Submitted:
08 August 2024
Posted:
09 August 2024
You are already at the latest version
Abstract
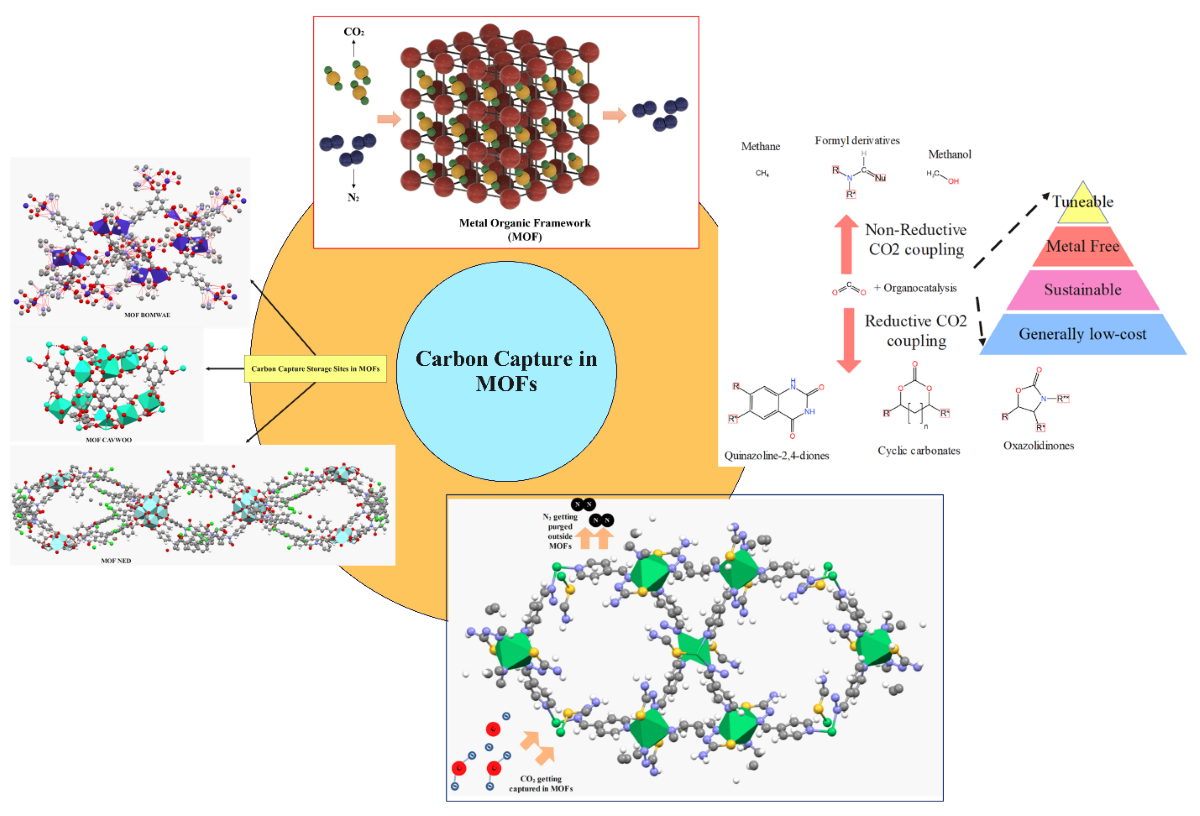
Keywords:
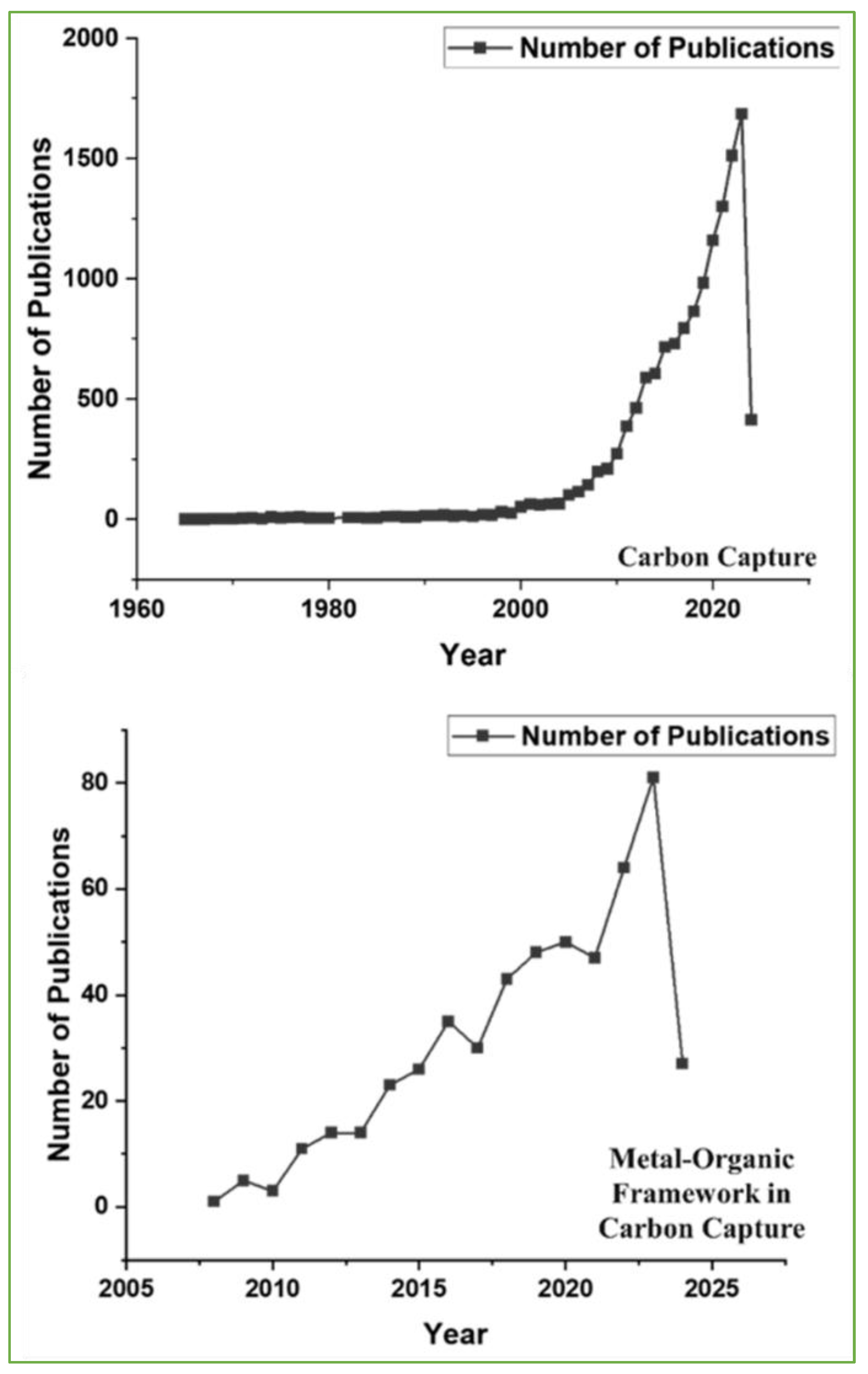
1. Introduction
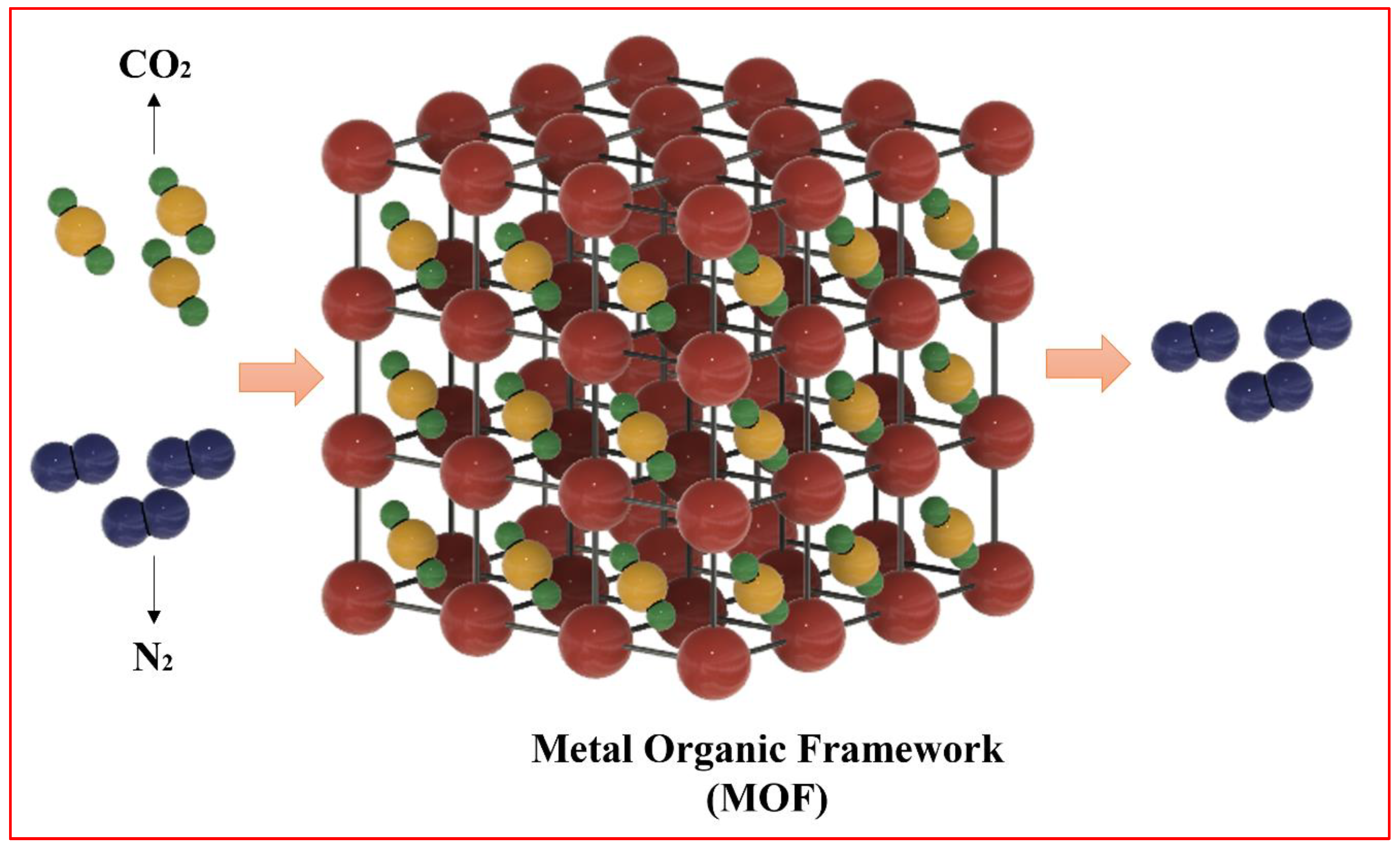
1.1. Role of MOFs in Carbon Capture
1.2. Pros and Cons of MOFs in Carbon Capture
2. Organic Properties and Carbon Dioxide Uptake Capacity in MOFs
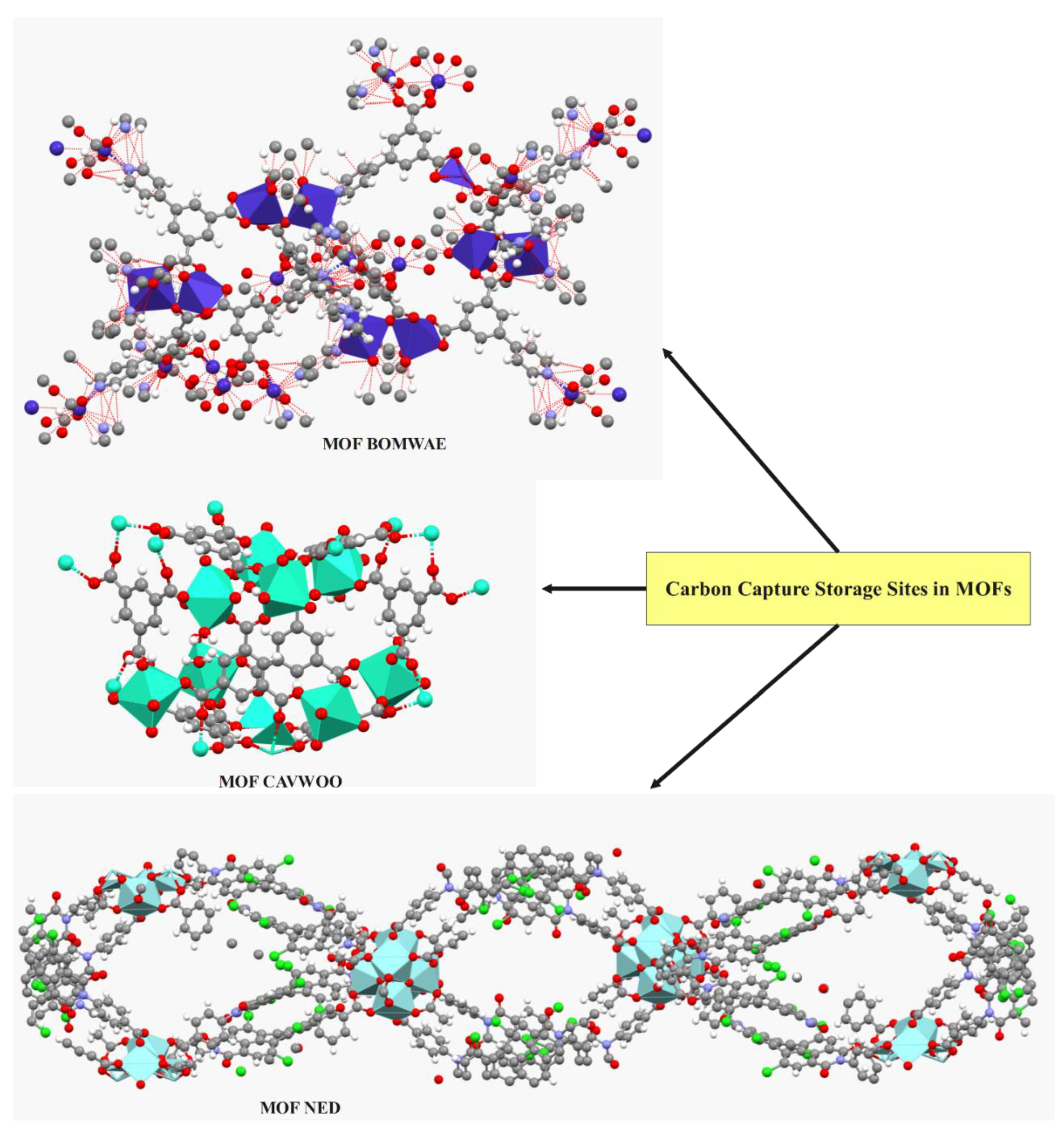
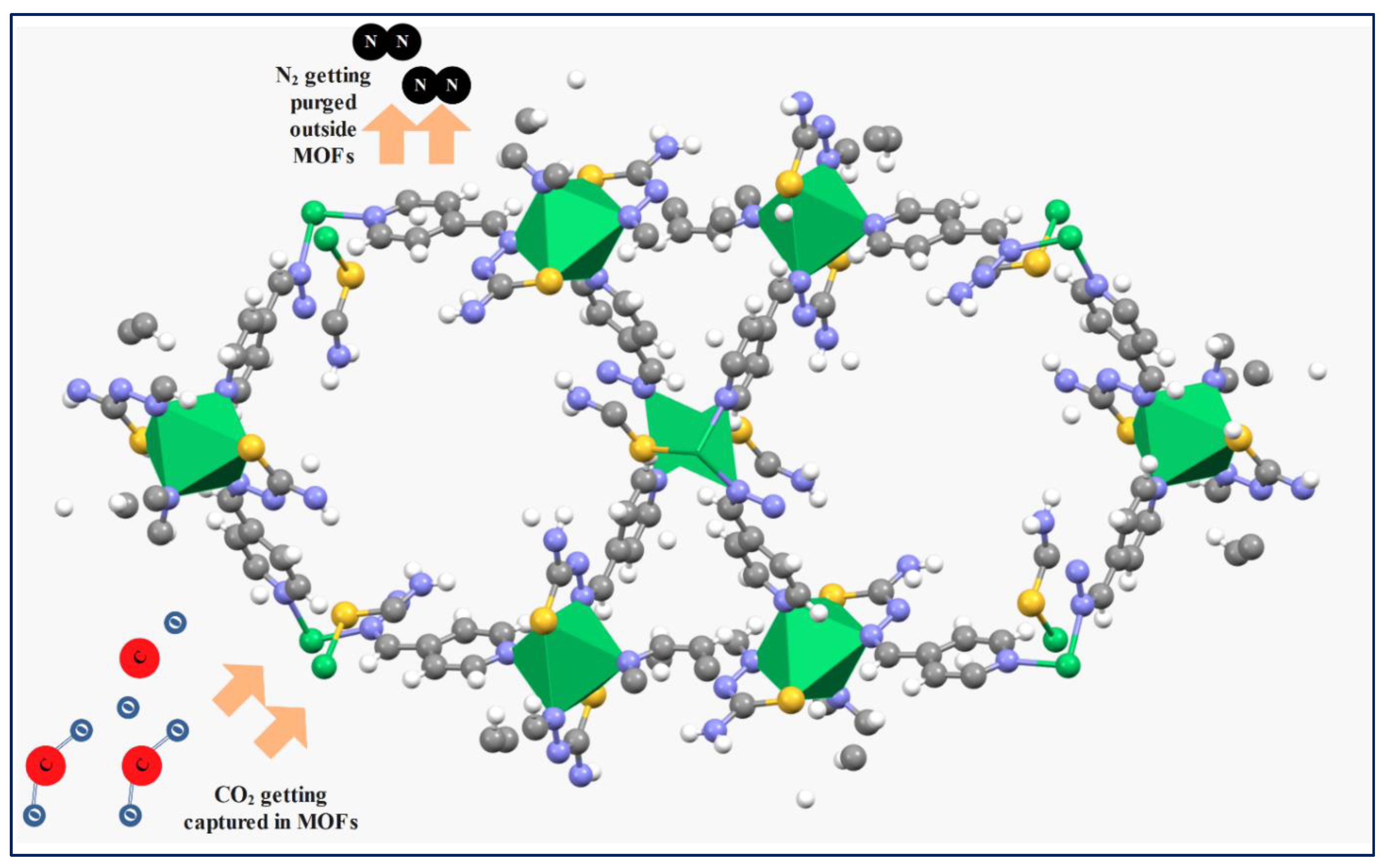
3. Carbon Dioxide Storage Sites in MOFs
4. Carbon Dioxide Conversion through Metal-Organic Frameworks
4.1. Organo-Catalysts Pathways through Carbon Capture in MOFs
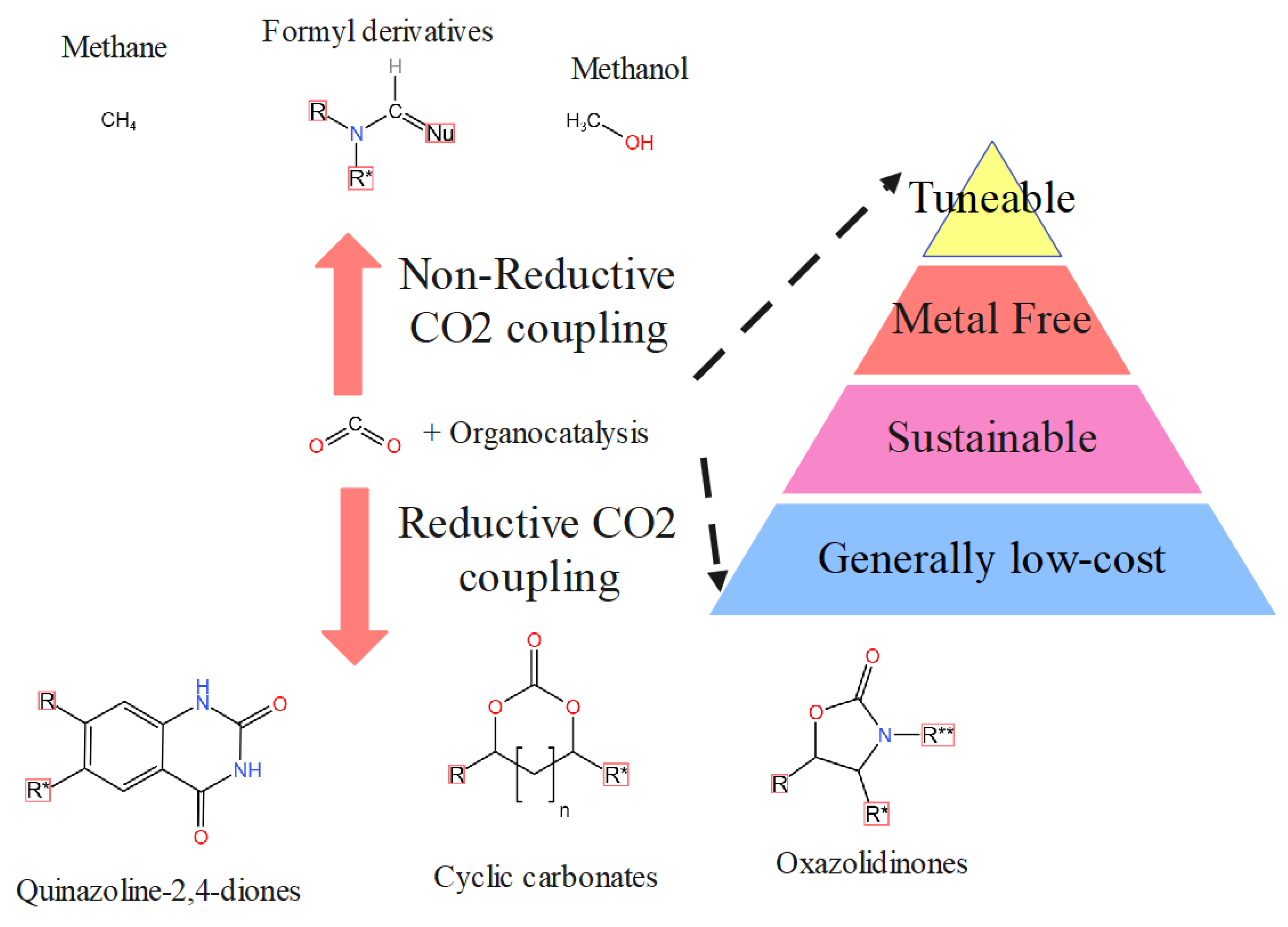
4.2. Carbon Dioxide Conversion to Organic Compounds


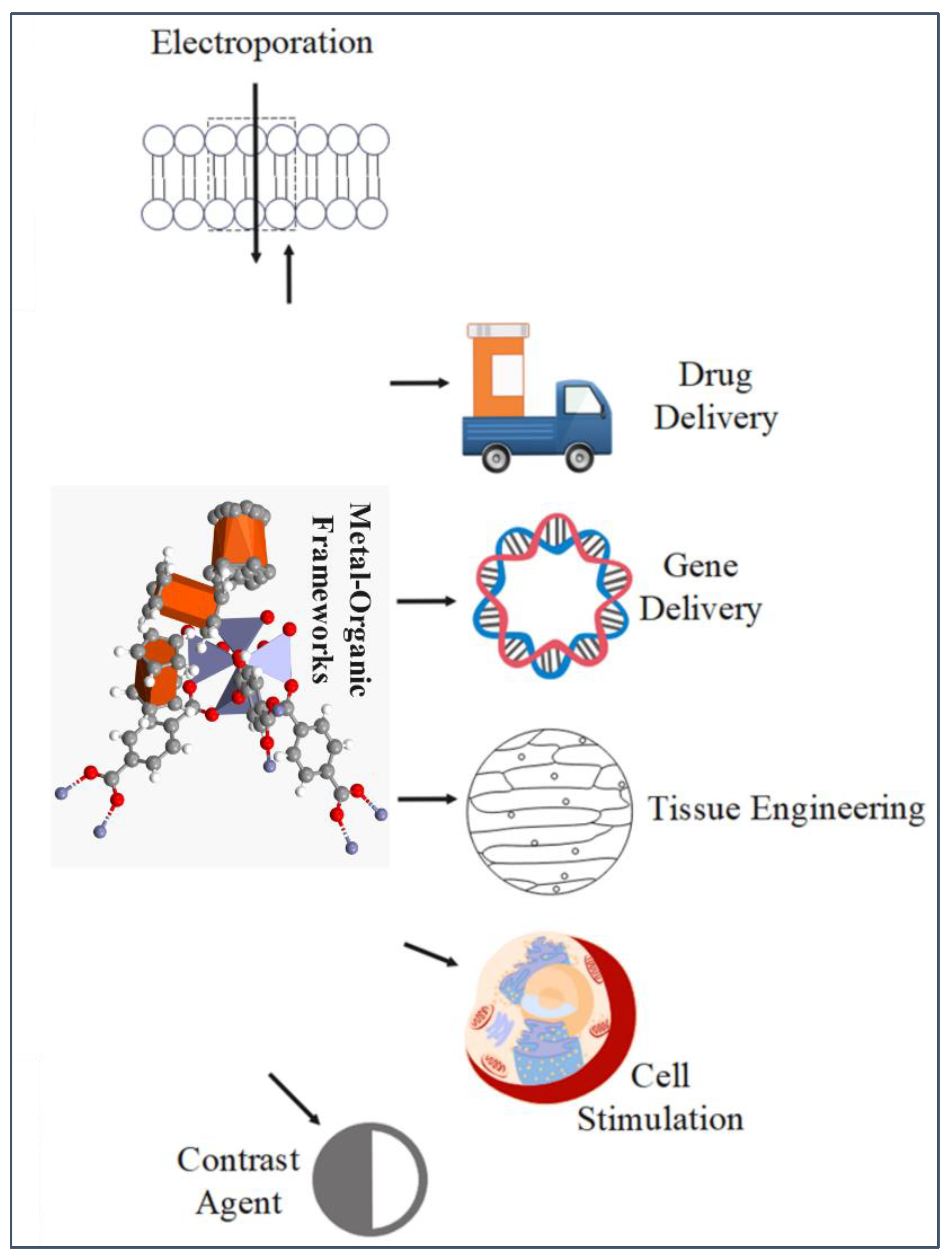
5. Applications of MOFs integrated with Carbon Capture
6. Conclusions
| UNSDGs | United Nations Sustainable Development Goals |
| CO2 | Carbon Dioxide |
| MOF | Metal Organics Framework |
| CC | Carbon Capture |
| ZIF | Zeolitic imidazolate frameworks |
| BET | Brunauer-Emmett-Teller |
| LBS | Lewis Primary Sites |
| USDOE | United States Department of Energy |
| DMC | Dimethyl Carbonate |
| DAC | Diallyl Carbonate |
| DEC | Diethyl Carbonate |
| DPC | Diphenyl Carbonate |
| EC | Ethylene Carbonate |
| PC | Propylene Carbonate |
| CC | Cyclohexene Carbonate |
| SC | Styrene Carbonate |
References
- Ahmed Ali, K., Ahmad, M. I., & Yusup, Y. (2020). Issues, Impacts, and Mitigations of Carbon Dioxide Emissions in the Building Sector. Sustainability, 12(18), 7427. [CrossRef]
- Ahmed, S. F., Mehejabin, F., Momtahin, A., Tasannum, N., Faria, N. T., Mofijur, M., Hoang, A. T., Vo, D.-V. N., & Mahlia, T. M. I. (2022). Strategies to improve membrane performance in wastewater treatment. Chemosphere, 306, 135527. [CrossRef]
- Alabadi, A., Razzaque, S., Yang, Y., Chen, S., & Tan, B. (2015). Highly porous activated carbon materials from carbonized biomass with high CO2 capturing capacity. Chemical Engineering Journal. [CrossRef]
- Álvarez-Murillo, A., Sabio, E., Ledesma, B., Román, S., & González-García, C. M. (2016). Generation of biofuel from hydrothermal carbonization of cellulose. Kinetics modelling. Energy, 94, 600–608. [CrossRef]
- Amaral, A. J. R., Coelho, J. F. J., & Serra, A. C. (2013). Synthesis of bifunctional cyclic carbonates from CO2 catalysed by choline-based systems. Tetrahedron Letters. [CrossRef]
- Ang, M. L., Oemar, U., Kathiraser, Y., Saw, E. T., Lew, C. H. K., Du, Y., Borgna, A., Wang, C.-H., & Kawi, S. (2015). High-temperature water–gas shift reaction over Ni/xK/CeO2 catalysts: Suppression of methanation via formation of bridging carbonyls. Journal of Catalysis. [CrossRef]
- Aresta, M., Dibenedetto, A., & Angelini, A. (2014). Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. technological use of CO2. Chemical Reviews. [CrossRef]
- Azdarpour, A., Guo, W., Asadullah, M., Mohammadian, E., Hamidi, H., Junin, R., & Karaei, M. A. (2015). A review on carbon dioxide mineral carbonation through pH-swing process. Chemical Engineering Journal. [CrossRef]
- Bhadra, M., Sasmal, H. S., Basu, A., Midya, S. P., Kandambeth, S., Pachfule, P., Balaraman, E., & Banerjee, R. (2017). Predesigned Metal-Anchored Building Block for In Situ Generation of Pd Nanoparticles in Porous Covalent Organic Framework: Application in Heterogeneous Tandem Catalysis. ACS Applied Materials & Interfaces. [CrossRef]
- Bhardwaj, R., Kumar, A., & Choudhury, J. (2022). An all-aqueous and phosphine-free integrated amine-assisted CO2 capture and catalytic conversion to formic acid. Chemical Communications. [CrossRef]
- Boycheva, S., Chakarova, K., Mihaylov, M., Hadjiivanov, K., & Popova, M. (2022). Effect of calcium on enhanced carbon capture potential of coal fly ash zeolites. Part II: A study on the adsorption mechanisms. Environmental Science: Processes and Impacts, 24(10), 1934–1944. [CrossRef]
