1. Introduction
The global rise of nonalcoholic fatty liver disease (NAFLD) over the past decades prompted researchers to invest more effort in developing reliable models, including in vitro models, which could unravel the unknown intricate mechanisms of this disease and lead to the development of effective therapies [
1]. NAFLD includes a spectrum of histological findings ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), which may lead to fibrosis and hepatocellular carcinoma [
2]. Various strategies have been employed to improve the reliability of 2D, 3D, and co-culture in vitro models of NAFLD, including using extracellular matrix proteins, such as collagen I, to mimic cell-to-extracellular matrix interactions [
3,
4,
5]. Although it is generally known that the extracellular matrix promotes cell adhesion, viability, differentiation, and proliferation in most cells [
6,
7], information regarding the effects of using collagen I to develop 2D or 3D in vitro NAFLD models is insufficient. Understanding the impact of collagen I in 2D in vitro models is essential before it is widely adopted for scaffold-based 3D in vitro NAFLD models.
As alternatives for using primary human hepatocytes, HepG2 and HepaRG cells are the most utilized cell lines for in vitro models of NAFLD [
1]. Our recently published study demonstrated that compared to HepG2 cells, HepaRG cells were more suitable for developing an early in vitro model of NASH [
8]. On the other hand, despite the limitations of HepG2 cell line such as low mitochondrial respiration and low expression of some nuclear receptors [
9], HepG2 cells exposed to palmitate (PA) exerted findings similar to those reported in patients with late stages of NASH, including increased apoptosis, ROS production and reduced mitochondrial respiration [
10,
11,
12]. Following the observation that exposure of HepG2 and HepaRG cells to PA reduced cell adhesion to cell culture vessels, it was hypothesized that culturing the cells on collagen I could improve cell adhesion and reduce PA-induced cell death. A study by Chethikkattuveli et al. [
13] has previously demonstrated that HepG2 cell adhesion was significantly enhanced by culturing them on collagen I. Collagen I is the most abundant extracellular protein in the human body and one of the most highly utilized extracellular matrices for cell culture [
14]. Collagen I is a 300 kDa molecule composed of two alpha-1 chains and one alpha-2 chain that combine to form a triple helix scaffold [
14]. Cells use various cell adhesion receptors, such as integrins, to bind to distinct types of collagens [
6]. Integrin α2β1 is the primary receptor that binds collagen I and is present in most epithelial cells [
15]. Previous studies have demonstrated that HepG2 cells cultured on collagen I exerted high proliferation correlated with increased expression of α2β1 receptors [
16,
17]. Although ligation of α2β1 receptors by collagen I does support the abovementioned advantages, unligated or antagonized α2β1 receptors have been found to activate apoptosis via integrin-mediated death [
18,
19]. While studies have demonstrated the significant benefits of culturing cells on collagen I as part of 2D or 3D in vitro platforms, its effects on HepG2 and HepaRG cells after exposure to PA have not been investigated. Patients diagnosed with NASH usually have a high serum concentration of PA [
20]. Thus, PA is commonly used to develop NAFLD/NASH in vitro models [
21,
22].
This study aimed to assess the effects of PA-induced lipotoxicity in HepG2 and HepaRG cells cultured in the absence or presence of collagen I. Understanding the interactions of collagen I with these commonly utilized cell lines may enable informed choices of whether to use collagen I to develop 2D or scaffold-based 3D in vitro NAFLD models.
2. Materials and Methods
2.1. Cell Culture
HepG2 cells (ECACC85011430, liver cancer cells) were purchased from ECACC, and cultured in Minimum Essential Medium (Merck & Co., Inc.) supplemented with 1% non-essential amino acids, 10% fetal bovine serum, a 1% mixture of penicillin (10,000 UI/ml) and streptomycin (10 mg/ml) and 1% sodium pyruvate. The cells were incubated at 37°C in a 5% CO2, 95% air-humidified atmosphere and passaged once a week at 75% confluency. HepaRG cells (HRP101, liver cancer cells) were purchased from Biopredic International and cultured at a recommended 26,600 cells/cm2 density. HepaRG were cultured in proliferation medium [William’s E Medium (Lonza Group, Ltd.) supplemented with 5 μg/ml insulin, 50 μM hydrocortisone, 1% L-glutamine, 1% mixture of penicillin (10,000 UI/ml), streptomycin (10 mg/ml) and 10% fetal bovine serum] for 14 days. After that, they were cultured in a differentiation medium (proliferation medium supplemented with 1.5% dimethyl sulfoxide) for another 14 days. They were incubated at 37°C in a 5% CO2, 95% air-humidified atmosphere, and the medium was changed three times a week.
