Submitted:
08 July 2024
Posted:
10 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Collection and Identification of Plant Material
Preparation of Methanolic and Aqueous Crude Extracts
Preparation of Terpenoid-Rich Fraction (IVLM DCM)
Determination of Total Phenols and Flavonoids Contents
Gas Chromatography/ Mass Spectrometry (GC/MS) Analysis
DPPH Free Radical Scavenging Assay
ABTS Radical Scavenging Assay
Cell Culture and MTT Cell Cytotoxicity Assay
DAPI Staining
Crystal Violet Staining
Western Blotting Analysis
Scratch/ Wound-Healing Assay
Statistical Analysis
3. Results
Extraction Yield, Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Antioxidant Capacity of Inula viscosa Leaves and Stems Extracts
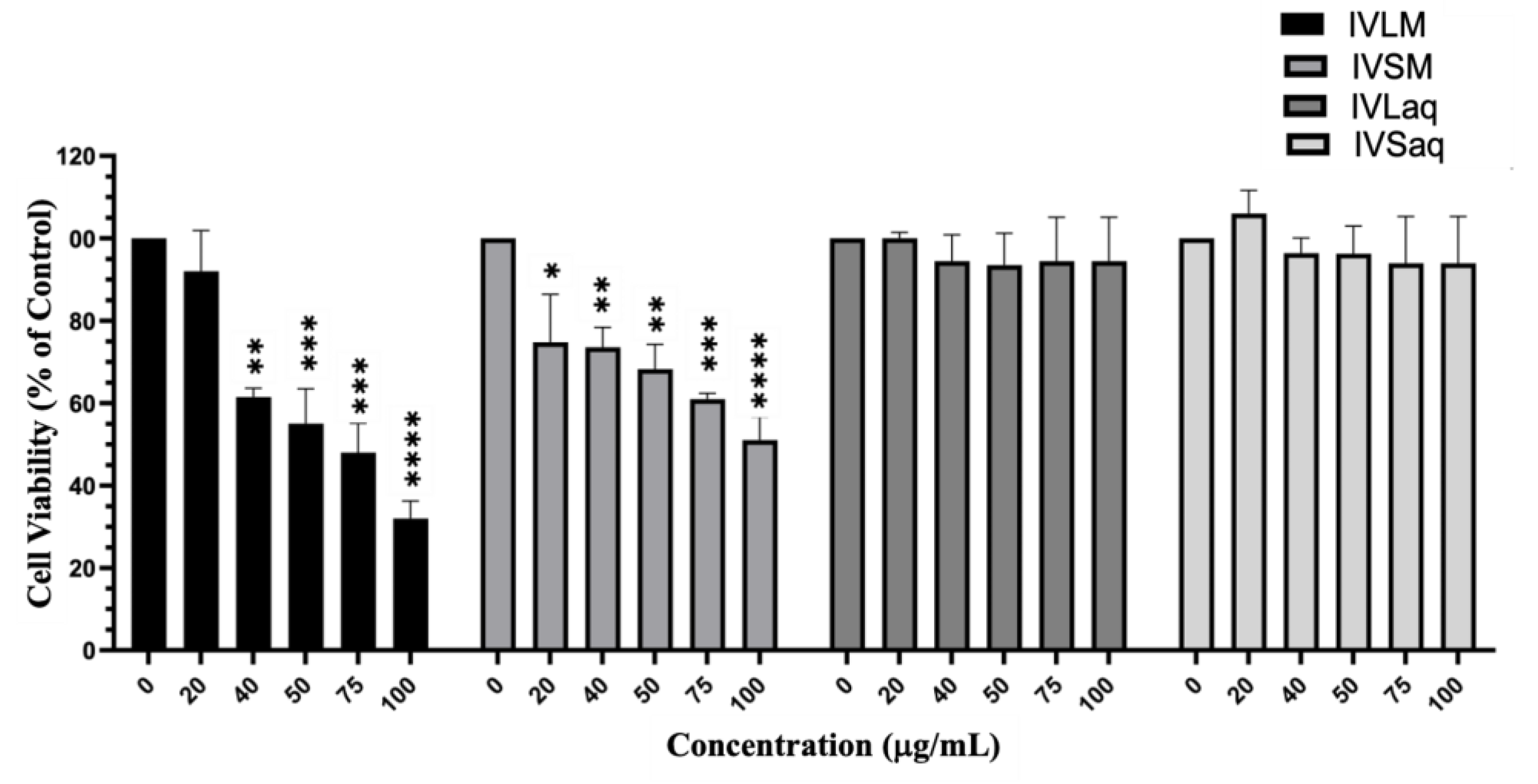
Inula viscosa Methanolic Extracts Reduced the Viability of A549 Lung Cancer Cells
Dichloromethane Fraction of Inula viscosa Leaves Methanolic Extract (IVL DCM) Reduced the Viability of A549 Lung Cancer Cells
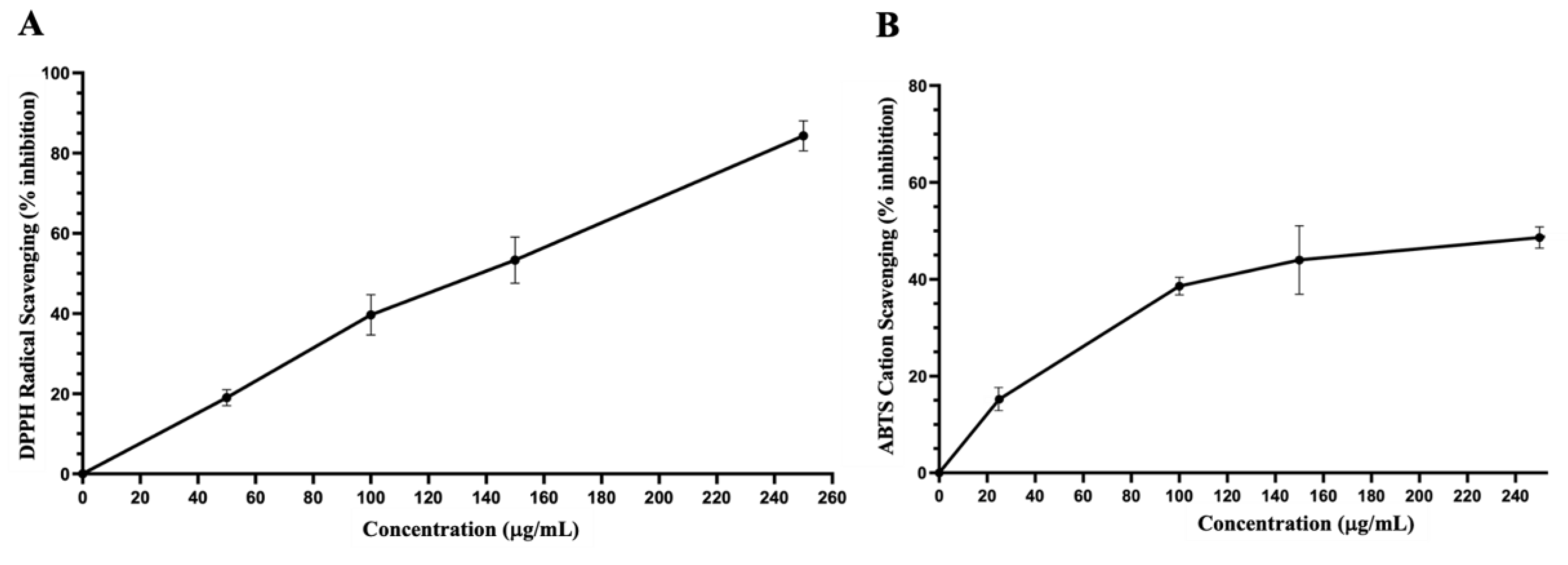
Antioxidant Capacity and Total Phenolic and Flavonoid Contents of Dichloromethane Fraction of Inula viscosa Leaves Methanolic Extract (IVL DCM)
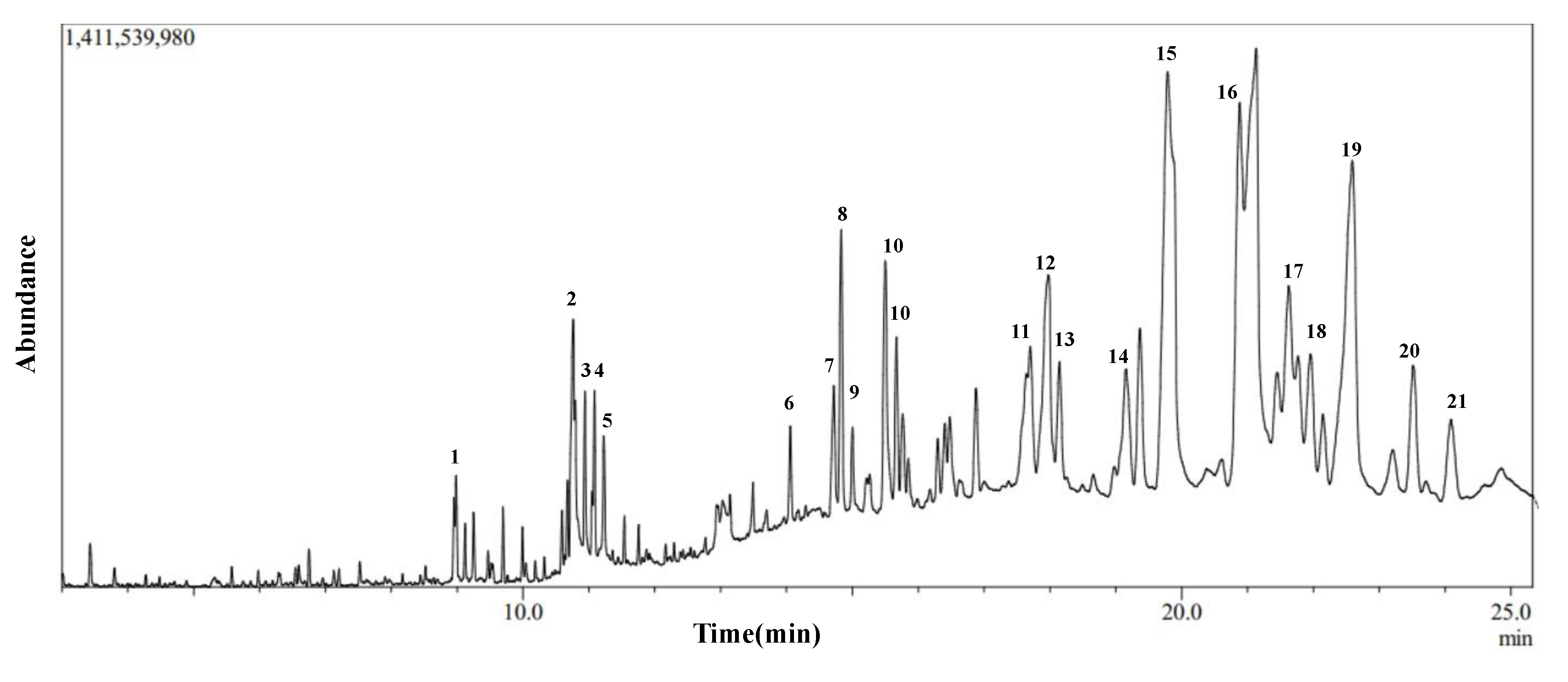
Gas Chromatography-Mass Spectroscopy (GC-MS) of Dichloromethane Fraction of Inula viscosa Leaves Methanolic Extract (IVL DCM)
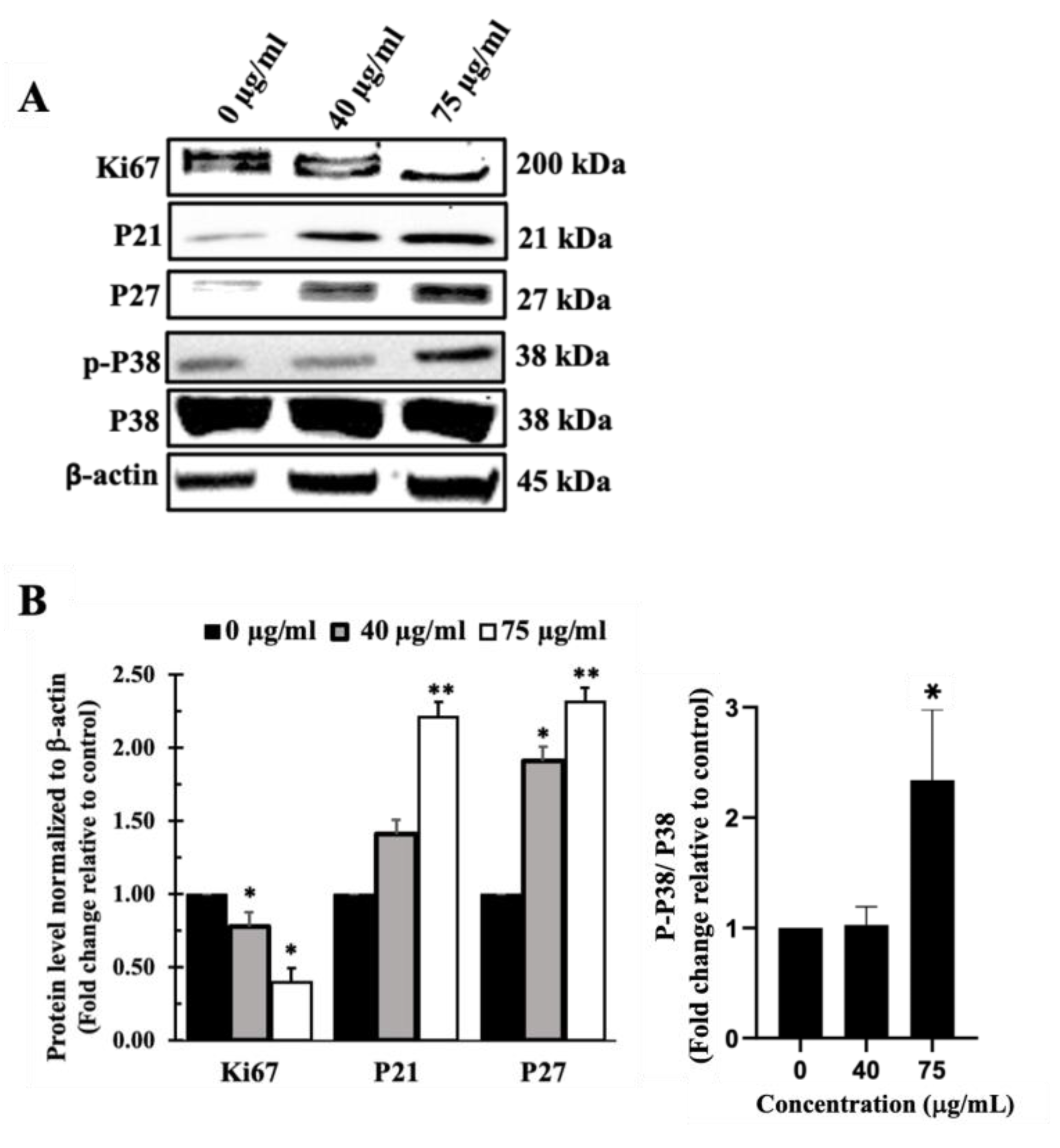
Inula viscosa Leaves Terpenoid-Rich Fraction (IVL DCM) Inhibited the Proliferation of A549 Lung Cancer Cells
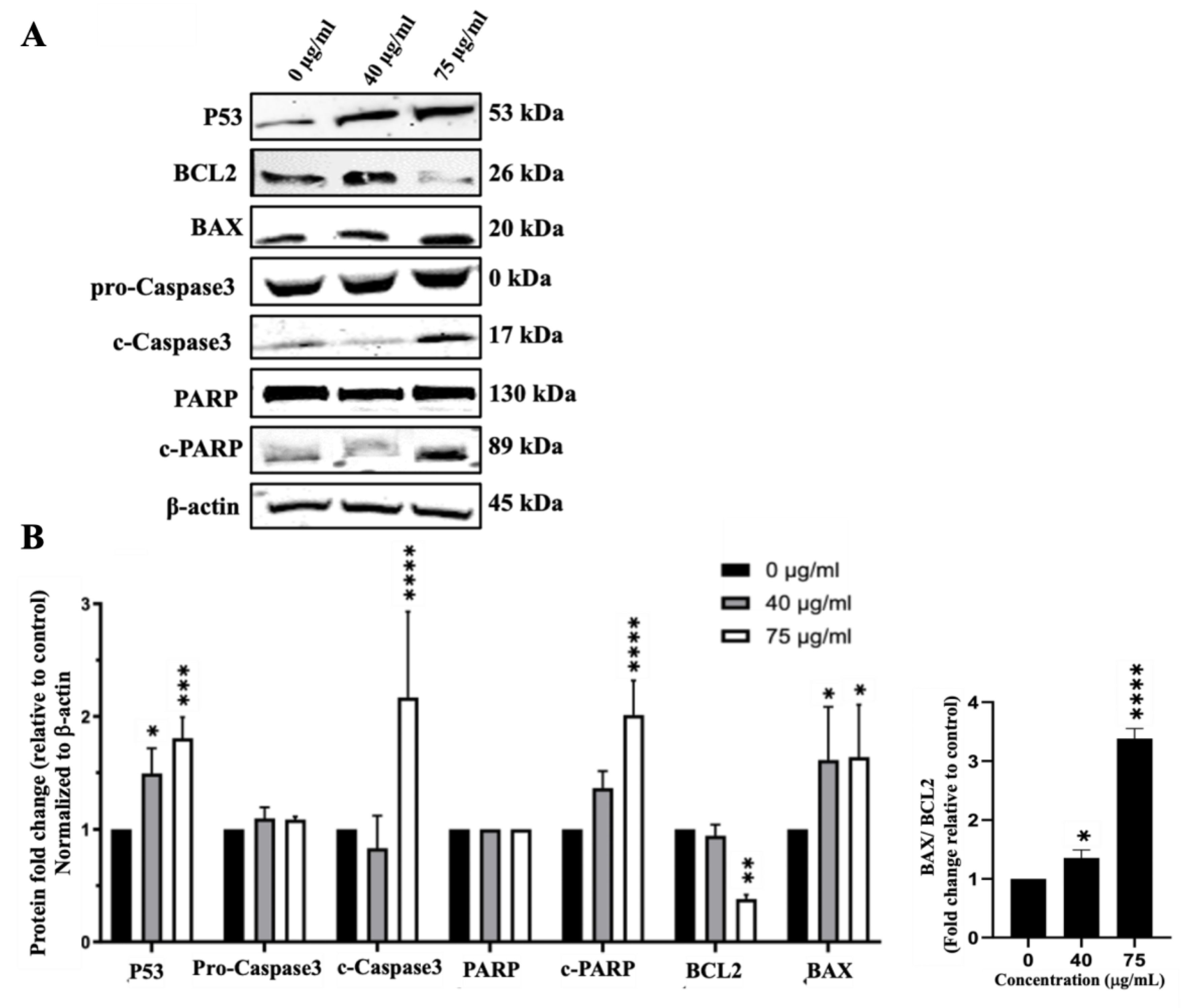
I. viscosa Leaves Terpenoid-Rich Fraction (IVL DCM) Induced Apoptosis of A549 Cells
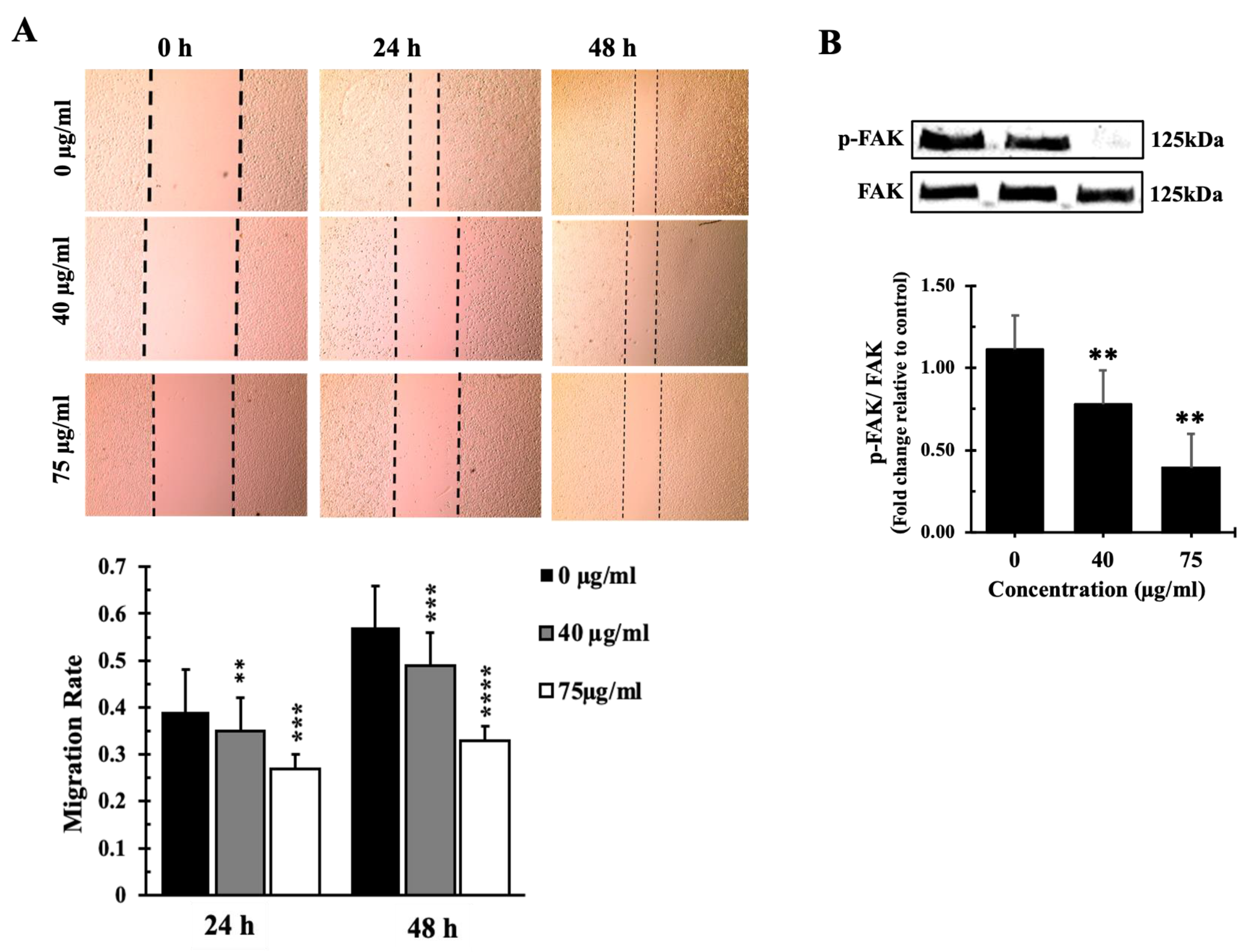
Inula viscosa Leaves Terpenoid Enriched Fraction (IVL DCM) Reduced the Migration of A549 Lung Cancer Cells through Reduction of FAK Activation
4. Discussion
Phenolic and Flavonoid Contents and Antioxidant Capacity of I. viscosa Parts
Inula viscosa Leaves Extracts Reduced the Viability of A549 Lung Cancer Cells
A Terpenoid-Rich Fraction of I. viscosa (IVL DCM)
The Terpenoid-Rich Fraction of I. viscosa Reduced the Proliferation and Migration and Induced Apoptosis of A549 Lung Cancer Cells
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung cancer statistics. Lung cancer and personalized medicine: current knowledge and therapies 2016, 1–19. [Google Scholar]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non–small cell lung cancer: epidemiology, screening, diagnosis, and treatment. In Proceedings of the Mayo Clinic Proceedings; 2019; pp. 1623–1640. [Google Scholar]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. International immunopharmacology 2018, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Mamdani, H.; Matosevic, S.; Khalid, A.B.; Durm, G.; Jalal, S.I. Immunotherapy in lung cancer: Current landscape and future directions. Frontiers in immunology 2022, 13, 823618. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Li, S.; Zhao, Y.; Cheng, K. Mechanisms of drug resistance to immune checkpoint inhibitors in non-small cell lung cancer. Frontiers in Immunology 2023, 14, 1127071. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Daoud, S.; Mahmod, A.I.; Hamed, R.A.; Awajan, D.; Abuarab, S.F.; Odeh, L.H.; Khater, S.; Al Kury, L.T. Plants as a Source of Anticancer Agents: From Bench to Bedside. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nature Reviews Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Yu, C.; Zhang, X.-D.; Liu, W.; Wu, Y.-C.; Gong, P.-X.; Li, H.-H.; Liu, Y.; Li, H.-J. Recent trends in anti-cancer activities of terrestrial plants-based polysaccharides: A review. Carbohydrate Polymer Technologies and Applications 2023, 6, 100341. [Google Scholar] [CrossRef]