- Calò, V., Nacci, A., Monopoli, A., & Fanizzi, A. (2002). Cyclic Carbonate Formation from Carbon Dioxide and Oxiranes in Tetrabutylammonium Halides as Solvents and Catalysts. Organic Letters. [CrossRef]
- Chatelet, B., Joucla, L., Joucla, L., Dutasta, J.-P., Martinez, A., Martinez, A., Szeto, K. C., & Dufaud, V. (2013). Azaphosphatranes as Structurally Tunable Organocatalysts for Carbonate Synthesis from CO2 and Epoxides. Journal of the American Chemical Society. [CrossRef]
- Chen, C., Mo, Q., Huang, Y., & Zhang, L. (2023). Selective Reduction of CO2 to Methanol via Hydrosilylation Boosted by a Porphyrinic Metal-Organic Framework. ACS Catalysis, 13(10), 6837–6845. [CrossRef]
- Clements, J. H. (2003). Reactive Applications of Cyclic Alkylene Carbonates. Industrial & Engineering Chemistry Research. [CrossRef]
- Da Costa, B. L., Rosa, I. L. A. A., Silva, V. H., Wu, Q., Samulewski, R. B., Scacchetti, F. A. P., Moisés, M. P., Lis, M. J., & Bezerra, F. M. (2022). Direct Synthesis of HKUST-1 onto Cotton Fabrics and Properties. Polymers, 14(20). [CrossRef]
- Daval, D. (2018). Carbon dioxide sequestration through silicate degradation and carbon mineralisation: Promises and uncertainties. In npj Materials Degradation (Vol. 2, Issue 1). Nature. [CrossRef]
- Decortes, A., Belmonte, M. M., Benet-Buchholz, J., & Kleij, A. W. (2010). Efficient carbonate synthesis under mild conditions through cycloaddition of carbon dioxide to oxiranes using a Zn(salphen) catalyst. Chemical Communications. [CrossRef]
- Ding, M., Flaig, R. W., Jiang, H.-L., & Yaghi, O. M. (2019). Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chemical Society Reviews. [CrossRef]
- Dweck, J., Büchler, P. M., Coelho, A. C. V., & Cartledge, F. K. (2000). Hydration of a Portland cement blended with calcium carbonate. Thermochimica Acta. [CrossRef]
- Escobar-Hernandez, H. U., Quan, Y., Papadaki, M. I., & Wang, Q. (2023). Life Cycle Assessment of Metal-Organic Frameworks: Sustainability Study of Zeolitic Imidazolate Framework-67. ACS Sustainable Chemistry and Engineering, 11(10), 4219–4225. [CrossRef]
- Fei, L., Dong, S., Xue, L., Liang, Q., & Yang, W. (2011). Energy consumption-economic growth relationship and carbon dioxide emissions in China. Energy Policy, 39(2), 568–574. [CrossRef]
- Geissler, C. H., & Maravelias, C. T. (2022). Analysis of alternative bioenergy with carbon capture strategies: Present and future. Energy and Environmental Science, 15(7), 2679–2689. [CrossRef]
- Genovese, C., Ampelli, C., Perathoner, S., & Centi, G. (2013). Electrocatalytic conversion of CO2 to liquid fuels using nanocarbon-based electrodes. Journal of Energy Chemistry. [CrossRef]
- Gong, K., Du, F., Xia, Z., Durstock, M. F., & Dai, L. (2009). Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science. [CrossRef]
- Guo, Y., Zhao, C., & Li, C. (2015). Thermogravimetric analysis of kinetic characteristics of K2CO3-impregnated mesoporous silicas in low concentration CO2. Journal of Thermal Analysis and Calorimetry. [CrossRef]
- Hänchen, M., Prigiobbe, V., Baciocchi, R., & Mazzotti, M. (2008). Precipitation in the Mg-carbonate system—Effects of temperature and CO2 pressure. Chemical Engineering Science. [CrossRef]
- Hu, B., Guild, C., & Suib, S. L. (2013). Thermal, electrochemical, and photochemical conversion of CO2 to fuels and value-added products. Journal of CO 2 Utilization. [CrossRef]
- Hu, L., Rexed, I., & Lagergren, C. (2014). Electrochemical performance of reversible molten carbonate fuel cells. International Journal of Hydrogen Energy. [CrossRef]
- Huang, K., Sun, C., & Shi, Z. (2011). Transition-metal-catalyzed C–C bond formation through the fixation of carbon dioxide. Chemical Society Reviews. [CrossRef]
- Hussain, I., Jalil, A. A., Hassan, N. S., & Hamid, M. Y. S. (2021). Recent advances in catalytic systems for CO2 conversion to substitute natural gas (SNG): Perspective and challenges. Journal of Energy Chemistry. [CrossRef]
