Submitted:
18 July 2024
Posted:
19 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Working Principle of Rechargeable Zinc Air Battery
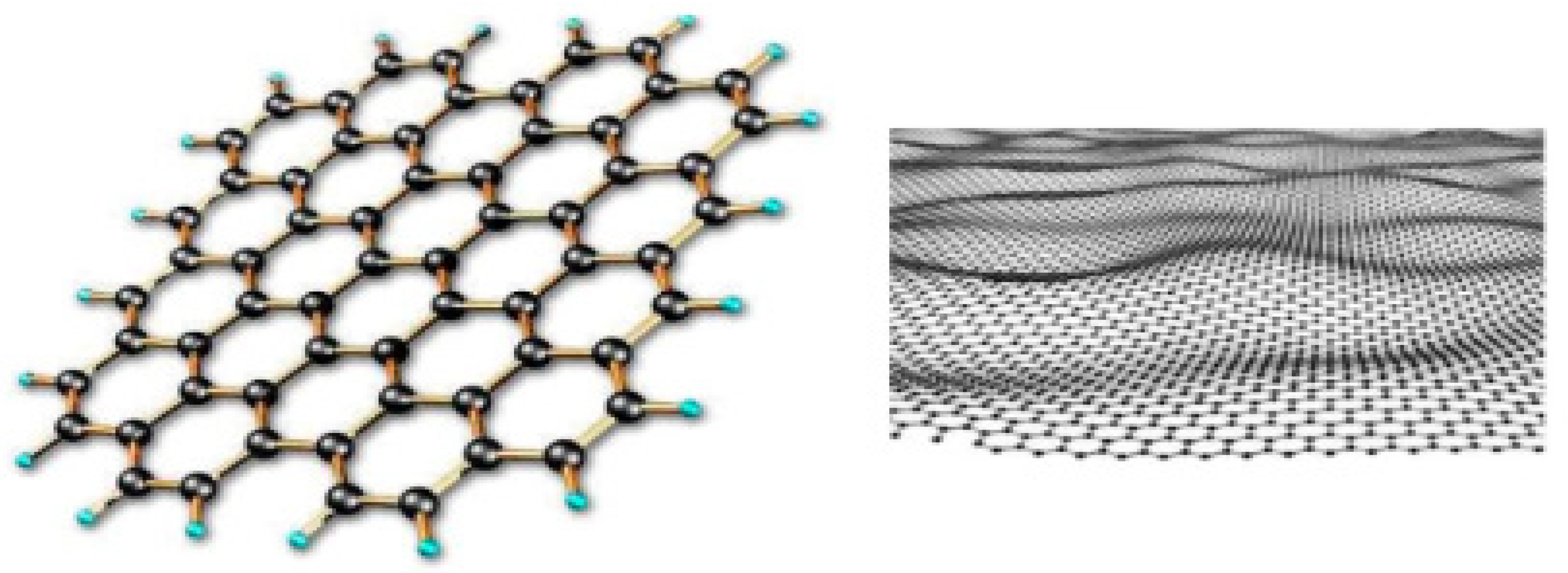
3. The Recent Advancement of Graphene
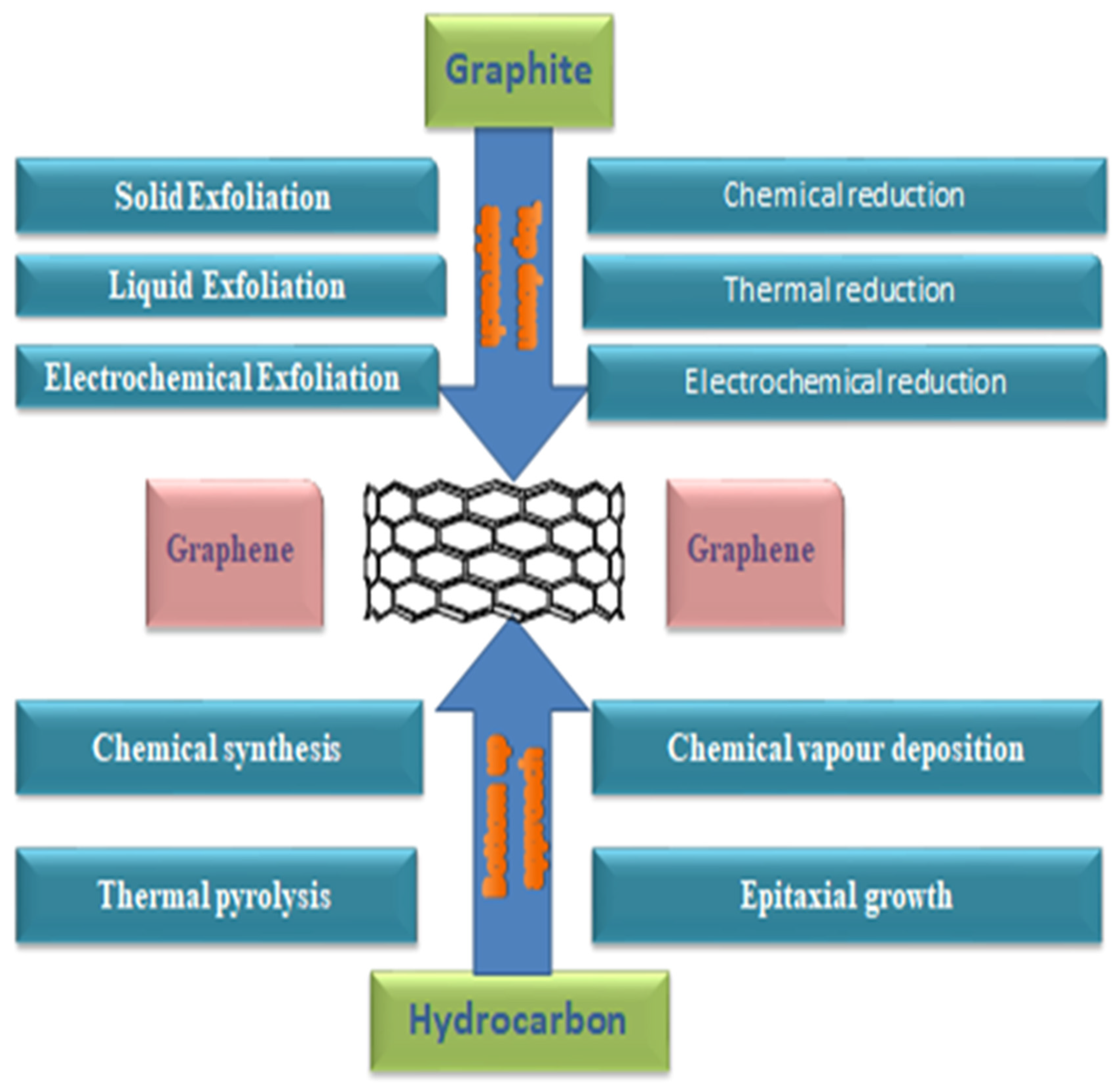
4. Preparation of Graphene
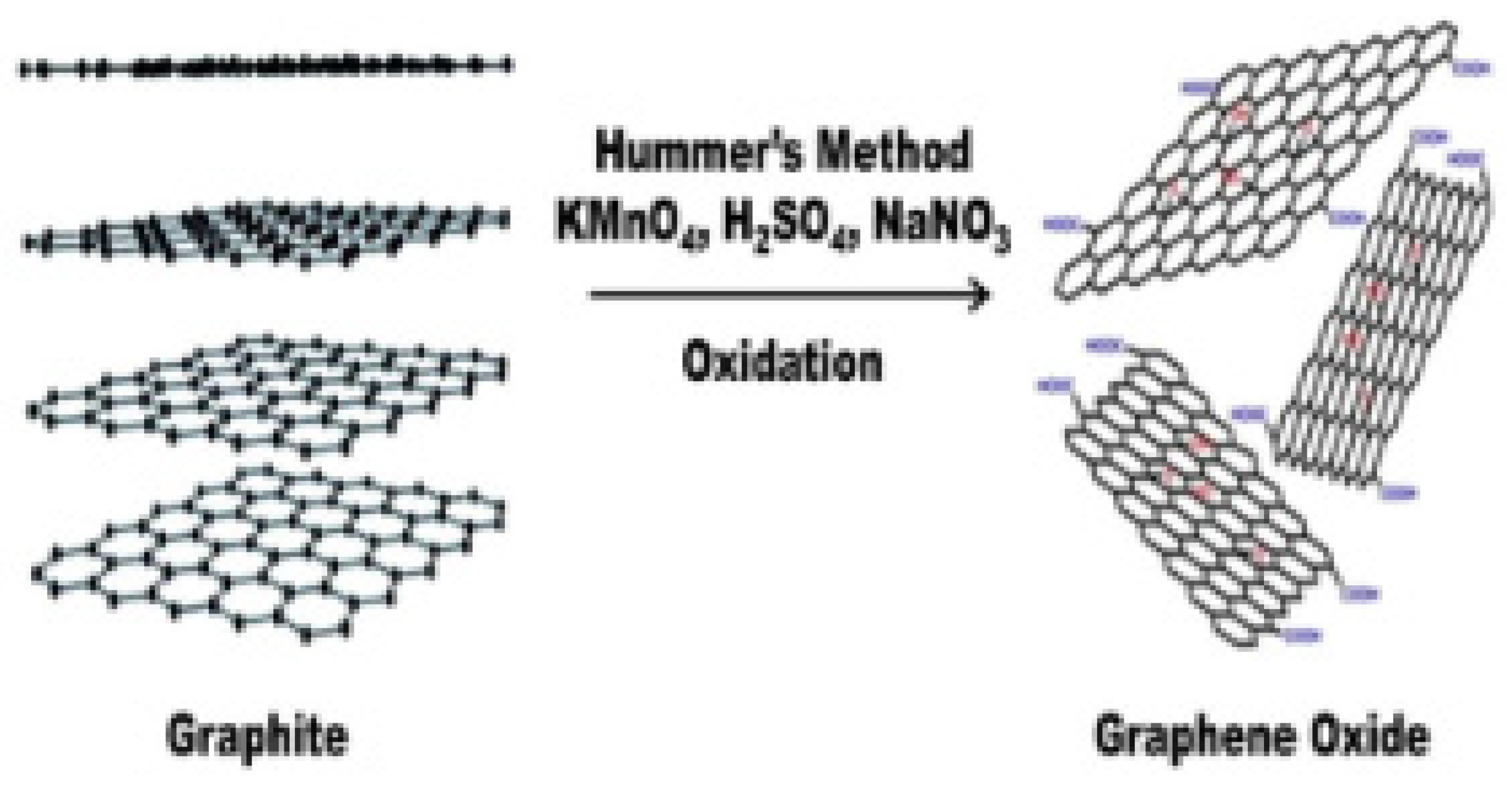
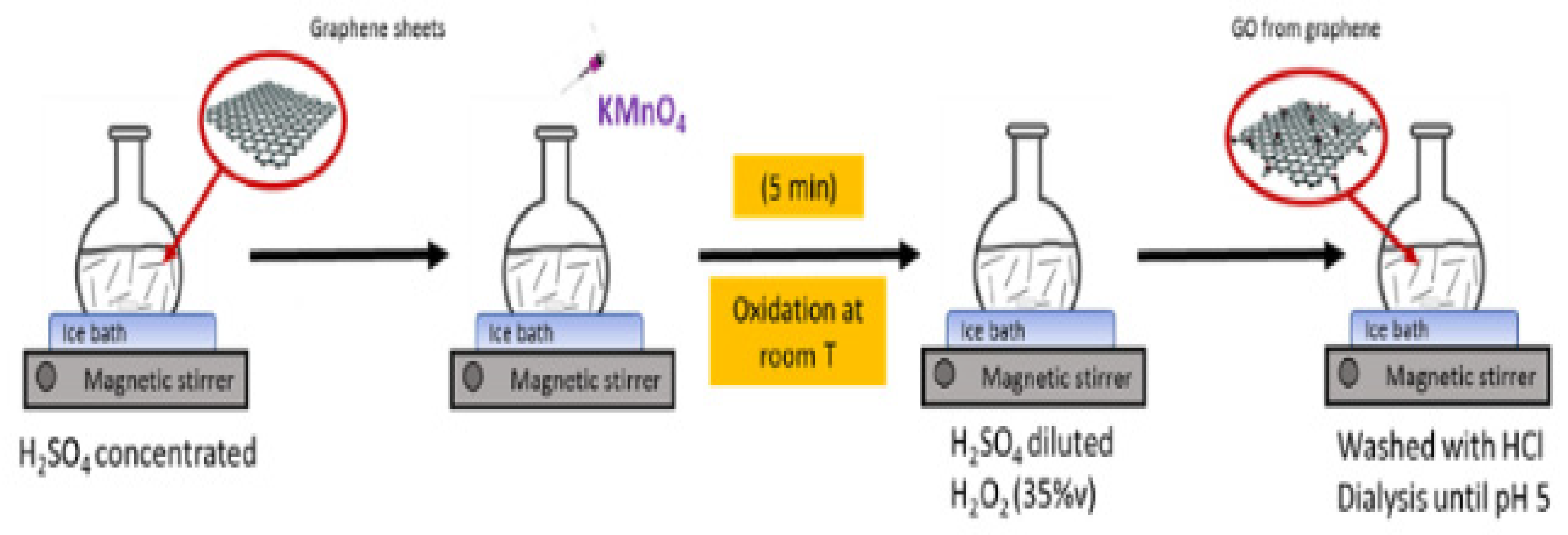
4.1. Synthesis of Graphene Oxide (GO)
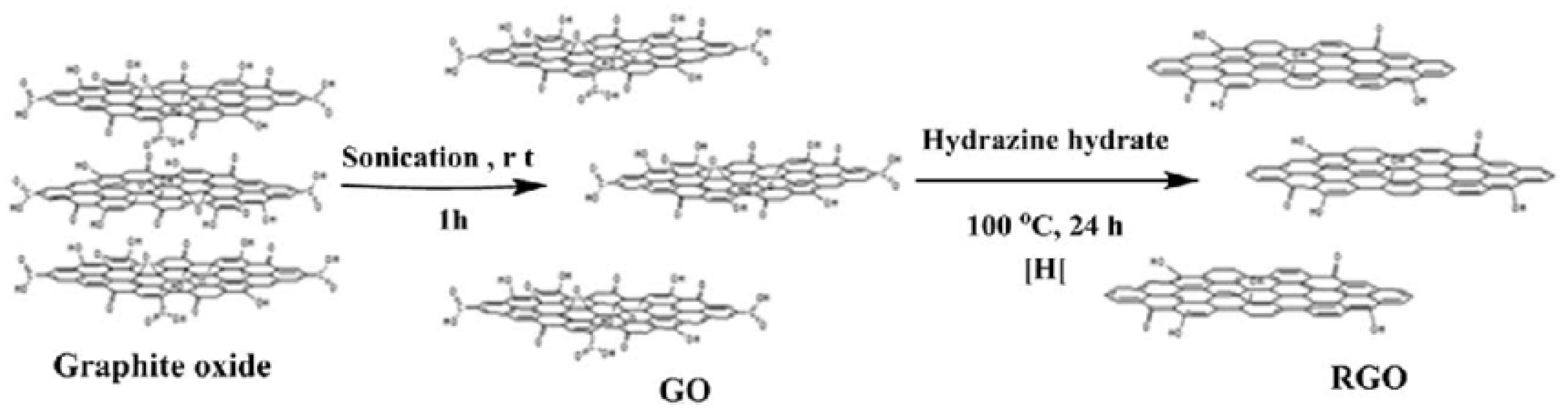
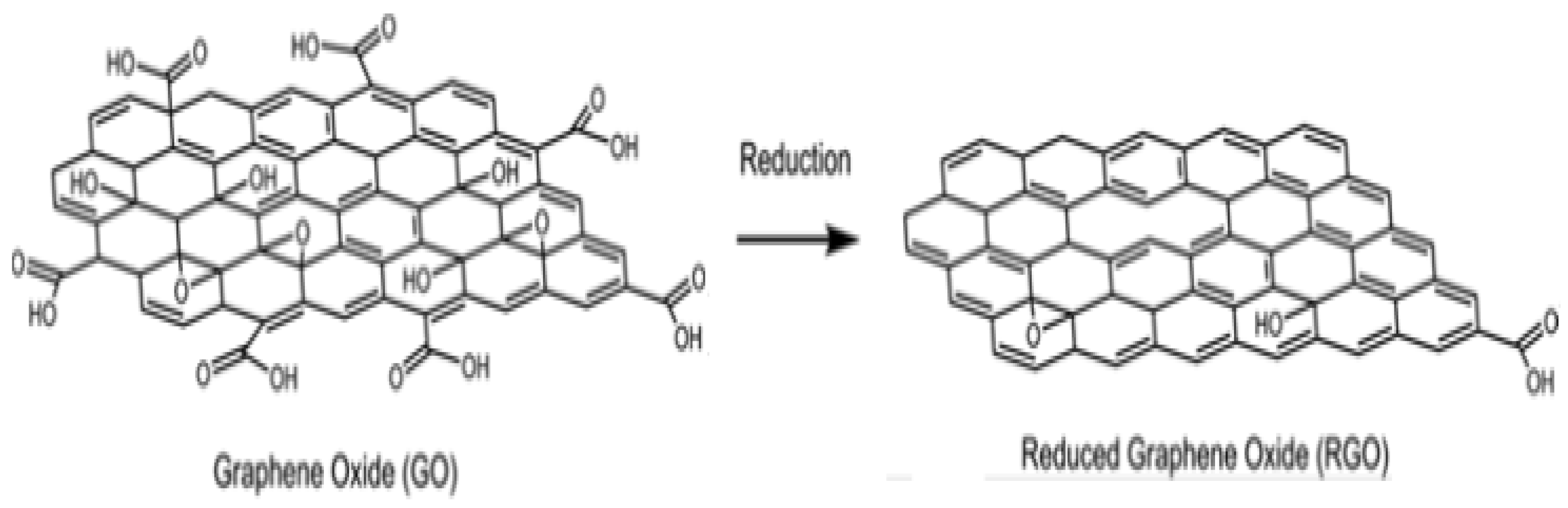
4.2. Synthesis of Reduced Graphene Oxide (RGO)
4.3. Chemical Reduction of GO to RGO
4.4. Thermal Reduction of GO to RGO
4.5. Photo-Thermal Reduction of GO to RGO
5. Electrocatalysts for ZABs
6. Graphene Based Cathode Material for Zinc-Air Battery
6.1. Nitrogen Doped Graphene (N/G (GO,rGO))
6.2. Metal Co-Doped Nitrogen Doped Graphene (M/N/G (GO, rGO))
6.3. Metal Oxide Co-Doped Nitrogen Doped Graphene (MOx/N/G (GO, rGO))
6.4. Bimetallic Metal Oxides Co-Doped Nitrogen Doped Graphene (M1M2Ox/N/G (GO,rGO))
7. Challenges and Prospectives
- Developing an extensive performance assessment of different graphene flaws in relation to OER/ORR activities is crucial. Systematic studies on the causes of altered OER/ORR performance resulting from different faults are still lacking. The explanation of each faulty site’s contribution is necessary to enhance comprehension of their respective responsibilities on graphene-based materials and enable their utilization in ZABs. Naturally, rigorous control studies on carbon materials with a single kind of defect are also required. Furthermore, it is important to understand how the location and quantity of dopants affect the degree of defect.
- In order to understand the electrocatalytic process of graphene-based catalysts for ZABs and to effectively develop and promote active sites, critical cathode electrode efficiency studies are desperately needed. Typically, catalyst recharge processes can result in structural changes to the active sites that are not detectable by standard characterisation techniques.
- Research on the functions of individual components in hybrid graphene-based ZAB catalysts is still needed. Researchers typically attribute these roles to the “synergistic effects” among the several components. In addition to proving the impact exists, we also need to clarify if the doping level and component location will affect this synergistic effect. Through comprehension and utilization of individual component contributions, we may enhance the efficiency of carbon-based cathodes for ZAB design.
- Since energy storage devices require rapid charging, the rate capability is a crucial technical requirement for applications involving rechargeable ZABs. Because of their enormous surface area and exceptional conductivity, graphene-based materials are commonly used in high-performance rechargeable zinc air batteries. Nevertheless, prior investigations have focused mostly on how using graphene materials in air cathodes can impact the rate capability of rechargeable ZABs.
- The inherent redox pathways of OER/ORR in air cathodes are the reason for the restricted gains in ZAB energy efficiency. In order to lower charge potentials, it is imperative to create more effective cathode systems, which might be motivated by the development of innovative cathodes made of carbon-based materials.
8. Conclusion
Data Availability Statement
Conflicts of Interest
References
- Z.L. Wang, D. Xu, J.J. Xu, X.B. Zhang, Oxygen electrocatalysts in metal-air batteries: From aqueous to nonaqueous electrolytes, Chem. Soc. Rev. 43 (2014) 7746–7786. [CrossRef]
- M. Asmare Alemu, A. Ketema Worku, M. Zegeye Getie, Recent Advancement of Electrically Rechargeable Di-Trivalent Metal-Air Batteries for Future Mobility, Results Chem. 6 (2023) 1199–1211. [CrossRef]
- J. Zhang, Q. Zhou, Y. Tang, L. Zhang, Y. Li, Zinc-air batteries: Are they ready for prime time?, Chem. Sci. 10 (2019) 8924–8929. [CrossRef]
- P. Chen, K. Zhang, D. Tang, W. Liu, F. Meng, Q. Huang, J. Liu, Recent Progress in Electrolytes for Zn–Air Batteries, Front. Chem. 8 (2020) 1–7. [CrossRef]
- X.W. Lv, Z. Wang, Z. Lai, Y. Liu, T. Ma, J. Geng, Z.Y. Yuan, Rechargeable Zinc–Air Batteries: Advances, Challenges, and Prospects, Small. (2023). [CrossRef]
- F. Santos, A. Urbina, J. Abad, R. López, C. Toledo, A.J. Fernández Romero, Environmental and economical assessment for a sustainable Zn/air battery, Chemosphere. 250 (2020) 126273. [CrossRef]
- P.A. García-salaberri, P.K. Das, A.M. Chaparro, Local oxygen transport resistance in polymer electrolyte fuel cells: origin, dependencies and mitigation, (2024) 1–18. [CrossRef]
- Vazhayal, J. Thomas, S. Ashok C, N. Thomas, A comprehensive review on the recent developments in transition metal-based electrocatalysts for oxygen evolution reaction, Appl. Surf. Sci. Adv. 6 (2021) 100184. [CrossRef]
- Haas, F. Holzer, K. Müller, S. Müller, Metal/air batteries: the zinc/air case, Handb. Fuel Cells. (2010) 1–27. [CrossRef]
- L. Jo, Bifunctional oxygen electrodes, J. Power Sources. 11 (1984) 215–220. [CrossRef]
- V.S. Velan, P. Karthika, N. Rajalakshmi, K.S. Dhathathreyan, A Novel Graphene Based Cathode for Metal–Air Battery, Graphene. 1 (2014) 86–92. [CrossRef]
- M.I. Jamesh, P. Moni, A.S. Prakash, M. Harb, ORR/OER activity and zinc-air battery performance of various kinds of graphene-based air catalysts, Mater. Sci. Energy Technol. 4 (2021) 1–22. [CrossRef]
- G. Nazir, A. Rehman, J.-H. Lee, C.-H. Kim, J. Gautam, K. Heo, S. Hussain, M. Ikram, A.A. AlObaid, S.-Y. Lee, S.-J. Park, A Review of Rechargeable Zinc–Air Batteries: Recent Progress and Future Perspectives, Nano-Micro Lett. 16 (2024) 138. [CrossRef]
- S.K. Yadav, D. Deckenbach, J.J. Schneider, Secondary Zinc–Air Batteries: A View on Rechargeability Aspects, Batteries. 8 (2022). [CrossRef]
- J. Fu, R. Liang, G. Liu, A. Yu, Z. Bai, L. Yang, Z. Chen, Recent Progress in Electrically Rechargeable Zinc–Air Batteries, Adv. Mater. 31 (2019) 1–13. [CrossRef]
- Q. Liu, P. Qiao, M. Tong, Y. Xie, X. Zhang, K. Lin, Z. Liang, L. Wang, H. Fu, Enhancing zinc–air battery performance by constructing three-dimensional N-doped carbon coating multiple valence Co and MnO heterostructures, Nano Res. (2024). [CrossRef]
