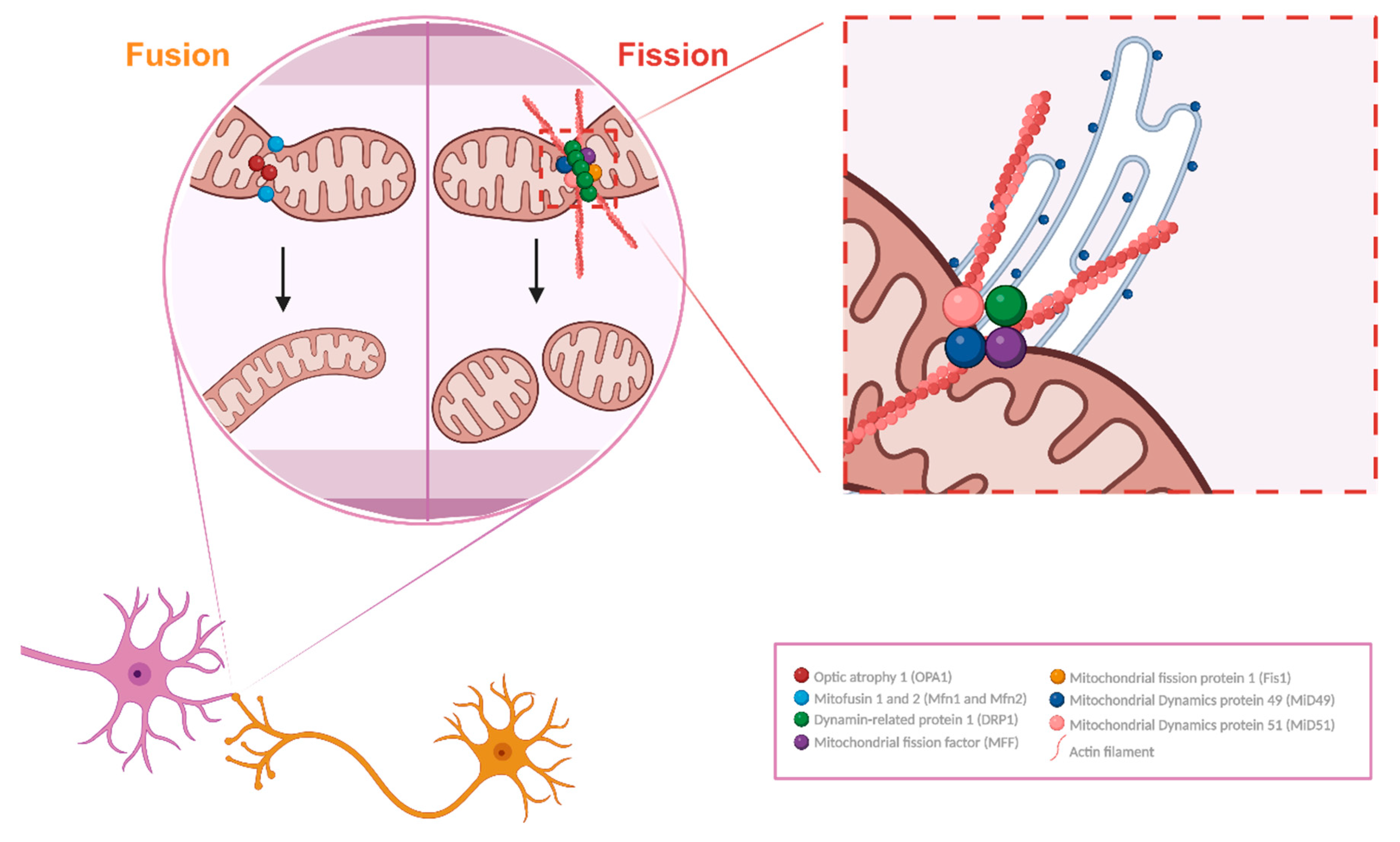
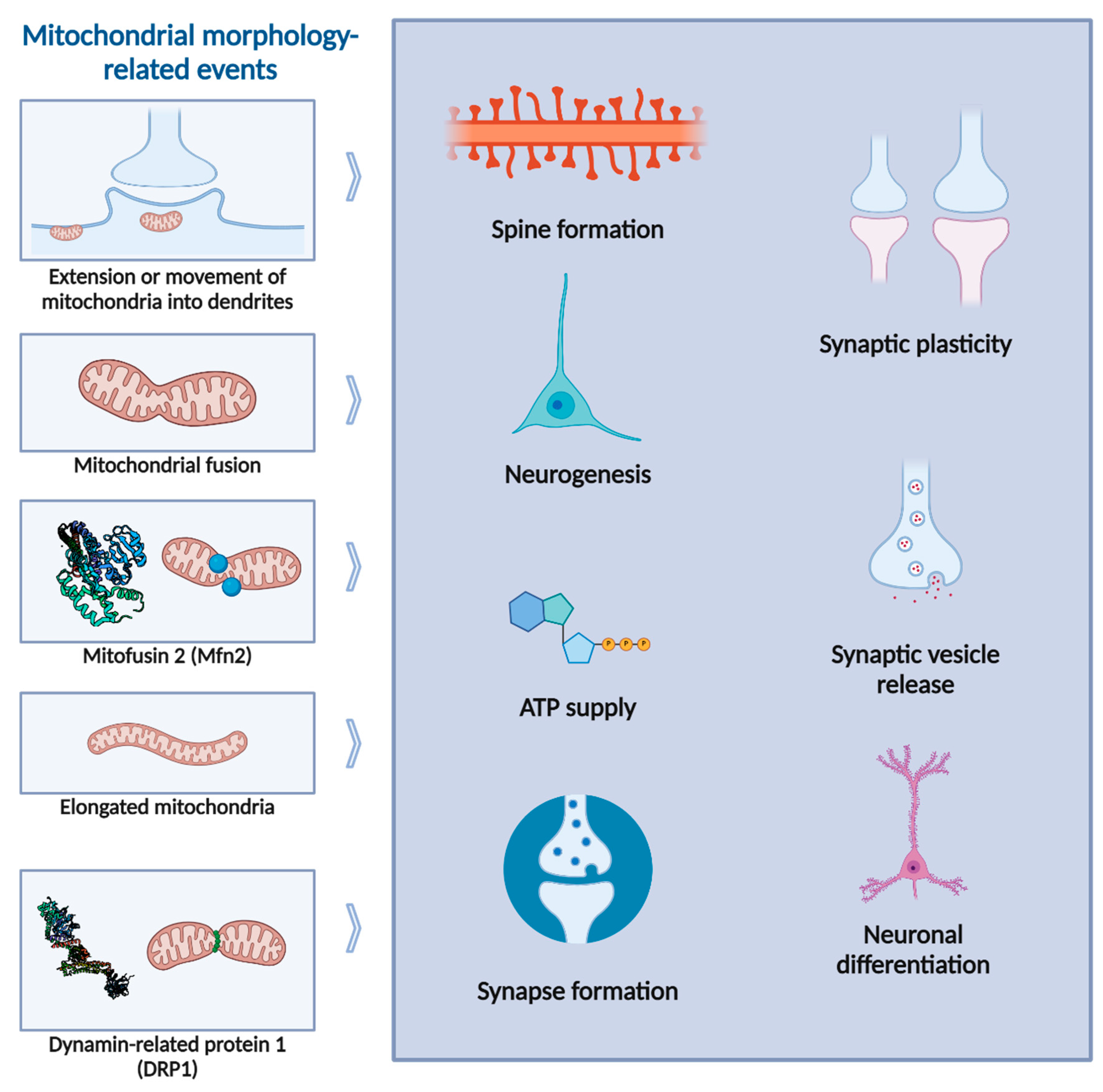
2. Mitochondrial Morphology and Dynamics
- a)
- Fusion
- b)
- Fission
3. Mitochondrial Morphology in the Synapse
4. Mitochondrial Morphology: A Role in Disease
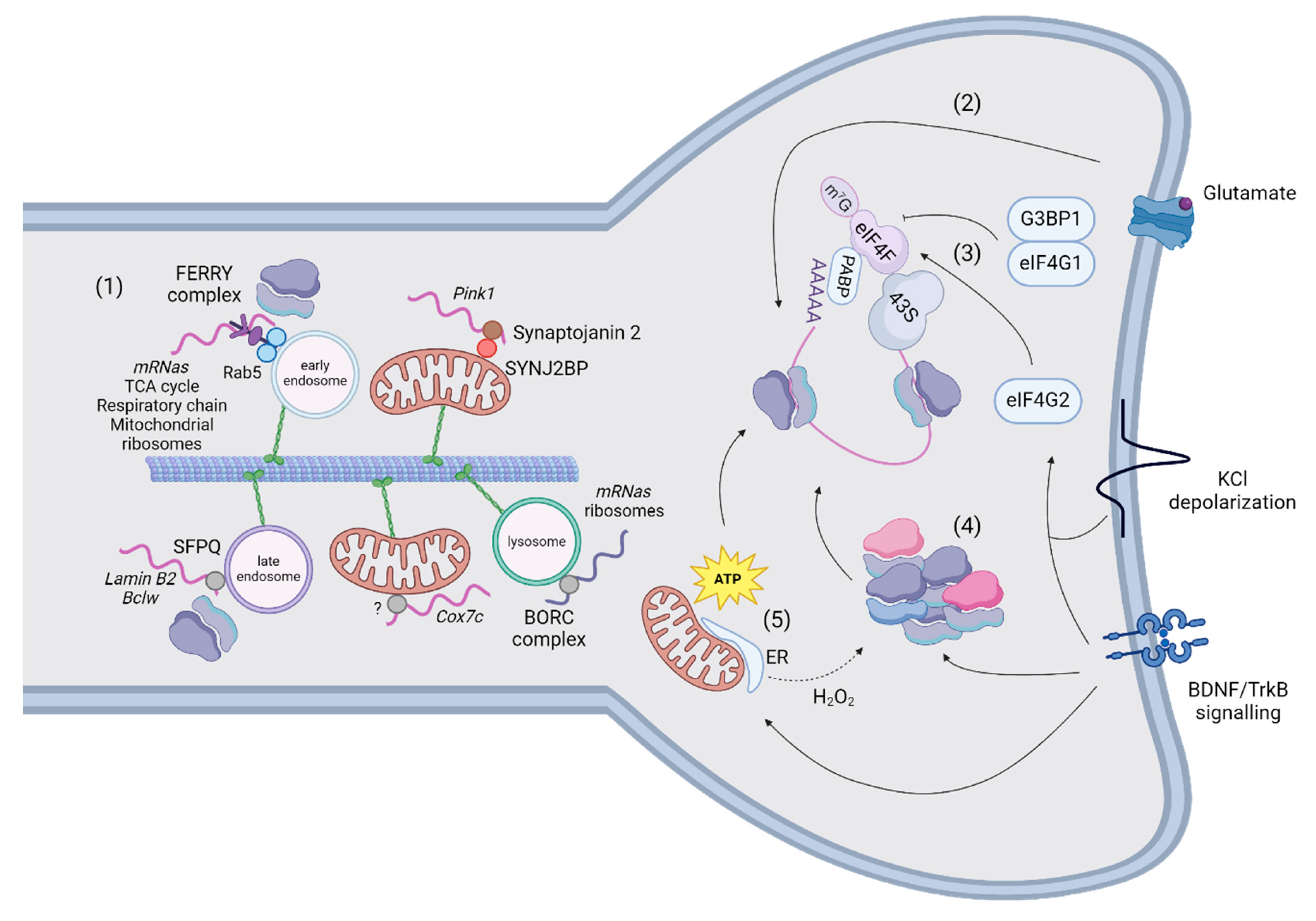
5. RNA-Binding Proteins and Nuclear-Encoded Mitochondrial Transcripts
- a)
- Mechanisms of mRNA transport
- b)
- Translation in axons and dendrites
6. Translation and mRNA Regulation of Mitochondria-Shaping Proteins in Synapses
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rossi, M.J.; Pekkurnaz, G. Powerhouse of the mind: mitochondrial plasticity at the synapse. Curr. Opin. Neurobiol. 2019, 57, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic Energy Use and Supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.C.; Bartol, T.M.; Phan, S.; Bushong, E.A.; Perkins, G.; Sejnowski, T.J.; Ellisman, M.H.; Skupin, A. Mitochondrial morphology provides a mechanism for energy buffering at synapses. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Duarte, F.V.; Ciampi, D.; Duarte, C.B. Mitochondria as central hubs in synaptic modulation. Cell. Mol. Life Sci. 2023, 80, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.-X.; Tan, X.; Fang, H.; Lau, P.