1. Introduction
Aortic stenosis (AS) is a common valvular heart disease that disproportionately affects older individuals, with severe cases often necessitating surgical intervention to mitigate symptoms and prolong life[
1]. For patients with symptomatic severe AS, surgical aortic valve replacement (SAVR) has long been considered the gold standard treatment, offering substantial improvements in quality of life and long-term survival[
2,
3,
4,
5,
6]. However, the overall success of SAVR can be hindered by patient-prosthesis mismatch (PPM), a phenomenon that occurs when the implanted prosthetic valve is too small in relation to the patient's body size and hemodynamic requirements[
7,
8,
9].
PPM can lead to a range of adverse consequences, including elevated transvalvular gradients, reduced left ventricular mass regression, and increased cardiac workload[
10,
11]. These hemodynamic abnormalities have been linked to poorer clinical outcomes, such as higher rates of mortality, rehospitalization, and heart failure symptoms[
12,
13]. While the impact of PPM on hemodynamics and clinical endpoints has been extensively studied, its potential influence on hemostatic parameters, particularly the von Willebrand factor, remains largely unexplored.
VWF is a multimeric glycoprotein that plays a pivotal role in primary hemostasis by mediating platelet adhesion and aggregation at sites of vascular injury[
14,
15,
16,
17]. Abnormalities in VWF levels and function have been implicated in various cardiovascular disorders, including AS, where they may contribute to the development of bleeding or thrombotic complications[
15,
16,
17,
18,
19,
20]. Given the complex interplay between hemodynamic factors and hemostatic pathways, it is plausible that PPM could modulate VWF dynamics in patients undergoing SAVR, potentially impacting their perioperative and long-term outcomes.
Despite the potential significance of this relationship, there is a paucity of data on the effects of PPM on postoperative VWF levels in SAVR patients. A better understanding of how PPM influences VWF dynamics could provide valuable insights into the hemostatic consequences of PPM and guide the development of tailored strategies for optimizing valve selection, perioperative management, and long-term follow-up in this high-risk population.
To address this knowledge gap, the present prospective study aims to investigate the association between PPM and postprocedural VWF levels in patients undergoing SAVR for severe AS. By elucidating the interplay between PPM and VWF, this study seeks to contribute to the growing body of evidence on the multifaceted impact of PPM and inform the development of personalized approaches for improving outcomes and minimizing complications in patients undergoing SAVR.
2. Materials and Methods
Patient Selection and Study Protocol
This prospective investigation recruited 31 patients consecutively diagnosed with severe aortic stenosis who subsequently underwent surgical aortic valve replacement. The study's inclusion criteria mandated the presence of severe AS, confirmed by echocardiographic evaluation, and the patient's suitability for SAVR.
Data Acquisition and Evaluation
A set of preoperative data was gathered for each patient, encompassing demographic information (age and gender), comorbid conditions (hypertension, diabetes, coronary artery disease, valvular pathologies, and aortic disorders), echocardiographic measurements (aortic valve area and mean transvalvular pressure gradient), and relevant laboratory parameters (hemoglobin levels, platelet counts, and coagulation profiles). Records were maintained regarding intraoperative details, particularly the type (biological or mechanical) and dimensions of the implanted prosthetic valve.
Echocardiographic Evaluation and Aortic Stenosis Grading
The severity of aortic stenosis was assessed using transthoracic echocardiography. Peak velocity, mean pressure gradient across the aortic valve, and aortic valve area were quantified upon admission. Established guidelines were employed to categorize the severity of aortic stenosis as mild, moderate, or severe[
10,
11].
Prosthetic Valve Characteristics and Patient-Prosthesis Mismatch Assessment
The effective orifice area (EOA) values corresponding to the implanted prosthetic aortic valves were sourced from manufacturer-provided charts and existing literature (
Table 1)[
12,
13,
15]. Each patient's body surface area (BSA) was calculated using the Dubois formula. The BSA was then used to determine the indexed EOA (EOAi) of the prosthetic valve and the indexed aortic valve area prior to the surgical intervention (SOAi).
The presence and extent of patient-prosthesis mismatch were evaluated based on the criteria put forth by Pibarot and Rahimtoola[
2,
15,
21]. The EOAi of the prosthetic valve was used to classify the severity of PPM into mild (EOAi > 0.85 cm²/m²), moderate (EOAi between 0.65 and 0.85 cm²/m²), and severe (EOAi < 0.65 cm²/m²) categories.
