Submitted:
30 July 2024
Posted:
31 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Hematologic Malignancies
2.1. Acute Myeloid Leukemia
2.2. Acute Lymphoblastic Leukemia
2.3. Lymphomas
2.4. Myelodysplastic Syndrome/ Myeloproliferative Neoplasms
2.5. Multiple Myeloma
3. CD147
3.1. CD147 Structure and Expression
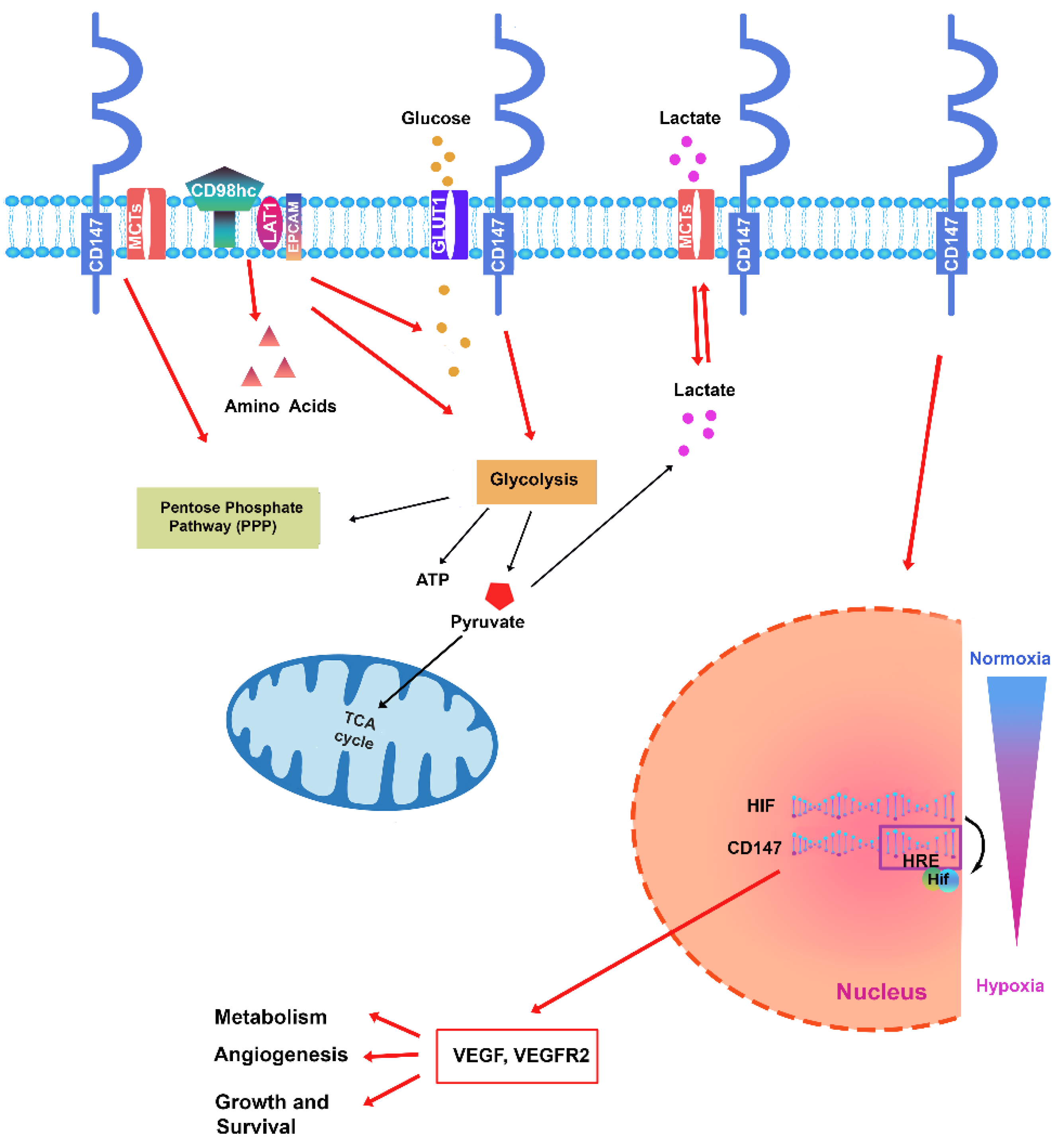
3.2. CD147 Functions: Its Interaction with MCTs and Other Partners
4. Regulation of Energy Metabolism in Hematological malignancies
4.1. Acute Myeloid Leukemia
4.2. Chronic Myeloid Leukemia
4.3. Lymphoma
4.4. Chronic Lymphocytic Leukemia
4.5. Multiple Myeloma and Myelodysplastic Syndrome
5. CD147 in Leukemic Microenvironment
6. Therapeutic Targeting of CD147
7. Conclusion and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Ramdass, B.; Chowdhary, A.; Koka, P.S. Hematological malignancies: disease pathophysiology of leukemic stem cells. J Stem Cells. 2013, 8, 151–187. [Google Scholar]
- Taylor, J.; Xiao, W.; Abdel-Wahab, O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood. 2017, 130, 410–423. [Google Scholar] [CrossRef]
- Traba, J.; Sack, M.N.; Waldmann, T.A.; Anton, OM. Immunometabolism at the Nexus of Cancer Therapeutic Efficacy and Resistance. Front Immunol, 2012; 12, 657293. [Google Scholar]
- Cordoba, R.; Eyre, T.A.; Klepin, H.D.; Wildes, T.M.; Goede, V. A comprehensive approach to therapy of haematological malignancies in older patients. Lancet Haematol. 2021, 8, e840–e852. [Google Scholar] [CrossRef]
- Tang, L.; Huang, Z.; Mei, H.; Hu, Y. Immunotherapy in hematologic malignancies: achievements, challenges and future prospects. Signal Transduct Target Ther. 2023, 8, 306. [Google Scholar] [CrossRef]
- Sochacka-Cwikła, A.; Maczynski, M.; Regiec, A. FDA-Approved Drugs for Hematological Malignancies-The Last Decade Review. Cancers 2022, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhou, X.; Wang, X. Metabolic Reprogramming in Hematologic Malignancies: Advances and Clinical Perspectives. Cancer Res. 2022, 82, 2955–2963. [Google Scholar] [CrossRef]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef] [PubMed]
- San-Millán, I.; Brooks, G.A. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Iacono, K.T.; Brown, A.L.; Greene, M.I.; Saouaf, S.J. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp Mol Pathol. 2007, 83, 283–295. [Google Scholar] [CrossRef]
- Muramatsu, T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J Biochem. 2016, 159, 481–490. [Google Scholar] [CrossRef]
- Spinello, I.; Saulle, E.; Quaranta, M.T.; Pasquini, L.; Pelosi, E.; Castelli, G.; Ottone, T.; Voso, M.T.; Testa, U.; Labbaye, C. The small-molecule compound AC-73 targeting CD147 inhibits leukemic cell proliferation, induces autophagy and increases the chemotherapeutic sensitivity of acute myeloid leukemia cells. Haematologica. 2019, 104, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Nyalali, A.M.K.; Leonard, A.U.; Xu, Y.; Li, H.; Zhou, J.; Zhang, X. Rugambwa, T.K., Shi, X. L,i F. CD147: an integral and potential molecule to abrogate hallmarks of cancer. In Front Oncol; 2023; Volume 13, p. 1238051. [Google Scholar]
- Zhu, D.; Wang, Z.; Zhao, J.J. The Cyclophilin A-CD147 complex promotes the proliferation and homing of multiple myeloma cells. Nat Med. 2015, 21, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Edwards, C.K. 3rd.; Zhou, L. The biological function and clinical utilization of CD147 in human diseases: a review of the current scientific literature. Int J Mol Sci. 2014, 15, 17411–17441. [Google Scholar] [CrossRef] [PubMed]
- Eichner, R.; Heider, M.; Fernández-Sáiz, V. Immunomodulatory drugs disrupt the cereblon-CD147-MCT1 axis to exert anti-tumor activity and teratogenicity. Nat Med. 2016, 22, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Landras, A.; Reger de Moura, C.; Jouenne, F.; Lebbe, C.; Menashi, S.; Mourah, S. CD147 Is a Promising Target of Tumor Progression and a Prognostic Biomarker. Cancers (Basel). 2019, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022, 140, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748, Erratum in: Leukemia 2023, 37, 1944–1951. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Erba, H.P.; Freeman, S.D.; Wei, A.H. Acute myeloid leukaemia. Lancet. 2023, 401, 2073–2086. [Google Scholar] [CrossRef]
- Mishra, S.K.; Millman, S.E.; Zhang, L. Metabolism in acute myeloid leukemia: mechanistic insights and therapeutic targets. Blood. 2023, 141, 1119–1135. [Google Scholar] [CrossRef]
