Submitted:
12 August 2024
Posted:
14 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Mixed polyolefin post-consumer plastic waste (MPO323)
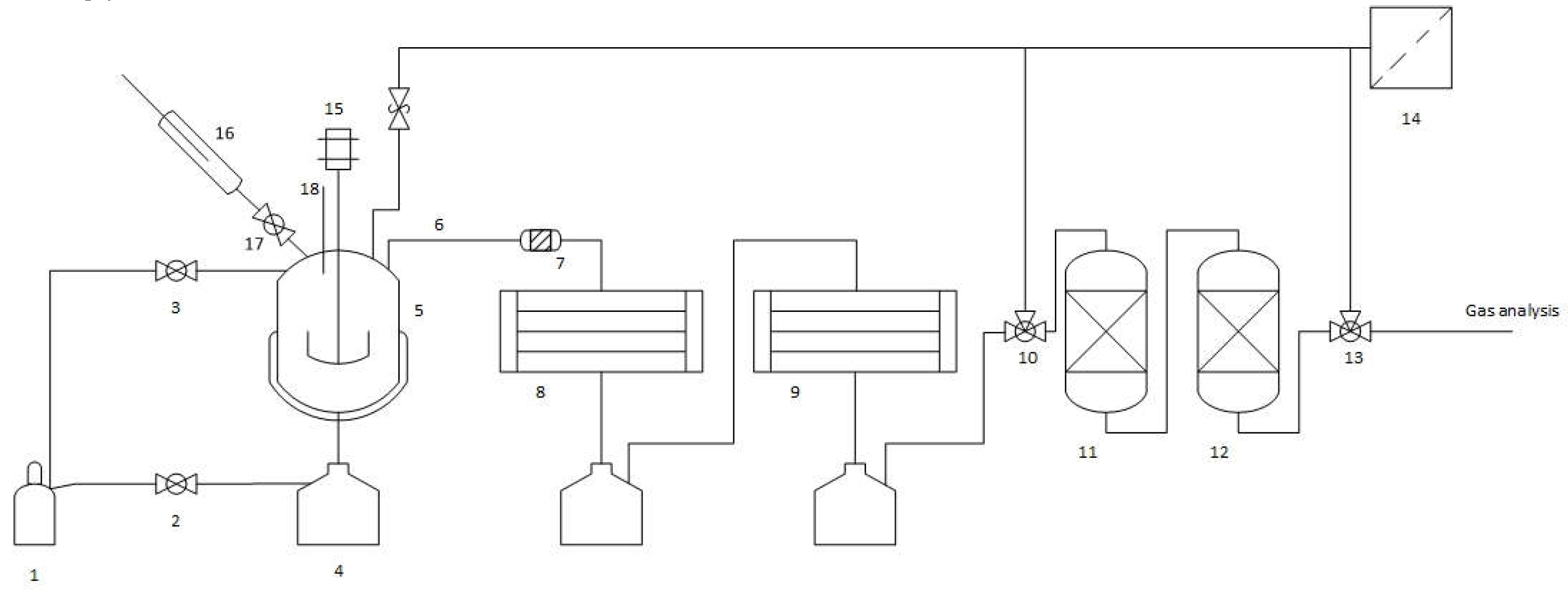
2.2. Thermo-Chemical Conversion
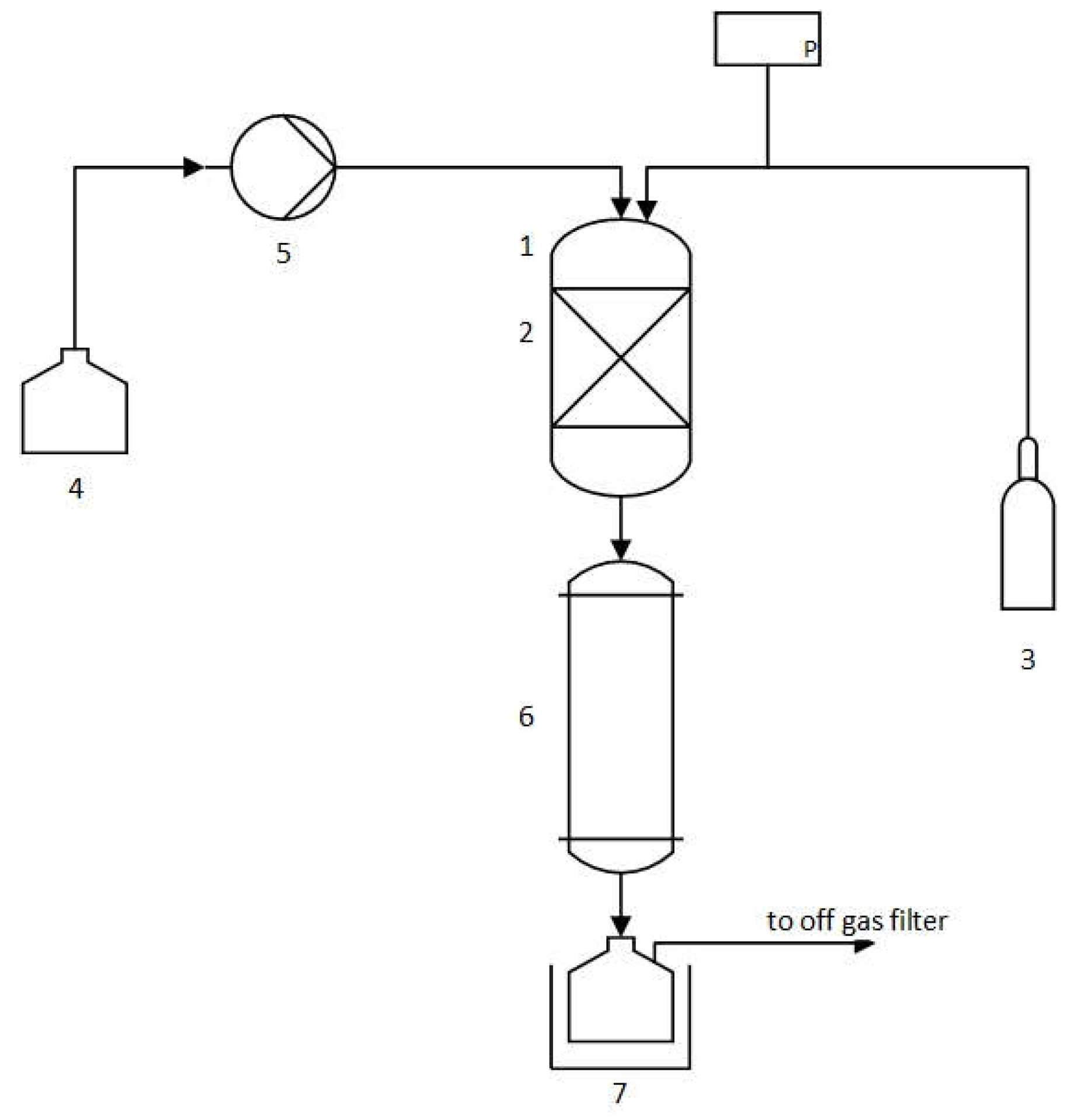
2.3. Auto-Catalytic Refomring Process
2.4. Analytical Methods
2.4.1. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.4.2. Elemental Analysis (CHNS)
2.4.3. Inductively Coupled Plasma with Optical Emission Spectrometry (ICP-OES)
2.4.2. Surface Area, Pore Size and Pore Volume