- K.U. Nisa, W. da Silva Freitas, J. Montero, A. D’Epifanio, B. Mecheri, Development and Optimization of Air-Electrodes for Rechargeable Zn–Air Batteries, Catalysts. 13 (2023). [CrossRef]
- S. Huang, H. Li, P. Pei, K. Wang, Y. Xiao, C. Zhang, C. Chen, A Dendrite-Resistant Zinc-Air Battery, IScience. 23 (2020) 101169. [CrossRef]
- Y. Liu, J. Lu, S. Xu, W. Zhang, D. Gao, Carbon-based composites for rechargeable zinc-air batteries: A mini review, Front. Chem. 10 (2022) 1–7. [CrossRef]
- G. Toussaint, P. Stevens, F. Moureau, R. Rouget, F. Fourgeot, Development of a Rechargeable Zinc-Air Battery, ECS Meet. Abstr. MA2010-01 (2010) 757–757. [CrossRef]
- A.G. Williams, E. Moore, A. Thomas, J.A. Johnson, Graphene-Based Materials in Dental Applications: Antibacterial, Biocompatible, and Bone Regenerative Properties, Int. J. Biomater. 2023 (2023). [CrossRef]
- A.R. Urade, I. Lahiri, K.S. Suresh, Graphene Properties, Synthesis and Applications: A Review, Jom. 75 (2023) 614–630. [CrossRef]
- F. Catania, E. Marras, M. Giorcelli, P. Jagdale, L. Lavagna, A. Tagliaferro, M. Bartoli, A review on recent advancements of graphene and graphene-related materials in biological applications, Appl. Sci. 11 (2021) 1–21. [CrossRef]
- Mondal, N.R. Jana, Graphene-Nanoparticle Composites and Their Applications in Energy, Environmental and Biomedical Science, Rev. Nanosci. Nanotechnol. 3 (2014) 177–192. [CrossRef]
- Frank, R. Blume, A. Rinaldi, A. Trunschke, R. Schlögl, Oxygen insertion catalysis by sp2 carbon, Angew. Chemie—Int. Ed. 50 (2011) 10226–10230. [CrossRef]
- Z. Yin, J. Zhu, Q. He, X. Cao, C. Tan, H. Chen, Q. Yan, H. Zhang, Graphene-Based materials for solar cell applications, Adv. Energy Mater. 4 (2014) 1–19. [CrossRef]
- S. Navalón, W.J. Ong, X. Duan, Sustainable catalytic processes driven by graphene-based materials, Processes. 8 (2020). [CrossRef]
- D.D. MacDonald, The history of the Point Defect Model for the passive state: A brief review of film growth aspects, Electrochim. Acta. 56 (2011) 1761–1772. [CrossRef]
- L. Wang, S. Ma, Z. Jiao, D. Yuan, Capability of defective graphene-supported Co 4 nanoparticle toward ammonia dehydrogenation, Appl. Surf. Sci. 465 (2019) 1–9. [CrossRef]
- Bashir, A. Mehvish, M. Khalil, Advanced Carbon Materials for Sustainable and Emerging Applications, 21st Century Adv. Carbon Mater. Eng. Appl.—A Compr. Handb. (2021) 1–19. [CrossRef]
- H. Etri, Graphene: A State-of-the-Art Review of Types, Properties and Applications in Different Sectors, Prabha Mater. Sci. Lett. 2 (2023) 98–139. [CrossRef]
- K. Ma, S. Zhang, B. Ye, J. Ouyang, G.H. Yue, A new view of graphene oxide biosafety in a water environment using an eatable fish as a model, RSC Adv. 6 (2016) 29619–29623. [CrossRef]
- P. Yang, X. Yang, W. Liu, R. Guo, Z. Yao, Graphene-based electrocatalysts for advanced energy conversion, Green Energy Environ. 8 (2023) 1265–1278. [CrossRef]
- S. Navalón, J.R. Herance, M. Álvaro, H. García, Frontispiece: Covalently Modified Graphenes in Catalysis, Electrocatalysis and Photoresponsive Materials, Chem.—A Eur. J. 23 (2017) 15275. [CrossRef]
- A.T. Lawal, Graphene-based nano composites and their applications. A review, Biosens. Bioelectron. 141 (2019) 111384. [CrossRef]
- Y. Tian, Z. Yu, L. Cao, X.L. Zhang, C. Sun, D.W. Wang, Graphene oxide: An emerging electromaterial for energy storage and conversion, J. Energy Chem. 55 (2021) 323–344. [CrossRef]
- D. Jana, C.L. Sun, L.C. Chen, K.H. Chen, Effect of chemical doping of boron and nitrogen on the electronic, optical, and electrochemical properties of carbon nanotubes, Prog. Mater. Sci. 58 (2013) 565–635. [CrossRef]
- C.N.R. Rao, K. Gopalakrishnan, A. Govindaraj, Synthesis, properties and applications of graphene doped with boron, nitrogen and other elements, Nano Today. 9 (2014) 324–343. [CrossRef]
- H. Wang, T. Maiyalagan, X. Wang, Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications, ACS Catal. 2 (2012) 781–794. [CrossRef]
- K.S. Kim, Y. Zhao, H. Jang, S.Y. Lee, J.M. Kim, K.S. Kim, J.H. Ahn, P. Kim, J.Y. Choi, B.H. Hong, Large-scale pattern growth of graphene films for stretchable transparent electrodes, Nature. 457 (2009) 706–710. [CrossRef]
- A.H. Castro Neto, F. Guinea, N.M.R. Peres, K.S. Novoselov, A.K. Geim, The electronic properties of graphene, Rev. Mod. Phys. 81 (2009) 109–162. [CrossRef]
- A.T. Smith, A.M. LaChance, S. Zeng, B. Liu, L. Sun, Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites, Nano Mater. Sci. 1 (2019) 31–47. [CrossRef]
- D. Wu, W. Song, L. Chen, X. Duan, Q. Xia, X. Fan, Y. Li, F. Zhang, W. Peng, S. Wang, High-performance porous graphene from synergetic nitrogen doping and physical activation for advanced nonradical oxidation, J. Hazard. Mater. 381 (2020) 1–10. [CrossRef]
- N. Bundaleska, J. Henriques, M. Abrashev, A.M. Botelho do Rego, A.M. Ferraria, A. Almeida, F.M. Dias, E. Valcheva, B. Arnaudov, K.K. Upadhyay, M.F. Montemor, E. Tatarova, Large-scale synthesis of free-standing N-doped graphene using microwave plasma, Sci. Rep. 8 (2018) 1–11. [CrossRef]
- D. Wei, Y. Liu, Y. Wang, H. Zhang, L. Huang, G. Yu, Synthesis of n-doped graphene by chemical vapor deposition and its electrical properties, Nano Lett. 9 (2009) 1752–1758. [CrossRef]
- Y. Shang, H. Xu, M. Li, G. Zhang, Preparation of N-Doped Graphene by Hydrothermal Method and Interpretation of N-Doped Mechanism, Nano. 12 (2017) 1–9. [CrossRef]
- N.E. Graphene, A. Ilnicka, M. Skorupska, P. Romanowski, P. Kamedulski, J.P. Lukaszewicz, Improving the Performance of Zn-Air Batteries with, (2020) 1–14.
- B.F. MacHado, P. Serp, Graphene-based materials for catalysis, Catal. Sci. Technol. 2 (2012) 54–75. [CrossRef]
- Ababay Ketema Worku, Delele Worku Ayele, Recent advances of graphene-based materials for emerging technologies, Results Chem. 5 (2023) 100971. [CrossRef]
- S. Navalon, A. Dhakshinamoorthy, M. Alvaro, M. Antonietti, H. García, Active sites on graphene-based materials as metal-free catalysts, Chem. Soc. Rev. 46 (2017) 4501–4529. [CrossRef]
- X. Chen, W. Da Oh, T.T. Lim, Graphene- and CNTs-based carbocatalysts in persulfates activation: Material design and catalytic mechanisms, Chem. Eng. J. 354 (2018) 941–976. [CrossRef]
