Submitted:
12 July 2024
Posted:
13 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
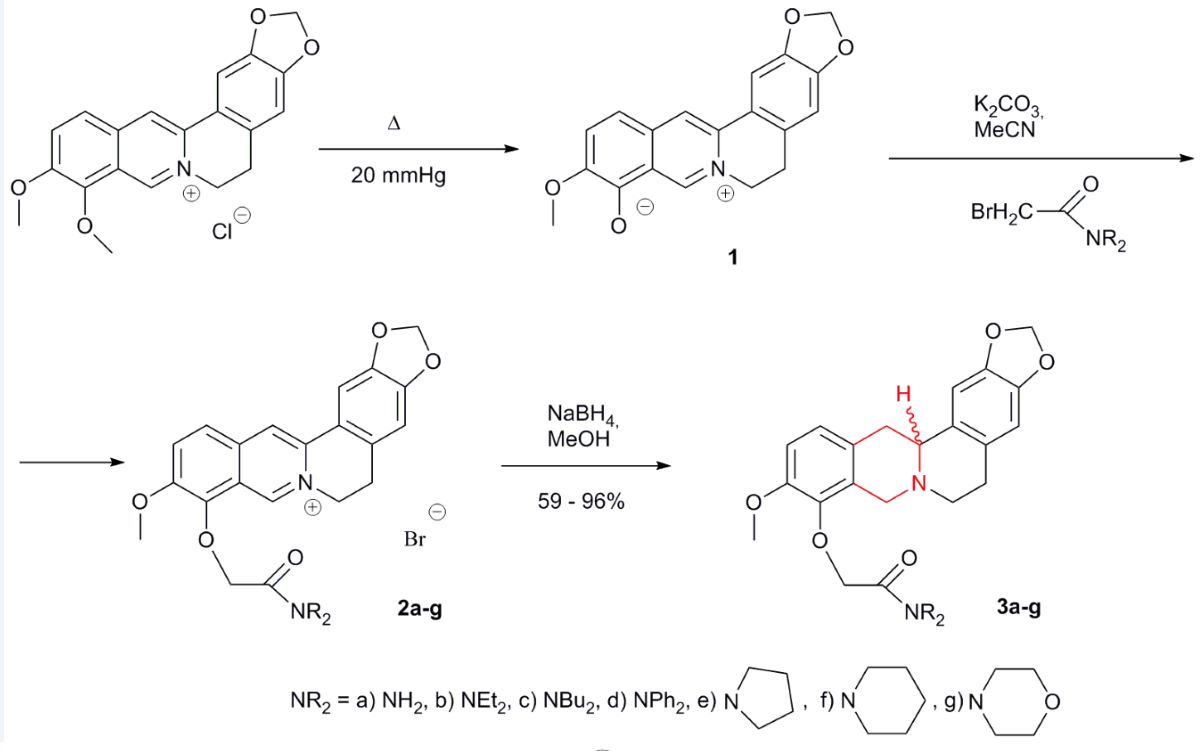
2.1. Synthesis of 3
2.2. Spectral Data of 3
3. Materials and Methods
3.1. General
3.2. Instrumentation and Analysis
3.3. General Procedure for Tetrahydroderivatives 3a-g Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Shen, W.; Shao, W.; Hu, H. Berberine alleviates cholesterol and bile acid metabolism disorders induced by high cholesterol diet in mice. Biochem. Biophys. Research Comm. 2024, 719, 150088. [Google Scholar] [CrossRef] [PubMed]
- Shams, G.; Allah, S.A.; Ezzat, R.; Said, M.A. Ameliorative effects of berberine and selenium against paracetamol-induced hepatic toxicity in rats. Open veterinary j. 2024, 14(1), 292–303. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiao, Y.; Zhou, J.; Mo, H.; Li, X.; Li, Y.; Wang, Y.; Zhong, M. Effects of Berberine on glucolipid metabolism among dehydroepiandrosterone-induced rats of polycystic ovary syndrome with insulin-resistance. Heliyon 2024, 10(2), e24338. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, F.; Kiani, S.; Rahimi, G.; Boskabady, M.H. Anti-inflammatory, antioxidant, and immunomodulatory effects of Berberis vulgaris and its constituent berberine, experimental and clinical, a review. Phytotherapy Research 2024, 38(4), 1882–1902. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Chen, J.; Ma, J.; Si, H.; Xia, D. Inhibition of inflammation by berberine: Molecular mechanism and network pharmacology analysis. Phytomedicine 2024, 128, 155258. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Mishra, D.; Singh, R.P. Cancer Pathways Targeted by Berberine: Role of microRNAs. Curr. Med. Chem. 2024. In press. [Google Scholar] [CrossRef] [PubMed]