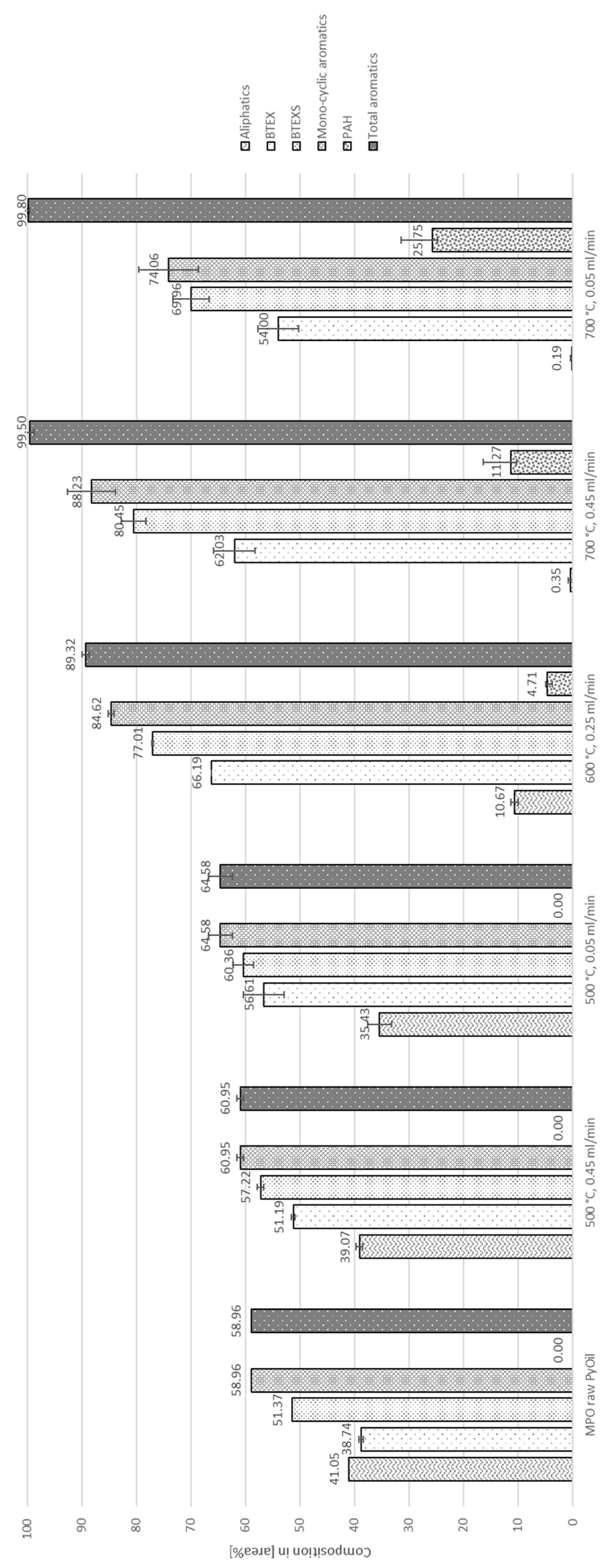
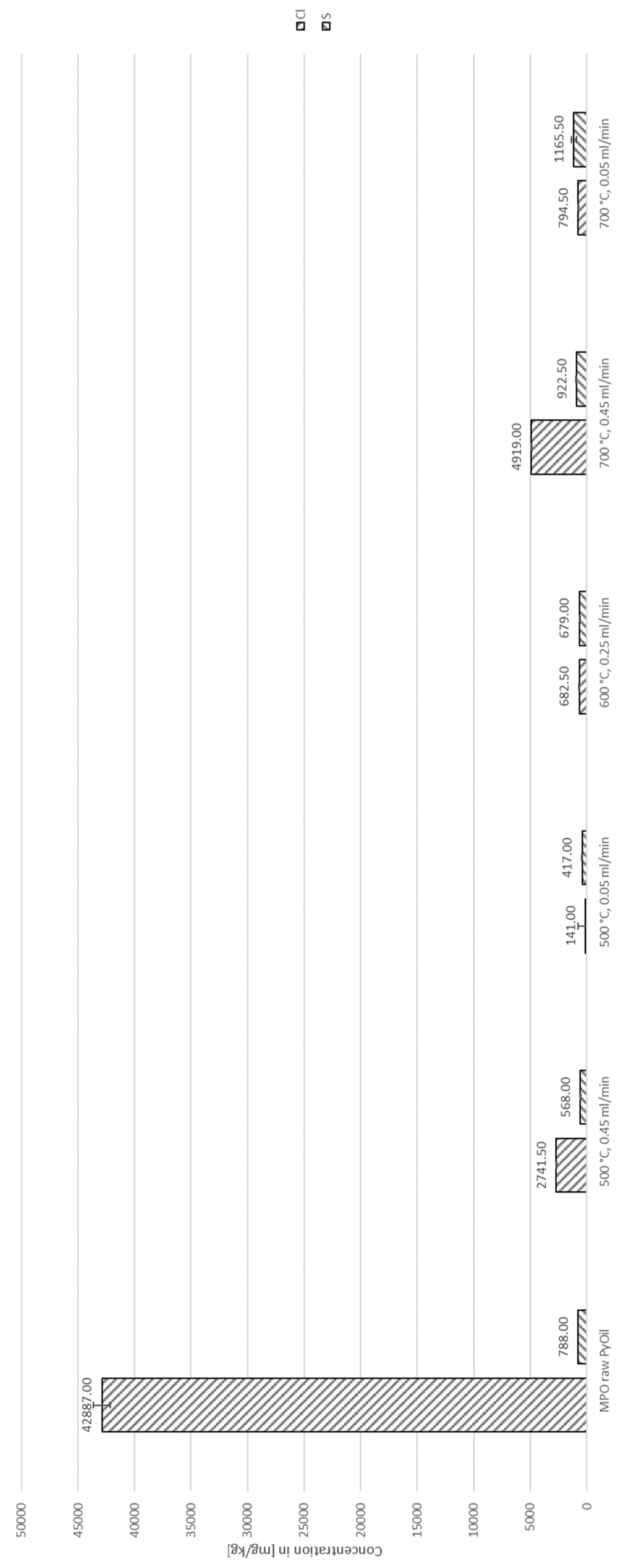
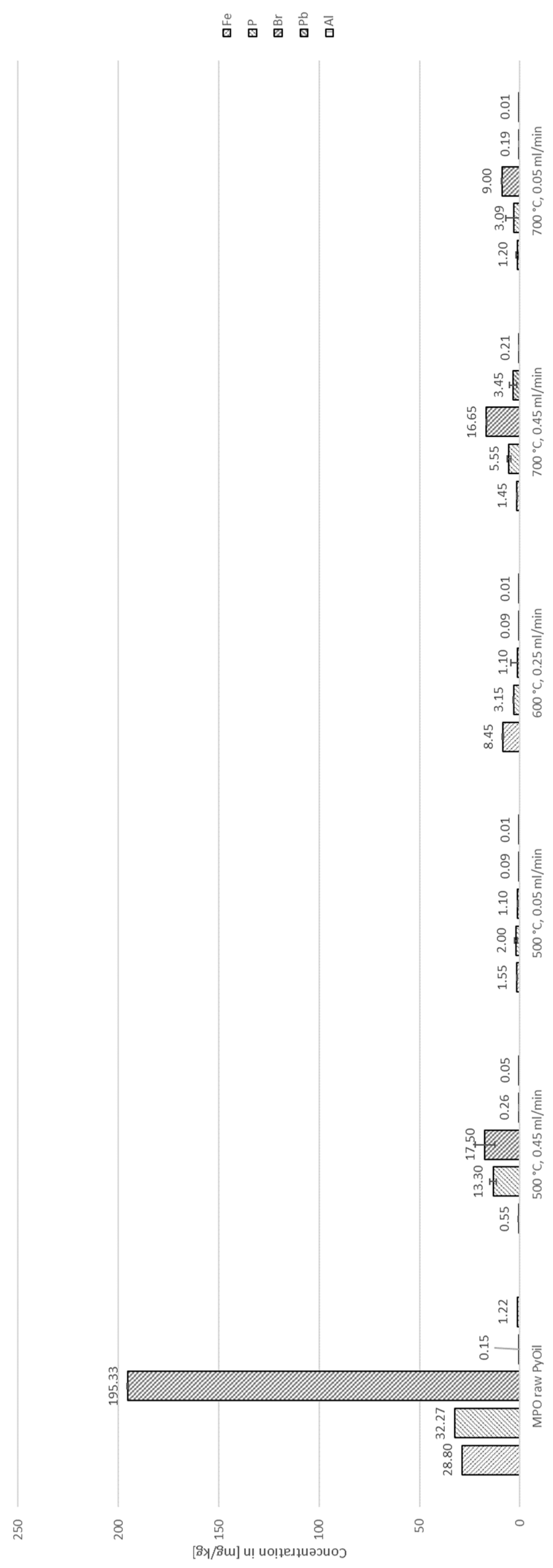
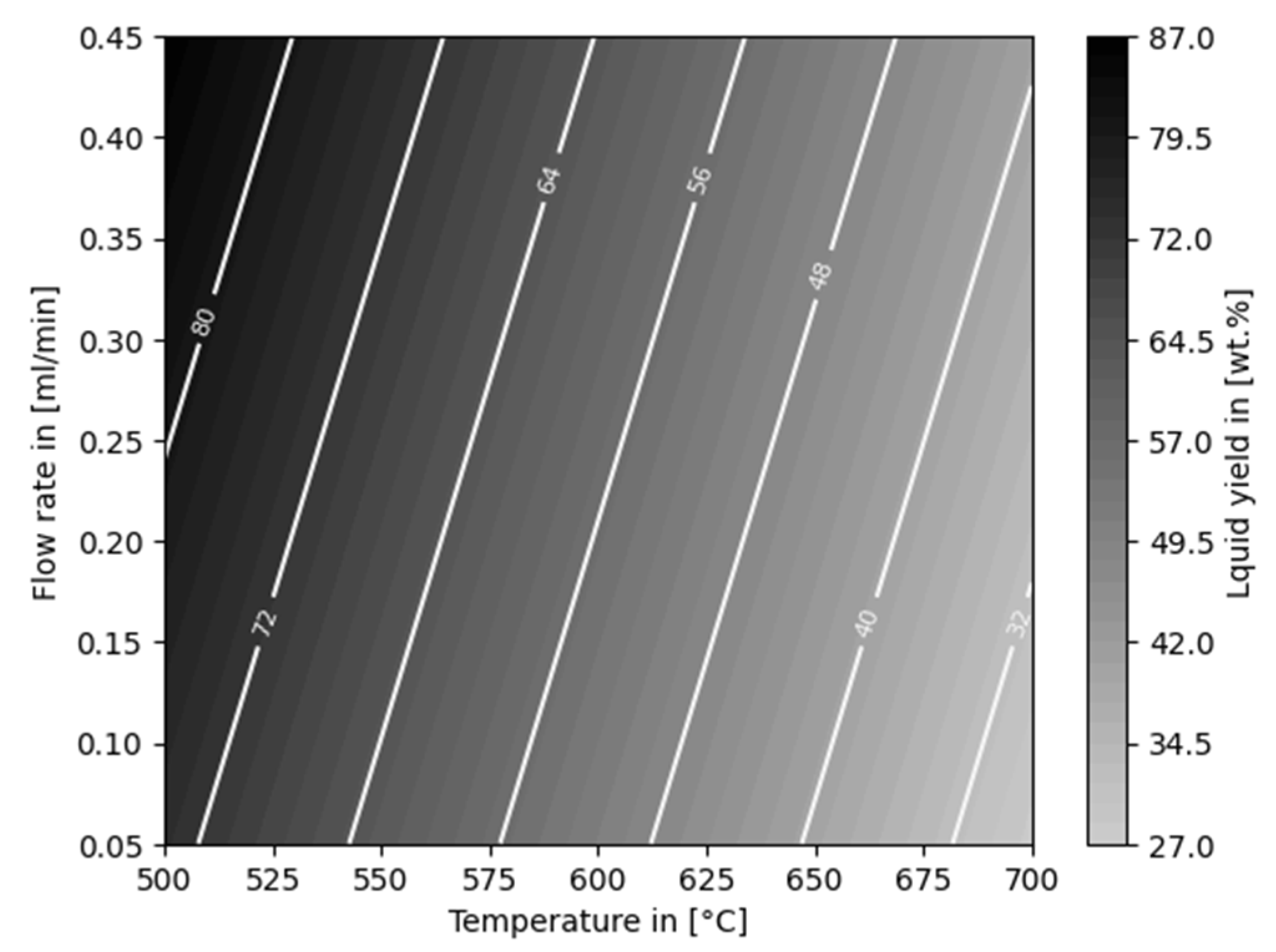
3. Results
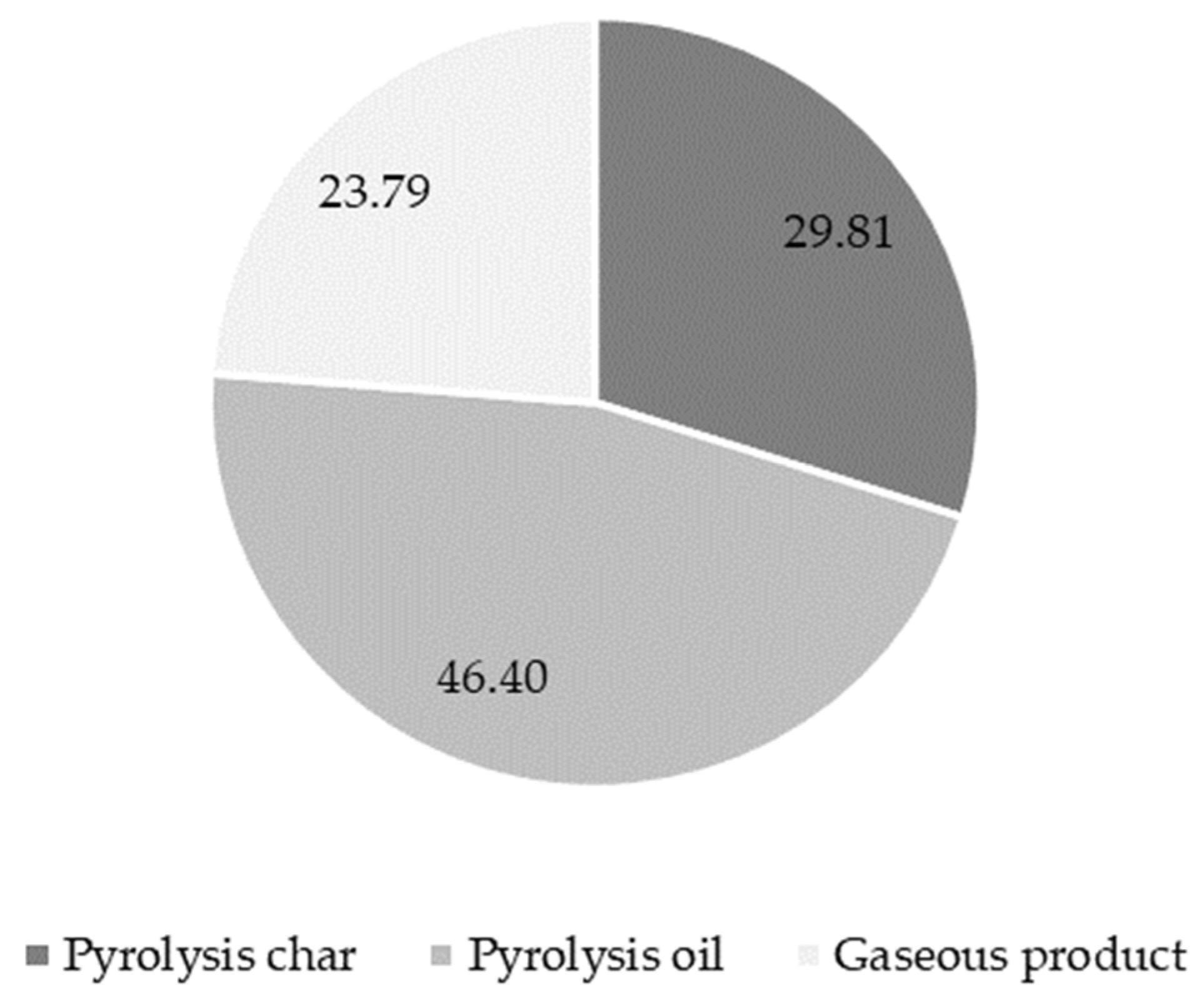
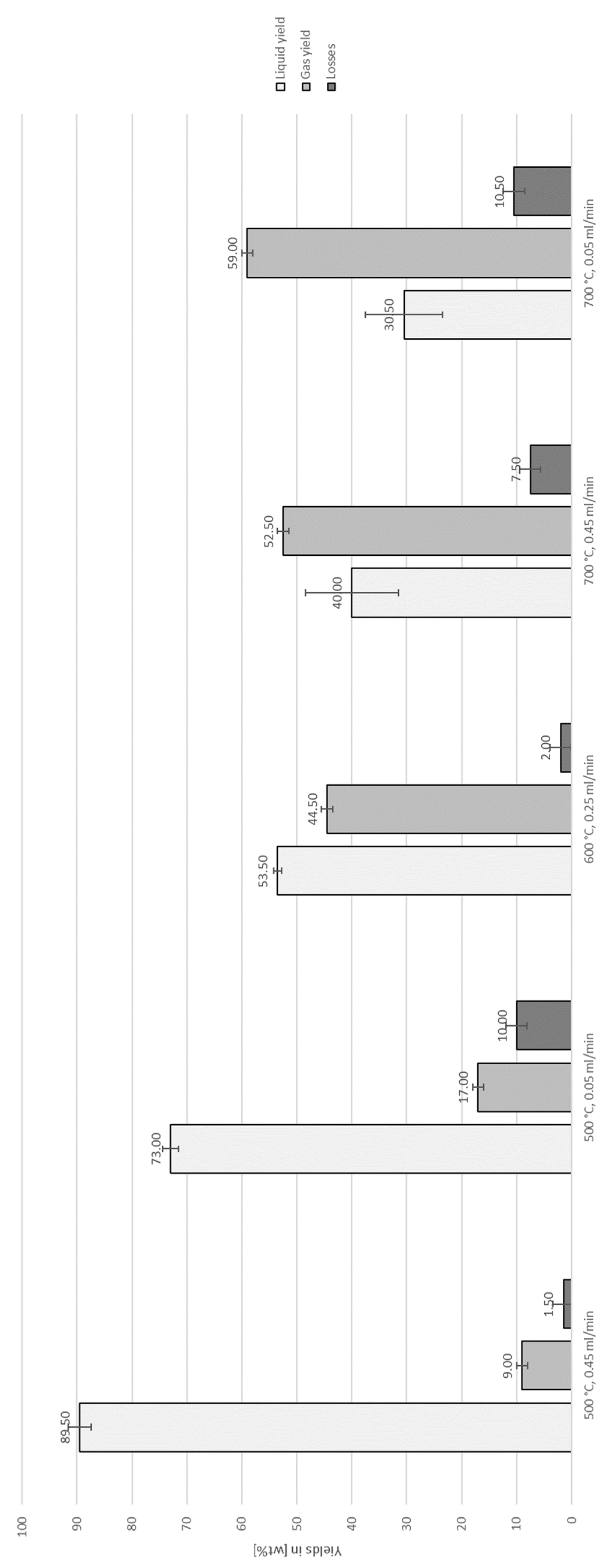
3.1. Thermo-Chemical Conversion
3.2. Auto-Catalytic Reforming Process
4. Discussion
4.1. Aromatization
4.2. Decontamination of Pyrolysis Oils
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, S.; Li, Z.; Zhang, F.; Chen, J. Upgrading waste plastics to value-added aromatics. Chem Catalysis 2024, 4, 100928. [Google Scholar] [CrossRef]
- Pachaly, B. Silicones: [creating tomorrow’s solutions]; Wiley-VCH: Weinheim, 2005; ISBN 978-3527307708. [Google Scholar]
- Ragaert, K.; Delva, L.; van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Management 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Svenja Grummt. Praxis der Sortierung und Verwertung von Verpackungen im Sinne des § 21 VerpackG 2020/2021. Teilbericht No. 125, 2022. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/1410/publikationen/2023-01-05_texte_125-2022_praxis_der_sortierung_und_verwertung_von_verpackungen.pdf (accessed on 29 July 2024).
- Der Grüne Punkt. Rohstofffraktionsspezifikation 323 Gemischte Polyolefin-Artikel DOC-23-50737, 2024. Available online: https://www.gruener-punkt.de/fileadmin/Dateien/Downloads/PDFs/Rohstofffraktionsspezifikationen2024/April/DOC-23-50737_-_Rohstofffraktionsspezifikation_323_Gemischte_Polyolefin-Artikel_-_v1.00.0001.pdf (accessed on 29 July 2024).