- Y. Jia, J. Zhang, D. Kong, C. Zhang, D. Han, J. Han, Y. Tao, W. Lv, Q.-H. Yang, Practical Graphene Technologies for Electrochemical Energy Storage, Adv. Funct. Mater. 32 (2022) 2204272. [CrossRef]
- B. Gürünlü, Ç. Taşdelen Yücedaǧ, M.R. Bayramoǧlu, Green Synthesis of Graphene from Graphite in Molten Salt Medium, J. Nanomater. 2020 (2020). [CrossRef]
- E. Lai, X. Yue, W. Ning, J. Huang, X. Ling, H. Lin, Three-Dimensional Graphene-Based Composite Hydrogel Materials for Flexible Supercapacitor Electrodes, Front. Chem. 7 (2019) 1–5. [CrossRef]
- N. Kumar, R. Salehiyan, V. Chauke, O. Joseph Botlhoko, K. Setshedi, M. Scriba, M. Masukume, S. Sinha Ray, Top-down synthesis of graphene: A comprehensive review, FlatChem. 27 (2021) 100224. [CrossRef]
- A.K.M.S. Islam, R.T. Hussain, M. Khairuddean, F.B. Mohd Suah, N.M. Ahmed, Innovative Approaches to Synthesize Novel Graphene Materials, Curr. Nanosci. 17 (2021) 829–843. [CrossRef]
- M.I. Kairi, S. Dayou, N.I. Kairi, S.A. Bakar, B. Vigolo, A.R. Mohamed, Toward high production of graphene flakes-a review on recent developments in their synthesis methods and scalability, J. Mater. Chem. A. 6 (2018) 15010–15026. [CrossRef]
- F.S. Mirza, Z.E.H. Aftab, M.D. Ali, A. Aftab, T. Anjum, H. Rafiq, G. Li, Green synthesis and application of GO nanoparticles to augment growth parameters and yield in mungbean (Vigna radiata L.), Front. Plant Sci. 13 (2022) 1–19. [CrossRef]
- M. Saeed, Y. Alshammari, S.A. Majeed, E. Al-Nasrallah, Chemical Vapour Deposition of Graphene—Synthesis, Characterisation, and Applications: A Review, Molecules. 25 (2020). [CrossRef]
- D. Chen, H. Feng, J. Li, Graphene oxide: Preparation, functionalization, and electrochemical applications, Chem. Rev. 112 (2012) 6027–6053. [CrossRef]
- D. Torres, J.L. Pinilla, R. Moliner, I. Suelves, On the oxidation degree of few-layer graphene oxide sheets obtained from chemically oxidized multiwall carbon nanotubes, Carbon N. Y. 81 (2015) 405–417. [CrossRef]
- D.A. Jasim, N. Lozano, K. Kostarelos, Synthesis of few-layered, high-purity graphene oxide sheets from different graphite sources for biology, 2D Mater. 3 (2016). [CrossRef]
- V. Palermo, I.A. Kinloch, S. Ligi, N.M. Pugno, Nanoscale Mechanics of Graphene and Graphene Oxide in Composites: A Scientific and Technological Perspective, Adv. Mater. 28 (2016) 6232–6238. [CrossRef]
- S.S. Sekhon, P. Kaur, Y.H. Kim, S.S. Sekhon, 2D graphene oxide–aptamer conjugate materials for cancer diagnosis, Npj 2D Mater. Appl. 5 (2021). [CrossRef]
- M.C.F. Costa, V.S. Marangoni, P.R. Ng, H.T.L. Nguyen, A. Carvalho, A.H. Castro Neto, Accelerated synthesis of graphene oxide from graphene, Nanomaterials. 11 (2021) 1–8. [CrossRef]
- J. McDonald-Wharry, M. Manley-Harris, K. Pickering, Carbonisation of biomass-derived chars and the thermal reduction of a graphene oxide sample studied using Raman spectroscopy, Carbon N. Y. 59 (2013) 383–405. [CrossRef]
- K.K.H. De Silva, H.H. Huang, R.K. Joshi, M. Yoshimura, Chemical reduction of graphene oxide using green reductants, Carbon N. Y. 119 (2017) 190–199. [CrossRef]
- A.T. Habte, D.W. Ayele, M. Hu, Synthesis and Characterization of Reduced Graphene Oxide (rGO) Started from Graphene Oxide (GO) Using the Tour Method with Different Parameters, Adv. Mater. Sci. Eng. 2019 (2019). [CrossRef]
- M. Kurian, Recent progress in the chemical reduction of graphene oxide by green reductants–A Mini review, Carbon Trends. 5 (2021) 100120. [CrossRef]
- U.N. Maiti, W.J. Lee, J.M. Lee, Y. Oh, J.Y. Kim, J.E. Kim, J. Shim, T.H. Han, S.O. Kim, 25th anniversary article: Chemically modified/doped carbon nanotubes & graphene for optimized nanostructures & nanodevices, Adv. Mater. 26 (2014) 40–67. [CrossRef]
- S. Sadhukhan, T.K. Ghosh, D. Rana, I. Roy, A. Bhattacharyya, G. Sarkar, M. Chakraborty, D. Chattopadhyay, Studies on synthesis of reduced graphene oxide (RGO) via green route and its electrical property, Mater. Res. Bull. 79 (2016) 41–51. [CrossRef]
- I.O. Faniyi, O. Fasakin, B. Olofinjana, A.S. Adekunle, T. V. Oluwasusi, M.A. Eleruja, E.O.B. Ajayi, The comparative analyses of reduced graphene oxide (RGO) prepared via green, mild and chemical approaches, SN Appl. Sci. 1 (2019) 1–7. [CrossRef]
- S.N. Tripathi, G.S.S. Rao, A.B. Mathur, R. Jasra, Polyolefin/graphene nanocomposites: A review, RSC Adv. 7 (2017) 23615–23632. [CrossRef]
- S.M.S. Al-Mufti, A. Almontasser, S.J.A. Rizvi, Single and double thermal reduction processes for synthesis reduced graphene oxide assisted by a muffle furnace: A facile robust synthesis and rapid approach to enhance electrical conductivity, AIP Adv. 12 (2022). [CrossRef]
- R. Hidayat, S. Wahyuningsih, A.H. Ramelan, Simple synthesis of rGO (reduced graphene oxide) by thermal reduction of GO (graphene oxide), IOP Conf. Ser. Mater. Sci. Eng. 858 (2020). [CrossRef]
- Fitrilawati, M.B. Perkasa, N. Syakir, A. Aprilia, L. Safriani, T. Saragi, Risdiana, S. Hidayat, A. Bahtiar, R. Siregar, R.R. Sihombing, A. Nugroho, Thermal Reduction Study of Graphene Oxide Paper, IOP Conf. Ser. Mater. Sci. Eng. 196 (2017). [CrossRef]
- F. Zhang, Y.H. Li, M.Y. Qi, Y.M.A. Yamada, M. Anpo, Z.R. Tang, Y.J. Xu, Photothermal catalytic CO2 reduction over nanomaterials, Chem Catal. 1 (2021) 272–297. [CrossRef]
- D. Mateo, J.L. Cerrillo, S. Durini, J. Gascon, Fundamentals and applications of photo-thermal catalysis, Chem. Soc. Rev. 50 (2021) 2173–2210. [CrossRef]
- E. Davari, A.D. Johnson, A. Mittal, M. Xiong, D.G. Ivey, Manganese-cobalt mixed oxide film as a bifunctional catalyst for rechargeable zinc-air batteries, Electrochim. Acta. 211 (2016) 735–743. [CrossRef]
- E. Davari, D.G. Ivey, Bifunctional electrocatalysts for Zn-air batteries, Sustain. Energy Fuels. 2 (2018) 39–67. [CrossRef]
- Y. Wang, E. Wang, X. Zhang, H. Yu, High-Voltage “single-Crystal” Cathode Materials for Lithium-Ion Batteries, Energy and Fuels. 35 (2021) 1918–1932. [CrossRef]
- C. Goswami, K.K. Hazarika, P. Bharali, Transition metal oxide nanocatalysts for oxygen reduction reaction, Mater. Sci. Energy Technol. 1 (2018) 117–128. [CrossRef]
- C. Simanjuntak, R. Siburian, H. Marpaung, Tamrin, Properties of Mg/graphite and Mg/graphene as cathode electrode on primary cell battery, Heliyon. 6 (2020) e03118. [CrossRef]
- B.K. Mandal, Sulfur Cathode for High-Capacity Lithium-Sulfur Cells, Energies. 15 (2022) 1–11.
