Submitted:
02 August 2024
Posted:
05 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Canola Genotypes and Sowing Density
2.3. Pre-Sowing Knockdown Herbicide and Sowing
2.4. Data Collection to Assess Weed Suppressive Ability of Canola Genotypes
2.5. Statistical Analysis
3. Results
3.1. Canola and Weed Biomass
3.2. Plant Functional Traits
3.3. Environmental Effect and Stability of Genotypes for Weed Suppression
4. Discussions
5. Conclusion
Author Contributions
Acknowledgments
Conflict of Interest
References
- Afuape, S.O.; Okocha, P.I.; Njoku, D. Multivariate assessment of the agro-morphological variability and yield components among sweet potato [Ipomoea batatas (L.) Lam] landraces. Afr. J. Plant Sci. 2011, 5, 123–132.
- Australian Oilseeds Federation (AOF). The State of Oilseed Industry in Australia: Annual Report 2023. Available online: https://www.graincentral.com/news/aof-forecasts-new-crop-canola-area-at-3-2mha/ (accessed on [Date]).
- Asaduzzaman, M.; Pratley, J.E.; An, M.; Luckett, D.J.; Lemerle, D. Metabolomics differentiation of canola genotypes: toward an understanding of canola allelochemicals. Front. Plant Sci. 2015, 5, 765–765. [CrossRef]
- Asaduzzaman, M.; Luckett, D.J.; Cowley, R.B.; An, M.; Pratley, J.E.; Lemerle, D. Canola cultivar performance in weed-infested field plots confirms allelopathy ranking from in vitro testing. Bio. Sci. Tech. 2014, 24, 1394–1411.
- Asaduzzaman, M.; An, M.; Pratley, J.E.; Luckett, D.J.; Lemerle, D. Canola (Brassica napus) germplasm shows variable allelopathic effects against annual ryegrass (Lolium rigidum). Plant Soil 2014, 380, 47–56. [CrossRef]
- Asseng, S.; Anderson, G.C.; Dunin, F.X.; Fillery, I.R.P.; Dolling, P.J.; Keating, B.A. Use of the APSIM wheat model to predict yield, drainage, and NO3- leaching for a deep sand. Aust. J. Agric. Res. 1998, 49, 363. [CrossRef]
- Beckie, H.J.; Harker, K.N.; Legere, A.; Morrison, M.J.; Swartz, G.S.; Falk, K.C. GM canola: the Canadian experience. Farm. Pol. 2011, 8, 43–49.
- Beckie, H.J.; Johnson, E.N.; Blackshaw, R.E.; Gan, Y. Weed Suppression by Canola and Mustard Cultivars. Weed Technol. 2008, 22, 182–185. [CrossRef]
- Belz, R.G.; Hurle, K. Differential Exudation of Two BenzoxazinoidsOne of the Determining Factors for Seedling Allelopathy of Triticeae Species. J. Agric. Food Chem. 2005, 53, 250–261. [CrossRef]
- Berkowitz, A.R. Competition for resources in weed-crop mixtures. In: Altieri, M.A.; Liebman, M., Eds.; Weed Management in Agroecosystems: Ecological Approaches; CRC Press Inc: Boca Raton, FL, USA, 1988; pp. 89–119.