-M.; Wang, X.; Cheng, H.; Bi, G.-Q. Dendritic mitoflash as a putative signal for stabilizing long-term synaptic plasticity. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Lee, A.; Hirabayashi, Y.; Kwon, S.-K.; Lewis, T.L.; Polleux, F. Emerging roles of mitochondria in synaptic transmission and neurodegeneration. Curr. Opin. Physiol. 2018, 3, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.L.; Kwon, S.-K.; Lee, A.; Shaw, R.; Polleux, F. MFF-dependent mitochondrial fission regulates presynaptic release and axon branching by limiting axonal mitochondria size. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Rangaraju, V.; Lauterbach, M.; Schuman, E.M. Spatially Stable Mitochondrial Compartments Fuel Local Translation during Plasticity. Cell 2019, 176, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Ivannikov, M.V.; Sugimori, M.; Llinás, R.R. Synaptic Vesicle Exocytosis in Hippocampal Synaptosomes Correlates Directly with Total Mitochondrial Volume. J. Mol. Neurosci. 2012, 49, 223–230. [Google Scholar] [CrossRef]
- R. L. Doser, K. M. R. L. Doser, K. M. Knight, E. W. Deihl, and F. J. Hoerndli, “Activity-dependent mitochondrial ROS signaling regulates recruitment of glutamate receptors to synapses,” Elife, vol. 13, Mar. 2024.