- L. Ji, P. Meduri, V. Agubra, X. Xiao, M. Alcoutlabi, Graphene-Based Nanocomposites for Energy Storage, Adv. Energy Mater. 6 (2016) 7–16. [CrossRef]
- L. Poolnapol, W. Kao-Ian, A. Somwangthanaroj, F. Mahlendorf, M.T. Nguyen, T. Yonezawa, S. Kheawhom, Silver decorated reduced graphene oxide as electrocatalyst for zinc–air batteries, Energies. 13 (2020). [CrossRef]
- H. Purwaningsih, W. Widiyastuti, H. Setyawan, Synthesis and characterization of magnetite/graphene nanocomposite material for electrocatalyst of zinc-Air battery chatode, AIP Conf. Proc. 2384 (2021). [CrossRef]
- M.E.S. Ali, H.A. Tariq, B. Moossa, Z.A. Qureshi, R. Kahraman, S. Al-Qaradawi, R.A. Shakoor, LiMn2O4—MXene nanocomposite cathode for high-performance lithium-ion batteries, Energy Reports. 11 (2024) 2401–2414. [CrossRef]
- Q. Liu, Z. Li, Y. Liu, H. Zhang, Y. Ren, C. Sun, W. Lu, Y. Zhou, L. Stanciu, E.A. Stach, J. Xie, electrochemical performance for Li-ion batteries, (2015) 1–10. [CrossRef]
- B. Campbell, J. Manning, The rise of victimhood culture: Microaggressions, safe spaces, and the new culture wars, Rise Vict. Cult. Microaggressions, Safe Spaces, New Cult. Wars. (2018) 1–265. [CrossRef]
- D.G. Papageorgiou, I.A. Kinloch, R.J. Young, Mechanical properties of graphene and graphene-based nanocomposites, Prog. Mater. Sci. 90 (2017) 75–127. [CrossRef]
- V. Lightcap, T.H. Kosel, P. V. Kamat, Anchoring semiconductor and metal nanoparticles on a two-dimensional catalyst mat. storing and shuttling electrons with reduced graphene oxide, Nano Lett. 10 (2010) 577–583. [CrossRef]
- W. Zhang, Y. Liu, L. Zhang, J. Chen, Recent advances in isolated single-atom catalysts for zinc air batteries: A focus review, Nanomaterials. 9 (2019) 1–18. [CrossRef]
- .Tang, W. Chen, H. Chai, G. Zhao, Y. Li, D. Ma, X. Dai, Metal- and Nonmetal-Atom-Modified Graphene as Efficient Catalysts for CO Oxidation Reactions, J. Phys. Chem. C. 123 (2019) 10926–10939. [CrossRef]
- C.B. Clemons, M.W. Roberts, J.P. Wilber, G.W. Young, A. Buldum, D.D. Quinn, Continuum plate theory and atomistic modeling to find the flexural rigidity of a graphene sheet interacting with a substrate, J. Nanotechnol. 2010 (2010). [CrossRef]
- N. Sharma, R. Dev Gupta, R. Chandmal Sharma, S. Dayal, A. Singh Yadav, Graphene: An overview of its characteristics and applications, Mater. Today Proc. 47 (2021) 2752–2755. [CrossRef]
- S. Tang, X. Zhou, N. Xu, Z. Bai, J. Qiao, J. Zhang, Template-free synthesis of three-dimensional nanoporous N-doped graphene for high performance fuel cell oxygen reduction reaction in alkaline media, Appl. Energy. 175 (2016) 405–413. [CrossRef]
- X. Duan, K. O’Donnell, H. Sun, Y. Wang, S. Wang, Sulfur and Nitrogen Co-Doped Graphene for Metal-Free Catalytic Oxidation Reactions, Small. 11 (2015) 3036–3044. [CrossRef]
- X. Cai, L. Lai, J. Lin, Z. Shen, Recent advances in air electrodes for Zn-air batteries: Electrocatalysis and structural design, Mater. Horizons. 4 (2017) 945–976. [CrossRef]
- L.L. Tian, J. Yang, M.Y. Weng, R. Tan, J.X. Zheng, H.B. Chen, Q.C. Zhuang, L.M. Dai, F. Pan, Fast Diffusion of O2 on Nitrogen-Doped Graphene to Enhance Oxygen Reduction and Its Application for High-Rate Zn-Air Batteries, ACS Appl. Mater. Interfaces. 9 (2017) 7125–7130. [CrossRef]
- C.H. Choi, H.K. Lim, M.W. Chung, J.C. Park, H. Shin, H. Kim, S.I. Woo, Long-range electron transfer over graphene-based catalyst for high-performing oxygen reduction reactions: Importance of size, n-doping, and metallic impurities, J. Am. Chem. Soc. 136 (2014) 9070–9077. [CrossRef]
- W. Tang, J. Mai, L. Liu, N. Yu, L. Fu, Y. Chen, Y. Liu, Y. Wu, T. van Ree, Recent advances of bifunctional catalysts for zinc air batteries with stability considerations: from selecting materials to reconstruction, Nanoscale Adv. 5 (2023) 4368–4401. [CrossRef]
- R. Cheng, Y. Min, H. Li, C. Fu, Electronic structure regulation in the design of low-cost efficient electrocatalysts: From theory to applications, Nano Energy. 115 (2023) 108718. [CrossRef]