- Jeffry, L., Ong, M. Y., Nomanbhay, S., Mofijur, M., Mubashir, M., & Show, P. L. (2021). Greenhouse gases utilization: A review. Fuel, 301, 121017. [CrossRef]
- Jutz, F., Jutz, F., Grunwaldt, J.-D., & Baiker, A. (2009). In situ XAS study of the Mn(III)(salen)Br catalyzed synthesis of cyclic organic carbonates from epoxides and CO2. Journal of Molecular Catalysis A-Chemical. [CrossRef]
- Kalyanasundaram, K., & Graetzel, M. (2010). Artificial photosynthesis: Biomimetic approaches to solar energy conversion and storage. Current Opinion in Biotechnology. [CrossRef]
- Lee, J. H., Lee, J. W., & Kang, Y. T. (2016). CO2 regeneration performance enhancement by nanoabsorbents for energy conversion application. Applied Thermal Engineering. [CrossRef]
- Lee, K., Liu, X., Vyawahare, P., Sun, P., Elgowainy, A., & Wang, M. (2022). Techno-economic performances and life cycle greenhouse gas emissions of various ammonia production pathways including conventional, carbon-capturing, nuclear-powered, and renewable production. Green Chemistry, 24(12), 4830–4844. [CrossRef]
- Liang, S., Liu, H., Jiang, T., Song, J., Yang, G., & Han, B. (2011). Highly efficient synthesis of cyclic carbonates from CO2 and epoxides over cellulose/KI. Chemical Communications. [CrossRef]
- Lu, L., Huang, Z., Rau, G. H., & Ren, Z. J. (2015). Microbial Electrolytic Carbon Capture for Carbon Negative and Energy Positive Wastewater Treatment. Environmental Science and Technology, 49(13), 8193–8201. [CrossRef]
- Lu, X.-B., & Darensbourg, D. J. (2012). Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates. Chemical Society Reviews. [CrossRef]
- Martín, C. F., Stöckel, E., Clowes, R., Adams, D. J., Cooper, A. I., Pis, J. J., Rubiera, F., & Pevida, C. (2011). Hypercrosslinked organic polymer networks as potential adsorbents for pre-combustion CO2 capture. Journal of Materials Chemistry. [CrossRef]
- Martín, C., Fiorani, G., & Kleij, A. W. (2015). Recent advances in the catalytic preparation of cyclic organic carbonates. ACS Catalysis. [CrossRef]
- Matter, J. M., Stute, M., Snæbjörnsdóttir, S. Ó., Oelkers, E. H., Gislason, S. R., Aradóttir, E. S., Sigfússon, B., Gunnarsson, I., Sigurdardottir, H., Gunnlaugsson, E., Axelsson, G., Alfredsson, H. A., Wolff-Boenisch, D., Mesfin, K. G., de la Reguera Taya, D. F., Hall, J., Dideriksen, K., & Broecker, W. S. (2016). Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science. [CrossRef]
- Matter, J. M., Stute, M., Snæbjörnsdottir, S. Ó., Oelkers, E. H., Gislason, S. R., Aradottir, E. S., Sigfusson, B., Gunnarsson, I., Sigurdardottir, H., Gunnlaugsson, E., Axelsson, G., Alfredsson, H. A., Wolff-Boenisch, D., Mesfin, K., Taya, D. F. de la R., Hall, J., Dideriksen, K., & Broecker, W. S. (2016). Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science, 352(6291), 1312–1314. [CrossRef]
- Mehrpooya, M., Moftakhari Sharifzadeh, M. M., Rajabi, M., Aghbashlo, M., Tabatabai, M., Hosseinpour, S., & Ramakrishna, S. (2017). Design of an integrated process for simultaneous chemical looping hydrogen production and electricity generation with CO 2 capture. International Journal of Hydrogen Energy, 42(12), 8486–8496. [CrossRef]
- Meng, W., Huang, Y., Fu, Y., Wang, Z., & Zhi, C. (2014). Polymer composites of boron nitride nanotubes and nanosheets. J. Mater. Chem. C, 2(47), 10049–10061. [CrossRef]
- Miller, Q. R. S., Thompson, C. J., Loring, J. S., Windisch, C. F., Windisch, C. F., Windisch, C. F., Windisch, C. F., Bowden, M. E., Hoyt, D. W., Hu, J. Z., Arey, B. W., Rosso, K. M., & Schaef, H. T. (2013). Insights into silicate carbonation processes in water-bearing supercritical CO2 fluids. International Journal of Greenhouse Gas Control. [CrossRef]
- Mittal, H., & Kushwaha, O. S. (2024a). Biogas and Biofuel Production from Biowaste: Modelling and Simulation Study. In From Waste to Wealth (pp. 379–400). Springer Nature Singapore. [CrossRef]
