Submitted:
10 July 2024
Posted:
11 July 2024
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. OC: Etiology, Epidemiology, Risk factors, Characteristics, and Prognosis
3. ROS: General Definition, Balance and Levels of ROS, and Positive Role of ROS
4. Generation of ROS and the Role of OS in OC Pathogenesis, and the Clinical Impact of ROS
5. OS and Signaling Pathways
5.1. Keap1-Nrf2-ARE Signaling Pathway
5.2. PI3K/AKT/mTOR Signaling Pathway
5.3. Wnt/β-Catenin Signaling Pathway
5.4. Notch Signaling Pathway
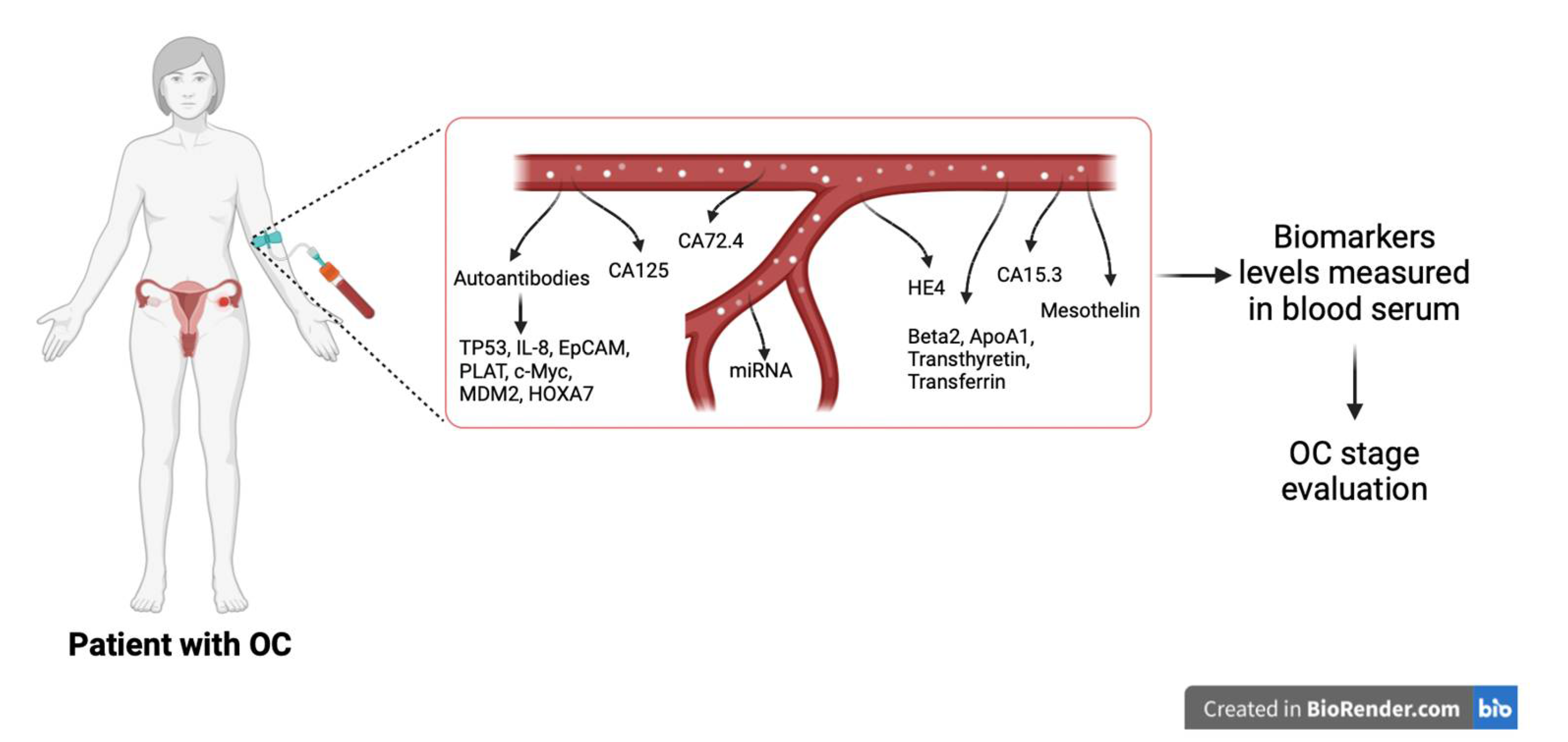
6. Diagnostic Methods
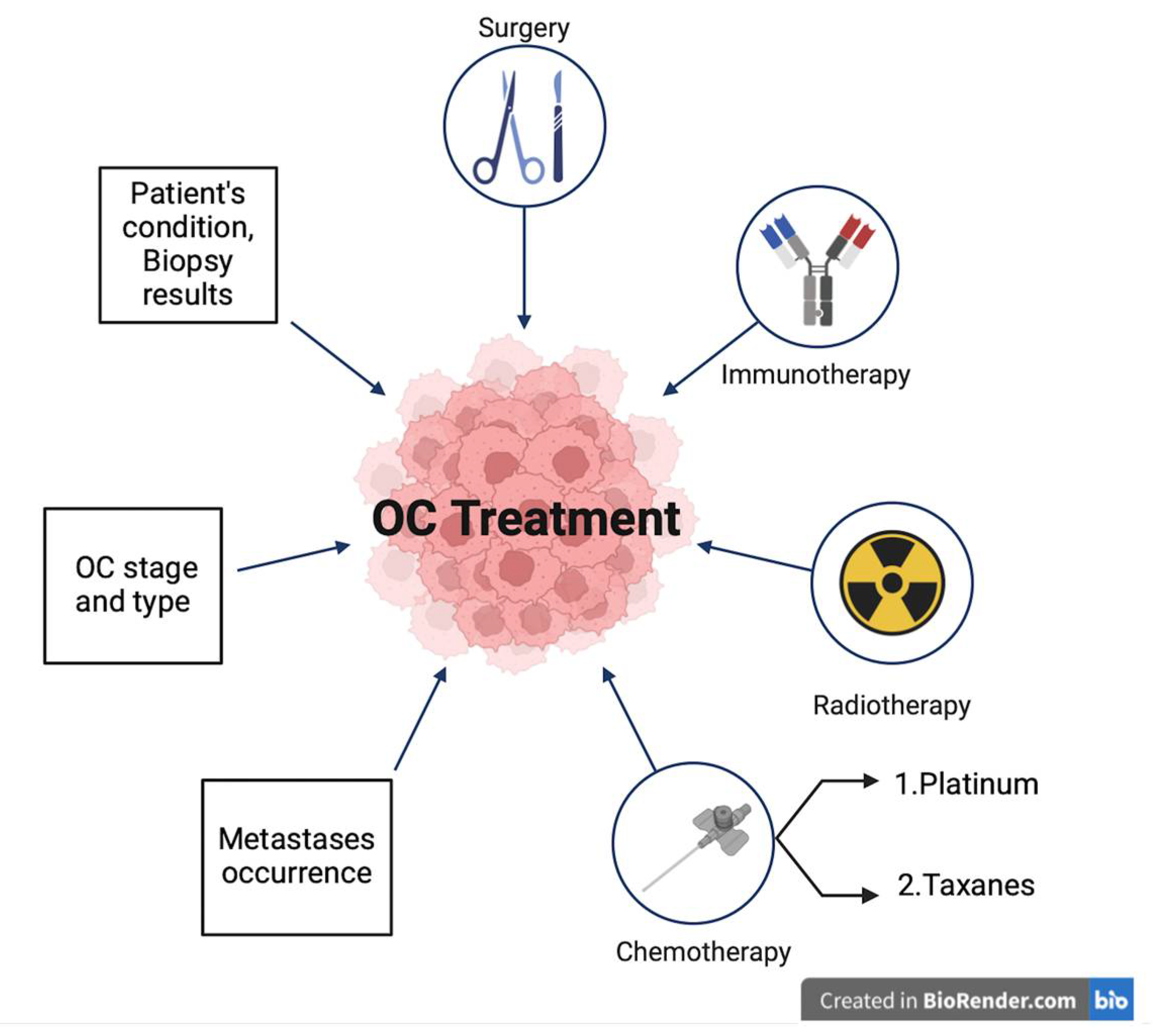
7. Treatment Options for OC and the Role of Oxidative Stress in Chemotherapy Resistance
8. Future Perspectives for OC Treatment
9. Conclusion
References
- Arora, T., S. Mullangi, and M.R. Lekkala, Ovarian cancer. 2021.