On the day of seeding, cell culture vessels were coated using collagen I (rat tail, Merck & Co., Inc.). Collagen I stock solution (300 μg/ml) was prepared in 0.02M acetic acid and allowed to dissolve for 24 hrs. Cell culture vessels were coated with collagen I for 30 min at 37°C. After coating the cell culture vessels, collagen I was aspirated and neutralized with phosphate-buffered saline (PBS) and allowed to dry for 30 min at 37°C before seeding the cells (HepG2 or HepaRG). The final concentration of collagen I used for coating was 5 μg/cm2. The cells were seeded for 24 hrs. in the absence or presence of collagen I before applying treatments.
2.2. Preparation of Palmitate
Sodium palmitate (PA) was purchased from Merck & Co., Inc. As previously described [
23], a 40 mM stock solution of PA was first prepared in 0.1 M NaOH (Merck & Co., Inc.), followed by conjugation to bovine serum albumin (BSA) (Merck & Co., Inc.). PA was dissolved at 70°C for 30 min and stored at −80°C for up to 3 months. To prepare the PA-BSA conjugation, 40 mM stock solution PA was dissolved and mixed with 20% BSA for 1 h to yield an 8-mM stock solution (pH 7.4), which was further dissolved in the culture medium (without fetal bovine serum) to yield 1 mM final concentration needed to treat cells. The 8-mM stock solution was sterile-filtered prior to use. The molar ratio between PA and BSA was 5.3, and 2.5% BSA was used as the control [
23]. Unless otherwise indicated, the cells were treated with 2.5% BSA and 1 mM PA for 8 hrs. (total culture of 32 hrs.) and 24 hrs. (total culture of 48 hrs.) (
Figure S1A) in all the methods described below. Where necessary, detached cells were collected from the culture medium at 940 x g, 4°C, 5 min.
2.3. Cell Viability/Proliferation Assay
Cell viability and proliferation were determined using tetrazolium salt WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) purchased from Roche, Ltd. The assay is based on the cleavage of the slightly red tetrazolium salt WST-1 to form a yellow formazan dye by metabolically active cells. The WST-1 test was performed according to the manufacturer’s recommendations. Briefly, cells cultured in 96-well plates were treated with 10% WST-1 (diluted in cell culture medium), and absorbance (440 nm) was measured at 0 and 60 min using Tecan Infinite M200 (Tecan Group, Ltd.).
Cell viability assay was also used to assess the effect of caspase inhibitors on PA-induced cell death. Following 24 hrs. of seeding, cells were treated for 30 min with 20 μM of inhibitors: [Z-DEVD-FMK, caspase 3, (Casp 3)], [Z-IETD-FMK, caspase 8, (Casp 8)], and [Z-LEHD-FMK TFA, caspase 9, (Casp 9)]. After that, cells were exposed to a combination of treatments (2.5% BSA and 1 mM PA) with the caspase inhibitors for a total culture period of 32 and 48 hrs. 10 mM stock solutions of inhibitors were prepared in dimethyl sulfoxide and stored at −20°C. All caspase inhibitors were purchased from MedChemExpress LLC.