- Mittal, H., & Kushwaha, O. S. (2024b). Machine Learning in Commercialized Coatings. In Functional Coatings (pp. 450–474). Wiley. [CrossRef]
- Mittal, H., & Kushwaha, O. S. (2024c). Policy Implementation Roadmap, Diverse Perspectives, Challenges, Solutions Towards Low-Carbon Hydrogen Economy. Green and Low-Carbon Economy. [CrossRef]
- Mittal, H., Verma, S., Bansal, A., & Singh Kushwaha, O. (2024). Low-Carbon Hydrogen Economy Perspective and Net Zero-Energy Transition through Proton Exchange Membrane Electrolysis Cells (PEMECs), Anion Exchange Membranes (AEMs) and Wind for Green Hydrogen Generation. Qeios. [CrossRef]
- Mofarahi, M., Khojasteh, Y., Khaledi, H., & Farahnak, A. (2008). Design of CO2 absorption plant for recovery of CO2 from flue gases of gas turbine. Energy. [CrossRef]
- Moh, P. Y., Cubillas, P., Anderson, M. W., & Attfield, M. P. (2011). Revelation of the Molecular Assembly of the Nanoporous Metal Organic Framework ZIF-8. Journal of the American Chemical Society. [CrossRef]
- Mohan, D., & Pittman, C. U. (2006). Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. Journal of Hazardous Materials. [CrossRef]
- Morales-Flórez, V., Santos, A., Romero-Hermida, I., & Esquivias, L. (2015). Hydration and carbonation reactions of calcium oxide by weathering: Kinetics and changes in the nanostructure. Chemical Engineering Journal. [CrossRef]
- Naranjo, T., Collado, L., Gomez-Mendoza, M., Pizarro, A. H., Barawi, M., Gándara, F., Liras, M., & de la Peña O’Shea, V. A. (2023). Solar-Driven Hydrogen Production Using a BODIPY Covalent Organic Framework Hybrid Photocatalyst. ACS Catalysis, 283–291. [CrossRef]
- North, M., Pasquale, R., & Young, C. (2010). Synthesis of cyclic carbonates from epoxides and CO2. Green Chemistry. [CrossRef]
- Olajire, A. A. (2013). A review of mineral carbonation technology in sequestration of CO2. Journal of Petroleum Science and Engineering. [CrossRef]
- Pio, D. T., Vilas-Boas, A. C. M., Rodrigues, N. F. C., & Mendes, A. (2022). Carbon neutral methanol from pulp mills towards full energy decarbonization: An inside perspective and critical review. In Green Chemistry (Vol. 24, Issue 14, pp. 5403–5428). Royal Society of Chemistry. [CrossRef]
- Prajapati, A., Sartape, R., Galante, M. T., Xie, J., Leung, S. L., Bessa, I., Andrade, M. H. S., Somich, R. T., Rebouças, M. V., Hutras, G. T., Diniz, N., & Singh, M. R. (2022). Fully-integrated electrochemical system that captures CO2 from flue gas to produce value-added chemicals at ambient conditions. Energy and Environmental Science, 15(12), 5105–5117. [CrossRef]
- Rahimi, M., Khurram, A., Hatton, T. A., & Gallant, B. (2022). Electrochemical carbon capture processes for mitigation of CO2 emissions. In Chemical Society Reviews (Vol. 51, Issue 20, pp. 8676–8695). Royal Society of Chemistry. [CrossRef]
- Ramasubramanian, B., Sundarrajan, S., Rao, R. P., Reddy, M. V., Chellappan, V., & Ramakrishna, S. (2022). Novel low-carbon energy solutions for powering emerging wearables, smart textiles, and medical devices. In Energy and Environmental Science (Vol. 15, Issue 12, pp. 4928–4981). Royal Society of Chemistry. [CrossRef]
- Roshan, K. R., Mathai, G., Kim, J., Tharun, J., Park, G.-A., & Park, D.-W. (2012). A biopolymer mediated efficient synthesis of cyclic carbonates from epoxides and carbon dioxide. Green Chemistry. [CrossRef]
- Sakakura, T., Choi, J.-C., & Yasuda, H. (2007). Transformation of carbon dioxide. Chemical Reviews. [CrossRef]
- Sanna, A., Gaubert, J., & Maroto-Valer, M. M. (2016). Alternative regeneration of chemicals employed in mineral carbonation towards technology cost reduction. Chemical Engineering Journal. [CrossRef]
- Sanna, A., Uibu, M., Caramanna, G., Kuusik, R., & Maroto-Valer, M. M. (2014). A review of mineral carbonation technologies to sequester CO2. Chemical Society Reviews. [CrossRef]
- Serna-Guerrero, R., Belmabkhout, Y., & Sayari, A. (2010). Further investigations of CO2 capture using triamine-grafted pore-expanded mesoporous silica. Chemical Engineering Journal. [CrossRef]