- Siegel, R.L. , et al., Cancer statistics, 2023. CA: A Cancer Journal for Clinicians, 2023. 73(1): p. 17-48.
- Morand, S. , et al., Ovarian Cancer Immunotherapy and Personalized Medicine. International Journal of Molecular Sciences, 2021. 22(12): p. 6532.
- Stewart, C., C. Ralyea, and S. Lockwood. Ovarian cancer: an integrated review. in Seminars in oncology nursing. 2019. Elsevier.
- Lheureux, S. , et al., Epithelial ovarian cancer. The Lancet, 2019. 393(10177): p. 1240-1253.
- Akter, S. , et al., Recent Advances in Ovarian Cancer: Therapeutic Strategies, Potential Biomarkers, and Technological Improvements. Cells, 2022. 11(4): p. 650.
- Garg, V. and A.M. Oza, Treatment of Ovarian Cancer Beyond PARP Inhibition: Current and Future Options. Drugs, 2023. 83(15): p. 1365-1385.
- Zapardiel, I., M. Diestro, and G. Aletti, Conservative treatment of early stage ovarian cancer: oncological and fertility outcomes. European Journal of Surgical Oncology (EJSO), 2014. 40(4): p. 387-393.
- Roett, M.A. and P. Evans, Ovarian cancer: an overview. Am Fam Physician, 2009. 80(6): p. 609-16.
- Cirillo, P.D.R. , et al., Multi-analytical test based on serum miRNAs and proteins quantification for ovarian cancer early detection. PLoS One, 2021. 16(8): p. e0255804.
- Zhang, R. , et al., Molecular Biomarkers for the Early Detection of Ovarian Cancer. International Journal of Molecular Sciences, 2022. 23(19): p. 12041.
- Clarke, A., Y. M. Chang, and K. McPherson, Removing organs “just in case”—is prophylactic removal of the ovaries a good thing? 2006, BMJ Publishing Group Ltd. p. 186-187.
- Garzon, S. , et al., Secondary and tertiary ovarian cancer recurrence: what is the best management? Gland Surgery, 2020. 9(4): p. 1118-1129.
- Oranratanaphan, S. , et al., Assessment of Diagnostic Values among CA-125, RMI, HE4, and ROMA for Cancer Prediction in Women with Nonfunctional Ovarian Cysts. Obstetrics and Gynecology International, 2018. 2018: p. 7821574.
- Renjen, P.N. , et al., Paraneoplastic Cerebellar Degeneration Associated With Ovarian Adenocarcinoma: A Case Report and Review of Literature. Annals of Indian Academy of Neurology, 2018. 21(4): p. 311-314.
- National Academies of Sciences, E. , et al., Ovarian Cancers: Evolving Paradigms in Research and Care. 2016: National Academies Press.
- Zheng, M. , et al., Oxidative Stress Response Biomarkers of Ovarian Cancer Based on Single-Cell and Bulk RNA Sequencing. Oxidative Medicine and Cellular Longevity, 2023. 2023: p. 1261039.
- Jayson, G.C. , et al., Ovarian cancer. The Lancet, 2014. 384(9951): p. 1376-1388.
- Siegel, R.L. , et al., Cancer statistics, 2022. CA: A Cancer Journal for Clinicians, 2022. 72(1): p. 7-33.
- Rooth, C. , Ovarian cancer: risk factors, treatment and management. Br J Nurs, 2013. 22(17): p. S23-30.
