Submitted:
17 July 2024
Posted:
18 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
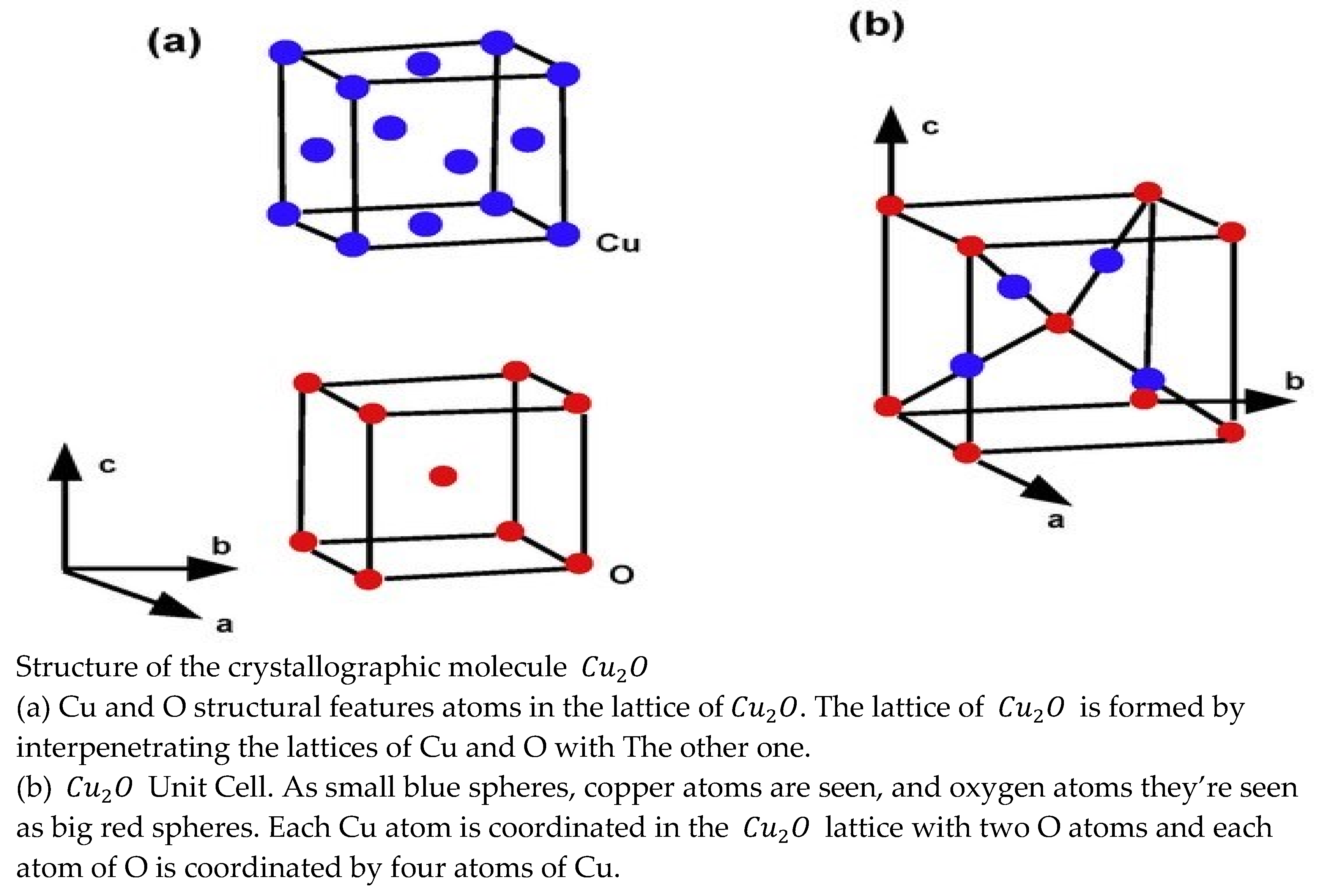
