Submitted:
24 July 2024
Posted:
25 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
The Genetic and Molecular Origins of Periodontitis
Periodontitis and Systemic Health
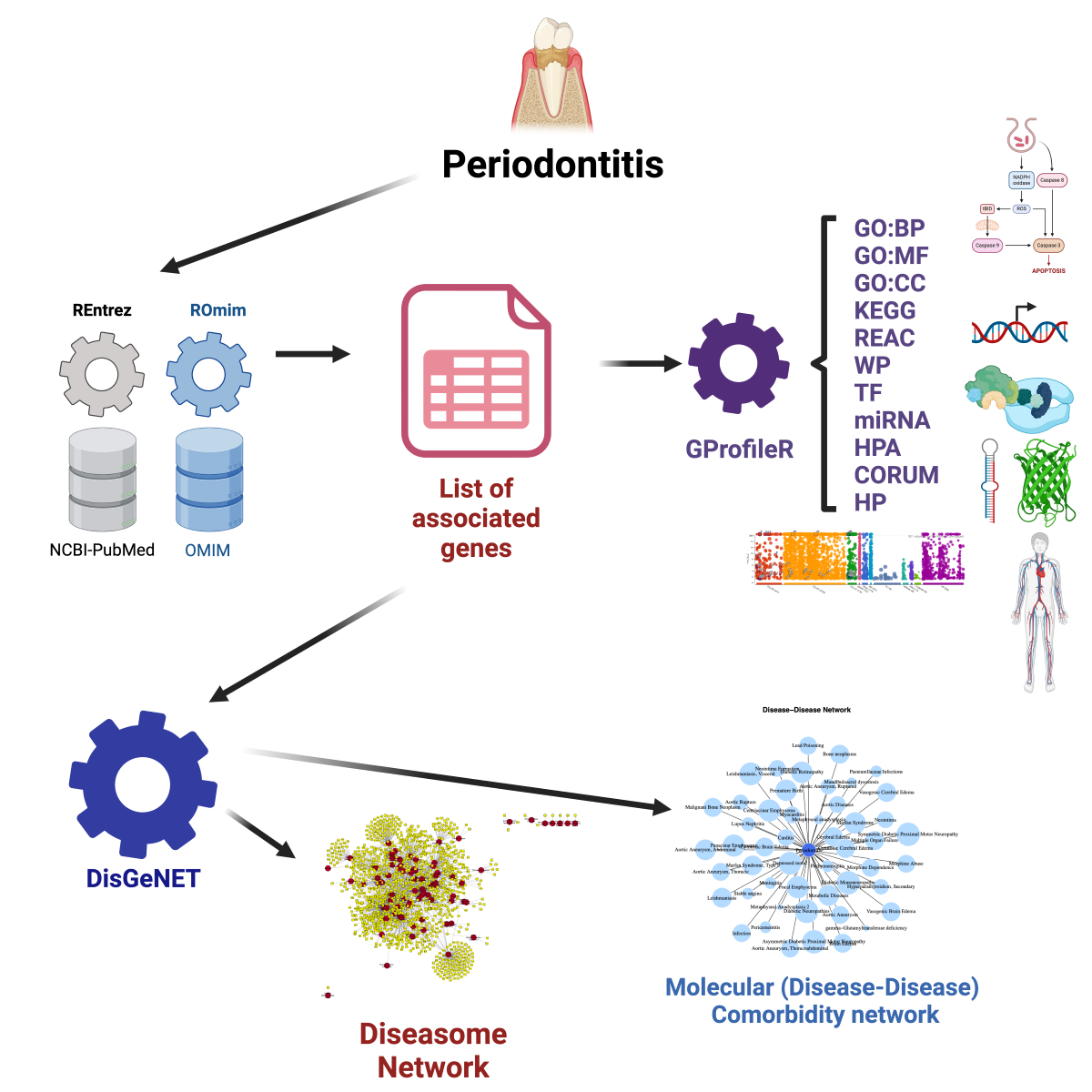
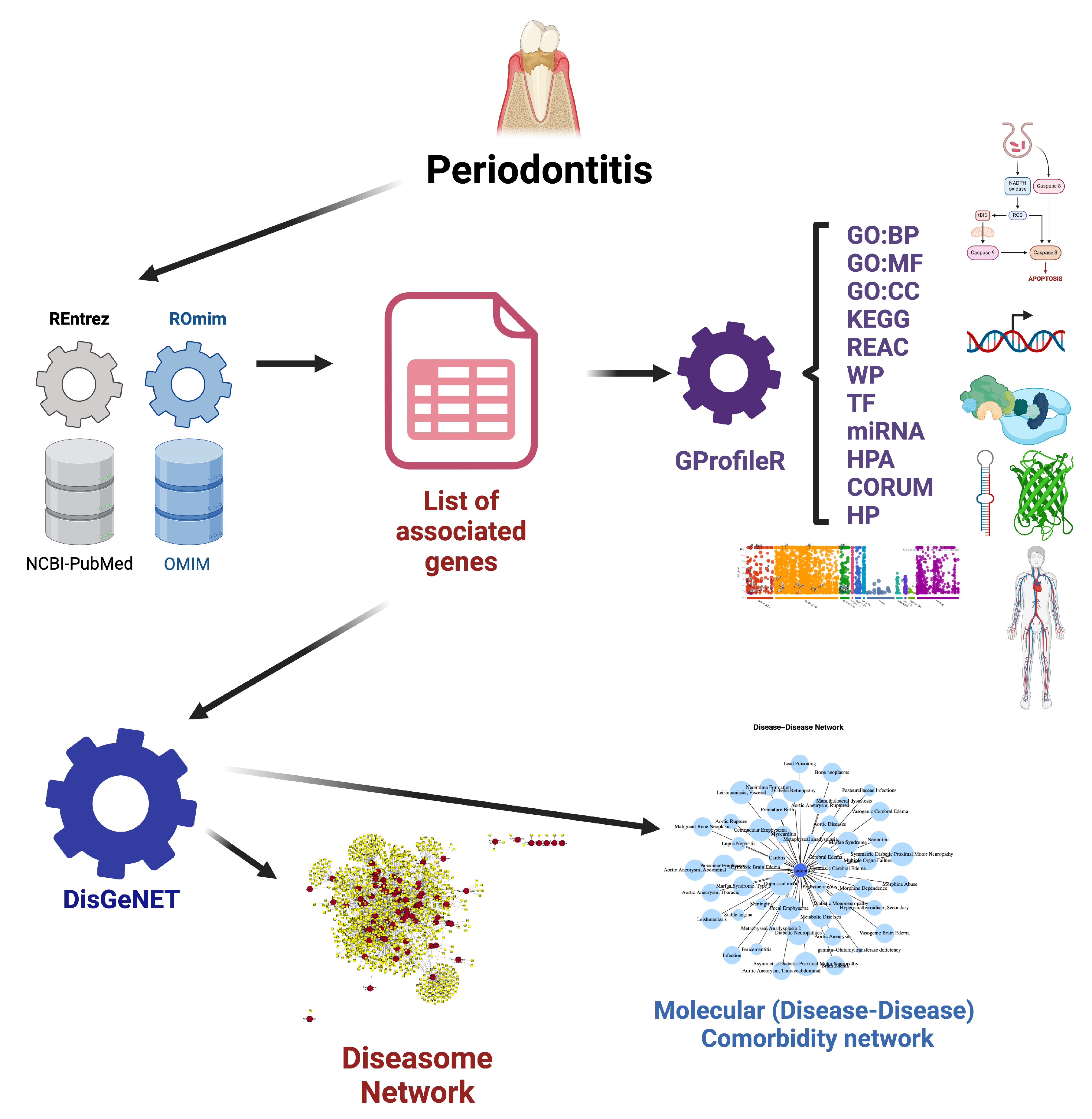
Materials and Methods
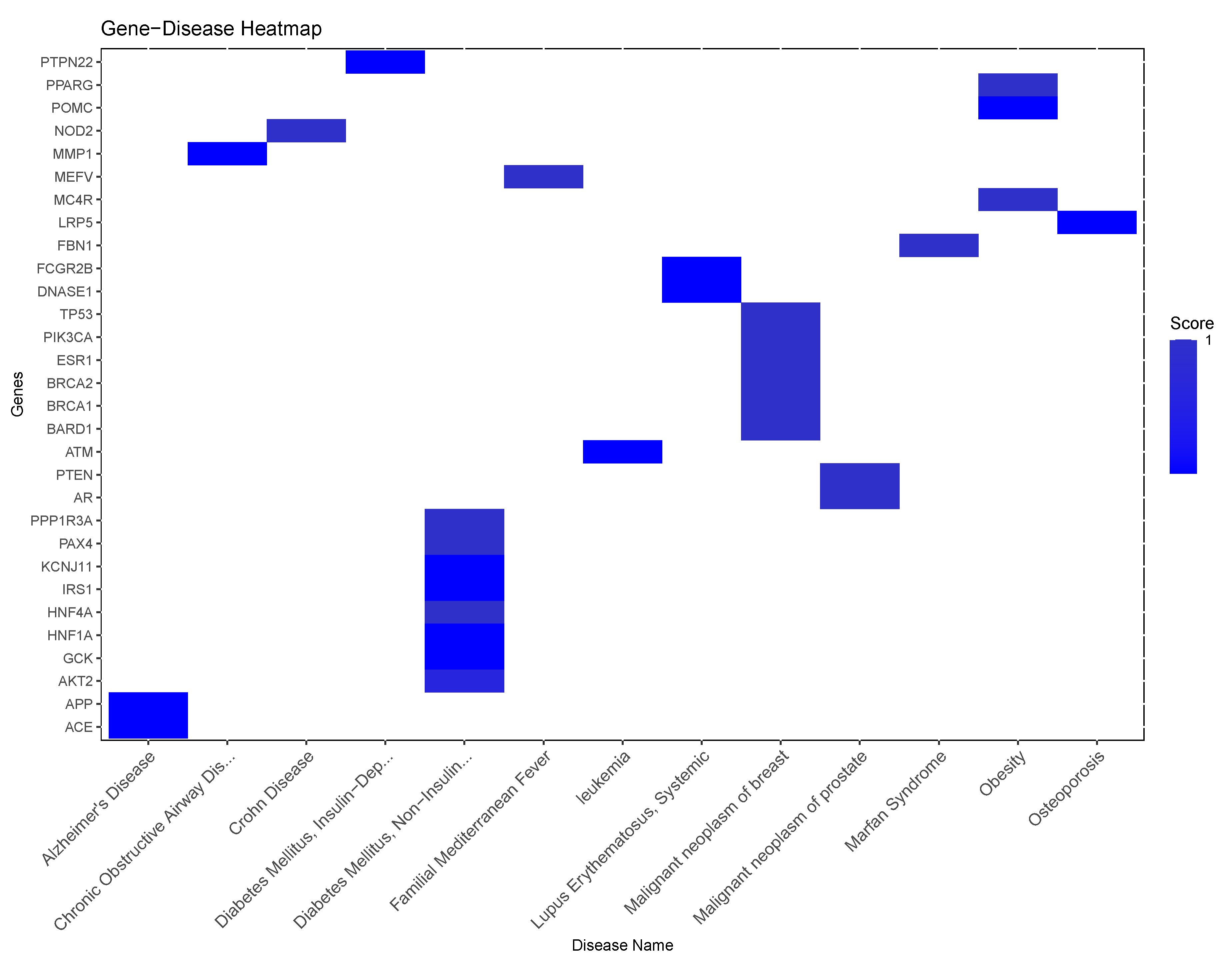
Molecular Association Analysis
Molecular Comorbidity and Diseasome Networks
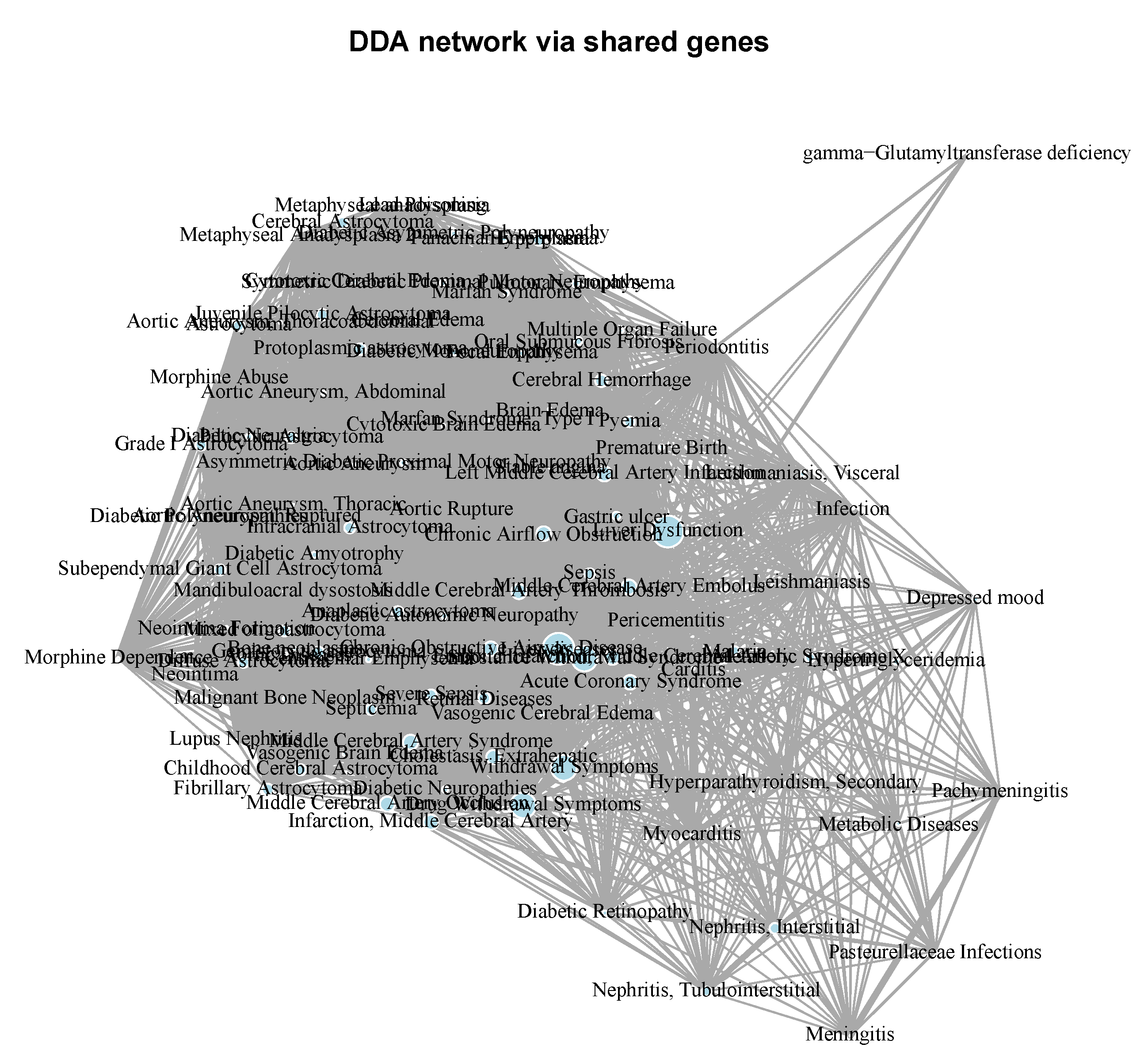
Molecular Comorbidity Network Reconstruction
Diseasome Network Reconstruction
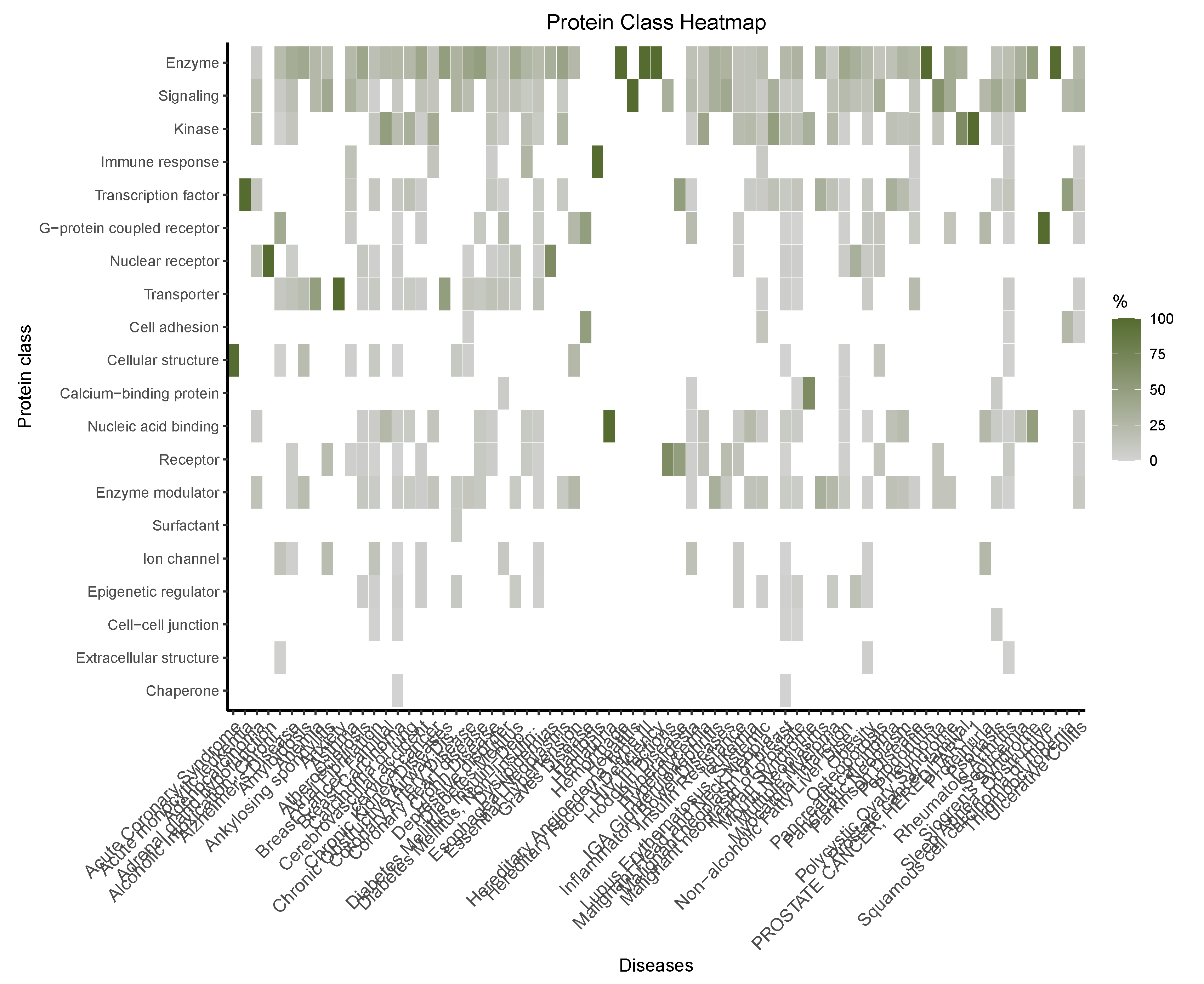
Functional Enrichment Analysis
Results
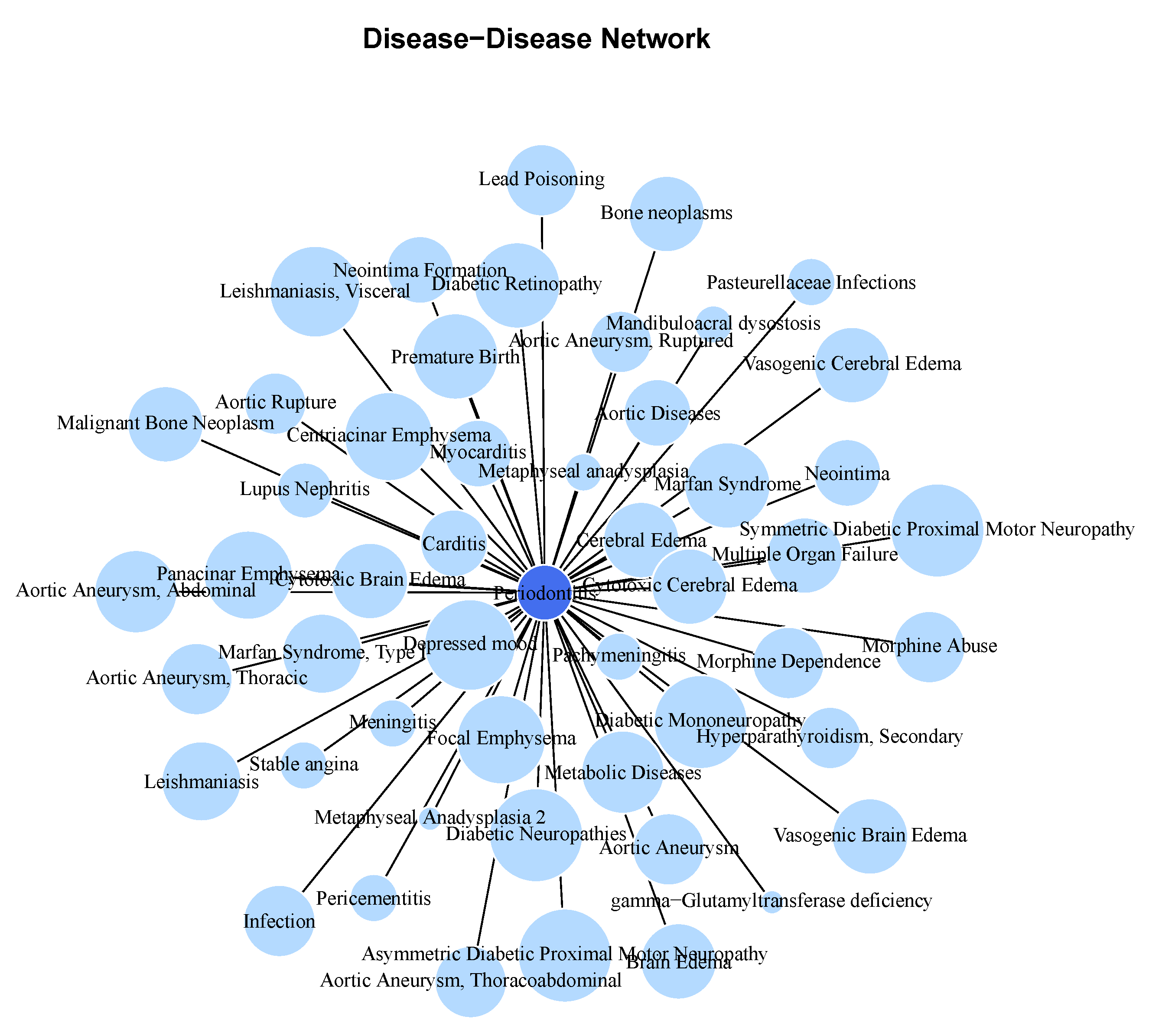
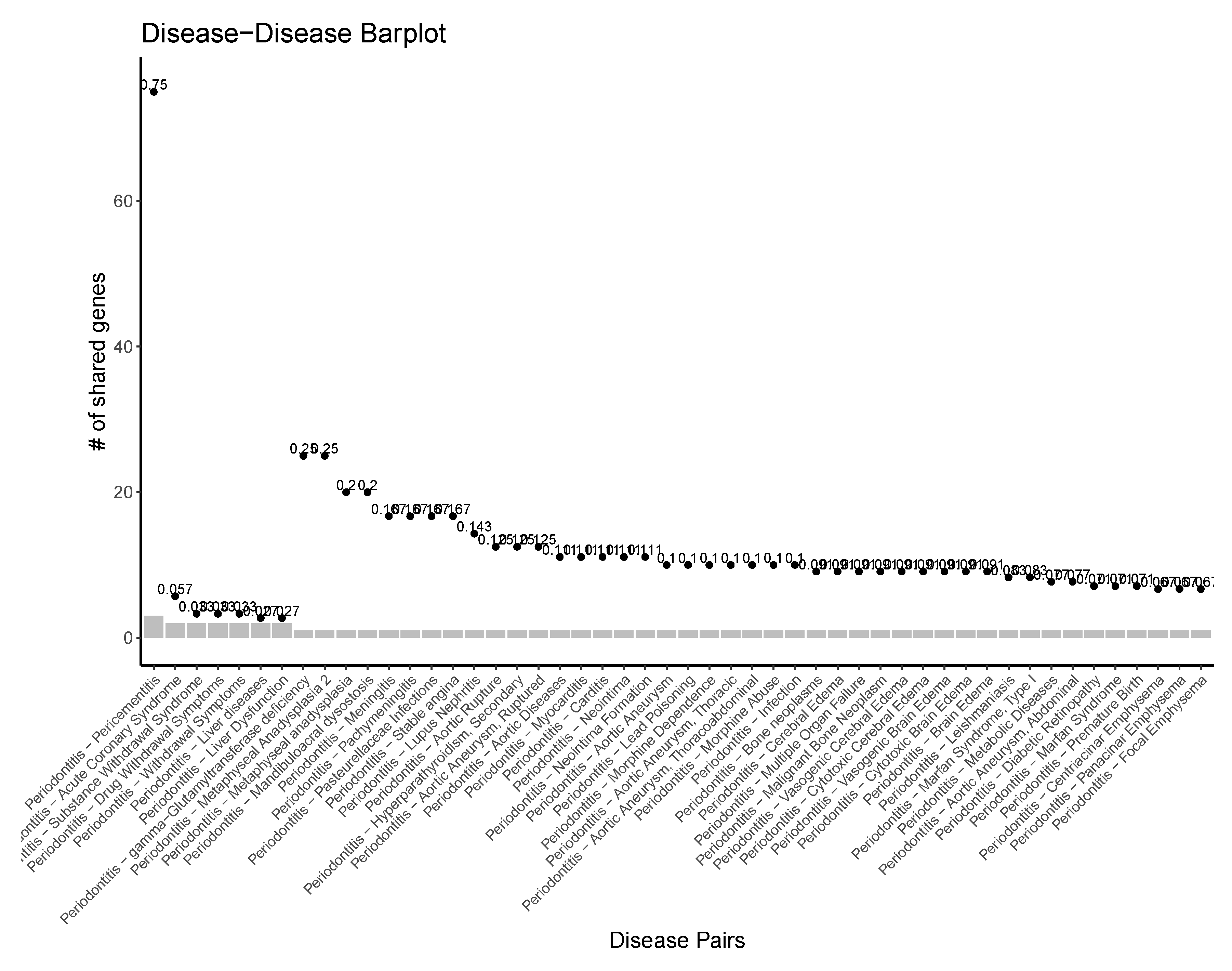
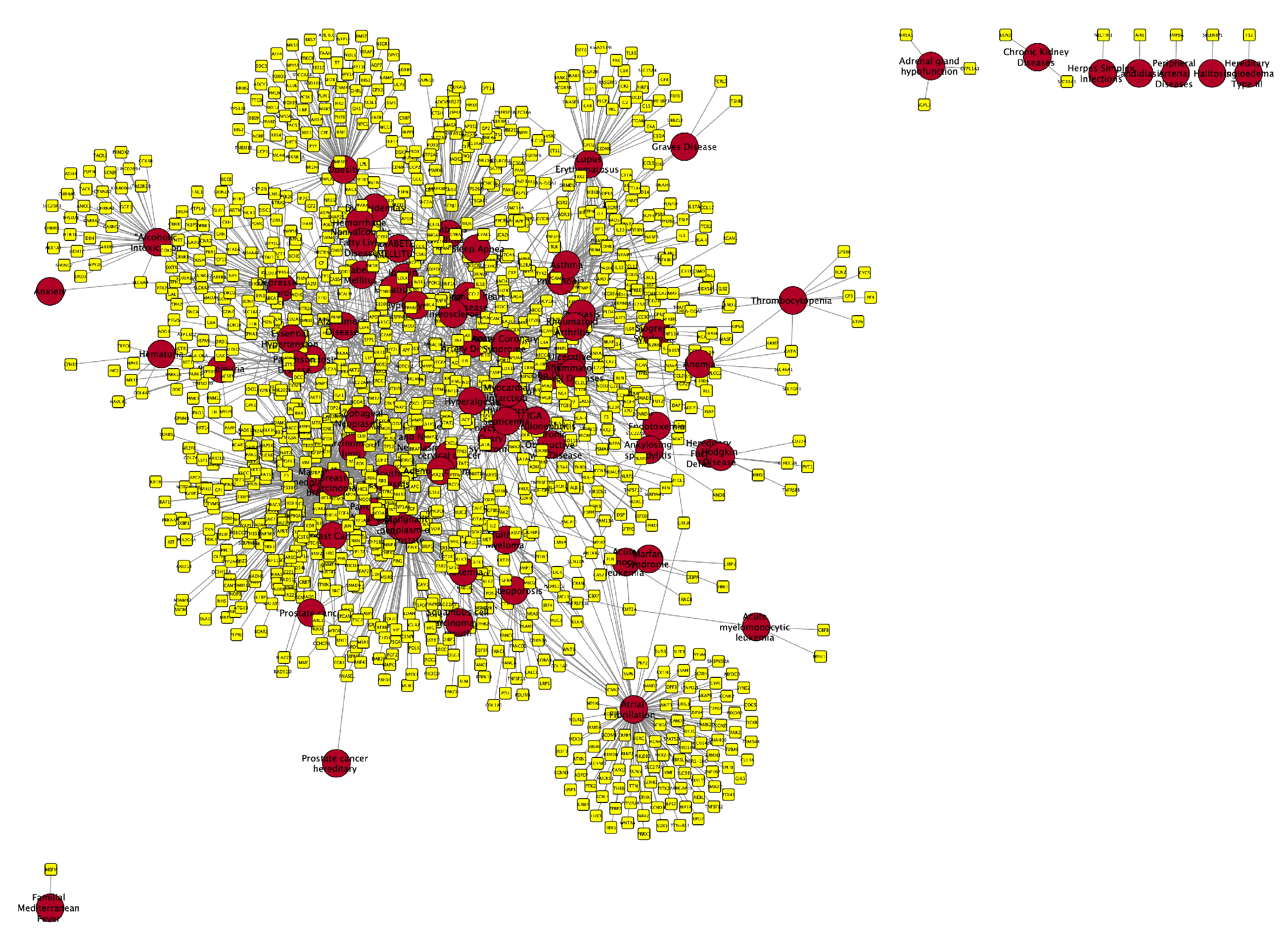
Molecular Comorbidity Network
Periodontitis Centered Disease Network
A Diseasome of Periodontitis Related Conditions
- Cardiometabolic Diseases: Diseases like Acute coronary syndrome, Atrial fibrillation, Coronary artery disease, and Coronary heart disease are all cardiovascular conditions that share a common underlying feature of vascular dysfunction or cardiac abnormalities. The presence of shared genes between these diseases (say, Myocardial infarction, Atrial fibrillation, Cerebrovascular accident, or Coronary artery disease) and periodontitis suggests potential molecular links related to inflammation, endothelial dysfunction, or immune dysregulation, which