- Ullah, S.; Khan, T.; Khan, T.; Ali, M.; Khaliq Jan, A.; Khan Shinwari, Z.; Khan, A.; Al-Farsi, M.; Waqas, M. Phytochemicals for Cancer Treatment: An Update on Plant-Derived Anti-Cancer Compounds and their Mechanisms of Action. Curr Top Med Chem 2024. [Google Scholar] [CrossRef]
- Shaito, A.A.; Omairi, I.; Al-Thani, N.; Seglab, F.; Ad-Darwish, E.; Kobeissy, F.; Nasreddine, S. Determination of Medicago orbicularis Antioxidant, Antihemolytic, and Anti-Cancerous Activities and Its Augmentation of Cisplatin-Induced Cytotoxicity in A549 Lung Cancer Cells. Plants (Basel) 2024, 13. [Google Scholar] [CrossRef]
- Qneibi, M.; Hanania, M.; Jaradat, N.; Emwas, N.; Radwan, S. Inula viscosa (L.) Greuter, phytochemical composition, antioxidant, total phenolic content, total flavonoids content and neuroprotective effects. European Journal of Integrative Medicine 2021, 42, 101291. [Google Scholar] [CrossRef]
- Jerada, R.; Er-Rakibi, A.; Hassani, A.C.; Benzeid, H.; El Ouardi, A.; Harhar, H.; Goh, B.H.; Yow, Y.-Y.; Ser, H.L.; Bouyahya, A. A comprehensive review on ethnomedicinal uses, phytochemistry, toxicology, and pharmacological activities of Dittrichia viscosa (L.) Greuter. Journal of Traditional and Complementary Medicine 2024. [Google Scholar] [CrossRef]
- Sladonja, B.; Poljuha, D.; Krapac, M.; Uzelac, M.; Mikulic-Petkovsek, M. Dittrichia viscosa: Native-Non Native Invader. Diversity 2021, 13, 380. [Google Scholar] [CrossRef]
- Ouahchia, C.; Hamaidi-Chergui, F.; Cherif, H.-S.; Hemma, R.; Negab, I.; Azine, K.; Saidi, F. Total Phenolic Content, Anti-Inflammatory, Analgesic, and Antipyretic Activities of Some Extracts of Inula viscosa (L.) from Algeria. Phytothérapie 2020, 18, 81–91. [Google Scholar] [CrossRef]
- Ben Sassi, A.; Harzallah-Skhiri, F.; Bourgougnon, N.; Aouni, M. Antiviral activity of some Tunisian medicinal plants against Herpes simplex virus type 1. Natural Product Research 2008, 22, 53–65. [Google Scholar] [CrossRef]
- Kurz, H.; Karygianni, L.; Argyropoulou, A.; Hellwig, E.; Skaltsounis, A.L.; Wittmer, A.; Vach, K.; Al-Ahmad, A. Antimicrobial effects of Inula viscosa extract on the in situ initial oral biofilm. Nutrients 2021, 13, 4029. [Google Scholar] [CrossRef]
- Ouari, S.; Benzidane, N. Chemical composition, biological activities, and molecular mechanism of Inula viscosa (L.) bioactive compounds: a review. Naunyn-Schmiedeberg’s Archives of Pharmacology 2024, 1–9. [Google Scholar] [CrossRef]
- Zeggwagh, N.A.; Ouahidi, M.L.; Lemhadri, A.; Eddouks, M. Study of hypoglycaemic and hypolipidemic effects of Inula viscosa L. aqueous extract in normal and diabetic rats. J Ethnopharmacol 2006, 108, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Migheli, R.; Virdis, P.; Galleri, G.; Arru, C.; Lostia, G.; Coradduzza, D.; Muroni, M.R.; Pintore, G.; Podda, L.; Fozza, C. Antineoplastic Properties by Proapoptotic Mechanisms Induction of Inula viscosa and Its Sesquiterpene Lactones Tomentosin and Inuviscolide. Biomedicines 2022, 10, 2739. [Google Scholar] [CrossRef]
- Virdis, P.; Migheli, R.; Galleri, G.; Fancello, S.; Cadoni, M.P.L.; Pintore, G.; Petretto, G.L.; Marchesi, I.; Fiorentino, F.P.; di Francesco, A. Antiproliferative and proapoptotic effects of Inula viscosa extract on Burkitt lymphoma cell line. Tumor Biology 2020, 42, 1010428319901061. [Google Scholar] [CrossRef] [PubMed]