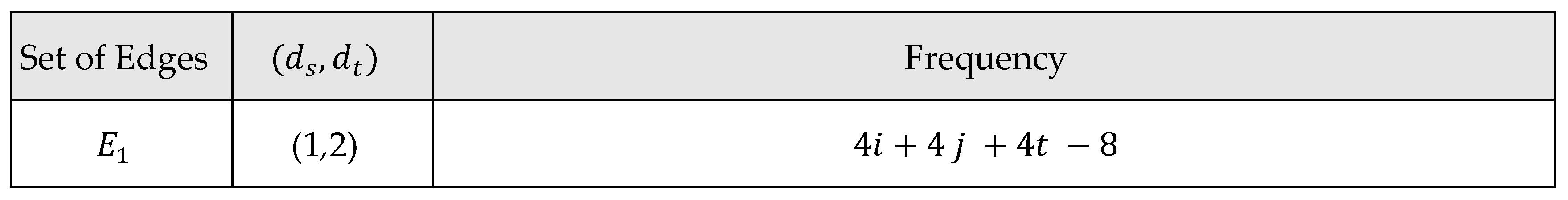
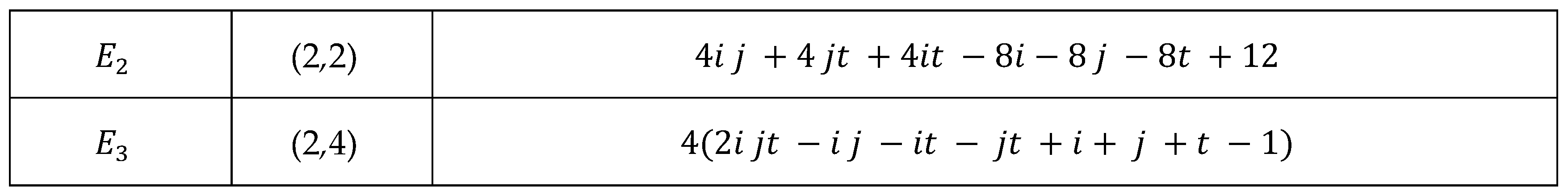