- Shiraishi, T., & Hirata, R. (2021). Estimation of carbon dioxide emissions from the megafires of Australia in 2019–2020. Scientific Reports, 11(1), 8267. [CrossRef]
- Sinha, A., Darunte, L. A., Jones, C. W., Realff, M. J., & Kawajiri, Y. (2017). Systems Design and Economic Analysis of Direct Air Capture of CO2 through Temperature Vacuum Swing Adsorption Using MIL-101(Cr)-PEI-800 and mmen-Mg2(dobpdc) MOF Adsorbents. Industrial & Engineering Chemistry Research. [CrossRef]
- Smith, C., Hill, A. K., & Torrente-Murciano, L. (2020). Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy and Environmental Science. [CrossRef]
- Song, Q.-W., He, L.-N., Wang, J., Yasuda, H., Sakakura, T., & Sakakura, T. (2013). Catalytic fixation of CO2 to cyclic carbonates by phosphonium chlorides immobilized on fluorous polymer. Green Chemistry. [CrossRef]
- Sun, J., Cheng, W., Yang, Z., Wang, J., Xu, T., Xin, J., & Zhang, S. (2014). Superbase/cellulose: An environmentally benign catalyst for chemical fixation of carbon dioxide into cyclic carbonates. Green Chemistry. [CrossRef]
- Sun, X., Mueller, S., Liu, Y., Shi, H., Haller, G. L., Sanchez-Sanchez, M., van Veen, A. C., & Lercher, J. A. (2014). On reaction pathways in the conversion of methanol to hydrocarbons on HZSM-5. Journal of Catalysis. [CrossRef]
- Tai, M. H., Gao, P., Tan, B. Y. L., Sun, D. D., & Leckie, J. O. (2014). Highly Efficient and Flexible Electrospun Carbon–Silica Nanofibrous Membrane for Ultrafast Gravity-Driven Oil–Water Separation. ACS Applied Materials & Interfaces, 6(12), 9393–9401. [CrossRef]
- Tan, C., Tao, F., & Xu, P. (2022). Direct carbon capture for the production of high-performance biodegradable plastics by cyanobacterial cell factories. Green Chemistry, 24(11), 4470–4483. [CrossRef]
- Theo, W. L., Lim, J. S., Hashim, H., Mustaffa, A. A., & Ho, W. S. (2016). Review of pre-combustion capture and ionic liquid in carbon capture and storage. Applied Energy. [CrossRef]
- Thiruvenkatachari, R., Su, S., An, H., & Yu, X. X. (2009). Post combustion CO2 capture by carbon fibre monolithic adsorbents. Progress in Energy and Combustion Science. [CrossRef]
- Trinh, H. H., Trinh, K. A., Trinh, N., & Haouas, I. (2022). Energy Technology RD&D Budgets, sustainable transition, carbon risk, and climate change: Fresh insights from IEA member countries under policy uncertainty.
- Tsutsumi, Y., Yamakawa, K., Yoshida, M., Ema, T., & Sakai, T. (2010). Bifunctional Organocatalyst for Activation of Carbon Dioxide and Epoxide To Produce Cyclic Carbonate: Betaine as a New Catalytic Motif. Organic Letters. [CrossRef]
- Veetil, S. P., Pasquier, L.-C., Blais, J.-F., Cecchi, E., Kentish, S. E., Mercier, G., & Mercier, G. (2015). Direct gas–solid carbonation of serpentinite residues in the absence and presence of water vapor: A feasibility study for carbon dioxide sequestration. Environmental Science and Pollution Research. [CrossRef]
- Wall, T., Liu, Y., Spero, C., Elliott, L., Khare, S., Rathnam, R. K., Zeenathal, F., Moghtaderi, B., Buhre, B. J. P., Sheng, C., Gupta, R., Yamada, T., Makino, K., & Yu, J. (2009). An overview on oxyfuel coal combustion—State of the art research and technology development. Chemical Engineering Research & Design. [CrossRef]
- Wang, H., Zhu, Q. L., Zou, R., & Xu, Q. (2017). Metal-Organic Frameworks for Energy Applications. In Chem (Vol. 2, Issue 1, pp. 52–80). Elsevier Inc. [CrossRef]
- Wang, W., Hu, M., Zheng, Y., Wang, P., & Ma, C. (2011). CO2 Fixation in Ca2+-/Mg2+-Rich Aqueous Solutions through Enhanced Carbonate Precipitation. Industrial & Engineering Chemistry Research. [CrossRef]
- Whiteoak, C. J., Henseler, A. H., Ayats, C., Kleij, A. W., & Pericàs, M. A. (2014). Conversion of oxiranes and CO2 to organic cyclic carbonates using a recyclable, bifunctional polystyrene-supported organocatalyst. Green Chemistry. [CrossRef]
