Submitted:
28 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.2.1. Tissue Microarray
2.2.2. Immunohistochemistry
2.2.3. Evaluation of Immunohistochemistry
2.2.4. TCGA PanCancer mRNA Data
2.2.5. Statistics
3. Results
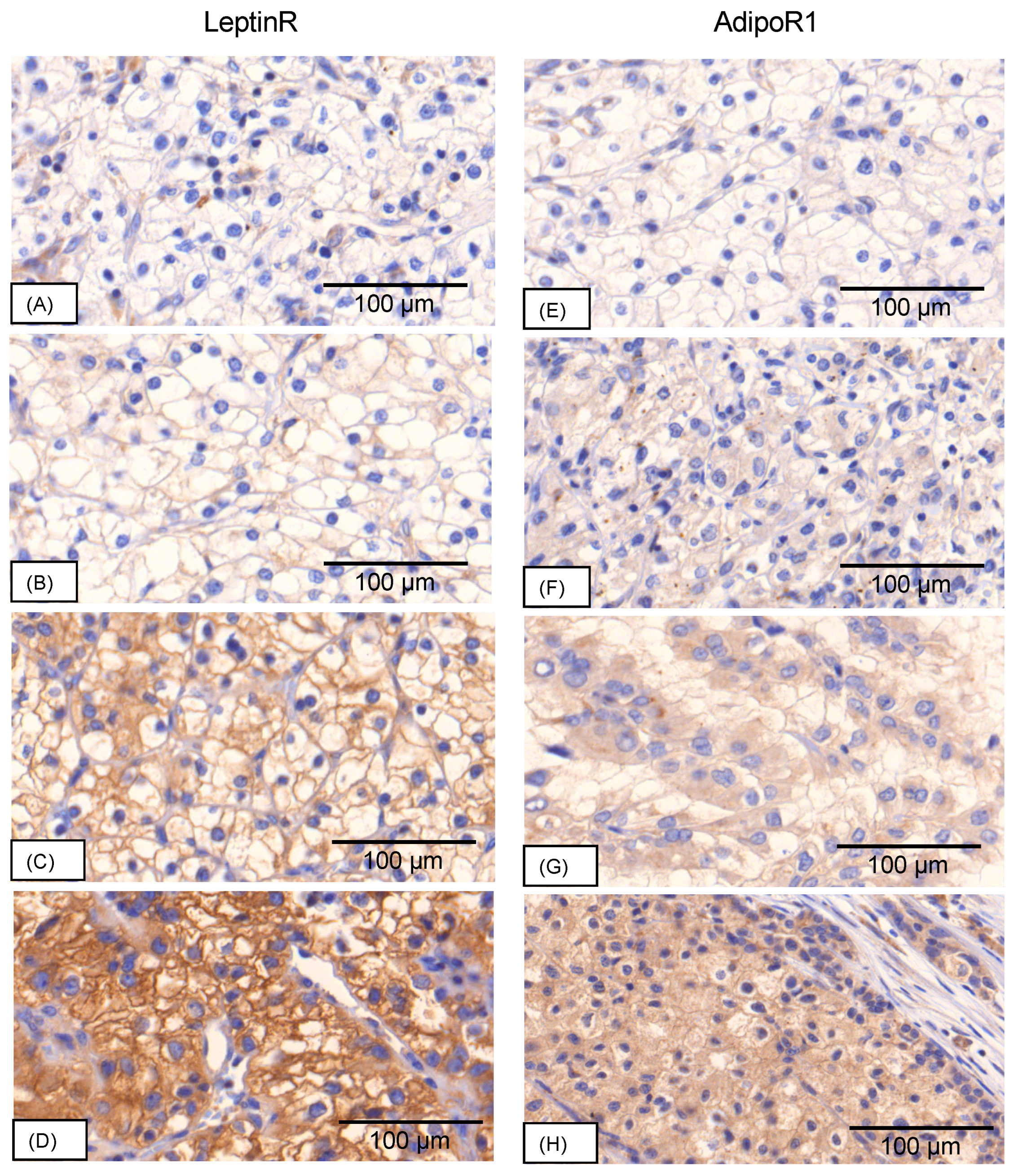
3.1. TMA Staining Evaluation
3.1.1. Staining Patterns leptinR
3.1.2. Staining Patterns adipoR1
3.3. Receptor Expression
3.3.1. LeptinR
3.3.2. AdipoR1
3.4. Associations and Correlations with Clinicopathological Parameters
3.4.1. LeptinR
RCC:
Cervical carcinoma:
Vulvar carcinoma:
3.4.2. AdipoR1RCC:
Cervical carcinoma:
Vulvar carcinoma:
Endometrial carcinoma:
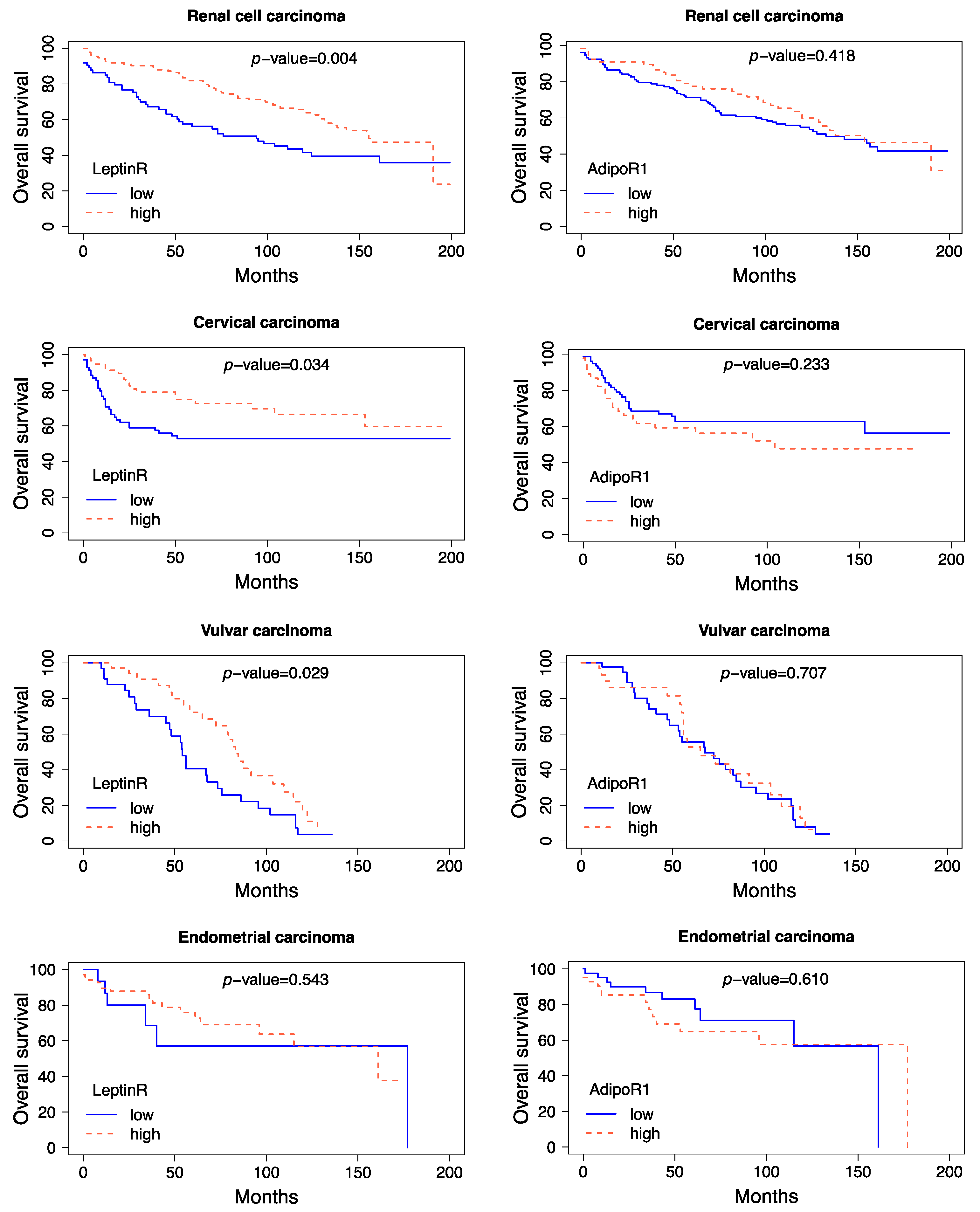
3.5. Survival Analysis of Immunohistochemical Data
3.5.1. LeptinR
3.5.2. AdipoR1
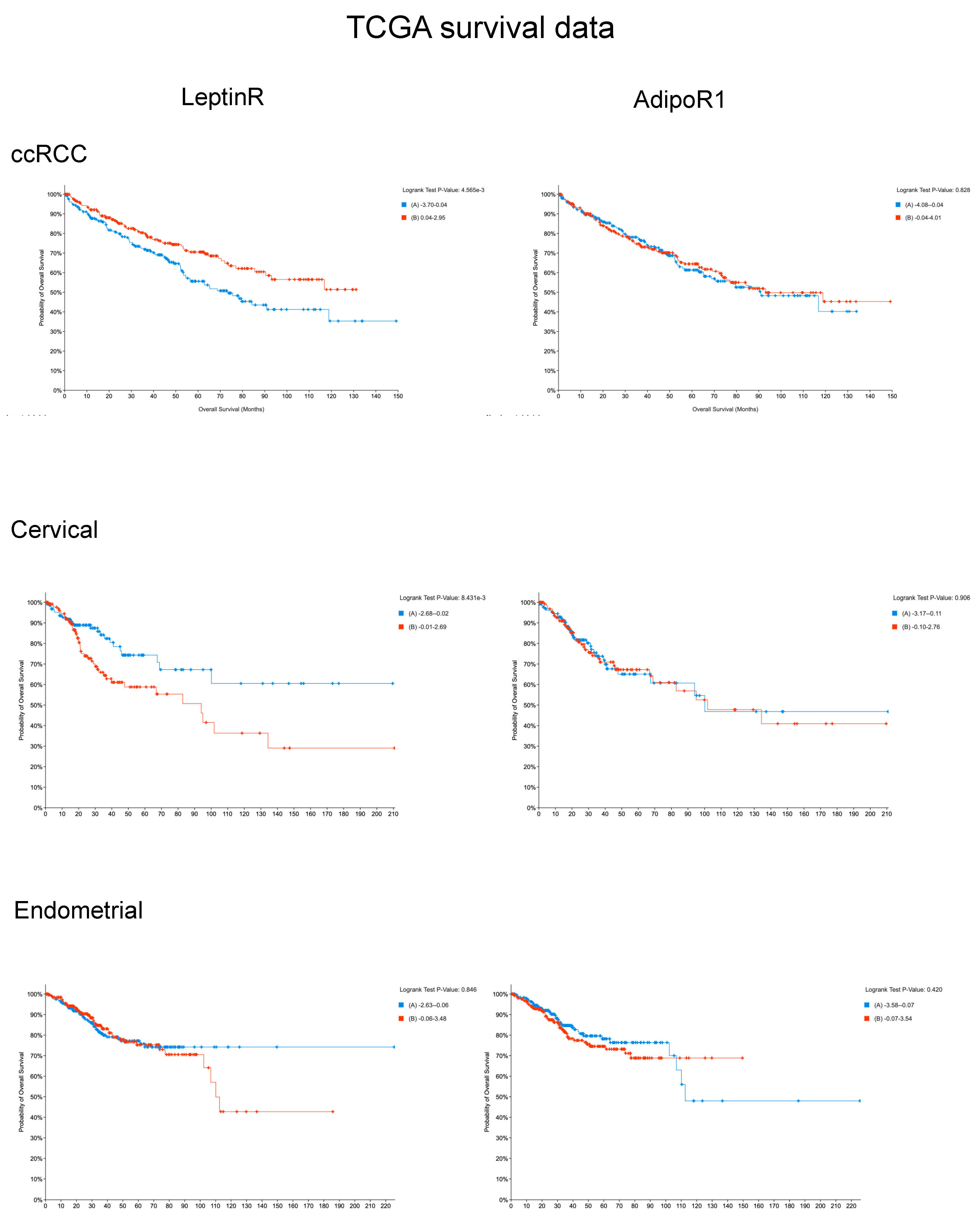
3.6. Prognostic Value of Receptor mRNA Expression Using TCGA Databases
3.6.1. LeptinR
3.6.2. AdipoR1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aravani, A.; Downing, A.; Thomas, J.D.; Lagergren, J.; Morris, E.J.A.; Hull, M.A. Obesity surgery and risk of colorectal and other obesity-related cancers: An English population-based cohort study. Cancer Epidemiol. 2018, 53, 99–104. [CrossRef]