- Khezri, M.R.; Mohammadipanah, S.; Ghasemnejad-Berenji, M. The pharmacological effects of Berberine and its therapeutic potential in different diseases: Role of the phosphatidylinositol 3-kinase/ AKT signaling pathway. Phytotherapy Research 2024, 38(1), 349–367. [Google Scholar] [CrossRef] [PubMed]
- Jivad, N.; Heidari-Soureshjani, S.; Bagheri, H.; Sherwin, C.M.; Rostamian, S. Anti-seizure Effects and Mechanisms of Berberine: A Systematic Review. Curr. pharmaceutical biotechnology 2024. In press. [Google Scholar] [CrossRef] [PubMed]
- El-Nahas, A.E.; Elbedaiwy, H.M.; Helmy, M.W.; El-Kamel, A.H. Simultaneous Estimation of Berberine and Piperine in a Novel Nanoformulation for Epilepsy Control via HPLC. J. Chromat. Sci. 2024, 62(2), 120–126. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Su, H.; Nie, K.; Wang, H.; Gao, Y.; Chen, S.; Lu, F.; Dong, H. Berberine exerts antidepressant effects in vivo and in vitro through the PI3K/AKT/CREB/BDNF signaling pathway. Biomedicine and Pharmacotherapy 2024, 170, 116012. [Google Scholar] [CrossRef]
- Gao, Y.; Nie, K.; Wang, H.; Dong, H.; Tang, Y. Research progress on antidepressant effects and mechanisms of berberine. Frontiers in Pharmacology 2024, 15, 1331440. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Gao, M.; Lin, Q. Integration of bioinformatics analysis, molecular docking and animal experiments to study the therapeutic mechanisms of berberine against allergic rhinitis. Scient. reports 2024, 14(1), 11999. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, S.V.; Staroverov, S.A.; Skvortsova, N.I.; Soldatov, D.A.; Chekunov, M.A.; Kozlov, E.S.; Artemev, D.A.; Chekunova, E.D.; Klyukina, A.D.; Rakhkho, V.; et al. Method of preparing water-soluble pharmaceutical composition based on berberine. Patent RU2814497C1.