- Palade, G.E. The fine structure of mitochondria. Anat. Rec. 1952, 114, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Cserép, C.; Pósfai, B.; Schwarcz, A.D.; Dénes. Mitochondrial Ultrastructure Is Coupled to Synaptic Performance at Axonal Release Sites. eneuro 2018, 5. [Google Scholar] [CrossRef]
- Freeman, D.W.; Petralia, R.S.; Wang, Y.-X.; Mattson, M.P.; Yao, P.J. Mitochondria in hippocampal presynaptic and postsynaptic compartments differ in size as well as intensity. Matters 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.; Medvedev, N.I.; Davies, H.A.; Stewart, M.G. Mitochondria form a filamentous reticular network in hippocampal dendrites but are present as discrete bodies in axons: A three-dimensional ultrastructural study. J. Comp. Neurol. 2005, 492, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Delgado, T.; Petralia, R.S.; Freeman, D.W.; Sedlacek, M.; Wang, Y.-X.; Brenowitz, S.D.; Sheu, S.-H.; Gu, J.W.; Kapogiannis, D.; Mattson, M.P.; et al. Comparing 3D ultrastructure of presynaptic and postsynaptic mitochondria. Biol. Open 2019, 8, bio044834. [Google Scholar] [CrossRef]
- J. Faitg et al., “3D neuronal mitochondrial morphology in axons, dendrites, and somata of the aging mouse hippocampus,” Cell Rep., vol. 36, no. 6, p. 109509, Aug. 2021.
- Badal, K.K.; Akhmedov, K.; Lamoureux, P.; Liu, X.-A.; Reich, A.; Fallahi-Sichani, M.; Swarnkar, S.; Miller, K.E.; Puthanveettil, S.V. Synapse Formation Activates a Transcriptional Program for Persistent Enhancement in the Bi-directional Transport of Mitochondria. Cell Rep. 2019, 26, 507–517. [Google Scholar] [CrossRef]
- Brown, M.R.; Sullivan, P.G.; Geddes, J.W. Synaptic Mitochondria Are More Susceptible to Ca2+Overload than Nonsynaptic Mitochondria. J. Biol. Chem. 2006, 281, 11658–11668. [Google Scholar] [CrossRef]
- Stauch, K.L.; Purnell, P.R.; Fox, H.S. Quantitative Proteomics of Synaptic and Nonsynaptic Mitochondria: Insights for Synaptic Mitochondrial Vulnerability. J. Proteome Res. 2014, 13, 2620–2636. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, S.V.; Waseem, T.V.; Puchkova, L.V. Biogenetic and morphofunctional heterogeneity of mitochondria: the case of synaptic mitochondria. Prog. Neurobiol. 2017, 28, 363–373. [Google Scholar] [CrossRef]
- Bomba-Warczak, E.; Edassery, S.L.; Hark, T.J.; Savas, J.N. Long-lived mitochondrial cristae proteins in mouse heart and brain. J. Cell Biol. 2021, 220. [Google Scholar] [CrossRef]
- Graham, L.C.; Eaton, S.L.; Brunton, P.J.; Atrih, A.; Smith, C.; Lamont, D.J.; Gillingwater, T.H.; Pennetta, G.; Skehel, P.; Wishart, T.M. Proteomic profiling of neuronal mitochondria reveals modulators of synaptic architecture. Mol. Neurodegener. 2017, 12, 1–16. [Google Scholar] [CrossRef]
- Bulovaite, E.; Qiu, Z.; Kratschke, M.; Zgraj, A.; Fricker, D.G.; Tuck, E.J.; Gokhale, R.; Koniaris, B.; Jami, S.A.; Merino-Serrais, P.; et al. A brain atlas of synapse protein lifetime across the mouse lifespan. Neuron 2022, 110, 4057–4073. [Google Scholar] [CrossRef] [PubMed]
- Seager, R.; Lee, L.; Henley, J.M.; Wilkinson, K.A. Mechanisms and roles of mitochondrial localisation and dynamics in neuronal function. Neuronal Signal. 2020, 4, NS20200008. [Google Scholar] [CrossRef]
- Pekkurnaz, G.; Wang, X. Mitochondrial heterogeneity and homeostasis through the lens of a neuron. Nat. Metab. 2022, 4, 802–812. [Google Scholar] [CrossRef]
- J. Bereiter-Hahn and M. Vöth, “Dynamics of mitochondria in living cells: Shape changes, dislocations, fusion, and fission of mitochondria,” Microsc. Res. Tech., vol. 27, no. 3, pp. 198–219, Feb. 1994.
- Protasoni, M.; Zeviani, M. Mitochondrial Structure and Bioenergetics in Normal and Disease Conditions. Int. J. Mol. Sci. 2021, 22, 586. [Google Scholar] [CrossRef]
- G. Chandhok, M. Lazarou, and B. Neumann, “Structure, function, and regulation of mitofusin-2 in health and disease,” Biol. Rev., vol. 93, no. 2, pp. 933–949, May 2018.
- Pernas, L.; Scorrano, L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu. Rev. Physiol. 2016, 78, 505–531. [Google Scholar] [CrossRef] [PubMed]
- von der, Malsburg; et al. , “Structural mechanism of mitochondrial membrane remodelling by human OPA1,” Nature, vol. 620, no. 7976, pp. 1101–1108, Aug. 2023.
- Quintana-Cabrera, R.; Scorrano, L. Determinants and outcomes of mitochondrial dynamics. Mol. Cell 2023, 83, 857–876. [Google Scholar] [CrossRef]
- Knott, A.B.; Perkins, G.; Schwarzenbacher, R.; Bossy-Wetzel, E. Mitochondrial fragmentation in neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 505–518. [Google Scholar] [CrossRef]
- Kraus, F.; Roy, K.; Pucadyil, T.J.; Ryan, M.T. Function and regulation of the divisome for mitochondrial fission. Nature 2021, 590, 57–66. [Google Scholar] [CrossRef]
- Atkins, K.; Dasgupta, A.; Chen, K.-H.; Mewburn, J.; Archer, S.L. The role of Drp1 adaptor proteins MiD49 and MiD51 in mitochondrial fission: implications for human disease. Clin. Sci. 2016, 130, 1861–1874. [Google Scholar] [CrossRef]
- Egner, J.M.; Nolden, K.A.; Harwig, M.C.; Bonate, R.P.; De Anda, J.; Tessmer, M.H.; Noey, E.L.; Ihenacho, U.K.; Liu, Z.; Peterson, F.C.; et al. Structural studies of human fission protein FIS1 reveal a dynamic region important for GTPase DRP1 recruitment and mitochondrial fission. J. Biol. Chem. 2022, 298, 102620. [Google Scholar] [CrossRef]
- Su, B.; Ji, Y.-S.; Sun, X.-L.; Liu, X.-H.; Chen, Z.-Y. Brain-derived Neurotrophic Factor (BDNF)-induced Mitochondrial Motility Arrest and Presynaptic Docking Contribute to BDNF-enhanced Synaptic Transmission. J. Biol. Chem. 2014, 289, 1213–1226. [Google Scholar] [CrossRef]
- Cheng, A.; Hou, Y.; Mattson, M.P. Mitochondria and neuroplasticity. ASN Neuro 2010, 2, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Faria-Pereira and V., A. Morais, “Synapses: The Brain’s Energy-Demanding Sites,” Int. J. Mol. Sci., vol. 23, no. 7, p. 3627, Mar. 2022.