- Kusenberg, M.; Eschenbacher, A.; Delva, L.; Meester, S. de; Delikonstantis, E.; Stefanidis, G.D.; Ragaert, K.; van Geem, K.M. Towards high-quality petrochemical feedstocks from mixed plastic packaging waste via advanced recycling: The past, present and future. Fuel Processing Technology 2022, 238, 107474. [Google Scholar] [CrossRef]
- Snow, J.; Kuráň, P.; Kašpárek, A.; Leštinský, P.; Suchopa, R. Virgin polymers via pyrolysis – A review of heteroatom removal options. Fuel Processing Technology 2024, 254, 108031. [Google Scholar] [CrossRef]
- Belbessai, S.; Azara, A.; Abatzoglou, N. Recent Advances in the Decontamination and Upgrading of Waste Plastic Pyrolysis Products: An Overview. Processes 2022, 10, 733. [Google Scholar] [CrossRef]
- Genuino, H.C.; Ruiz, M.P.; Heeres, H.J.; Kersten, S.R. Pyrolysis of mixed plastic waste (DKR-350): Effect of washing pre-treatment and fate of chlorine. Fuel Processing Technology 2022, 233, 107304. [Google Scholar] [CrossRef]
- Lee, J.-K.; Shin, J.-H. Triboelectrostatic separation of pvc materials from mixed plastics for waste plastic recycling. Korean J. Chem. Eng. 2002, 19, 267–272. [Google Scholar] [CrossRef]
- Burat, F.; Güney, A.; Olgaç Kangal, M. Selective separation of virgin and post-consumer polymers (PET and PVC) by flotation method. Waste Management 2009, 29, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, T.; Yan, W.; Yuan, L. Dechlorination of PVC wastes by hydrothermal treatment using alkaline additives. Environ. Technol. 2018, 39, 977–985. [Google Scholar] [CrossRef]
- Lu, J.; Borjigin, S.; Kumagai, S.; Kameda, T.; Saito, Y.; Fukushima, Y.; Yoshioka, T. Practical dehalogenation of automobile shredder residue in NaOH/ethylene glycol with an up-scale ball mill reactor. J Mater Cycles Waste Manag 2020, 22, 1620–1629. [Google Scholar] [CrossRef]
- Rieger, T.; Oey, J.C.; Palchyk, V.; Hofmann, A.; Franke, M.; Hornung, A. Chemical Recycling of WEEE Plastics—Production of High Purity Monocyclic Aromatic Chemicals. Processes 2021, 9, 530. [Google Scholar] [CrossRef]
- Hegedüs, Balázs, and Zsolt Dobó. Gasoline like fuel from plastic waste pyrolysis and hydrotreatment. nalecta Technica Szegedinensia 58–63.
- Ware, R.L.; Rodgers, R.P.; Marshall, A.G.; Mante, O.D.; Dayton, D.C.; Verdier, S.; Gabrielsen, J.; Rowland, S.M. Tracking Elemental Composition through Hydrotreatment of an Upgraded Pyrolysis Oil Blended with a Light Gas Oil. Energy Fuels 2020, 34, 16181–16186. [Google Scholar] [CrossRef]
- Kusenberg, M.; Eschenbacher, A.; Djokic, M.R.; Zayoud, A.; Ragaert, K.; Meester, S. de; van Geem, K.M. Opportunities and challenges for the application of post-consumer plastic waste pyrolysis oils as steam cracker feedstocks: To decontaminate or not to decontaminate? Waste Manag. 2022, 138, 83–115. [Google Scholar] [CrossRef]
- Zhu, H.M.; Jiang, X.G.; Yan, J.H.; Chi, Y.; Cen, K.F. TG-FTIR analysis of PVC thermal degradation and HCl removal. Journal of Analytical and Applied Pyrolysis 2008, 82, 1–9. [Google Scholar] [CrossRef]
- Park, K.-B.; Choi, M.-J.; Chae, D.-Y.; Jung, J.; Kim, J.-S. Separate two-step and continuous two-stage pyrolysis of a waste plastic mixture to produce a chlorine-depleted oil. Energy 2022, 244, 122583. [Google Scholar] [CrossRef]
- Cho, M.-H.; Jung, S.-H.; Kim, J.-S. Pyrolysis of Mixed Plastic Wastes for the Recovery of Benzene, Toluene, and Xylene (BTX) Aromatics in a Fluidized Bed and Chlorine Removal by Applying Various Additives. Energy Fuels 2010, 24, 1389–1395. [Google Scholar] [CrossRef]