- Chen, C.; Xie, M.; Yan, Y.; Li, Y.; Li, Z.; Zhang, T.; Gao, Z.; Deng, L.; Wang, H. Preparation of berberine hydrochloride-Ag nanoparticle composite antibacterial dressing based on 3D printing technology. J. Biomaterials Appl. 2024, 38(7), 808–820. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Agharazi, F.; Hosseinzadeh, S.A.; Mashayekhi, M.; Saffari, Z.; Shafiei, M.; Nader, S.; Ebrahimi-Rad, M.; Sadeghi, M. Gold nanoparticle conjugation enhances berberine’s antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Talanta 2024, 268 Part 1, 125358. [Google Scholar] [CrossRef]
- Mehra, M.; Sheorain, J.; Bakshi, J.; Thakur, R.; Grewal, S.; Dhingra, D.; Kumari, S. Synthesis and evaluation of berberine loaded chitosan nanocarrier for enhanced in-vitro antioxidant and anti-inflammatory potential. Carbohydrate Polymer Techn. and Appl. 2024, 7, 100474. [Google Scholar] [CrossRef]
- Guo, S.; Shen, C.; Chen, T.; Zhao, L.; Qiao, R.; Li, C. A stimuli-responsive demethyleneberberine-conjugated carboxylmethyl chitosan prodrug for treatment of inflammatory bowel diseases. Materials Lett. 2024, 357, 135730. [Google Scholar] [CrossRef]
- Saleh, S.R.; Abd-Elmegied, A.; Aly, M.S.; Khattab, S.N.; Sheta, E.; Elnozahy, F.Y.; Mehanna, R.A.; Ghareeb, D.A.; Abd-Elmonem, N.M. Brain-targeted Tet-1 peptide-PLGA nanoparticles for berberine delivery against STZ-induced Alzheimer′s disease in a rat model: Alleviation of hippocampal synaptic dysfunction, Tau pathology, and amyloidogenesis. Int. J. Pharmaceutics 2024, 658, 124218. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ye, T.; Chen, X.; Li, B.; Wei, Y.; Zheng, H.; Piao, J.-G.; Li, F. A self-assembly active nanomodulator based on berberine for photothermal immunotherapy of breast cancer via dual regulation of immune suppression. Int. J. Pharmaceutics 2024, 653, 123898. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Zh.; Zhang, W.; Liu, Y.; Wang, X.; Sun, Meng; Fang, X.; Han, We. The study on synthesis and vitro hypolipidemic activity of novel berberine derivatives nitric oxide donors. Fitoterapia 2024, 176, 105964. [CrossRef] [PubMed]
- Khvostov, M.V.; Gladkova, E.D.; Borisov, S.A.; Zhukova, N.A.; Marenina, M.K.; Meshkova, Y.V.; Luzina, O.A.; Tolstikova, T.G.; Salakhutdinov, N.F. Discovery of the First in Class 9-N-Berberine Derivative as Hypoglycemic Agent with Extra-Strong Action. Pharmaceutics 2021, 13, 2138. [Google Scholar] [CrossRef] [PubMed]
- Teng, Q.; Meng, Q.; Zhu, X.; Jiang, W.; Miao, C.; Yang, H. Synthesis and use of 9-O-aryl substituted berberine derivatives and its application in antibacterial drugs. Patent CN10923 2557.
- Valipour, M.; Zakeri, K.Z.; Abdollahi, E.; Ayati, A. Recent Applications of Protoberberines as Privileged Starting Materials for the Development of Novel Broad-Spectrum Antiviral Agents: A Concise Review (2017-2023). ACS Pharmacology and Translat. Sci. 2024, 7(1), 48–71. [Google Scholar] [CrossRef] [PubMed]
- Afroozandeh, Z.; Rashidi, R.P.; Khoobi, M.; Forootanfar, H.; Ameri, A.; Foroumadi, A. New Berberine Conjugates with Self-Assembly and Improved Antioxidant/Neuroprotection Properties: Effect of the Anchored Part on CMC, Shape and Size of the Nanomicelles. J. Cluster Sci. 2024, 35(5), 1305–1315. [Google Scholar] [CrossRef]