- Mrid, R.B.; Bouchmaa, N.; Kabach, I.; Zouaoui, Z.; Chtibi, H.; Maadoudi, M.E.; Kounnoun, A.; Cacciola, F.; Majdoub, Y.O.E.; Mondello, L.; et al. Dittrichia viscosa L. Leaves: A Valuable Source of Bioactive Compounds with Multiple Pharmacological Effects. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Zarga, M.H.; Mahasneh, A.M. Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscosa. Molecules 2012, 17, 3291–3303. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shalom, R.; Bergman, M.; Grossman, S.; Azzam, N.; Sharvit, L.; Fares, F. Inula viscosa extract inhibits growth of colorectal cancer cells in vitro and in vivo through induction of apoptosis. Frontiers in oncology 2019, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Kheyar, N.; Bellik, Y.; Serra, A.T.; Kheyar, F.; Bedjou, F. Inula viscosa phenolic extract suppresses colon cancer cell proliferation and ulcerative colitis by modulating oxidative stress biomarkers. BioTechnologia (Pozn) 2022, 103, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Anglana, C.; Rojas, M.; Girelli, C.R.; Barozzi, F.; Quiroz-Troncoso, J.; Alegría-Aravena, N.; Montefusco, A.; Durante, M.; Fanizzi, F.P.; Ramírez-Castillejo, C.; et al. Methanolic Extracts of D. viscosa Specifically Affect the Cytoskeleton and Exert an Antiproliferative Effect on Human Colorectal Cancer Cell Lines, According to Their Proliferation Rate. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Benbacer, L.; Merghoub, N.; El Btaouri, H.; Gmouh, S.; Attaleb, M.; Morjani, H.; Amzazi, S.; El Mzibri, M. Antiproliferative effect and induction of apoptosis by Inula viscosa L. and Retama monosperma L. extracts in human cervical cancer cells. In Topics on Cervical Cancer With an Advocacy for Prevention.; Rajamanickam, Rajkumar, Ed.; InTech, 2012; pp. 267–284. [Google Scholar]
- Colak, D.K.; Egeli, U.; Eryilmaz, I.E.; Aybastier, O.; Malyer, H.; Cecener, G.; Tunca, B. The Anticancer Effect of Inula viscosa Methanol Extract by miRNAs’ Re-regulation: An in vitro Study on Human Malignant Melanoma Cells. Nutrition and Cancer 2022, 74, 211–224. [Google Scholar] [CrossRef] [PubMed]
- El Yaagoubi, O.M.; Lahmadi, A.; Bouyahya, A.; Filali, H.; Samaki, H.; El Antri, S.; Aboudkhil, S. Antitumor Effect of Inula viscosa Extracts on DMBA-Induced Skin Carcinoma Are Mediated by Proteasome Inhibition. Biomed Res Int 2021, 2021, 6687589. [Google Scholar] [CrossRef] [PubMed]
- Rechek, H.; Haouat, A.; Hamaidia, K.; Pinto, D.; Boudiar, T.; Válega, M.; Pereira, D.M.; Pereira, R.B.; Silva, A.M.S. Inula viscosa (L.) Aiton Ethanolic Extract Inhibits the Growth of Human AGS and A549 Cancer Cell Lines. Chem Biodivers 2023, 20, e202200890. [Google Scholar] [CrossRef]
- Abu Zarga, M.H.; Hamed, E.M.; Sabri, S.S.; Voelter, W.; Zeller, K.-P. New sesquiterpenoids from the Jordanian medicinal plant Inula viscosa. Journal of natural products 1998, 61, 798–800. [Google Scholar] [CrossRef]
- Lauro, L.; Rolih, C. Observations and research on an extract of Inula viscosa Ait. Bollettino della Societa Italiana di Biologia Sperimentale 1990, 66, 829–834. [Google Scholar]