- Whiteoak, C. J., Kielland, N., Laserna, V., Escudero-Adán, E. C., Martin, E., & Kleij, A. W. (2013). A Powerful Aluminum Catalyst for the Synthesis of Highly Functional Organic Carbonates. Journal of the American Chemical Society. [CrossRef]
- Whiteoak, C. J., Nova, A., Maseras, F., & Kleij, A. W. (2012). Merging Sustainability with Organocatalysis in the Formation of Organic Carbonates by Using CO2 as a Feedstock. Chemsuschem. [CrossRef]
- Wörmeyer, K., & Smirnova, I. (2013). Adsorption of CO2, moisture and ethanol at low partial pressure using aminofunctionalised silica aerogels. Chemical Engineering Journal. [CrossRef]
- Yokozeki, A., Shiflett, M. B., Junk, C. P., Grieco, L. M., & Foo, T. (2008). Physical and chemical absorptions of carbon dioxide in room-temperature ionic liquids. Journal of Physical Chemistry B. [CrossRef]
- Younas, M., Rezakazemi, M., Daud, M., Wazir, M. B., Ahmad, S., Ullah, N., Inamuddin, & Ramakrishna, S. (2020). Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Progress in Energy and Combustion Science, 80, 100849. [CrossRef]
- Yu, M., Wang, K., & Vredenburg, H. (2021). Insights into low-carbon hydrogen production methods: Green, blue and aqua hydrogen. International Journal of Hydrogen Energy. [CrossRef]
- Zhang, Z., Lees, E. W., Habibzadeh, F., Salvatore, D. A., Ren, S., Simpson, G. L., Wheeler, D. G., Liu, A., & Berlinguette, C. P. (2022). Porous metal electrodes enable efficient electrolysis of carbon capture solutions. Energy and Environmental Science, 15(2), 705–713. [CrossRef]
- Zhao, C., Chen, X., Anthony, E. J., Jiang, X., Duan, L., Wu, Y., Dong, W., & Zhao, C. (2013). Capturing CO2 in flue gas from fossil fuel-fired power plants using dry regenerable alkali metal-based sorbent. Progress in Energy and Combustion Science. [CrossRef]
- Zheng, J., Mi, Z., Coffman, D., Milcheva, S., Shan, Y., Guan, D., & Wang, S. (2019). Regional development and carbon emissions in China. Energy Economics, 81, 25–36. [CrossRef]
- Zhong, N., Liu, H., Luo, X., Al-Marri, M. J., Benamor, A., Idem, R., Tontiwachwuthikul, P., & Liang, Z. (2016). Reaction Kinetics of Carbon Dioxide (CO2) with Diethylenetriamine and 1-Amino-2-propanol in Nonaqueous Solvents Using Stopped-Flow Technique. Industrial and Engineering Chemistry Research, 55(27), 7307–7317. [CrossRef]
| Synthesis Methods | Classifications | Advantages | Disadvantages |
|---|---|---|---|
| Conventional Methods | Solvothermal Method | Significant structures, crystal sizes and good yields. | Expensive energy costs and higher durations. |
| Electrochemical Method | Faster Synthesis | Fewer surface areas, higher electricity needs, and weak crystal structures. | |
| Flow chemical methods | Fewer energy needs, sustainable and great yields. | Variable durations on specific reactions. | |
| Modern Methods | Microwave Synthesis | Greater Crystal Sizes, Faster Synthesis and Tunability. | Less yield and scalability issues. |
| Sonochemical Method | Faster Synthesis, Tunability | Lesser yields | |
| Spray Drying | Less energy needs | Longer synthesis duration |
| Metal-Organic Framework | Carbon Dioxide Uptake Capacity (mmol/g) | Temperature (K) |
Pressure (atm) |
References |
|---|---|---|---|---|
| pip-γCD-MOF | 0.0273 | 333 | 1.1 | (Zhong et al., 2016) |
| PVAm(0.4)@MIL-101 | 3 | 298 | 1 | (Zhong et al., 2016) |
| PVAm(0.7)@MIL-101 | 3.3 | 298 | 1 | (Zhong et al., 2016) |
| PVAm(1.0)@MIL-101 | 2.8 | 298 | 1 | (Zhong et al., 2016) |
| Cu-BTC | 9.59 | 273 | 1 | (K. Lee et al., 2022) |
| Cu-BTC/GO2 | 9.05 | 273 | 1 | (K. Lee et al., 2022) |
| Cu-BTC/GO5 | 8.46 | 273 | 1 | (K. Lee et al., 2022) |
| Cu-BTC/GO10 | 9.59 | 273 | 1 | (K. Lee et al., 2022) |
| HKUST-1 | 3.3 | 298 | 1 | (Zhong et al., 2016) |
| PAN/HKUST-1(40 %) fibers | 1.4 | 298 | 1 | (Prajapati et al., 2022) |
| PAN/HKUST-1(60 %) fibers | 2.5 | 298 | 1 | (Prajapati et al., 2022) |
| NH2-β-CD-MOF | 0.549 | 273 | 1 | (L. Lu et al., 2015) |