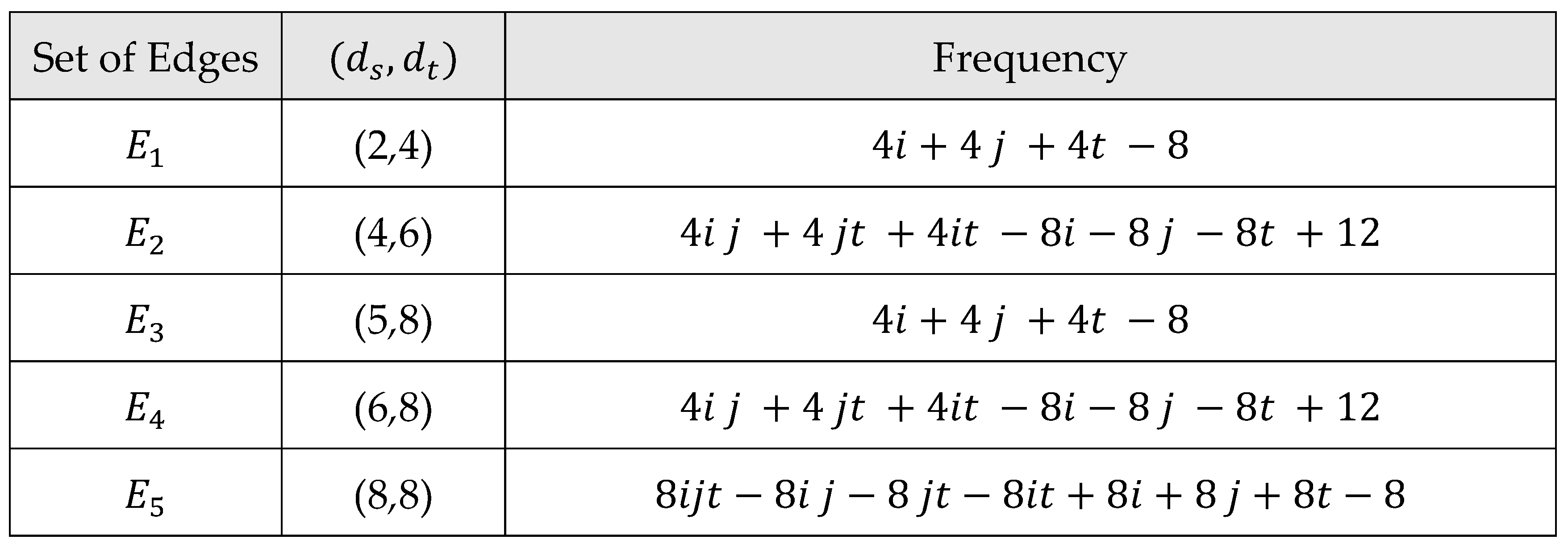
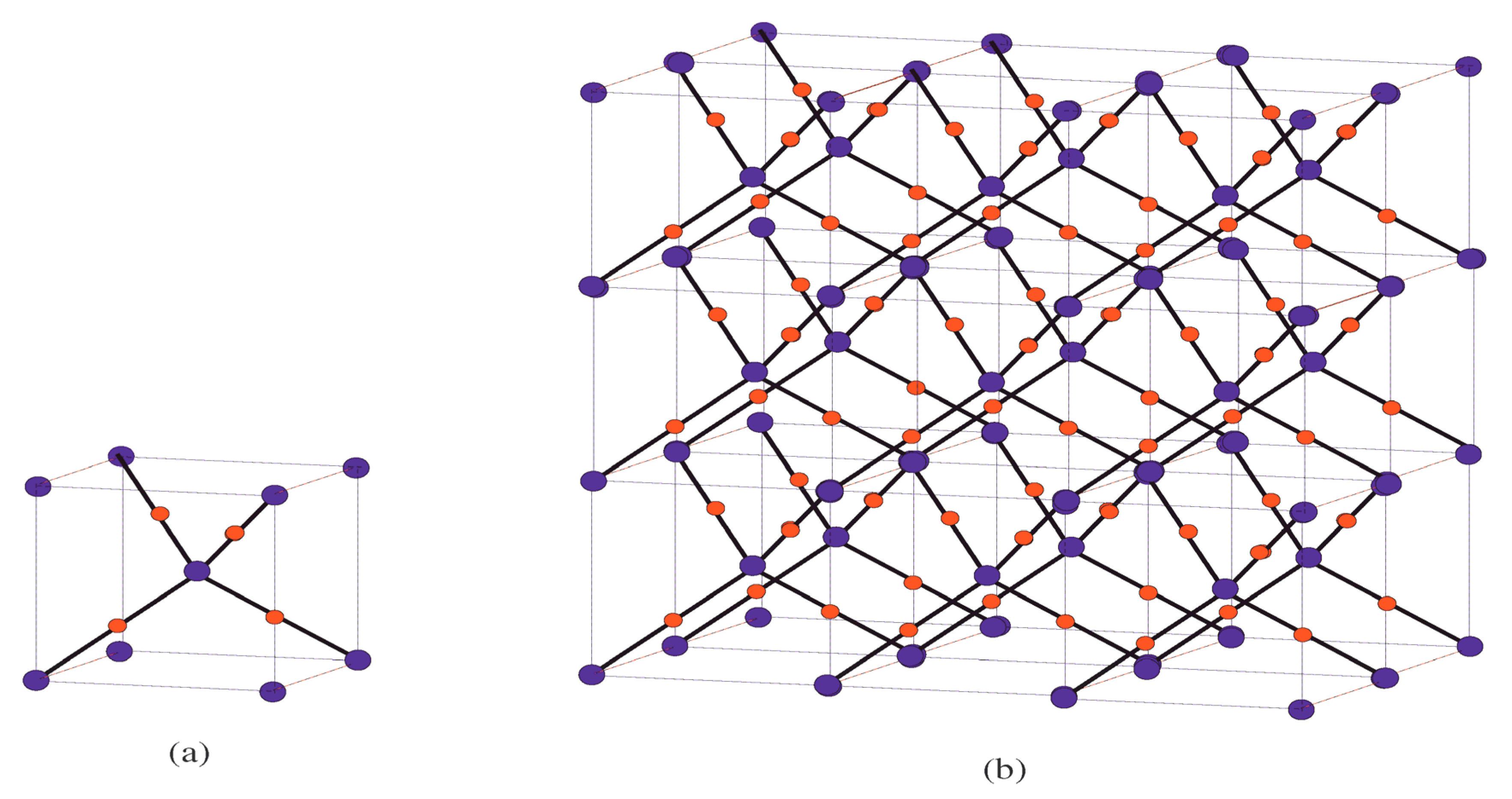
Main Results
Graphical Comparison of the Results:
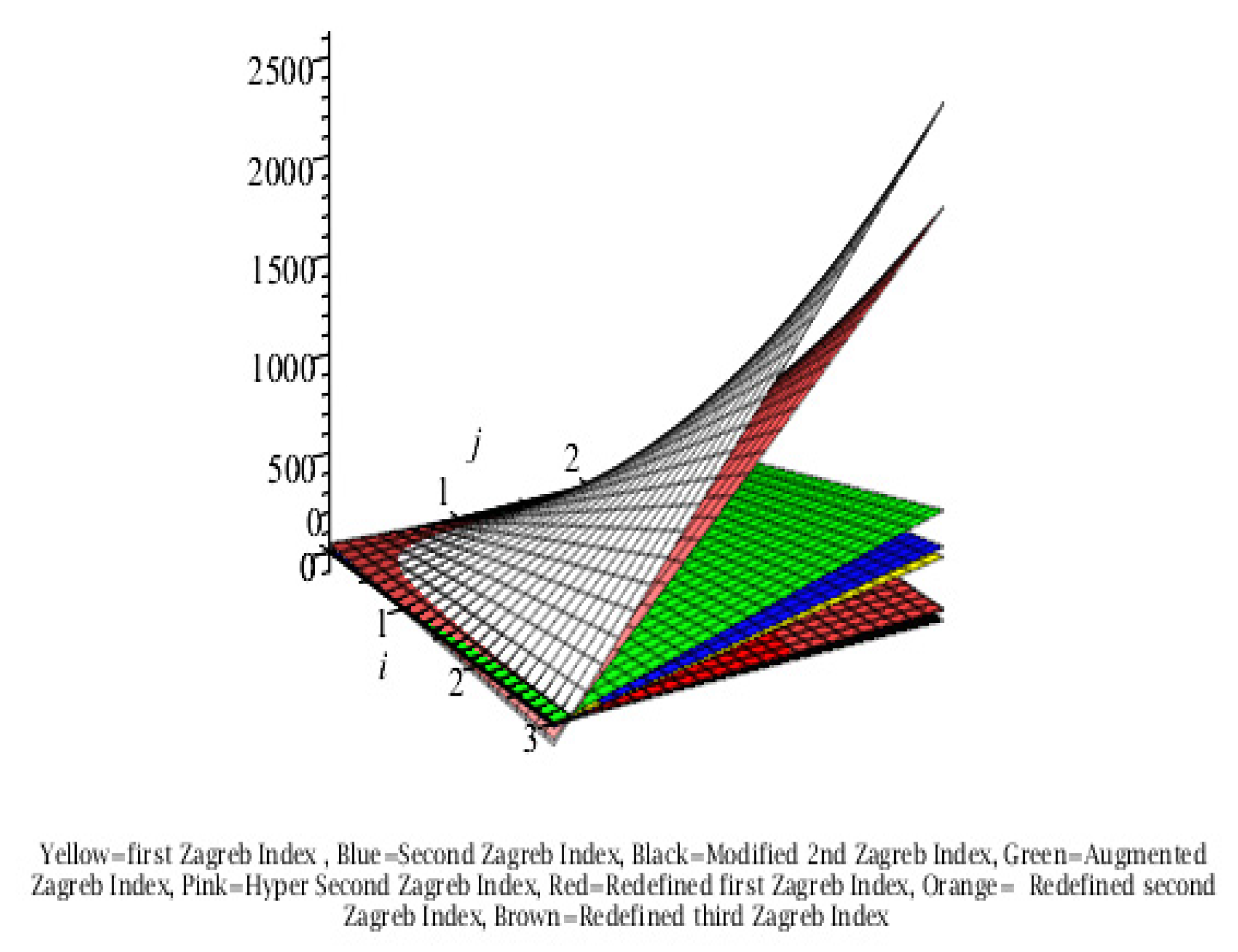
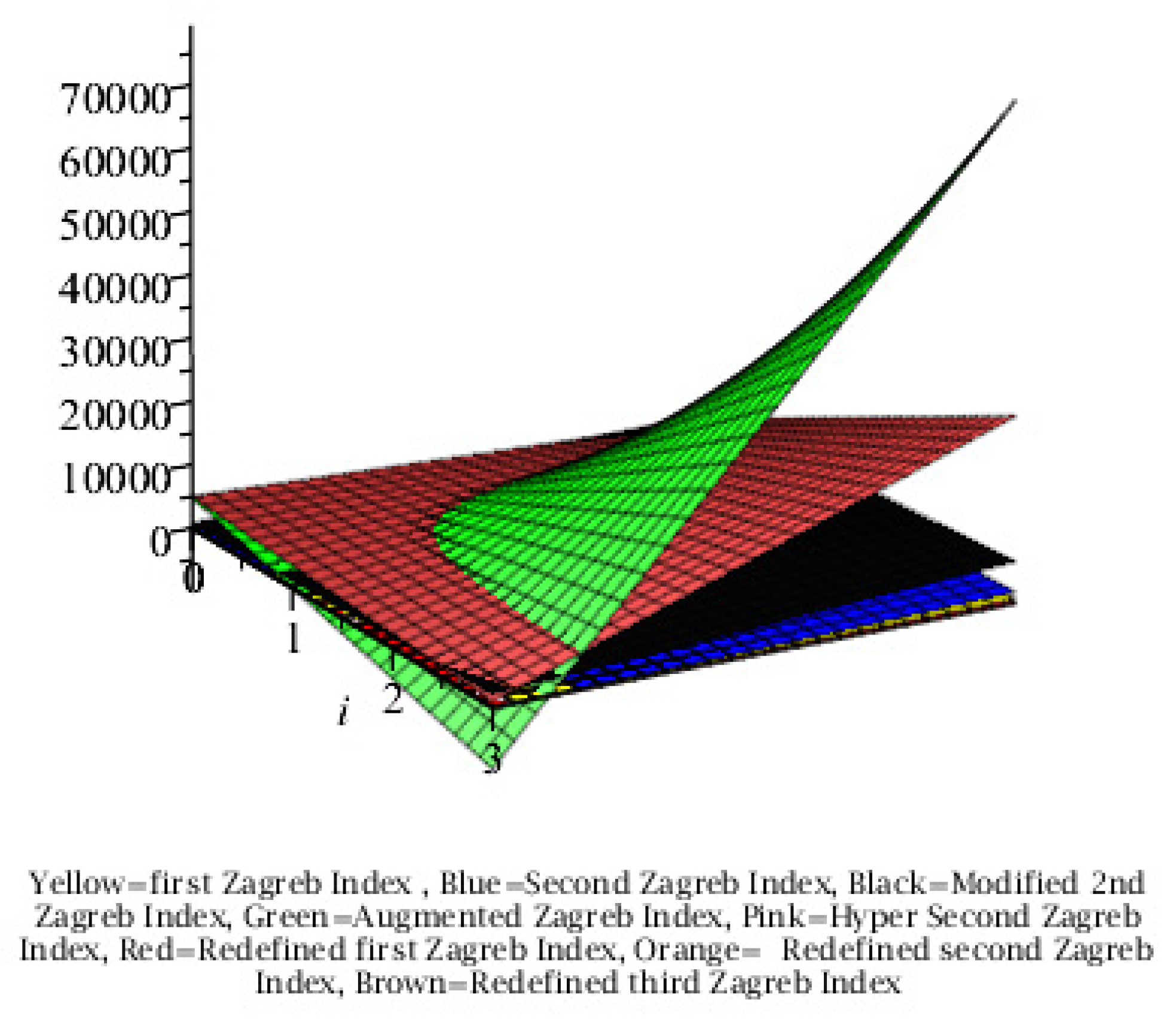
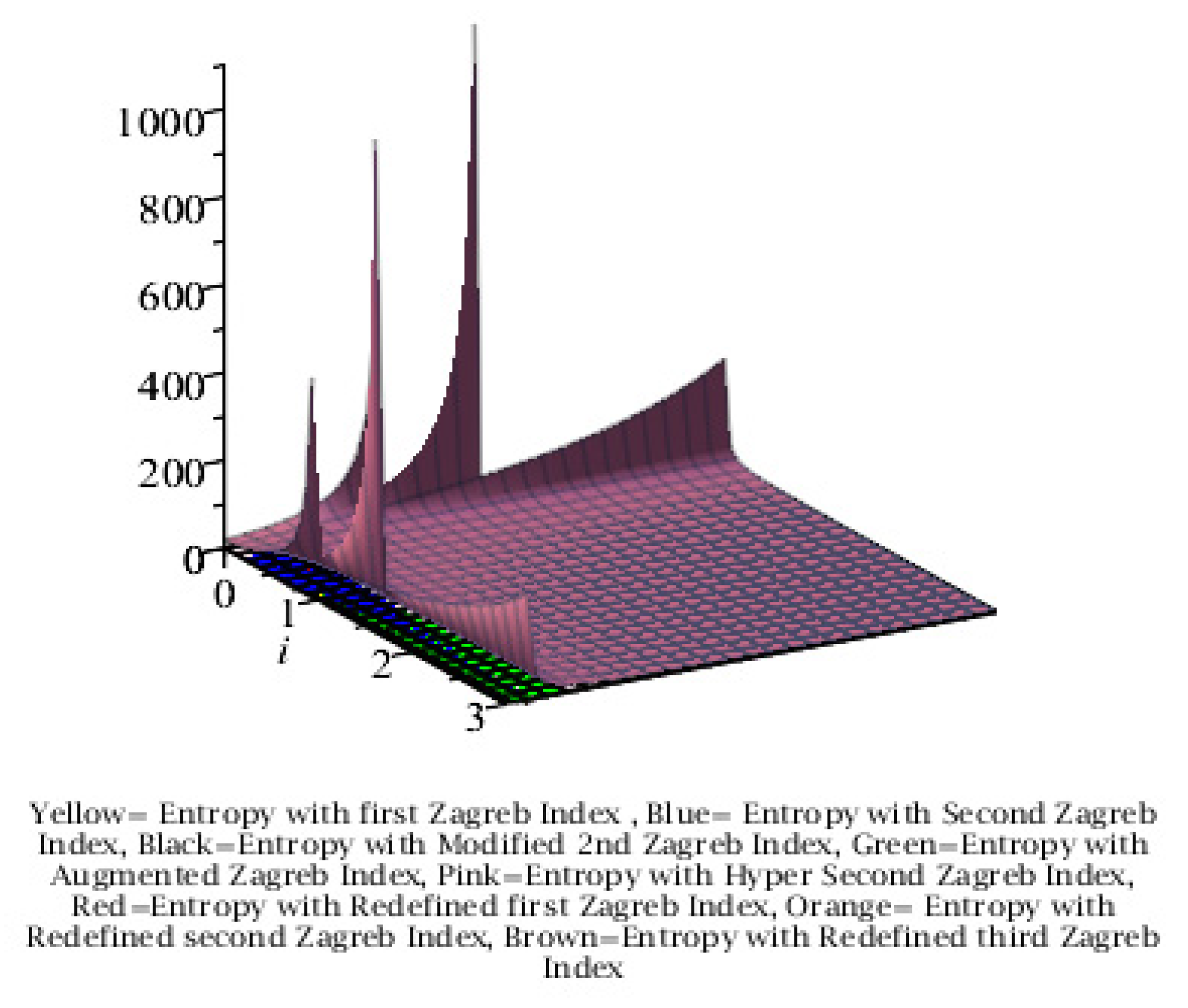