- Eruygur, N.; Tuzcu, N.; Tugay, O.; Yilmaz, M.A.; Cakir, O. Phytochemical characterization and biological activities of Inula viscosa L. Aiton: a promising plant from Turkey. International Journal of Environmental Health Research 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kheyar-Kraouche, N.; Boucheffa, S.; Bellik, Y.; Farida, K.; Brahmi-Chendouh, N. Exploring the potential of Inula viscosa extracts for antioxidant, antiproliferative and apoptotic effects on human liver cancer cells and a molecular docking study. Biotechnologia 2023, 104, 183. [Google Scholar] [CrossRef] [PubMed]
- Nikolakaki, A.; Christodoulakis, N. Leaf structure and cytochemical investigation of secretory tissues in Inula viscosa. Botanical Journal of the Linnean Society 2004, 144, 437–448. [Google Scholar] [CrossRef]
- Grande, M.; Torres, P.; Piera, F.; Bellido, I.S. Triterpenoids from Dittrichia viscosa. Phytochemistry 1992, 31, 1826–1828. [Google Scholar] [CrossRef]
- Santos, S.A.P.; Mota, L.; Malheiro, R.; Silva, F.; Campos, M.; de Pinho, P.G.; Pereira, J.A. Changes in volatile compounds of Dittrichia viscosa caused by the attack of the gall-forming dipteran Myopites stylatus. Industrial Crops and Products 2016, 87, 71–77. [Google Scholar] [CrossRef]
- González-Hernández, R.A.; Valdez-Cruz, N.A.; Trujillo-Roldán, M.A. Factors that influence the extraction methods of terpenes from natural sources. Chemical Papers 2024, 78, 2783–2810. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Natural Product Communications 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Lage, H.; Duarte, N.; Coburger, C.; Hilgeroth, A.; Ferreira, M.J.U. Antitumor activity of terpenoids against classical and atypical multidrug resistant cancer cells. Phytomedicine 2010, 17, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.; Alshawsh, M.A. Therapeutic potential of certain terpenoids as anticancer agents: a scoping review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef] [PubMed]
- El-Baba, C.; Baassiri, A.; Kiriako, G.; Dia, B.; Fadlallah, S.; Moodad, S.; Darwiche, N. Terpenoids’ anti-cancer effects: Focus on autophagy. Apoptosis 2021, 26, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.I.M.; Chua, L.S. Solvent Partition for Terpenoid Rich Fraction From Crude Extract of Eurycoma longifolia. 2020-12-30T14:22:47.000Z, 2020; pp. 62-67.
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American journal of Enology and Viticulture 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of food and drug analysis 2002, 10. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food science and Technology 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free radical biology and medicine 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Araghi, M.; Mannani, R.; Heidarnejad Maleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell Int 2023, 23, 162. [Google Scholar] [CrossRef]
- Prisa, D. Possible use of Inula viscosa (Dittrichia viscosa L.) for biostimulation of Oscularia deltoides and Corpuscolaria lehmanii plants and protection against Aphis nerii. GSC Biological and Pharmaceutical Sciences 2019, 9, 069–075. [Google Scholar] [CrossRef]
- De Laurentis, N.; Losacco, V.; Milillo, M.A.; Lai, O. Chemical investigations of volatile constituents of Inula viscosa (L.) Aiton (Asteraceae) from different areas of Apulia, Southern Italy. Delpinoa 2002, 44, 115–119. [Google Scholar]
- Hwija, E.; Mossa, Y.; Hasan, M. Chemical composition of essential oils extracted from flowers of Taion plant (Inula viscosa L.) from two different regions of Lattakia–Syria. Tishreen University Journal-Basic Sciences Series 2017, 39. [Google Scholar]