Blood Sample Collection and von Willebrand Factor Analysis
Blood samples were obtained within a 24-hour window prior to surgery and on the seventh postoperative day. Enzyme-linked immunosorbent assay (ELISA) techniques were employed to quantify serum levels of Factor VIII (Manufacturer: Antibodies-online, Jones Boulevard 321, Limerick PA USA), von Willebrand factor antigen (VWF:Ag - Manufacturer: Antibodies-online, Jones Boulevard 321, Limerick PA USA), and ristocetin-induced von Willebrand factor activity (VWF:RCo - Manufacturer: VWF:RCo– HemosIL Reagents 180 Hartwell Road Bedford, MA USA).
Statistical Methodology
The normality of the data was evaluated using the Shapiro-Wilk test. Continuous variables were reported as mean ± standard deviation and interquartile range, while categorical variables were expressed as frequencies and percentages. Spearman's rank test (Spearman's Rho) was used to assess correlations between variables. Univariate analyses were conducted using Student's t-test or Mann-Whitney U-test for continuous variables and chi-squared test for categorical data. Multivariable analyses, including linear regression and analysis of variance (ANOVA), were performed to identify independent variables influencing continuous outcomes. Statistical significance was set at a p-value < 0.05. All statistical computations were carried out using StataBE version 17.0 (StataCorp LLC, College Station, TX, USA).
Ethical Considerations
The study protocol was designed in accordance with the principles outlined in the Declaration of Helsinki and received approval from the Institutional Ethics Committee of the Institute of Cardiovascular Diseases Timisoara (nr.33/09.12.2019). Written informed consent was obtained from all participants prior to their inclusion in the study, acknowledging their agreement to participate and the use of their data in future scientific publications.
3. Results
Patient Characteristics and Baseline Data
Among the 31 patients who underwent SAVR, the majority were female (54.84%), with a mean age of 66.8 ± 9.15 years (range: 46-79 years). All patients had severe aortic stenosis, characterized by a mean aortic orifice area of 0.81 ± 0.15 cm² (indexed aortic orifice area: 0.43 ± 0.08 cm²/m² BSA) and a mean transvalvular gradient of 52.24 ± 13.79 mmHg. The average aortic annulus diameter was 2.25 ± 0.20 cm (range: 1.9-2.7 cm), and the mean ejection fraction was 48.44 ± 8%.
No significant correlations were found between demographic factors (age, gender, ethnicity) or comorbidities (smoking, hypertension, diabetes mellitus, chronic pulmonary disease, extracardiac arteriopathy, renal function impairment) and perioperative changes in von Willebrand factor levels.
Table 2.
Echographic assesment.
Table 2.
Echographic assesment.
| Variable |
Mean |
Min |
Max |
| Ao. Anulus (cm) |
2.25 |
1.9 |
2.7 |
| Pmax (mmHg) |
80.35 |
16 |
134 |
| Pmed (mmHg) |
52.24 |
36 |
90 |
| Valve area (cm2) |
0.81 |
0.55 |
1.2 |
| Indexed valve area (cm2) |
0.43 |
0.28 |
0.58 |
| EF (%) |
48 |
25 |
55 |
| VTD (ml) |
109.87 |
70 |
215 |
Surgical Procedure and Prosthetic Valve Characteristics
The mean prosthesis size was 22.35 mm. The average cardiopulmonary bypass (CPB) time and aortic cross-clamp time were 103.13 minutes [IQR: 78-110.5] and 62.35 minutes [IQR: 45-78.5], respectively. The mean intensive care unit stay was 4 days.
Bioprosthetic valves were implanted in 51.61% (n=16), while mechanical valves were used in 48.39% (n=15). The Carbomedics Top Hat was the most frequently utilized valve model (51.61%, n=16), followed by the Edwards Lifesciences CE Perimount (32.26%, n=10). The mean effective orifice area (EOA) of the implanted prostheses was 1.52 cm² [IQR: 1.3-1.7], with a mean indexed EOA of 0.79 cm²/m² [IQR: 0.71-0.92].
Table 3.
Intraprocedural and postprocedural outcomes.
Table 3.
Intraprocedural and postprocedural outcomes.
| |
Mean |
Median |
Q1 |
Q3 |
| Prosthesis size |
22.35 |
23 |
21 |
23 |
| CBP time (min) |
103.13 |
94.5 |
78 |
110.5 |
| Cross-clamp time (min) |
62.35 |
57 |
45 |
78.5 |
| Drainage (ml) |
377.69 |
310 |
230 |
450 |
| Days ICU |
4 |
2 |
1 |
3 |
| Days postprocedural |
5.96 |
6 |
5 |
7 |
In our cohort postoperative bleeding was quantified within the first 24 hours following the procedure. The mean drainage was 377.69 ml [IQR: 230-450]. Notably, our analysis revealed a significant inverse relationship between preoperative von Willebrand factor antigen levels and postoperative drainage. Specifically, baseline vWF:Ag emerged as an independent negative predictor of bleeding volume.