- Pagliaro, L.; Chen, S.J.; Herranz, D.; Mecucci, C.; Harrison, C.J.; Mullighan, C.G.; Zhang, M.; Chen, Z.; Boissel, N.; Winter, S.S.; et al. Acute lymphoblastic leukaemia. Nat Rev Dis Primers. 2024, 10, 41. [Google Scholar] [CrossRef]
- Chan, L.N.; Chen, Z.; Braas, D.; Lee, J.W.; Xiao, G.; Geng, H.; Cosgun, K.N.; Hurtz, C.; Shojaee, S.; Cazzaniga, V.; et al. Metabolic gatekeeper function of B-lymphoid transcription factors. Nature. 2017, 542, 479–483. [Google Scholar] [CrossRef]
- Momotow, J.; Borchmann, S.; Eichenauer, D.A.; Engert, A.; Sasse, S. Hodgkin Lymphoma-Review on Pathogenesis, Diagnosis, Current and Future Treatment Approaches for Adult Patients. J Clin Med. 2021, 10, 1125. [Google Scholar] [CrossRef]
- Bowzyk Al-Naeeb, A.; Ajithkumar, T.; Behan, S.; Hodson, D.J. Non-Hodgkin lymphoma. BMJ, 2018; 362, 3204. [Google Scholar]
- Kluckova, K.; D'Avola, A.; Riches, J.C. Advances in Understanding of Metabolism of B-Cell Lymphoma: Implications for Therapy. Cancers (Basel). 2022, 14, 5552. [Google Scholar] [CrossRef] [PubMed]
- Sekeres, M.A.; Taylor, J. Diagnosis and Treatment of Myelodysplastic Syndromes: A Review. JAMA 2022, 328, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Gerke, M.B.; Christodoulou, I.; Karantanos, T. Definitions, Biology, and Current Therapeutic Landscape of Myelodysplastic/Myeloproliferative Neoplasms. Cancers (Basel). 2023, 15, 3815. [Google Scholar] [CrossRef] [PubMed]
- Gulla, A.; Anderson, K.C. Multiple myeloma: the (r)evolution of current therapy and a glance into future. Haematologica 2020, 105, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Binder, M.; Nandakumar, B.; Rajkumar, S.V.; Kapoor, P.; Buadi, F.K.; Dingli, D.; Lacy, M.Q.; Gertz, M.A.; Hayman, S.R.; Leung, N.; et al. Mortality trends in multiple myeloma after the introduction of novel therapies in the United States. Leukemia 2022, 36, 801–808. [Google Scholar] [CrossRef]
- Butera, A.; Quaranta, M.T.; Crippa, L.; Spinello, I.; Saulle, E.; Di Carlo, N.; Campanile, D.; Boirivant, M.; Labbaye, C. CD147 Targeting by AC-73 Induces Autophagy and Reduces Intestinal Fibrosis Associated with TNBS Chronic Colitis. J Crohns Colitis. 2022, 16, 1751–1761. [Google Scholar] [CrossRef]
- Chuliá-Peris, L.; Carreres-Rey, C.; Gabasa, M.; Alcaraz, J.; Carretero, J.; Pereda, J. Matrix Metalloproteinases and Their Inhibitors in Pulmonary Fibrosis: EMMPRIN/CD147 Comes into Play. Int. J. Mol. Sci. 2022, 23, 6894. [Google Scholar] [CrossRef]
- Pushkarsky, T.; Zybarth, G.; Dubrovsky, L.; Yurchenko, V.; Tang, H.; Guo, H.; Toole, B.; Sherry, B.; Bukrinsky, M. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc Natl Acad Sci U S A. 2001, 98, 6360–6365. [Google Scholar] [PubMed]
- Spinello, I.; Saulle, E.; Quaranta, M.T.; Pelosi, E.; Castelli, G.; Cerio, A.; Pasquini, L.; Morsilli, O.; Dupuis, M.L.; Labbaye, C. AC-73 and Syrosingopine Inhibit SARS-CoV-2 Entry into Megakaryocytes by Targeting CD147 and MCT4. Viruses, 2024, 16, 82. [Google Scholar] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015, 15, 540–555. [Google Scholar] [PubMed]
- Cui, J.; Huang, W.; Wu, B.; Jin, J.; Jing, L.; Shi, W.P.; Liu, Z.Y.; Yuan, L.; Luo, D.; Li. L.; et al. N-glycosylation by N-acetylglucosaminyltransferase V enhances the interaction of CD147/basigin with integrin β1 and promotes HCC metastasis. J Pathol. 2018, 245, 41–52. [Google Scholar]
- Li, W.; Wang, D.; Ge, Y.; Zhang, L.; Wu, J.; Liu, D. Discovery and Biological Evaluation of CD147 N-Glycan Inhibitors: A New Direction in the Treatment of Tumor Metastasis. Molecules. 2020, 26, 33. [Google Scholar]
- Kato, N.; Yuzawa, Y.; Kosugi, T.; Hobo, A.; Sato, W.; Miwa, Y.; Sakamoto, K.; Matsuo, S.; Kadomatsu, K. The E-selectin ligand basigin/CD147 is responsible for neutrophil recruitment in renal ischemia/reperfusion. J Am Soc Nephrol. 2009, 20, 1565–1576. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, W.; Ma, L.T.; Jiang, J.; Chen, Z.N. Importance of N-glycosylation on CD147 for its biological functions. Int J Mol Sci. 2014, 15, 6356–6377. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chang, S.B.; Hemler, M.E. Links between CD147 function, glycosylation, and caveolin-1. Mol Biol Cell. 2004, 15, 4043–4050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, Y.; Jiang, L.; Miao, X.; Zhou, H.; Jia, L. Glycomic alterations are associated with multidrug resistance in human leukemia. Int J Biochem Cell Biol. 2012, 44, 1244–1253. [Google Scholar] [CrossRef]