- Miskolczi, N.; Ateş, F.; Borsodi, N. Comparison of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part II: contaminants, char and pyrolysis oil properties. Bioresour. Technol. 2013, 144, 370–379. [Google Scholar] [CrossRef]
- Areeprasert, C.; Khaobang, C. Pyrolysis and catalytic reforming of ABS/PC and PCB using biochar and e-waste char as alternative green catalysts for oil and metal recovery. Fuel Processing Technology 2018, 182, 26–36. [Google Scholar] [CrossRef]
- Kusenberg, M.; Zayoud, A.; Roosen, M.; Thi, H.D.; Abbas-Abadi, M.S.; Eschenbacher, A.; Kresovic, U.; Meester, S. de; van Geem, K.M. A comprehensive experimental investigation of plastic waste pyrolysis oil quality and its dependence on the plastic waste composition. Fuel Processing Technology 2022, 227, 107090. [Google Scholar] [CrossRef]
- Kopinke, F.D.; Zimmermann, G.; Reyniers, G.C.; Froment, G.F. Relative rates of coke formation from hydrocarbons in steam cracking of naphtha. 3. Aromatic hydrocarbons. Ind. Eng. Chem. Res. 1993, 32, 2620–2625. [Google Scholar] [CrossRef]
- Kondyli, A.; Schrader, W. Understanding “Fouling” in Extremely Complex Petroleum Mixtures. ACS Appl. Energy Mater. 2020, 3, 7251–7256. [Google Scholar] [CrossRef]
- Muhammad, C.; Onwudili, J.A.; Williams, P.T. Catalytic pyrolysis of waste plastic from electrical and electronic equipment. Journal of Analytical and Applied Pyrolysis 2015, 113, 332–339. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, J.; Ma, P.; Cheng, Y.; Yildiz, G. Unveiling the conditioning correlation in ex-situ catalytic pyrolysis of waste polyolefins towards designated conversion into valuable products. Journal of Analytical and Applied Pyrolysis 2024, 181, 106639. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Aromatic fuel oils produced from the pyrolysis-catalysis of polyethylene plastic with metal-impregnated zeolite catalysts. Journal of the Energy Institute 2019, 92, 195–202. [Google Scholar] [CrossRef]
- Akin, O.; Varghese, R.J.; Eschenbacher, A.; Oenema, J.; Abbas-Abadi, M.S.; Stefanidis, G.D.; van Geem, K.M. Chemical recycling of plastic waste to monomers: Effect of catalyst contact time, acidity and pore size on olefin recovery in ex-situ catalytic pyrolysis of polyolefin waste. Journal of Analytical and Applied Pyrolysis 2023, 172, 106036. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, M.; Chen, Z.; Zhang, X.; Yang, H.; Wang, X.; Feng, H.; Yu, J.; Gao, S. Production of monocyclic aromatics and light olefins through ex-situ catalytic pyrolysis of low-density polyethylene over Ga/P/ZSM-5 catalyst. Journal of the Energy Institute 2023, 108, 101235. [Google Scholar] [CrossRef]
- Sun, K.; Themelis, N.J.; Bourtsalas, A.C.; Huang, Q. Selective production of aromatics from waste plastic pyrolysis by using sewage sludge derived char catalyst. Journal of Cleaner Production 2020, 268, 122038. [Google Scholar] [CrossRef]
- Qian, K.; Tian, W.; Li, W.; Wu, S.; Chen, D.; Feng, Y. Catalytic Pyrolysis of Waste Plastics over Industrial Organic Solid-Waste-Derived Activated Carbon: Impacts of Activation Agents. Processes 2022, 10, 2668. [Google Scholar] [CrossRef]
- Sun, K.; Huang, Q.; Ali, M.; Chi, Y.; Yan, J. Producing Aromatic-Enriched Oil from Mixed Plastics Using Activated Biochar as Catalyst. Energy Fuels 2018, 32, 5471–5479. [Google Scholar] [CrossRef]