- Mahmoudi, H.; Hosni, K.; Zaouali, W.; Amri, I.; Zargouni, H.; Hamida, N.B.; Kaddour, R.; Hamrouni, L.; Nasri, M.B.; Ouerghi, Z. Comprehensive phytochemical analysis, antioxidant and antifungal activities of Inula viscosa Aiton leaves. Journal of Food Safety 2016, 36, 77–88. [Google Scholar] [CrossRef]
- Haoui, I.E.; Derriche, R.; Madani, L.; Oukali, Z. Analysis of the chemical composition of essential oil from Algerian Inula viscosa (L.) Aiton. Arabian Journal of Chemistry 2015, 8, 587–590. [Google Scholar] [CrossRef]
- Ainseba, N.; Soulimane, A.; DIB, M.E.A.; Djabou, N.; Muselli, A. Comparative Study of the Antioxidant, Antimicrobial and Anti-Inflammatory Activity between Essential Oil and Hydrosol Extract of the Aerial Parts of Inula viscosa L. Journal of Applied Biotechnology Reports 2023, 10, 1169–1175. [Google Scholar]
- Sriti Eljazi, J.; Selmi, S.; Zarroug, Y.; Wesleti, I.; Aouini, B.; Jallouli, S.; Limam, F. Essential oil composition, phenolic compound, and antioxidant potential of Inulaviscosa as affected by extraction process. International journal of food properties 2018, 21, 2309–2319. [Google Scholar] [CrossRef]
- Ounoughi, A.; Ramdani, M.; Lograda, T.; Chalard, P. Chemotypes and antibacterial activities of Inula viscosa essential oils from Algeria. Biodiversitas Journal of Biological Diversity 2020, 21. [Google Scholar] [CrossRef]
- Karamenderes, C.; Zeybek, U. COMPOSITION OF THE ESSENTIAL OILS OF IAJULA VISCOSA, I. GRAVEOLENS AND I. HELENIUM SUBSP. TURCORACEMOSA. Journal of Faculty of Pharmacy of Istanbul University 2000, 33, 1–6. [Google Scholar]
- Hammal, A.; Al-Duihi, H.A.-H.; Alchab, L. Preparation of Nano Hydroxyapatite Loaded with Syrian Inula Extract Against Dental Caries. SSRN Social Science Netwrok 2024. [Google Scholar] [CrossRef]
- Blanc, M.-C.; Bradesi, P.; Gonçalves, M.J.; Salgueiro, L.; Casanova, J. Essential oil of Dittrichia viscosa ssp. viscosa: analysis by 13C-NMR and antimicrobial activity. Flavour and Fragrance Journal 2006, 21, 324–332. [Google Scholar] [CrossRef]
- Madani, L.; Derriche, R.; Haoui, I.E. Essential oil of Algerian Inula viscosa leaves. Journal of Essential Oil Bearing Plants 2014, 17, 164–168. [Google Scholar] [CrossRef]
- Faria, J.M.; Barbosa, P.; Bennett, R.N.; Mota, M.; Talbeueiredo, A.C. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from essential oils, essential oils fractions and decoction waters. Phytochemistry 2013, 94, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Rhimi, W.; Salem, I.B.; Iatta, R.; Chaabane, H.; Saidi, M.; Boulila, A.; Cafarchia, C. Dittrichia viscosa L. leaves lipid extract: An unexploited source of essential fatty acids and tocopherols with antifungal and anti-inflammatory properties. Industrial crops and products 2018, 113, 196–201. [Google Scholar] [CrossRef]
- Bentarhlia, N.; Kartah, B.E.; Fadil, M.; El Harkaoui, S.; Matthäus, B.; Abboussi, O.; Abdelmoumen, H.; Bouhnik, O.; El Monfalouti, H. Exploring the wound-healing and antimicrobial potential of Dittrichia viscosa L lipidic extract: Chemical composition and in vivo evaluation. Fitoterapia 2024, 172, 105707. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, R.; Shaito, A.A.; Badran, A.M.; Baydoun, S.; Sobeh, M.; Ouchari, W.; Sahri, N.; Eid, A.H.; Mesmar, J.E.; Baydoun, E. Fractionation and phytochemical composition of an ethanolic extract of Ziziphus nummularia leaves: antioxidant and anticancerous properties in triple negative human breast cancer cells. Frontiers in Pharmacology, 15, 1331843.