- Y. Irmawati, F. Balqis, P.B. Persada, F. Destyorini, R. Yudianti, F. Iskandar, A. Sumboja, Iron-Decorated Nitrogen/Boron co-Doped Reduced Graphene Oxide Aerogel for Neutral Rechargeable Zn-Air Batteries, Batteries. 9 (2023). [CrossRef]
- T. Huang, S. Mao, M. Qiu, O. Mao, C. Yuan, J. Chen, Nitrogen-boron Dipolar-doped Nanocarbon as a High-efficiency Electrocatalyst for Oxygen Reduction Reaction, Electrochim. Acta. 222 (2016) 481–487. [CrossRef]
- L.S. Panchakarla, K.S. Subrahmanyam, S.K. Saha, A. Govindaraj, H.R. Krishnamurthy, U. V Waghmare, C.N.R. Rao, Synthesis, Structure, and Properties of Boron- and, Adv. Mater. 27 (2009) 4726–4730.
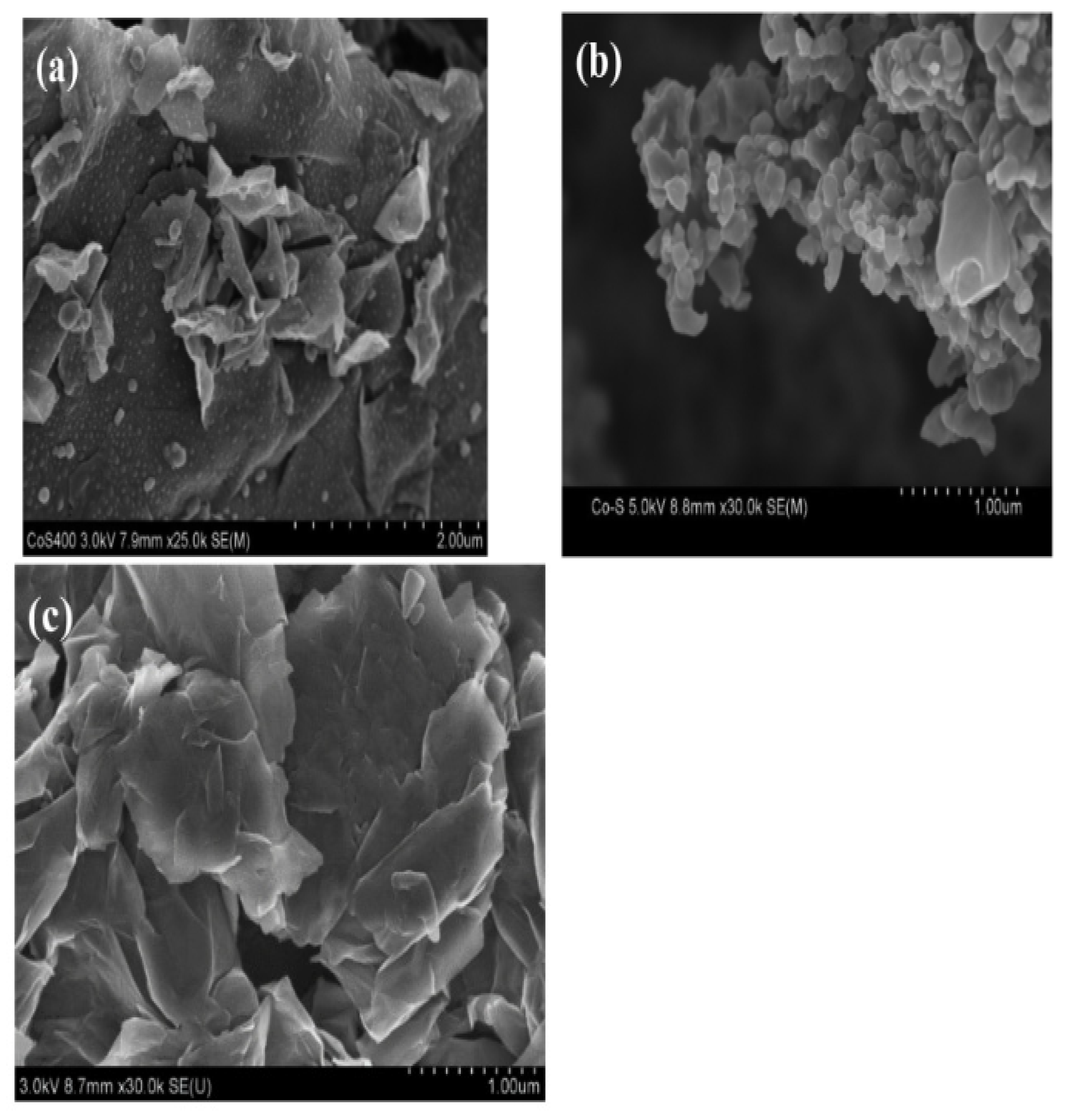
- D. Geng, N.N. Ding, T.S.A. Hor, S.W. Chien, Z. Liu, Y. Zong, Cobalt sulfide nanoparticles impregnated nitrogen and sulfur co-doped graphene as bifunctional catalyst for rechargeable Zn-air batteries, RSC Adv. 5 (2015) 7280–7284. [CrossRef]
- M. Sun, F. Yuan, R. Li, S. Dong, Y. Zhao, W. Zhong, C. Shen, J. Wu, H. Zheng, Fe-Fe3C Functionalized Few-Layer Graphene Sheet Nanocomposites for an Efficient Electrocatalyst of the Oxygen Reduction Reaction, ACS Omega. 7 (2022) 25458–25465. [CrossRef]
- Y. Hu, J.O. Jensen, W. Zhang, L.N. Cleemann, W. Xing, N.J. Bjerrum, Q. Li, Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts, Angew. Chemie—Int. Ed. 53 (2014) 3675–3679. [CrossRef]
- S. Calabuig-Mompó, D. Cazorla-Amorós, E. Morallón, Electrocatalysts based on graphene oxide and its buckypaper for enhanced Zn-air battery performance, J. Electroanal. Chem. 955 (2024). [CrossRef]
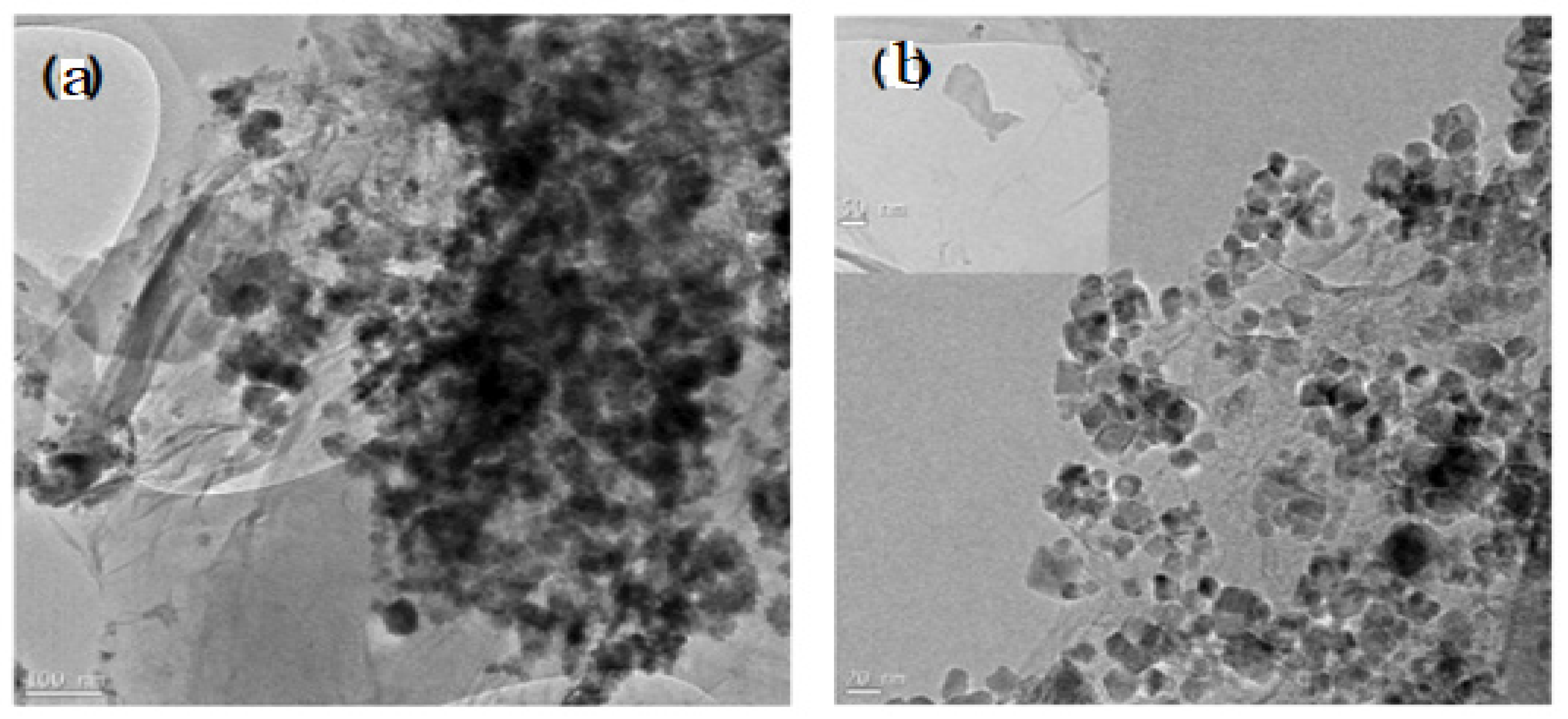
- Y. Chen, H. Wang, F. Liu, H. Gai, S. Ji, V. Linkov, R. Wang, Hydrophobic 3D Fe/N/S doped graphene network as oxygen electrocatalyst to achieve unique performance of zinc-air battery, Chem. Eng. J. 353 (2018) 472–480. [CrossRef]
- S. Kattel, P. Atanassov, B. Kiefer, Catalytic activity of Co-Nx/C electrocatalysts for oxygen reduction reaction: A density functional theory study, Phys. Chem. Chem. Phys. 15 (2013) 148–153. [CrossRef]
- Niu, B. Yang, J. Cui, J. Jin, X. Fu, Q. Zhao, J. Zhang, Graphene-based non-noble-metal Co/N/C catalyst for oxygen reduction reaction in alkaline solution, J. Power Sources. 243 (2013) 65–71. [CrossRef]
- Y. Balasooriya, P. Samarasekara, C.M. Lim, Y.F.C. Chau, M.R.R. Kooh, R. Thotagamuge, Cu—Nitrogen doped graphene (Cu–N/Gr) nanocomposite as cathode catalyst in fuel cells—DFT study, Heliyon. 9 (2023) e15989. [CrossRef]
- S. Liu, X. Wan, Y. Sun, S. Li, X. Guo, M. Li, R. Yin, Q. Kong, J. Kong, J. Zhang, Cobalt-based multicomponent nanoparticles supported on N-doped graphene as advanced cathodic catalyst for zinc—air batteries, Int. J. Miner. Metall. Mater. 29 (2022) 2212–2220. [CrossRef]
- H.L. Jia, J. Zhao, L. Gu, Z.J. Peng, Z.L. Bao, X.L. Sun, M.Y. Guan, Highly active Co-N-doped graphene as an efficient bifunctional electrocatalyst (ORR/HER) for flexible all-solid-state zinc-air batteries, Sustain. Energy Fuels. 4 (2020) 6165–6173. [CrossRef]
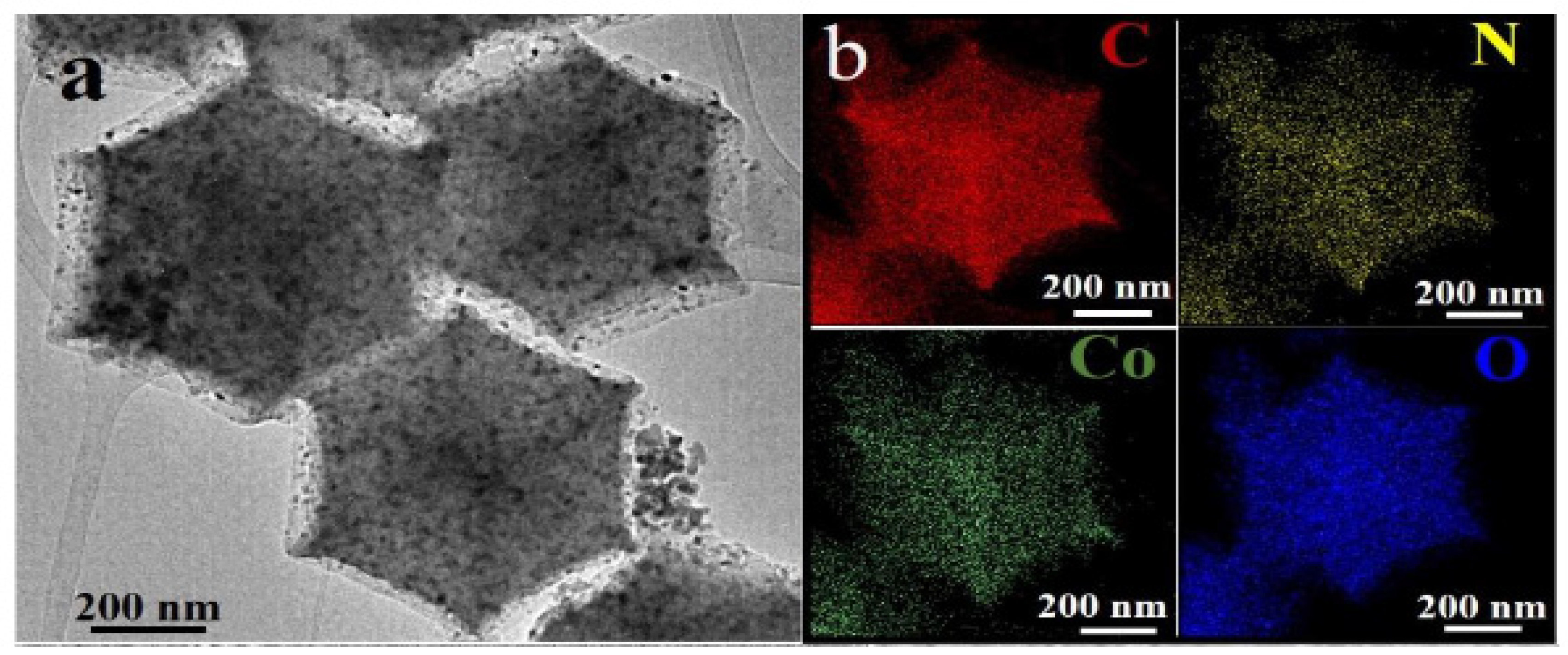
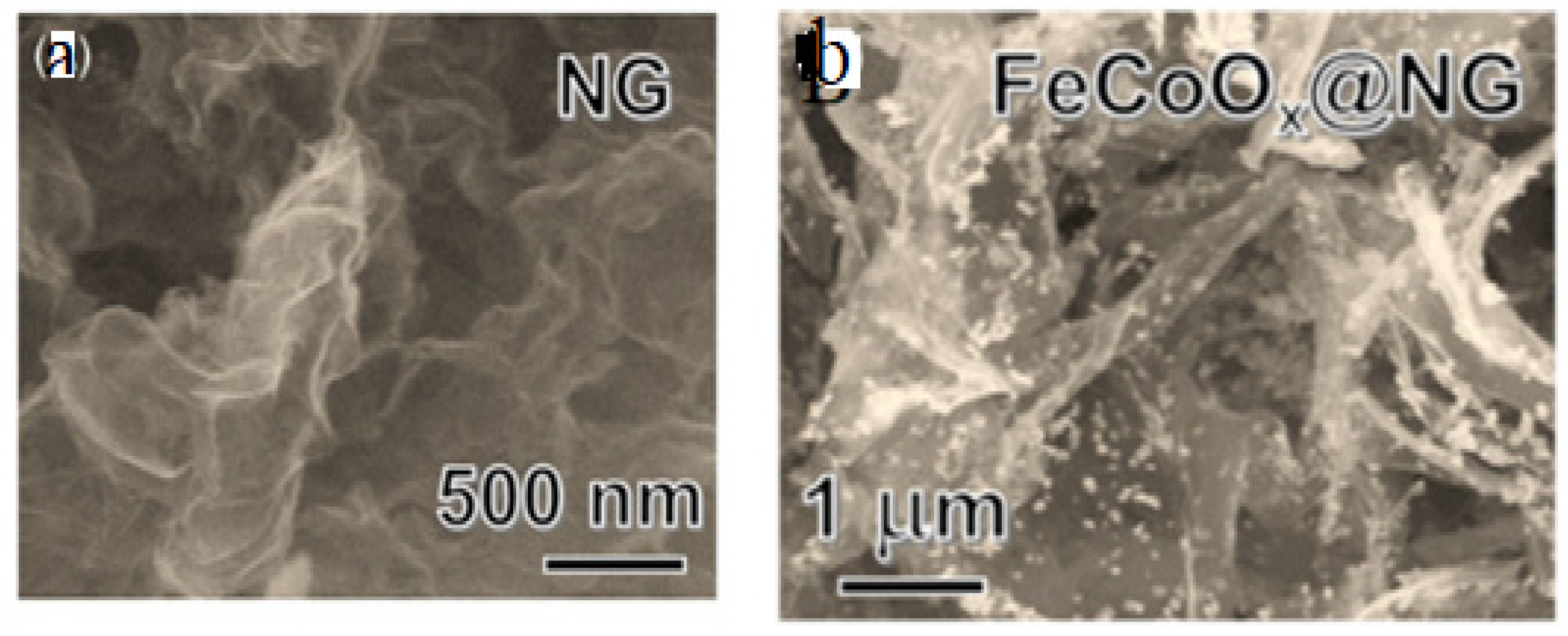
- Z. Zheng, C. Wang, P. Mao, Y. Zhu, R. Ran, W. Zhou, K. Liao, Z. Shao, In situ formation of self-antistacking FeCoOx on N-doped graphene: A 3D-on-2D nanoarchitecture for long-life Zn–air batteries, Carbon Energy. 5 (2023). [CrossRef]
- Xu, C. Wang, D. Deng, Y. Tian, X. He, G. Lu, J. Qian, S. Yuan, H. Li, Cobalt Oxide Nanoparticles/Nitrogen-Doped Graphene as the Highly Efficient Oxygen Reduction Electrocatalyst for Rechargeable Zinc-Air Batteries, ACS Sustain. Chem. Eng. 8 (2020) 343–350. [CrossRef]
- Wang, Y. Hou, R.C.T. Slade, J. Wang, D. Shi, D. Wexler, H. Liu, J. Chen, Core-shell Co/CoO integrated on 3D nitrogen doped reduced graphene oxide aerogel as an enhanced electrocatalyst for the oxygen reduction reaction, Front. Chem. 4 (2016) 1–10. [CrossRef]
- Q. Shen, J. Yang, K.L. Chen, H. Wang, J.B. Liu, H. Yan, Co3O4 nanorods–graphene composites as catalysts for rechargeable zinc-air battery, J. Solid State Electrochem. 20 (2016) 3331–3336. [CrossRef]
- Yu, Y. Chen, Highly efficient Co3O4 / Co @ NCs bifunctional oxygen electrocatalysts for long life rechargeable Zn-air batteries, (2020).