- Fan, W.; Tahir, M.H.; Chen, D.; Hong, L.; Yin, L.; Yu, H. High quality oil and H2-rich gas production from municipal solid wastes through pyrolysis and catalytic reforming: Comparison of differently modified waste char-based catalysts. Journal of Analytical and Applied Pyrolysis 2024, 178, 106382. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, L.; Wang, Y.; Su, S.; Sun, L.; Xu, B.; He, L.; Xiang, J. Effects of inherent alkali and alkaline earth metallic species on biomass pyrolysis at different temperatures. Bioresour. Technol. 2015, 192, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sima, J.; Wang, X.; Zhou, Y.; Huang, Q. BTEX recovery from waste rubbers by catalytic pyrolysis over Zn loaded tire derived char. Waste Manag. 2021, 131, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manage. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Conversion and Management 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Armenise, S.; SyieLuing, W.; Ramírez-Velásquez, J.M.; Launay, F.; Wuebben, D.; Ngadi, N.; Rams, J.; Muñoz, M. Plastic waste recycling via pyrolysis: A bibliometric survey and literature review. Journal of Analytical and Applied Pyrolysis 2021, 158, 105265. [Google Scholar] [CrossRef]
- Liu, D.; Cao, L.; Zhang, G.; Zhao, L.; Gao, J.; Xu, C. Catalytic conversion of light alkanes to aromatics by metal-containing HZSM-5 zeolite catalysts—A review. Fuel Processing Technology 2021, 216, 106770. [Google Scholar] [CrossRef]
- Lok, C.M.; van Doorn, J.; Aranda Almansa, G. Promoted ZSM-5 catalysts for the production of bio-aromatics, a review. Renewable and Sustainable Energy Reviews 2019, 113, 109248. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Safety and Environmental Protection 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Conversion and Management 2022, 254, 115243. [Google Scholar] [CrossRef]
- Brebu, M.; Bhaskar, T.; Murai, K.; Muto, A.; Sakata, Y.; Uddin, M.A. Removal of nitrogen, bromine, and chlorine from PP/PE/PS/PVC/ABS–Br pyrolysis liquid products using Fe- and Ca-based catalysts. Polymer Degradation and Stability 2005, 87, 225–230. [Google Scholar] [CrossRef]
- Hubáček, J.; Lederer, J.; Kuráň, P.; Koutník, P.; Gholami, Z.; Zbuzek, M.; Bačiak, M. Dechlorination during pyrolysis of plastics: The potential of stepwise pyrolysis in combination with metal sorbents. Fuel Processing Technology 2022, 231, 107226. [Google Scholar] [CrossRef]
- Jeong, Y.-S.; Park, K.-B.; Kim, J.-S. Kinetics and characteristics of activator-assisted pyrolysis of municipal waste plastic and chlorine removal using hot filter filled with absorbents. Energy 2022, 238, 121814. [Google Scholar] [CrossRef]
- Brebu, M.; Bhaskar, T.; Murai, K.; Muto, A.; Sakata, Y.; Uddin, M. Thermal degradation of PE and PS mixed with ABS-Br and debromination of pyrolysis oil by Fe- and Ca-based catalysts. Polymer Degradation and Stability 2004, 84, 459–467. [Google Scholar] [CrossRef]
- Bhaskar, T.; Kaneko, J.; Muto, A.; Sakata, Y.; Jakab, E.; Matsui, T.; Uddin, M. Pyrolysis studies of PP/PE/PS/PVC/HIPS-Br plastics mixed with PET and dehalogenation (Br, Cl) of the liquid products. Journal of Analytical and Applied Pyrolysis 2004, 72, 27–33. [Google Scholar] [CrossRef]
- Sakata, Y.; Bhaskar, T.; Uddin, M.A.; Muto, A.; Matsui, T. Development of a catalytic dehalogenation (Cl, Br) process for municipal waste plastic-derived oil. J Mater Cycles Waste Manag 2003, 5, 113–124. [Google Scholar] [CrossRef]
- Hornung, A.; Apfelbacher Andreas, Ouadi, Miloud, Neumann, Johannes. Pyrolysis oil and method for producing same.


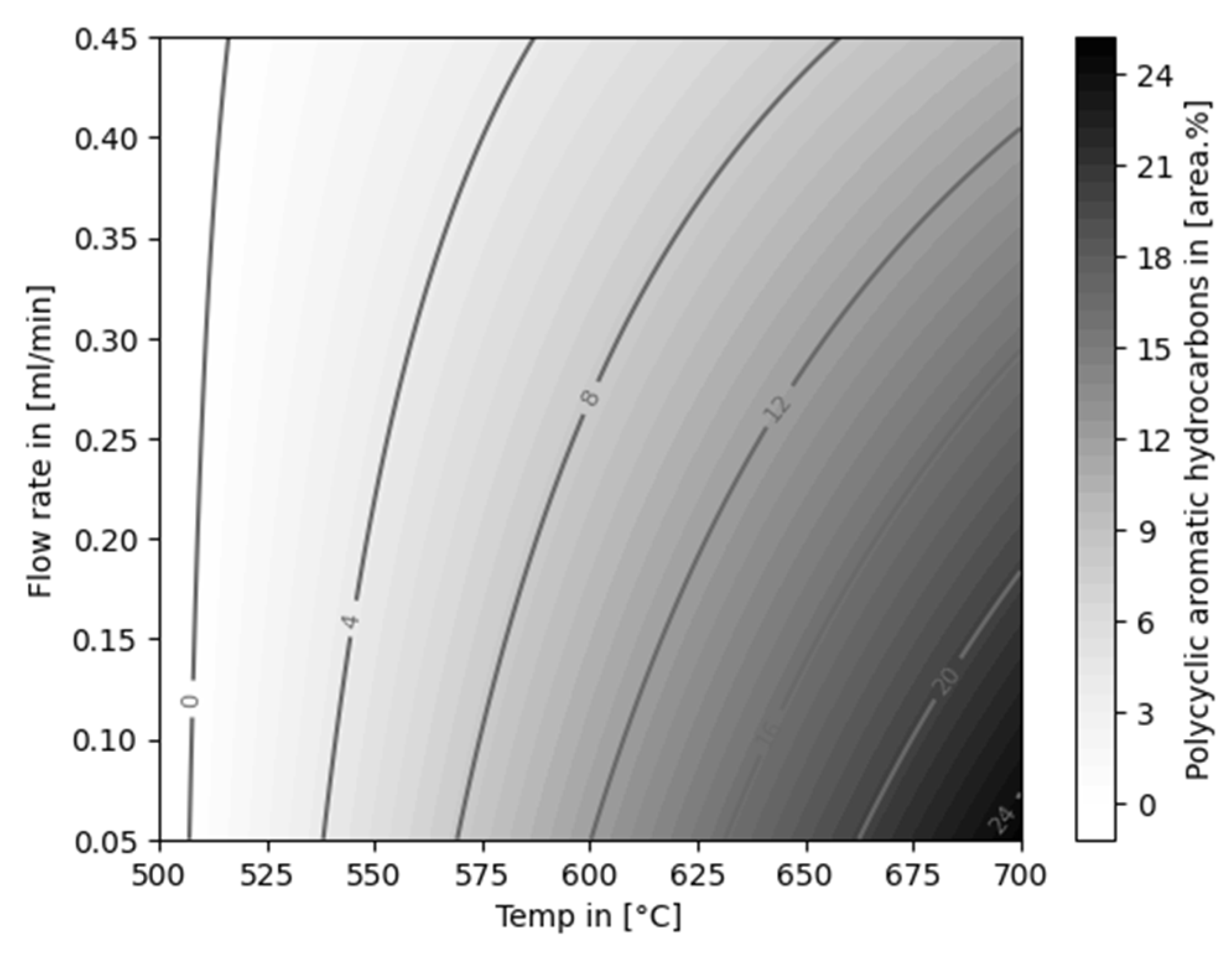
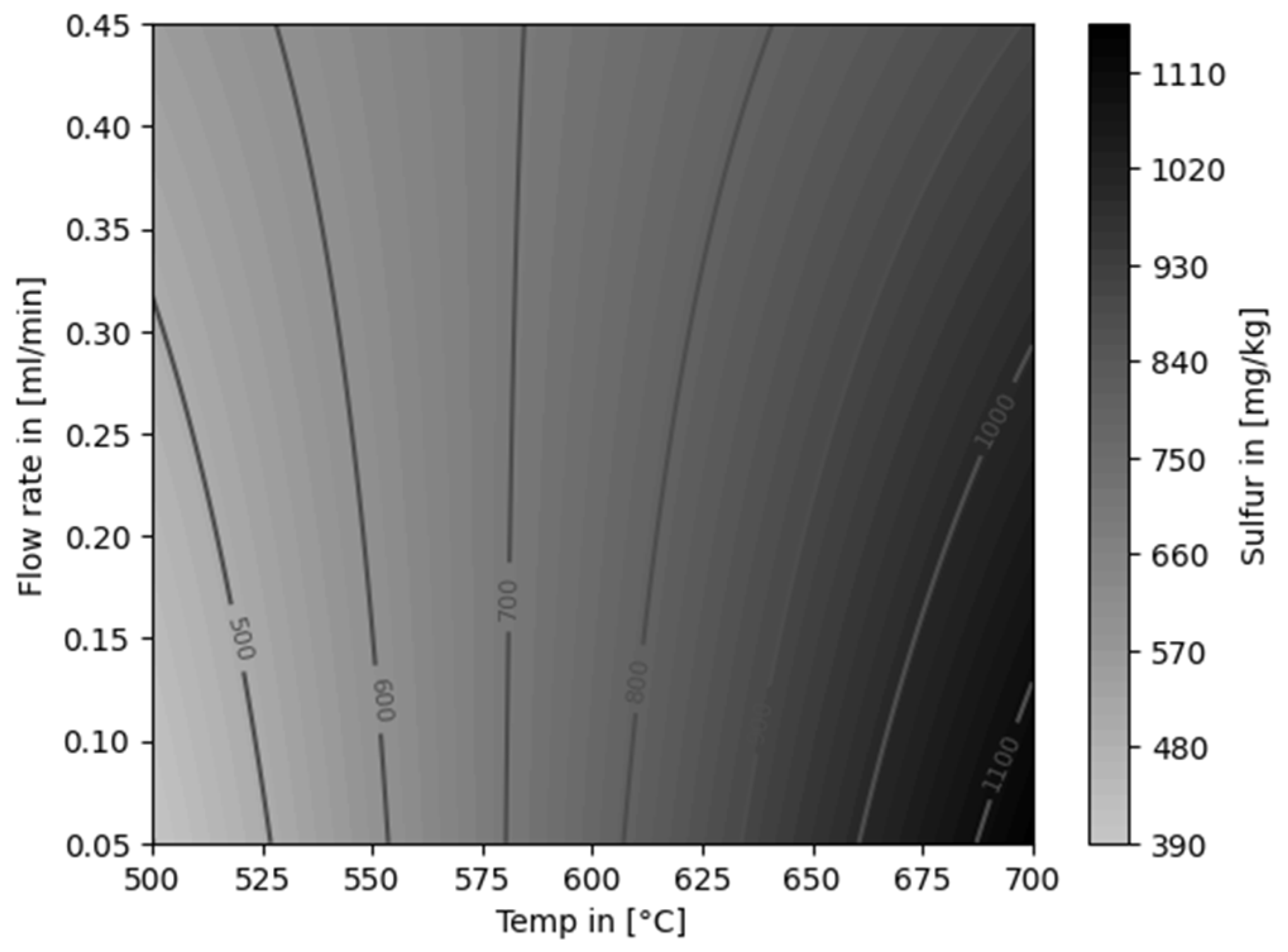