- Brahmi-Chendouh, N.; Piccolella, S.; Crescente, G.; Pacifico, F.; Boulekbache, L.; Hamri-Zeghichi, S.; Akkal, S.; Madani, K.; Pacifico, S. A nutraceutical extract from Inula viscosa leaves: UHPLC-HR-MS/MS based polyphenol profile, and antioxidant and cytotoxic activities. J Food Drug Anal 2019, 27, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Kheyar-Kraouche, N.; da Silva, A.B.; Serra, A.T.; Bedjou, F.; Bronze, M.R. Characterization by liquid chromatography-mass spectrometry and antioxidant activity of an ethanolic extract of Inula viscosa leaves. J Pharm Biomed Anal 2018, 156, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med 2018, 13, 20. [Google Scholar] [CrossRef]
- Kaczorová, D.; Karalija, E.; Dahija, S.; Bešta-Gajević, R.; Parić, A.; Ćavar Zeljković, S. Influence of Extraction Solvent on the Phenolic Profile and Bioactivity of Two Achillea Species. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Autor, E.; Cornejo, A.; Bimbela, F.; Maisterra, M.; Gandía, L.M.; Martínez-Merino, V. Extraction of Phenolic Compounds from Populus Salicaceae Bark. Biomolecules 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Jaafar, H.Z.; Ghasemzadeh, A.; Ibrahim, M.H. Light intensity effects on production and antioxidant activity of flavonoids and phenolic compounds in leaves, stems and roots of three varieties of Labisia pumila Benth. Aust J Crop Sci 2013, 7, 1016. [Google Scholar]
- Kumari, R.; Singh, S.; Agrawal, S. Effects of supplemental ultraviolet-B radiation on growth and physiology of Acorus calamus L.(sweet flag). Acta Biol. Cracoviensia, Ser. Bot 2009, 51, 19–27. [Google Scholar]
- Nguyen, K.Q.; Scarlett, C.J.; Vuong, Q.V. Assessment and comparison of phytochemicals and antioxidant properties from various parts of the Australian maroon bush (Scaevola spinescens). Heliyon 2021, 7, e06810. [Google Scholar] [CrossRef] [PubMed]
- Larbat, R.; Paris, C.; Le Bot, J.; Adamowicz, S. Phenolic characterization and variability in leaves, stems and roots of Micro-Tom and patio tomatoes, in response to nitrogen limitation. Plant Sci 2014, 224, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Martin-Puzon, J.J.R.; Rivera, W.L. Free-radical scavenging activity and bioactive secondary metabolites from various extracts of Glinus oppositifolius (L.) Aug. DC. (Molluginaceae) roots, stems and leaves. Asian Pacific Journal of Tropical Disease 2015, 5, 711–715. [Google Scholar] [CrossRef]
- NUGROHO, L.H.; NURINGTYAS, T.R. Anatomical structure, flavonoid content, and antioxidant activity of Rhodomyrtus tomentosa leaves and fruits on different age and maturity level. Biodiversitas Journal of Biological Diversity 2019, 20. [Google Scholar]
- Nawaz, H. 2020.
- Seglab, F.; Hamia, C.; Khacheba, I.; Djeridane, A.; Yousfi, M. High in vitro antioxidant capacities of Algerian Cleome arabica leaves’ extracts. Phytothérapie 2021, 19, 16–24. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food chemistry 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2 - Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds, Campos, M.R.S., Ed.; Woodhead Publishing: 2019; pp. 33-50.
- Asraoui, F.; Kounnoun, A.; Cacciola, F.; El Mansouri, F.; Kabach, I.; Oulad El Majdoub, Y.; Alibrando, F.; Arena, K.; Trovato, E.; Mondello, L. Phytochemical profile, antioxidant capacity, α-amylase and α-glucosidase inhibitory potential of wild Moroccan inula viscosa (L.) aiton leaves. Molecules 2021, 26, 3134. [Google Scholar] [CrossRef]
- Chahmi, N.; Anissi, J.; Jennan, S.; Farah, A.; Sendide, K.; Hassouni, M.E. Antioxidant activities and total phenol content of Inula viscosa extracts selected from three regions of Morocco. Asian Pacific Journal of Tropical Biomedicine 2015, 5, 228–233. [Google Scholar] [CrossRef]
- Gökbulut, A.; Ozhan, O.; Satilmiş, B.; Batçioğlu, K.; Günal, S.; Sarer, E. Antioxidant and antimicrobial activities, and phenolic compounds of selected Inula species from Turkey. Nat Prod Commun 2013, 8, 475–478. [Google Scholar] [CrossRef]
- Hepokur, C.; Budak, Y.; Karayel, H.B.; Selvi, B.; Yaylım, İ. Investigation of cytotoxic effects of Inula viscosa extract. Cumhuriyet Science Journal 2019, 40, 578–582. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed Pharmacother 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: a narrative review. Transl Cancer Res 2020, 9, 7619–7631. [Google Scholar] [CrossRef] [PubMed]
- Mesmar, J.; Abdallah, R.; Hamade, K.; Baydoun, S.; Al-Thani, N.; Shaito, A.; Maresca, M.; Badran, A.; Baydoun, E. Ethanolic extract of Origanum syriacum L. leaves exhibits potent anti-breast cancer potential and robust antioxidant properties. Front Pharmacol 2022, 13, 994025. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- AlKahlout, A.; Fardoun, M.; Mesmar, J.; Abdallah, R.; Badran, A.; Nasser, S.A.; Baydoun, S.; Kobeissy, F.; Shaito, A.; Iratni, R.; et al. Origanum syriacum L. Attenuates the Malignant Phenotype of MDA-MB231 Breast Cancer Cells. Front Oncol 2022, 12, 922196. [Google Scholar] [CrossRef] [PubMed]
- Bonta, R.K. Dietary Phenolic Acids and Flavonoids as Potential Anti-Cancer Agents: Current State of the Art and Future Perspectives. Anticancer Agents Med Chem 2020, 20, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev 2014, 8, 122–146. [Google Scholar] [CrossRef] [PubMed]
- Fatma, H.; Jameel, M.; Siddiqui, A.J.; Kuddus, M.; Buali, N.S.; Bahrini, I.; Siddique, H.R. Chemotherapeutic Potential of Lupeol Against Cancer in Pre-Clinical Model: A Systematic Review and Meta-Analysis. Phytomedicine 2024, 155777. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharmacological research 2021, 164, 105373. [Google Scholar] [CrossRef]
- Mitra, D.; Saha, D.; Das, G.; Mukherjee, R.; Banerjee, S.; Alam, N.; Mustafi, S.M.; Nath, P.; Majumder, A.; Majumder, B.; et al. Lupeol synergizes with 5-fluorouracil to combat c-MET/EphA2 mediated chemoresistance in triple negative breast cancer. iScience 2023, 26, 108395. [Google Scholar] [CrossRef]
- Adepoju, F.O.; Duru, K.C.; Li, E.; Kovaleva, E.G.; Tsurkan, M.V. Pharmacological Potential of Betulin as a Multitarget Compound. Biomolecules 2023, 13. [Google Scholar] [CrossRef]
- Król, S.K.; Kiełbus, M.; Rivero-Müller, A.; Stepulak, A. Comprehensive review on betulin as a potent anticancer agent. Biomed Res Int 2015, 2015, 584189. [Google Scholar] [CrossRef]
- Shabana, S.M.; Gad, N.S.; Othman, A.I.; Mohamed, A.F.; El-Missiry, M.A. β caryophyllene oxide induces apoptosis and inhibits proliferation of A549 lung cancer cells. Medical Oncology 2023, 40, 189. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Nam, D.; Yun, H.M.; Lee, S.G.; Jang, H.J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett 2011, 312, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Medicine 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Hanušová, V.; Caltová, K.; Svobodová, H.; Ambrož, M.; Skarka, A.; Murínová, N.; Králová, V.; Tomšík, P.; Skálová, L. The effects of β-caryophyllene oxide and trans-nerolidol on the efficacy of doxorubicin in breast cancer cells and breast tumor-bearing mice. Biomedicine & Pharmacotherapy 2017, 95, 828–836. [Google Scholar] [CrossRef]