- Prabu, P. Ramakrishnan, S. Shanmugam, CoMn2O4 nanoparticles anchored on nitrogen-doped graphene nanosheets as bifunctional electrocatalyst for rechargeable zinc-air battery, Electrochem. Commun. 41 (2014) 59–63. [CrossRef]
- J. Hu, Q. Liu, Z. Shi, L. Zhang, H. Huang, LaNiO3-nanorod/graphene composite as an efficient bi-functional catalyst for zinc-air batteries, RSC Adv. 6 (2016) 86386–86394. [CrossRef]
- J. Hu, L. Wang, L. Shi, H. Huang, Electrochimica Acta Oxygen reduction reaction activity of LaMn 1-x Co x O 3 -graphene nanocomposite for zinc-air battery, 161 (2015) 115–123. [CrossRef]
- Ganesan, P. Ramakrishnan, M. Prabu, S. Shanmugam, Electrochimica Acta Nitrogen and Sulfur Co-doped Graphene Supported Cobalt Sul fi de Nanoparticles as an Ef fi cient Air Cathode for Zinc-air Battery, Electrochim. Acta. (2015). [CrossRef]
- Ganesan, P. Ramakrishnan, M. Prabu, S. Shanmugam, Nitrogen and Sulfur Co-doped Graphene Supported Cobalt Sulfide Nanoparticles as an Efficient Air Cathode for Zinc-air Battery, Electrochim. Acta. 183 (2015) 63–69. [CrossRef]
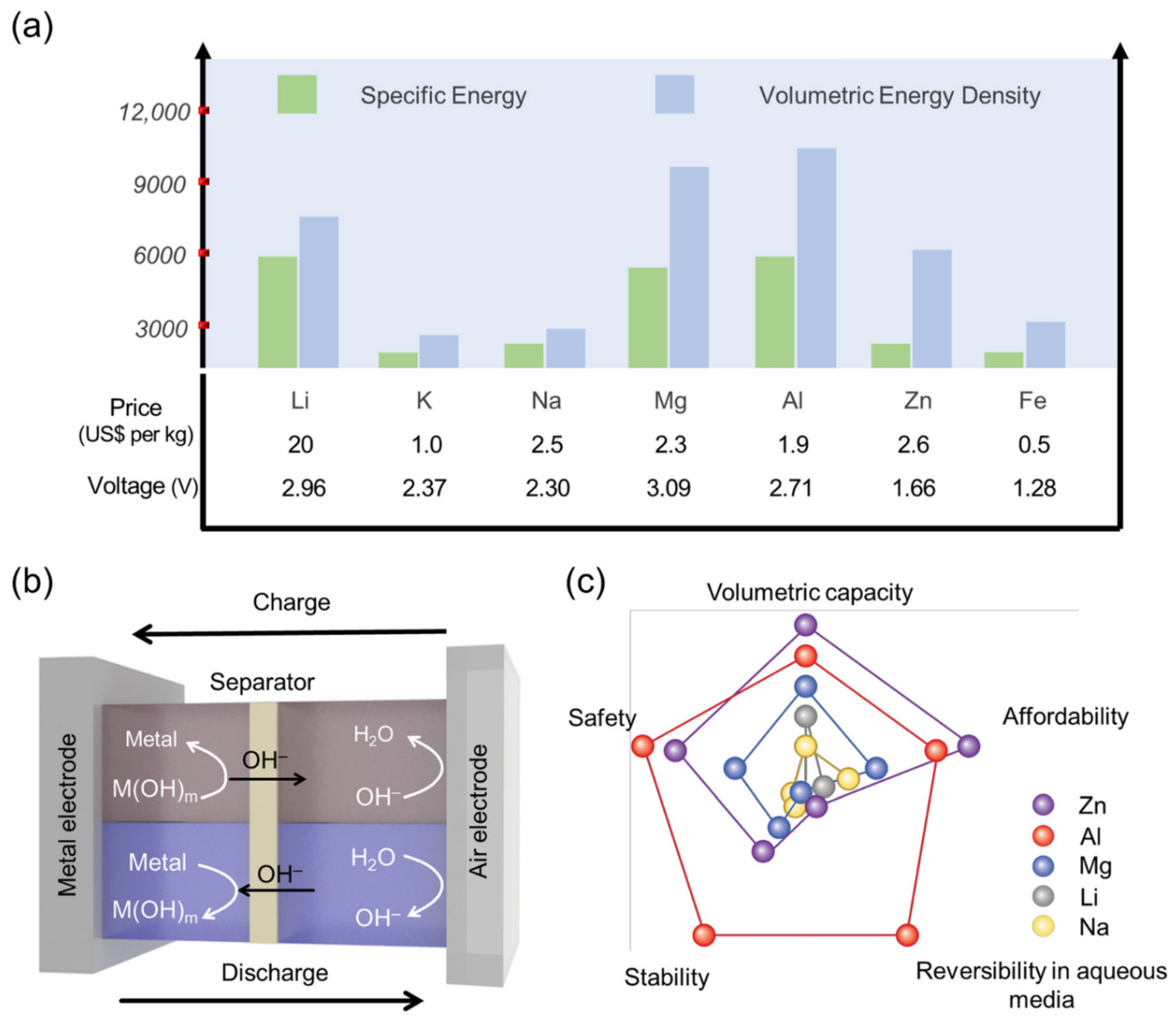
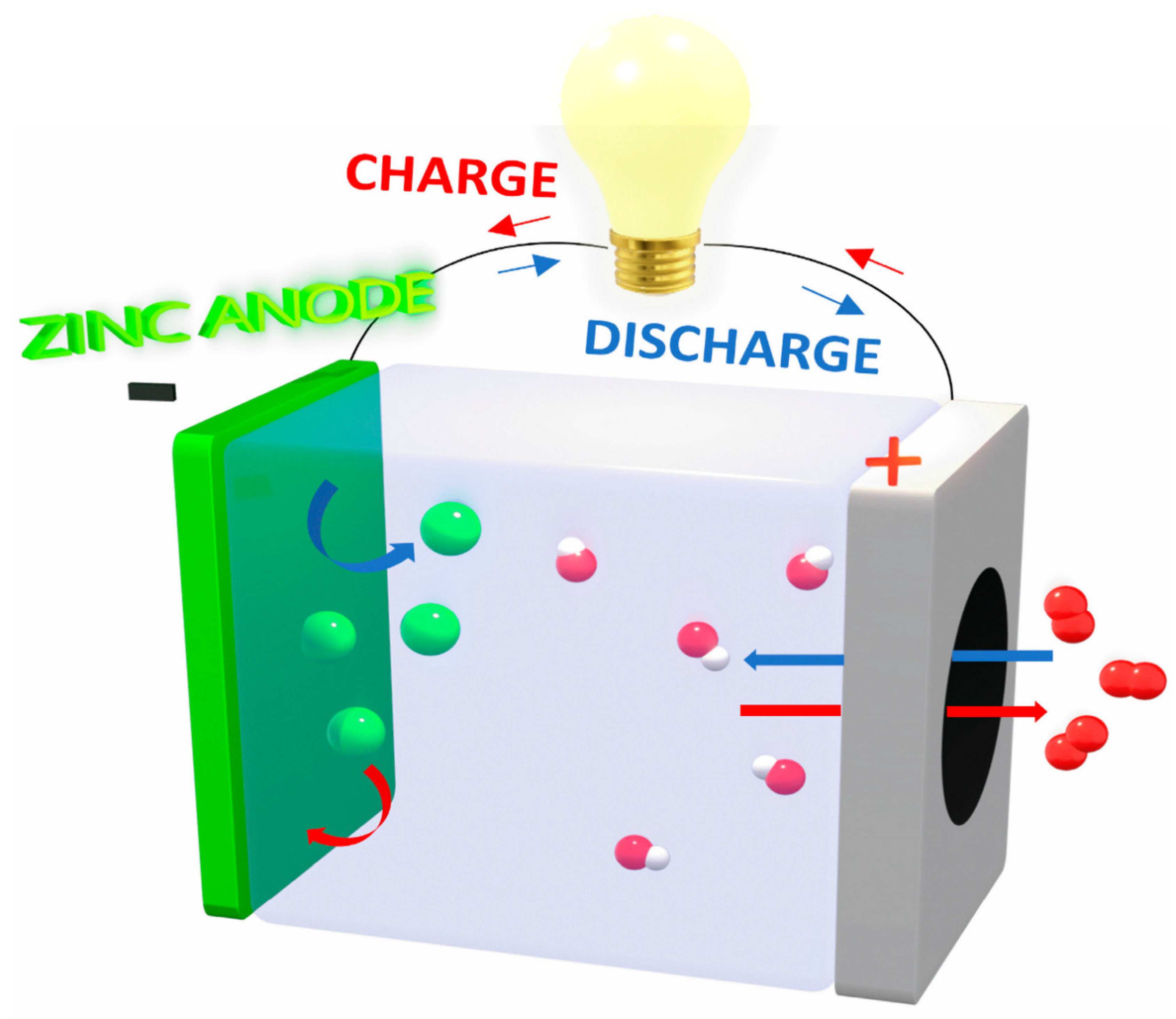
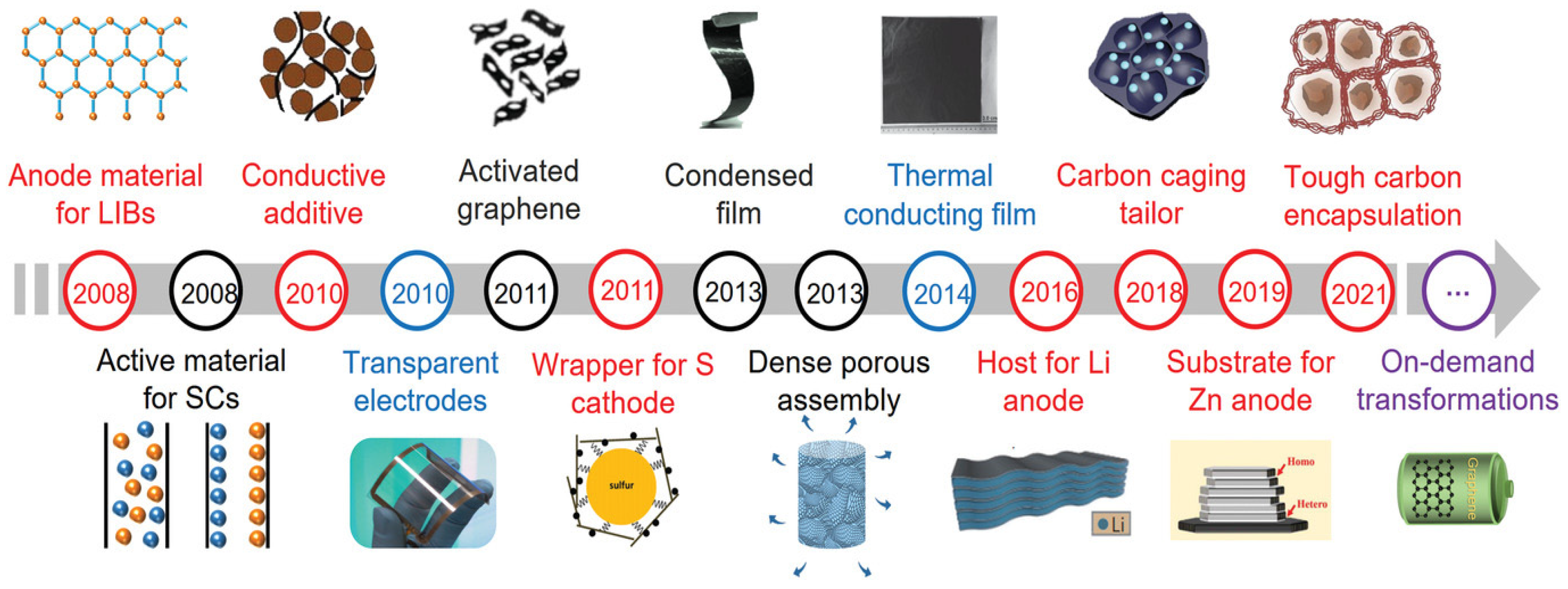
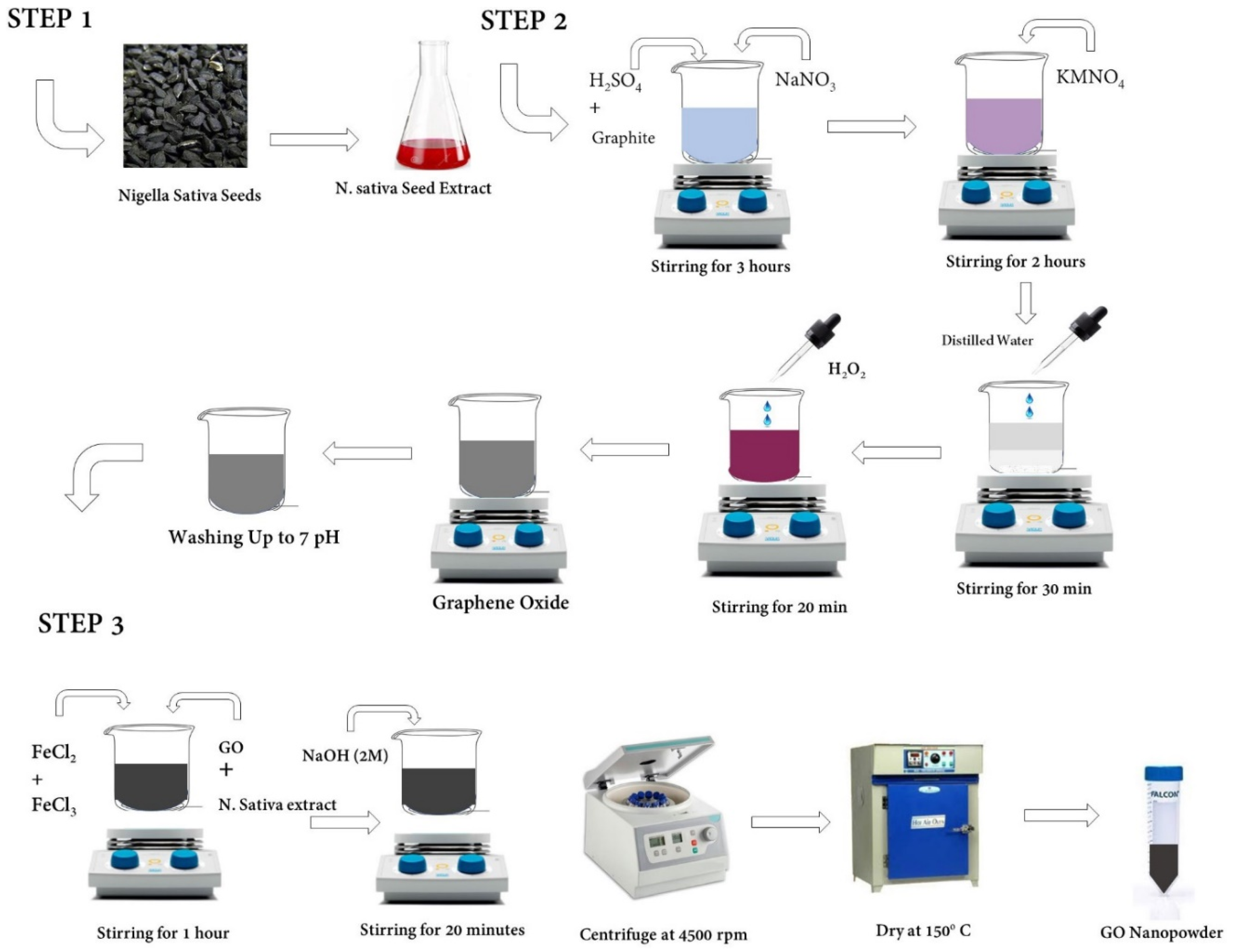
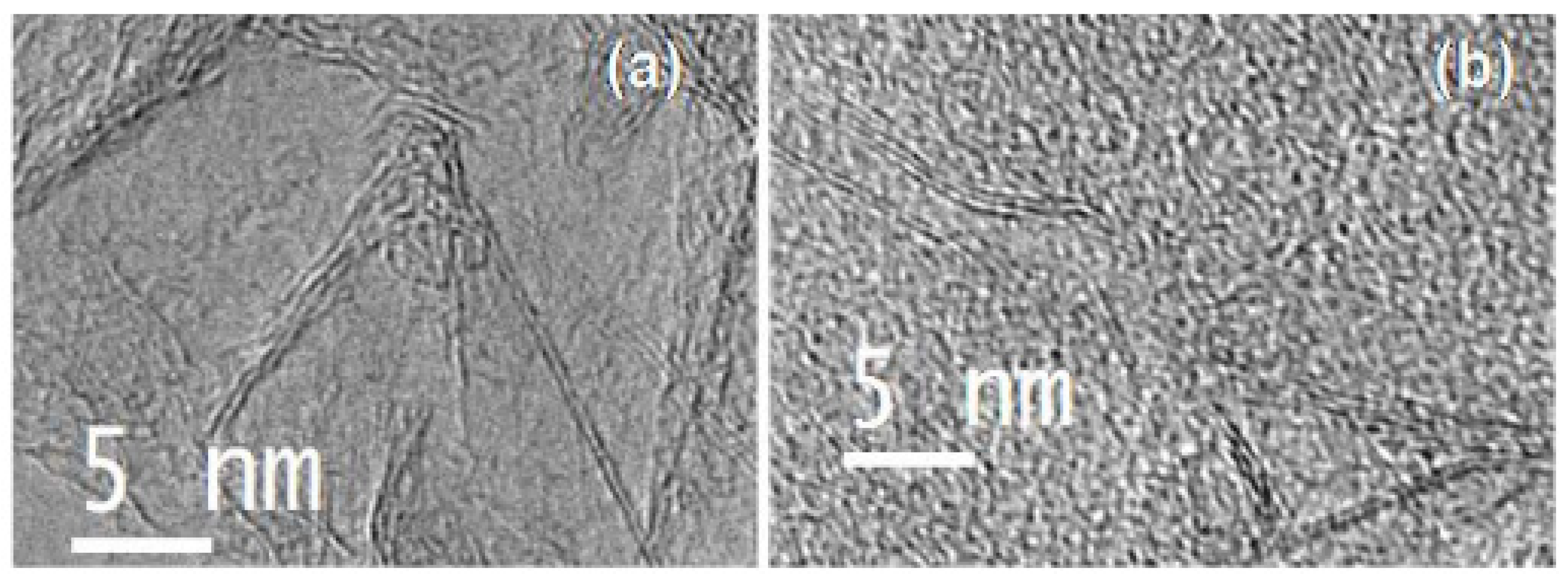