are known to contribute to both periodontal disease and cardiovascular disorders. Other conditions related to metabolism associated with cardiovascular health and present in the molecular (gene) comorbidity space of periodontitis are: Diabetes mellitus, non-insulin-dependent, Non-alcoholic fatty liver disease and Dyslipidemias, as well as Essential hypertension and Peripheral arterial diseases.
- Inflammatory Disorders and Immunological Diseases: Conditions such as Rheumatoid arthritis, Crohn’s disease, Chronic obstructive airway disease, and Asthma are all characterized by chronic inflammation and immune dysregulation. These diseases share overlapping inflammatory pathways with periodontitis, indicating potential common mechanisms contributing to their co-occurrence. The shared genes between these inflammatory disorders and periodontitis suggest that immune-mediated processes may play a critical role in the interplay between oral health and systemic inflammation as exemplified for instance, in Sjogren’s Syndrome, Rheumatoid arthritis and Lupus erythematosus systemic, but also in Psoriasis, Glomerulonephritis, and Graves disease .
- Cancer: Several types of cancer are represented in the list, including Breast cancer, Cervical cancer, Carcinoma of lung, and Adenocarcinoma. Although cancer types vary in their tissue origins and molecular profiles, they all involve dysregulated cell growth and proliferation. The presence of shared genes between these cancers and periodontitis may reflect shared molecular pathways related to cell cycle regulation, tumor progression, or immune evasion, highlighting potential links between periodontal health and cancer development such is the case of Breast carcinoma, Pancreatic Neoplasm, and Cervical cancer, Prostate cancer, as well as Multiple myeloma, Hodgkin disease, and some types of Leukemia.
- Neurological and Psychiatric Disorders: Diseases like Alzheimer’s disease and Depressive disorder represent neurological and psychiatric conditions that have been associated with chronic inflammation and immune activation. The shared genes between these disorders and periodontitis suggest potential bidirectional relationships, where systemic inflammation may contribute to neuroinflammation and cognitive decline, while neurological conditions may influence systemic inflammatory responses and periodontal health.
- Other Diseases: There are other less-general conditions such as Pneumonia, Osteoporosis, Amyloidosis, aside from hormonal diseases like Polycystic ovary syndrome, Adrenal gland hypofunction, Hereditary angioedema type III, as well as Chronic kidney diseases and connective tissue diseases like Marfan syndrome that also share relevant biomolecular players with Periodontitis.