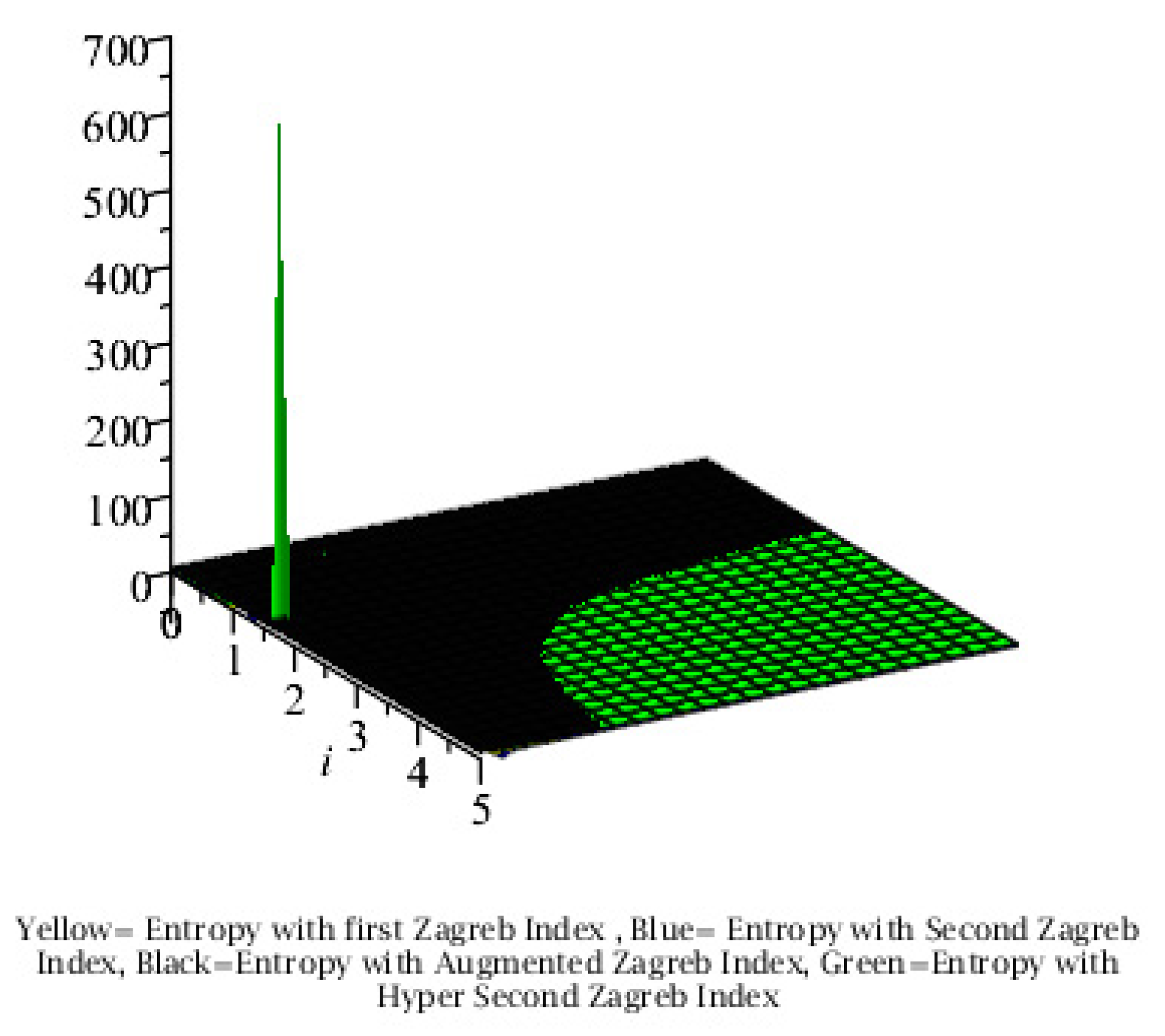
Conclusion
Data Availability Statement
Conflicts of Interest
References
- Gutman I., Some properties of the Wiener polynomials, Graph Theory, Notes New York, 1993, 125, 1318.