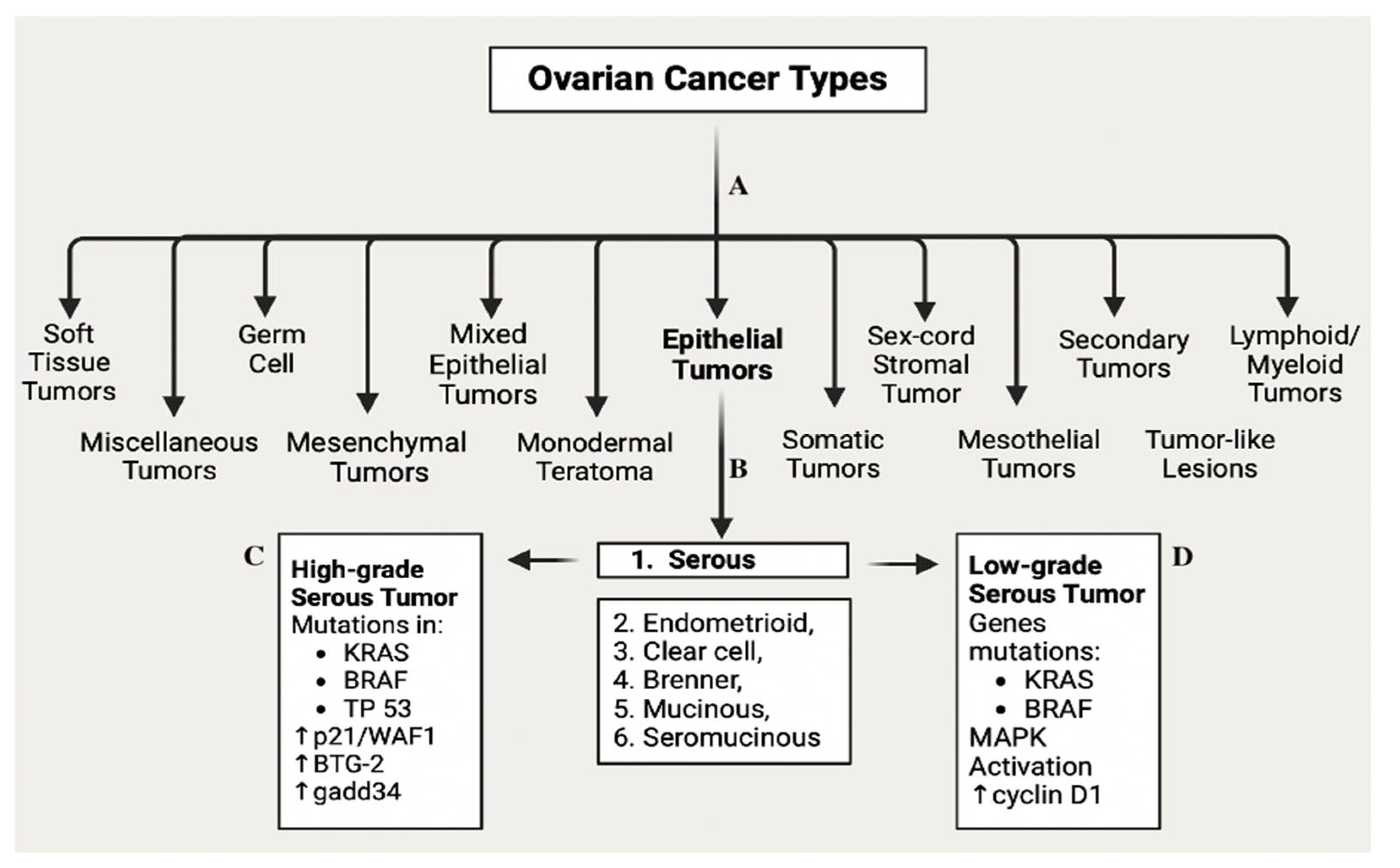
- Zamwar, U.M. and A.P. Anjankar, Aetiology, Epidemiology, Histopathology, Classification, Detailed Evaluation, and Treatment of Ovarian Cancer. Cureus, 2022. 14(10): p. e30561.
- Kossaï, M. , et al., Ovarian Cancer: A Heterogeneous Disease. Pathobiology, 2017. 85(1-2): p. 41-49.
- Murali, R. , et al., Deregulated Metabolic Pathways in Ovarian Cancer: Cause and Consequence. Metabolites, 2023. 13(4): p. 560.
- Meinhold-Heerlein, I. , et al., Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene, 2005. 24(6): p. 1053-1065.
- Singer, G. , et al., Mutations in BRAF and KRAS Characterize the Development of Low-Grade Ovarian Serous Carcinoma. JNCI: Journal of the National Cancer Institute, 2003. 95(6): p. 484-486.
- Suszynska, M., M. Ratajska, and P. Kozlowski, BRIP1, RAD51C, and RAD51D mutations are associated with high susceptibility to ovarian cancer: mutation prevalence and precise risk estimates based on a pooled analysis of ~30,000 cases. J Ovarian Res, 2020. 13(1): p. 50.
- Reid, B.M., J. B. Permuth, and T.A. Sellers, Epidemiology of ovarian cancer: a review. Cancer Biology and Medicine, 2017. 14(1): p. 9-32.
- Matulonis, U.A. , et al., Ovarian cancer. Nature reviews Disease primers, 2016. 2(1): p. 1-22.
- Webb, P.M. and S.J. Jordan, Epidemiology of epithelial ovarian cancer. Best practice & research Clinical obstetrics & gynaecology, 2017. 41: p. 3-14.
- Torre, L.A. , et al., Ovarian cancer statistics, 2018. CA: A Cancer Journal for Clinicians, 2018. 68(4): p. 284-296.
- Huber, D. , et al., Use of oral contraceptives in BRCA mutation carriers and risk for ovarian and breast cancer: a systematic review. Archives of Gynecology and Obstetrics, 2020. 301(4): p. 875-884.
- Goldzieher, J.W. and F.Z. Stanczyk, Oral contraceptives and individual variability of circulating levels of ethinyl estradiol and progestins. Contraception, 2008. 78(1): p. 4-9.
- Lacey, J. , James V., et al., Menopausal Hormone Replacement Therapy and Risk of Ovarian Cancer. JAMA, 2002. 288(3): p. 334-341.
- Orchard, S.G. , et al., Association of metformin, aspirin, and cancer incidence with mortality risk in adults with diabetes. JNCI Cancer Spectr, 2023. 7(2).
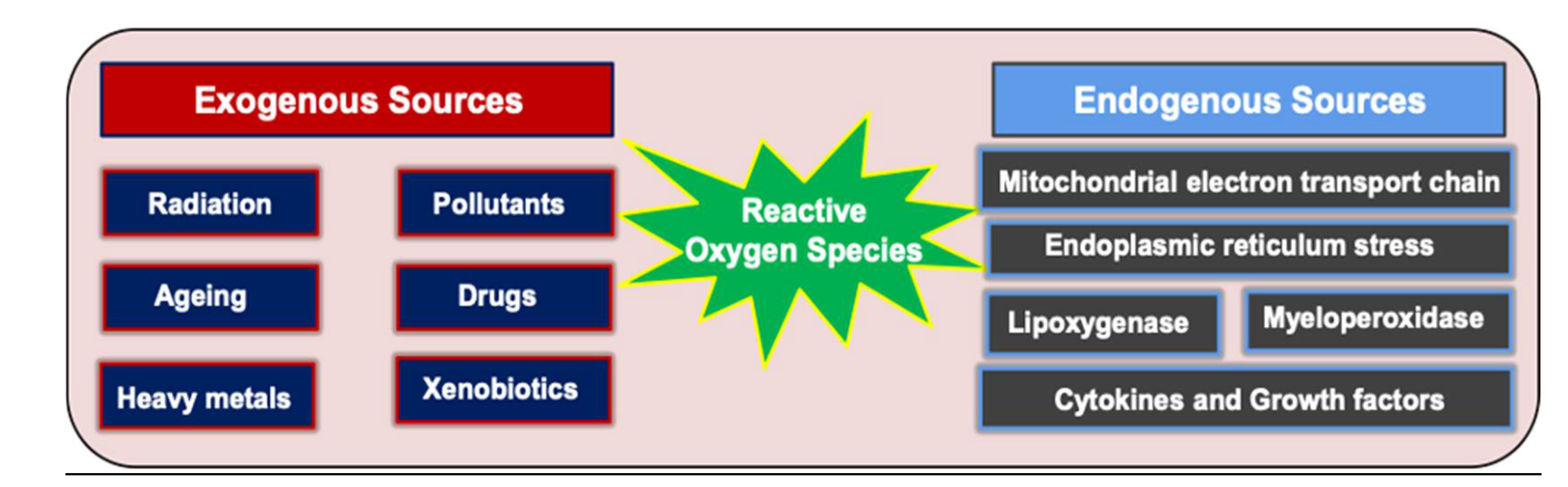
- Hensley, K. and R.A. Floyd, Reactive oxygen species and protein oxidation in aging: a look back, a look ahead. Archives of biochemistry and biophysics, 2002. 397(2): p. 377-383.
