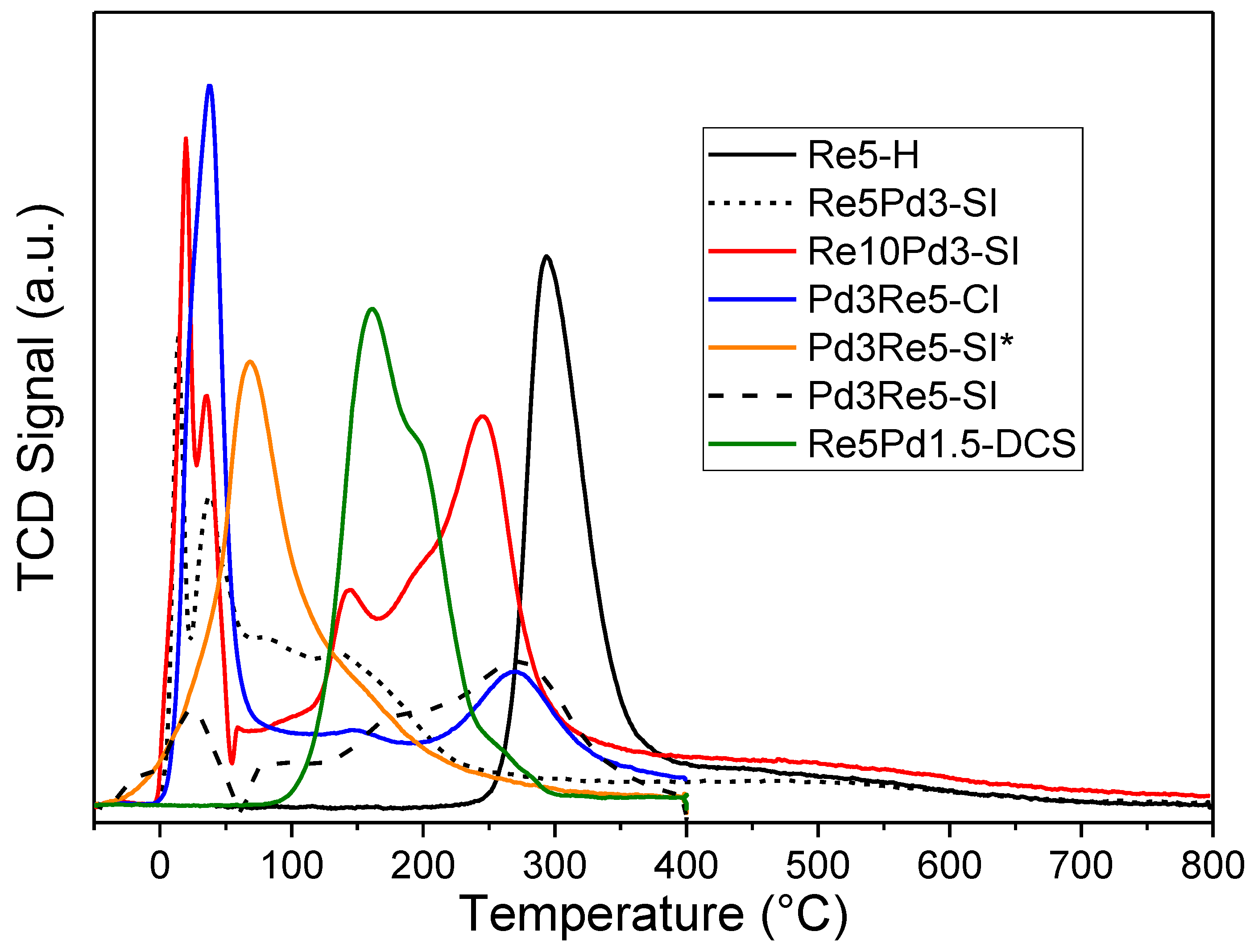
3.1. Catalyst Composition and Pulse CO Chemisorption
The metal precursor(s), calcination conditions and metal loadings (as determined by ICP-OES) of the catalysts are provided in
Table 1. These catalysts (except Pd3Re5-SI*) are the same ones described in our previous publications on selective furfural hydrogenation [
10,
11]. The catalyst nomenclature is consistent with that used in previous publications [
10,
12] and indicates the metal(s), nominal weight loadings, precursors (monometallics and DCS) and order of impregnation (for SI catalysts only). The BET surface areas of selected catalysts (
Table 2) are equivalent (or nearly so) to that of the γ-Al
2O
3 support (183 ± 10 m
2/g).
Pulse CO chemisorption data (
Table 2) were measured after in situ reduction in flowing 5% H
2/He at 400°C for 1 h and cooling to 35°C to avoid potential re-oxidation of Re during
in vacuo heating for volumetric chemisorption measurements. When compared to previous volumetric CO chemisorption results [
10,
11], the CO/M values are ~25% greater for the Pd/Al
2O
3 and PdRe/Al
2O
3 catalysts; however, the pulse CO/Re value for Re5-H is ~100% higher than the volumetric value (0.098). The CO/Re ratio of Re5-H suggests a low dispersion and relatively large supported Re particles; however, we will demonstrate (
vide infra) that the low CO/Re ratio is because of other factors (e.g., incomplete reduction, metal-support interactions). In situ CO DRIFTS experiments (
Figure S1, Supplementary Materials) indicate that heating the reduced Re5-H catalyst to 400°C in flowing He results in ~50% loss of CO chemisorption capacity. This behavior is consistent with oxidation of the supported Re nanoparticles by surface hydroxyl groups [
26,
27]. We suggest that
in vacuo heating to 400°C during the volumetric chemisorption experiments similarly resulted in oxidation of supported Re nanoparticles and loss of CO chemisorption capacity. Previous in situ DRIFTS studies have evidenced linear and doubly bridged CO species on Pd and linear CO species on Re [
10,
11]. The pulse CO chemisorption uptakes also include contributions from segregated Re species (atoms, clusters, nanoparticles) on γ-Al
2O
3 which were not included in volumetric uptake measurements. We speculate that Re associated with Pd particles mainly contributed to volumetric CO chemisorption by the bimetallic catalysts [
10,
11].
The pulse CO chemisorption data reveal that the Pd precursor has a determining influence on the dispersions of the Pd/Al
2O
3 and PdRe/Al
2O
3 catalysts. The CO/Pd ratios for Pd3-TA and Pd3-N indicate that the former has a much higher dispersion that we attribute to strong electrostatic adsorption of [Pd(NH
3)
4]
2+[
28]. The smaller Pd particle size in Pd3-TA was confirmed by HAADF-STEM imaging of the catalysts (
Figure S2, Supplemental Materials). The Pd3-N catalyst contains 10-nm and larger Pd nanoparticles, whereas the Pd3-TA catalyst contains a bimodal distribution of small 1- and ~4-nm Pd nanoparticles. For Pd1.5-TA, the combined effects of the TA precursor and its lower loading result in the highest dispersion. The CO uptakes of bimetallic catalysts prepared from the N precursor are greater than that of Pd3-N, but less than the sum of the CO uptakes of the respective monometallic catalysts (Pd3-N and Re5-H). In contrast, the CO uptakes of the bimetallic catalysts prepared from the TA precursor are equivalent to (or slightly less than) that of the respective Pd/Al
2O
3 catalysts (Pd3-TA and Pd1.5-TA). Consequently, the CO/M (M = Pd + Re) ratios of the Pd3Re5-CI and Pd3Re5-SI catalysts are equivalent to that of Pd3-N, whereas the CO/M values for Re5Pd3-SI and Re5Pd1.5-DCS are lower than that of Pd3-TA and Pd1.5-TA, respectively. Moreover, the Pd3Re5-CI and Pd3Re5-SI catalysts have lower CO/M ratios than the Re5Pd3-SI and Re5Pd1.5-DCS catalysts. Interestingly, the CO/Pd ratios of Re5Pd3-SI and Re5Pd1.5-DCS are closely similar to those of Pd3-TA and Pd1.5-TA, respectively.