- Beesley, A.H.; Weller, R.E.; Kees, U.R. The role of BSG (CD147) in acute lymphoblastic leukaemia and relapse. Br J Haematol. 2008, 142, 1000–1002. [Google Scholar] [CrossRef]
- Belton, R.J. Jr.; Chen, L.; Mesquita, F.S.; Nowak, R.A. Basigin-2 is a cell surface receptor for soluble basigin ligand. J. Biol. Chem. 2008, 283, 17805–17814. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Guo, T.; Wang, S.J.; Zhao, P.; Dong, Z.S.; Zhang, Y.; Jiang, J.L.; Chen, Z.N.; Yu, XL. Dimerization is essential for HAb18G/CD147 promoting tumor invasion via MAPK pathway. Biochem Biophys Res Commun. 2012, 419, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.S.; Mengistab, A.T.; Tauscher, A.N.; LaVail, J.; Basbaum, C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene 2004, 23, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Rode, A.; Nicoll, A.; Maczurek, A.E.; Lim, L.; Lim, S.; Angus, P.; Kronborg, I.; Arachchi, N.; Gorelik, A.; et al. Circulating CD147 predicts mortality in advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2016, 31, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.H.; Liu, Y.J.; Tang, L.L.; Wang, S.M.; Yan, G.J.; Liao, L.Q. Plasma soluble cluster of differentiation 147 levels are increased in breast cancer patients and associated with lymph node metastasis and chemoresistance. Hong Kong Med J. 2018, 24, 252–260. [Google Scholar] [CrossRef]
- Rurali, E.; Perrucci, G.L.; Gaetano, R.; Pini, A.; Moschetta, D.; Gentilini, D.; Nigro, P.; Pompilio, G. Soluble EMMPRIN levels discriminate aortic ectasia in Marfan syndrome patients. Theranostics. 2019, 9, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Łacina, P.; Butrym, A.; Turlej, E.; Stachowicz-Suhs, M.; Wietrzyk, J.; Mazur, G.; Bogunia-Kubik, K. BSG (CD147) Serum Level and Genetic Variants Are Associated with Overall Survival in Acute Myeloid Leukaemia. J Clin Med. 2022, 11, 332. [Google Scholar] [CrossRef]
- Łacina, P.; Butrym, A.; Frontkiewicz, D.; Mazur, G.; Bogunia-Kubik, K. Soluble CD147 (BSG) as a Prognostic Marker in Multiple Myeloma. Curr Issues Mol Biol. 2022, 44, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Kasinrerk, W.; Fiebiger, E.; Stefanová, I.; Baumruker, T.; Knapp, W.; Stockinger, H. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J Immunol. 1992, 149, 847–854. [Google Scholar] [CrossRef]
- Saulle, E. , Spinello, I., Quaranta, MT., Pasquini, L., Pelosi, E., Iorio, E., Castelli, G., Chirico, M., Pisanu, M.E., Ottone, T., Voso, M.T., Testa, U., Labbaye, C. Targeting Lactate Metabolism by Inhibiting MCT1 or MCT4 Impairs Leukemic Cell Proliferation, Induces Two Different Related Death-Pathways and Increases Chemotherapeutic Sensitivity of Acute Myeloid Leukemia Cells. Front Oncol. 2021, 1, 621458. [Google Scholar]
- Zou, S.; Parfenova, E.; Vrdoljak, N.; Minden, M.D.; Spagnuolo, P.A. Pseudolaric Acid B Targets CD147 to Selectively Kill Acute Myeloid Leukemia Cells. Int J Mol Sci. 2024, 25, 6517. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Bonzheim, I.; Steinhilber, J.; Montes-Mojarro, I.A.; Ortiz-Hidalgo, C.; Klapper, W.; Fend, F.; Quintanilla-Martínez, L. EMMPRIN (CD147) is induced by C/EBPβ and is differentially expressed in ALK+ and ALK- anaplastic large-cell lymphoma. Lab Invest. 2017, 97, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Panchabhai, S.; Schlam, I.; Sebastian, S.; Fonseca, R. PKM2 and other key regulators of Warburg effect positively correlate with CD147 (EMMPRIN) gene expression and predict survival in multiple myeloma. Leukemia 2017, 31, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Caudroy, S.; Polette, M.; Nawrocki-Raby, B.; Cao, J.; Toole, B.P.; Zucker, S.; Birembaut, P. EMMPRIN-mediated MMP regulation in tumor and endothelial cells. Clin Exp Metastasis. 2002, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Ghandour, F.; Kassem, S.; Simanovich, E.; Rahat, M.A. Glucose Promotes EMMPRIN/CD147 and the Secretion of Pro-Angiogenic Factors in a Co-Culture System of Endothelial Cells and Monocytes. Biomedicines 2024, 12, 706. [Google Scholar] [CrossRef] [PubMed]