- Fruehauf, J.P. and F.L. Meyskens, Jr., Reactive Oxygen Species: A Breath of Life or Death? Clinical Cancer Research, 2007. 13(3): p. 789-794.
- Ciani, F. , et al., Influence of ROS on ovarian functions. New discoveries in embryology, 2015: p. 41-73.
- Collin, F. , Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. International Journal of Molecular Sciences, 2019. 20(10): p. 2407.
- Aldred, E.M. , Pharmacology: a handbook for complementary healthcare professionals. 2008: Elsevier Health Sciences.
- Baker, M.A., J. Netherton, and R.J. Aitken, From past to present: an historical overview of the concept of spermatozoa, reactive oxygen species, and male-factor infertility, in Oxidants, antioxidants and impact of the oxidative status in male reproduction. 2019, Elsevier. p. 17-26.
- Fujii, J., Y. Iuchi, and F. Okada, Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reproductive Biology and Endocrinology, 2005. 3(1): p. 43.
- Singh, R. and P.P. Manna, Reactive oxygen species in cancer progression and its role in therapeutics. Exploration of Medicine, 2022. 3(1): p. 43-57.
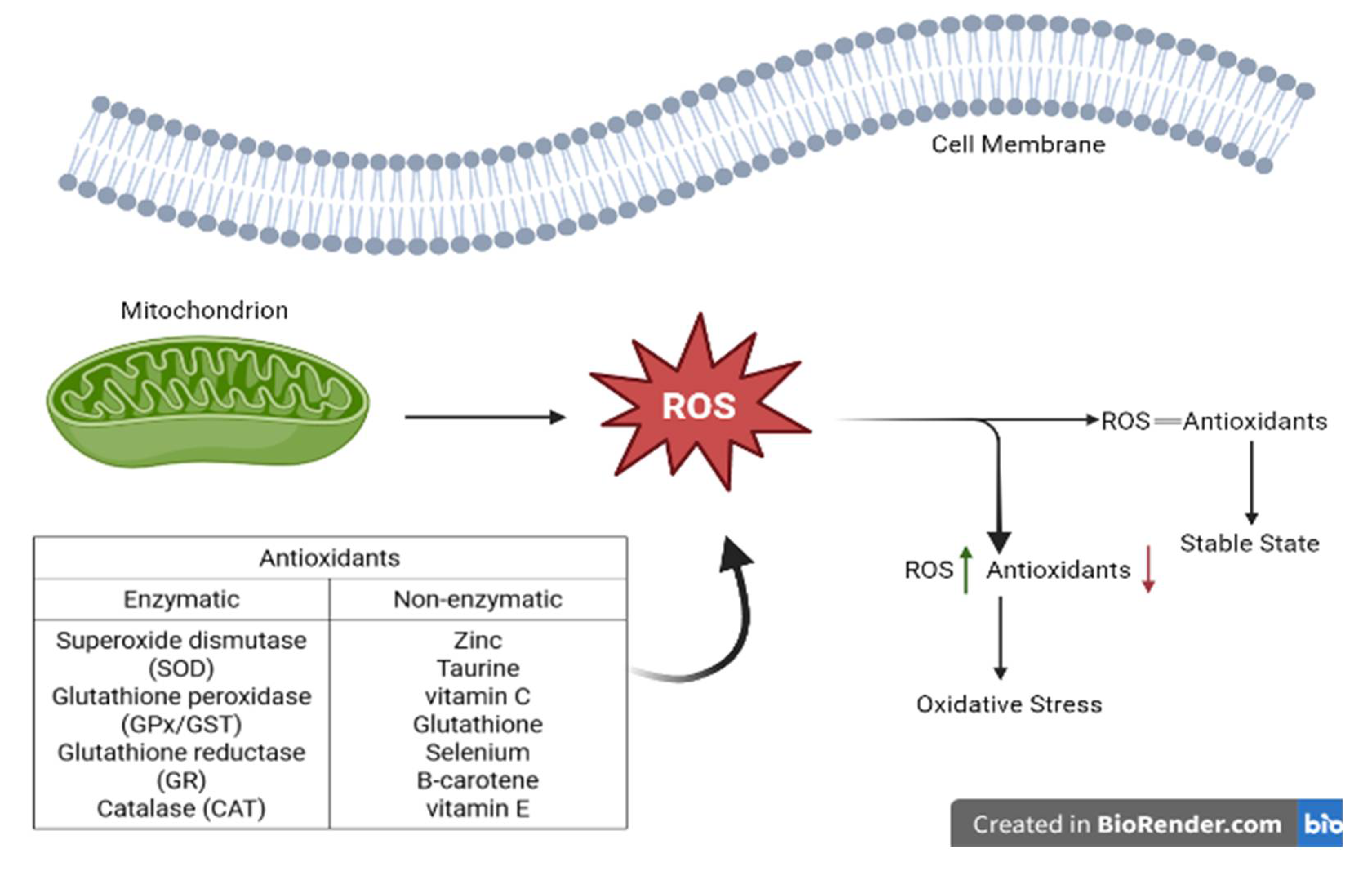
- Lushchak, V.I. , Free radicals, reactive oxygen species, oxidative stress and its classification. Chemico-biological interactions, 2014. 224: p. 164-175.
- Al-Dalaen, S.M. and A.I. Al-Qtaitat, Oxidative stress versus antioxidants. American journal of Bioscience and Bioengineering, 2014. 7(1): p. 60-71.
- Patekar, D. , et al., Antioxidant defence system. Oral & Maxillofacial Pathology Journal, 2013. 4(1).
- Moussa, Z., Z. Judeh, and S.A. Ahmed, Nonenzymatic exogenous and endogenous antioxidants. Free radical medicine and biology, 2019. 1: p. 11-22.
- Owoade, A.O. and A. Olorunnisola, The supportive role of dietary antioxidants in antioxidant defence system. Advanced in Life Science and Technology, 2019. 73: p. 53-9.
- Irato, P. and G. Santovito, Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants, 2021. 10(4): p. 579.
- Jeeva, J.S. , et al., Enzymatic antioxidants and its role in oral diseases. Journal of Pharmacy and Bioallied Sciences, 2015. 7(Suppl 2): p. S331-S333.
- Asakura, H. and T. Kitahora, Antioxidants in Inflammatory Bowel Disease, Ulcerative Colitis, and Crohn. Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease: Bioactive Foods in Chronic Disease States, 2013: p. 37.
- Katiyar, S.K., F. Afaq, and H. Mukhtar, Effects of solar radiation on detoxification mechanisms in the skin, in Comprehensive Series in Photosciences. 2001, Elsevier. p. 419-436.