- Bertholdsson, N. Use of multivariate statistics to separate allelopathic and competitive factors influencing weed suppression ability in winter wheat. Weed Res. 2011, 51, 273–283. [CrossRef]
- Bertholdsson, N. Breeding spring wheat for improved allelopathic potential. Weed Res. 2010, 50, 49–57. [CrossRef]
- Bertholdsson, N. Early vigour and allelopathy – two useful traits for enhanced barley and wheat competitiveness against weeds. Weed Res. 2005, 45, 94–102. [CrossRef]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [CrossRef]
- Bond, W.; Grundy, A.C. Non-chemical weed management in organic farming systems. Weed Res. 2001, 41, 383–405. [CrossRef]
- Chauvel, B.; Guillemin, J.-P.; Gasquez, J.; Gauvrit, C. History of chemical weeding from 1944 to 2011 in France: Changes and evolution of herbicide molecules. Crop. Prot. 2012, 42, 320–326. [CrossRef]
- Chen, Y.-T.; Wang, Y.; Yeh, K.-C. Role of root exudates in metal acquisition and tolerance. Curr. Opin. Plant Biol. 2017, 39, 66–72. [CrossRef]
- de Dorlodot, S.; Forster, B.; Pagès, L.; Price, A.; Tuberosa, R.; Draye, X. Root system architecture: opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 2007, 12, 474–481. [CrossRef]
- Dilday, R.H.; Yan, W.G.; Moldenhauer, K.A.K.; Gravois, K.A. Allelopathic activity in rice for controlling major aquatic weeds. In: Olofsdotter, M., Ed.; Allelopathy in Rice; International Rice Research Institute: Los Baños, Philippines, 1998; pp. 7–26.
- Dilday, R.H.; Gealy, D.R.; Mattice, J.D.; Moldenhauer, K.A. Allelopathy in rice as a weed control strategy. In: Abstracts of the 3rd International Weed Science Congress; International Weed Science Society: 2000; pp. 33–34.
- Dilday, R.H.; Mattice, J.D.; Moldenhauer, K.A.; Yan, W. Allelopathic Potential in Rice Germplasm Against Ducksalad, Redstem and Barnyard Grass. J. Crop. Prod. 2001, 4, 287–301. [CrossRef]
- Eberhart, S.A.; Russell, W.A. Stability Parameters for Comparing Varieties. Crop. Sci. 1966, 6, 36–40. [CrossRef]
- Gahoonia, T.S.; Nielsen, N.E. Root traits as tools for creating phosphorus efficient crop varieties. Plant Soil 2004, 260, 47–57. [CrossRef]
- Gealy, D.; Ottis, B.; Talbert, R.; Moldenhauer, K.; Yan, W. Evaluation and improvement of allelopathic rice germplasm at Stuttgart, Arkansas, USA. In: Proceedings of Fourth World Congress on Allelopathy; Charles Sturt University: Wagga Wagga, Australia, 2005; August 21–26. Published on CD-ROM.
- Harker, K.N.; Clayton, G.W.; Blackshaw, R.E.; O’donovan, J.T.; Stevenson, F.C. Seeding rate, herbicide timing and competitive hybrids contribute to integrated weed management in canola (Brassica napus). Can. J. Plant Sci. 2003, 83, 433–440. [CrossRef]
- Harker, K.N.; O’Donovan, J.T. Recent weed control, weed management, and integrated weed management. Weed Technol. 2013, 27, 1–110.
- Harker, K.N.; O'Donovan, J.T.; Turkington, T.K.; Blackshaw, R.E.; Lupwayi, N.Z.; Smith, E.G.; Klein-Gebbinck, H.; Dosdall, L.M.; Hall, L.M.; Willenborg, C.J.; et al. High-yield no-till canola production on the Canadian prairies. Can. J. Plant Sci. 2012, 92, 221–233. [CrossRef]
- Huang, Z.; Xu, W.; Yu, K. Bidirectional LSTM-CRF models for sequence tagging. arXiv preprint arXiv:1508.01991.
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop. Prot. 2015, 72, 57–65. [CrossRef]
- Lemerle, D.; Blackshaw, R.E.; Smith, A.B.; Potter, T.D.; Marcroft, S.J. Comparative survey of weeds surviving in triazine-tolerant and conventional canola crops in south-eastern Australia. Plant Protect. Quart. 2001, 16(1), 37–40.
- Lemerle, D.; Luckett, D.J.; Lockley, P.; Koetz, E.; Wu, H. Competitive ability of Australian canola (Brassica napus L.) genotypes for weed management. Crop Pasture Sci. 2014, 65, 1300–1310.
- Lemerle, D.; Luckett, D.J.; Wu, H.; Widderick, M.J. Agronomic interventions for weed management in canola (Brassica napus L.) – A review. Crop. Prot. 2017, 95, 69–73. [CrossRef]
- Lemerle, D.; Verbeek, B.; Coombes, N.; Loss, S.; van Burgel, A. Impact of weeds on crop production in Australia. Weed Technol. 2011, 25(2), 195–202.