- Song, M.; Giovannucci, E. Estimating the Influence of Obesity on Cancer Risk: Stratification by Smoking Is Critical. J. Clin. Oncol. 2016, 34, 3237–3239. [CrossRef]
- Da Lee, Y.; Lee, T.S. Associations between metabolic syndrome and gynecologic cancer. Obstet. Gynecol. Sci. 2020, 63, 215–224. [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [CrossRef]
- Mancuso, P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016, 5, 47–56. [CrossRef]
- Pergola, G. de; Silvestris, F. Obesity as a major risk factor for cancer. J. Obes. 2013, 2013, 291546. [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [CrossRef]
- Kazeminasab, F.; Behzadnejad, N.; Cerqueira, H.S.; Santos, H.O.; Rosenkranz, S.K. Effects of intermittent fasting combined with exercise on serum leptin and adiponectin in adults with or without obesity: a systematic review and meta-analysis of randomized clinical trials. Front. Nutr. 2024, 11, 1362731. [CrossRef]
- Katsiki, N.; Mantzoros, C.; Mikhailidis, D.P. Adiponectin, lipids and atherosclerosis. Curr. Opin. Lipidol. 2017, 28, 347–354. [CrossRef]
- Grossmann, M.E.; Cleary, M.P. The balance between leptin and adiponectin in the control of carcinogenesis - focus on mammary tumorigenesis. Biochimie 2012, 94, 2164–2171. [CrossRef]
- Chen, Z.; Yang, H.; Ren, Y.; Yang, Z.; Huang, J.; Li, C.; Xiong, Y.; Yu, B. Distinct roles of ADIPOR1 and ADIPOR2: A pan-cancer analysis. Front. Endocrinol. (Lausanne) 2023, 14, 1119534. [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [CrossRef]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18. [CrossRef]
- Dana, N.; Ferns, G.A.; Nedaeinia, R.; Haghjooy Javanmard, S. Leptin signaling in breast cancer and its crosstalk with peroxisome proliferator-activated receptors α and γ. Clin. Transl. Oncol. 2023, 25, 601–610. [CrossRef]
- Sanders, C.; Hamad, A.S.M.; Ng, S.; Hosni, R.; Ellinger, J.; Klümper, N.; Ritter, M.; Stephan, C.; Jung, K.; Hölzel, M.; et al. CD103+ Tissue Resident T-Lymphocytes Accumulate in Lung Metastases and Are Correlated with Poor Prognosis in ccRCC. Cancers (Basel) 2022, 14, 1541. [CrossRef]
- Condic, M.; Ralser, D.J.; Klümper, N.; Ellinger, J.; Qureischi, M.; Egger, E.K.; Kristiansen, G.; Mustea, A.; Thiesler, T. Comprehensive Analysis of N6-Methyladenosine (m6A) Writers, Erasers, and Readers in Cervical Cancer. Int. J. Mol. Sci. 2022, 23, 7165. [CrossRef]
- Hecking, T.; Thiesler, T.; Schiller, C.; Lunkenheimer, J.-M.; Ayub, T.H.; Rohr, A.; Condic, M.; Keyver-Paik, M.-D.; Fimmers, R.; Kirfel, J.; et al. Tumoral PD-L1 expression defines a subgroup of poor-prognosis vulvar carcinomas with non-viral etiology. Oncotarget 2017, 8, 92890–92903. [CrossRef]
- Hecking, T.; Thiesler, T.; Halbe, J.; Otten, L.; Recker, F.; Gevensleben, H.; Müller, T.; Schiller, C.; Egger, E.K.; Fimmers, R.; et al. Programmed Cell Death Ligand-1 (PDL-1) Correlates With Tumor Infiltration by Immune Cells and Represents a Promising Target for Immunotherapy in Endometrial Cancer. Anticancer Res. 2022, 42, 1367–1376. [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [CrossRef]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291-304.e6. [CrossRef]
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [CrossRef]
- Lindblad, P.; Wolk, A.; Bergström, R.; Persson, I.; Adami, H.O. The role of obesity and weight fluctuations in the etiology of renal cell cancer: a population-based case-control study. Cancer Epidemiol. Biomarkers Prev. 1994, 3, 631–639.