2.4. Cell Adhesion, Proliferation, and Cytotoxicity
The xCELLigence real-time cell analysis (RTCA) system (Agilent Technologies, Inc.) measured cell impedance and automatically calculated cell index values, which provided information about cell adhesion, proliferation, and cytotoxicity. Half of the E-plate (96-well plate) was coated with collagen I, and after carrying out cell density titration measurements, the optimal seeding number of 10,000 cells per well was determined. After seeding, the E-plates were loaded on the RTCA station and placed in a CO2 incubator at 37°C for 24 hrs. Following 24 hrs. of attachment, the culture medium was removed. The cells were treated with a culture medium containing 2.5% BSA and 1 mM PA, and cell impedance was continuously measured for the next 4 days. The cells treated with 5% DMSO were used as positive control. Evaluations were performed using xCELLigence 1.2.1 software (Agilent Technologies, Inc.).
2.5. Mitochondrial Respiration
The extracellular flux analyzer Seahorse XFe-96 (Agilent Technologies, Inc.) measured oxygen consumption rate (OCR) in real-time and provided information on mitochondrial respiration. Before the measurement, the culture media containing treatments (2.5% BSA and 1 mM PA) were replaced with assay medium [bicarbonate-free XF DMEM pH 7.4 (Agilent Technologies, Inc.) supplemented with 4 mM L-glutamine, 1 mM pyruvate and 1 g/l D-glucose] and the cells were incubated in a CO2-free incubator for 1 h at 37°C. The seeding density was 20,000 cells per well (96-well plates). Following the measurement of basal respiration, a mitochondrial stress test was performed by sequential additions of 1 μM oligomycin, 1.2 μM carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP), and 1 μM rotenone and antimycin A. Differences of OCR values in response to respiratory modulators were used to calculate various mitochondrial parameters (basal, and maximal respiration, ATP-linked respiration, spare respiratory capacity, and proton leak respiration); however, only maximal respiration was illustrated. Unless stated otherwise, the materials used here were purchased from Merck & Co., Inc.
2.6. Caspase Assays
Apoptosis was determined by monitoring the activities of caspases 3/7, caspase 8, and caspase 9 (Caspase-Glo Assays, Promega Corporation). The assays were used per the manufacturer’s recommendations at 32 and 48 hrs. of culture. The caspase-Glo Reagent results in cell lysis, followed by caspase cleavage of the substrates and the generation of luminescent signals. After 1 hr. of incubation at room temperature (in the dark), luminescence from all the substrates was measured using Tecan Infinite M200 (Tecan Group, Ltd.).
In other experiments, caspase 3 activity was measured using a fluorescent probe (Ac-DEVD-AMC, Life Sciences, Inc.). The cells were lysed in lysis buffer (50 mM HEPES, 5 mM CHAPS, and 5 mM DTT) and stored at −80°C. The cell culture medium was also collected and stored at −80°C. Samples were quantified in assay buffer containing 20 mM HEPES, 0.1% CHAPS, 5 mM DTT, 2 mM EDTA, and Ac-DEVD-AMC. Activated caspase enzymes cleave the probe to release fluorescent 7-amino-4-methylcoumarin (λex=360 nm, λem=465 nm), which was detected using Tecan Infinite M200 (Tecan Group, Ltd.). Unless stated otherwise, the materials used here were purchased from Merck & Co., Inc.
2.7. Lactate Dehydrogenase (LDH) Assay
To evaluate plasma membrane integrity, the LDH assay kit (Diagnostic Systems GmbH) was used to quantify LDH activity (Tecan Infinite M200, Tecan Group, Ltd.) in cell culture medium and cell lysates according to the manufacturer’s instructions. LDH leakage (%) was then calculated from the measured LDH activities.
2.8. Reactive Oxygen Species (ROS) Production
CM-H2DCFDA (Invitrogen; Thermo Fisher Scientific, Inc.) was used to evaluate the increased production of ROS. Its acetate groups are cleaved by intracellular esterases followed by subsequent oxidation yielding a fluorescent adduct (λex=485 nm, and λem=535 nm) that gets trapped inside the cells. In brief, the HepG2 cells were incubated with 40 μM of the indicator at room temperature for 30 min. The cells were washed with phosphate-buffered saline, and fluorescence was quantified using Tecan Infinite M200 (Tecan Group, Ltd.). Data were normalized to the protein concentration (BCA assay, Thermo Fisher Scientific, Inc.).