- Li, Y.; Allen, V.G.; Chen, J.; Hou, F.; Brown, C.P.; Green, P. Allelopathic Influence of a Wheat or Rye Cover Crop on Growth and Yield of No-Till Cotton. Agron. J. 2013, 105, 1581–1587. [CrossRef]
- Mohler, C.L. Enhancing the competitive ability of crops. In Liebman, M., Ed.; Ecological Management of Agricultural Weeds; Cambridge University Press: Cambridge, UK, 2001, pp. 302–305.
- Olivoto, T.; Lúcio, A.D. metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [CrossRef]
- Olofsdotter, M.; Jensen, L.B.; Courtois, B. Improving crop competitive ability using allelopathy — an example from rice. Plant Breed. 2002, 121, 1–9. [CrossRef]
- Reckling, M. Methods of yield stability analysis in long-term field experiments. A review. Agron. Sustain. Dev. 2021, 41, 27.
- Rice, E.L. Allelopathy; Academic Press: Orlando, FL, 1984.
- Rivoal, A.; Fernandez, C.; Greff, S.; Montes, N.; Vila, B. Does competition stress decrease allelopathic potential? Biochem. Syst. Ecol. 2011, 39, 401–407.
- Rogers, E.D.; Benfey, P.N. Regulation of plant root system architecture: implications for crop advancement. Curr. Opin. Biotechnol. 2015, 32, 93–98. [CrossRef]
- Rueda-Ayala, V.P.; Rasmussen, J.; Gerhards, R.; E Fournaise, N. The influence of post-emergence weed harrowing on selectivity, crop recovery and crop yield in different growth stages of winter wheat. Weed Res. 2011, 51, 478–488. [CrossRef]
- Sabri, R.S.; Rafii, M.Y.; Ismail, M.R.; Yusuff, O.; Chukwu, S.C.; Hasan, N. Assessment of Agro-Morphologic Performance, Genetic Parameters and Clustering Pattern of Newly Developed Blast Resistant Rice Lines Tested in Four Environments. Agronomy 2020, 10, 1098. [CrossRef]
- Sarif, H.M.; Rafii, M.Y.; Ramli, A.; Oladosu, Y.; Musa, H.M.; Rahim, H.A.; Zuki, Z.M.; Chukwu, S.C. Genetic diversity and variability among pigmented rice germplasm using molecular marker and morphological traits. Biotechnol. Biotechnol. Equip. 2020, 34, 747–762. [CrossRef]
- A Salisbury, P.; Potter, T.D.; Gurung, A.M.; Mailer, R.J.; Williams, W.M. Potential impact of weedy Brassicaceae species on oil and meal quality of oilseed rape (canola) in Australia. Weed Res. 2018, 58, 200–209. [CrossRef]
- Seal, A.N.; Pratley, J.E.; Haig, T.; Lewin, L.G. Screening rice varieties for allelopathic potential against arrowhead (Sagittaria montevidensis), an aquatic weed infesting Australian Riverina rice crops. Aust. J. Agric. Res. 2004, 55, 673–680. [CrossRef]
- Smith, J.; Jones, A.; Doe, B. A gravimetric method for measuring stem-specific density in forestry research. J. Frost Sci. 2018, 15(3), 123–135.