3.3. Temperature-Programmed Hydride Decomposition (TPHD)
Supported Pd nanoparticles form bulk hydride phases (α- and β-PdH
x) [
30]. The stoichiometry depends on particle surface-to-volume ratio and hence particle size [
31]. The H/Pd ratio increases with Pd particle size [
31,
32] ultimately saturating at the bulk value (β-PdH
x ~0.65). The H/Pd ratio is diminished by alloying Pd with another transition metal, such as Au, Ag, or Re [
15,
30]. Ziemecki and coworkers first exploited this effect to infer that PdRe alloy particles were formed in PdRe/Al
2O
3 catalysts [
15]; however, Re and Pd are only partially miscible in the bulk, and the bulk PdRe alloy phase(s) contains a maximum of ~16% Re. More recently, Malinowski, et al. [
19] used TPHD (referred to as differential TPR) to characterize a series of PdRe/Al
2O
3 catalysts having a wide range of compositions prepared by SI using a procedure similar to that employed for Pd3Re5-SI. In this section, we use TPHD to detect disruption of β-PdH
x formation associated with Pd-Re interactions (i.e., alloy and near-surface alloy formation) in a series of PdRe/Al
2O
3 catalysts.
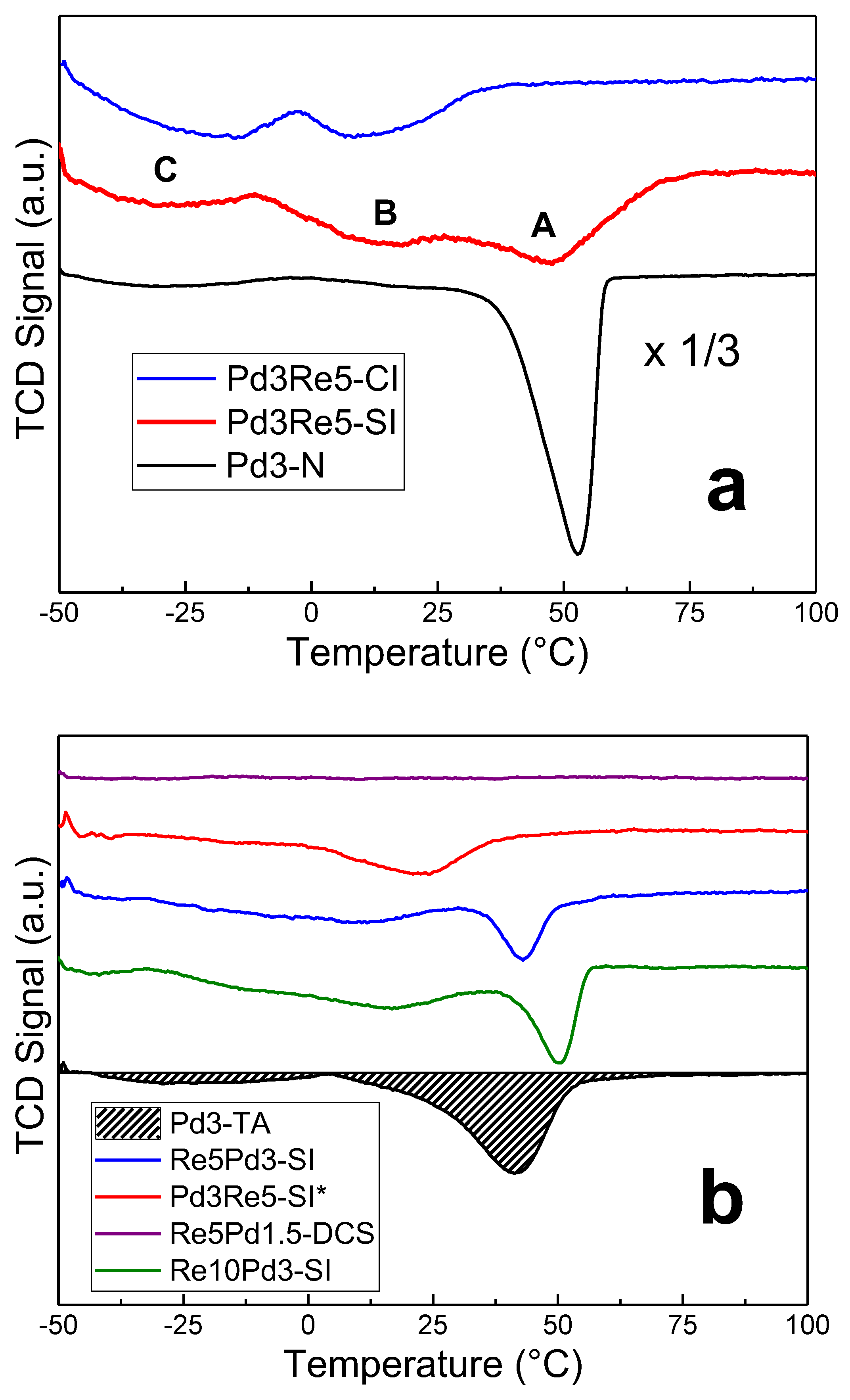
The large asymmetric H
2 evolution peak at 50-55°C in the TPHD profile of Pd3-N (
Figure 2a) is assigned to β-PdH
x decomposition [
15,
19]. The corresponding peak for Pd3-TA (
Figure 2b) is smaller, and the hydride decomposes at lower temperature (~40°C) indicating lower stability and consistent with a smaller Pd particle size. Moreover, the main peak is broad and strongly skewed toward lower temperatures, and there is a weak low-temperature H
2 evolution feature at approximately -25°C. The TPHD spectrum of Pd1.5-TA (not shown) contains an even smaller β-PdH
x decomposition peak at ~45°C. The quantitative H/Pd ratios for the main β-PdH
x decomposition peak (at >0°C) and the total hydrogen evolved in TPHD are given in
Table 3. Because Peaks A and B (
Figure 2a) overlap in some catalysts (e.g., Pd3-TA), we have chosen to combine the areas and report the sum (as >0°C) in
Table 3. For the Pd/Al
2O
3 catalysts, there is a very strong linear correlation of the H/Pd (>0°C) ratio with the pulse chemisorption CO/Pd ratio with a slope of -1.0 and an intercept of 0.71 (near bulk maximum)—consistent with the expected Pd particle size effect [
31,
32].
The TPHD spectra of the PdRe/Al
2O
3 catalysts prepared using the N precursor (
Figure 2a) show strong suppression of Peak A (when compared to Pd3-N) and the appearance Peaks B and C at approximately 15 and -25°C, respectively. H
2 evolution at these low temperatures evidences decomposition of less stable hydrides, e.g., those associated with Pd-Re alloy or bimetallic nanoparticles. For Pd3Re5-SI (prepared by impregnating a reduced Pd3-N catalyst with HReO
4), Peak A is strongly suppressed but not completely eliminated indicating that some unalloyed Pd particles remain in this catalyst; however, the ~2/3 reduction in the H/Pd ratio (Peak A) indicates that these are a minority. In contrast, Peak A is entirely eliminated in the TPHD spectrum of Pd3Re5-CI leaving only Peaks B and C which we infer are associated with Pd-Re alloy nanoparticles with different compositions. Thus, co-impregnation provides for better intermixing of Pd and Re in this catalyst; however, the alloy particles are relatively large consistent with the pulse CO chemisorption data and as seen previously in HAADF-STEM images [
10].
