1. Introduction
Transcatheter aortic valve replacement (TAVR) has emerged as a well-established therapeutic modality for the treatment of symptomatic severe aortic stenosis (AS) [
1]. In the initial PARTNER A trial, the most prevalent access to aortic valve was via femoral artery and was achieved by surgical cut-down in over two thirds of the patients [
2,
3]. Advancements in TAVR techniques and its expanded application have underscored the necessity for a highly effective, fully percutaneous access approach, based on the decreasing sheath size and the option of complete hemostasis with vascular closure devices (VCDs).
The difference of the two strategies, i.e. surgical cut-down of the femoral artery and percutaneous access, mainly lies up on the invasiveness of the first. The percutaneous approach is less invasive with lower procedural time, in contrast to less vascular complications and better control of them in the surgical approach [
4]. This study aims to compare the association of the surgical cut-down approach and fully percutaneous with in-hospital outcomes and the hospital length of stay.
2. Materials and Methods
This is a retrospective observational cohort study that includes data from medical records of all patients that underwent transfemoral TAVR in our structural heart disease expert center, from January 2019 to December 2023. The study has been approved by the Institutional Review Board of our center, in compliance with the Declaration of Helsinki.
2.1. Inclusion Criteria
All patients with severe symptomatic aortic stenosis that underwent transfemoral TAVR in the Hybrid Operating Room at Interbalkan Medical Center were included. The decision to carry out the transcatheter procedure was made by the local Heart Team, according to the 2017 or 2021 ECS / EACTS guidelines [
1,
5]. From January 2019 to December 2020, every transfemoral TAVR was performed via fully percutaneous approach, using the Perclose Proglide 6 F Suture-Mediated Closure System (Abbott Vascular, Santa Clara, CA, USA) or the MANTA (Essential Medical Inc., Malvern, PA, USA) VCD for complete hemostasis. After January 2021, the surgical cut-down of the femoral artery approach was adopted, operated by the same vascular surgeon and his team.
2.2. Percutaneous Technique
The percutaneous technique for transfemoral TAVR in our center involves a series of meticulously planned steps to ensure minimal invasiveness and optimal patient outcomes. Initially, the patient is positioned, and local anesthesia is administered to the femoral access site. Ultrasound guidance is employed to precisely locate the common femoral artery. A micropuncture needle is used to access the artery, followed by the insertion of a guidewire. Sequential dilation of the artery is performed to accommodate the introducer sheath, typically ranging from 14Fr to 18Fr, depending on the device specifications.
Vascular closure devices (VCDs) such as ProGlide or MANTA are pre-placed to ensure effective hemostasis post-procedure. The TAVR sheath is then introduced over a stiff guidewire, advanced to the aortic arch, and positioned for valve deployment. During the procedure, continuous hemodynamic monitoring and fluoroscopic guidance are utilized to ensure accurate placement of the valve. Upon successful valve deployment and verification of adequate function and positioning via angiography and echocardiography, the sheath is carefully removed.
Hemostasis is achieved by activating the pre-placed VCDs, ensuring complete closure of the femoral artery puncture site. To confirm the effectiveness of the closure, elective angiography is performed to ensure complete hemostasis and proper functioning of the VCDs. The access site is then monitored for any signs of bleeding or complications. Patients are typically observed in the intensive care unit (ICU) post-procedure for close monitoring and early detection of any adverse events.
2.3. Surgical Cut-Down Technique
Surgical cut-down technique in our series has some special characteristics regarding the type of surgical incision, the size, the use of special retractors, and most important the long term experience of a vascular team performing the cut-down technique for over 20 year period for femoral artery access for endovascular repair of abdominal aortic aneurysm using local anesthesia. Vascular surgeons perform a 3-5 cm transverse incision, just above femora-inguinal line at the anatomical site of common femoral artery bifurcation. A Gelpi retractor is used to open the surgical field and common femoral artery is prepared and controlled using proximal and distal vascular loops. Following direct arterial puncture a 6Fr (11cm) sheath is introduced and angiographic wire is then advanced to the aortic arch. Using a pig-tail catheter a stiff wire is exchanged and the final aortic valve introducing sheath is advanced into the abdominal aorta. After the sheath is sutured on patient, interventional cardiology team proceed with the TAVR procedure. Following completion of TAVR procedure, vascular surgery team then retrieve the introducing sheath, check proximal and distal arterial flow and proceed with arterial suture and closure of surgical trauma. Additional procedures regarding arterial access whether that involve iliac arteries’ stenosis or other arterial pathology need to be addressed, are performed during or before completion of the TAVR procedure. Those may involve angioplasty of the iliac arteries or even of the abdominal aortic bifurcation. Distal arterial flow is checked with doppler ultrasound before patient is transferred to intensive care unit (ICU).