| MIL-101(Cr, Mg) | 1.9 | 301 | 1 | (L. Lu et al., 2015) |
| 5% PEI-MIL-101(Cr, Mg) | 2.5 | 301 | 1 | (Álvarez-Murillo et al., 2016) |
| 10 % PEI-MIL-101(Cr, Mg) | 3.1 | 301 | 1 | (Álvarez-Murillo et al., 2016) |
| 20% PEI-MIL-101(Cr, Mg) | 3 | 301 | 1 | (Álvarez-Murillo et al., 2016) |
| 30% PEI-MIL-101(Cr, Mg) | 2.6 | 301 | 1 | (Tsutsumi et al., 2010) |
| 40 % PEI-MIL-101(Cr, Mg) | 2.4 | 301 | 1 | (Tsutsumi et al., 2010) |
| MIL-101 Cr | 1.5 | 298 | 1 | (Álvarez-Murillo et al., 2016) |
| TEPA-MIL-101 | 3.5 | 298 | 1 | (Tsutsumi et al., 2010) |
| PEI-MIL-101 | 2 | 298 | 1 | (Tsutsumi et al., 2010) |
| UiO-66/FA_mod | 1.5 | 298 | 1 | (Tsutsumi et al., 2010) |
| Qc-5-Cu | 2.48 | 298 | 1 | (Álvarez-Murillo et al., 2016) |
| SIFSIX-3-Cu | 1.02 | 298 | 1 | (Álvarez-Murillo et al., 2016) |
| Zn(im-P-im) | 3.54 | 298 | 1 | (Álvarez-Murillo et al., 2016) |
| Ni-4PyC | 3.11 | 298 | 1 | (Smith et al., 2020) |
| 30% PEI-Zn/Co ZIF@450 ◦C | 1.4 | 298 | 1 | (Alabadi et al., 2015) |
| 40% PEI-Zn/Co ZIF@450 ◦C | 1.8 | 298 | 1 | (Alabadi et al., 2015) |
| f-MWCNTs@Zn/Co-ZIF | - | 298 | 1 | (L. Lu et al., 2015) |
| N-MWCNTs@ZIF-8 | - | 298 | 1 | (L. Lu et al., 2015) |
| N-MWCNTs@ZIF-67 | - | 298 | 1 | (L. Lu et al., 2015) |
| N-MWCNTs@Zn/Co-ZIF | - | 298 | 1 | (L. Lu et al., 2015) |
| PM24@ MIL-101 | 2.9 | 298 | 1 | (North et al., 2010) |
| PM36@ MIL-101 | 2.7 | 298 | 1 | (North et al., 2010) |
| R-PM24@ MIL-101 | 3.6 | 298 | 1 | (North et al., 2010) |
| NH2-ZIF-8 | 49.1 | 298 | 1 | (Olajire, 2013) |
| 18% NH2-ZIF-8 | 53.57 | 298 | 1 | (Olajire, 2013) |
| ZIF-90 | 2.2 | 323 | 1 | (Olajire, 2013) |
| UiO-66 | 2.32 | 298 | 1 | (Olajire, 2013) |
| Cu3(BTC)2 | 4.4 | 298 | 1 | (Olajire, 2013) |
| NH2-UiO-66 | 3.32 | 298 | 1 | (Zhong et al., 2016) |
| NH2-Cu3(BTC)2 | 3.86 | 298 | 1 | (Zhong et al., 2016) |
| UiO-66 | 2.27 | 298 | 1 | (Veetil et al., 2015) |
| UiO-66/GO | 3.37 | 298 | 1 | (Chatelet et al., 2013) |
| UiO-66-NH2 | 2.59 | 298 | 1 | (Hänchen et al., 2008) |
| UiO-66-NH2/GO | 3.8 | 298 | 1 | (Hänchen et al., 2008) |
| 30TEPA/UiO-66 | 3.7 | 348 | 1 | (Hänchen et al., 2008) |
| NH2-UiO-66 | 3.15 | 298 | 1 | (Zhong et al., 2016) |
| GMA-UiO-66 | 4.28 | 298 | 1 | (Hänchen et al., 2008) |
| MOF-200 | 1.17 | 298 | 1 | (C. Martín et al., 2015) |
| MOF-200/GO | 1.34 | 298 | 1 | (C. Martín et al., 2015) |
| GO@ZIF-8 | 0.8 | 298 | 1 | (C. Martín et al., 2015) |
| MH-0 | 4.12 | 298 | 1 | (Hänchen et al., 2008) |
| MH-1 | 3.7 | 298 | 1 | (Hänchen et al., 2008) |
| MH-2 | 4.64 | 298 | 1 | (Hänchen et al., 2008) |
| MH-3 | 4.38 | 298 | 1 | (Hänchen et al., 2008) |
| Fe(pz)[Pt(CN)4] | 4.7 | 298 | 1 | (Zhong et al., 2016) |
| MIL-101(Cr)-NH2 | 3.4 | 308 | 1 | (Zhong et al., 2016) |
| UiO-66(Hf) | 1.5 | 298 | 1 | (Zhong et al., 2016) |
| UiO-66(Hf)-NH2 | 2.8 | 298 | 1 | (Zhong et al., 2016) |
| UiO-66(Hf)-(OH)2 | 4.06 | 298 | 1 | (North et al., 2010) |
| UiO-66(Hf)-(COOH)2 | 1.2 | 298 | 1 | (North et al., 2010) |
| UiO-66(Hf)-(F)4 | 0.82 | 298 | 1 | (North et al., 2010) |
| GO-TAc/MOF-60 | 5.62 | 298 | 1 | (North et al., 2010) |
| Meso-Tetraphenyl Porphinato–Cu(II) | 1.74 | 298 | 40 | (North et al., 2010) |
| PPIA-MOF-5(40 %) | 3.5 | 298 | 1 | (North et al., 2010) |
| Ni(II)-MOF | 2.69 | 298 | 27 | (Amaral et al., 2013) |
| PAN/HK@HK3-A NFM | 3.9 | 273 | 1 | (Amaral et al., 2013) |
| Bz@InOF-1 | 2 | 298 | 1 | (Amaral et al., 2013) |
| MIL-96(Al)–Ca1 | 10.22 | 273 | 9.3 | (Amaral et al., 2013) |
| MIL-96(Al)–Ca2 | 9.38 | 273 | 9.3 | (Amaral et al., 2013) |
| 50PEI@meso-UiO66− 0.2Cu | 1.39 | 298 | 1 | (Amaral et al., 2013) |
| Zn(Bmic)(AT) | 3.53 | 353 | 5 | (Amaral et al., 2013) |
| Zn(BPZ) | 5.1 | 298 | 1 | (North et al., 2010) |
| PEI(50)@NU-1000 | 1.75 | 298 | 1 | (North et al., 2010) |
| Ca3L2(H2O)2(DMA)2 | 4.32 | 298 | 1 | (Sanna et al., 2014) |
| PCN-250(Fe2Co) | 2.23 | 298 | 1 | (Sanna et al., 2014) |
| ACN1/3@Cu-BTC | 4.32 | 298 | 1 | (da Costa et al., 2022) |