- Nguyen, P.V.; Atwood, H.L.; Marin, L. Altered impulse activity modifies synaptic physiology and mitochondria in crayfish phasic motor neurons. J. Neurophysiol. 1994, 72, 2944–2955. [Google Scholar] [CrossRef] [PubMed]
- Alnaes, E.; Rahamimoff, R. On the role of mitochondria in transmitter release from motor nerve terminals. J. Physiol. 1975, 248, 285–306. [Google Scholar] [CrossRef]
- Tang, Y.-G.; Zucker, R.S. Mitochondrial Involvement in Post-Tetanic Potentiation of Synaptic Transmission. Neuron 1997, 18, 483–491. [Google Scholar] [CrossRef]
- Billups, B.; Forsythe, I.D. Presynaptic Mitochondrial Calcium Sequestration Influences Transmission at Mammalian Central Synapses. J. Neurosci. 2002, 22, 5840–5847. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Okamoto, K.-I.; Hayashi, Y.; Sheng, M. The Importance of Dendritic Mitochondria in the Morphogenesis and Plasticity of Spines and Synapses. Cell 2004, 119, 873–887. [Google Scholar] [CrossRef]
- Mattson, M.P. Mitochondrial Regulation of Neuronal Plasticity. Neurochem. Res. 2006, 32, 707–715. [Google Scholar] [CrossRef] [PubMed]
- C. I. Thomas, M. A. C. I. Thomas, M. A. Ryan, N. Kamasawa, and B. Scholl, “Postsynaptic mitochondria are positioned to support functional diversity of dendritic spines,” Elife, vol. 12, pp. 1–17, Dec. 2023.
- Fang, D.; Yan, S.; Yu, Q.; Chen, D.; Yan, S.S. Mfn2 is Required for Mitochondrial Development and Synapse Formation in Human Induced Pluripotent Stem Cells/hiPSC Derived Cortical Neurons. Sci. Rep. 2016, 6, 31462. [Google Scholar] [CrossRef]
- Kochan, S.M.; Malo, M.C.; Jevtic, M.; Jahn-Kelleter, H.M.; Wani, G.A.; Ndoci, K.; Pérez-Revuelta, L.; Gaedke, F.; Schäffner, I.; Lie, D.C.; et al. Enhanced mitochondrial fusion during a critical period of synaptic plasticity in adult-born neurons. Neuron 2024, 112, 1997–2014. [Google Scholar] [CrossRef]
- Virga, D.M.; Hamilton, S.; Osei, B.; Morgan, A.; Kneis, P.; Zamponi, E.; Park, N.J.; Hewitt, V.L.; Zhang, D.; Gonzalez, K.C.; et al. Activity-dependent compartmentalization of dendritic mitochondria morphology through local regulation of fusion-fission balance in neurons in vivo. Nat. Commun. 2024, 15, 1–21. [Google Scholar] [CrossRef]
- Ishihara, N.; Nomura, M.; Jofuku, A.; Kato, H.; Suzuki, S.O.; Masuda, K.; Otera, H.; Nakanishi, Y.; Nonaka, I.; Goto, Y.-I.; et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 2009, 11, 958–966. [Google Scholar] [CrossRef]
- Singh, M.; Denny, H.; Smith, C.; Granados, J.; Renden, R. Presynaptic loss of dynamin-related protein 1 impairs synaptic vesicle release and recycling at the mouse calyx of Held. J. Physiol. 2018, 596, 6263–6287. [Google Scholar] [CrossRef]
- Cardanho-Ramos, C.; Faria-Pereira, A.; Morais, V.A. Orchestrating mitochondria in neurons: Cytoskeleton as the conductor. Cytoskeleton 2019, 77, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Peng, H.B. The Function of Mitochondria in Presynaptic Development at the Neuromuscular Junction. Mol. Biol. Cell 2008, 19, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Cai, Q.; Lu, W.; Sheng, Z.-H.; Mochida, S. KIF5B Motor Adaptor Syntabulin Maintains Synaptic Transmission in Sympathetic Neurons. J. Neurosci. 2009, 29, 13019–13029. [Google Scholar] [CrossRef]
- Rizzuto, R. Intracellular Ca2+ pools in neuronal signalling. Curr. Opin. Neurobiol. 2001, 11, 306–311. [Google Scholar] [CrossRef]
- Devine, M.J.; Kittler, J.T. Mitochondria at the neuronal presynapse in health and disease. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef]
- Sun, T.; Qiao, H.; Pan, P.-Y.; Chen, Y.; Sheng, Z.-H. Motile Axonal Mitochondria Contribute to the Variability of Presynaptic Strength. Cell Rep. 2013, 4, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Lores-Arnaiz, S.; Bustamante, J. Age-related alterations in mitochondrial physiological parameters and nitric oxide production in synaptic and non-synaptic brain cortex mitochondria. Neuroscience 2011, 188, 117–124. [Google Scholar] [CrossRef]
- Davey, G.P.; Peuchen, S.; Clark, J.B. Energy Thresholds in Brain Mitochondria. J. Biol. Chem. 1998, 273, 12753–12757. [Google Scholar] [CrossRef]
- Li, S.; Xiong, G.-J.; Huang, N.; Sheng, Z.-H. The cross-talk of energy sensing and mitochondrial anchoring sustains synaptic efficacy by maintaining presynaptic metabolism. Nat. Metab. 2020, 2, 1077–1095. [Google Scholar] [CrossRef] [PubMed]
- E. Schuman and D. Chan, “Fueling synapses.,” Cell, vol. 119, no. 6, pp. 738–40, Dec. 2004.
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Chicurel, M.E.; Harris, K.M. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J. Comp. Neurol. 1992, 325, 169–182. [Google Scholar] [CrossRef]
- Cameron, H.A.; Kaliszewski, C.K.; Greer, C.A. Organization of mitochondria in olfactory bulb granule cell dendritic spines. Synapse 1991, 8, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Engmann, O.; Teylan, M.A.; Nairn, A.C.; Greengard, P.; Kim, Y. WAVE1 controls neuronal activity-induced mitochondrial distribution in dendritic spines. Proc. Natl. Acad. Sci. 2008, 105, 3112–3116. [Google Scholar] [CrossRef]
- AP Silva, C.; Yalnizyan-Carson, A.; Busch, M.V.F.; van Zwieten, M.; Verhage, M.; Lohmann, C.; Neurogenomics, C.F. ; Netherlands Activity-dependent regulation of mitochondrial motility in developing cortical dendrites. eLife 2021, 10. [Google Scholar] [CrossRef]
- MacAskill, A.F.; Rinholm, J.E.; Twelvetrees, A.E.; Arancibia-Carcamo, I.L.; Muir, J.; Fransson, A.; Aspenstrom, P.; Attwell, D.; Kittler, J.T. Miro1 Is a Calcium Sensor for Glutamate Receptor-Dependent Localization of Mitochondria at Synapses. Neuron 2009, 61, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Rintoul, G.L.; Filiano, A.J.; Brocard, J.B.; Kress, G.J.; Reynolds, I.J. Glutamate Decreases Mitochondrial Size and Movement in Primary Forebrain Neurons. J. Neurosci. 2003, 23, 7881–7888. [Google Scholar] [CrossRef]
- MacAskill, A.F.; Kittler, J.T. Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 2009, 20, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Bapat, O.; Purimetla, T.; Kruessel, S.; Shah, M.; Fan, R.; Thum, C.; Rupprecht, F.; Langer, J.D.; Rangaraju, V. VAP spatially stabilizes dendritic mitochondria to locally support synaptic plasticity. Nat. Commun. 2024, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, V.; Devine, M.J.; Higgs, N.F.; Kittler, J.T. Miro1-dependent mitochondrial positioning drives the rescaling of presynaptic Ca 2+ signals during homeostatic plasticity. Embo Rep. 2016, 18, 231–240. [Google Scholar] [CrossRef] [PubMed]
- R. S. Zucker and W. G. Regehr, “Short-Term Synaptic Plasticity,” Annu. Rev. Physiol., vol. 64, no. 1, pp. 355–405, Mar. 2002.