- Mir, R.A. and M.A. Khah, Recent progress in enzymatic antioxidant defense system in plants against different environmental stresses. Improving Stress Resilience in Plants, 2024: p. 203-224.
- Janků, M., L. Luhová, and M. Petřivalský, On the Origin and Fate of Reactive Oxygen Species in Plant Cell Compartments. Antioxidants, 2019. 8(4): p. 105.
- Yang, Y. , et al., Reactive oxygen species in the immune system. International reviews of immunology, 2013. 32(3): p. 249-270.
- Liang, J. , et al., Reactive oxygen species and ovarian diseases: Antioxidant strategies. Redox Biology, 2023. 62: p. 102659.
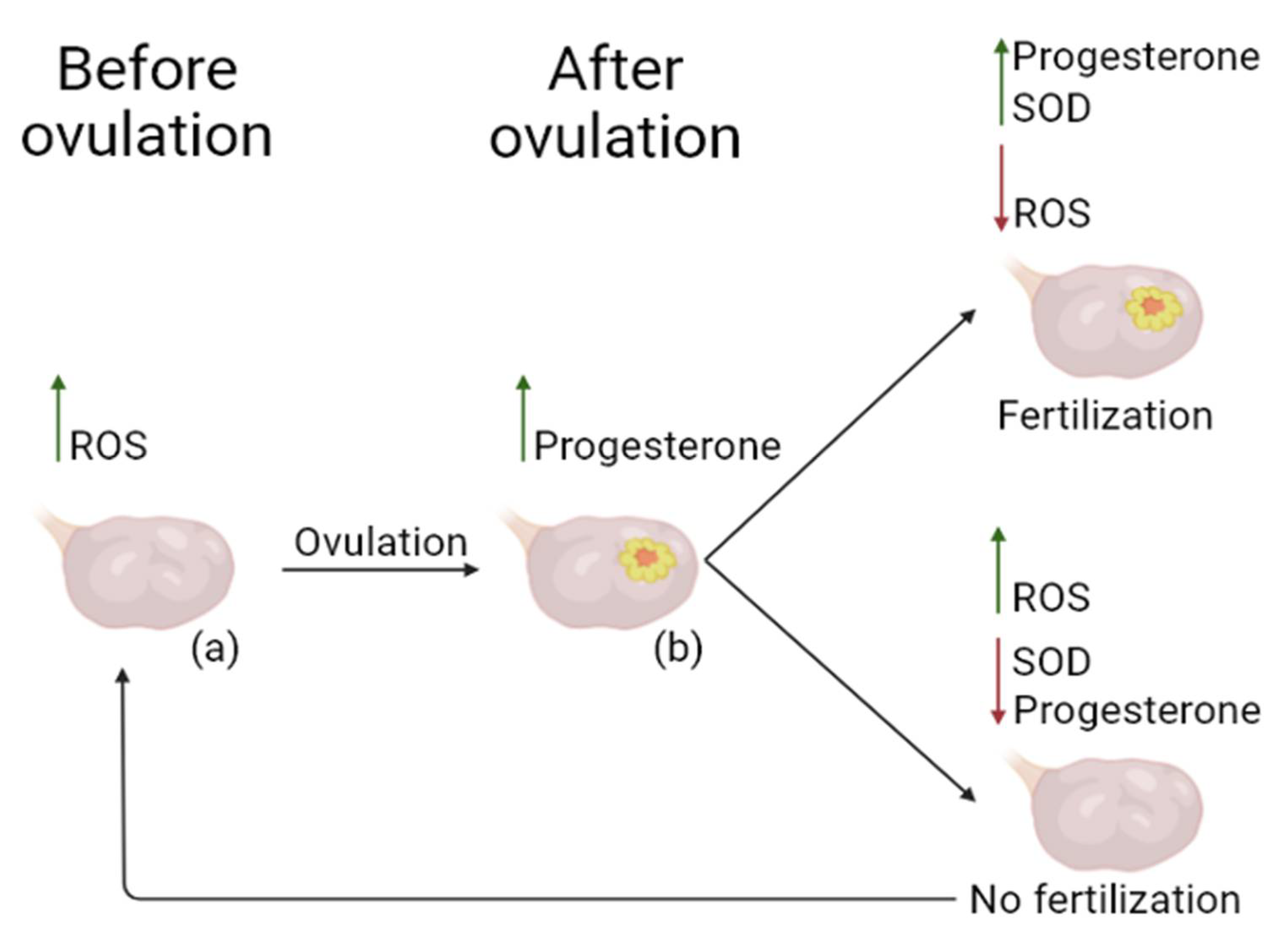
- Shkolnik, K. , et al., Reactive oxygen species are indispensable in ovulation. Proceedings of the National Academy of Sciences, 2011. 108(4): p. 1462-1467.
- SUGINO, N. , Roles of reactive oxygen species in the corpus luteum. Animal Science Journal, 2006. 77(6): p. 556-565.
- Sugino, N. , et al., Superoxide dismutase expression in the human corpus luteum during the menstrual cycle and in early pregnancy. Molecular Human Reproduction, 2000. 6(1): p. 19-25.
- Stocco, C., C. Telleria, and G. Gibori, The Molecular Control of Corpus Luteum Formation, Function, and Regression. Endocrine Reviews, 2007. 28(1): p. 117-149.
- Pizzino, G. , et al., Oxidative Stress: Harms and Benefits for Human Health. Oxidative Medicine and Cellular Longevity, 2017. 2017: p. 8416763.
- Wang, Q. , et al., Mechanistic study of TRPM2-Ca2+-CAMK2-BECN1 signaling in oxidative stress-induced autophagy inhibition. Autophagy, 2016. 12(8): p. 1340-1354.
- Berlett, B.S. and E.R. Stadtman, Protein oxidation in aging, disease, and oxidative stress. Journal of Biological Chemistry, 1997. 272(33): p. 20313-20316.
- Brieger, K. , et al., Reactive oxygen species: from health to disease. Swiss Medical Weekly, 2012. 142(3334): p. w13659.
- Sullivan, L.B. and N.S. Chandel, Mitochondrial reactive oxygen species and cancer. Cancer & metabolism, 2014. 2: p. 1-12.
- Moloney, J.N. and T.G. Cotter, ROS signalling in the biology of cancer. Semin Cell Dev Biol, 2018. 80: p. 50-64.
- Jelic, M.D. , et al., Oxidative stress and its role in cancer. J Cancer Res Ther, 2021. 17(1): p. 22-28.
- Marí-Alexandre, J. , et al., Interplay Between MicroRNAs and Oxidative Stress in Ovarian Conditions with a Focus on Ovarian Cancer and Endometriosis. International Journal of Molecular Sciences, 2019. 20(21): p. 5322.
- Bartel, D.P. , MicroRNAs: target recognition and regulatory functions. Cell, 2009. 136(2): p. 215-33.