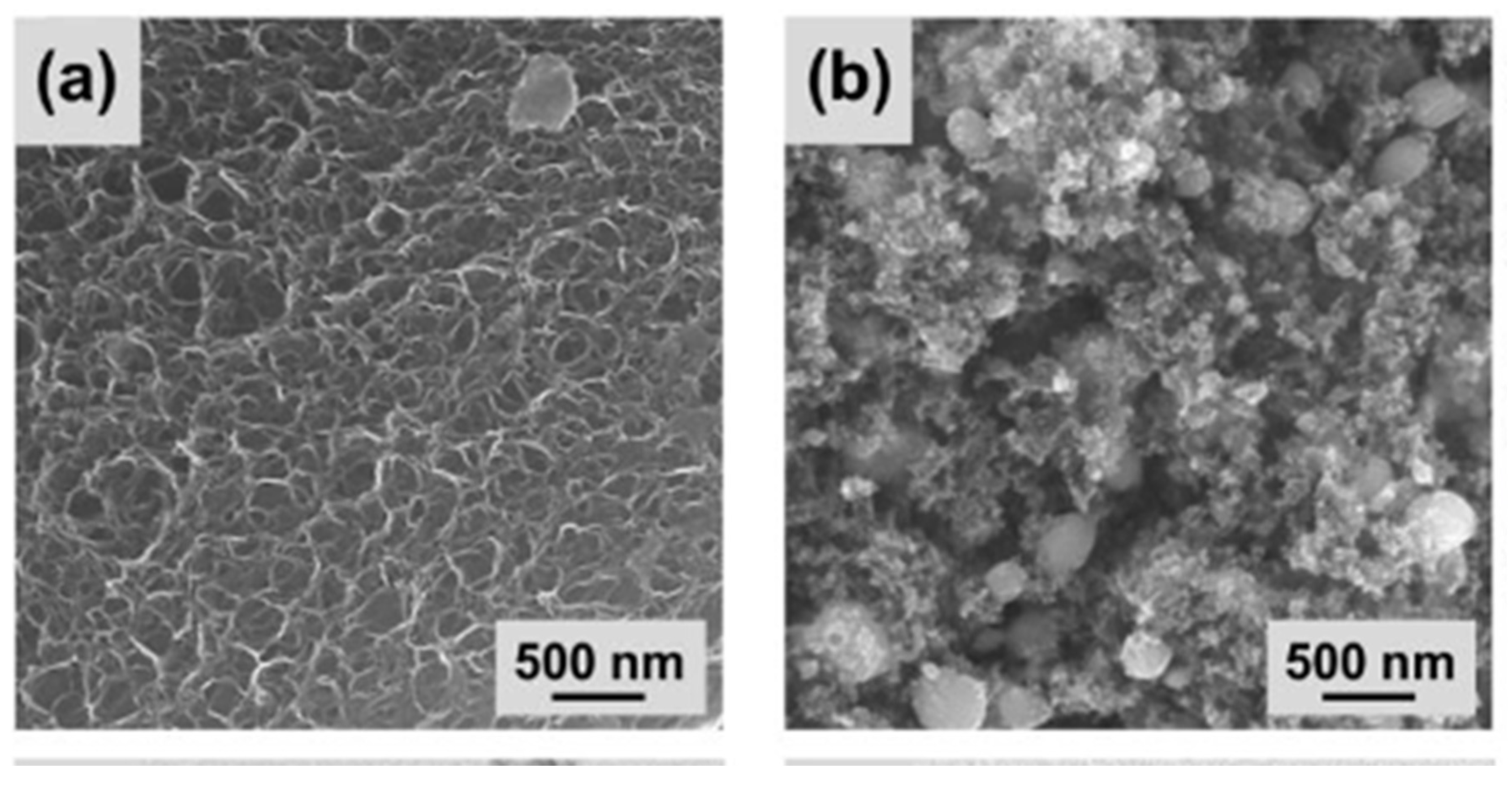
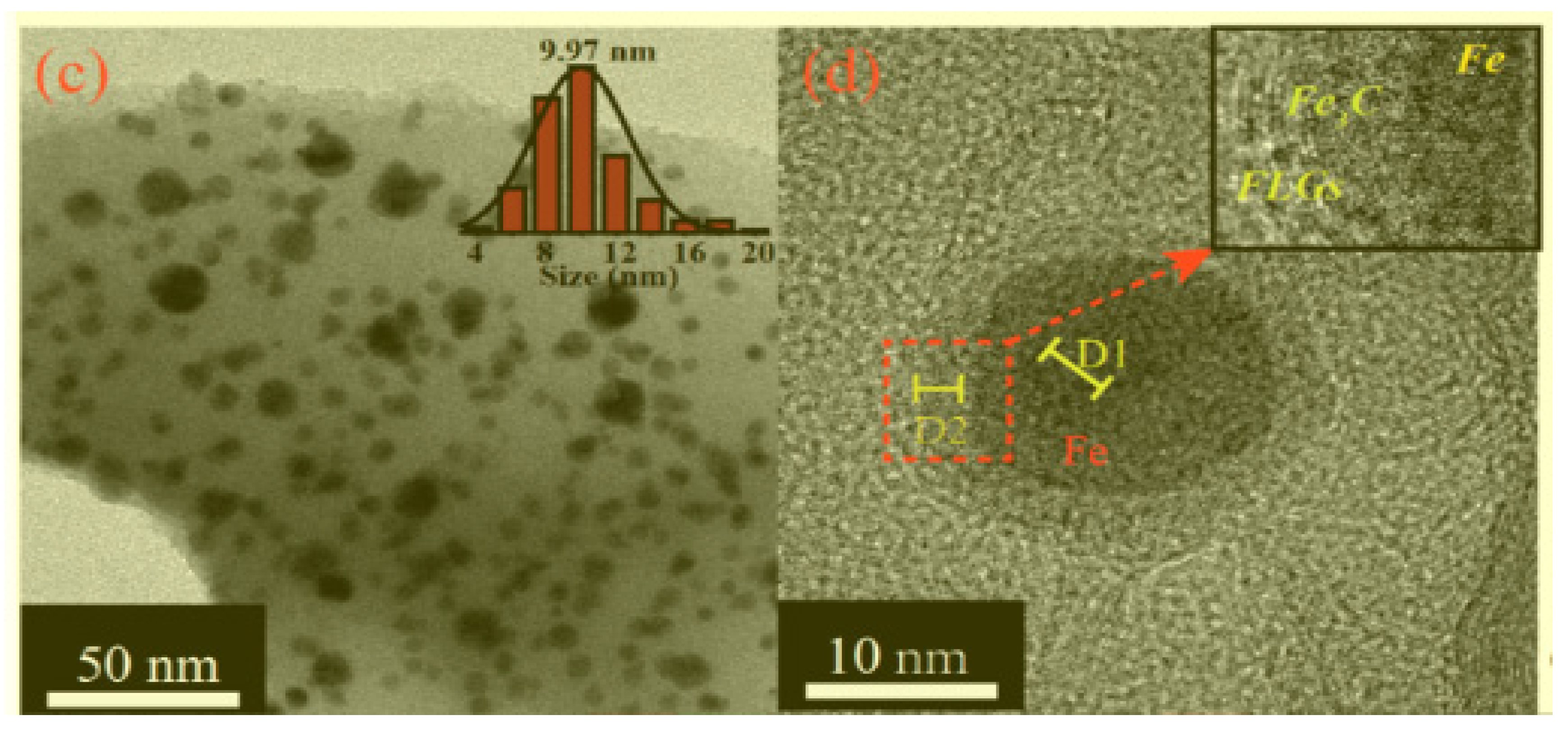
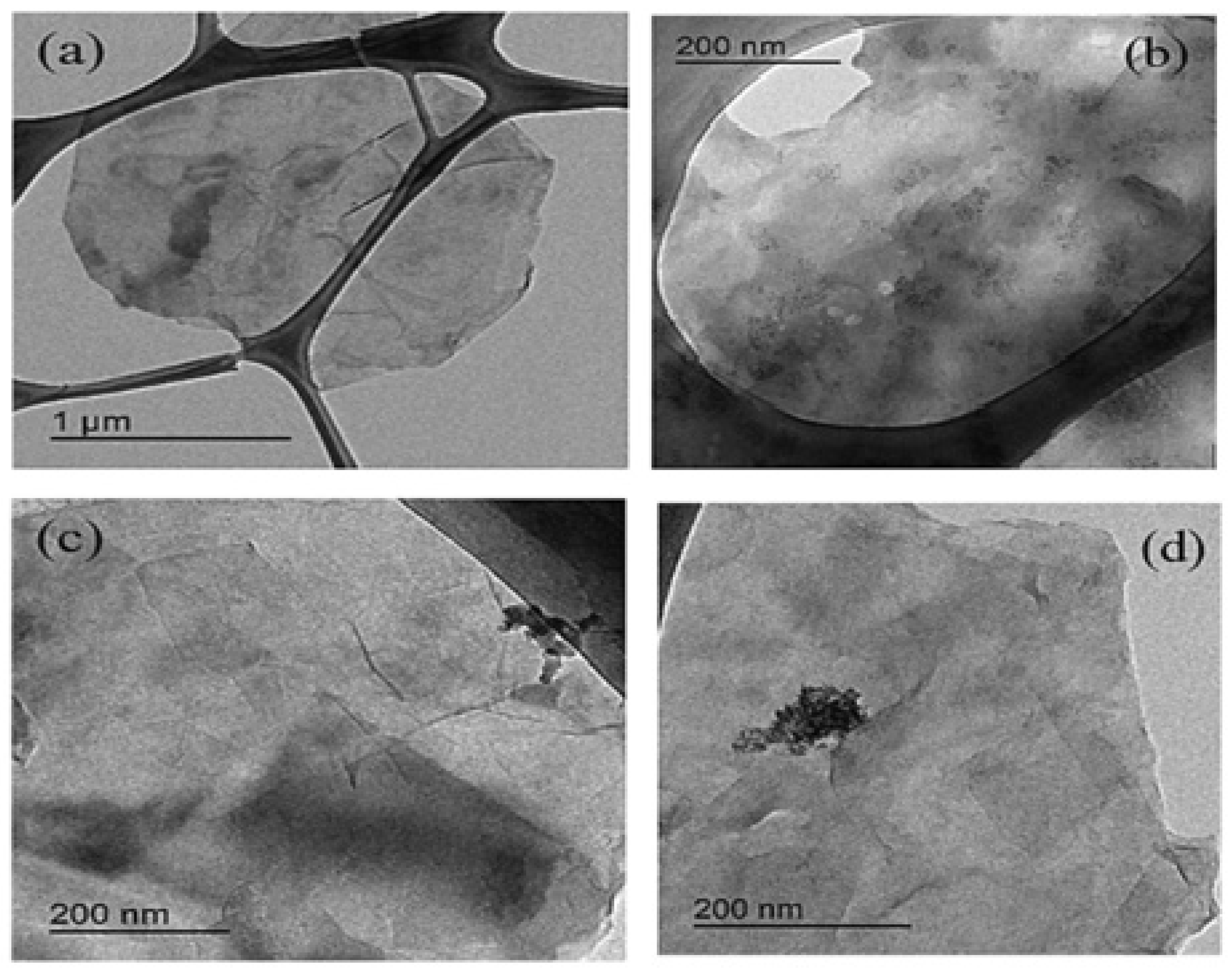

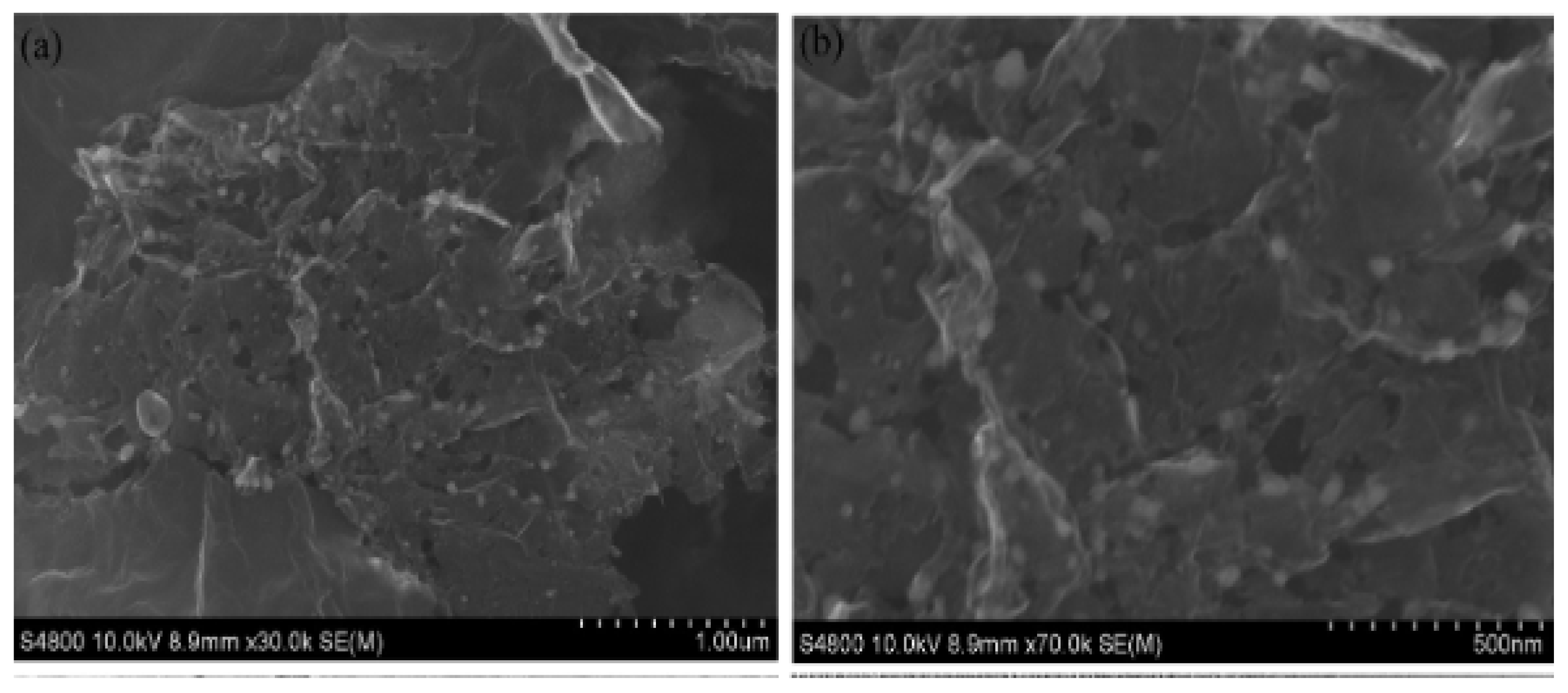
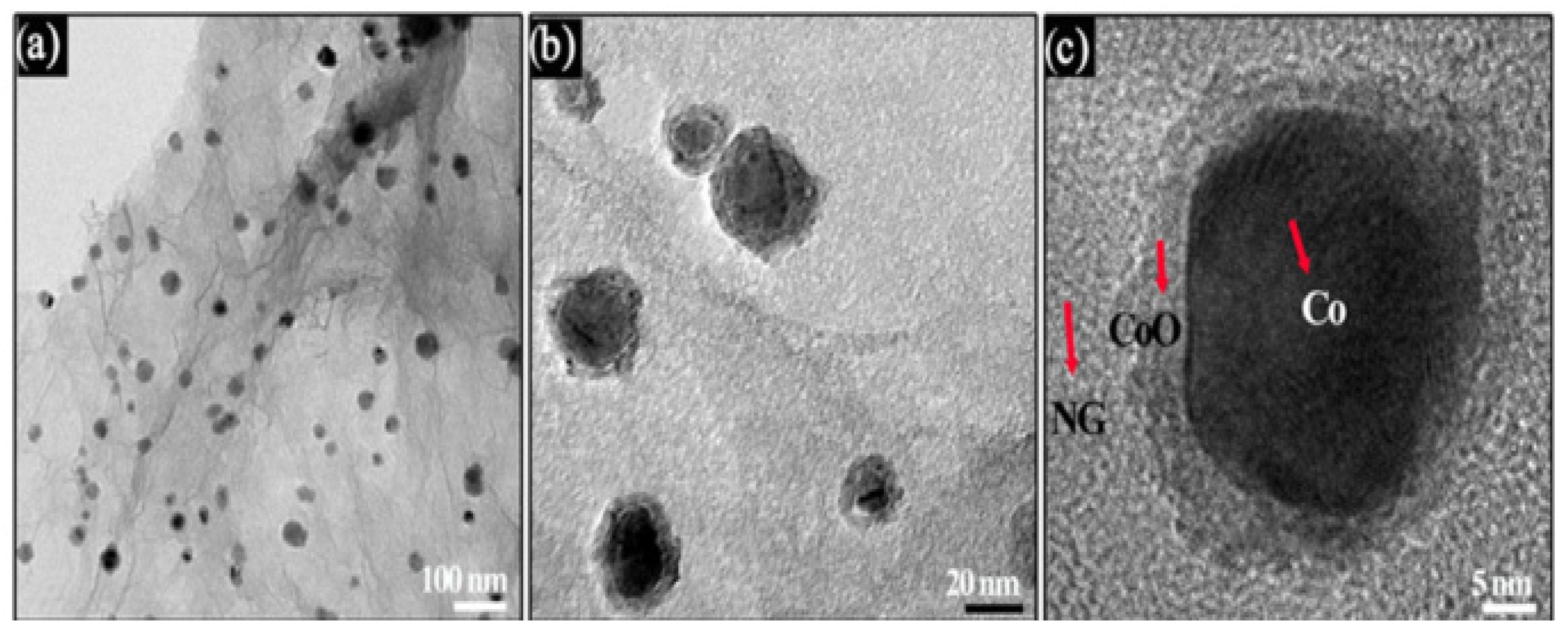
| Catalyst | Eonseta | E ½ | Electron transfer number br | jlim b | Tafel slope |
|---|---|---|---|---|---|
| (V) | (V) | (mAcm-2) | (mV dec-1) | ||
| GO/FePc | 0.95 | 0.87 | 4 | -6.9 | -31 |
| GO/MnPc | 0.87 | 0.69 | 3.7 | -4.4 | -61 |
| GO/CoPc | 0.84 | 0.59 | 3.6 | -4.7 | -86 |
| Pt/C | 0.99 | 0.87 | 4 | -6.4 | -52 |
| GO | 0.80 | 0.66 | 2.7 | -2.1 | -81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