- Divakaruni, S.S.; Van Dyke, A.M.; Chandra, R.; LeGates, T.A.; Contreras, M.; Dharmasri, P.A.; Higgs, H.N.; Lobo, M.K.; Thompson, S.M.; Blanpied, T.A. Long-Term Potentiation Requires a Rapid Burst of Dendritic Mitochondrial Fission during Induction. Neuron 2018, 100, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Oettinghaus, B.; Schulz, J.M.; Restelli, L.M.; Licci, M.; Savoia, C.; Schmidt, A.; Schmitt, K.; Grimm, A.; Morè, L.; Hench, J.; et al. Synaptic dysfunction, memory deficits and hippocampal atrophy due to ablation of mitochondrial fission in adult forebrain neurons. Cell Death Differ. 2016, 23, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Shields, L.Y.; Kim, H.; Zhu, L.; Haddad, D.; Berthet, A.; Pathak, D.; Lam, M.; Ponnusamy, R.; Diaz-Ramirez, L.G.; Gill, T.M.; et al. Dynamin-related protein 1 is required for normal mitochondrial bioenergetic and synaptic function in CA1 hippocampal neurons. Cell Death Dis. 2015, 6, e1725–e1725. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Engeln, M.; Schiefer, C.; Patton, M.H.; Martin, J.A.; Werner, C.T.; Riggs, L.M.; Francis, T.C.; McGlincy, M.; Evans, B.; et al. Drp1 Mitochondrial Fission in D1 Neurons Mediates Behavioral and Cellular Plasticity during Early Cocaine Abstinence. Neuron 2017, 96, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Tian, R.; Han, H.; Slone, J.; Wang, C.; Ke, X.; Zhang, T.; Li, X.; He, Y.; Liao, P.; et al. PINK1-mediated Drp1S616 phosphorylation modulates synaptic development and plasticity via promoting mitochondrial fission. Signal Transduct. Target. Ther. 2022, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, B.; Lee, H.-G.; Li, X.; Perry, G.; Smith, M.A.; Zhu, X. Impaired Balance of Mitochondrial Fission and Fusion in Alzheimer's Disease. J. Neurosci. 2009, 29, 9090–9103. [Google Scholar] [CrossRef]
- Wang, W.; Yin, J.; Ma, X.; Zhao, F.; Siedlak, S.L.; Wang, Z.; Torres, S.; Fujioka, H.; Xu, Y.; Perry, G.; et al. Inhibition of mitochondrial fragmentation protects against Alzheimer’s disease in rodent model. Hum. Mol. Genet. 2017, 26, 4118–4131. [Google Scholar] [CrossRef]
- Fischer, T.D.; Hylin, M.J.; Zhao, J.; Moore, A.N.; Waxham, M.N.; Dash, P.K. Altered Mitochondrial Dynamics and TBI Pathophysiology. Front. Syst. Neurosci. 2016, 10, 29–29. [Google Scholar] [CrossRef]
- Zhao, H.; Pan, W.; Chen, L.; Luo, Y.; Xu, R. Nur77 promotes cerebral ischemia–reperfusion injury via activating INF2-mediated mitochondrial fragmentation. Histochem. J. 2018, 49, 599–613. [Google Scholar] [CrossRef]
- Kumar, R.; Bukowski, M.J.; Wider, J.M.; Reynolds, C.A.; Calo, L.; Lepore, B.; Tousignant, R.; Jones, M.; Przyklenk, K.; Sanderson, T.H. Mitochondrial dynamics following global cerebral ischemia. Mol. Cell. Neurosci. 2016, 76, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Luo, Y.; Chen, L.; Zhang, Z.; Shen, C.; Li, Y.; Xu, R. Sirt3 inhibits cerebral ischemia-reperfusion injury through normalizing Wnt/β-catenin pathway and blocking mitochondrial fission. Cell Stress Chaperon- 2018, 23, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Zeigler, M.; Mizuno, S.; Morrison, R.S.; Totah, R.A.; Barker-Haliski, M. Reductions in Hydrogen Sulfide and Changes in Mitochondrial Quality Control Proteins Are Evident in the Early Phases of the Corneally Kindled Mouse Model of Epilepsy. Int. J. Mol. Sci. 2022, 23, 1434. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Park, H.; Kim, T.-H.; Kang, T.-C. LONP1 Regulates Mitochondrial Accumulations of HMGB1 and Caspase-3 in CA1 and PV Neurons Following Status Epilepticus. Int. J. Mol. Sci. 2021, 22, 2275. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Sun, P.; Zhang, H.; Yang, H. Mitochondrial quality control in the brain: The physiological and pathological roles. Front. Neurosci. 2022, 16, 1075141. [Google Scholar] [CrossRef] [PubMed]
- Verrigni, D.; Di Nottia, M.; Ardissone, A.; Baruffini, E.; Nasca, A.; Legati, A.; Bellacchio, E.; Fagiolari, G.; Martinelli, D.; Fusco, L.; et al. Clinical-genetic features and peculiar muscle histopathology in infantileDNM1L-related mitochondrial epileptic encephalopathy. Hum. Mutat. 2019, 40, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Vanstone, J.R.; Smith, A.M.; McBride, S.; Naas, T.; Holcik, M.; Antoun, G.; Harper, M.-E.; Michaud, J.; Sell, E.; Chakraborty, P.; et al. DNM1L-related mitochondrial fission defect presenting as refractory epilepsy. Eur. J. Hum. Genet. 2015, 24, 1084–1088. [Google Scholar] [CrossRef]
- Waterham, H.R.; Koster, J.; van Roermund, C.W.; Mooyer, P.A.; Wanders, R.J.; Leonard, J.V. A Lethal Defect of Mitochondrial and Peroxisomal Fission. New Engl. J. Med. 2007, 356, 1736–1741. [Google Scholar] [CrossRef]
- Misko, A.L.; Sasaki, Y.; Tuck, E.; Milbrandt, J.; Baloh, R.H. Mitofusin2 Mutations Disrupt Axonal Mitochondrial Positioning and Promote Axon Degeneration. J. Neurosci. 2012, 32, 4145–4155. [Google Scholar] [CrossRef]
- Pham, A.H.; Meng, S.; Chu, Q.N.; Chan, D.C. Loss of Mfn2 results in progressive, retrograde degeneration of dopaminergic neurons in the nigrostriatal circuit. Hum. Mol. Genet. 2012, 21, 4817–4826. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Sterky, F.H.; Mourier, A.; Terzioglu, M.; Cullheim, S.; Olson, L.; Larsson, N.-G. Mitofusin 2 is necessary for striatal axonal projections of midbrain dopamine neurons. Hum. Mol. Genet. 2012, 21, 4827–4835. [Google Scholar] [CrossRef] [PubMed]
- E Kushnareva, Y.; A Gerencser, A.; Bossy, B.; Ju, W.-K.; White, A.D.; Waggoner, J.; Ellisman, M.H.; Perkins, G.; Bossy-Wetzel, E. Loss of OPA1 disturbs cellular calcium homeostasis and sensitizes for excitotoxicity. Cell Death Differ. 2012, 20, 353–365. [Google Scholar] [CrossRef]
- Zaninello, M.; Palikaras, K.; Sotiriou, A.; Tavernarakis, N.; Scorrano, L. Sustained intracellular calcium rise mediates neuronal mitophagy in models of autosomal dominant optic atrophy. Cell Death Differ. 2021, 29, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Zaninello, M.; Palikaras, K.; Naon, D.; Iwata, K.; Herkenne, S.; Quintana-Cabrera, R.; Semenzato, M.; Grespi, F.; Ross-Cisneros, F.N.; Carelli, V.; et al. Inhibition of autophagy curtails visual loss in a model of autosomal dominant optic atrophy. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- J. Koch et al., “Disturbed mitochondrial and peroxisomal dynamics due to loss of MFF causes Leigh-like encephalopathy, optic atrophy and peripheral neuropathy,” J. Med. Genet., vol. 53, no. 4, pp. 270–278, Apr. 2016.