- Hanahan, D. and R.A. Weinberg, The hallmarks of cancer. Cell, 2000. 100(1): p. 57-70.
- Hart, P.C. , et al., MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nature communications, 2015. 6(1): p. 6053.
- Kim, J., J. Kim, and J.-S. Bae, ROS homeostasis and metabolism: a critical liaison for cancer therapy. Experimental & molecular medicine, 2016. 48(11): p. e269-e269.
- Wang, Y. , et al., The double-edged roles of ROS in cancer prevention and therapy. Theranostics, 2021. 11(10): p. 4839.
- Chiarugi, P. , et al., Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. The Journal of cell biology, 2003. 161(5): p. 933-944.
- Takeuchi, T., M. Nakajima, and K. Morimoto, Relationship between the intracellular reactive oxygen species and the induction of oxidative DNA damage in human neutrophil-like cells (vol 17, pg 1543, 1996). 1997, OXFORD UNIV PRESS GREAT CLARENDON ST, OXFORD, ENGLAND OX2 6DP. p. 1683-1683.
- Douma, S. , et al., Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature, 2004. 430(7003): p. 1034-9.
- Stieg, D.C. , et al., ROS and miRNA Dysregulation in Ovarian Cancer Development, Angiogenesis and Therapeutic Resistance. International Journal of Molecular Sciences, 2022. 23(12): p. 6702.
- Xia, C. , et al., Reactive Oxygen Species Regulate Angiogenesis and Tumor Growth through Vascular Endothelial Growth Factor. Cancer Research, 2007. 67(22): p. 10823-10830.
- Caneba, C.A. , et al., Nitric oxide is a positive regulator of the Warburg effect in ovarian cancer cells. Cell Death Dis, 2014. 5(6): p. e1302.
- Galadari, S. , et al., Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic Biol Med, 2017. 104: p. 144-164.
- Dodson, M., R. Castro-Portuguez, and D.D. Zhang, NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol, 2019. 23: p. 101107.
- Redza-Dutordoir, M. and D.A. Averill-Bates, Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta, 2016. 1863(12): p. 2977-2992.
- Ding, D.N. , et al., Insights into the Role of Oxidative Stress in Ovarian Cancer. Oxid Med Cell Longev, 2021. 2021: p. 8388258.
- Sharifi-Rad, M. , et al., Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol, 2020. 11: p. 694.
- Franco, R. , et al., Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett, 2008. 266(1): p. 6-11.
- Xu, M. , et al., Taurine alleviates oxidative stress in porcine mammary epithelial cells by stimulating the Nrf2-MAPK signaling pathway. Food Sci Nutr, 2023. 11(4): p. 1736-1746.
- Rojo de la Vega, M., E. Chapman, and D.D. Zhang, NRF2 and the Hallmarks of Cancer. Cancer Cell, 2018. 34(1): p. 21-43.
- Kerins, M.J. and A. Ooi, A catalogue of somatic NRF2 gain-of-function mutations in cancer. Sci Rep, 2018. 8(1): p. 12846.
- Nguyen, T., P. Nioi, and C.B. Pickett, The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem, 2009. 284(20): p. 13291-5.
- Dinkova-Kostova, A.T., W. D. Holtzclaw, and N. Wakabayashi, Keap1, the sensor for electrophiles and oxidants that regulates the phase 2 response, is a zinc metalloprotein. Biochemistry, 2005. 44(18): p. 6889-99.
- Sun, Z. , et al., KPNA6 (Importin {alpha}7)-mediated nuclear import of Keap1 represses the Nrf2-dependent antioxidant response. Mol Cell Biol, 2011. 31(9): p. 1800-11.
- Ma, Q. , Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol, 2013. 53: p. 401-26.
- Klaunig, J.E., L. M. Kamendulis, and B.A. Hocevar, Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol, 2010. 38(1): p. 96-109.
- Kumar, B. , et al., Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res, 2008. 68(6): p. 1777-85.
- Mateescu, B. , et al., miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat Med, 2011. 17(12): p. 1627-35.
- Chen, Z. , et al., MAP kinases. Chem Rev, 2001. 101(8): p. 2449-76.
- Innocenti, M. , et al., Phosphoinositide 3-kinase activates Rac by entering in a complex with Eps8, Abi1, and Sos-1. J Cell Biol, 2003. 160(1): p. 17-23.
- Pimienta, G. and J. Pascual, Canonical and alternative MAPK signaling. Cell Cycle, 2007. 6(21): p. 2628-32.
- Qiu, X. , et al., COX2 and PGE2 mediate EGF-induced E-cadherin-independent human ovarian cancer cell invasion. Endocr Relat Cancer, 2014. 21(4): p. 533-43.
- Fruman, D.A. and C. Rommel, PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov, 2014. 13(2): p. 140-56.
- Levine, D.A. , et al., Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res, 2005. 11(8): p. 2875-8. [CrossRef]
- Philp, A.J. , et al., The phosphatidylinositol 3'-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res, 2001. 61(20): p. 7426-9.
- Sakamoto, K. , et al., Role of the tumor suppressor PTEN in antioxidant responsive element-mediated transcription and associated histone modifications. Mol Biol Cell, 2009. 20(6): p. 1606-17.
- van der Reest, J. , et al., Proteome-wide analysis of cysteine oxidation reveals metabolic sensitivity to redox stress. Nat Commun, 2018. 9(1): p. 1581.
- Wu, W.S. , The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev, 2006. 25(4): p. 695-705.
- Kinross, K.M. , et al., An activating Pik3ca mutation coupled with Pten loss is sufficient to initiate ovarian tumorigenesis in mice. J Clin Invest, 2012. 122(2): p. 553-7.
- Arend, R.C. , et al., The Wnt/β-catenin pathway in ovarian cancer: a review. Gynecol Oncol, 2013. 131(3): p. 772-9.
- Jung, Y.S. and J.I. Park, Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp Mol Med, 2020. 52(2): p. 183-191.
- Wiese, K.E., R. Nusse, and R. van Amerongen, Wnt signalling: conquering complexity. Development, 2018. 145(12).
- Groden, J. , et al., Identification and characterization of the familial adenomatous polyposis coli gene. Cell, 1991. 66(3): p. 589-600.
- van Schie, E.H. and R. van Amerongen, Aberrant WNT/CTNNB1 Signaling as a Therapeutic Target in Human Breast Cancer: Weighing the Evidence. Front Cell Dev Biol, 2020. 8: p. 25.
- Gatcliffe, T.A. , et al., Wnt signaling in ovarian tumorigenesis. Int J Gynecol Cancer, 2008. 18(5): p. 954-62. [CrossRef]
- Wu, R. , et al., Diverse mechanisms of beta-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res, 2001. 61(22): p. 8247-55.
- Nusse, R. and H. Clevers, Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell, 2017. 169(6): p. 985-999.
- Nguyen, V.H.L. , et al., Wnt/β-catenin signalling in ovarian cancer: Insights into its hyperactivation and function in tumorigenesis. J Ovarian Res, 2019. 12(1): p. 122.