As reported previously, the TPHD spectrum of Re5Pd1.5-DCS (
Figure 2b) does not contain any peaks indicating complete suppression of Pd hydride formation in the very small bimetallic (alloy) clusters [
11]. In contrast, the β-PdH
x decomposition feature at ~45°C (
Figure 2b) in the PdRe/Al
2O
3 catalysts prepared using the TA precursor is not suppressed completely. For Re5Pd3-SI and Re10Pd3-SI, Peak A is narrower and shifted to higher temperatures relative to Pd3-TA, and the H/Pd ratios are lower and higher than Pd3-TA, respectively. We infer from these results that Re5Pd3-SI contains small Pd nanoparticles (similar to Pd3-TA) and PdRe alloy nanoparticles. The Pd particles in Re10Pd3-SI are larger, as confirmed by HAADF-STEM [
10] and EXAFS spectroscopy (
vide infra). In contrast, the TPHD spectrum of Pd3Re5-SI* comprises a strong Peak B and a very weak Peak C indicating that this catalyst contains primarily PdRe alloy nanoparticles.
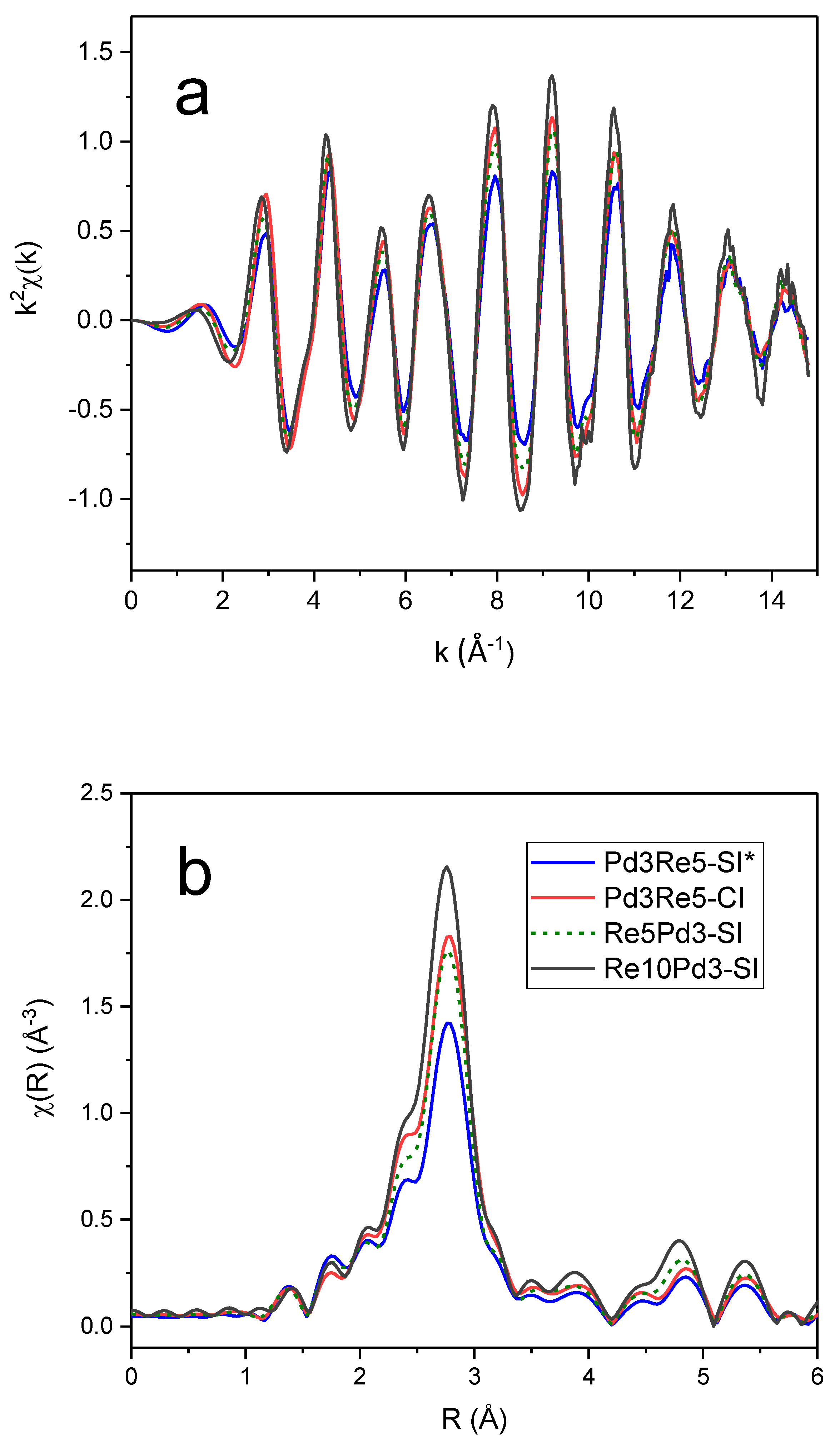
3.5. EXAFS Spectroscopy
The
k2-weighted EXAFS spectra and corresponding Fourier transform (FT) magnitudes of the PdRe/Al
2O
3 catalysts at the Pd K edge (
Figure 5) exhibit very similar features. The main peak corresponds to the first-shell Pd-Pd distance in the metal; however, there are significant low-R side lobes. The most evident differences are the
chi amplitudes and corresponding FT magnitudes that decrease in order Re10Pd3-SI > Pd3Re5-CI > Re5Pd3-SI > Pd3Re5-SI*. The Pd higher shells exhibit a similar trend. In comparison, the
k2-weighted
chi data and FTs of the Re/Al
2O
3 and PdRe/Al
2O
3 catalysts at the Re L
III edge (
Figure 6) indicate the presence of multiple backscatterers (e.g., Re, Pd, O) in the first coordination shell. The lower EXAFS amplitudes at high
k (
Figure 6a) for the bimetallic catalysts arise from interference between the Re-Re and Re-Pd backscattering contributions. Destructive interference is maximum near the node at ~11-Å
-1. Of course, smaller Re nanoparticles with lower Re-Re coordination numbers will also have lower the high-
k amplitudes. The FT peaks at ~2.4 and 2.8 Å are comprised of Re-Re (major) and Re-Pd (minor) contributions, and strong interference affects their magnitudes and shapes. To illustrate, simulated FTs were generated using FEFF6 and an
fcc model by replacing Pd atoms with Re atoms in the first coordination shell at a weighted average bond distance (
Figure S3a), and vice versa at the Re edge (
Figure S3b). These simulations are meant only for semiquantitative illustration, and fits to the experimental data are discussed later; however, it is clear that the greatest destructive interference occurs with the insertion of ~3 Pd atoms within the 12-member first coordination shell of Re. The analogous spectra generated at the Pd K-edge show that significant destructive interference only exists for Re backscatterers about a Pd absorber at high Re:Pd ratios, i.e., 1:1 or greater. A small fraction of Re around a Pd absorber only creates minimal destructive interference consistent with the experimental spectra in
Figure 5. Because the bulk thermodynamics of PdRe alloys limits the heteroatom concentration to ~16% [
13], strong destructive interference is only expected at the Re L
III edge. The Re L
III EXAFS spectra of the catalysts are complicated further by the presence of O backscatterers in the first coordination shell. The peak at ~1.9 Å in the FT of Re5-H is indicative of Re-O bonding; smaller Re-O contributions are found also in the EXAFS spectra of the bimetallic catalysts.