- Munir M., Nazeer W., Shahzadi S., Kang S.M., Some invariants of circulant graphs, Symmetry, 2016, 8(11), 134, 10.3390/ sym8110134.
- Gutman, I., and Trinajstic, N. (1972). Graph theory and molecular orbitals. Total f- electron energy of alternant hydrocarbons. Chemical physics letters, 17(4), 535-538.
- Milicevic, A., Nikolic, S., and Trinajstic, N. (2004). On reformulated Zagreb indices. Molecular diversity, 8(4), 393-399. [CrossRef]
- Furtula, B., Graovac, A., and Vukicevic, D. (2010). Augmented Zagreb index. Journal of mathematical chemistry, 48(2), 370-380.
- Shirdel, G. H., Rezapour, H., and Sayadi, A. M. (2013). The hyper-Zagreb index of graph operations.
- Ranjini, P. S., Lokesha, V., and Usha, A. (2013). Relation between phenylene and hexagonal squeeze using harmonic an index. Int. J. Graph Theory, 1(4), 116-121.
- Vukicevic, D., and Gaperov, M. (2010). Bond additive modeling 1. Adriatic indices. Croatica chemical Acta, 83(3), 243-260.
- Di Crescenzo, A., and Longobardi, M. (2007). On weighted residual and past entropies. ArXiv preprint math/0703489.
- Araujo, O., and De La Pena, J. A. (1998). The connectivity index of a weighted graph. Linear algebra and its applications, 283(1-3), 171-177.
- Gutman, I., and Trinajstic, N. (1972). Graph theory and molecular orbitals. Total f- electron energy of alternant hydrocarbons. Chemical physics letters, 17(4), 535-538.
- Ullah, A., Zaman, S., Hamraz, A., & Saeedi, G. (2022). Network-based modeling of the molecular topology of fuchsine acid dye with respect to some irregular molecular descriptors. Journal of Chemistry, 2022. [CrossRef]
- Ullah, A., Qasim, M., Zaman, S., & Khan, A. (2022). Computational and comparative aspects of two carbon nanosheets with respect to some novel topological indices. Ain Shams Engineering Journal, 13(4), 101672. [CrossRef]
- Zaman, S., Yaqoob, H. S. A., Ullah, A., & Sheikh, M. (2023). QSPR Analysis of Some Novel Drugs Used in Blood Cancer Treatment Via Degree Based Topological Indices and Regression Models. Polycyclic Aromatic Compounds, 1-17. [CrossRef]
- Zaman, S., Ullah, A., & Shafaqat, A. (2023). Structural modeling and topological characterization of three kinds of dendrimer networks. The European Physical Journal E, 46(5), 36.
- Ali, N., Kousar, Z., Safdar, M., Tolasa, F. T., & Suleiman, E. (2023). Mapping Connectivity Patterns: Degree-Based Topological Indices of Corona Product Graphs. Journal of Applied Mathematics, 2023.
- Zaman, S., & Ullah, A. (2023). Kemeny’s constant and global mean first passage time of random walks on octagonal cell network. Mathematical Methods in the Applied Sciences, 46(8), 9177-9186.
- Randic, M. (1975). Characterization of molecular branching. Journal of the American Chemical Society, 97(23), 6609-6615.
- Hayat, S., Khan, S., Khan, A., & Liu, J. B. (2022). Valency-based molecular descriptors for measuring the π-electronic energy of lower polycyclic aromatic hydrocarbons. Polycyclic Aromatic Compounds, 42(4), 1113-1129. [CrossRef]
- Hayat, S., Khan, S., Khan, A., & Imran, M. (2020). Distance-based topological descriptors for measuring the π-electronic energy of benzenoid hydrocarbons with applications to carbon nanotubes. Mathematical Methods in the Applied Sciences. [CrossRef]
- Malik, M. Y. H., Hayat, S., Khan, S., & Binyamin, M. A. (2021). Predictive potential of spectrum-based topological descriptors for measuring the π-electronic energy of benzenoid hydrocarbons with applications to boron triangular and boron α-nanotubes. Mathematical Methods in the Applied Sciences.
- Tomescu, I., Arshad, M., and Jamil, M. K. (2015). Extremal topological indices for graphs of given connectivity. Filomat, 29(7), 1639-1643.