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
To quantify the integrin receptor subunit (ITGA2) the cells were lysed in RIPA lysis buffer (Merck & Co., Inc.). To quantify phosphorylated focal adhesion kinase (P-FAK), the cells were lysed in the recommended buffer (Invitrogen; Thermo Fisher Scientific, Inc.) consisting of 10 mM Tris (pH 7.4), 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, 1 mM PMSF and protease inhibitor cocktail. All the ELISA kits were purchased from Invitrogen Thermo Fisher Scientific, Inc., and were used according to the manufacturer’s recommendations. TECAN Infinite M200 (Tecan, Group, Ltd.) was used to measure absorbance at 450 nm. The data were normalized to protein concentration using the Bradford assay (Thermo Fisher Scientific, Inc.). Unless stated otherwise, the materials used here were purchased from Merck & Co., Inc.
2.10. RNA Isolation and Reverse Transcription-Quantitative Polymerase Chain Reaction
Total cellular RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the manufacturer’s methodology. The isolated RNA was reverse-transcribed into complementary DNA (cDNA) using a cDNA Reverse Transcription Kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). Quantification of individual gene expressions was performed using TaqMan Gene Expression Assays. The list of used gene expression assays includes the following: Apoptosis regular BAX (BAX) Hs00180269_m1, BCL2 apoptosis regular (BCL2) Hs04986394_s1, BH3 interacting domain death agonist (BID) Hs00609632_m1, Cyclin-dependent kinase inhibitor 1B (CDKN1B) Hs00153277_m1, Fas-associated via death domain (FADD) Hs00538709_m1, Integrin subunit alpha 2 (ITGA2) Hs00158127_m1, Integrin subunit beta 1 (ITGB1) Hs01127536_m1, PPARG coactivator 