Table 4.
Multivariable analysis of variables affecting bleeding in surgical patients.
Table 4.
Multivariable analysis of variables affecting bleeding in surgical patients.
| Variable |
Coefficient |
95% CI |
p |
| Preoperative VWF:Ag levels |
-1.12 |
-2.09 - -0.14 |
0.02 |
| Preoperative fVIII levels |
0.80 |
-0.38 - 1.98 |
0.17 |
Patient-Prosthesis Mismatch
PPM, defined as an indexed EOA < 0.85 cm²/m² BSA, was observed in 61.29% (n=19) of patients. The presence of PPM did not significantly influence postoperative VWF antigen levels (285.43 IU/dL [IQR: 135.65-382.9] vs. 293.30 IU/dL [IQR: 222.9-345.4], p=0.88), VWF activity (178.33% [IQR: 119.2-130.9] vs. 204.76% [IQR: 115.8-399.2], p=0.56), or Factor VIII levels (100.38 IU/dL [IQR: 75.3-111.5] vs. 97.10 IU/dL [IQR: 80.4-111.2], p=0.79).
Von Willebrand Factor
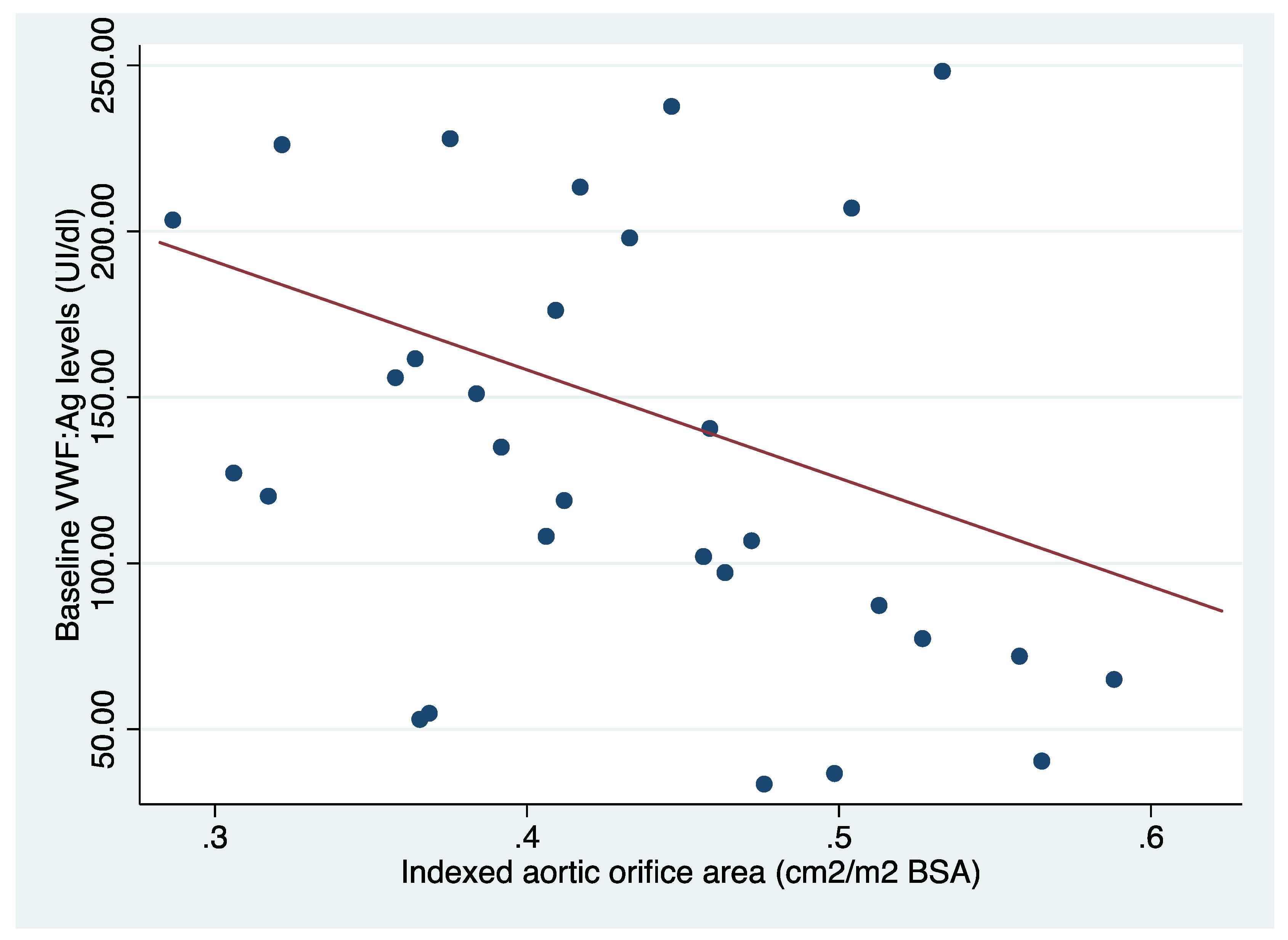
The mean preoperative VWF antigen (VWF:Ag) level was 131.37 ± 64.82 IU/dL [IQR: 77.3-198]. Preoperative VWF:Ag levels showed a significant inverse correlation with the indexed aortic valve surface area (rho=-0.36, p<0.04). Following SAVR, VWF:Ag levels increased significantly to 311.01 IU/dL [IQR: 172.2-387] (p<0.01), and no correlation was found with the indexed EOA of the implanted prosthesis (rho=-0.01, p=0.95).
VWF activity also increased significantly from 79.25% [IQR: 45.9-122] at baseline to 190.41% [IQR: 120-135.4] (p<0.01). However, no significant differences were observed in Factor VIII levels (95.3 IU/dL [IQR: 61.9-105.4] vs. 100.18 IU/dL [IQR: 79-111.2], p=0.21) or VWF:Ag/VWF:Activity ratio (0.66 [IQR: 0.43-0.78] vs. 0.75 [IQR: 0.38-0.79], p=0.33) following the procedure.
Figure 1.
Relationship between preoperative VWF antigen levels and indexed aortic valve area.
Figure 1.
Relationship between preoperative VWF antigen levels and indexed aortic valve area.