- Garca, Domenech, R., Glvez, J., de Julin-Ortiz, J. V., and Pogliani, L. (2008). Some new trends in chemical graph theory. Chemical Reviews, 108(3), 1127-1169.
- Ali, N., Siddiqui, H. M. A., & Qureshi, M. I. (2023). A Graph-Theoretic Approach to Ring Analysis: Dominant Metric Dimensions in Zero-Divisor Graphs. arXiv preprint arXiv:2312.16005. [CrossRef]
- Ullah, A., Zaman, S., Hamraz, A., & Muzammal, M. (2023). On the construction of some bioconjugate networks and their structural modeling via irregularity topological indices. The European Physical Journal E, 46(8), 72. [CrossRef]
- Zaman, S., Jalani, M., Ullah, A., & Saeedi, G. (2022). Structural analysis and topological characterization of sudoku nanosheet. Journal of Mathematics, 2022. [CrossRef]
- Ullah, A., Zeb, A., & Zaman, S. (2022). A new perspective on the modeling and topological characterization of H-Naphtalenic nanosheets with applications. Journal of Molecular Modeling, 28(8), 211.
- Bozkurt, S. B., Gngr, A. D., Gutman, I., and Cevik, A. S. (2010). Randic matrix and Randic energy. MATCH Commun. Math. Compute. Chem, 64(1), 239-250.
- Kier, L. B., and Hall, L. H. (1986). Molecular connectivity in structure-activity analysis. Research studies.
- Hayat, S., Suhaili, N., & Jamil, H. (2023). Statistical significance of valency-based topological descriptors for correlating thermodynamic properties of benzenoid hydrocarbons with applications. Computational and Theoretical Chemistry, 1227, 114259. [CrossRef]
- Hayat, Sakander. “Distance-based graphical indices for predicting thermodynamic properties of benzenoid hydrocarbons with applications.” Computational Materials Science 230 (2023): 112492. [CrossRef]
- Hayat, S., Khan, A., Ali, K., & Liu, J. B. (2024). Structure-property modeling for thermodynamic properties of benzenoid hydrocarbons by temperature-based topological indices. Ain Shams Engineering Journal, 15(3), 102586. [CrossRef]
- Pogliani, L. (2000). From molecular connectivity indices to semi-empirical connectivity terms: Recent trends in graph-theoretical descriptors. Chemical Reviews, 100(10), 3827-3858.
- Todeschini, R., and Consonni, V. (2008). Handbook of molecular descriptors (Vol. 11). John Wiley and Sons.
- Van, Z.B. (2017) Section 2.3: Energy Bands. Principles of Semiconductor Devices. Ph.D. Thesis, Electrical, Computer, Energy Engineering Department, University of Colorado, Boulder, CO, USA.
- Malik, M. Y. H., Binyamin, M. A., & Hayat, S. (2022). Correlation ability of degree-based topological indices for physicochemical properties of polycyclic aromatic hydrocarbons with applications. Polycyclic Aromatic Compounds, 42(9), 6267-6281. [CrossRef]
- Hayat, S., Khan, S., Imran, M., & Liu, J. B. (2020). Quality testing of distance-based molecular descriptors for benzenoid hydrocarbons. Journal of Molecular Structure, 1222, 128927. [CrossRef]
- Hayat, S., Khan, S., & Imran, M. (2021). Quality testing of spectrum-based distance descriptors for polycyclic aromatic hydrocarbons with applications to carbon nanotubes and nanocones. Arabian Journal of Chemistry, 14(3), 102994. [CrossRef]
- Safdar, M., Mushtaq, T., Ali, N., & Akgül, A. (2023). On study of flow features of hybrid nanofluid subjected to oscillatory disk. International Journal of Modern Physics B, 2450356. [CrossRef]
- Zakir, M. S., Arshad, M., Naseer, M. K., Cancan, M. One Study of Weighted Entropy Based on Topological Indices for Triazine-Based Dendrimer: A Mathematical Chemistry Approach. Eur. Chem. Bull. 2023, 12(Special Issue 1, Part-B), 2770-2788.
- MAHBOOB, A., HUSSAIN, T., AKRAM, M., MAHBOOB, S., ALI, N., & RAZA, A. (2020). CHARACTERIZATIONS OF CHEVALLEY GROUPS USING ORDER OF THE FINITE GROUPS. Journal of Prime Research in Mathematics, 16(1), 46-51.
- Zakir, M. S., Arshad, M., Naseer, M. K., Kanwal, Z., Ahmad, I., & Cancan, M. EVALUATION OF WEIGHTED ENTROPIES BASED ON TOPOLOGICAL INDICES FOR POLY PROPYL ETHER IMINE DENDRIMERS. Eur. Chem. Bull. 2023, 12(Issue 8), 7120-7130.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).