- Andreassi, C.; Zimmermann, C.; Mitter, R.; Fusco, S.; De Vita, S.; Saiardi, A.; Riccio, A. An NGF-responsive element targets myo-inositol monophosphatase-1 mRNA to sympathetic neuron axons. Nat. Neurosci. 2010, 13, 291–301. [Google Scholar] [CrossRef]
- Jung, J.; Ohk, J.; Kim, H.; Holt, C.E.; Park, H.J.; Jung, H. mRNA transport, translation, and decay in adult mammalian central nervous system axons. Neuron 2022, 111, 650–668. [Google Scholar] [CrossRef]
- C. Glock et al., “The translatome of neuronal cell bodies, dendrites, and axons,” Proc. Natl. Acad. Sci., vol. 118, no. 43, pp. 1–11, Oct. 2021.
- Fernandopulle, M.S.; Lippincott-Schwartz, J.; Ward, M.E. RNA transport and local translation in neurodevelopmental and neurodegenerative disease. Nat. Neurosci. 2021, 24, 622–632. [Google Scholar] [CrossRef]
- Voigt, A.; Herholz, D.; Fiesel, F.C.; Kaur, K.; Müller, D.; Karsten, P.; Weber, S.S.; Kahle, P.J.; Marquardt, T.; Schulz, J.B. TDP-43-Mediated Neuron Loss In Vivo Requires RNA-Binding Activity. PLOS ONE 2010, 5, e12247. [Google Scholar] [CrossRef]
- Daigle, J.G.; Lanson, J.N.A.; Smith, R.B.; Casci, I.; Maltare, A.; Monaghan, J.; Nichols, C.D.; Kryndushkin, D.; Shewmaker, F.; Pandey, U.B. RNA-binding ability of FUS regulates neurodegeneration, cytoplasmic mislocalization and incorporation into stress granules associated with FUS carrying ALS-linked mutations. Hum. Mol. Genet. 2012, 22, 1193–1205. [Google Scholar] [CrossRef]
- H. Ederle et al., “Nuclear egress of TDP-43 and FUS occurs independently of Exportin-1/CRM1,” Sci. Rep., vol. 8, no. 1, p. 7084, May 2018.
- Tsai, Y.-L.; Mu, Y.C.; Manley, J.L. Nuclear RNA transcript levels modulate nucleocytoplasmic distribution of ALS/FTD-associated protein FUS. Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Ziff, O.J.; Harley, J.; Wang, Y.; Neeves, J.; Tyzack, G.; Ibrahim, F.; Skehel, M.; Chakrabarti, A.M.; Kelly, G.; Patani, R. Nucleocytoplasmic mRNA redistribution accompanies RNA binding protein mislocalization in ALS motor neurons and is restored by VCP ATPase inhibition. Neuron 2023, 111, 3011–3027. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-L.; Coady, T.H.; Lu, L.; Zheng, D.; Alland, I.; Tian, B.; Shneider, N.A.; Manley, J.L. ALS/FTD-associated protein FUS induces mitochondrial dysfunction by preferentially sequestering respiratory chain complex mRNAs. Genes Dev. 2020, 34, 785–805. [Google Scholar] [CrossRef] [PubMed]
- Altman, T.; Ionescu, A.; Ibraheem, A.; Priesmann, D.; Gradus-Pery, T.; Farberov, L.; Alexandra, G.; Shelestovich, N.; Dafinca, R.; Shomron, N.; et al. Axonal TDP-43 condensates drive neuromuscular junction disruption through inhibition of local synthesis of nuclear encoded mitochondrial proteins. Nat. Commun. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Pazyra-Murphy, M.F.; Silagi, E.S.; Tasdemir-Yilmaz, O.E.; Li, Y.; Rose, L.; Yeoh, Z.C.; Vangos, N.E.; Geffken, E.A.; Seo, H.-S.; et al. Binding and transport of SFPQ-RNA granules by KIF5A/KLC1 motors promotes axon survival. J. Cell Biol. 2020, 220. [Google Scholar] [CrossRef] [PubMed]
- Baumann, S.; Komissarov, A.; Gili, M.; Ruprecht, V.; Wieser, S.; Maurer, S.P. A reconstituted mammalian APC-kinesin complex selectively transports defined packages of axonal mRNAs. Sci. Adv. 2020, 6, eaaz1588. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.C.; Grawenhoff, J.; Baumann, S.J.; Lorenzon, N.; Maurer, S.P. Mammalian Neuronal mRNA Transport Complexes: The Few Knowns and the Many Unknowns. Front. Integr. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
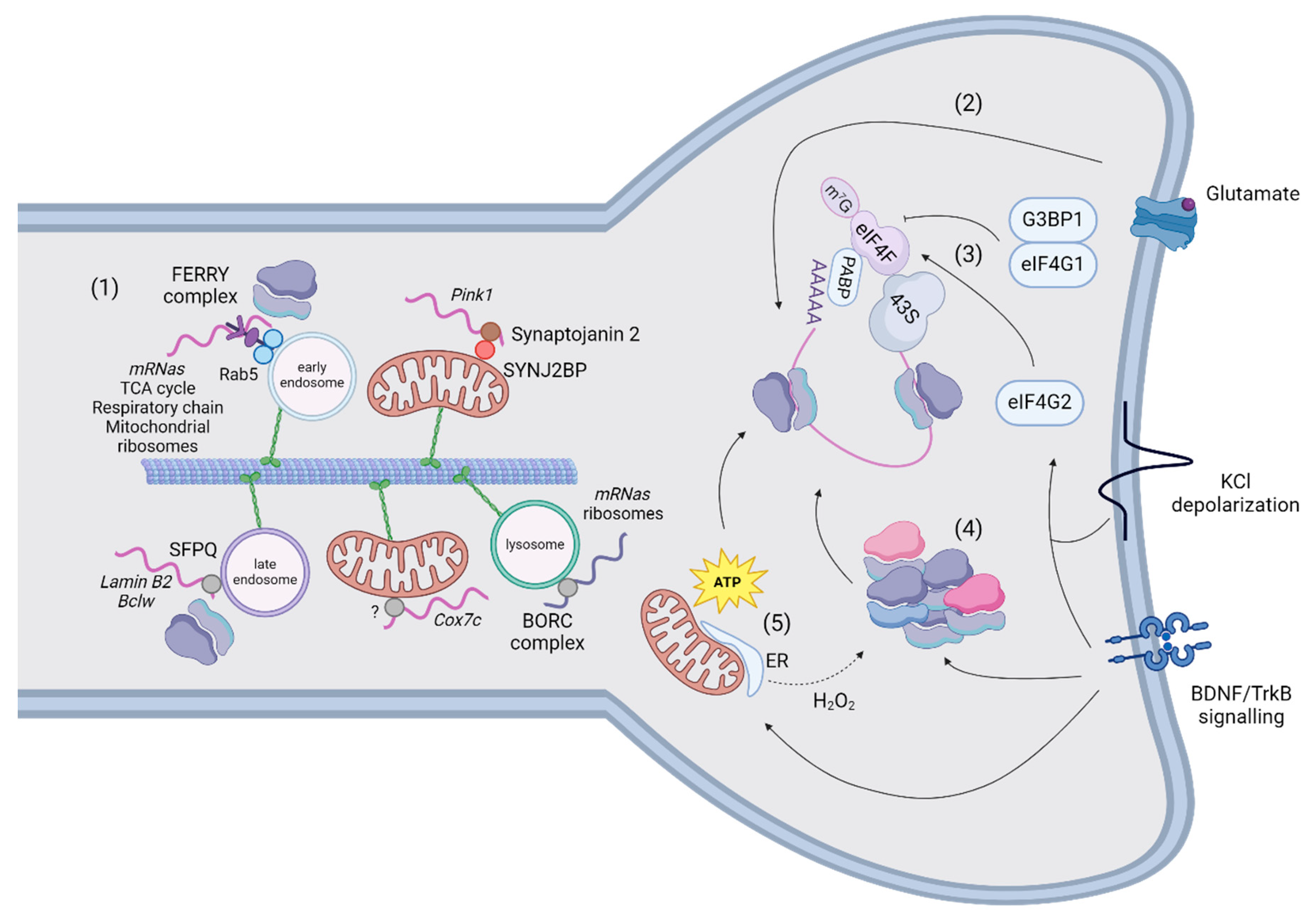
- Hacisuleyman, E.; Hale, C.R.; Noble, N.; Luo, J.-D.; Fak, J.J.; Saito, M.; Chen, J.; Weissman, J.S.; Darnell, R.B. Neuronal activity rapidly reprograms dendritic translation via eIF4G2:uORF binding. Nat. Neurosci. 2024, 27, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.F.; Oleynikov, Y.; Kislauskis, E.H.; Taneja, K.L.; Singer, R.H. Characterization of a β-Actin mRNA Zipcode-Binding Protein. Mol. Cell. Biol. 1997, 17, 2158–2165. [Google Scholar] [CrossRef]
- Hüttelmaier, S.; Zenklusen, D.; Lederer, M.; Dictenberg, J.; Lorenz, M.; Meng, X.; Bassell, G.J.; Condeelis, J.; Singer, R.H. Spatial regulation of β-actin translation by Src-dependent phosphorylation of ZBPNature 2005, 438, 512–515. [CrossRef]