The Re L
III FT EXAFS spectrum of Re10Pd5-SI most closely resembles that of Re5-H, indicating the presence of segregated metallic Re species, as previously inferred from TPR and HAADF-STEM [
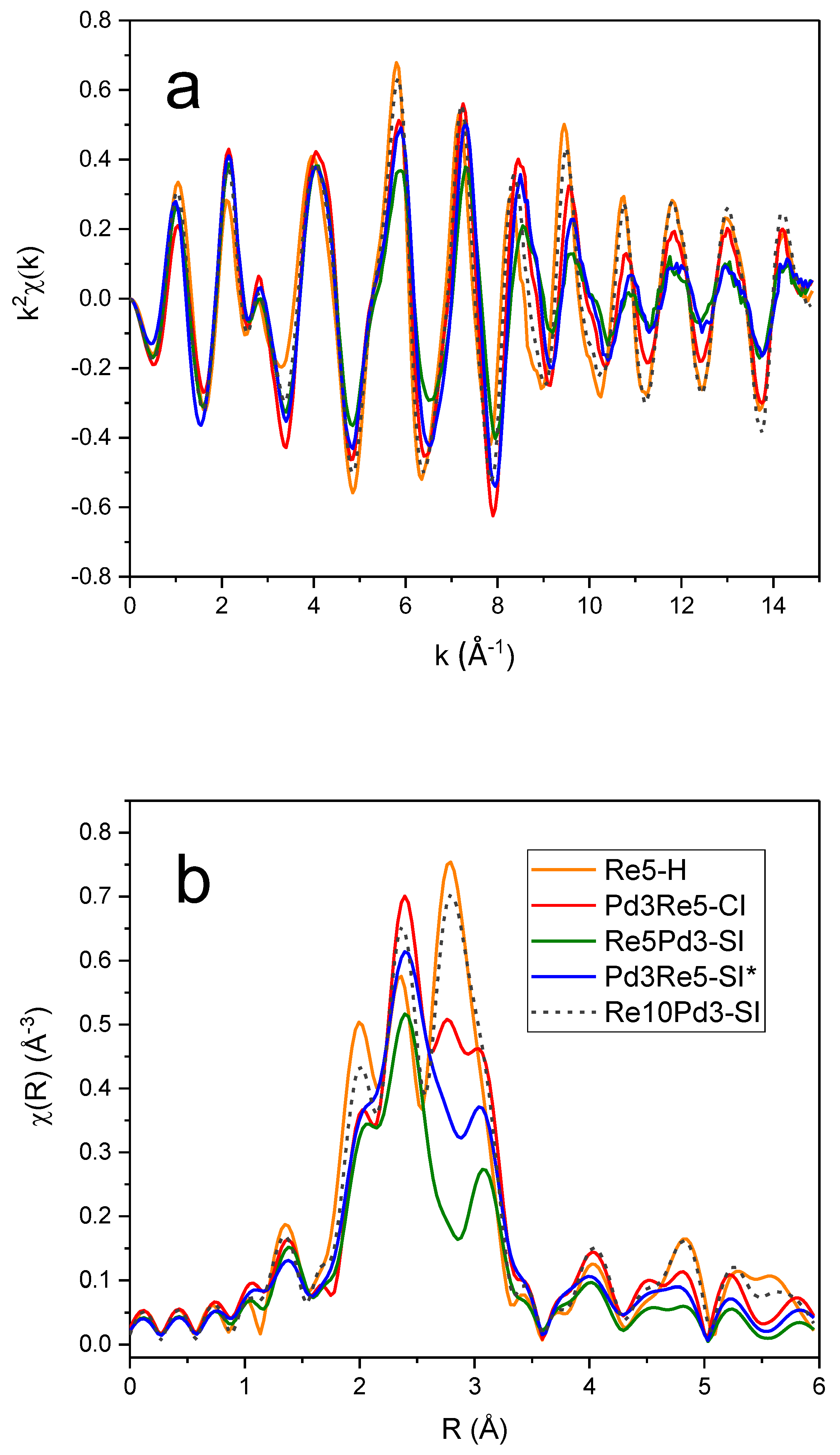
10]. The 2.8-Å peak appears broader with lower intensity in the spectrum of Pd3Re5-CI. This peak is diminished further in the FT spectra of Pd3Re5-SI* and Re5Pd3-SI, and the residual intensity shifts to ~3.0 Å. Conversely, the peak at ~2.4 Å has maximum intensity for Pd3Re5-CI and remains relatively strong for Pd3Re5-SI* and Re5Pd3-SI. The peak at ~1.9 Å associated with Re-O contributions is very similar for the three 1:1 Pd:Re bimetallic catalysts. The higher coordination shell peaks (i.e., features at >3.5 Å) are more intense for Re5-H and Re10Pd3-SI indicating the presence of larger Re nanoparticles in these catalysts. To a first approximation, the magnitudes of the higher shell peaks scale with the first-shell peak at 2.8 Å. Next, we will discuss quantitative fitting of the EXAFS spectra using FEFF6 models for the Re-O, Re-Re and Re-Pd paths.