- Venkatesh, N.; Martini, A.; McQuade, J.L.; Msaouel, P.; Hahn, A.W. Obesity and renal cell carcinoma: Biological mechanisms and perspectives. Semin. Cancer Biol. 2023, 94, 21–33. [CrossRef]
- Urbute, A.; Frederiksen, K.; Thomsen, L.T.; Kesmodel, U.S.; Kjaer, S.K. Overweight and obesity as risk factors for cervical cancer and detection of precancers among screened women: A nationwide, population-based cohort study. Gynecol. Oncol. 2024, 181, 20–27. [CrossRef]
- Klapdor, R.; Hillemanns, P.; Woelber, L.L.; Jueckstock, J.K.; Hilpert, F.; Gregorio, N. de; Hasenburg, A.; Sehouli, J.; Ignatov, A.; Fuerst, S.; et al. The influence of obesity on tumor recurrence in vulvar cancer patients. JCO 2019, 37, e17130-e17130. [CrossRef]
- Lindemann, K.; Vatten, L.J.; Ellstrøm-Engh, M.; Eskild, A. Body mass, diabetes and smoking, and endometrial cancer risk: a follow-up study. Br. J. Cancer 2008, 98, 1582–1585. [CrossRef]
- Kölbl, H.; Bartl, T. Obesity in Gynecologic Oncology. Geburtshilfe Frauenheilkd. 2020, 80, 1205–1211. [CrossRef]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [CrossRef]
- Fan, W.-L.; Yeh, Y.-M.; Liu, T.-T.; Lin, W.-M.; Yang, T.-Y.; Lee, C.-W.; Lin, T.-C. Leptin Is Associated with Poor Clinical Outcomes and Promotes Clear Cell Renal Cell Carcinoma Progression. Biomolecules 2021, 11. [CrossRef]
- Horiguchi, A.; Sumitomo, M.; Asakuma, J.; Asano, T.; Zheng, R.; Asano, T.; Nanus, D.M.; Hayakawa, M. Increased serum leptin levels and over expression of leptin receptors are associated with the invasion and progression of renal cell carcinoma. J. Urol. 2006, 176, 1631–1635. [CrossRef]
- Perumal, K.; Mun, K.S.; Yap, N.Y.; Razack, A.H.A.; Gobe, G.C.; Ong, T.A.; Kuppusamy, S.; Rajandram, R. A Study on the Immunohistochemical Expressions of Leptin and Leptin Receptor in Clear Cell Renal Cell Carcinoma. Biomed Res. Int. 2020, 2020, 3682086. [CrossRef]
- Lowrance, W.T.; Thompson, R.H.; Yee, D.S.; Kaag, M.; Donat, S.M.; Russo, P. Obesity is associated with a higher risk of clear-cell renal cell carcinoma than with other histologies. BJU Int. 2010, 105, 16–20. [CrossRef]
- Callahan, C.L.; Hofmann, J.N.; Corley, D.A.; Zhao, W.K.; Shuch, B.; Chow, W.-H.; Purdue, M.P. Obesity and renal cell carcinoma risk by histologic subtype: A nested case-control study and meta-analysis. Cancer Epidemiol. 2018, 56, 31–37. [CrossRef]
- Zhang, Y.; Liu, L.; Li, C.; Ai, H. Correlation analysis between the expressions of leptin and its receptor (ObR) and clinicopathology in endometrial cancer. Cancer Biomark. 2014, 14, 353–359. [CrossRef]
- Boroń, D.; Nowakowski, R.; Grabarek, B.O.; Zmarzły, N.; Opławski, M. Expression Pattern of Leptin and Its Receptors in Endometrioid Endometrial Cancer. J. Clin. Med. 2021, 10. [CrossRef]