- Loedige, I.; Baranovskii, A.; Mendonsa, S.; Dantsuji, S.; Popitsch, N.; Breimann, L.; Zerna, N.; Cherepanov, V.; Milek, M.; Ameres, S.; et al. mRNA stability and m6A are major determinants of subcellular mRNA localization in neurons. Mol. Cell 2023, 83, 2709–2725. [Google Scholar] [CrossRef]
- Zaninello, M.; Bean, C. Highly Specialized Mechanisms for Mitochondrial Transport in Neurons: From Intracellular Mobility to Intercellular Transfer of Mitochondria. Biomolecules 2023, 13, 938. [Google Scholar] [CrossRef]
- Harbauer, A.B.; Hees, J.T.; Wanderoy, S.; Segura, I.; Gibbs, W.; Cheng, Y.; Ordonez, M.; Cai, Z.; Cartoni, R.; Ashrafi, G.; et al. Neuronal mitochondria transport Pink1 mRNA via synaptojanin 2 to support local mitophagy. Neuron 2022, 110, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- J.-M. M. Cioni et al., “Late Endosomes Act as mRNA Translation Platforms and Sustain Mitochondria in Axons,” Cell, vol. 176, no. 1–2, pp. 56-72.e15, Jan. 2019. [CrossRef]
- Cohen, B.; Altman, T.; Golani-Armon, A.; Savulescu, A.F.; Ibraheem, A.; Mhlanga, M.M.; Perlson, E.; Arava, Y.S. Co-transport of the nuclear-encoded Cox7c mRNA with mitochondria along axons occurs through a coding-region-dependent mechanism. J. Cell Sci. 2022, 135. [Google Scholar] [CrossRef] [PubMed]
- Hees, J.T.; Wanderoy, S.; Lindner, J.; Helms, M.; Mahadevan, H.M.; Harbauer, A.B. Insulin signalling regulates Pink1 mRNA localization via modulation of AMPK activity to support PINK1 function in neurons. Nat. Metab. 2024, 6, 514–530. [Google Scholar] [CrossRef] [PubMed]
- Zaninello, M.; Schlegel, T.; Nolte, H.; Pirzada, M.; Savino, E.; Barth, E.; Klein, I.; Wüstenberg, H.; Uddin, T.; Wolff, L.; et al. CLUH maintains functional mitochondria and translation in motoneuronal axons and prevents peripheral neuropathy. Sci. Adv. 2024, 10, eadn2050. [Google Scholar] [CrossRef] [PubMed]
- Schuhmacher, J.S.; Dieck, S.T.; Christoforidis, S.; Landerer, C.; Gallesio, J.D.; Hersemann, L.; Seifert, S.; Schäfer, R.; Giner, A.; Toth-Petroczy, A.; et al. The Rab5 effector FERRY links early endosomes with mRNA localization. Mol. Cell 2023, 83, 1839–1855. [Google Scholar] [CrossRef] [PubMed]
- E Cosker, K.; Fenstermacher, S.J.; Pazyra-Murphy, M.F.; Elliott, H.L.; A Segal, R. The RNA-binding protein SFPQ orchestrates an RNA regulon to promote axon viability. Nat. Neurosci. 2016, 19, 690–696. [Google Scholar] [CrossRef]
- R. De Pace et al., “Messenger RNA transport on lysosomal vesicles maintains axonal mitochondrial homeostasis and prevents axonal degeneration,” Nat. Neurosci., vol. 27, no. 6, pp. 1087–1102, Jun. 2024.
- Campbell, D.S.; E Holt, C. Chemotropic Responses of Retinal Growth Cones Mediated by Rapid Local Protein Synthesis and Degradation. Neuron 2001, 32, 1013–1026. [Google Scholar] [CrossRef]
- Taylor, A.M.; Wu, J.; Tai, H.-C.; Schuman, E.M. Axonal Translation of β-Catenin Regulates Synaptic Vesicle Dynamics. J. Neurosci. 2013, 33, 5584–5589. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.F.; Martínez, J.C.; Hengst, U. Intra-axonal Synthesis of SNAP25 Is Required for the Formation of Presynaptic Terminals. Cell Rep. 2017, 20, 3085–3098. [Google Scholar] [CrossRef]
- Sun, C.; Nold, A.; Fusco, C.M.; Rangaraju, V.; Tchumatchenko, T.; Heilemann, M.; Schuman, E.M. The prevalence and specificity of local protein synthesis during neuronal synaptic plasticity. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Smart, F.M.; Edelman, G.M.; Vanderklish, P.W. BDNF induces translocation of initiation factor 4E to mRNA granules: Evidence for a role of synaptic microfilaments and integrins. Proc. Natl. Acad. Sci. 2003, 100, 14403–14408. [Google Scholar] [CrossRef]
- Kang, H.; Schuman, E.M. A Requirement for Local Protein Synthesis in Neurotrophin-Induced Hippocampal Synaptic Plasticity. Science 1996, 273, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Querido, J.B.; Díaz-López, I.; Ramakrishnan, V. The molecular basis of translation initiation and its regulation in eukaryotes. Nat. Rev. Mol. Cell Biol. 2023, 25, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Li, X.; Flores, A.D.; Lai, K.-O. The translation initiating factor eIF4E and arginine methylation underlie G3BP1 function in dendritic spine development of neurons. J. Biol. Chem. 2023, 299, 105029. [Google Scholar] [CrossRef]
- S. Callegari, L. D. Cruz-Zaragoza, and P. Rehling, “From TOM to the TIM23 complex – handing over of a precursor,” Biol. Chem., vol. 401, no. 6–7, pp. 709–721, May 2020.