- Smith, A.N.; Reberg-Horton, S.C.; Place, G.T.; Meijer, A.D.; Arellano, C.; Mueller, J.P. Rolled Rye Mulch for Weed Suppression in Organic No-Tillage Soybeans. Weed Sci. 2011, 59, 224–231. [CrossRef]
- Starling, A.P.; Umbach, D.M.; Kamel, F.; Long, S.; Sandler, D.P.; A Hoppin, J. Pesticide use and incident diabetes among wives of farmers in the Agricultural Health Study. Occup. Environ. Med. 2014, 71, 629–635. [CrossRef]
- Shukla, G.K. Some statistical aspects of partitioning genotype-environmental components of variability. Heredity 1972, 29, 237–245. [CrossRef]
- Vencill, W.K.; Nichols, R.L.; Webster, T.M.; Soteres, J.K.; Mallory-Smith, C.; Burgos, N.R.; Johnson, W.G.; McClelland, M.R. Herbicide Resistance: Toward an Understanding of Resistance Development and the Impact of Herbicide-Resistant Crops. Weed Sci. 2012, 60, 2–30. [CrossRef]
- Worthington, M.; Reberg-Horton, C. Breeding Cereal Crops for Enhanced Weed Suppression: Optimizing Allelopathy and Competitive Ability. J. Chem. Ecol. 2013, 39, 213–231. [CrossRef]
- Wu, H.; Haig, T.; Pratley, J.; Lemerle, D.; An, M. Allelochemicals in Wheat (Triticum aestivum L.): Variation of Phenolic Acids in Shoot Tissues. J. Chem. Ecol. 2001, 27, 125–135. [CrossRef]
- Wu, H.; Pratley, J.; Lemerle, D.; Haig, T. Crop cultivars with allelopathic capability. Weed Res. 1999, 39, 171–180.
- York, L.M.; Nord, E.A.; Lynch, J.P. Integration of root phenes for soil resource acquisition. Front. Plant Sci. 2013, 4, 355. [CrossRef]