At baseline, patients with blood group O had significantly lower VWF activity levels compared to those with a non-O blood group (1.20 ± 0.57 vs. 2.48 ± 1.98, p=0.03). However, no significant differences were found in preoperative VWF:Ag levels (99.51 IU/dL [IQR: 56.2-137.4] vs. 142.45 IU/dL [IQR: 87.3-207], p=0.09) or Factor VIII levels (76.4 IU/dL [IQR: 55.5-91.3] vs. 101.87 IU/dL [IQR: 66.6-120.6], p=0.42) between the two groups. These differences in VWF activity disappeared one week after SAVR (2.38 [IQR: 1.04-3.37] vs. 2.08 [IQR: 1.27-2.60], p=0.48), and no significant differences were observed in postoperative VWF:Ag levels (393.25 IU/dL [IQR: 212.3-506] vs. 282.40 IU/dL [IQR: 167.8-318.4], p=0.13) or Factor VIII levels (94 IU/dL [IQR: 69.5-92.3] vs. 102.33 IU/dL [IQR: 83.8-113.2], p=0.56) between blood group O and non-O patients.
4. Discussion
Aortic stenosis is a well-known cause of acquired von Willebrand syndrome (AVWS), particularly type 2A, which is characterized by a deficiency in high-molecular-weight multimers (HMWM) of von Willebrand factor[
15,
16,
17,
22]. The elevated shear stress caused by the narrowed valve orifice in AS leads to structural changes in the VWF molecule, making it more susceptible to proteolysis[
15,
16,
17,
22]. As a result, patients with severe AS may experience a significant reduction in HMWM VWF levels, which can increase the risk of bleeding complications[
15,
16,
17,
23]. In fact, studies have shown that individuals with severe AS may have up to a 50% decrease in HMWM VWF levels compared to healthy individuals[
15,
18].
The severity of AVWS in AS patients has been found to correlate with the degree of valve stenosis and the pressure gradient across the aortic valve[
18,
24]. This highlights the crucial role of high shear stress in the degradation of HMWM VWF.
Interestingly, structural abnormalities in VWF have been linked to the pressure gradient across prosthetic valves following aortic valve replacement[
15]. This suggests that patients who develop PPM after SAVR may continue to experience elevated shear stress, even if the prosthetic valve is functioning properly. Consequently, PPM could potentially contribute to the persistence or recurrence of AVWS even after valve replacement surgery[
15].