| mmen-Mg2(dobpdc) | 3.33 | 298 | 1 | (da Costa et al., 2022) |
| sod-ZMOF-chitosan | 22.23 | 298 | 1 | (da Costa et al., 2022) |
| {[(CH3)2NH2][Zn2(L) (H2O)PO4]⋅2DMF}n | 4.99 | 298 | 1 | (da Costa et al., 2022) |
| MOF-505@5GO | 3.94 | 298 | 1 | (da Costa et al., 2022) |
| UTSA-16 | 4.5 | 333 | 1 | (Naranjo et al., 2023) |
| Imi1/3@Cu-BTC | 4.4 | 298 | 1 | (Naranjo et al., 2023) |
| NbOFFIVE-1-Ni | 1.3 | 298 | 1 | (Ang et al., 2015) |
| Tb-L | 1.84 | 298 | 1 | (Aresta et al., 2014) |
| Cu-BTC-PEI-2.5 | 4.15 | 298 | 1 | (Aresta et al., 2014) |
| [Ni-4PyC, Ni9(mH2O)4(H2O)2(C6NH4O2) 18.solvent] | 8.2 | 298 | 10 | (Aresta et al., 2014) |
| LDH@ZIF-67 | 0.52 | 303 | 1 | (Sanna et al., 2014) |
| ZIF-8− 90 100 % | 5.22 | 273 | 1 | (Sanna et al., 2014) |
| MOF-505 | 5.51 | 273 | 1 | (Aresta et al., 2014) |
| HNUST-7 | 26.1 | 273 | 1 | (Sanna et al., 2014) |
| opt-UiO-66(Zr)-(OH)2 | 5.63 | 298 | 1 | (Calò et al., 2002) |
| [Zn2(NH2BDC)2(dpNDI)]n | 1.26 | 298 | 1 | (Calò et al., 2002) |
| [Zn5(btz)6(bdc)2(H2O)2]⋅7DMA | 2.16 | 298 | 1 | (Calò et al., 2002) |
| MIL-53 | 0.05 | 298 | 1 | (Calò et al., 2002) |
| MWCNT@MIL-53 | 0.3 | 298 | 1 | (Calò et al., 2002) |
| CNF@MIL-53 | 0.1 | 298 | 1 | (Calò et al., 2002) |
| MWCNT@MIL-101 | 0.003 | 298 | 1 | (Calò et al., 2002) |
| 1⋅MeCN | 0.82 | 298 | 1 | (Calò et al., 2002) |
| 1-mmen | 4.13 | 298 | 1 | (Decortes et al., 2010) |
| 1-en | 2.63 | 298 | 1 | (Sanna et al., 2014) |
| 1-ppz | 3.15 | 298 | 1 | (Sanna et al., 2014) |
| 1000-as | 3.31 | 298 | 1 | (Sanna et al., 2014) |
| 1000- clean | 3.22 | 298 | 1 | (Sanna et al., 2014) |
| MOF-888 | 1.07 | 298 | 800 torr | (Sanna et al., 2016) |
| MOF-889 | 2.46 | 298 | 800 torr | (Sanna et al., 2016) |
| MOF-890 | 2.59 | 298 | 800 torr | (Sanna et al., 2016) |
| MOF-891 | 2.59 | 298 | 800 torr | (Sanna et al., 2016) |
| 476-MOF | 1.68 | 293 | 1 | (Whiteoak et al., 2013) |
| 477-MOF | 1.92 | 293 | 1 | (Whiteoak et al., 2013) |
| ɤ-CD-MOF | 0.55 | 303 | 1 | (Whiteoak et al., 2013) |
| NPC-6 | 4.83 | 293 | 1 | (Whiteoak et al., 2013) |
| TMOF-1 | 1.45 | 298 | 1 | (Whiteoak et al., 2013) |
| [Cu2L(H2O)2]• 4H2O•2DMF | 6.65 | 273 | 1 | (Whiteoak et al., 2013) |
| Cr-MIL-101-SO3H | 2.28 | 313 | 150 mbar | (Miller et al., 2013) |
| MIL-91(Al) | - | 303 | 1 | (W. Wang et al., 2011) |
| Co2L2(AzoD)2⋅2DMF (1) | 0.56 | 298 | 1 | (W. Wang et al., 2011) |
| Al-soc-MOF-1 | - | 298 | 1 | (W. Wang et al., 2011) |
| PN@MOF-5 | 3.48 | - | 1 | (W. Wang et al., 2011) |
| Metal-Organic Framework Type | MOF Site of Reaction | Reaction Type | Reactants | References |
|---|---|---|---|---|
| Pd@[Zn4O(BDC)3] (MOF-5) | MOF as a classical support | Hydrogenation | Cyclooctene | (Chen et al., 2023) |
| Ru@[Zn4O(BDC)3] (MOF-5) | Oxidation | Benzyl alcohol +O2 | (Jeffry et al., 2021; Mehrpooya et al., 2017; Sinha et al., 2017) | |
| Cu@[Zn4O(BDC)3] (MOF-5) | Methanol synthesis | Syngas | (Ding et al., 2019; Sinha et al., 2017) | |
| Pd@[Zn4O(BDC)3] (MOF-5) | Hydrogenation | Styrene+H2 | (Ahmed Ali et al., 2020; Fei et al., 2011) | |
| Pd@[Zn4O(BDC)3] (MOF-5) | Hydrogenation | Ethyl cinnamate+H2 | (Ahmed Ali et al., 2020; Fei et al., 2011) | |
| Cr3(F,OH)(en)2O(BDC)3(ED-MIL-101) | Post-synthetic modification of the framework | Heck condensation | Iodobenzene+acrylic acid | (Shiraishi & Hirata, 2021) |
| Cr3(F,OH)(en)2O(BDC)3(ED-MIL-101) | Knoevenagel condensation | Benzaldehyde+ethyl cyanoacetate | (Shiraishi & Hirata, 2021) | |
| [Ni(L-aspartate)bpy0.5]HCl0.9 MeOH0.5 | Methanolysis of epoxides | Cis-2,3-epoxybutane | (Shiraishi & Hirata, 2021) | |
| Ti(OiPr)4[Cd3Cl6(L1)3] 4DMF 6MeOH 3H2O | Active side in organic ligand | Addition to carbonyls | ZnEt2 + aromatic aldehyde | (Ahmed et al., 2022) |
| [Zn2(BPDC)2(L2)] 10DMF 8H2O | Epoxidation | 2,1-Dimethyl-2H-chromene + (tert-buthylsulfonyl) iodosilybenzene | (Chen et al., 2023; Escobar-Hernandez et al., 2023) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