2.4. In-Hospital Outcomes
In-hospital outcomes were reported according to the Valve Academic Research Consortium-2 (VARC-2) consensus [
6]. The main outcomes were in-hospital mortality, myocardial infarction (MI), stroke, bleeding events with needed transfusion blood units, acute kidney injury (AKI), vascular complications, atrial fibrillation (AF), pacemaker implantation, and paravalvular leak (PVL).
2.5. Statistical Analysis
Continuous variables following normal distribution were presented as mean and standard deviation (SD), while variables that were not distributed normally were presented as median and interquartile range (IQR). Normality of distribution was assessed by comparing mean and median values, graphical representation of the distribution of the variables and by using the Kolmogorov–Smirnov test. Qualitative variables were summarized using absolute and relative frequencies [n/N (%)]. Propensity score matching was performed to reduce the risk of bias in the approach selection and potential confounders. A propensity score was estimated by fitting a logistic-regression model adjusted for age, sex, New York Heart Association (NYHA) functional class (III versus IV), heart failure (HF), type of valve, sheath size, mean aortic gradient (mean PG), and left ventricular ejection fraction (LVEF %). Nearest-neighbor matching without replacement was utilized to establish one-to-one pair matching between the two groups. Covariate balances were assessed by comparing standardized mean differences (SMD) before and after matching. A SMD <0.1 was deemed indicative of a minimal imbalance between the two groups Statistical comparisons of continuous variables that exhibited normal distribution were performed using the student t-test, while the Wilcoxon rank-sum test was employed for variables that did not follow a normal distribution. Categorical variables were compared with the χ2 test or the Fisher exact test if cell counts were small (≤5). The Kruskal-Wallis test was used for comparison of continuous variables between more than two independent samples. For the observation of the hospital length of stay comparison between the two groups, the cohort was divided into one-year intervals, with 2019 to 2020 representing the percutaneous group and 2021 to 2023 the surgical group. All statistical analyses were performed on RStudio version 2023.03.0+386.
3. Results
3.1. Baseline Characteristics
In total 251 patients that underwent transfemoral TAVR were included (55% female) with median (IQR) age of 80 (11) years. The propensity-matching algorithm resulted in a match for 154 of the 251 patients. A comprehensive overview of the baseline characteristics of the patients according to the femoral access strategy is presented in
Table 1. Patients in the percutaneous group had higher Euroscore II and right ventricular systolic pressure (RVSP) with lower LVEF. No difference was reported in NYHA class and aortic valve area (AVA) or hemodynamics, i.e. mean and maximum aortic pressure gradient. In the matched cohort, no statistically significant difference in the baseline characteristics was reported between the two groups.
3.2. In-Hospital Outcomes
In the total cohort, 11 (4.5%) patients were deceased, 37 (15%) had a bleeding event, 3 (1.2%) stroke, 5 (2%) vascular complications, 15 (6%) AF, 17 (7%) were implanted a pacemaker and no MIs were reported.
Table 2 presents the in-hospital outcomes in the matched cohort. Surgical cut-down technique for the femoral access led to significantly less vascular complications, less bleeding cases with no major or life threatening incidents, and less blood units needed for transfusion. No death was reported in the surgical group in contrast to 7 (9.1%) in the percutaneous group (p: 0.014). No stroke, AF or need for pacemaker was observed in the surgical group, in contrast to 3 (3.9%), 15 (19%) and 15 (19%) in the percutaneous group, respectively (p: 0.02, p< 0.001, p< 0.001). No difference was reported in AKI (p> 0.9) or PVL incidence after TAVR (p: 0.1).