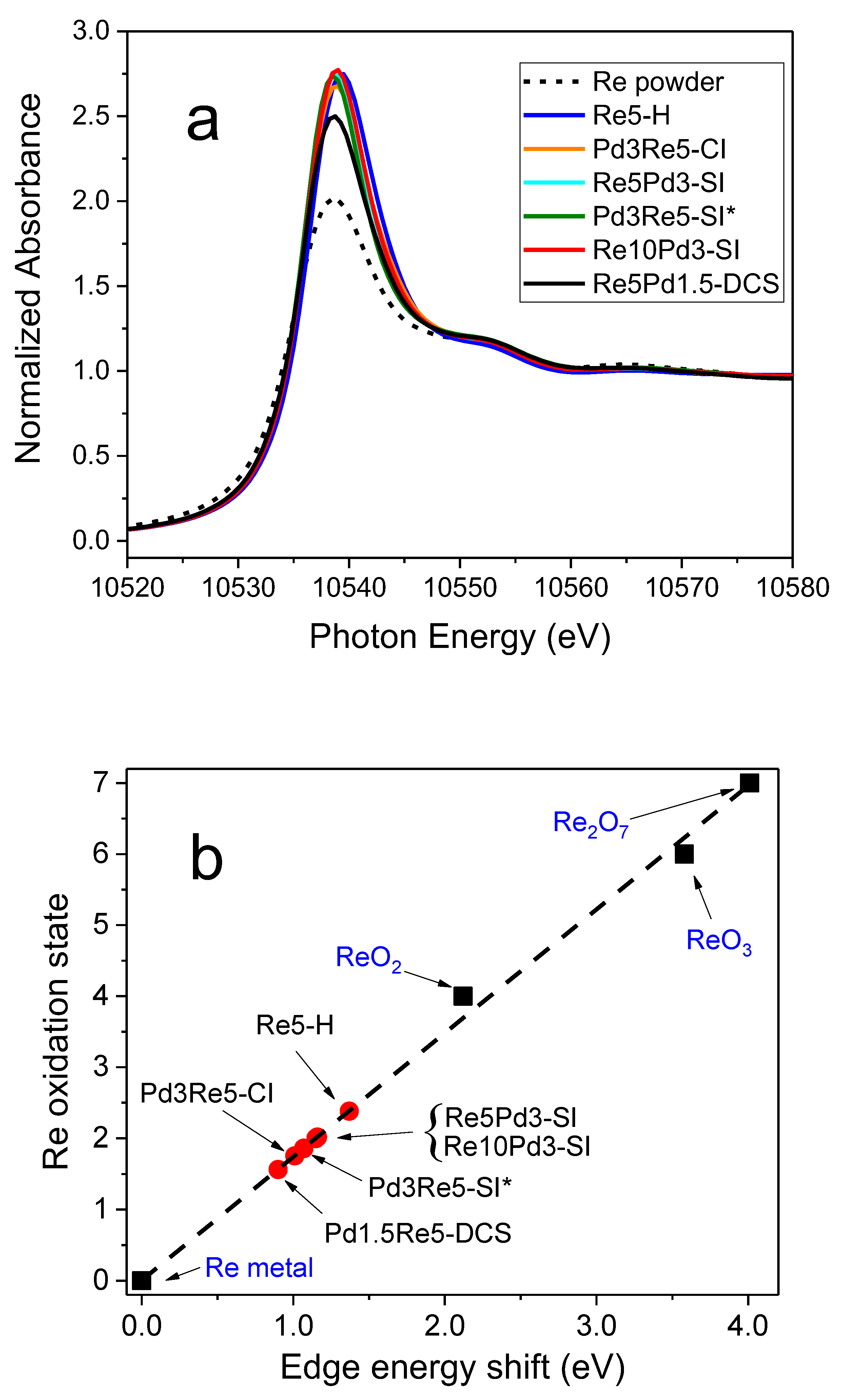
The Re L
III first-shell EXAFS spectrum of the as-prepared Re5-H catalyst (after calcination and measured in air) could be fit very well using 4 Re-O backscatterers at 1.73 Å consistent with the structure of perrhenate ion, [ReO
4]
- (
Table 4). The overall best fit to the Re L
III first-shell EXAFS spectrum of Re5-H after reduction in flowing 3.5% H
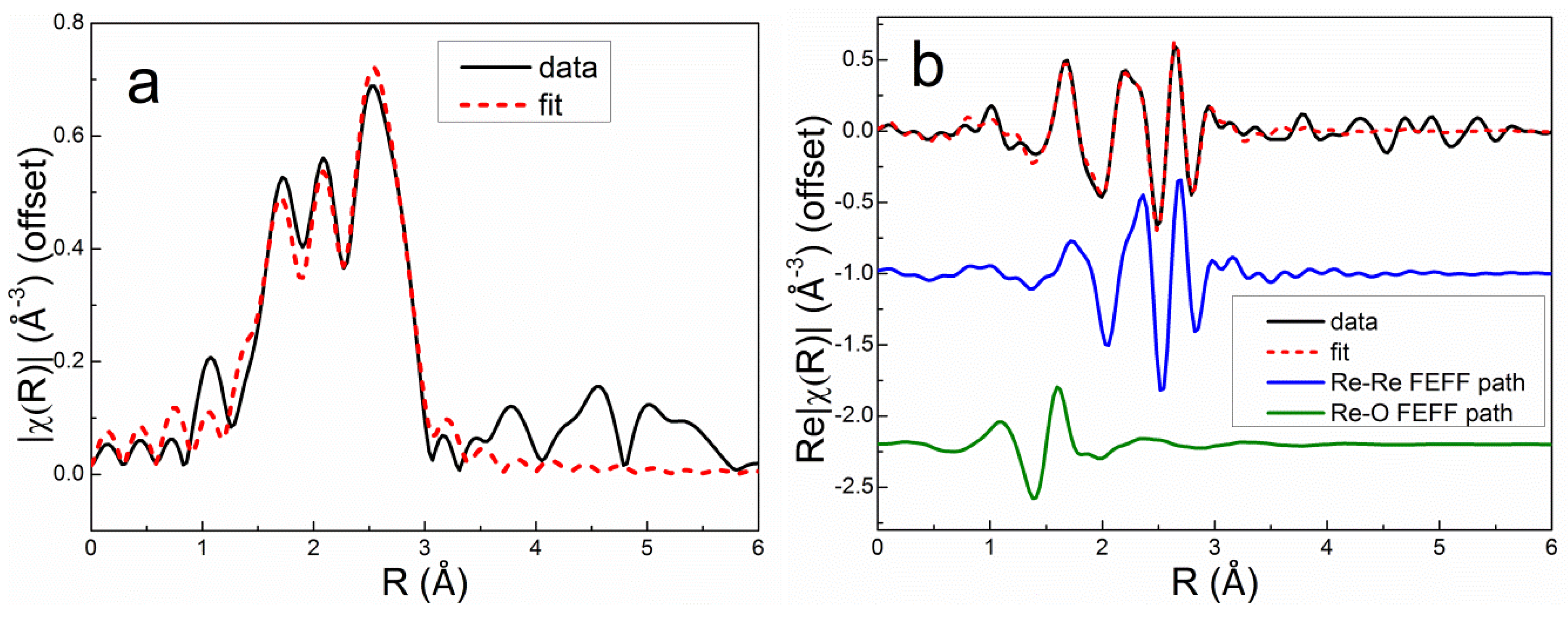
2 at 400°C for 1 h is compared with the experimental data in
r space in
Figure 7 [FT magnitude (a) and real (Re) part (b)]. This fit was achieved using only the Re-Re and Re-O1 paths shown. The fitting parameters (
Table 4) indicate a mixture of small metallic Re particles and ReO
x species. The average Re-Re distance (2.74 Å) is equal to the average of the two Re-Re distances in the
hcp metal. The Re-O1 distance (2.01 Å) is equivalent to that found in bulk ReO
2 suggesting that Re
4+ is most prevalent non-zero oxidation state. Incomplete reduction of Re
7+ to Re
4+ has been observed in previous studies of Re/Al
2O
3 catalysts by electron spin resonance [
33] and XPS [
34]. A similar Re-O distance was also reported by Bare, et al. [
3] and Chen, et al. [
5]. Adding a second Re-O2 path with a bond distance corresponding to Re
7+ oxide (1.73 Å) improved the fit slightly (
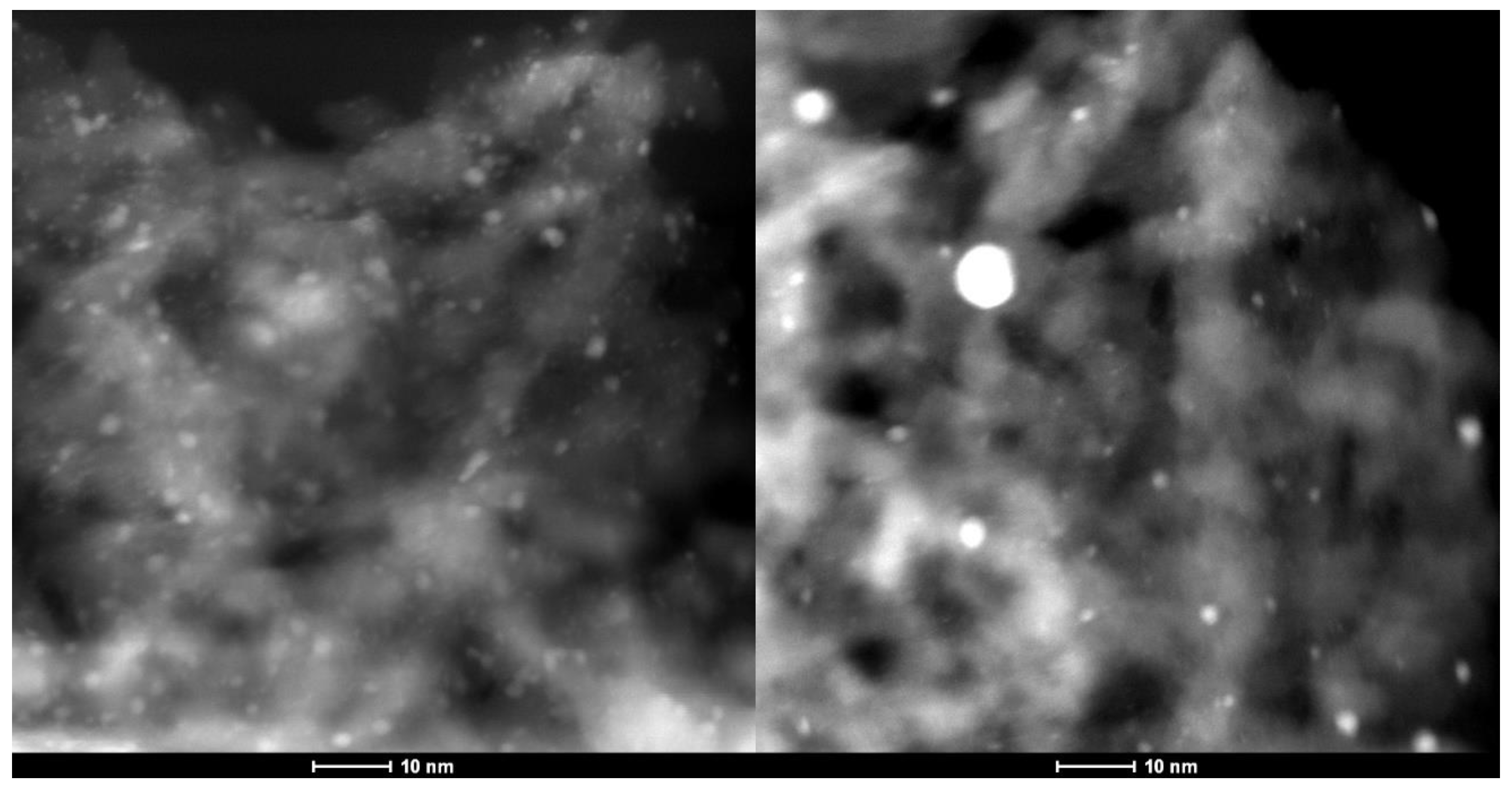
Table 4). The EXAFS spectroscopy results for Re5-H are consistent with HAADF-STEM images of this catalyst showing ~1-nm Re clusters (
Figure 8a) and some larger Re nanoparticles (
Figure 8b). The Re clusters are relatively uniform in size. The composite particle size distribution derived from these images is shown in
Figure S4.
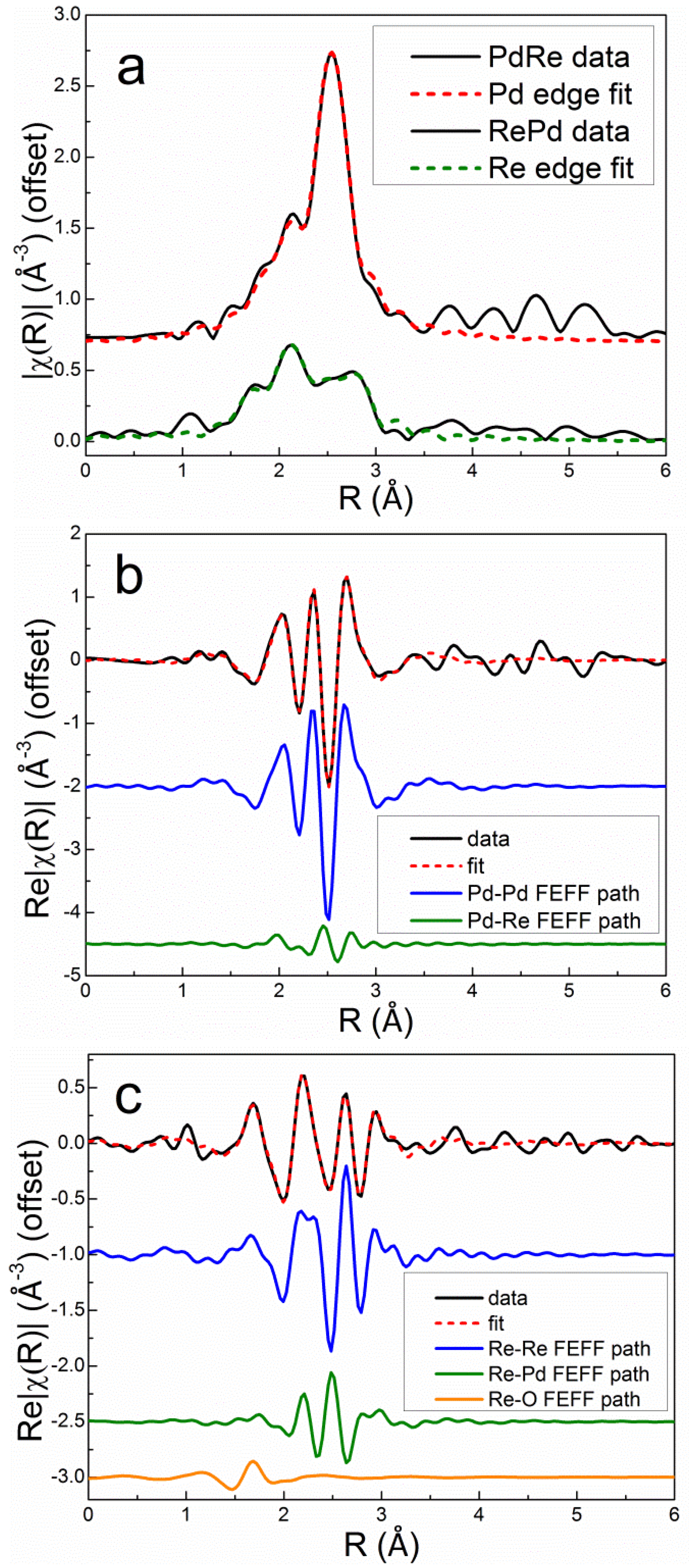
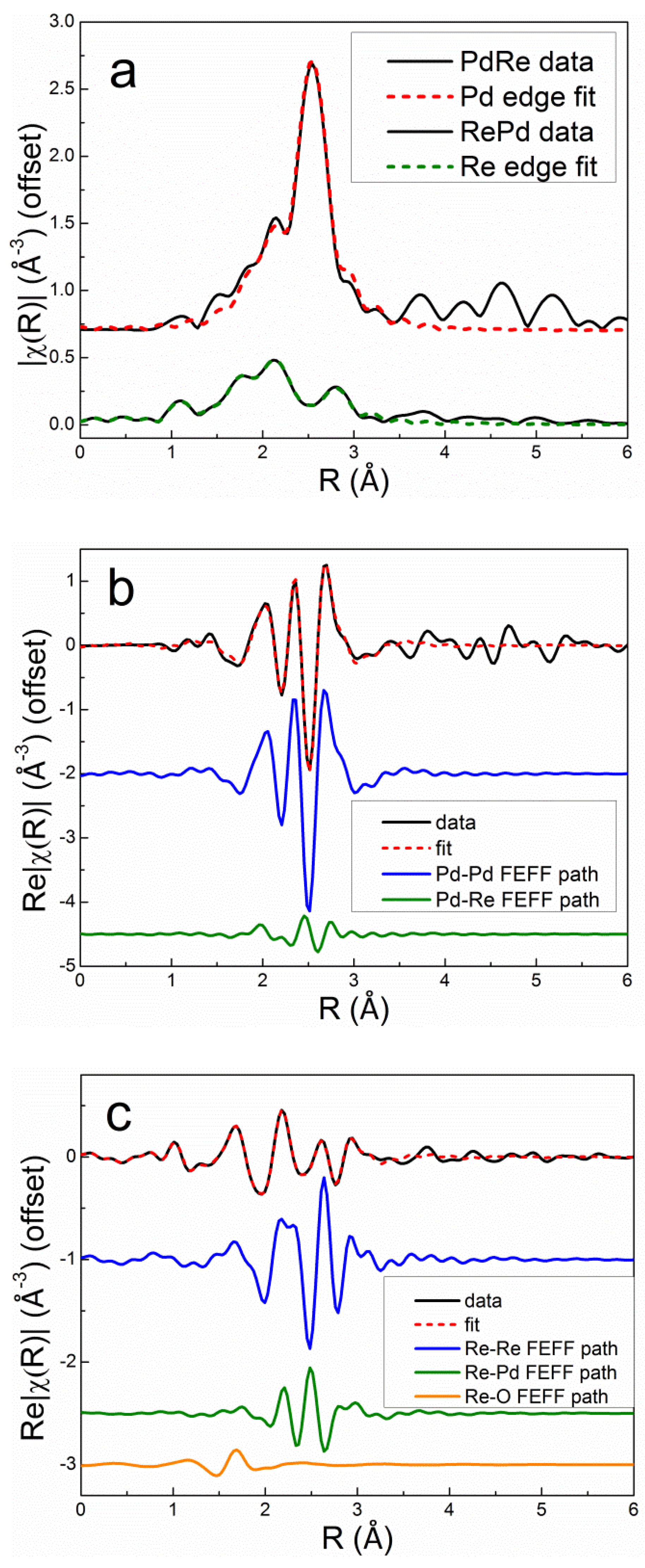
Excellent simultaneous fits at the Pd K and Re L