The VWF Dynamics after SAVR
We observed a significant increase in VWF antigen levels following SAVR, indicating an improvement in hemostatic function. The mean preoperative VWF level was low at 131.37 ± 64.82 IU/dL [IQR: 77.3-198], reflecting the hemostatic impairment caused by AS. After surgery, VWF levels increased to 311.01 UI/dl ± 176.77 IU/dL [IQR: 172.2-387], suggesting a substantial recovery. This finding aligns with previous studies that have shown that the removal of the stenotic valve reduces high shear stress, thereby decreasing VWF degradation [
7,
8,
15,
18].
It is worth noting that prolonged cardiopulmonary bypass time, particularly in combined surgical procedures, can independently increase the risk of bleeding due to CPB-induced platelet dysfunction[
25]. Although this factor does not directly influence VWF levels, it highlights the complex nature of hemostasis management in patients undergoing SAVR.
Impact of Patient-Prosthesis Mismatch
Patient-prosthesis mismatch is a significant concern in aortic valve replacement procedures, as it can lead to suboptimal hemodynamics, increased shear stress on the prosthetic valve, and a higher risk of acquired von Willebrand factor deficiency[
26,
27,
28]. PPM occurs when the effective orifice area of the implanted valve is smaller than that of the native stenotic valve, a concept first introduced by Rahimtoola in 1978[
26,
27,
28]. The prevalence of moderate PPM is estimated to range between 20% and 70%, while severe PPM occurs in 2% to 10% of cases[
29].
When selecting the optimal prosthesis size, the effective orifice area is considered a more reliable measure than the geometric orifice area[
2]. Bioprosthetic valves typically have smaller diameters and EOAs compared to mechanical prostheses or stentless prostheses[
15,
26,
30].
Our study of 31 patients undergoing surgical aortic valve replacement revealed a significant incidence of patient-prosthesis mismatch, affecting more than half of the cohort. This high prevalence of PPM can be attributed to a complex interplay of patient characteristics and surgical considerations.
The group presented with distinctive anthropometric features that contributed to the challenge of achieving optimal prosthesis sizing. With a mean height of 1.67 ± 0.08 m, weight of 82.25 ± 20.9 kg, and resultant body surface area (BSA) of 1.90 ± 0.24 m², these patients had relatively large body sizes. Crucially, their mean aortic annulus diameter was 2.25 ± 0.20 cm, which is comparatively small in relation to their BSA (
Table 5). This disparity between a smaller native aortic annulus and larger body dimensions significantly complicated the prosthesis selection process.
To address the varied needs of our SAVR patients, we employed a range of prosthetic valves. Bioprosthetic options included the Hancock II (Medtronic, Minneapolis, MN, USA) and the Edwards Perimount (Edwards Lifesciences, Irvine, CA, USA). The Hancock II, a porcine valve, was used in 23 mm and 25 mm sizes, offering effective orifice areas of 1.3 cm² and 1.5 cm², respectively. The Edwards Perimount, a bovine pericardial valve, was implanted in sizes ranging from 19 mm to 25 mm, with EOAs spanning from 1.1 cm² to 1.8 cm². For patients receiving mechanical valves, we utilized the Carbomedics Standard and Top Hat models (LivaNova, London, UK) in sizes from 21 mm to 25 mm, providing EOAs between 1.5 cm² and 2.3 cm².
The selection of appropriate valve size involved a delicate balance between minimizing PPM and avoiding more extensive surgical procedures like aortic root enlargement, which could potentially increase perioperative risks. Our approach prioritized overall patient safety and clinical benefit, sometimes accepting a degree of PPM when alternatives posed greater risks.
Interestingly, our analysis revealed that PPM did not significantly impact postoperative von Willebrand factor levels, vWF activity, or factor VIII levels in the short term. This finding contradicts some previous studies[
7,
9,
15] that suggested a relationship between PPM and VWF dynamics. The discrepancy might be attributed to the immediate hemostatic benefits of aortic valve replacement overshadowing any potential short-term effects of PPM on these parameters.
To further explore the implications of PPM in our cohort, we conducted additional analyses. We stratified patients based on the severity of PPM and examined correlations between the degree of mismatch and various clinical outcomes. These included postoperative bleeding, transfusion requirements, length of hospital stay, and early postoperative complications.
Our findings underscore the complexity of managing PPM. While the short-term VWF levels appeared unaffected, the long-term implications of PPM on hemostatic function, valve durability, and overall clinical outcomes remain uncertain. This highlights the need for extended follow-up studies to elucidate the full impact of PPM over time.