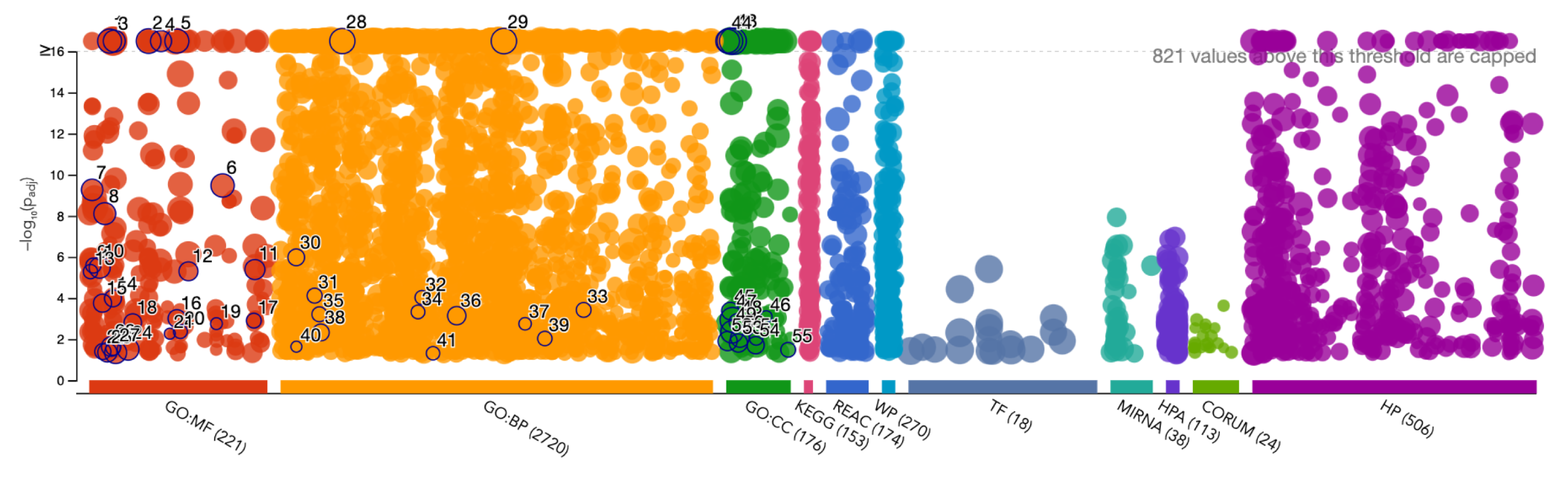
Functional Enrichment of the Diseasome Genes
Discussion
Cardiometabolic Diseases
Inflammatory and Immunological Disorders
Cancer
Neurological and Psychiatric Diseases
Respiratory Diseases
Musculoskeletal and connective tissue diseases
Hormonal Diseases
Kidney Diseases
Some Open-Ended Avenues for Future Inquiry
Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Martínez-García, M.; Hernández-Lemus, E. Periodontal inflammation and systemic diseases: an overview. Frontiers in physiology 2021, 12, 709438. [Google Scholar] [CrossRef] [PubMed]
- Bui, F.Q.; Almeida-da Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomedical journal 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- de Molon, R.S.; Rossa, C., Jr.; Thurlings, R.M.; Cirelli, J.A.; Koenders, M.I. Linkage of periodontitis and rheumatoid arthritis: current evidence and potential biological interactions. International journal of molecular sciences 2019, 20, 4541. [Google Scholar] [CrossRef] [PubMed]
- Alrayyes, S.; Hart, T.C. Periodontal disease in children. Disease-a-Month 2011, 57, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.A.; Paz, H.E.d.S.; Monteiro, M.d.F.; Casati, M.Z.; Steiner-Oliveira, C.; Pascon, F.M.; Casarin, R.C.V. Early Manifestation of Periodontal Disease in Children and Its Association with Familial Aggregation. Journal of Dentistry for Children 2021, 88, 140–143. [Google Scholar] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nature Reviews Disease Primers 2017, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Graves, D. Cytokines that promote periodontal tissue destruction. Journal of periodontology 2008, 79, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Balta, M.G.; Loos, B.G.; Nicu, E.A. Emerging concepts in the resolution of periodontal inflammation: a role for resolvin E1. Frontiers in immunology 2017, 8, 1682. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, D.E.; Hariyani, N.; Indrawati, R.; Ridwan, R.D.; Diyatri, I. Cytokines and chemokines in periodontitis. European Journal of Dentistry 2020, 14, 483. [Google Scholar] [CrossRef] [PubMed]
- Cecoro, G.; Annunziata, M.; Iuorio, M.T.; Nastri, L.; Guida, L. Periodontitis, Low-Grade Inflammation and Systemic Health: A Scoping Review. Medicina 2020, 56, 272. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.M.; Morford, L.A.; Nibali, L. Periodontal health and disease: The contribution of genetics. Periodontology 2000 2021, 85, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Haworth, S.; Kho, P.F.; Holgerson, P.L.; Hwang, L.D.; Timpson, N.J.; Rentería, M.E.; Johansson, I.; Cuellar-Partida, G. Assessment and visualization of phenome-wide causal relationships using genetic data: an application to dental caries and periodontitis. European Journal of Human Genetics 2021, 29, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Laine, M.L.; Crielaard, W.; Loos, B.G. Genetic susceptibility to periodontitis. Periodontology 2000 2012, 58, 37–68. [Google Scholar] [CrossRef] [PubMed]
- Michalowicz, B.S.; Diehl, S.R.; Gunsolley, J.C.; Sparks, B.S.; Brooks, C.N.; Koertge, T.E.; Califano, J.V.; Burmeister, J.A.; Schenkein, H.A. Evidence of a substantial genetic basis for risk of adult periodontitis. Journal of periodontology 2000, 71, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Shungin, D.; Haworth, S.; Divaris, K.; Agler, C.S.; Kamatani, Y.; Lee, M.K.; Grinde, K.; Hindy, G.; Alaraudanjoki, V.; Pesonen, P.; others. Genome-wide analysis of dental caries and periodontitis combining clinical and self-reported data. Nature communications 2019, 10, 1–13. [CrossRef]
- da Silva, M.K.; de Carvalho, A.C.G.; Alves, E.H.P.; da Silva, F.R.P.; Pessoa, L.d.S.; Vasconcelos, D.F.P. Genetic factors and the risk of periodontitis development: findings from a systematic review composed of 13 studies of meta-analysis with 71,531 participants. International journal of dentistry 2017, 2017.
- Aarabi, G.; Zeller, T.; Seedorf, H.; Reissmann, D.; Heydecke, G.; Schaefer, A.; Seedorf, U. Genetic susceptibility contributing to periodontal and cardiovascular disease. Journal of dental research 2017, 96, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G.; Van Dyke, T.E. The role of inflammation and genetics in periodontal disease. Periodontology 2000 2020, 83, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Küchler, E.C.; Hannegraf, N.D.; Lara, R.M.; Reis, C.L.B.; de Oliveira, D.S.B.; Mazzi-Chaves, J.F.; Andrades, K.M.R.; de Lima, L.F.; Salles, A.G.; Antunes, L.A.A.; others. Investigation of Genetic Polymorphisms in BMP2, BMP4, SMAD6, and RUNX2 and Persistent Apical Periodontitis. Journal of Endodontics 2021, 47, 278–285.
- Mezzavilla, M.; Navarra, C.O.; Di Lenarda, R.; Gasparini, P.; Bevilacqua, L.; Robino, A. Runs of homozygosity are associated with staging of periodontitis in isolated populations. Human Molecular Genetics 2021, 30, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Ari, G.; Cherukuri, S.; Namasivayam, A. Epigenetics and periodontitis: a contemporary review. Journal of clinical and diagnostic research: JCDR 2016, 10, ZE07. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.; Mullersman, A.; Huang, H.; Wallet, S.; Langaee, T.; Aukhil, I. Epigenetic regulation of inflammation in localized aggressive periodontitis. Clinical epigenetics 2017, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Bunte, K.; Beikler, T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. International journal of molecular sciences 2019, 20, 3394. [Google Scholar] [CrossRef]
- Kavrikova, D.; Borilova Linhartova, P.; Lucanova, S.; Poskerova, H.; Fassmann, A.; Izakovicova Holla, L. Chemokine Receptor 2 (CXCR2) Gene Variants and Their Association with Periodontal Bacteria in Patients with Chronic Periodontitis. Mediators of Inflammation 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Khumaedi, A.I.; Purnamasari, D.; Wijaya, I.P.; Soeroso, Y. The relationship of diabetes, periodontitis and cardiovascular disease. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2019, 13, 1675–1678. [Google Scholar]
- Cardoso, E.M.; Reis, C.; Manzanares-Céspedes, M.C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgraduate medicine 2018, 130, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Pradeep, A.R.; Kanoriya, D.; Garg, V. Human soluble receptor for advanced glycation end products and tumor necrosis factor-α as gingival crevicular fluid and serum markers of inflammation in chronic periodontitis and type 2 diabetes. Journal of oral science 2016, 58, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Torrungruang, K.; Ongphiphadhanakul, B.; Jitpakdeebordin, S.; Sarujikumjornwatana, S. Mediation analysis of systemic inflammation on the association between periodontitis and glycaemic status. Journal of clinical periodontology 2018, 45, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Li, B.; Gong, Q.; Chen, X.; Wang, Q. Co-culture of bone marrow stem cells and macrophages indicates intermediate mechanism between local inflammation and innate immune system in diabetic periodontitis. Experimental and Therapeutic Medicine 2016, 12, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Taylor, J.J.; Jaedicke, K.M.; De Jager, M.; Bikker, J.W.; Selten, W.; Bissett, S.M.; Whall, K.M.; van de Merwe, R.; Areibi, A.; others. Treatment of periodontitis reduces systemic inflammation in type 2 diabetes. Journal of Clinical Periodontology 2020, 47, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.S.; Kim, S.; Boström, K.I.; Wang, C.Y.; Kim, R.H.; Park, N.H. Periodontitis-induced systemic inflammation exacerbates atherosclerosis partly via endothelial–mesenchymal transition in mice. International journal of oral science 2019, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schenkein, H.A.; Papapanou, P.N.; Genco, R.; Sanz, M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontology 2000 2020, 83, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Leira, Y.; Rodríguez-Yáñez, M.; Arias, S.; López-Dequidt, I.; Campos, F.; Sobrino, T.; D’Aiuto, F.; Castillo, J.; Blanco, J. Periodontitis is associated with systemic inflammation and vascular endothelial dysfunction in patients with lacunar infarct. Journal of periodontology 2019, 90, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart, Lung and Circulation 2018, 27, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A.; Hasturk, H.; Kantarci, A.; Serhan, C.N.; Van Dyke, T. Atherosclerosis, periodontal disease, and treatment with resolvins. Current atherosclerosis reports 2017, 19, 57. [Google Scholar] [CrossRef]
- Furutama, D.; Matsuda, S.; Yamawaki, Y.; Hatano, S.; Okanobu, A.; Memida, T.; Oue, H.; Fujita, T.; Ouhara, K.; Kajiya, M.; others. IL-6 Induced by Periodontal Inflammation Causes Neuroinflammation and Disrupts the Blood–Brain Barrier. Brain sciences 2020, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: a two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Papapanou, P.N. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nature Reviews Endocrinology 2011, 7, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Hu, B.; Sun, J.; Yang, Z.; Yu, W.; He, Z.; Gao, X.; Song, J. Identification of cross-talk pathways and ferroptosis-related genes in periodontitis and type 2 diabetes mellitus by bioinformatics analysis and experimental validation. Frontiers in immunology 2022, 13, 1015491. [Google Scholar] [CrossRef] [PubMed]
- Leite, R.S.; Marlow, N.M.; Fernandes, J.K.; Hermayer, K. Oral health and type 2 diabetes. The American journal of the medical sciences 2013, 345, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Agrafioti, P.; Morin-Baxter, J.; Tanagala, K.K.; Dubey, S.; Sims, P.; Lalla, E.; Momen-Heravi, F. Decoding the role of macrophages in periodontitis and type 2 diabetes using single-cell RNA-sequencing. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 2022, 36, e22136. [Google Scholar] [CrossRef] [PubMed]
- Almubarak, A.; Tanagala, K.K.K.; Papapanou, P.N.; Lalla, E.; Momen-Heravi, F. Disruption of monocyte and macrophage homeostasis in periodontitis. Frontiers in immunology 2020, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.N.d.A.; Casarin, M.; Monajemzadeh, S.; Bezerra, B.d.B.; Lux, R.; Pirih, F.Q. The microbiome in periodontitis and diabetes. Frontiers in oral health 2022, 3, 859209. [Google Scholar] [CrossRef] [PubMed]