- Wu, Y. , et al., Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett, 2014. 345(2): p. 164-73.
- Yang, H.Y. , et al., Tankyrase Promotes Aerobic Glycolysis and Proliferation of Ovarian Cancer through Activation of Wnt/β-Catenin Signaling. Biomed Res Int, 2019. 2019: p. 2686340.
- Radtke, F. and K. Raj, The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nature Reviews Cancer, 2003. 3(10): p. 756-767.
- El-Sehemy, A. , et al., Notch activation augments nitric oxide/soluble guanylyl cyclase signaling in immortalized ovarian surface epithelial cells and ovarian cancer cells. Cell Signal, 2013. 25(12): p. 2780-7.
- Rose, S.L. , et al., Notch 1 signaling is active in ovarian cancer. Gynecol Oncol, 2010. 117(1): p. 130-3.
- Park, J.T. , et al., Notch3 overexpression is related to the recurrence of ovarian cancer and confers resistance to carboplatin. Am J Pathol, 2010. 177(3): p. 1087-94.
- Gupta, N. , et al., Notch3 induces epithelial-mesenchymal transition and attenuates carboplatin-induced apoptosis in ovarian cancer cells. Gynecol Oncol, 2013. 130(1): p. 200-6.
- Lu, C. , et al., Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Res, 2007. 67(4): p. 1757-68.
- Elias, K.M., J. Guo, and R.C. Bast, Jr., Early Detection of Ovarian Cancer. Hematol Oncol Clin North Am, 2018. 32(6): p. 903-914.
- Sundar, S., R. D. Neal, and S. Kehoe, Diagnosis of ovarian cancer. Bmj, 2015. 351: p. h4443.
- Charkhchi, P. , et al., CA125 and Ovarian Cancer: A Comprehensive Review. Cancers (Basel), 2020. 12(12).
- Matte, I. , et al., Ascites from ovarian cancer patients stimulates MUC16 mucin expression and secretion in human peritoneal mesothelial cells through an Akt-dependent pathway. BMC Cancer, 2019. 19(1): p. 406.
- Zhang, M. , et al., Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta Rev Cancer, 2021. 1875(2): p. 188503.
- Timmerman, D. , et al., Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. Bmj, 2010. 341: p. c6839.
- Skates, S.J. , Ovarian cancer screening: development of the risk of ovarian cancer algorithm (ROCA) and ROCA screening trials. Int J Gynecol Cancer, 2012. 22 Suppl 1(Suppl 1): p. S24-6.
- Folkins, A.K. , et al., A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol, 2008. 109(2): p. 168-73.
- Nelson, S.J. , et al., Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C] pyruvate. Science translational medicine, 2013. 5(198): p. 198ra108-198ra108.
- Adolphi, N.L. , et al., Imaging of Her2-targeted magnetic nanoparticles for breast cancer detection: comparison of SQUID-detected magnetic relaxometry and MRI. Contrast Media Mol Imaging, 2012. 7(3): p. 308-19.
- James, N.E., C. Chichester, and J.R. Ribeiro, Beyond the Biomarker: Understanding the Diverse Roles of Human Epididymis Protein 4 in the Pathogenesis of Epithelial Ovarian Cancer. Front Oncol, 2018. 8: p. 124.
- Yang, W.L., Z. Lu, and R.C. Bast, Jr., The role of biomarkers in the management of epithelial ovarian cancer. Expert Rev Mol Diagn, 2017. 17(6): p. 577-591.
- Chandra, A. , et al., Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med, 2019. 8(16): p. 7018-7031.
- Vang, R. , et al., Molecular Alterations of TP53 are a Defining Feature of Ovarian High-Grade Serous Carcinoma: A Rereview of Cases Lacking TP53 Mutations in The Cancer Genome Atlas Ovarian Study. Int J Gynecol Pathol, 2016. 35(1): p. 48-55.
- Cohen, J.D. , et al., Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science, 2018. 359(6378): p. 926-930.
- Xu, S. , et al., MiR-497 decreases cisplatin resistance in ovarian cancer cells by targeting mTOR/P70S6K1. Oncotarget, 2015. 6(28): p. 26457-71.
- Kan, C.W. , et al., Elevated levels of circulating microRNA-200 family members correlate with serous epithelial ovarian cancer. BMC Cancer, 2012. 12: p. 627.
- Iorio, M.V. , et al., MicroRNA signatures in human ovarian cancer. Cancer Res, 2007. 67(18): p. 8699-707.
- Jiajie, T. , et al., Conserved miR-10 family represses proliferation and induces apoptosis in ovarian granulosa cells. Sci Rep, 2017. 7: p. 41304.
- Vescarelli, E. , et al., MiR-200c sensitizes Olaparib-resistant ovarian cancer cells by targeting Neuropilin 1. J Exp Clin Cancer Res, 2020. 39(1): p. 3.
- Choi, P.W. and S.W. Ng, The Functions of MicroRNA-200 Family in Ovarian Cancer: Beyond Epithelial-Mesenchymal Transition. Int J Mol Sci, 2017. 18(6).
- Zhou, J. , et al., miR-183 modulated cell proliferation and apoptosis in ovarian cancer through the TGF-β/Smad4 signaling pathway. Int J Mol Med, 2019. 43(4): p. 1734-1746.
- Lv, Y., F. -L. Li, and P.-S. Liu, MiR-151 promotes ovarian cancer through activation of akt/mTOR signaling pathway by decreasing RhoGDIA. Int. J. Clin. Exp. Pathol, 2016. 9: p. 11222-11229.
- Nakayama, I. , et al., Loss of HOXD10 expression induced by upregulation of miR-10b accelerates the migration and invasion activities of ovarian cancer cells. Int J Oncol, 2013. 43(1): p. 63-71.
- Staicu, C.E. , et al., Role of microRNAs as Clinical Cancer Biomarkers for Ovarian Cancer: A Short Overview. Cells, 2020. 9(1).
- Orr, B. and R.P. Edwards, Diagnosis and treatment of ovarian cancer. Hematology/Oncology Clinics, 2018. 32(6): p. 943-964.
- Cummings, M., O. Nicolais, and M. Shahin, Surgery in Advanced Ovary Cancer: Primary versus Interval Cytoreduction. Diagnostics (Basel), 2022. 12(4).
- Bacalbasa, N. , et al., The Risk of Para-Aortic Lymph Node Metastases in Apparent Early Stage Ovarian Cancer. Medicina (Kaunas), 2020. 56(3).
- Kurnit, K.C., G. F. Fleming, and E. Lengyel, Updates and New Options in Advanced Epithelial Ovarian Cancer Treatment. Obstet Gynecol, 2021. 137(1): p. 108-121.
- Herrera, F.G. , et al., Rational combinations of immunotherapy with radiotherapy in ovarian cancer. Lancet Oncol, 2019. 20(8): p. e417-e433.