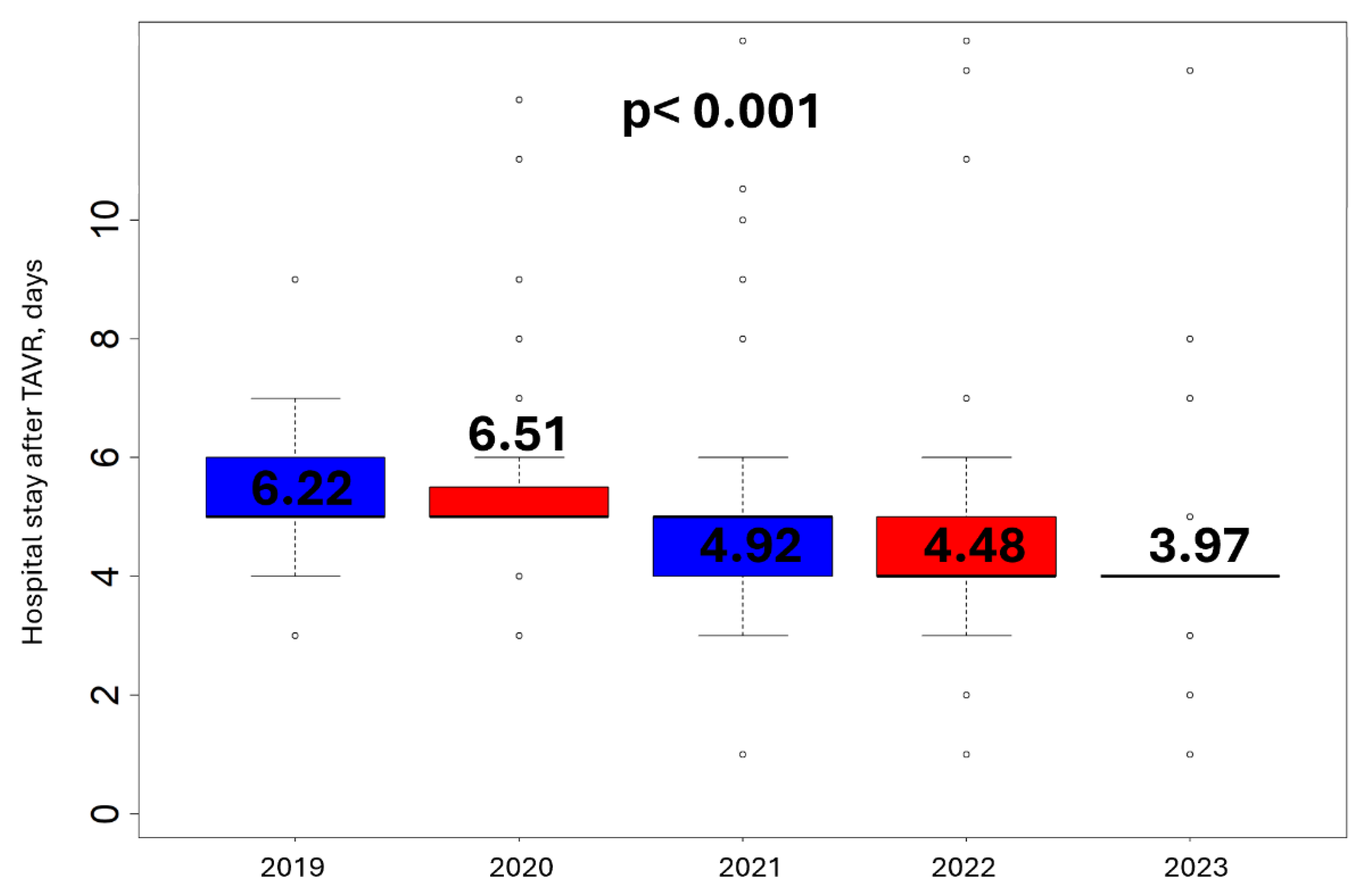
3.3. Hospital Length of Stay
Figure 1 presents the temporal variance of hospital length of stay of all transfemoral TAVR patients from 2019 to 2023. From 2021 and after, every femoral artery access was achieved by surgical cut-down. A reduction in mean days of hospitalization is reported in the total cohort over the years (p< 0.001), more prevalent after 2021, when the surgical approach was adopted by our center, with mean ±SD days of hospitalization 6.40 ±6.46 versus 4.34 ±1.61, in the percutaneous and surgical group respectively (p< 0.001).
4. Discussion
This retrospective study underlines the superiority of the surgical cut-down access to femoral artery in TAVR versus fully percutaneous approach in terms of in-hospital outcomes. Furthermore, it verifies the increasing procedure experience over the years, as it is reflected in the temporal reduction in hospital length of stay, in conjunction with the confirmation of the dominance of the surgical approach in this field, too.