- Kanekura, T. CD147/Basigin Is Involved in the Development of Malignant Tumors and T-Cell-Mediated Immunological Disorders via Regulation of Glycolysis. Int. J. Mol. Sci. 2023, 24, 17344. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, W. CD147-mediated reprogrammed glycolytic metabolism potentially induces immune escape in the tumor microenvironment (Review). Oncol Rep. 2019, 41, 2945–2956. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.; Wilson, M.C.; Heddle, C.; Brown, M.H.; Barclay, A.N.; Halestrap, A.P. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000, 19, 3896–3904. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. The monocarboxylate transporter family--Structure and functional characterization. IUBMB Life. 2012, 64, 1–9. [Google Scholar] [CrossRef]
- Aït-Ali, N.; Fridlich, R.; Millet-Puel, G.; Clérin, E.; Delalande, F.; Jaillard, C.; Blond, F.; Perrocheau, L.; Reichman, S.; Byrne, L.C.; et al. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell. 2015, 161, 817–832. [Google Scholar] [CrossRef]
- Almeida, L.M.C.A.; Silva, R.; Cavadas, B.; Lima, J.; Pereira, L.; Soares, P.; Sobrinho-Simões, M.; Lopes, J.M.; Máximo, V. GLUT1, MCT1/4 and CD147 overexpression supports the metabolic reprogramming in papillary renal cell carcinoma. Histol Histopathol. 2017, 32, 1029–1040. [Google Scholar] [PubMed]
- Huang, X.Q.; Chen, X.; Xie, X.X.; Zhou, Q.; Li, K.; Li, S.; Shen, L.F.; Su, J. Co-expression of CD147 and GLUT-1 indicates radiation resistance and poor prognosis in cervical squamous cell carcinoma. Int J Clin Exp Pathol. 2014, 7, 1651–1666. [Google Scholar] [PubMed]
- Su, J.; Gao, T.; Jiang, M.; Wu, L.; Zeng, W.; Zhao, S.; Peng, C.; Chen, X. CD147 silencing inhibits tumor growth by suppressing glucose transport in melanoma. Oncotarget. 2016, 7, 64778–64784. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Zabala, M.; Ramakrishnan, R.; Reinbach. ; Ghosh, S.; Oburoglu, L.; Falqués-Costa, A.; Bellamkonda, K.; Ehinger, M.; Peña-Martínez, P.; Puente-Moncada, N.; et al. Combined GLUT1 and OXPHOS inhibition eliminates acute myeloid leukemia cells by restraining their metabolic plasticity. Blood Adv. 2023, 7, 5382–5395. [Google Scholar] [CrossRef]
- Hartmann, S.; Agostinelli, C.; Diener, J.; Döring, C.; Fanti, S.; Zinzani, P.L.; Gallamini, A.; Bergmann, L.; Pileri, S.; Hansmann, M.L. GLUT1 expression patterns in different Hodgkin lymphoma subtypes and progressively transformed germinal centers. BMC Cancer. 2012, 12, 586. [Google Scholar] [CrossRef]
- Shim, H.K.; Lee, W.W.; Park, S.Y.; Kim, H.; Kim, S.E. Relationship between FDG uptake and expressions of glucose transporter type 1, type 3, and hexokinase-II in Reed-Sternberg cells of Hodgkin lymphoma. Oncol Res. 2009, 17, 331–337. [Google Scholar] [CrossRef]
- McBrayer, S.K.; Cheng, J.C.; Singhal, S.; Krett, N.L.; Rosen, S.T.; Shanmugam, M. Multiple myeloma exhibits novel dependence on GLUT4, GLUT8, and GLUT11: implications for glucose transporter-directed therapy. Blood. 2012, 119, 4686–4697. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Jimi, S.; Migita, K.; Takamatsu, Y.; Hara, S. Inhibition of glucose transporter 1 induces apoptosis and sensitizes multiple myeloma cells to conventional chemotherapeutic agents. Leuk Res. 2016, 41, 103–110. [Google Scholar] [CrossRef]
- Berditchevski, F.; Chang, S.; Bodorova, J.; Hemler, M.E. Generation of monoclonal antibodies to integrin-associated proteins. Evidence that alpha3beta1 complexes with EMMPRIN/basigin/OX47/M6. J Biol Chem. 1997, 272, 29174–29180. [Google Scholar] [CrossRef]
- Xu, D.; Hemler, M.E. Metabolic activation-related CD147-CD98 complex. Mol Cell Proteomics 2005, 4, 1061–1071. [Google Scholar] [CrossRef]
- Bajaj, J.; Konuma, T.; Lytle, N.K.; Kwon, H.Y.; Ablack, J.N.; Cantor, J.M.; Rizzieri, D.; Chuah, C.; Oehler, V.G.; Broome, E.H.; et al. CD98-Mediated Adhesive Signaling Enables the Establishment and Propagation of Acute Myelogenous Leukemia. Cancer Cell 2016, 30, 792–805. [Google Scholar] [CrossRef]
- Devés, R.; Boyd, C.A. Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. J Membr Biol. 2000, 173, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Ikeda, S.; Yaga, M.; Watanabe, K.; Urakawa, R.; Iehara, A.; Iwai, M.; Hashiguchi, S.; Morimoto, S.; Fujiki, F.; et al. Selective targeting of multiple myeloma cells with a monoclonal antibody recognizing the ubiquitous protein CD98 heavy chain. Sci Transl Med. 2022, 14, eaax7706. [Google Scholar] [CrossRef] [PubMed]