1 alpha (PPARGC1A) Hs00173304_m1 and Tumor protein p53 (TP53) Hs_00153349_m1. Gene expression analysis was performed using the Quant studio 6 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific). The gene expression analysis results were normalized to RNA polymerase II subunit A (POLR2A) (Hs00172187_m1) RNA expression. Gene expression levels were calculated using a comparative Ct (ΔΔCt) method.
2.11. Statistical Analysis
All experiments consisted of a minimum of three independent replicates. Statistical analysis was performed using GraphPad Prism 9.2.0 (GraphPad Software Inc.). Data are expressed as the mean ± SD. Following normality tests, two-way ANOVA followed by Tukey’s post hoc multiple comparison tests was used to assess significance among experimental groups. A P-value <0.05 was considered to indicate a statistically significant difference.
4. Discussion
Collagen I is one of the most used cell adhesion materials in cell culture, and its utility has significantly increased owing to the need to improve 2D models and increased recommendations for transformation to 3D models [
5,
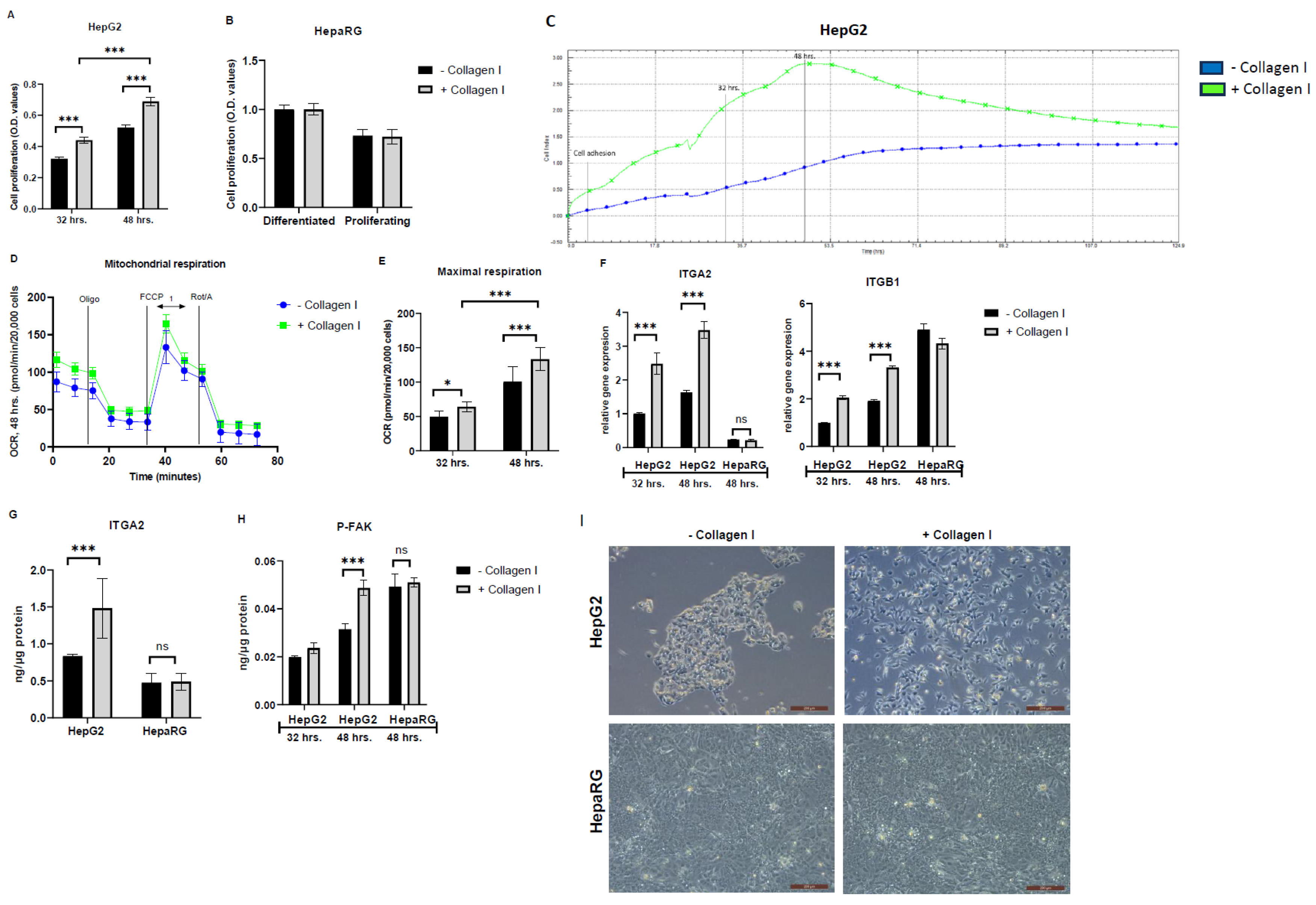
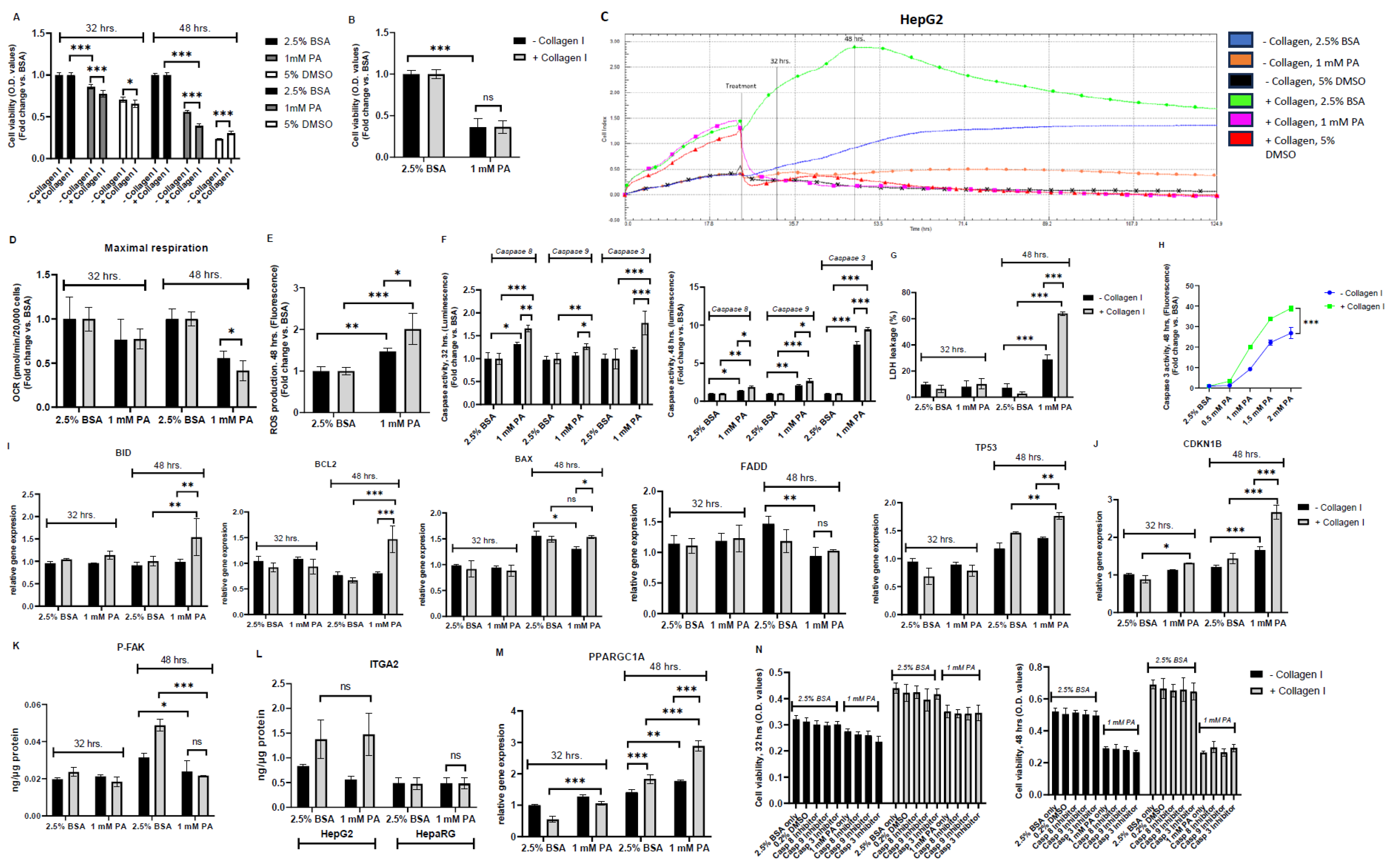
14]. This study assessed PA-induced cell death in HepG2 cells cultured in the absence or presence of collagen I. For the first time, this study revealed that culturing HepG2 cells in the presence of collagen I was associated with increased PA-induced cell death compared to culturing in the absence of collagen I.
Numerous ways have been adopted to improve 2D models to be more suitable for NAFLD in vitro studies. For example, using coculture 2D models, selecting suitable primary cells and cell lines, and enhancing cell adhesion and extracellular communication of utilized cells [