III edges were obtained for the bimetallic catalysts using two homometallic backscattering paths (Pd-Pd and Re-Re), two heterometallic paths (Pd-Re and Re-Pd), and a single Re-O path. Debye-Waller factors were evaluated for each homometallic bond. The heterometallic bond distances and Debye-Waller factors were constrained to be equal at the Pd K and Re L
III edges. The Pd-Re partial coordination number was constrained to be equal to the Re-Pd partial coordination number multiplied by the molar ratio of Re to Pd in the catalyst, i.e., the Via criterion [
35], ensuring consistency in fitting the heterometallic coordination numbers at each edge. The Debye-Waller factor for Re-O was set to 0.005 Å
2 based on experience and fitting the EXAFS spectrum of the calcined Re5-H catalyst. The overall best fits to the Pd K edge and Re L
III edge first-shell EXAFS spectra after reduction in flowing 3.5% H
2 at 400°C for 1 h are compared with the experimental data in
r space for Pd3Re5-CI and Re5Pd3-SI in
Figure 9 and
Figure 10, respectively. The fits clearly evidence that the homometallic backscattering paths are the dominant contributions; however, the heterometallic paths are significant, especially at the Re L
III edge. Destructive interference between the Re-Re and Re-Pd paths can be seen by comparing the real parts of the Fourier transforms. The Re-O backscattering paths are minor contributions for both catalysts.
The quantitative fitting results (
Table 4) show good consistency in the homometallic and heterometallic distances and Debye-Waller factors across the series of bimetallic catalysts. The R factors (one at each edge) evidence excellent agreement with the experimental data. In agreement with previous reports [
15,
16,
17,
19], the heterometallic and Re-Re distances are shorter than in the bulk metals. An early EXAFS spectroscopy investigation of a PdRe/Al