- Delgado, C.; Mendez-Callejas, G.; Celis, C. Caryophyllene Oxide, the Active Compound Isolated from Leaves of Hymenaea courbaril L. (Fabaceae) with Antiproliferative and Apoptotic Effects on PC-3 Androgen-Independent Prostate Cancer Cell Line. Molecules 2021, 26, 6142. [Google Scholar] [CrossRef]
- Velu, P. TNF-α regulated inflammatory pathway by Isopulegol in human lung adenocarcinoma (A549) cells through ROS generation. Aust. J. Sci. Technol 2020, 4, 348–352. [Google Scholar]
- Jaafari, A.; Tilaoui, M.; Mouse, H.A.; M’bark, L.A.; Aboufatima, R.; Chait, A.; Lepoivre, M.; Zyad, A. Comparative study of the antitumor effect of natural monoterpenes: relationship to cell cycle analysis. Revista Brasileira de Farmacognosia 2012, 22, 534–540. [Google Scholar] [CrossRef]
- Jiang, Q. Different Roles of Tocopherols and Tocotrienols in Chemoprevention and Treatment of Prostate Cancer. Advances in Nutrition 2024, 15, 100240. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Ahmed Jum, A.D.; Attallah, Z.S.; Jallad, M.S.; Al Kury, L.T.; Hadi, R.W.; Mahmod, A.I. Role of vitamins A, C, D, E in cancer prevention and therapy: therapeutic potentials and mechanisms of action. Front Nutr 2023, 10, 1281879. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Linsalata, M.; Tutino, V.; D′Attoma, B.; Notarnicola, M.; Russo, F. Vitamin K1 Exerts Antiproliferative Effects and Induces Apoptosis in Three Differently Graded Human Colon Cancer Cell Lines. BioMed Research International 2015, 2015, 296721. [Google Scholar] [CrossRef] [PubMed]
- Linsalata, M.; Orlando, A.; Tutino, V.; Notarnicola, M.; D’Attoma, B.; Russo, F. Inhibitory effect of vitamin K1 on growth and polyamine biosynthesis of human gastric and colon carcinoma cell lines. Int J Oncol 2015, 47, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Pai, J.T.; Hsu, M.W.; Leu, Y.L.; Chang, K.T.; Weng, M.S. Induction of G2/M Cell Cycle Arrest via p38/p21(Waf1/Cip1)-Dependent Signaling Pathway Activation by Bavachinin in Non-Small-Cell Lung Cancer Cells. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer cell international 2021, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell death & differentiation 2018, 25, 104–113. [Google Scholar]
- Masood, N.; Dubey, V.; Luqman, S. Activation of Caspase-3 by terpenoids and flavonoids in different types of cancer cells. Current Topics in Medicinal Chemistry 2020, 20, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol 2013, 14, 32. [Google Scholar] [CrossRef]
- Cao, F.; Chu, C.; Qin, J.-J.; Guan, X. Research progress on antitumor mechanisms and molecular targets of Inula sesquiterpene lactones. Chinese Medicine 2023, 18, 164. [Google Scholar] [CrossRef]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr Physiol 2012, 2, 2369–2392. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef]
- Siraj, M.A.; Islam, M.A.; Al Fahad, M.A.; Kheya, H.R.; Xiao, J.; Simal-Gandara, J. Cancer chemopreventive role of dietary terpenoids by modulating Keap1-Nrf2-ARE signaling system—a comprehensive update. Applied Sciences 2021, 11, 10806. [Google Scholar] [CrossRef]
- Harmankaya, A.; Çınar, İ.; Yayla, M.; Harmankaya, S.; Beytur, M.; Öziç, C. In vitro evaluation of the effects of Inula viscosa’s different extracts on wound healing and oxidative stress in mouse L929 fibroblast cell line. Fabad Journal of Pharmaceutical Sciences 2023, 49, 129–142. [Google Scholar] [CrossRef]
- Lee, C.M.; Lee, J.; Nam, M.J.; Choi, Y.S.; Park, S.H. Tomentosin Displays Anti-Carcinogenic Effect in Human Osteosarcoma MG-63 Cells via the Induction of Intracellular Reactive Oxygen Species. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Virdis, P.; Migheli, R.; Bordoni, V.; Fiorentino, F.P.; Sanna, L.; Marchesi, I.; Pintore, G.; Galleri, G.; Muroni, M.R.; Bagella, L.; et al. Clarifying the molecular mechanism of tomentosin--induced antiproliferative and proapoptotic effects in human multiple myeloma via gene expression profile and genetic interaction network analysis. Int J Mol Med 2021, 48. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lin, H.H.; Hsu, C.H.; Wang, C.J.; Chiang, T.A.; Chen, J.H. Inhibitory effects of andrographolide on migration and invasion in human non-small cell lung cancer A549 cells via down-regulation of PI3K/Akt signaling pathway. Eur J Pharmacol 2010, 632, 23–32. [Google Scholar] [CrossRef]
- Tai, Y.L.; Chen, L.C.; Shen, T.L. Emerging roles of focal adhesion kinase in cancer. Biomed Res Int 2015, 2015, 690690. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Cao, B.; Wang, Y.; Ma, C.; Zeng, Z.; Liu, L.; Li, X.; Tao, D.; Gong, J.; Xie, D. Hippo component YAP promotes focal adhesion and tumour aggressiveness via transcriptionally activating THBS1/FAK signalling in breast cancer. J Exp Clin Cancer Res 2018, 37, 175. [Google Scholar] [CrossRef]
- Luo, M.; Guan, J.L. Focal adhesion kinase: a prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett 2010, 289, 127–139. [Google Scholar] [CrossRef]
- Lai, H.; Zhao, X.; Qin, Y.; Ding, Y.; Chen, R.; Li, G.; Labrie, M.; Ding, Z.; Zhou, J.; Hu, J.; et al. FAK-ERK activation in cell/matrix adhesion induced by the loss of apolipoprotein E stimulates the malignant progression of ovarian cancer. J Exp Clin Cancer Res 2018, 37, 32. [Google Scholar] [CrossRef] [PubMed]
- Weiner, T.M.; Liu, E.T.; Craven, R.J.; Cance, W.G. Expression of focal adhesion kinase gene and invasive cancer. Lancet 1993, 342, 1024–1025. [Google Scholar] [CrossRef] [PubMed]
- Taliaferro-Smith, L.; Oberlick, E.; Liu, T.; McGlothen, T.; Alcaide, T.; Tobin, R.; Donnelly, S.; Commander, R.; Kline, E.; Nagaraju, G.P.; et al. FAK activation is required for IGF1R-mediated regulation of EMT, migration, and invasion in mesenchymal triple negative breast cancer cells. Oncotarget 2015, 6, 4757–4772. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Kim, H.S.; Jin, T.; Hwang, E.H.; Jung, M.; Moon, W.K. Overexpression of the miR-141/200c cluster promotes the migratory and invasive ability of triple-negative breast cancer cells through the activation of the FAK and PI3K/AKT signaling pathways by secreting VEGF-A. BMC Cancer 2016, 16, 570. [Google Scholar] [CrossRef] [PubMed]
- TIAN, R.; LIU, Z.-M.; JIN, H.-W.; ZHANG, L.-R.; LIN, W.-H. Target identification of isomalabaricane terpenes extracted from sponges. Acta Physico-Chimica Sinica 2011, 27, 1214–1222. [Google Scholar]
- Xu, L.; Bi, Y.; Xu, Y.; Zhang, Z.; Xu, W.; Zhang, S.; Chen, J. Oridonin inhibits the migration and epithelial-to-mesenchymal transition of small cell lung cancer cells by suppressing FAK-ERK1/2 signalling pathway. J Cell Mol Med 2020, 24, 4480–4493. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Uckaya, F. Anti-aging and Antioxidant Activities of Ethanol and Three-phase Partitioned Extracts of Inula viscosa. 2022.