- Zimdahl, R.L. Weed-Crop Competition: A Review; Blackwell Publishing: Ames, 2004.
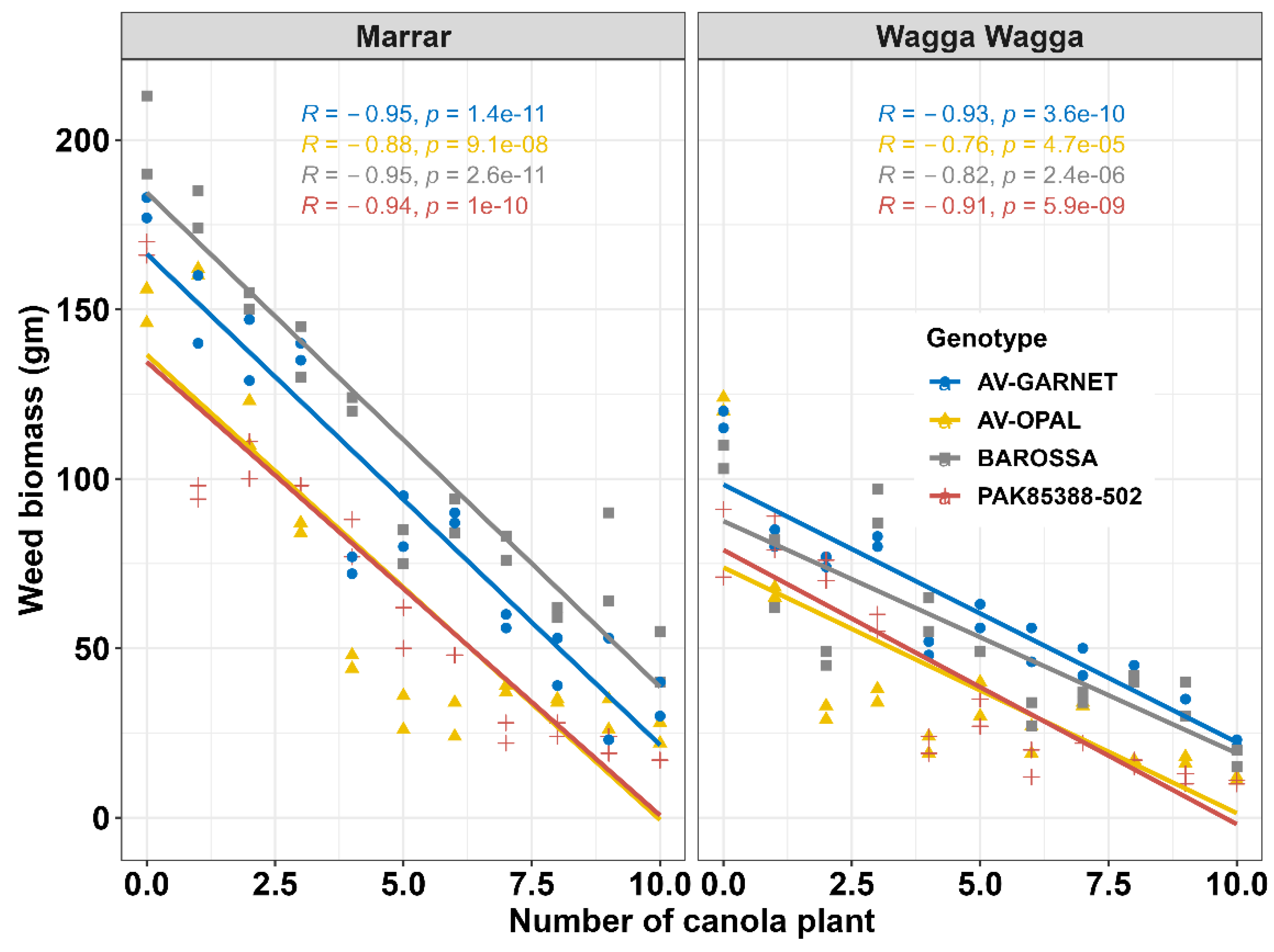
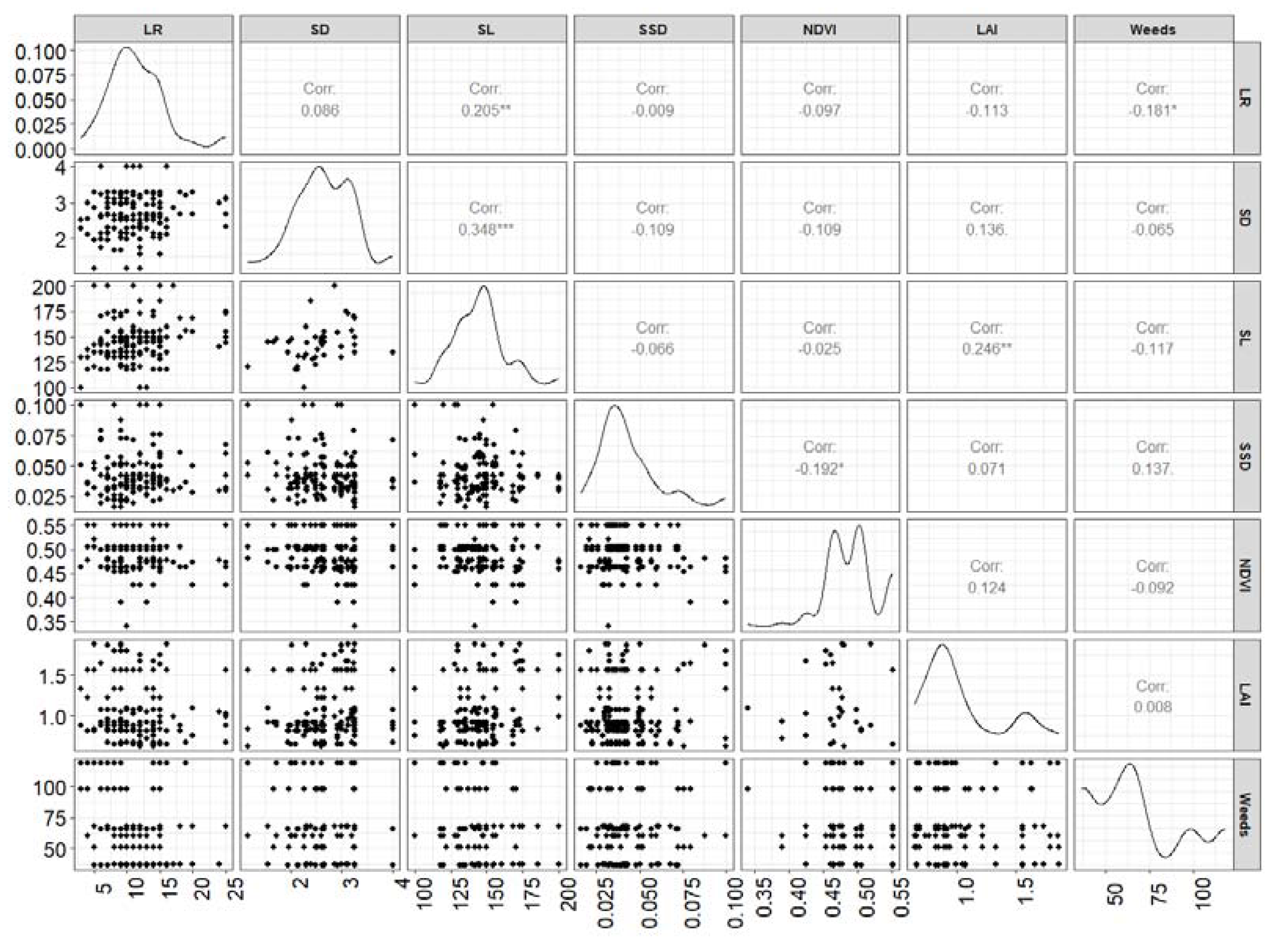
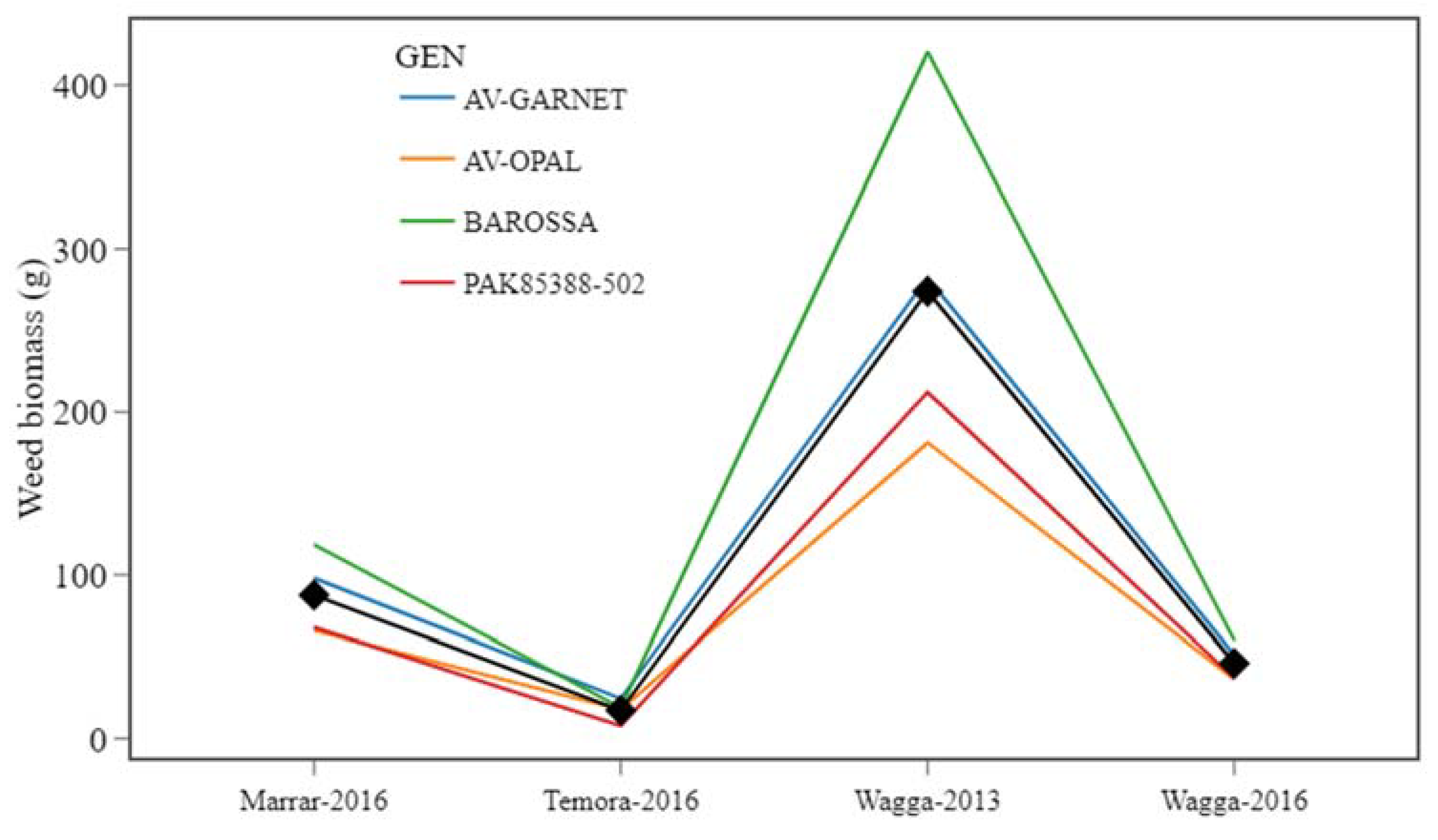
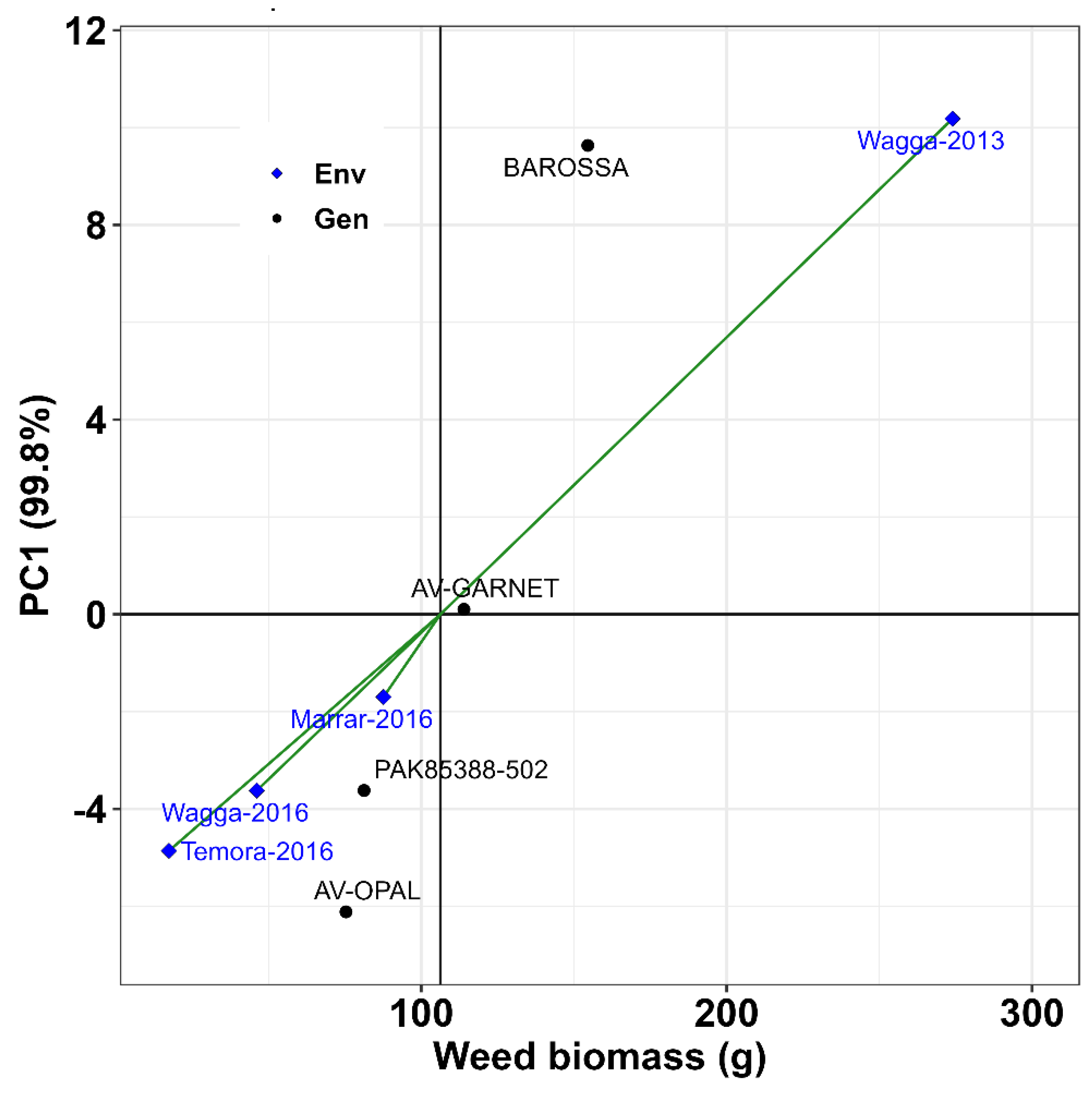
| Name | Units | Where measured | Site |
|---|---|---|---|
| Weed number | plants/m2 | 2 x quads (0.5 m x 0.5 m per plot | Wagga Wagga 2016, and 2013, Temora 2016 |
| Weed biomass (drymatter) | g/m2 | 2 x quads (0.5 m x 0.5 m per plot | Wagga Wagga 2016, and 2013, Temora 2016 |
| NDVI (greenness index) | unit less | Whole plot length | Wagga Wagga 2016, Marrar 2016 |
| Ceptometer (LAI and PAR) | unit less | Whole plot length | Wagga Wagga 2016, and Marrar 2016 |