- Xiao, E.; Mattos, M.; Vieira, G.H.A.; Chen, S.; Corrêa, J.D.; Wu, Y.; Albiero, M.L.; Bittinger, K.; Graves, D.T. Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell host & microbe 2017, 22, 120–128. [Google Scholar]
- Lu, C.; Zhao, Q.; Deng, J.; Chen, K.; Jiang, X.; Ma, F.; Ma, S.; Li, Z. Salivary microbiome profile of diabetes and periodontitis in a Chinese population. Frontiers in Cellular and Infection Microbiology 2022, 12, 933833. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.z.; Yuan, Y.h.; Liu, H.h.; Li, S.s.; Zhang, B.w.; Chen, W.; An, Z.j.; Chen, S.y.; Wu, Y.z.; Han, B.; others. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Bitencourt, F.; Nascimento, G.; Costa, S.; Andersen, A.; Sandbæk, A.; Leite, F. Co-occurrence of periodontitis and diabetes-related complications. Journal of Dental Research 2023, 102, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Kocher, T.; König, J.; Borgnakke, W.S.; Pink, C.; Meisel, P. Periodontal complications of hyperglycemia/diabetes mellitus: epidemiologic complexity and clinical challenge. Periodontology 2000 2018, 78, 59–97. [Google Scholar] [CrossRef] [PubMed]
- Gurav, A.N. Periodontitis and insulin resistance: casual or causal relationship? Diabetes & metabolism journal 2012, 36, 404–411. [Google Scholar]
- Bains, V.K.; Mahendra, J.; Mahendra, L.; Mittal, M.; Valli, G. Markers, pathways, and current evidence for periodontitis-associated insulin resistance: a narrative review. Journal of International Society of Preventive and Community Dentistry 2022, 12, 475–487. [Google Scholar] [PubMed]
- SKM, A.I.; Seo, M.; Lee, Y.S.; Moon, S.S. Association of periodontitis with insulin resistance, β-cell function, and impaired fasting glucose before onset of diabetes. Endocrine journal 2015, 62, 981–989. [Google Scholar]
- Zhao, P.; Xu, A.; Leung, W.K. Obesity, bone loss, and periodontitis: the interlink. Biomolecules 2022, 12, 865. [Google Scholar] [CrossRef] [PubMed]
- Arboleda, S.; Vargas, M.; Losada, S.; Pinto, A. Review of obesity and periodontitis: an epidemiological view. British dental journal 2019, 227, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.M.; Lee, S.; Hwang, W.; Son, E.; Kim, T.W.; Kim, K.; Kim, Y.H. Obesity and periodontitis: A systematic review and updated meta-analysis. Frontiers in Endocrinology 2022, 13, 999455. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, L.L.C.; Nascimento, G.G.; Leite, F.R.M.; Alves-Costa, S.; Barbosa, J.M.A.; Alves, C.M.C.; Thomaz, E.B.A.F.; Batista, R.F.L.; Ribeiro, C.C.C. Obesity, insulin resistance, caries, and periodontitis: syndemic framework. Nutrients 2023, 15, 3512. [Google Scholar] [CrossRef] [PubMed]
- Moura-Grec, P.G.d.; Marsicano, J.A.; Carvalho, C.A.P.d.; Sales-Peres, S.H.d.C. Obesity and periodontitis: systematic review and meta-analysis. Ciencia & saude coletiva 2014, 19, 1763–1772. [Google Scholar]
- Gomes-Filho, I.S.; Oliveira, M.T.; Cruz, S.S.d.; Cerqueira, E.d.M.M.; Trindade, S.C.; Vieira, G.O.; Couto Souza, P.H.; Adan, L.F.F.; Hintz, A.M.; Passos-Soares, J.d.S.; others. Periodontitis is a factor associated with dyslipidemia. Oral diseases 2022, 28, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, F.V.; Nascimento, G.G.; Costa, S.A.; Orrico, S.R.P.; Ribeiro, C.C.C.; Leite, F.R.M. The role of dyslipidemia in periodontitis. Nutrients 2023, 15, 300. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, W.; Liu, X.; Zhang, W.; Li, Y. Interrelationship between diabetes and periodontitis: role of hyperlipidemia. Archives of oral biology 2015, 60, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, S.S.; Al-Windy, S.A.; Al-Nami, M.S.; Al Kuraishy, H.M.; Al Gareeb, A.I. Statins improve periodontal disease–induced inflammatory changes and associated lipid peroxidation in patients with dyslipidemia: Two birds by one stone. Journal of International Oral Health 2020, 12, 66–73. [Google Scholar]
- Akinkugbe, A.A.; Slade, G.D.; Barritt, A.S.; Cole, S.R.; Offenbacher, S.; Petersmann, A.; Kocher, T.; Lerch, M.M.; Mayerle, J.; Völzke, H.; others. Periodontitis and Non-alcoholic Fatty Liver Disease, a population-based cohort investigation in the Study of Health in Pomerania. Journal of clinical periodontology 2017, 44, 1077–1087.
- Hatasa, M.; Yoshida, S.; Takahashi, H.; Tanaka, K.; Kubotsu, Y.; Ohsugi, Y.; Katagiri, T.; Iwata, T.; Katagiri, S. Relationship between NAFLD and periodontal disease from the view of clinical and basic research, and immunological response. International Journal of Molecular Sciences 2021, 22, 3728. [Google Scholar] [PubMed]
- Alakhali, M.S.; Al-Maweri, S.A.; Al-Shamiri, H.M.; Al-Haddad, K.; Halboub, E. The potential association between periodontitis and non-alcoholic fatty liver disease: a systematic review. Clinical oral investigations 2018, 22, 2965–2974. [Google Scholar] [CrossRef]
- Qiao, F.; Li, X.; Liu, Y.; Zhang, S.; Liu, D.; Li, C. Periodontitis and NAFLD-related diseases: A bidirectional two-sample Mendelian randomization study. Oral Diseases 2023. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, Z.; Yao, L.; Xue, B.; Xi, H.; Wang, X.; Sun, S. Exploration of shared gene signatures and molecular mechanisms between periodontitis and nonalcoholic fatty liver disease. Frontiers in genetics 2022, 13, 939751. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Redd, K.; Trivedi, T.; Moss, K.; Alonso, A.; Soliman, E.Z.; Magnani, J.W.; Chen, L.Y.; Gottesman, R.F.; Rosamond, W.; others. Periodontal disease, atrial fibrillation and stroke. American heart journal 2021, 235, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Nishi, H.; Ouhara, K.; Tokuyama, T.; Okubo, Y.; Okamura, S.; Miyamoto, S.; Oguri, N.; Uotani, Y.; Takasaki, T.; others. Relationship between periodontitis and atrial fibrosis in atrial fibrillation: histological evaluation of left atrial appendages. Clinical Electrophysiology 2023, 9, 43–53. [CrossRef]
- Leelapatana, P.; Limpuangthip, N. Association between oral health and atrial fibrillation: A systematic review. Heliyon 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Shrihari, T. Potential correlation between periodontitis and coronary heart disease—An overview. Gen Dent 2012, 60, 20–4. [Google Scholar] [PubMed]
- Bokhari, S.A.H.; Khan, A.A.; Butt, A.K.; Hanif, M.; Izhar, M.; Tatakis, D.N.; Ashfaq, M. Periodontitis in coronary heart disease patients: strong association between bleeding on probing and systemic biomarkers. Journal of clinical periodontology 2014, 41, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, P.P.; Drangsholt, M.; Spiekerman, C.; DeRouen, T.A. Periodontal disease and coronary heart disease risk. Jama 2000, 284, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. American heart journal 2007, 154, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Nocini, R.; Favaloro, E.J.; Sanchis-Gomar, F.; Lippi, G. Periodontitis, coronary heart disease and myocardial infarction: treat one, benefit all. Blood Coagulation & Fibrinolysis 2020, 31, 339–345. [Google Scholar]
- Franek, E.; Klamczynska, E.; Ganowicz, E.; Blach, A.; Budlewski, T.; Gorska, R. Association of chronic periodontitis with left ventricular mass and central blood pressure in treated patients with essential hypertension. American journal of hypertension 2009, 22, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, P.; Mikołajczyk, T.P.; Cześnikiewicz-Guzik, M.; Guzik, T.J. Periodontitis as an inflammatory trigger in hypertension: From basic immunology to clinical implications. Polish Heart Journal (Kardiologia Polska) 2021, 79, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Munoz Aguilera, E.; Suvan, J.; Buti, J.; Czesnikiewicz-Guzik, M.; Barbosa Ribeiro, A.; Orlandi, M.; Guzik, T.J.; Hingorani, A.D.; Nart, J.; D’Aiuto, F. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovascular research 2020, 116, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Leong, X.F.; Ng, C.Y.; Badiah, B.; Das, S. Association between hypertension and periodontitis: possible mechanisms. The Scientific World Journal 2014, 2014, 768237. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.A.C.; Rodrigues, J.V.S.; Cláudio, M.M.; Franciscon, J.P.S.; Mulinari-Santos, G.; Cirelli, T.; de Molon, R.S.; Gouveia Garcia, V.; Theodoro, L.H. The relationship between hypertension and periodontitis: a cross-sectional study. Journal of Clinical Medicine 2023, 12, 5140. [Google Scholar] [CrossRef] [PubMed]
- Surma, S.; Romańczyk, M.; Witalińska-Łabuzek, J.; Czerniuk, M.R.; Łabuzek, K.; Filipiak, K.J. Periodontitis, blood pressure, and the risk and control of arterial hypertension: epidemiological, clinical, and pathophysiological aspects—review of the literature and clinical trials. Current Hypertension Reports 2021, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, L.S.; Cai, C.; Shi, Q.; Wen, N.; Xu, J. Association between periodontitis and peripheral artery disease: a systematic review and meta-analysis. BMC cardiovascular disorders 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Geng, X.; Sun, J.; Zhang, S.; Yu, W.; Zhang, X.; Liu, H. The risk of periodontitis for peripheral vascular disease: a systematic review. Reviews in Cardiovascular Medicine 2019, 20, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Song, I.S.; Choi, J.; Gwon, J.G. Risk of peripheral arterial disease in patients with periodontitis: A nationwide, population-based, matched cohort study. Atherosclerosis 2020, 297, 96–101. [Google Scholar] [CrossRef]
- Kure, K.; Sato, H.; Aoyama, N.; Izumi, Y. Accelerated inflammation in peripheral artery disease patients with periodontitis. Journal of periodontal & implant science 2018, 48, 337–346. [Google Scholar] [CrossRef]
- Jacobi, N.; Walther, C.; Borof, K.; Heydecke, G.; Seedorf, U.; Lamprecht, R.; Beikler, T.; Debus, S.E.; Waldeyer, C.; Blankenberg, S.; others. The association of periodontitis and peripheral arterial occlusive disease in a prospective population-based cross-sectional cohort study. Journal of clinical medicine 2021, 10, 2048. [CrossRef]
- Berlin-Broner, Y.; Febbraio, M.; Levin, L. Apical periodontitis and atherosclerosis: is there a link? Review of the literature and potential mechanism of linkage. Quintessence Int 2017, 48, 527–53. [Google Scholar] [CrossRef]