In our study, we investigated the potential relationship between PPM and VWF levels. Interestingly, we found that the presence of PPM did not significantly alter the pattern of VWF level changes observed in patients with different blood groups. Previous studies have shown that individuals with blood group O typically have lower baseline VWF levels compared to other blood groups[
31,
32,
33]. In our cohort, this blood group-related difference in VWF levels was evident preoperatively. However, following surgery, these differences became less pronounced, regardless of the presence or absence of PPM.
This observation suggests that the hemostatic recovery process following SAVR may be robust enough to overcome both blood group-related variations in VWF levels and any potential influences of PPM. It's important to note that this finding contradicts some earlier hypotheses that PPM might interfere with the normalization of hemostatic parameters post-surgery.
Our analysis revealed that baseline VWF levels, rather than the presence of PPM, served as an independent negative predictor of postoperative bleeding. Specifically, patients with higher preoperative vWF levels tended to experience less postoperative bleeding, irrespective of whether they developed PPM.
5. Conclusions
In conclusion, our study provides valuable insights into the impact of patient-prosthesis mismatch on von Willebrand factor dynamics in patients undergoing surgical aortic valve replacement for severe aortic stenosis. While we did not observe a significant association between PPM and postoperative VWF levels in the short term, it is crucial to acknowledge that the persistent high shear stress caused by PPM could potentially lead to the re-emergence of acquired von Willebrand syndrome over time. Future research with longer follow-up periods is warranted to elucidate the long-term implications of PPM on hemostatic function and clinical outcomes. Furthermore, our findings highlight the importance of careful preoperative planning and prosthesis selection to minimize the risk of PPM, particularly in patients with smaller aortic roots and larger body surface areas.
6. Limitations
Our investigation into patient-prosthesis mismatch among the 31 surgical aortic valve replacement patients faced several constraints that warrant consideration when interpreting the results.
A primary limitation was the short-term nature of our follow-up. This restricted timeframe allowed us to capture only the immediate post-operative effects of PPM, potentially missing longer-term hemostatic and clinical implications. The acute post-surgical period may not fully reflect the ongoing impact of PPM on valve function, hemodynamics, and patient outcomes. Extended observation periods in future studies could reveal whether PPM leads to progressive changes in von Willebrand factor levels or clinical endpoints that were not apparent in our short-term analysis.
The modest sample size of 31 patients, while providing valuable insights, limits the statistical power and generalizability of our findings. This constraint may have obscured subtle associations between PPM and VWF levels or clinical outcomes. Larger cohorts would enable more robust subgroup analyses, potentially uncovering PPM effects that vary based on factors such as prosthesis type, size, or patient characteristics.
Author Contributions
Conceptualization, A.E.G. and H.F..; methodology, A.E.G., H.F., A.A.,; software, H.F., A.E.G..; validation, A.E.G., H.F., A.A.; formal analysis, A.A.,H.F.; investigation, A.E.G., H.F..; resources, A.A., H.F.; data curation, A.E.G.; writing—original draft preparation, A.E.G., H.F.; writing—review and editing, A.E.G, H.F., A.A.,; visualization, A.E.G., H.F.; supervision, H.F., A.A; project administration, A.E.G.; funding acquisition, U.M.F.V.B.T.. All authors have read and agreed to the published version of the manuscript.
Funding
Part of the necessary reagent for this study were funded by the University of Medicine and Pharmacy “Victor Babes” Timisoara. The APC was funded by the University of Medicine and Pharmacy “Victor Babes” Timisoara.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Institute of Cardiovascular Diseases and University of Medicine “Victor Babes” Timisoara (no.33/09.12.2019).
Informed Consent Statement
Informed consent was obtained from all participants prior to their inclusion in the study, with approval for their participation and agreement to any future scientific publications.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baumgartner, H.; Walther, T. Aortic Stenosis. In The ESC Textbook of Cardiovascular Medicine; Baumgartner, H., Camm, A.J., Lüscher, T.F., Maurer, G., Serruys, P.W., Eds.; Oxford University Press, 2018; p. 0 ISBN 978-0-19-878490-6.