- Koshiba, H.; Kitawaki, J.; Ishihara, H.; Kado, N.; Kusuki, I.; Tsukamoto, K.; Honjo, H. Progesterone inhibition of functional leptin receptor mRNA expression in human endometrium. Mol. Hum. Reprod. 2001, 7, 567–572. [CrossRef]
- Nagel, G.; Concin, H.; Bjørge, T.; Rapp, K.; Manjer, J.; Hallmans, G.; Diem, G.; Häggström, C.; Engeland, A.; Almquist, M.; et al. Metabolic syndrome and rare gynecological cancers in the metabolic syndrome and cancer project (Me-Can). Ann. Oncol. 2011, 22, 1339–1345. [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [CrossRef]
- Yuan, Y.; Zhang, J.; Cai, L.; Ding, C.; Wang, X.; Chen, H.; Wang, X.; Yan, J.; Lu, J. Leptin induces cell proliferation and reduces cell apoptosis by activating c-myc in cervical cancer. Oncol. Rep. 2013, 29, 2291–2296. [CrossRef]
- Rasmussen, M.S.; Lihn, A.S.; Pedersen, S.B.; Bruun, J.M.; Rasmussen, M.; Richelsen, B. Adiponectin receptors in human adipose tissue: effects of obesity, weight loss, and fat depots. Obesity (Silver Spring) 2006, 14, 28–35. [CrossRef]
- Wang, H.; Wu, J.; Gu, W.; Wang, B.; Wan, F.; Dai, B.; Zhang, H.; Shi, G.; Shen, Y.; Zhu, Y.; et al. Serum Adiponectin Level May be an Independent Predictor of Clear Cell Renal Cell Carcinoma. J. Cancer 2016, 7, 1340–1346. [CrossRef]
- Kleinmann, N.; Duivenvoorden, W.C.M.; Hopmans, S.N.; Beatty, L.K.; Qiao, S.; Gallino, D.; Lhotak, S.; Daya, D.; Paschos, A.; Austin, R.C.; et al. Underactivation of the adiponectin-adiponectin receptor 1 axis in clear cell renal cell carcinoma: implications for progression. Clin. Exp. Metastasis 2014, 31, 169–183. [CrossRef]
- Pinthus, J.H.; Kleinmann, N.; Tisdale, B.; Chatterjee, S.; Lu, J.-P.; Gillis, A.; Hamlet, T.; Singh, G.; Farrokhyar, F.; Kapoor, A. Lower plasma adiponectin levels are associated with larger tumor size and metastasis in clear-cell carcinoma of the kidney. Eur. Urol. 2008, 54, 866–873. [CrossRef]
- Ito, R.; Narita, S.; Huang, M.; Nara, T.; Numakura, K.; Takayama, K.; Tsuruta, H.; Maeno, A.; Saito, M.; Inoue, T.; et al. The impact of obesity and adiponectin signaling in patients with renal cell carcinoma: A potential mechanism for the "obesity paradox". PLoS One 2017, 12, e0171615. [CrossRef]
- Gandhi, R.; Sharma, A.; Kapoor, M.; Sundararajan, K.; Perruccio, A.V. Racial Differences in Serum Adipokine and Insulin Levels in a Matched Osteoarthritis Sample: A Pilot Study. J. Obes. 2016, 2016, 8746268. [CrossRef]
- Gavrila, A.; Peng, C.-K.; Chan, J.L.; Mietus, J.E.; Goldberger, A.L.; Mantzoros, C.S. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J. Clin. Endocrinol. Metab. 2003, 88, 2838–2843. [CrossRef]