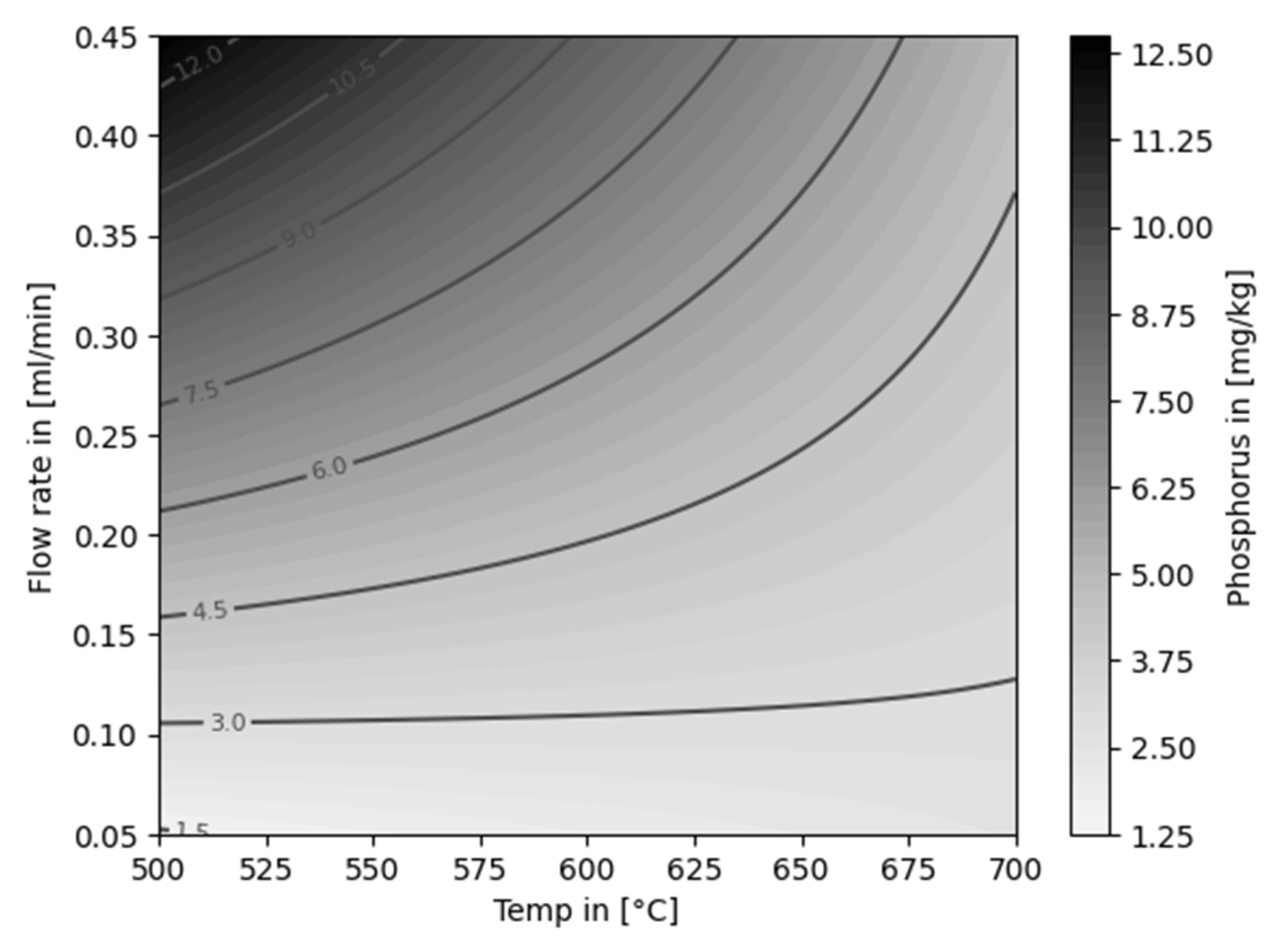
| Experiment | Catalytically active material | Temperature [°C] |
Flow rate [ml/min] |
Feed material |
|---|---|---|---|---|
| CR1_MPO_1 | Raw MPO323 char | 500 | 0.05 | MPO323 oil |
| CR1_MPO_2 | Raw MPO323 char | 500 | 0.05 | MPO323 oil |
| CR2_MPO_1 | Raw MPO323 char | 500 | 0.45 | MPO323 oil |
| CR2_MPO_2 | Raw MPO323 char | 500 | 0.45 | MPO323 oil |
| CR3_MPO_1 | Raw MPO323 char | 600 | 0.25 | MPO323 oil |
| CR3_MPO_2 | Raw MPO323 char | 600 | 0.25 | MPO323 oil |
| CR4_MPO_1 | Raw MPO323 char | 700 | 0.05 | MPO323 oil |
| CR4_MPO_2 | Raw MPO323 char | 700 | 0.05 | MPO323 oil |
| CR5_MPO_1 | Raw MPO323 char | 700 | 0.45 | MPO323 oil |
| CR5_MPO_2 | Raw MPO323 char | 700 | 0.45 | MPO323 oil |
| Area% | Ret. Time | Name |
| 19.10 | 7.468 | Ethylbenzene |
| 12.82 | 8.111 | Styrene |
| 11.10 | 5.486 | Toluene |
| 8.73 | 6.994 | 2,4-Dimethyl-1-heptene |
| 6.87 | 3.591 | Benzene |
| 3.54 | 10.000 | 1-Decene |
| 3.34 | 5.242 | AH C8 |
| 3.19 | 8.055 | AH C9 |
| 3.11 | 6.801 | AH C8 |
| 2.98 | 15.020 | AH C13 |
| 2.20 | 8.752 | Benzene, (1-methylethyl)- |
| 2.09 | 11.775 | 1-Undecene |
| 2.06 | 5.957 | 1-Octene |
| 1.99 | 9.574 | AH C10 |
| 1.88 | 14.906 | 1-Tridecene |
| 1.69 | 13.398 | 1-Dodecene |
| 1.64 | 4.010 | 1-Heptene |
| 1.62 | 9.893 | alpha.-Methylstyrene |
| 1.61 | 7.665 | Xylene |
| 1.59 | 15.267 | AH C13 |
| 1.59 | 16.317 | 1-Tetradecene |
| 1.47 | 17.646 | 1-Pentadecene |
| 1.31 | 11.506 | AH C11 |
| 1.27 | 9.650 | Mesitylene |
| 1.22 | 5.435 | AH C8 |
| Species | Concentration [ppm] | Standard deviation [ppm] |
| Cl | 42,887.00 | 747.24 |
| S | 788.00 | 3.46 |
| Br | 195.33 | 3.51 |
| P | 32.27 | 0.15 |
| Fe | 28.80 | 4.92 |
| Al | 1.22 | 0.43 |
| Pb | 0.15 | 0.06 |
| Species | Concentration [wt.%] | Species | Concentration [wt.%] |
| Al | 11.00 | Mn | 0.05 |
| Ba | 0.73 | N | 0.67 |
| C | 63.66 | Na | 1.60 |
| Ca | 12.00 | Ni | 0.06 |
| Cu | 2.10 | P | 0.34 |
| Cr | 0.06 | Pb | 0.05 |
| Fe | 1.80 | S | 0.14 |
| H | 3.14 | Si | 8.30 |
| K | 0.67 | Ti | 3.10 |
| Mg | 1.20 | Zn | 2.70 |
| Name, Property | Unit | MPO323 char | Biochar [22] |
Electronic waste char [22] |
Fe/Biochar [22] |
Fe/ Electronic char [22] |
|---|---|---|---|---|---|---|
| SiO2 | [wt.%] | 7.30 | 10.88 | 0.676 | 33.3 | 2.28 |
| Al2O3 | [wt.%] | 9.30 | 1.28 | 0.165 | 5.46 | 0.627 |
| CaO | [wt.%] | 23.0 | 1.28 | 1.89 | 5.13 | 7.55 |
| Fe2O3 | [wt.%] | 2.20 | 1.12 | 1.21 | 17.2 | 8.92 |
| TiO2 | [wt.%] | 2.50 | 0.06 | 0 | 0.236 | 2.29 |
| Na2O | [wt.%] | 0.93 | 0.82 | 0.112 | 3.33 | 1.5 |
| MgO | [wt.%] | 0.80 | 0.48 | 0.421 | 2.03 | 0 |
| ZnO | [wt.%] | 1.8 | - | - | - | - |
| BET | [m2/g] | 5.3 | 4.2 | 4.5 | 52.4 | 10.8 |
| Pore volume | [cm2/g] | 0.013 | 0.008 | 0.006 | 0.055 | 0.02 |
| Pore size | [nm] | 10.2 | 7.65 | 5.43 | 4.2 | 7.34 |
| Initial pyrolysis oil | Auto-catalytically reformed at 600 C, 0.25 ml/min | ||
|---|---|---|---|
| Area% | Name | Area% | Name |
| 6.87 | Benzene | 14.63 | Benzene |
| 11.1 | Toluene | 22.17 | Toluene |
| 19.1 | Ethylbenzene | 23.69 | Ethylbenzene |
| 1.61 | Xylene | 6.08 | Xylene |
| 12.82 | Styrene | 10.52 | Styrene |
| 2.2 | Benzene, (1-methylethyl)- | 1.73 | Benzene, (1-methylethyl)- |
| 0.88 | Benzene, 1-ethyl-3-methyl- | ||
| 0.68 | Benzene, 1-ethyl-2-methyl- | ||
| 0.99 | Benzene, 1,2,3-trimethyl- | ||
| 1.62 | .alpha.-Methylstyrene | 1.23 | .alpha.-Methylstyrene |
| 1.27 | Mesitylene | 2.37 | Mesitylene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