- Hickey, N.A.; Shalamanova, L.; Whitehead, K.A.; Dempsey-Hibbert, N.; van der Gast, C.; Taylor, R.L. Exploring the putative interactions between chronic kidney disease and chronic periodontitis. Critical Reviews in Microbiology 2020, 46, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, F.; Saccucci, M.; Di Carlo, G.; Lucchetti, R.; Pilloni, A.; Pranno, N.; Luzzi, V.; Valesini, G.; Polimeni, A. Periodontitis and rheumatoid arthritis: the same inflammatory mediators? Mediators of inflammation 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Pütz, N.; Jurianz, E.; Schaller, H.G.; Reichert, S. Are there any common genetic risk markers for rheumatoid arthritis and periodontal diseases? A case-control study. Mediators of inflammation 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bañuelos, E.; Mukherjee, A.; Darrah, E.; Andrade, F. Rheumatoid arthritis-associated mechanisms of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. Journal of clinical medicine 2019, 8, 1309. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L.; Aleti, G.; Choudhury, S.; Nguyen, D.; Yaskell, T.; Zhang, Y.; Li, W.; Nelson, K.E.; Neto, L.L.S.; Sant’Ana, A.C.; others. Host-microbial interactions in systemic lupus erythematosus and periodontitis. Frontiers in immunology 2019, 10, 2602. [CrossRef]
- Lira-Junior, R.; Figueredo, C.M. Periodontal and inflammatory bowel diseases: Is there evidence of complex pathogenic interactions? World journal of gastroenterology 2016, 22, 7963. [Google Scholar] [CrossRef] [PubMed]
- Piras, V.; Usai, P.; Mezzena, S.; Susnik, M.; Ideo, F.; Schirru, E.; Cotti, E. Prevalence of apical periodontitis in patients with inflammatory bowel diseases: a retrospective clinical study. Journal of Endodontics 2017, 43, 389–394. [Google Scholar] [CrossRef]
- Hajishengallis, G. New developments in neutrophil biology and periodontitis. Periodontology 2000 2020, 82, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.B.; Shetty, I.P.; Jain, S.; Padakannaya, I.; Acharya, S.; Shettar, L.; Thakur, S. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in chronic periodontitis before and after nonsurgical therapy. Journal of Indian Society of Periodontology 2019, 23, 419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.; Pan, K.; Li, H.; Shang, S.; Wang, Y.; Tang, G.; Han, X. In-vivo imaging revealed antigen-directed gingival B10 infiltration in experimental periodontitis. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2020, 1867, 165991. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.C.; Neville, B.W.; Krayer, J.W.; Gonsalves, W.C. Oral manifestations of systemic disease. American family physician 2010, 82, 1381–1388. [Google Scholar] [PubMed]
- Sharma, A.; Raman, A.; Pradeep, A. Association of chronic periodontitis and psoriasis: periodontal status with severity of psoriasis. Oral Diseases 2015, 21, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Wijarnpreecha, K.; Wetter, D. Periodontitis and risk of psoriasis: A systematic review and meta-analysis. Journal of the European Academy of Dermatology and Venereology 2017, 31, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Dalmády, S.; Kemény, L.; Antal, M.; Gyulai, R. Periodontitis: a newly identified comorbidity in psoriasis and psoriatic arthritis. Expert review of clinical immunology 2020, 16, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.S.; Cota, L.O.M.; Costa, A.A.; Oliveira, A.M.S.D.; Costa, F.O. Periodontitis as another comorbidity associated with psoriasis: A case-control study. Journal of periodontology 2019, 90, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Misaki, T.; Nagasawa, Y.; Nomura, R.; Naka, S.; Fukunaga, A.; Matsuoka, D.; Matayoshi, S.; Matsumoto-Nakano, M.; Nakano, K. Porphyromonas gingivalis infection in the oral cavity is associated with elevated galactose-deficient IgA1 and increased nephritis severity in IgA nephropathy. Clinical and Experimental Nephrology 2024, 28, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, Y.; Misaki, T.; Ito, S.; Naka, S.; Wato, K.; Nomura, R.; Matsumoto-Nakano, M.; Nakano, K. Title IgA nephropathy and oral bacterial species related to dental caries and periodontitis. International journal of molecular sciences 2022, 23, 725. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Lin, X.P. Detection and significance of IL-6 and TNF-α in patients with Graves disease and periodontitis. Shanghai kou Qiang yi xue= Shanghai Journal of Stomatology 2018, 27, 43–47. [Google Scholar] [PubMed]
- Gao, Y.; Huang, D.; Liu, Y.; Qiu, Y.; Lu, S. Periodontitis and thyroid function: A bidirectional Mendelian randomization study. Journal of Periodontal Research 2024, 59, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Inchingolo, A.D.; Fatone, M.C.; Ferrante, L.; Avantario, P.; Fiore, A.; Palermo, A.; Amenduni, T.; Galante, F.; others. Bidirectional Association between Periodontitis and Thyroid Disease: A Scoping Review. International Journal of Environmental Research and Public Health 2024, 21, 860. [CrossRef]
- Yang, B.; Pang, X.; Guan, J.; Liu, X.; Li, X.; Wang, Y.; Chen, Z.; Cheng, B. The association of periodontal diseases and Sjogren’s syndrome: a systematic review and meta-analysis. Frontiers in Medicine 2023, 9, 904638. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, D.N.; Popescu, D.M.; Dinescu, S.C.; Boldeanu, M.V.; Surlin, P.; Vreju, F.; Ciurea, P.L. Clinical Evaluation of Periodontal Status and IL-6 Gingival Fluid Level in Patients with Sjogren’s Syndrome. Current health sciences journal 2023, 49, 163. [Google Scholar] [PubMed]
- Moreno-Quispe, L.A.; Serrano, J.; Virto, L.; Sanz, M.; Ramírez, L.; Fernández-Castro, M.; Hernández, G.; López-Pintor, R.M. Association of salivary inflammatory biomarkers with primary Sjögren’s syndrome. Journal of Oral Pathology & Medicine 2020, 49, 940–947. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, N.; Sun, P.; Liu, Y.; Hua, W. Periodontitis and Sjogren’s syndrome: a bidirectional two-sample mendelian randomization study. BMC Oral Health 2024, 24, 380. [Google Scholar] [CrossRef] [PubMed]
- Maarse, F.; Jager, D.H.J.; Alterch, S.; Korfage, A.; Forouzanfar, T.; Vissink, A.; Brand, H.S. Sjogren’s syndrome is not a risk factor for periodontal disease: a systematic review. Clinical and experimental rheumatology 2019, 37, S225–S233. [Google Scholar]
- Wu, S.Y.; Wu, C.Y.; Chen, M.H.; Huang, H.Y.; Chen, Y.h.; Tsao, Y.P.; Lai, Y.L.; Lee, S.Y. Periodontal conditions in patients with Sjögren’s syndrome: a meta-analysis. Journal of Dental Sciences 2021, 16, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Galvão-Moreira, L.V.; da Cruz, M.C.F.N. Oral microbiome, periodontitis and risk of head and neck cancer. Oral oncology 2016, 53, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, P.P.; Drangsholt, M.; Spiekerman, C.; Weiss, N.S. An exploration of the periodontitis–cancer association. Annals of epidemiology 2003, 13, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.G.; Katz, J. The association between periodontal disease and cancer: a review of the literature. Journal of dentistry 2010, 38, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Choung, H.; Lee, J.; Rhyu, I.; Kim, H. Association of periodontitis with oral cancer: a case-control study. Journal of dental research 2019, 98, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wu, L.; Leng, W.D.; Fang, C.; Zhu, Y.J.; Jin, Y.H.; Zeng, X.T. Periodontal disease and breast cancer: a meta-analysis of 1, 73,162 participants. Frontiers in oncology 2018, 8, 601. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal disease, tooth loss, and cancer risk. Epidemiologic reviews 2017, 39, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Amar, S.; Lowenfels, A.B. Periodontal disease, edentulism, and pancreatic cancer: a meta-analysis. Annals of Oncology 2017, 28, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.; Chadwick, J.; Sun, C.; Parbhakar, K.; Khoury, N.; Barbour, A.; Goldberg, M.; Tenenbaum, H.; Glogauer, M. Periodontal Inflammation Primes the Systemic Innate Immune Response. Journal of Dental Research, 2020; 0022034520963710. [Google Scholar] [CrossRef]
- Heikkilä, P.; But, A.; Sorsa, T.; Haukka, J. Periodontitis and cancer mortality: Register-based cohort study of 68,273 adults in 10-year follow-up. International journal of cancer 2018, 142, 2244–2253. [Google Scholar] [CrossRef]
- Cheng, R.; Billet, S.; Liu, C.; Haldar, S.; Choudhury, D.; Tripathi, M.; Hav, M.; Merchant, A.; Hu, T.; Huang, H.; others. Periodontal inflammation recruits distant metastatic breast cancer cells by increasing myeloid-derived suppressor cells. Oncogene 2020, 39, 1543–1556. [CrossRef]
- da Silva, A.P.B.; Alluri, L.S.C.; Bissada, N.F.; Gupta, S. Association between oral pathogens and prostate cancer: building the relationship. American journal of clinical and experimental urology 2019, 7, 1. [Google Scholar] [PubMed]
- Kaur, T.; Uppoor, A.; Naik, D. Parkinson’s disease and periodontitis–the missing link? A review. Gerodontology 2016, 33, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Sansores-España, D.; Carrillo-Avila, A.; Melgar-Rodriguez, S.; Díaz-Zuñiga, J.; Martínez-Aguilar, V. Periodontitis and Alzheimers disease. Med. Oral Patol. Oral Cir. Bucal 2020, 23, 23940. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Thangavelu, S.R.; Merciaro, I.; D’Orazio, M.; Bramanti, P.; Mazzon, E.; Trubiani, O. Porphyromonas gingivalis lipopolysaccharide stimulation in human periodontal ligament stem cells: role of epigenetic modifications to the inflammation. European journal of histochemistry: EJH 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Gil Montoya, J.A.; Barrios, R.; Sanchez-Lara, I.; Ramos, P.; Carnero, C.; Fornieles, F.; Montes, J.; Santana, S.; Luna, J.d.D.; Gonzalez-Moles, M.A. Systemic inflammatory impact of periodontitis on cognitive impairment. Gerodontology 2020, 37, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Leira, Y.; Carballo, Á.; Orlandi, M.; Aldrey, J.M.; Pías-Peleteiro, J.M.; Moreno, F.; Vázquez-Vázquez, L.; Campos, F.; D’Aiuto, F.; Castillo, J.; others. Periodontitis and systemic markers of neurodegeneration: A case–control study. Journal of Clinical Periodontology 2020, 47, 561–571. [CrossRef]