- Spyridopoulos, T.N.; Petridou, E.T.; Skalkidou, A.; Dessypris, N.; Chrousos, G.P.; Mantzoros, C.S. Low adiponectin levels are associated with renal cell carcinoma: a case-control study. Int. J. Cancer 2007, 120, 1573–1578. [CrossRef]
- Petridou, E.; Mantzoros, C.; Dessypris, N.; Koukoulomatis, P.; Addy, C.; Voulgaris, Z.; Chrousos, G.; Trichopoulos, D. Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J. Clin. Endocrinol. Metab. 2003, 88, 993–997. [CrossRef]
- Xie, L.; Wang, Y.; Wang, S.; Wu, N.; Chen, Y.; Yan, J. Adiponectin induces growth inhibition and apoptosis in cervical cancer HeLa cells. Biologia 2011, 66, 712–720. [CrossRef]
- Chou, S.H.; Tseleni-Balafouta, S.; Moon, H.-S.; Chamberland, J.P.; Liu, X.; Kavantzas, N.; Mantzoros, C.S. Adiponectin receptor expression in human malignant tissues. Horm. Cancer 2010, 1, 136–145. [CrossRef]
- Modesitt, S.C.; van Nagell, J.R. The impact of obesity on the incidence and treatment of gynecologic cancers: a review. Obstet. Gynecol. Surv. 2005, 60, 683–692. [CrossRef]
- Moon, H.-S.; Chamberland, J.P.; Aronis, K.; Tseleni-Balafouta, S.; Mantzoros, C.S. Direct role of adiponectin and adiponectin receptors in endometrial cancer: in vitro and ex vivo studies in humans. Mol. Cancer Ther. 2011, 10, 2234–2243. [CrossRef]
- Yamauchi, N.; Takazawa, Y.; Maeda, D.; Hibiya, T.; Tanaka, M.; Iwabu, M.; Okada-Iwabu, M.; Yamauchi, T.; Kadowaki, T.; Fukayama, M. Expression levels of adiponectin receptors are decreased in human endometrial adenocarcinoma tissues. Int. J. Gynecol. Pathol. 2012, 31, 352–357. [CrossRef]
| Proteins | Staining Intensity | N (Low/High) | Confounders | Hazard Ratio | 95% CI | p-Value (cox) | Likelihood-Ratio-Test | |
|---|---|---|---|---|---|---|---|---|
| RCC | LeptinR | 0–1+/2–3+ | 73/133 | age, BMI, pT, subtype, grade | 0.62 | 0.40 - 0.96 | 0.03 | <0.001 |
| AdipoR1 | 0–1+/2–3+ | 133/67 | age, BMI, pT, subtype, grade | 1.02 | 0.63 - 1.63 | 0.95 | <0.001 | |
| ccRCC | LeptinR | 0–1+/2–3+ | 65/95 | age, BMI, pT, grade | 0.64 | 0.40 - 1.01 | 0.06 | <0.001 |
| AdipoR1 | 0–1+/2–3+ | 116/41 | age, BMI, pT, grade | 0.97 | 0.56 - 1.67 | 0.92 | <0.001 | |
| Cervical cancer | LeptinR | 0–1+/2–3+ | 69/57 | age, BMI, pT, grade | 1.05 | 0.49 - 2.28 | 0.90 | <0.001 |
| AdipoR1 | 0–1+/2–3+ | 73/48 | age, BMI, pT, grade | 0.93 | 0.42 - 2.05 | 0.85 | <0.001 | |
| Vulvar cancer | LeptinR | 0–1+/2–3+ | 42/47 | age, BMI, pT, grade | 0.37 | 0.18 - 0.79 | 0.01 | 0.02 |
| AdipoR1 | 0–1+/2–3+ | 55/36 | age, BMI, pT, grade | 1.13 | 0.52 - 2.45 | 0.76 | 0.6 | |
| Endometrial cancer | LeptinR | 0–1+/2–3+ | 15/67 | age, BMI, pT, grade | 1.52 | 0.36 - 6.39 | 0.56 | 0.004 |
| AdipoR1 | 0–1+/2–3+ | 40/42 | age, BMI, pT, grade | 1.42 | 0.54 - 3.73 | 0.48 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