- Hayes, G.M.; Chinn, L.; Cantor, J.M.; Cairns, B.; Levashova, Z.; Tran, H.; Velilla, T.; Duey, D.; Lippincott, J.; Zachwieja, J.; et al. Antitumor activity of an anti-CD98 antibody. Int J Cancer 2015, 137, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, V.; Constant, S.; Eisenmesser, E.; Bukrinsky, M. Cyclophilin-CD147 interactions: a new target for anti-inflammatory therapeutics. Clin Exp Immunol. 2010, 160, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, V.; Zybarth, G.; O'Connor, M.; Dai, W.W.; Franchin, G.; Hao, T.; Guo, H.; Hung, H.C.; Toole, B.; Gallay, P.; et al. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002, 277, 22959–22965. [Google Scholar] [CrossRef]
- Wang, W.J.; Li, Q.Q.; Xu, J.D.; Cao, X.X.; Li, H.X.; Tang, F.; Chen, Q.; Yang, J.M.; Xu, Z.D.; Liu, X.P. Interaction between CD147 and P-glycoprotein and their regulation by ubiquitination in breast cancer cells. Chemotherapy, 2008; 54, 291–301. [Google Scholar]
- Seizer, P.; Borst, O.; Langer, H.F.; Bültmann, A.; Münch, G.; Herouy, Y.; Stellos, K.; Krämer, B.; Bigalke, B.; Büchele, B.; et al. EMMPRIN (CD147) is a novel receptor for platelet GPVI and mediates platelet rolling via GPVI-EMMPRIN interaction. Thromb Haemost. 2009, 101, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Wang, J.H.; Zhao, A.H.; Xu, X.; Wang, Y.H.; Chen, T.L.; Li, J.M.; Mi, J.Q.; Zhu, Y.M.; Liu, Y.F.; et al. A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood. 2014, 124, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, M.T.; Panagopoulou, P.; Paramera, E.; Pechlivanis, A.; Virgiliou, C.; Papakonstantinou, E.; Palabougiouki, M.; Ioannidou, M.; Vasileiou, E.; Tragiannidis, A.; et al. Metabolic Fingerprint in Childhood Acute Lymphoblastic Leukemia. Diagnostics (Basel). 2024, 14, 682. [Google Scholar]
- Maiso, P.; Huynh, D.; Moschetta, M.; Sacco, A.; Aljawai, Y.; Mishima, Y.; Asara, J.M.; Roccaro, A.M.; Kimmelman, A.C.; Ghobrial, I.M. Metabolic signature identifies novel targets for drug resistance in multiple myeloma. Cancer Res. 2015, 75, 2071–2082. [Google Scholar] [CrossRef]
- Balaian, E.; Wobus, M.; Bornhäuser, M.; Chavakis, T.; Sockel, K. Myelodysplastic Syndromes and Metabolism. Int. J. Mol. Sci. 2021, 22, 11250. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, M.; Szczudło, J.; Pietrzyk, A.; Shah, J.; Trojan, S.E.; Ostrowska, B.; Kocemba-Pilarczyk, K.A. The Warburg effect: a score for many instruments in the concert of cancer and cancer niche cells. Pharmacol Rep. 2023, 75, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Pereira, O.; Teixeira, A.; Sampaio-Marques, B.; Castro, I.; Girao, H.; Ludovico, P. Signalling mechanisms that regulate metabolic profile and autophagy of acute myeloid leukaemia cells. J. Cell Mol. Med. 2018, 22, 4807–4817. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, N.; Poulain, L.; Birsen, R.; Tamburini, J.; Bouscary, D. Rationale for Targeting Deregulated Metabolic Pathways as a Therapeutic Strategy in Acute Myeloid Leukemia. Front Oncol. 2019, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz Concepción, B.; Bartolo-García, L.D.; Tizapa-Méndez, M.D.; Martínez-Vélez, M.; Valerio-Diego, J.J.; Illades-Aguiar, B.; Salmerón-Bárcenas, E.G.; Ortiz-Ortiz, J.; Torres-Rojas, F.I.; Mendoza-Catalán, M.Á.; et al. EMMPRIN is an emerging protein capable of regulating cancer hallmarks. Eur Rev Med Pharmacol Sci. 2022, 26, 6700–6724. [Google Scholar] [PubMed]
- Kendrick, A.A.; Schafer, J.; Dzieciatkowska, M.; Nemkov, T.; D'Alessandro, A.; Neelakantan, D.; Ford, H.L.; Pearson, C.G.; Weekes, C.D.; Hansen, K.C.; et al. CD147: a small molecule transporter ancillary protein at the crossroad of multiple hallmarks of cancer and metabolic reprogramming. Oncotarget 2017, 8, 6742–6762. [Google Scholar] [CrossRef] [PubMed]
- Grønningsæter, I.S.; Reikvam, H.; Aasebø, E.; Bartaula-Brevik, S.; Tvedt, T.H.; Bruserud, Ø.; Hatfield, K.J. Targeting Cellular Metabolism in Acute Myeloid Leukemia and The Role of Patient Heterogeneity. Cells 2020, 9, 1155. [Google Scholar] [CrossRef]
- Kui, S.; Xiaojun, X.; Li, X.; Xiao, Z.; Fuhua, Z.; Ke, Z.; Meiqing, W.; Ren, L.; Qifa, L. The Expression Profile of Glycolysis Associated Molecules and Chemoresistance in Acute Myeloid Leukemia. Blood 2012, 120, 4318. [Google Scholar]
- Song, K.; Li, M.; Xu, X.; Xuan, L.I.; Huang, G.; Liu, Q. Resistance to chemotherapy is associated with altered glucose metabolism in acute myeloid leukemia. Oncol Lett. 2016, 12, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Fox, D.A.; Horejsi, V.; Sagawa, K.; Skubitz, K.M.; Katz, D.R.; Chain, B. The functional interactions between CD98, beta1-integrins, and CD147 in the induction of U937 homotypic aggregation. Blood 2001, 98, 374–382. [Google Scholar] [CrossRef] [PubMed]