- Zhang, C. , et al., Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics, 2022. 12(5): p. 2115-2132.
- Erol, A., M. Niemira, and A.J. Krętowski, Novel Approaches in Ovarian Cancer Research against Heterogeneity, Late Diagnosis, Drug Resistance, and Transcoelomic Metastases. Int J Mol Sci, 2019. 20(11).
- Chehelgerdi, M. , et al., Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Mol Cancer, 2023. 22(1): p. 169.
- Wang, Q. , et al., The prognostic factor for recurrence in advanced-stage high-grade serous ovarian cancer after complete clinical remission: a nested case-control study. J Ovarian Res, 2021. 14(1): p. 179.
- Qian, S. , et al., The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front Oncol, 2022. 12: p. 985363.
- Kaloni, D. , et al., BCL-2 protein family: attractive targets for cancer therapy. Apoptosis, 2023. 28(1-2): p. 20-38.
- Mobahat, M., A. Narendran, and K. Riabowol, Survivin as a preferential target for cancer therapy. Int J Mol Sci, 2014. 15(2): p. 2494-516.
- Zhang, Y. , et al., Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer, 2018. 17(1): p. 45.
- Surowiak, P. , et al., Significance of cyclooxygenase 2 and MDR1/P-glycoprotein coexpression in ovarian cancers. Cancer Lett, 2006. 235(2): p. 272-80.
- Januchowski, R. , et al., Analysis of MDR genes expression and cross-resistance in eight drug resistant ovarian cancer cell lines. J Ovarian Res, 2016. 9(1): p. 65.
- Vella, V. , et al., Insulin/IGF Axis and the Receptor for Advanced Glycation End Products: Role in Meta-inflammation and Potential in Cancer Therapy. Endocr Rev, 2023. 44(4): p. 693-723.
- Bilyk, O. , et al., Epithelial-to-Mesenchymal Transition in the Female Reproductive Tract: From Normal Functioning to Disease Pathology. Front Oncol, 2017. 7: p. 145.
- Singh, S. , et al., Small Molecule Targeting Immune Cells: A Novel Approach for Cancer Treatment. Biomedicines, 2023. 11(10).
- Galluzzi, L. , et al., Classification of current anticancer immunotherapies. Oncotarget, 2014. 5(24): p. 12472-508.
- Macpherson, A.M. , et al., Epithelial ovarian cancer and the immune system: biology, interactions, challenges and potential advances for immunotherapy. Journal of clinical medicine, 2020. 9(9): p. 2967.
- Drugs Approved for Ovarian, Fallopian Tube, or Primary Peritoneal Cancer. 2022; Available from: https://www.cancer.gov/about-cancer/treatment/drugs/ovarian.
- Davis-Perry, S. , et al., Melphalan for the treatment of patients with recurrent epithelial ovarian cancer. Am J Clin Oncol, 2003. 26(4): p. 429-33.
- Carboplatin. 2023; Available from: https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin.
- Collins, I.M. , et al., Carboplatin dosing in ovarian cancer: problems and pitfalls. Int J Gynecol Cancer, 2011. 21(7): p. 1213-8.
- Handolias, D. , et al., Oral cyclophosphamide in recurrent ovarian cancer. Asia Pac J Clin Oncol, 2016. 12(1): p. e154-60.
- Lihua, P., X. Y. Chen, and T.X. Wu, Topotecan for ovarian cancer. Cochrane Database Syst Rev, 2008. 2008(2): p. Cd005589.
- Song, M., M. Cui, and K. Liu, Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur J Med Chem, 2022. 232: p. 114205.
- Doxorubicin Hydrochloride. 2023; Available from: https://www.cancer.gov/about-cancer/treatment/drugs/doxorubicinhydrochloride.
- Gemcitabine Hydrochloride. 2023; Available from: https://www.cancer.gov/about-cancer/treatment/drugs/gemcitabinehydrochloride.
- Weaver, B.A. , How Taxol/paclitaxel kills cancer cells. Mol Biol Cell, 2014. 25(18): p. 2677-81.
- Nakai, H. and N. Matsumura, The roles and limitations of bevacizumab in the treatment of ovarian cancer. Int J Clin Oncol, 2022. 27(7): p. 1120-1126.
- Bevacizumab. 2023; Available from: https://www.cancer.gov/about-cancer/treatment/drugs/bevacizumab.
- Mirvetuximab Soravtansine-gynx. 2024; Available from: https://www.cancer.gov/about-cancer/treatment/drugs/mirvetuximab-soravtansine-gynx.
- Dilawari, A. , et al., FDA Approval Summary: Mirvetuximab Soravtansine-Gynx for FRα-Positive, Platinum-Resistant Ovarian Cancer. Clin Cancer Res, 2023. 29(19): p. 3835-3840.
- Moore, K. , et al., Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med, 2018. 379(26): p. 2495-2505.
- Dockery, L.E., C. C. Gunderson, and K.N. Moore, Rucaparib: the past, present, and future of a newly approved PARP inhibitor for ovarian cancer. Onco Targets Ther, 2017. 10: p. 3029-3037.
- Schmid, B.C. and M.K. Oehler, New perspectives in ovarian cancer treatment. Maturitas, 2014. 77(2): p. 128-36.
- Gogineni, V. , et al., Current Ovarian Cancer Maintenance Strategies and Promising New Developments. J Cancer, 2021. 12(1): p. 38-53.
- Rocconi, R.P. , et al., Maintenance vigil immunotherapy in newly diagnosed advanced ovarian cancer: Efficacy assessment of homologous recombination proficient (HRP) patients in the phase IIb VITAL trial. Journal of Clinical Oncology, 2021. 39(15_suppl): p. 5502-5502.
- Wang, X. , et al., A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood, 2011. 118(5): p. 1255-63.
- Rob, L. , et al., Safety and efficacy of dendritic cell-based immunotherapy DCVAC/OvCa added to first-line chemotherapy (carboplatin plus paclitaxel) for epithelial ovarian cancer: a phase 2, open-label, multicenter, randomized trial. J Immunother Cancer, 2022. 10(1).
- Xiao, Y.F. , et al., Peptide-Based Treatment: A Promising Cancer Therapy. J Immunol Res, 2015. 2015: p. 761820.
- Hoare, J., N. Campbell, and E. Carapuça, Oncolytic virus immunotherapies in ovarian cancer: moving beyond adenoviruses. Porto Biomed J, 2018. 3(1): p. e7.
- Pal, S. , et al., Targeting cancer-specific metabolic pathways for developing novel cancer therapeutics. Front Immunol, 2022. 13: p. 955476.
- Basudan, A.M. , The Role of Immune Checkpoint Inhibitors in Cancer Therapy. Clin Pract, 2022. 13(1): p. 22-40.
- Pirš, B. , et al., Overview of Immune Checkpoint Inhibitors in Gynecological Cancer Treatment. Cancers (Basel), 2022. 14(3).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