1,
4]. Collagen I-sandwiched primary rat hepatocytes maintain the secretion of important hepatic markers, such as fibrinogen, albumin, and bile acids, for up to 6 weeks [
33]. 3D cell cultures of HepG2 cells using collagen I have also shown increased albumin and urea secretion and higher expression/activities of some xenobiotic-metabolizing enzymes [
34,
35]. Consistent with the previous studies [
16,
17], the current study also demonstrated that culturing HepG2 cells in the presence of collagen I resulted in increased cell adhesion, which was correlated with increased cell proliferation, mitochondrial respiration, higher levels of integrin receptors and P-FAK and improved cell spreading. The findings from HepG2 cells cultured on collagen I were further validated by the data from HepaRG cells, showing that lack of enhanced cell proliferation on collagen I was correlated with unchanged levels of integrin receptors, P-FAK, and no change in cellular morphology. Having confirmed the benefits of culturing HepG2 in the presence of collagen I, it was hypothesized that seeding HepG2 on collagen I could improve cell adhesion and reduce PA-induced cell death. To test our hypotheses, this study measured and compared various markers of cell death (cell viability, cell adhesion, caspase activities, LDH leakage, pro and anti-apoptotic genes, and gene expression of CNKN1B and TP53), mitochondrial respiration and biogenesis, P-FAK, gene and protein levels of integrin receptor subunits, ROS production and TAG accumulation in PA-treated HepG2 cultured in the absence or presence of collagen I. Some of the parameters mentioned above were also assessed in PA-treated HepaRG cultured in the absence or presence of collagen I.