- de la Ballina, L.R.; Cano-Crespo, S.; González-Muñoz, E.; Bial, S.; Estrach, S.; Cailleteau, L.; Tissot, F.; Daniel, H.; Zorzano, A.; Ginsberg, M.H.; et al. Amino Acid Transport Associated to Cluster of Differentiation 98 Heavy Chain (CD98hc) Is at the Cross-road of Oxidative Stress and Amino Acid Availability. J Biol Chem. 2016, 291, 9700–9711. [Google Scholar] [CrossRef] [PubMed]
- Gayatri, M.B.; Kancha, R.K.; Patchva, D.; Velugonda, N.; Gundeti, S.; Reddy, A.B.M. Metformin exerts antileukemic effects by modulating lactate metabolism and overcomes imatinib resistance in chronic myelogenous leukemia. FEBS J. 2023, 290, 4480–4495. [Google Scholar] [CrossRef]
- Fukuchi, K.; Nanai, K.; Yuita, H.; Maru, C.; Tsukada, J.; Ishigami, M.; Nagai, Y.; Nakano, Y.; Yoshimura, C.; Yoneda, K.; et al. Novel Antibody Exerts Antitumor Effect through Downregulation of CD147 and Activation of Multiple Stress Signals. J Oncol. 2022, 2022, 3552793. [Google Scholar] [CrossRef] [PubMed]
- Thorns, C.; Feller, A.C.; Merz, H. EMMPRIN (CD 147) is expressed in Hodgkin's lymphoma and anaplastic large cell lymphoma. An immunohistochemical study of 60 cases. Anticancer Res. 2002, 22, 1983–1986. [Google Scholar] [PubMed]
- Nabeshima, K.; Suzumiya, J.; Nagano, M.; Ohshima, K.; Toole, B.P.; Tamura, K.; Iwasaki, H.; Kikuchi, M. Emmprin, a cell surface inducer of matrix metalloproteinases (MMPs), is expressed in T-cell lymphomas. J Pathol. 2004, 202, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Montes-Mojarro, I.A.; Steinhilber, J.; Griessinger, C.M.; Rau, A.; Gersmann, A.-K.; Kohlhofer, U.; Fallier-Becker, P.; Liang, H.-C.; Hofmann, U.; Haag, M.; et al. CD147 a direct target of miR-146a supports energy metabolism and promotes tumor growth in ALK+ ALCL. Leukemia 2022, 36, 2050–2063. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, J.; Chang, J.; Kanekura, T.; Li, J.; Kuang, Y.H.; Peng, S.; Yang, F.; Lu, H.; Zhang, J.L. Inhibition of CD147 gene expression via RNA interference reduces tumor cell proliferation, activation, adhesion, and migration activity in the human Jurkat T-lymphoma cell line. Cancer Investig. 2008, 26, 689–697. [Google Scholar] [CrossRef]
- Sakamoto, M.; Miyagaki, T.; Kamijo, H.; Oka, T.; Boki, H.; Takahashi-Shishido, N.; Suga, H.; Sugaya, M.; Sato, S. CD147-Cyclophilin a Interactions Promote Proliferation and Survival of Cutaneous T-Cell Lymphoma. Int. J. Mol. Sci. 2021, 22, 7889. [Google Scholar] [CrossRef]
- Donnelly, R.P; Finlay, D.K. Glucose, glycolysis and lymphocyte responses. Mol Immunol. 2015, 68 Pt C, 513–519. [Google Scholar] [CrossRef]
- Prieto, D.; Sotelo, N.; Seija, N.; Sernbo,S. ; Abreu, C.; Duran, R.; Gil, M.; Sicco, E.; Irigoin, V.; Oliver, C.; Landoni, A.I.; Gabus, R.; Dighiero, G.; Oppezzo, P. S100-A9 protein in exosomes from chronic lymphocytic leukemia cells promotes NF-kappaB activity during disease progression. Blood 2017, 130, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.M.; Gilardoni, M.B.; Remedi, M.M.; Sastre, D.; Heller, V.; Pellizas, C.G.; Donadio, A.C. Tumor-stroma interaction increases CD147 expression in neoplastic B lymphocytes in chronic lymphocytic leukemia. Blood Cells Mol Dis. 2020, 82, 102405. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.K.; Arendt, B.K.; Jelinek, D.F. CD147 regulates the expression of MCT1 and lactate export in multiple myeloma cells. Cell Cycle, 2013; 12, 3175–3183. [Google Scholar]
- Arendt, B.K.; Walters, D.K.; Wu, X.; Tschumper, R.C.; Huddleston, P.M.; Henderson, K.J.; Dispenzieri, A.; Jelinek, D.F. Increased expression of extracellular matrix metalloproteinase inducer (CD147) in multiple myeloma: Role in regulation of myeloma cell proliferation. Leukemia 2012, 26, 2286–2296. [Google Scholar] [CrossRef] [PubMed]
- Łacina, P.; Butrym, A.; Mazur, G.; Bogunia-Kubik, K. BSG and MCT1 Genetic Variants Influence Survival in Multiple Myeloma Patients. Genes (Basel) 2018, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Bolomsky, A.; Hübl, W.; Spada, S.; Müldür, E.; Schlangen, K.; Heintel, D.; Rocci, A.; Weißmann, A.; Fritz, V.; Willheim, M.; et al. IKAROS expression in distinct bone marrow cell populations as a candidate biomarker for outcome with lenalidomide-dexamethasone therapy in multiple myeloma. Am J Hematol. 2017, 92, 269–278. [Google Scholar] [PubMed]