2O
3 catalyst prepared by co-impregnation observed contraction of the Pd-Re and Re-Re bonds from 2.74 Å in the metals to 2.72 Å for Re-Re and 2.67 Å for Pd-Re [
17]. Our fitting parameters evidence heterometallic distances of 2.69 to 2.71 Å depending on the preparation. Only in Re10Pd3-SI, which contains segregated Re nanoparticles, is the Re-Re distance equivalent to the bulk metal (or nearly so). Ziemecki, et al. employed in situ XRD of reduced PdRe/Al
2O
3 (prepared by impregnating Pd/Al
2O
3 with Re
2O
7) and observed two distinct alloy phases, both with smaller lattice parameters (3.84 and 3.88 Å) than Pd (3.89 Å) [
15,
16]. These lattice parameters correspond to metal-metal distances of 2.71 and 2.73 Å, respectively, which are equivalent to the Pd-Re and Pd-Pd bond distances from EXAFS spectroscopy of Pd3Re5-CI. A shorter Pd-Re distance (relative to the bulk metals) also was reported by Malinowski, et al. [
19] based on XRD. Interestingly, static and dynamic disorder (as measured by Debye-Waller factors) is consistently lower for the heterometallic backscattering paths than for the homometallic paths. Combining these data with the TPHD results, the Pd3Re5-CI and Pd3Re5-SI* catalysts appear to contain PdRe alloy nanoparticles albeit with different compositions.
The total first-shell coordination numbers, heteroatom compositions and Cowley short-range order parameters at the Pd K edge and Re L
III edge are provided in
Table 5. Data based on our previous EXAFS analysis of Re5Pd1.5-DCS are included for comparison [
11]. The first observation is that the Pd total coordination number (
NPd-M) is higher (and in most cases significantly) than the Re total coordination number (
NRe-M) for each catalyst,
except Re5Pd1.5-DCS where the total coordination numbers are equivalent.