- Muneretto, C.; Bisleri, G.; Negri, A.; Manfredi, J. The Concept of Patient-Prosthesis Mismatch. J Heart Valve Dis 2004, 13, 5. [Google Scholar]
- Chan, J.; Dimagli, A.; Fudulu, D.P.; Sinha, S.; Narayan, P.; Dong, T.; Angelini, G.D. Trend and Early Outcomes in Isolated Surgical Aortic Valve Replacement in the United Kingdom. Front. Cardiovasc. Med. 2023, 9, 1077279. [Google Scholar] [CrossRef]
- De La Morena-Barrio, M.E.; Corral, J.; López-García, C.; Jiménez-Díaz, V.A.; Miñano, A.; Juan-Salvadores, P.; Esteve-Pastor, M.A.; Baz-Alonso, J.A.; Rubio, A.M.; Sarabia-Tirado, F.; et al. Contact Pathway in Surgical and Transcatheter Aortic Valve Replacement. Front. Cardiovasc. Med. 2022, 9, 887664. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, J.; Chen, L.; Li, R.; Ma, L.; Ni, Y.; Zhao, H. Impact of Prosthesis–Patient Mismatch on Short-Term Outcomes after Aortic Valve Replacement: A Retrospective Analysis in East China. J Cardiothorac Surg 2017, 12, 42. [Google Scholar] [CrossRef]
- Živković, I.; Vuković, P.; Krasić, S.; Milutinović, A.; Milić, D.; Milojević, P. MINIMALLY INVASIVE AORTIC VALVE REPLACEMENT VS AORTIC VALVE REPLACEMENT THROUGH MEDIAL STERNOTOMY: PROSPECTIVE RANDOMIZED STUDY. AMM 2019, 58, 97–102. [Google Scholar] [CrossRef]
- Yoshida, K.; Tobe, S.; Kawata, M.; Yamaguchi, M. Acquired and Reversible von Willebrand Disease With High Shear Stress Aortic Valve Stenosis. The Annals of Thoracic Surgery 2006, 81, 490–494. [Google Scholar] [CrossRef]
- Vincentelli, A.; Susen, S.; Le Tourneau, T.; Six, I.; Fabre, O.; Juthier, F.; Bauters, A.; Decoene, C.; Goudemand, J.; Prat, A.; et al. Acquired von Willebrand Syndrome in Aortic Stenosis. N Engl J Med 2003, 349, 343–349. [Google Scholar] [CrossRef]
- Blackshear, J.L.; McRee, C.W.; Safford, R.E.; Pollak, P.M.; Stark, M.E.; Thomas, C.S.; Rivera, C.E.; Wysokinska, E.M.; Chen, D. Von Willebrand Factor Abnormalities and Heyde Syndrome in Dysfunctional Heart Valve Prostheses. JAMA Cardiology 2016, 1, 198. [Google Scholar] [CrossRef]
- Messika-Zeitoun, D. ; Lloyd’, ’Guy Aortic Valve Stenosis: Evaluation and Management of Patients with Discordant Gra. European Society of Cardiology 2018, 15, 8. [Google Scholar]
- Manzo, R.; Ilardi, F.; Nappa, D.; Mariani, A.; Angellotti, D.; Immobile Molaro, M.; Sgherzi, G.; Castiello, D.; Simonetti, F.; Santoro, C.; et al. Echocardiographic Evaluation of Aortic Stenosis: A Comprehensive Review. Diagnostics 2023, 13, 2527. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the Imaging Assessment of Prosthetic Heart Valves: A Report from the European Association of Cardiovascular Imaging Endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging †. European Heart Journal – Cardiovascular Imaging 2016, 17, 589–590. [Google Scholar] [CrossRef]
- Hahn, R.T.; Leipsic, J.; Douglas, P.S.; Jaber, W.A.; Weissman, N.J.; Pibarot, P.; Blanke, P.; Oh, J.K. Comprehensive Echocardiographic Assessment of Normal Transcatheter Valve Function. JACC: Cardiovascular Imaging 2019, 12, 25–34. [Google Scholar] [CrossRef]
- Peyvandi, F.; Garagiola, I.; Baronciani, L. Role of von Willebrand Factor in the Haemostasis. Blood Transfusion 2011, s3–s8. [Google Scholar] [CrossRef]
- Grigorescu, A.E.; Anghel, A.; Buriman, D.G.; Feier, H. Acquired Von Willebrand Factor Deficiency at Patient-Prosthesis Mismatch after AVR Procedure—A Narrative Review. Medicina 2023, 59, 954. [Google Scholar] [CrossRef]
- Vučić, M.; Veličković, S.; Lilić, B. BLEEDING ASSESSMENT TOOLS. AMM 2023, 62, 117–126. [Google Scholar] [CrossRef]
- Milić, D.; Lazarević, M.; Golubović, M.; Perić, V.; Kamenov, A.; Stojiljković, V.; Stošić, M.; Živić, S.; Milić, I.; Spasić, D. THE SIGNIFICANCE OF IMPEDANCE AGGREGOMETRY IN CARDIAC SURGERY. AMM 2024, 63, 05–13. [Google Scholar] [CrossRef]