- Teng, Q.; Zhu, X.; Guo, Q.; Jiang, W.; Liu, J.; Meng, Q. Synthesis of 9-O-arylated berberines via copper-catalyzed CAr–O coupling reactions. Beilstein J. Org. Chem. 2019, 15, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.; Hoffmann, R.S.; Mueller, M.; Schoenherr, H.; Ihmels, H. Fluorimetric Cell Analysis with 9-Aryl-Substituted Berberine Derivatives as DNA-Targeting Fluorescent Probes. ChemBioChem 2024, 25(2), e202300761. [Google Scholar] [CrossRef] [PubMed]
- Nechepurenko, I.V.; Komarova, N.I.; Vasil’ev, V.G.; Salakhutdinov, N.F. Synthesis of berberine bromide analogs containing tertiary amides of acetic acid in the 9-O-position. Chem. Natural Compounds 2013, 48, 1047–1053. [Google Scholar] [CrossRef]
- Nechepurenko, I.V.; Komarova, N.I.; Shernyukov, A.V.; Vasil’ev, V.G.; Salakhutdinov, N.F. Smiles rearrangements in a series of berberine analogues containing a secondary acetamide fragment. Tetrahedron Lett. 2014, 55, 6125–6127. [Google Scholar] [CrossRef]
- Lai, R.; Lin, Z.; Yang, C.; Hai, L.; Yang, Z.; Guo, L.; Nie, R.; Wu, Y. Novel berberine derivatives as p300 histone acetyltransferase inhibitors in combination treatment for breast cancer. Eur. J. Med. Chem. 2024, 266, 116116. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Huang, N.; Li, M.; Guan, F.; Chen, L.; Liao, Y.; Xie, X.; Li, Y.; Su, Z.; Chen, J.; et al. Tetrahydroberberine alleviates high-fat diet-induced hyperlipidemia in mice via augmenting lipoprotein assembly-induced clearance of low-density lipoprotein and intermediate-density lipoprotein. Eur. J. Pharmacology 2024, 968, 176433. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, K.; Sui, D.; Ouyang, Z.; Xu, H.; Wei, Y. Effects of tetrahydroberberine and tetrahydropalmatine on hepatic cytochrome P450 expression and their toxicity in mice. Chemico-Biological Interactions 2017, 268, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Nechepurenko, I.V.; Shirokova, E.D., Khvostov, M.V.; Frolova, T.S.; Sinitsyna, O.I.; Maksimov, A.M.; Bredikhin, R.A.; Komarova, N.I.; Fadeev, D.S.; Luzina, O.A.; et al. Synthesis, hypolipidemic and antifungal activity of tetrahydroberberrubine sulfonates. Russ. Chem. Bul. Intern. Ed. 2019, 68, 1052–1060. [CrossRef]
- Kong, Y.; Yi, Y.; Liu, X.-Q.; Yu, P.; Zhao, L.-G.; Li, D.-D. Discovery and structural optimization of 9-O-phenylsulfonyl-berberines as new lipid-lowering agents. Bioorg. Chem. 2022, 121, 105665. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Zhang, H.; Han, W. Review of synthesis and activity of tetrahydroberberine derivatives. Zhongguo Linchuang Yaolixue Zazhi 2021, 37(18), 138–140. [Google Scholar] [CrossRef]
- Mari, G.; De Crescentini, L.; Benedetti, S.; Palma, F.; Santeusanio, S.; Mantellini, F. Synthesis of new dihydroberberine and tetrahydroberberine analogues and evaluation of their antiproliferative activity on NCI-H1975 cells. Beilstein J. Org. Chem. 2020, 16, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Sun, H. Preparation of tetrahydroberberine thiazolidinedione compound and application as antibacterial and/or antifungal agents. Patent CN10865 8971.
- Gladkova, E.D.; Nechepurenko, I.V.; Bredikhin, R.A.; Chepanova, A.A.; Zakharenko, A.L.; Luzina, O.A.; Ilina, E.S.; Dyrkheeva, N.S.; Mamontova, E.M.; Anarbaev, R.O.; et al. The First Berberine-Based Inhibitors of Tyrosyl-DNA Phosphodiesterase 1 (Tdp1), an Important DNA Repair Enzyme. Int. J. Mol. Sci. 2020, 21, 7162. [Google Scholar] [CrossRef] [PubMed]
- Kalinowsky, H.-O.; Berger, S.; Brawn, S. 13-C NMR Spectroscopy, Chichister: John Wiley & Sons, 1988; pp. 198–219.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