| Canola root length, lateral roots and relative root growth | cm, no/plant | 20 plants/genotype | Wagga Wagga 2016, and Marrar 2016 |
| Canola shoot length, diameter and stem-specific density | cm, g cm–3 | 20 plants/genotype | Wagga Wagga 2016 and Marrar 2016 |
| Genotype | LR | RRG | SL (cm) | SSD | NDVI | LAI | PAR |
|---|---|---|---|---|---|---|---|
| AV-GARNET | 9.80 (±0.63) | 2.50 (±0.40) M 3.0 (±0.34) W |
142.60 (±3.47) | 0.04 (±0.002) | 0.46 M 0.49 W |
1.10 | 0.54 |
| AV-OPAL | 12.60 (±0.60) | 1.10 (±0.19) M 1.80 (±0.24) W |
137.60 (±4.00) | 0.04 (±0.002) | 0.49 M 0.46 W |
1.00 | 0.53 |
| BAROSSA | 10.55 (±0.63) | 3.18 (±0.49) M 2.00 (±0.21) W |
136.73 (±6.50) | 0.05 (±0.003) | 0.45 M 0.49 W |
1.20 | 0.50 |
| PAK85388-502 | 14.35 (±0.58) | 2.37 (±0.25) M 1.92 (±0.25) W |
164.00 (±4.80) | 0.03(±0.002) | 0.47 M 0.51 W |
1.10 | 0.48 |
| Genotype | Analysis for all environments | Analysis for unfavourable environments | Analysis for favourable environments | Shukla stability | Regression parameters | ||||||
| Weed biomass (g) | Rank | Weed biomass (g) | Rank | Weed biomass (g) | Rank | Rank | b0 | b1 | R2 | RMSE | |
| AV-GARNET | 114 | 3 | 282 | 3 | 57.8 | 3 | 3 | 114 | 1.01 | 1.00 | 1.87 |
| AV-OPAL | 75.3 | 1 | 181 | 1 | 40.1 | 2 | 2 | 75.3 | 0.63 | 0.99 | 1.45 |
| BAROSSA | 155.0 | 4 | 420 | 4 | 65.9 | 4 | 4 | 155 | 1.58 | 0.99 | 4.15 |
| PAK85388-502 | 81.3 | 2 | 212 | 2 | 37.7 | 1 | 1 | 81.3 | 0.78 | 0.99 | 2.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