- Hashioka, S.; Inoue, K.; Hayashida, M.; Wake, R.; Oh-Nishi, A.; Miyaoka, T. Implications of systemic inflammation and periodontitis for major depression. Frontiers in neuroscience 2018, 12, 483. [Google Scholar] [CrossRef]
- Teixeira, F.B.; Saito, M.T.; Matheus, F.C.; Prediger, R.D.; Yamada, E.S.; Maia, C.S.; Lima, R.R. Periodontitis and Alzheimer’s disease: a possible comorbidity between oral chronic inflammatory condition and neuroinflammation. Frontiers in aging neuroscience 2017, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Toraman, A.; Toraman, E.; Özkaraca, M.; Budak, H. Increased nociceptive sensitivity is associated with periodontal inflammation and expression of chronic pain genes in gingival tissues of male rats. Chemico-biological interactions 2022, 366, 110128. [Google Scholar] [CrossRef] [PubMed]
- Toraman, A.; Toraman, E.; Özkaraca, M.; Budak, H. Evaluated periodontal tissues and oxidative stress in rats with neuropathic pain-like behavior. Molecular Biology Reports 2023, 50, 9315–9322. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nie, X.; Siddiqui, Y.; Wang, X.; Arora, V.; Fan, X.; Thumbigere-Math, V.; Chung, M. Nociceptor neurons magnify host responses to aggravate periodontitis. Journal of Dental Research 2022, 101, 812–820. [Google Scholar] [CrossRef] [PubMed]
- de Avila, E.D.; de Molon, R.S.; de Godoi Gonçalves, D.A.; Camparis, C.M. Relationship between levels of neuropeptide S ubstance P in periodontal disease and chronic pain: a literature review. Journal of investigative and clinical dentistry 2014, 5, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, I.S.; Cruz, S.S.d.; Trindade, S.C.; Passos-Soares, J.d.S.; Carvalho-Filho, P.C.; Figueiredo, A.C.M.G.; Lyrio, A.O.; Hintz, A.M.; Pereira, M.G.; Scannapieco, F. Periodontitis and respiratory diseases: A systematic review with meta-analysis. Oral diseases 2020, 26, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Muthu, J.; Muthanandam, S. Periodontitis and respiratory diseases: what does the recent evidence point to? Current Oral Health Reports 2018, 5, 63–69. [Google Scholar] [CrossRef]
- Molina, A.; Huck, O.; Herrera, D.; Montero, E. The association between respiratory diseases and periodontitis: A systematic review and meta-analysis. Journal of Clinical Periodontology 2023, 50, 842–887. [Google Scholar] [CrossRef] [PubMed]
- Moraschini, V.; Calasans-Maia, J.d.A.; Calasans-Maia, M.D. Association between asthma and periodontal disease: A systematic review and meta-analysis. Journal of periodontology 2018, 89, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.K.M.; de Oliveira Ferreira, R.; Castro, M.M.L.; Magno, M.B.; Carvalho, A.P.C.P.S.; Fagundes, N.C.F.; Maia, L.C.; Lima, R.R.; others. Is there an association between asthma and periodontal disease among adults? Systematic review and meta-analysis. Life Sciences 2019, 223, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Jung, H.J.; Park, M.W.; Choi, H.G.; Kim, H.; Wee, J.H. Association between Asthma and Periodontitis. Diagnostics 2023, 13, 3637. [Google Scholar] [CrossRef] [PubMed]
- Trindade, S.C.; others. Humoral Immunity in the Context of the Relationship between Periodontitis and Severe Asthma Literature Review. EC Dental Science 2019, 18, 82–90.
- Mainas, G.; Nibali, L.; Ide, M.; Mahmeed, W.A.; Al-Rasadi, K.; Al-Alawi, K.; Banach, M.; Banerjee, Y.; Ceriello, A.; Cesur, M.; others. Associations between periodontitis, COVID-19, and cardiometabolic complications: molecular mechanisms and clinical evidence. Metabolites 2022, 13, 40.
- Jeronimo, L.S.; Abreu, L.G.; Cunha, F.A.; Lima, R.P.E. Association between periodontitis and nosocomial pneumonia: a systematic review and meta-analysis of observational studies. Oral Health and Preventive Dentistry 2019. [Google Scholar] [CrossRef]
- Hutomo, S.; Putri, D.U. The role of the immune response in the development of pneumonia arising from oral periodontitis: a literature review. Bali Medical Journal 2023, 12, 2527–2531. [Google Scholar] [CrossRef]
- Loke, W.; Girvan, T.; Ingmundson, P.; Verrett, R.; Schoolfield, J.; Mealey, B.L. Investigating the association between obstructive sleep apnea and periodontitis. Journal of periodontology 2015, 86, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Lembo, D.; Caroccia, F.; Lopes, C.; Moscagiuri, F.; Sinjari, B.; D’Attilio, M. Obstructive sleep apnea and periodontal disease: a systematic review. Medicina 2021, 57, 640. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yuan, X.; Zhang, Y.; Wei, F.; Hou, Y.; Zhang, Y. A meta-analysis on the association between obstructive sleep apnea and periodontitis. Sleep and Breathing 2023, 27, 641–649. [Google Scholar] [CrossRef]
- Gamsiz-Isik, H.; Kiyan, E.; Bingol, Z.; Baser, U.; Ademoglu, E.; Yalcin, F. Does obstructive sleep apnea increase the risk for periodontal disease? A case-control study. Journal of periodontology 2017, 88, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ge, S.; Zhai, G.; Yu, S.; Cui, Z.; Si, S.; Chou, X. Incidence and risk of periodontitis in obstructive sleep apnea: A meta-analysis. Plos one 2022, 17, e0271738. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fu, Y.; Ziebolz, D.; Li, S.; Schmalz, G.; Li, F. Transcriptomic analysis reveals pathophysiological relationship between chronic obstructive pulmonary disease (COPD) and periodontitis. BMC medical genomics 2022, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Sapey, E.; Yonel, Z.; Edgar, R.; Parmar, S.; Hobbins, S.; Newby, P.; Crossley, D.; Usher, A.; Johnson, S.; Walton, G.M.; others. The clinical and inflammatory relationships between periodontitis and chronic obstructive pulmonary disease. Journal of clinical periodontology 2020, 47, 1040–1052. [CrossRef]
- Takeuchi, K.; Matsumoto, K.; Furuta, M.; Fukuyama, S.; Takeshita, T.; Ogata, H.; Suma, S.; Shibata, Y.; Shimazaki, Y.; Hata, J.; others. Periodontitis is associated with chronic obstructive pulmonary disease. Journal of Dental Research 2019, 98, 534–540. [CrossRef]
- Ramesh, A.; Varghese, S.S.; Jayakumar, N.; Malaiappan, S. Chronic obstructive pulmonary disease and periodontitis–unwinding their linking mechanisms. Journal of Oral Biosciences 2016, 58, 23–26. [Google Scholar] [CrossRef]
- Yu, B.; Wang, C.Y. Osteoporosis and periodontal diseases–An update on their association and mechanistic links. Periodontology 2000 2022, 89, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Straka, M.; Straka-Trapezanlidis, M.; Deglovic, J.; Varga, I. Periodontitis and osteoporosis. Neuroendocrinology Letters 2015, 36. [Google Scholar]
- Wang, C.W.J.; McCauley, L.K. Osteoporosis and periodontitis. Current osteoporosis reports 2016, 14, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Yang, B.E.; Yoo, D.M.; Kim, S.J.; Choi, H.G.; Byun, S.H. Analysis of the relationship between periodontitis and osteoporosis/fractures: a cross-sectional study. BMC oral health 2021, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sufaru, I.G.; Solomon, S.M.; Pasarin, L.; Martu-Stefanache, M.A.; Oanta, A.C.; Martu, I.; Ciocan-Pendefunda, A.; Martu, S. Study regarding the quantification of RANKL levels in patients with chronic periodontitis and osteoporosis. Romanian Journal of Oral Rehabilitation 2016, 8, 42–46. [Google Scholar]
- Penoni, D.; Vettore, M.; Torres, S.; Farias, M.; Leão, A. An investigation of the bidirectional link between osteoporosis and periodontitis. Archives of osteoporosis 2019, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gil-Montoya, J.A.; Barrios, R.; Santana, S.; Sanchez-Lara, I.; Pardo, C.C.; Fornieles-Rubio, F.; Montes, J.; Ramírez, C.; González-Moles, M.A.; Burgos, J.S. Association between periodontitis and amyloid β peptide in elderly people with and without cognitive impairment. Journal of periodontology 2017, 88, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Guzmán, I.C. Comparison of serum amyloid A protein and C-reactive protein levels as inflammatory markers in periodontitis. Journal of Periodontal & Implant Science 2015, 45, 14–22. [Google Scholar] [CrossRef]
- Fentoğlu, Ö.; Dinç, G.; Doğru, A.; Karahan, N.; İlhan, İ.; Kırzıoğlu, F.Y.; Şentürk, M.F.; Orhan, H. Serum, salivary, and tissue levels of plasminogen in familial Mediterranean fever, amyloidosis, and chronic periodontitis. Journal of Periodontology 2018, 89, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Furusho, H.; Kawashima, N.; Xu, S.; de Beer, M.; Battaglino, R.; Van Dyke, T.; Stashenko, P.; Sasaki, H. Serum amyloid A contributes to chronic apical periodontitis via TLR2 and TLR4. Journal of dental research 2019, 98, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.i.; Aoyama, N.; Izumi, Y.; Isobe, M.; Komuro, I.; Hirata, Y. Effect of periodontitis on cardiovascular manifestations in Marfan syndrome critical common role of TGF-β. International heart journal 2015, 56, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Venza, N.; Danesi, C.; Contò, D.; Fabi, F.; Mampieri, G.; Sangiuolo, F.; Laganà, G. Periodontal condition in growing subjects with Marfan Syndrome: A case-control study. PeerJ 2019, 7, e6606. [Google Scholar] [CrossRef]
- Cervino, G.; Cicciù, M.; De Stefano, R.; Falcomatà, D.; Bianchi, A.; Crimi, S.; Laino, L.; Herford, A.S.; Gaeta, M.; Fiorillo, L. Oral health in patients with Marfan syndrome. Archives of Oral Biology 2020, 116, 104745. [Google Scholar] [CrossRef] [PubMed]
- Handa, K.; Abe, S.; Suresh, V.V.; Fujieda, Y.; Ishikawa, M.; Orimoto, A.; Kobayashi, Y.; Yamada, S.; Yamaba, S.; Murakami, S.; others. Fibrillin-1 insufficiency alters periodontal wound healing failure in a mouse model of Marfan syndrome. Archives of Oral Biology 2018, 90, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, M.; Wiemann, S.; Jung, S.; Kleinheinz, J.; Bohner, L. Oral health-related quality of life in people with rare hereditary connective tissue disorders: Marfan syndrome. International Journal of Environmental Research and Public Health 2018, 15, 2382. [Google Scholar] [CrossRef]
- da Costa, T.A.; Silva, M.J.B.; Alves, P.M.; Chica, J.E.L.; Barcelos, E.Z.; Giani, M.A.A.; Garlet, G.P.; da Silva, J.S.; Rodrigues Júnior, V.; Rodrigues, D.B.R.; others. Inflammation biomarkers of advanced disease in nongingival tissues of chronic periodontitis patients. Mediators of Inflammation 2015, 2015, 983782. [Google Scholar] [CrossRef] [PubMed]