The intricate interplay between vascular complications and bleeding risks significantly shapes the clinical landscape of TAVR, with major impact on outcomes [
7,
8]. So far, the two large published meta-analyses found similar rates of vascular complications and bleeding risks between the surgical and percutaneous group, with a possible superiority of the surgical group in minor vascular complications, while for the hospital stay duration the results were conflicting [
4,
9]. Our results suggest that surgical approach outweighs percutaneous in almost the whole spectrum of in-hospital outcomes.
We documented a total in-hospital mortality rate of only 4.5%, the lowest reported, with no in-hospital deaths occurring in the surgical group since our center adopted this method in 2021. Additionally, the incidence of vascular complications, bleeding events, and stroke was remarkably low, with no occurrences of myocardial infarctions. This improvement in TAVR procedures at our center is further reflected in the substantial temporal reduction in hospital length of stay, particularly post-2021. The large discrepancy of our results comparing to those of previous published studies, could be attributed to the surgical team’s extensive experience and the different surgical technique adopted for the femoral access, highlighting the necessity for every structural heart disease center to incorporate a skillful and experienced vascular surgeon team.
A key limitation of our study is the relatively small sample size. Furthermore, the single-center design may restrict the generalizability of our findings to a broader population. However, it's noteworthy that our center serves as one of the largest centers for structural heart disease patients in northern Greece, which mitigates potential selection bias. Moreover, while the retrospective nature of the study may introduce biases related to data collection and patient selection, the use of propensity score matching helped achieve a sufficient balance of baseline characteristics.
5. Conclusions
In summary, our study underscores the clear advantages of utilizing a surgical cut-down approach to femoral artery access in TAVR procedures over fully percutaneous methods. Furthermore, it reflects a notable progression in procedural expertise over time, evident in the reduction of hospital length of stay, which, also, confirms the superiority of the surgical approach. Looking ahead, our study reinforces the need for continued research and refinement in TAVR techniques to optimize patient outcomes and procedural efficiency.
Author Contributions
Conceptualization, G.P. and V.N.; methodology, G.P., E.L. and V.N.; software, G.P. and V.N.; validation, G.P. and V.N.; formal analysis, G.P. and V.N.; investigation, E.L., K.P., K.K., I.N., S.E., A.I. and V.N.; resources, G.P. and V.N.; data curation, G.P., E.L. and V.N.; writing—original draft preparation, G.P.; writing—review and editing, V.N.; visualization, G.P. and V.N.; supervision, V.N.; project administration, V.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Interbalkan Medical Center (Protocol Umber 2313/02.08.2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). European Heart Journal 2021, 43, 561–632. [Google Scholar]
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010, 363, 1597–607. [Google Scholar] [CrossRef] [PubMed]
- Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011, 364, 2187–98. [Google Scholar] [CrossRef] [PubMed]
- Ando T, Briasoulis A, Holmes AA, Takagi H, Slovut DP. Percutaneous versus surgical cut-down access in transfemoral transcatheter aortic valve replacement: A meta-analysis. J Card Surg. 2016, 31, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. European Heart Journal. 2017, 38, 2739–91. [Google Scholar] [CrossRef] [PubMed]
- Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012, 60, 1438–54. [Google Scholar] [CrossRef] [PubMed]
- Généreux P, Webb JG, Svensson LG, Kodali SK, Satler LF, Fearon WF, et al. Vascular Complications After Transcatheter Aortic Valve Replacement: Insights From the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial. Journal of the American College of Cardiology 2012, 60, 1043–52. [Google Scholar] [CrossRef] [PubMed]
- Kochman J, Rymuza B, Huczek Z, Kołtowski Ł, Ścisło P, Wilimski R, et al. Incidence, Predictors and Impact of Severe Periprocedural Bleeding According to VARC-2 Criteria on 1-Year Clinical Outcomes in Patients After Transcatheter Aortic Valve Implantation. International Heart Journal. 2016, 57, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz HK, Megaly M, Debski M, Rahbi H, Kamal D, Saad M, et al. Meta-Analysis Comparing Percutaneous to Surgical Access in Trans-Femoral Transcatheter Aortic Valve Implantation. Am J Cardiol. 2020, 125, 1239–48. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).