NPd-M >
NRe-M indicates that either Re is segregated to the surface (and Pd to the core) of alloy nanoparticles and/or segregated Pd nanoparticles are larger than Re nanoparticles (or clusters). The former hypothesis appears to explain the data for Pd3Re5-CI and Pd3Re5-SI, and the latter the data for Re5Pd3-SI and Re10Pd3-SI. For Pd3Re5-CI, the coordination numbers are indicative of relatively large nanoparticles and are consistent with HAADF-STEM images showing (up to 30-nm) alloy particles [
10]. For Pd3Re5-SI*, both
NPd-M and
NRe-M are smaller suggesting small Pd-Re alloy nanoparticles (consistent with TPHD and CO chemisorption) with Re surface segregation. For Re5Pd3-SI,
NPd-Pd is much greater than
NRe-Re consistent with a mixture of Pd nanoparticles and small Re clusters (consistent with TPHD and CO chemisorption) as observed by HAADF-STEM [
10]. The segregated Re particles are largest in Re10Pd3-SI and smallest in Re5Pd3-SI as evidenced by the decreasing 2.9-Å peak in
Figure 6b. For the 1:1 Pd:Re catalysts, there are ~11% Re nearest neighbors (
NN) in the first coordination shell of Pd and ~15% Pd
NN in the first coordination shell of Re. These values are also consistent with Pd-Re alloy formation via Re dissolution in Pd with either Re surface enrichment and/or the presence of segregated Re species (clusters, nanoparticles). The %Re
NN (Pd) is similar for Re10Pd3-SI; however, the %Pd
NN (Re) is lower than expected which we attribute to the prevalence of segregated Re nanoparticles. In contrast, the Re5Pd1.5-DCS catalyst has a much higher %Re
NN (Pd) and a somewhat higher %Pd
NN (Re) indicating greater intermixing in these smaller bimetallic clusters than in bulk PdRe alloys [
11]. The Cowley short-range order parameter (
α) was calculated from the partial coordination numbers and overall composition, as follows:
where
NAB is the partial coordination number of Type A atoms (by Type B atoms),
NAM is total coordination number of Type A atoms (M = A + B), and
is the mole fraction of type B atoms [
36]. The Cowley short-range order parameter can vary on the interval: -1 <
α < 1 indicating homogeneous alloying to complete segregation of the metals. The crossover point,
α = 0, distinguishes between homogeneous and heterogeneous nanoalloys. The
αPd-Re) and
αRe-Pd) values for the bimetallic catalysts prepared by co-impregnation and sequential impregnation are 0.67 to 0.85 (
Table 5) indicating heterogeneity in local composition (e.g., segregation and clustering of like atoms). The heterogeneity seems to be slightly greater for Re atoms around Pd and for the Re10Pd5-SI catalyst. In contrast, the Cowley parameters are much smaller (but still positive) for the Re5Pd1.5-DCS catalyst indicating a tendency toward homogeneity; however, the bimetallic clusters remain heterogeneous on the local composition scale as evidenced by HAADF-STEM [
11].
As evidenced by TPR and XANES, not all Re in these catalysts is reduced to metal at 400°C, and this is reflected in the Re-O contributions. The Re-O bond length is nearly constant at 2.01-2.03 Å consistent with the average first-shell Re-O bond in ReO
2 [
37]. This Re-O bond is similar to that found by Tomishige and coworkers in fitting the Re L
III-edge spectra of RhRe-, PtRe-, and IrRe/SiO
2 catalysts [
5,
6,
7,
8]. Adding a Re-O path with a bond length of 1.73 Å (corresponding to perrhenate ion) did not improve our fits. When a second unrestricted Re-O path was added and the fit repeated, two bond lengths were found: 1.91 Å and 2.08 Å—the equatorial and axial Re-O distances in ReO
2. The Re-O coordination number decreases in the order: Re5-H > Re10Pd3-SI > Pd3Re5-SI* > Re5Pd3-SI > Pd3Re5-CI > Re5Pd1.5-DCS. Average Re oxidation states were calculated using the Re-O coordination numbers assuming the ReO
2 structure with octahedral coordination of Re
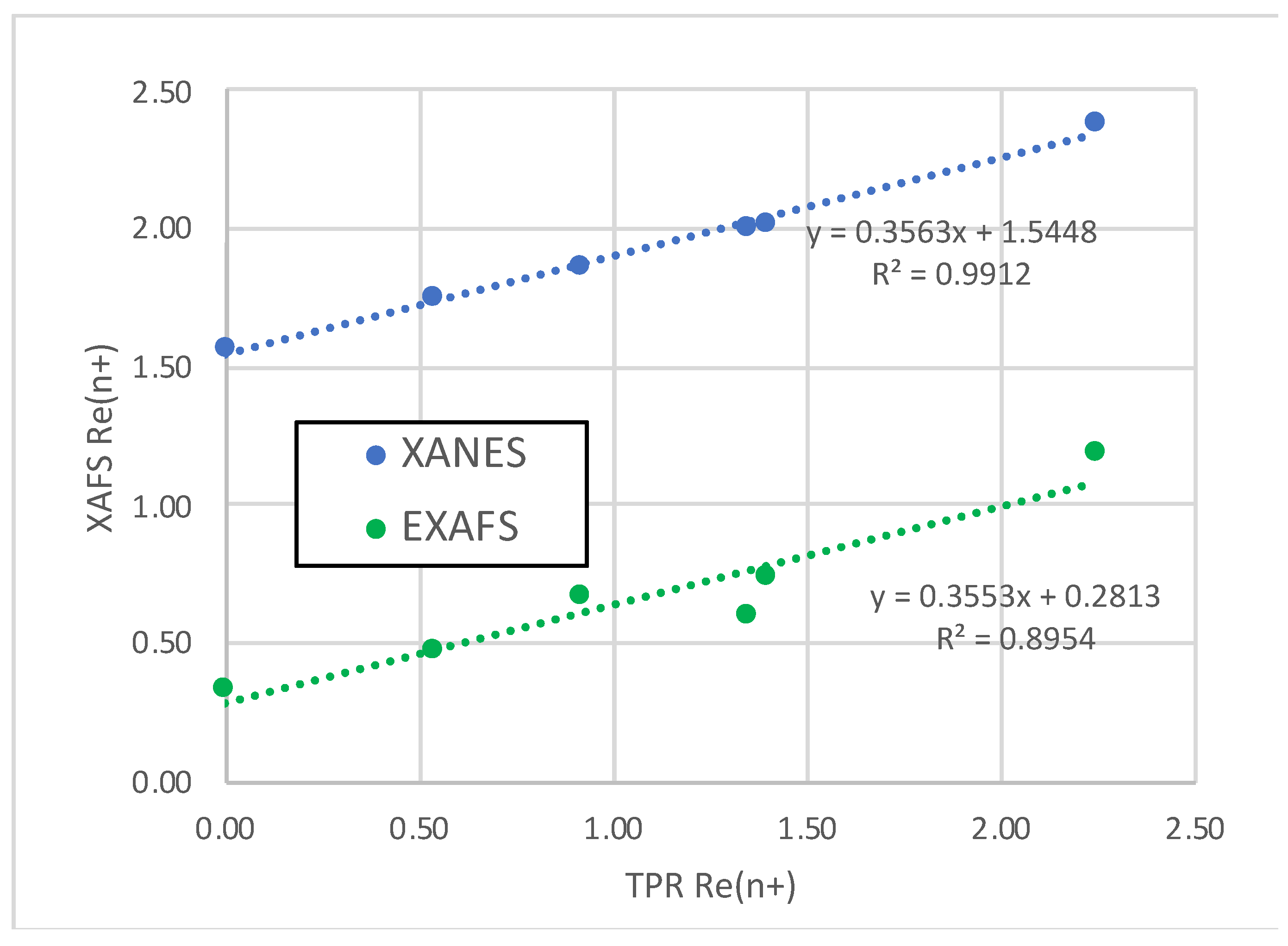
4+ by oxygen. The results (including a datum for Re5-H) are plotted using oxidation states determining by TPR on the abscissa in
Figure 4. We find a linear correlation with a closely similar slope to that found using the XANES-derived oxidation states; however, the
y intercept (+0.29) is much smaller consistent with a very small residual concentration of Re
4+ in Re5Pd1.5-SI. The difference in the
y intercepts (+1.26) suggests a net positive charge on the supported Re clusters (including potentially single atoms). Electrostatic interaction (charge transfer) between small Re particles (clusters) and the alumina support has been posited by Bare, et al. [
3], to explain edge shifts and higher-than-expected white line intensities for Re/MOR/Al
2O
3 catalysts reduced at 500 and 700°C in dry H
2. We suggest that these cationic Re species may explain the higher oxidation state inferred from Re L
III XANES edge shifts despite the relatively low Re-O coordination numbers indicated by EXAFS spectroscopy.