- Elburki, M.; Moore, D.; Terezakis, N.; Zhang, Y.; Lee, H.M.; Johnson, F.; Golub, L. A novel chemically modified curcumin reduces inflammation-mediated connective tissue breakdown in a rat model of diabetes: periodontal and systemic effects. Journal of periodontal research 2017, 52, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2000 2020, 84, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Shcherba, V.; Kyryliv, M.; Bekus, I.; Krynytska, I.; Marushchak, M.; Korda, M. A comparative study of connective tissue metabolism indices in experimental comorbidity-free periodontitis and periodontitis combined with thyroid dysfunction. Journal of medicine and life 2020, 13, 219. [Google Scholar] [CrossRef]
- Dionigi, C.; Larsson, L.; Difloe-Geisert, J.C.; Zitzmann, N.U.; Berglundh, T. Cellular expression of epigenetic markers and oxidative stress in periodontitis lesions of smokers and non-smokers. Journal of Periodontal Research 2022, 57, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Kocaman, G.; Altinoz, E.; Erdemli, M.; Gul, M.; Erdemli, Z.; Zayman, E.; Bag, H.; Aydın, T. Crocin attenuates oxidative and inflammatory stress-related periodontitis in cardiac tissues in rats. Advances in clinical and experimental medicine: official organ Wroclaw Medical University 2021, 30. [Google Scholar] [CrossRef] [PubMed]
- Dionigi, C.; Larsson, L.; Carcuac, O.; Berglundh, T. Cellular expression of DNA damage/repair and reactive oxygen/nitrogen species in human periodontitis and peri-implantitis lesions. Journal of Clinical Periodontology 2020, 47, 1466–1475. [Google Scholar] [CrossRef]
- Machado, V.; Escalda, C.; Proença, L.; Mendes, J.J.; Botelho, J. Is there a bidirectional association between polycystic ovarian syndrome and periodontitis? A systematic review and meta-analysis. Journal of clinical medicine 2020, 9, 1961. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Xin, J.; Zhou, P.; Tang, J.; Xie, H.; Fan, W.; Zhang, Z.; Wu, D. Bidirectional association between polycystic ovary syndrome and periodontal diseases. Frontiers in Endocrinology 2023, 14, 1008675. [Google Scholar] [CrossRef] [PubMed]
- Young, H.E.; Ward, W.E. The relationship between polycystic ovarian syndrome, periodontal disease, and osteoporosis. Reproductive Sciences 2021, 28, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, X.; Zhou, P.; Zhang, W.; Li, D.; Lv, M.; Liao, X. Assessment of bidirectional relationships between polycystic ovary syndrome and periodontitis: Insights from a mendelian randomization analysis. Frontiers in Genetics 2021, 12, 644101. [Google Scholar] [CrossRef] [PubMed]
- Dănciulescu Miulescu, R.E.; Guja, L.; Socea, B.; Dumitriu, A.; Paunica, S.; Ștefănescu, E. Oral pathology induced by excess or deficiency of glucocorticoids in adults. Journal of Mind and Medical Sciences 2020, 7, 141–147. [Google Scholar] [CrossRef]
- Bugălă, N.M.; Carsote, M.; Stoica, L.E.; Albulescu, D.M.; Ţuculină, M.J.; Preda, S.A.; Boicea, A.R.; Alexandru, D.O. New approach to addison disease: oral manifestations due to endocrine dysfunction and comorbidity burden. Diagnostics 2022, 12, 2080. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, J.; Mahendra, L.; Janani, M.; Sharma, V. Influence of Systemic Conditions on Periodontium-Exploring the Oral Systemic Connection: A Mini-Review. International Journal Of Drug Research And Dental Science 2022, 4, 61–75. [Google Scholar]
- Tunheim, E.G.; Skallevold, H.E.; Rokaya, D. Role of hormones in bone remodeling in the craniofacial complex: A review. Journal of oral biology and craniofacial research 2023, 13, 210–217. [Google Scholar] [CrossRef]
- Singh, U.; Lumry, W.R.; Busse, P.; Wedner, H.J.; Banerji, A.; Craig, T.J.; Li, H.H.; Tachdjian, R.; Jacobs, J.S.; Riedl, M.A.; others. Association between self-reported dental hygiene practices and dental procedure-related recurrent angioedema attacks in HAE subjects: a multicenter survey. The Journal of Allergy and Clinical Immunology: In Practice 2020, 8, 3162–3169. [CrossRef]
- Hanisch, M.; Hoffmann, T.; Bohner, L.; Hanisch, L.; Benz, K.; Kleinheinz, J.; Jackowski, J. Rare diseases with periodontal manifestations. International Journal of Environmental Research and Public Health 2019, 16, 867. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Complement and periodontitis. Biochemical pharmacology 2010, 80, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, Y.; Zhi, Y. Throat microbiota alterations in patients with hereditary angioedema. World Allergy Organization Journal 2022, 15, 100694. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: a key system for immune surveillance and homeostasis. Nature immunology 2010, 11, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Reis, E.S.; Mastellos, D.C.; Ricklin, D.; Lambris, J.D. Novel mechanisms and functions of complement. Nature immunology 2017, 18, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.S.; Mastellos, D.C.; Hajishengallis, G.; Lambris, J.D. New insights into the immune functions of complement. Nature Reviews Immunology 2019, 19, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lambris, J.D. Crosstalk pathways between Toll-like receptors and the complement system. Trends in immunology 2010, 31, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Jia, J.; Yang, Y.; Bian, Z.; Ji, Y. A follicle-stimulating hormone exacerbates the progression of periapical inflammation through modulating the cytokine release in periodontal tissue. Inflammation 2020, 43, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Fenton, A.; Dias, I.H.; Heaton, B.; Brown, C.L.; Sidhu, A.; Rahman, M.; Griffiths, H.R.; Cockwell, P.; Ferro, C.J.; others. Oxidative stress links periodontal inflammation and renal function. Journal of Clinical Periodontology 2021, 48, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.L.; Liu, X.Y.; Meng, X.; Zhao, R.Q.; Ou, L.L.; Li, B.Z.; Xing, T. Periodontitis exacerbates and promotes the progression of chronic kidney disease through oral flora, cytokines, and oxidative stress. Frontiers in microbiology 2021, 12, 656372. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Liu, H.; Zheng, Y.; Zhang, Z. Role of oxidative stress in the relationship between periodontitis and systemic diseases. Frontiers in physiology 2023, 14, 1210449. [Google Scholar] [CrossRef] [PubMed]
- Palathingal, P.; Mahendra, J.; Annamalai, P.T.; Varma, S.S.; Mahendra, L.; Thomas, L.; Baby, D.; Jose, A.; Srinivasan, S.; Ambily, R. A cross-sectional study of serum glutathione peroxidase: An antioxidative marker in chronic periodontitis and chronic kidney disease. Cureus 2022, 14. [Google Scholar] [CrossRef]
- Ademowo, O.S.; Sharma, P.; Cockwell, P.; Reis, A.; Chapple, I.L.; Griffiths, H.R.; Dias, I.H. Distribution of plasma oxidised phosphatidylcholines in chronic kidney disease and periodontitis as a co-morbidity. Free Radical Biology and Medicine 2020, 146, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Ganimusa, I.; Chew, E.; Lu, E.M.C. Vitamin D deficiency, Chronic kidney disease and Periodontitis. Medicina 2024, 60, 420. [Google Scholar] [CrossRef] [PubMed]
- Serni, L.; Caroti, L.; Barbato, L.; Nieri, M.; Serni, S.; Cirami, C.L.; Cairo, F. Association between chronic kidney disease and periodontitis. A systematic review and metanalysis. Oral diseases 2023, 29, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, J.d.S.; De Azambuja, C.B.; Cunha, G.R.; Cavagni, J.; Rösing, C.K.; Haas, A.N.; Thomé, F.S.; Fiorini, T. Association between severe periodontitis and chronic kidney disease severity in predialytic patients: A cross-sectional study. Oral diseases 2020, 26, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Narváez, R.V.; Valenzuela-Narváez, D.R.; Valenzuela-Narváez, D.A.O.; Córdova-Noel, M.E.; Mejía-Ruiz, C.L.; Salcedo-Rodríguez, M.N.; Gonzales-Aedo, O. Periodontal disease as a predictor of chronic kidney disease (CKD) stage in older adults. Journal of International Medical Research 2021, 49, 03000605211033266. [Google Scholar] [CrossRef] [PubMed]
- Priyamvara, A.; Dey, A.K.; Maddi, A.; Teich, S. The relationship between periodontal and kidney disease: a critical review. Quintessence International 2022, 53. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nature Reviews Immunology 2021, 21, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontology 2000 2022, 89, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Sanui, T.; Nishimura, F.; Shapira, L. Diabetes as a risk factor for periodontal disease—plausible mechanisms. Periodontology 2000 2020, 83, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Li, X.; Divaris, K.; Chavakis, T. Maladaptive trained immunity and clonal hematopoiesis as potential mechanistic links between periodontitis and inflammatory comorbidities. Periodontology 2000 2022, 89, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Larvin, H.; Kang, J.; Aggarwal, V.; Pavitt, S.; Wu, J. Systemic multimorbidity clusters in people with periodontitis. Journal of Dental Research 2022, 101, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, M.C.; Furgal, A.; Furst, W.; Ramakrishnan, M.; Capizzano, N.; Sen, A.; Klinkman, M. The Prevalence of Periodontitis Among US Adults with Multimorbidity Using NHANES Data 2011–2014. The Journal of the American Board of Family Medicine 2023, 36, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Larvin, H.; Kang, J.; Aggarwal, V.R.; Pavitt, S.; Wu, J. Periodontitis and risk of immune-mediated systemic conditions: A systematic review and meta-analysis. Community Dentistry and Oral Epidemiology 2023, 51, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.; Watt, R.; Heilmann, A.; Stennett, M.; Singh, A. Social Disadvantage and Multimorbidity Including Oral Conditions in the United States. Journal of Dental Research 2024, 103, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, H.M.; Koroukian, S.M.; Stange, K.C.; Schiltz, N.K.; Bissada, N.F. Investigating the influence of periodontal disease on the association between complex multimorbidity and health: A cross-sectional study. Journal of International Society of Preventive and Community Dentistry 2023, 13, 247–255. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