- Frank, R.D.; Lanzmich, R.; Haager, P.K.; Budde, U. Severe Aortic Valve Stenosis: Sustained Cure of Acquired von Willebrand Syndrome After Surgical Valve Replacement. Clin Appl Thromb Hemost 2017, 23, 229–234. [Google Scholar] [CrossRef]
- Casonato, A.; Galletta, E.; Cella, G.; Barbon, G.; Daidone, V. Acquired von Willebrand Syndrome Hiding Inherited von Willebrand Disease Can Explain Severe Bleeding in Patients With Aortic Stenosis. ATVB 2020, 40, 2187–2194. [Google Scholar] [CrossRef]
- Bańka, P.; Wybraniec, M.; Bochenek, T.; Gruchlik, B.; Burchacka, A.; Swinarew, A.; Mizia-Stec, K. Influence of Aortic Valve Stenosis and Wall Shear Stress on Platelets Function. JCM 2023, 12, 6301. [Google Scholar] [CrossRef]
- Pibarot, P.; Magne, J.; Leipsic, J.; Côté, N.; Blanke, P.; Thourani, V.H.; Hahn, R. Imaging for Predicting and Assessing Prosthesis-Patient Mismatch After Aortic Valve Replacement. JACC: Cardiovascular Imaging 2019, 12, 149–162. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services National Institutes of Health, N.H.L. and B.I. The Diagnosis, Evaluation, and Management of von Willebrand Disease; 2007; p. 126.
- Hillegass, W.B.; Limdi, N.A. Valvular Heart Disease and Acquired Type 2A von Willebrand Syndrome: The “Hemostatic” Waring Blender Syndrome. JAMA Cardiology 2016, 1, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Horiuchi, H.; Imai, M.; Tada, T.; Shiomi, H.; Kuroda, M.; Nishimura, S.; Takahashi, Y.; Yoshikawa, Y.; Tsujimura, A.; et al. Unexpectedly High Prevalence of Acquired von Willebrand Syndrome in Patients with Severe Aortic Stenosis as Evaluated with a Novel Large Multimer Index. J Atheroscler Thromb 2015, 22, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, M.; Milić, D.; Golubović, M.; Kostić, T.; Djordjević, M. MONITORING OF HEMOSTASIS DISORDERS IN CARDIAC SURGERY. AMM 2019, 58, 141–151. [Google Scholar] [CrossRef]
- Pibarot, P. Prosthesis-Patient Mismatch: Definition, Clinical Impact, and Prevention. Heart 2006, 92, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Rahimtoola, S.H. The Problem of Valve Prosthesis-Patient Mismatch. Circulation 1978, 58, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D.P. Prosthesis Patient Mismatch in Aortic Valve Replacement: Possible but Pertinent?The Opinions Expressed in This Article Are Not Necessarily Those of the Editors of the European Heart Journal or of the European Society of Cardiology. European Heart Journal 2006, 27, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Dumesnil, J.G. Prosthetic Heart Valves: Selection of the Optimal Prosthesis and Long-Term Management. Circulation 2009, 119, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Bonderman, D.; Graf, A.; Kammerlander, A.A.; Kocher, A.; Laufer, G.; Lang, I.M.; Mascherbauer, J. Factors Determining Patient-Prosthesis Mismatch after Aortic Valve Replacement – A Prospective Cohort Study. PLoS ONE 2013, 8, e81940. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.L.; Salton, G.D.; Bandinelli, E.; Oliveira, A.R.; Roisenberg, I. The Effect of ABO Blood Group on von Willebrand Response to Exercise. Clin Appl Thromb Hemost 2008, 14, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Capra, F.; Targher, G.; Montagnana, M.; Lippi, G. Relationship between ABO Blood Group and von Willebrand Factor Levels: From Biology to Clinical Implications. Thrombosis J 2007, 5, 14. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Soltani, S.; McDonald, J.; Grezchnik, E.; Easton, L.; Favaloro, J.W.C. Reassessment of ABO Blood Group, Sex, and Age on Laboratory Parameters Used to Diagnose von Willebrand Disorder: Potential Influence on the Diagnosis vs the Potential Association With Risk of Thrombosis. Am J Clin Pathol 2005, 124, 910–917. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).