In general, the findings concerning lipotoxicity due to PA exposure in HepG2 and HepaRG, irrespective of the absence or presence of collagen I, were consistent with previous in vitro studies or clinical studies of late stages of NASH, demonstrating increased cell death, decreased mitochondrial respiration and high ROS production [
10,
11,
12,
36]. However, contradictory to our expectations, these parameters were all exacerbated in HepG2 cultured in the presence of collagen I. These unexpected findings demanded a possible explanation; therefore, another hypothesis was formulated. After considering all the data from HepG2 and HepaRG, we hypothesized that increased cell death in HepG2 cultured in the presence of collagen I was due to integrin-mediated death. Previous studies have demonstrated that unligated or antagonized integrins recruit caspase 8 to the cell membrane and activate apoptosis through integrin-mediated death [
18,
27,
37]. Noting the correlation between increased activity of caspase 8 in PA-treated HepG2 cells cultured on collagen and high levels of the α2β1 receptors, it was speculated that PA may have exacerbated cell death by somehow antagonizing excess α2β1 receptors. This hypothesis was further supported by data showing that constant expression of the α2β1 receptors in HepaRG cells was associated with constant PA-induced cell death. Additionally, the data from gene expression analysis confirmed the established link between activated caspase 8 and cleavage of BID [
28]. This may explain the adaptive increase of BID gene expression, as most of it is cleaved to amplify mitochondrial-driven apoptosis seen in PA-treated HepG2 cultured on collagen I. As described elsewhere [
17,
38,
39,
40], our findings support the crosstalk involving amplified antagonized α2β1 receptors, increased apoptosis, reduced levels of P-FAK, increased gene expression of TP53, and CDKN1B.
Since integrin-mediated death has not been described in NAFLD [
41], the findings from this study imply that that PA-induced lipotoxicity was incorrectly enhanced by culturing HepG2 on collagen I. Moreover, the data from this study could suggest that the EC
50 and IC
50 of some drugs tested in HepG2 in the presence of collagen I may be overestimated, especially if those drugs interact with integrin receptors. Therefore, researchers may need to compare the effects of drug agents in the absence or presence of collagen I in HepG2 or other cell lines. Although the data from this study suggest that integrin-mediated death is likely responsible for the outcomes observed, future research is necessary to provide robust evidence by overexpressing or knocking down the ITGA2 or ITGB1 subunits in HepG2 to determine the effects of PA-induced cell death. In addition, future studies may also determine how PA interacts with the α2β1 receptors using fluorescent or radiolabelled PA and receptor subunits.
In conclusion, the present study demonstrated for the first time that collagen I-cultured HepG2 exhibited enhanced cell death following exposure to PA through the mechanism of integrin-mediated death. The findings from this study may serve as a caution to those using 2D models or 3D scaffold-based models of HepG2 in the presence of collagen I.
Author Contributions
Conceptualization, T.E.M., Z.Č. and O.K.; methodology, T.E.M., E.P., M.E., D.K., and J.M.; software, E.M. and D.K.; validation, T.E.M., P.S., H.L. and O.K.; formal analysis, T.E.M..; investigation, T.E.M., V.Š. and R.M.; data curation, Z.Č. and O.K.; writing—original draft preparation, T.E.M.; writing—review and editing, T.E.M., E.P., M.E., D.K., J.M., P.S., H.L., Z.Č. and O.K.; visualization, T.E.M.; supervision, O.K.; project administration, Z.Č. and O.K.; funding acquisition, T.E.M., Z.Č. and O.K. All authors have read and agreed to the published version of the manuscript.