- Winkler, I.G.; Barbier, V.; Nowlan, B.; et al. The vascular niche E-selectin regulates hematopoietic stem cell dormancy, self-renewal and chemoresistance. Nat Med. 2012, 18, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, A. ; Hsu, Y-M. S.; Day, R.B.; Schuettpelz, L.G.; Christopher, M.J.; Borgerding, J.N.; Nagasawa, T.; Link, D.C. CXCL12 in early mesenchymal progenitors is required for the maintenance of hematopoietic stem cells. Nature, 2013; 495, 227–230. [Google Scholar]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar]
- Wang, Y.H.; Israelsen, W.J.; Lee, D.; Yu, V.W.; Jeanson, N.T.; Clish, C.B.; et al. Specific metabolic dependence of cell status in hematopoiesis and leukemogenesis. Cell 2014, 158, 1309–1323. [Google Scholar] [CrossRef]
- Saito, Y.; Chapple, R.H.; Lin, A.; Kitano, A.; Nakada, D. AMPK protects the cells that initiate leukemia in myeloid leukemias from metabolic stress in the bone marrow. Cell Stem cell 2015, 17, 585–596. [Google Scholar]
- Zhang, W.; Trachootham, D.; Liu, J.; Chen, G.; Pelicano, H.; Garcia-Prieto, C.; et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukemia. Nat Cell Biol. 2012, 14, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Rovida, E.; Peppicelli, S.; Bono, S.; Bianchini, F.; Tusa, I.; Cheloni, G.; et al. The metabolically modulated stem cell niche: a dynamic scenario that regulates cancer cell phenotype and resistance to therapy. Cell cycle 2014, 13, 3169–3175. [Google Scholar] [CrossRef] [PubMed]
- Amati, E.; Perbellini, O.; Rotta, G.; et al. High-throughput immunophenotypic characterization of bone marrow-derived mesenchymal stromal cells and umbilical cord blood reveals common and differentially expressed markers: identification of angiotensin-converting enzyme (CD143) as a differentially expressed marker between adult and perinatal tissue sources. Stem Cell Res Ther. 2018, 9, 10. [Google Scholar] [PubMed]
- Ke, X.; Chen, Y.; Wang, P.; Xing, J.; Chen, Z. Upregulation of CD147 protects hepatocellular carcinoma cells from apoptosis through glycolytic switch via HIF-1 and MCT-4 in hypoxia. Hepatol Int. 2014, 8, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Bougatef, F.; Menashi, S.; Khayati, F.; Naïmi, B.; Porcher, R.; Podgorniak, MP.; et al. EMMPRIN promotes the malignant properties of melanoma cells through HIF-2alpha-mediated upregulation of the VEGF-2 receptor. PloS One 2010, 5, e12265. [Google Scholar] [CrossRef]
- Wang, C.H.; Yao, H.; Chen, L.N.; Jia, J.F.; Wang, L.; Dai, J.Y.; et al. CD147 induces angiogenesis through a vascular endothelial growth factor and a hypoxia-inducible transcription factor 1α-mediated pathway in rheumatoid arthritis. Arthritis Rheum. 2012, 64, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Bougatef, F. , Eougatef, F.; Quemener, C.; Kellouche, S.; Naimi, B.; Podgorniak, M.P.; Millot G.; Gabison, E.E.; Calvo, F.; Dosquet, C.; Lebbe, C.; et al. EMMPRIN promotes angiogenesis through hypoxia-inducible factor-2-mediated regulation of soluble VEGF isoforms and their VEGFR-2 receptor. Blood 2009, 114, 5547–5556. [Google Scholar]
- Lopes-Coelho, F.; Nunes, C.; Gouveia-Fernandes, S.; Rosas, R.; Silva, F.; Gameiro, P.; Carvalho, T.; Gomes da Silva, M.; Cabeçadas, J.; Dias, S.; Gonçalves, LG.; Serpa, J. Monocarboxylate transporter 1 (MCT1), a tool to stratify patients with acute myeloid leukemia (AML) and a vehicle to kill cancer cells. Oncotarget 2017, 8, 82803–82823. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Hata, K.; Hirose, K.; Okui, T.; Toyosawa, S.; Uzawa, N.; Nishimura, R.; Yoneda, T. The GPR81 lactate sensor regulates the glycolysis and tumor growth of breast cancer. Scientific reports, 2022; 12, 6261. [Google Scholar]
- Brown, TP.; Bhattacharjee, P.; Ramachandran, S.; et al. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene 2020, 39, 3292–3304. [Google Scholar] [CrossRef]
- Soto, C. A.; Lesch, M.L.; Sharipol, A.; Khan, A.; Schafer, L. X.; Becker, M. W.; Munger, J. C.; Frisch B., J. Elevated Lactate in Acute Myeloid Leukemia Bone Marrow Microenvironment Dysfunction, with a Dual Role of GPR81 in Macrophage Polarization and Leukemia Cell Growth. BioRxiv 2023, 11, 566874. [Google Scholar]