- Ozkan, E.; Karakas, F.P.; Yildirim, A.; Tas, I.; Eker, I.; Yavuz, M.Z.; Turker, A.U. Promising medicinal plant Inula viscosa L.: Antiproliferative, antioxidant, antibacterial and phenolic profiles. Prog. Nutr 2019, 21, 652–661. [Google Scholar]
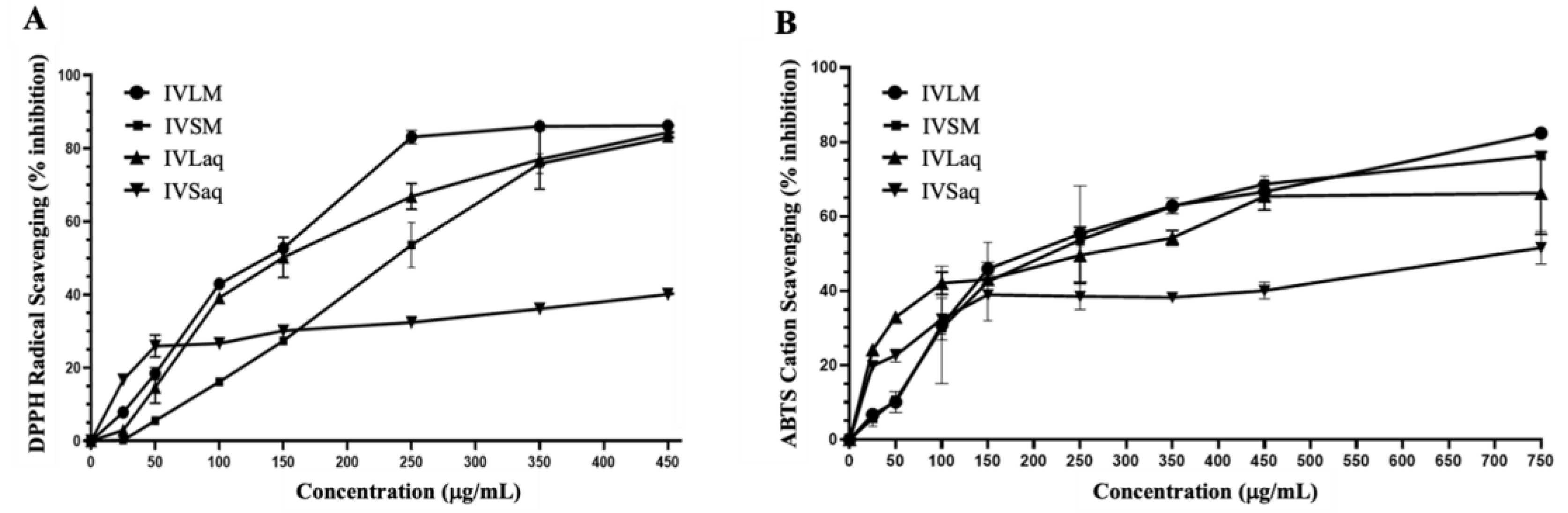

| Plant extract | Extraction yield (%) | TPC (µg GAE/ g) |
TFC (µg QE/ g) |
DPPH EC50 (µg/ mL) |
ABTS EC50 (µg/ mL) |
|---|---|---|---|---|---|
| IVLM | 16.5 | 726.4 ± 1.1 | 303.3± 8.8 | 145.7 ± 2.6 | 236.9 ±22.2 |
| IVSM | 4.4 | 532.0 ±10.3 | 114.0 ± 4.4 | 229.7 ±3.1 | 239.0 ± 5.5 |
| IVLaq | 3.5 | 212.0 ± 1.5 | 79.8 ± 2.9 | 155.8 ±6.1 | 268.9 ± 3.7 |
| IVSaq | 2 | 174.3± 0.7 | 45.2 ± 0.1 | 693.8 ± 3.4 | 791.0 ± 14.5 |
| L-Ascorbic acid | ─ | ─ | ─ | 27.5 ±1.3 | 93.4 ± 0.9 |
| Cell line | IC50 (μg/mL) |
|---|---|
| SK-OV-3 | 80.0 ± 5.7 |
| MCF-7 | 54.0 ± 4.9 |
| HepG2 | 59.9 ± 7.5 |
| HCT116 | 39.2 ± 6.1 |
| IC50 (μg/mL) | |||
|---|---|---|---|
| Cell line | 24 h | 48 h | 72 h |
| SK-OV-3 | 110.2 ±6.9 | 96.5 ± 4.0 | 52.95 ±6.7 |
| MCF-7 | 84.4 ±6.1 | 42.5 ± 2.9 | 29.32 ± 1.2 |
| MDA-MB-231 | 86.8 ±4.8 | 69.3 ±3.3 | 49.09 ±1.8 |
| HepG2 | NT | 20.2 ± 5.2 | NT |
| HCT116 | NT | 19.7± 3.7 | NT |
| Plant extract |
TPC (µg GAE/ g) |
TFC (µg QE/ g) |
DPPH EC50 (µg/ mL) |
ABTS EC50 (µg/ mL) |
|---|---|---|---|---|
| IVL DCM | 724.4 ± 12.1 | 235.4 ± 5.1 | 143.0 ± 1.4 | 241.6± 9.7 |
| No. | Compound Name | Chemical Nature | RT (min) | Molecular Formula | Molecular Weight | Reference |
|---|---|---|---|---|---|---|
| 1 | 2-Hexyldecan-1-ol | Alcohol | 8.995 | C16H34O | 242 | NR |
| 2 | Pyridine, 1-acetyl-1,2,3,4-tetrahydro-5-(2-piperidinyl)- (Ammodendrine) | Pyridine alkaloid | 10.77 | C12H20N2O | 208 | NR |
| 3 | Isopulegol | Monoterpene | 10.95 | C10H18O | 154 | NR |
| 4 | Linoleic acid ethyl ester | Fatty acid derivative | 11.06 | C20H36O2 | 308 | [52,53,54] |
| 5 | Caryophyllene oxide | Oxygenated sesquiterpene | 11.10 | C15H24O | 220 | [54,55,56,57,58,59,60,61,62,63] |
| 6 | 3,25-bis(acetyloxy)-5-hydroxyergostan-6-one | Steroid | 13.90 | C32H52O6 | 532 | NR |
| 7 | Citronellal | Monoterpene | 14.60 | C10H18O | 154 | [64] |
| 8 | Lup-20(29)-en-3-one (Lupenone) | Triterpenoid | 14.85 | C30H48O | 424 | NR |
| 9 | δ-Tocopherol | Vitamin E | 15.23 | C27H46O2 | 402 | [65,66] |
| 10 | Lupeol, trifluoroacetate | Triterpene | 15.69 | C32H49F3O2 | 522 | NR |
| 11 | Linalyl propionate | Monoterpene | 17.73 | C13H22O2 | 210 | [54,61] |
| 12 | Betulin | Triterpenoid | 18.01 | C30H50O2 | 442 | NR |
| 13 | Phytyl palmitate | Fatty acid/ diterpene derivative | 18.20 | C36H70O2 | 534 | NR |
| 14 | Campesterol | Phytosterol | 19.41 | C28H48O | 400 | [66] |
| 15 | 6-Octadecenoic acid derivative | Fatty acid derivative | 19.83 | C22H41NO | 335 | NR |
| 16 | Norcodeine | Alkaloid | 21.10 | C17H19NO3 | 285 | NR |
| 17 | Phytonadione (Phylloquinone) | Vitamin K | 21.50 | C31H46O2 | 450 | NR |
| 18 | Lup-20(29)-en-3beta-ol, acetate (20(29)- (Lupenol acetate) | Triterpenoid | 21.67 | C32H52O2 | 468 | NR |
| 19 | Lupeol | Triterpenoid | 22.63 | C30H50O | 426 | NR |
| 20 | 9,19-Cyclolanostan-3-ol acetate | Triterpenoid | 23.77 | C32H54O2 | 470 | NR |
| 21 | 2-Hexadecyloxirane | Oxirane | 24.16 | C18H36O | 268 | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