- L. Van Haute et al., “Deficient methylation and formylation of mt-tRNAMet wobble cytosine in a patient carrying mutations in NSUN3,” Nat. Commun., vol. 7, no. 1, p. 12039, Jun. 2016.
- Graber, T.E.; Hébert-Seropian, S.; Khoutorsky, A.; David, A.; Yewdell, J.W.; Lacaille, J.-C.; Sossin, W.S. Reactivation of stalled polyribosomes in synaptic plasticity. Proc. Natl. Acad. Sci. 2013, 110, 16205–16210. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Moradi, M.; Reinhard, S.; Ji, C.; Jablonka, S.; Hennlein, L.; Lüningschrör, P.; Doose, S.; Sauer, M.; Sendtner, M. Dynamic remodeling of ribosomes and endoplasmic reticulum in axon terminals of motoneurons. J. Cell Sci. 2021, 134. [Google Scholar] [CrossRef] [PubMed]
- Fusco, C.M.; Desch, K.; Dörrbaum, A.R.; Wang, M.; Staab, A.; Chan, I.C.W.; Vail, E.; Villeri, V.; Langer, J.D.; Schuman, E.M. Neuronal ribosomes exhibit dynamic and context-dependent exchange of ribosomal proteins. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- E Ostroff, L.; Fiala, J.C.; Allwardt, B.; Harris, K.M. Polyribosomes Redistribute from Dendritic Shafts into Spines with Enlarged Synapses during LTP in Developing Rat Hippocampal Slices. Neuron 2002, 35, 535–545. [Google Scholar] [CrossRef]
- Chirillo, M.A.; Waters, M.S.; Lindsey, L.F.; Bourne, J.N.; Harris, K.M. Local resources of polyribosomes and SER promote synapse enlargement and spine clustering after long-term potentiation in adult rat hippocampus. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Biever, A.; Glock, C.; Tushev, G.; Ciirdaeva, E.; Dalmay, T.; Langer, J.D.; Schuman, E.M. Monosomes actively translate synaptic mRNAs in neuronal processes. Science 2020, 367, 526. [Google Scholar] [CrossRef]
- Baranov, S.V.; Baranova, O.V.; Yablonska, S.; Suofu, Y.; Vazquez, A.L.; Kozai, T.D.Y.; Cui, X.T.; Ferrando, L.M.; Larkin, T.M.; Tyurina, Y.Y.; et al. Mitochondria modulate programmed neuritic retraction. Proc. Natl. Acad. Sci. 2018, 116, 650–659. [Google Scholar] [CrossRef]
- Lores-Arnaiz, S.; Lombardi, P.; Karadayian, A.G.; Orgambide, F.; Cicerchia, D.; Bustamante, J. Brain cortex mitochondrial bioenergetics in synaptosomes and non-synaptic mitochondria during aging. Neurochem. Res. 2016, 41, 353–363. [Google Scholar] [CrossRef]
- C. Espino de la Fuente-Muñoz, M. C. Espino de la Fuente-Muñoz, M. Rosas-Lemus, P. Moreno-Castilla, F. Bermúdez-Rattoni, S. Uribe-Carvajal, and C. Arias, “Age-Dependent Decline in Synaptic Mitochondrial Function Is Exacerbated in Vulnerable Brain Regions of Female 3xTg-AD Mice,” Int. J. Mol. Sci., vol. 21, no. 22, p. 8727, Nov. 2020.
- Misgeld, T.; Schwarz, T.L. Mitostasis in Neurons: Maintaining Mitochondria in an Extended Cellular Architecture. Neuron 2017, 96, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Spillane, M.; Ketschek, A.; Merianda, T.T.; Twiss, J.L.; Gallo, G. Mitochondria Coordinate Sites of Axon Branching through Localized Intra-axonal Protein Synthesis. Cell Rep. 2013, 5, 1564–1575. [Google Scholar] [CrossRef]
- Cheng, A.; Wan, R.; Yang, J.-L.; Kamimura, N.; Son, T.G.; Ouyang, X.; Luo, Y.; Okun, E.; Mattson, M.P. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 2012, 3, 1250. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Wang, W.; Hwang, J.; Namgung, U.; Min, K.-T. Increased ER–mitochondria tethering promotes axon regeneration. Proc. Natl. Acad. Sci. 2019, 116, 16074–16079. [Google Scholar] [CrossRef]
- Cajigas, I.J.; Tushev, G.; Will, T.J.; Dieck, S.T.; Fuerst, N.; Schuman, E.M. The Local Transcriptome in the Synaptic Neuropil Revealed by Deep Sequencing and High-Resolution Imaging. Neuron 2012, 74, 453–466. [Google Scholar] [CrossRef]
- Nijssen, J.; Aguila, J.; Hoogstraaten, R.; Kee, N.; Hedlund, E. Axon-Seq Decodes the Motor Axon Transcriptome and Its Modulation in Response to ALS. Stem Cell Rep. 2018, 11, 1565–1578. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Cao, W.; Wang, Y.; Zhu, Q.; Luo, J.; Wang, B.; Zheng, H.; Weitz, D.A.; Zong, C. Droplet-based transcriptome profiling of individual synapses. Nat. Biotechnol. 2023, 41, 1332–1344. [Google Scholar] [CrossRef]
- Gonzalez-Lozano, M.A.; Koopmans, F.; Sullivan, P.F.; Protze, J.; Krause, G.; Verhage, M.; Li, K.W.; Liu, F.; Smit, A.B. Stitching the synapse: Cross-linking mass spectrometry into resolving synaptic protein interactions. Sci. Adv. 2020, 6, eaax5783. [Google Scholar] [CrossRef] [PubMed]
- M. van Oostrum et al., “The proteomic landscape of synaptic diversity across brain regions and cell types,” Cell, vol. 186, no. 24, pp. 5411-5427.e23, Nov. 2023.
- Sen, A.; Cox, R.T. Clueless is a conserved ribonucleoprotein that binds the ribosome at the mitochondrial outer membrane. Biol. Open 2016, 5, 195–203. [Google Scholar] [CrossRef]
- Gao, J.; Schatton, D.; Martinelli, P.; Hansen, H.; Pla-Martin, D.; Barth, E.; Becker, C.; Altmueller, J.; Frommolt, P.; Sardiello, M.; et al. CLUH regulates mitochondrial biogenesis by binding mRNAs of nuclear-encoded mitochondrial proteins. J. Cell Biol. 2014, 207, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sibilla, C.; Liu, R.; Yun, J.; Hay, B.A.; Blackstone, C.; Chan, D.C.; Harvey, R.J.; Guo, M. Clueless/CLUH regulates mitochondrial fission by promoting recruitment of Drp1 to mitochondria. Nat. Commun. 2022, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