- Ishikawa, F.; Yoshida, S.; Saito,Y; Hijikata A. ; Kitamura, H.; Tanaka, S.; et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007, 25, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Nick van Gastel, N.; Spinelli, J.B.; Sharda, A.; Schajnovitz, A.; Baryawno, N.; Rhee, C.; Oki, T.; Grace, E.; Soled, H.J.; Milosevic, J.; Sykes, D.B.; Hsu, P.P.; Vander Heiden, M.G.; Vidoudez, C.; Trauger, S.A.; Haigis, M.C.; Scadden, D.T. Induction of a timed metabolic collapse to overcome cancer chemoresistance. Cell Metab. 2020, 32, 391–403. [Google Scholar] [CrossRef]
- Ng, K.P.; Manjeri, A.; Lee, K.L.; Huang, W.; Tan, S.Y.; Chuah, C.T.; Poellinger, L.; Ong, S.T. Physiologic hypoxia promotes maintenance of CML stem cells despite effective BCR-ABL1 inhibition. Blood 2014, 123, 3316–3326. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Jiang, Q.; Han, Y.; Peng, J.; Wang, C. shRNA-mediated EMMPRIN silencing inhibits human leukemic monocyte lymphoma U937 cell proliferation and increases chemosensitivity to adriamycin. Cell Biochem Biophys. 2015, 71, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.M.; Silva, C.; Wang, J.; Tong, J.P.; Yong, V.W. A novel anti-EMMPRIN function-blocking antibody reduces T cell proliferation and neurotoxicity: Relevance to multiple sclerosis. J Neuroinflamm. 2012, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Somno, A.; Anuchapreeda, S.; Chruewkamlow, N.M.; Pata, S.; Kasinrerk, W.; Chiampanichayakul, S. Involvement of CD147 on multidrug resistance through the regulation of P-glycoprotein expression in K562/ADR leukemic cell line. Leuk Res Rep. 2016, 6, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Simanovich, E.; Brod, V.; Rahat, M.M.; Drazdov, E.; Walter, M.; Shakya, J.; Rahat, M.A. Inhibition of tumor growth and metastasis by EMMPRIN multiple antigenic peptide (MAP) vaccination is mediated by immune modulation. Oncoimmunology 2016, 6, e1261778. [Google Scholar] [CrossRef]
- Wang, L.; Ku, X.M.; Li, Y.; Bian, H.J.; Zhang, S.H.; Ye, H.; Yao, X.Y.; Li, B.H.; Yang, X.M.; Liao, C.G.; Chen, Z.N. Regulation of matrix metalloproteinase production and tumor cell invasion by four monoclonal antibodies against different epitopes of HAb18G/CD147 extracellular domain. Hybridoma (Larchmt) 2006, 25, 60–67. [Google Scholar] [CrossRef]
- Chen, Z.N.; Mi, L.; Xu, J.; Song, F.; Zhang, Q.; Zhang, Z.; et al. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) metuximab injection: clinical phase I/II trials. Int J Radiat Oncol Biol Phys. 2006, 65, 435–444. [Google Scholar] [CrossRef]
- Panich, T.; Tragoolpua, K.; Pata, S.; Tayapiwatana, C.; Intasai, N. Downregulation of extracellular matrix metalloproteinase inducer by scFv-M6-1B9 intrabody suppresses cervical cancer invasion through inhibition of urokinase-type plasminogen activator. Cancer Biother Radiopharm. 2017, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Sun, Q.; Feng, F.; Huhe, M.; Mi, L.; Chen, Z. Preclinical pharmacokinetics, tolerability, and pharmacodynamics of metuzumab, a novel CD147 human-mouse chimeric and glycoengineered antibody. Mol Cancer Ther. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.S.; Zhao, X.Y; Wei, D.; Miao, J.L; Liu, Z.K.; Yong, Y.L.; et al. CD147-specific chimeric antigen receptor T cells effectively inhibit T cell acute lymphoblastic leukemia. Cancer Lett. 2022, 542, 215762. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Dai, L.; Bratoeva, M.; Slomiany, M.G.; Toole, B.P.; Parsons, C. Cooperative roles for emmprin and LYVE-1 in the regulation of chemoresistance for primary effusion lymphoma. Leukemia 2011, 25, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
| Name |
Mechanism of Action |
Cell lines / tumor xenograft | Effects | References |
|---|---|---|---|---|
| siRNA | silencing | AML cell line | Inhibits leukemic cell proliferation, increases chemosensitivity to Adriamycin. | 132 |
| siRNA | silencing | Lymphoma Cell Line | increases chemosensitivity | 141 |
| AC-73 | Disrupts CD147 dimerization |
Cell lines and primary AML blasts |
Inhibits leukemic cell proliferation, increases chemosensitivity to ARA-C and ATO | 12 |
| PAB | CD147 inhibitor | Cell lines and primary AML blasts |
AML cell apoptosis | 54 |
| h4#147D | humanized anti-CD147 antibody | xenograft CML mouse models | strong antitumor effects with tumour regression | 99 |
| MEM-M6/6 | monoclonal CD147 antibodies (mAb) | CML cell line | Decreased expression of CD147 and caused downregulation of P-gp | 134 |
| CD147-CAR T cells | (CAR) T cell therapy | T-ALL cell line and T-ALL blasts | anti-tumor activity | 140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





