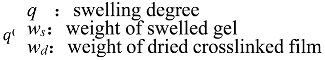1. Introduction
Natural polymers are vital and appealing materials for numerous application fields, including the food industry, agriculture, biomedicine, and tissue engineering. With the growing demand for biomedical materials, there is increasing focus on customizing the structure, properties, and function of natural polymers [
1]. Gels are formed when hydrophilic polymer chains undergo chemical or physical cross-linking, resulting in an interconnected network [
2,
3,
4]. Hydrogels,
i.e., gels interspersed with water, have been extensively researched and applied due to their extended shelf life in the form of xerogels, water retention efficiency, and enhanced mechanical properties [
5,
6]. The hydrogels possess functional groups, such as -NH
2, -SO
3H, -COOH, -OH, and -NHCOCH
3, making them highly hydrophilic. Moreover, hydrogels exhibit properties akin to animal tissues due to these configurations [
7,
8,
9].
Gelatine is a natural biopolymer containing functional groups, including amines, carboxylates, and hydroxyls. Its exceptional biodegradability and biocompatibility have made it a popular choice for a variety of biomedical applications, such as wound dressings, surgical treatments, and tissue engineering [
10,
11]. However, the practical use of gelatine is limited by its stability only at lower temperatures (35-43 °C). Upon exposure to higher temperatures (49-60 °C), the secondary bonding structure tends to weaken, causing the physical network to break down, resulting in poor mechanical and thermal properties of gelatine hydrogels [
12]. To overcome these limitations and expand the range of applications of gelatine, stabilization of its hydrogels is critical. This can be achieved through chemical modification or by blending gelatine with other biopolymers [
13,
14].
Sacran, a polysaccharide extracted from
Aphanothece sacrum, is characterized by its multifunctional anionic chains. With an impressive molecular weight of up to 1.6 x 10
7 g/mol, sacran demonstrates super water absorbance [
15]. Additionally, sacran exhibits valuable properties such as anti-inflammatory, anti-allergic, and wound-healing abilities, making it highly suitable for diverse biomedical applications [
16,
17,
18,
19]. Sacran formed polyion complexes with collagens [
20,
21]. Sacran has been blended with cellulose nanofiber (CNF-TEMPO) and Ag bestowing a synergetic effect on curbing bacterial and microbial infections [
22,
23]. It has never been investigated to modify sacran in order to add dialdehyde functionality for gelatine crosslinking. This article will investigate the novel approach of examining the performance of crosslinked gelatine gels containing dialdehyde moieties and sacran.
Polysaccharide dialdehyde [
24] is one of the reactive derivatives of polysaccharides, obtained after periodate or TEMPO oxidation, which contains polyaldehyde structures, similar to other cross-linking agents like glutaraldehyde, alginate dialdehyde (ADA), dialdehyde cellulose (DAC), dextran dialdehyde (DDA), and xanthan gum (OXG) [
25,
26,
27,
28,
29,
30]. These active moieties in polysaccharide dialdehydes are capable of crosslinking with free amino groups in the gelatine. Nevertheless, there has not been much research published on the application of SDA as a crosslinking agent to stabilize gelatin hydrogels.
A review article examined various natural polymers that have been explored for enhancing PVOH-based films used in food packaging. It specifically highlights starch, chitosan, cellulose, and gelatin, due to their low cost, renewability, abundance, sustainability, biocompatibility, and biodegradability. Additionally, this review briefly discussed the use of PVOH in conjunction with bio-waste-based films. Finally, it elaborates on the current research trends (2016–2021) regarding the combined use of PVOH-based and natural polymer films for food packaging applications [
1].
This study aims to explore the potential use of sacran aldehyde (SDA) as a natural cross-linker in enhancing the stability of gelatine hydrogels. The cross-linking density, swelling properties, and surficial morphology of cross-linked gelatine hydrogels were analyzed and discussed using various characterization techniques. This cross-linked network is responsible for the improved rigidity and mechanical strength observed in the hydrogels. By utilizing sacran as a cross-linker and confirming the formation of sacran-aldehyde through FT-IR analysis, the study successfully demonstrated a strategy to enhance the stability of gelatine hydrogels. This advancement has significant implications for various biomedical applications, where stable and robust hydrogels are crucial for ensuring desired performance and functionality.
2. Materials and Methods
2.1. Materials
The gelatine from porcine skin was purchased from Sigma Aldrich, Japan. Sacran (Mw 1.6 x 107 g/mol) was obtained from Green Science Materials Inc. (Kumamoto, Japan). Sodium periodate was purchased from Nacalai Tesque, Japan. Ninhydrin (2,2-dihydroxy-1,3-indanedione) was purchased from TCI, Japan. All chemicals were used as received. Dialysis membrane was purchased from Funakoshi, Japan (MWCO 1KDa; ɸ 29 x 45 x 5 mm).
2.2. Experimental
2.2.1. Preparation of Sacran Aldehyde
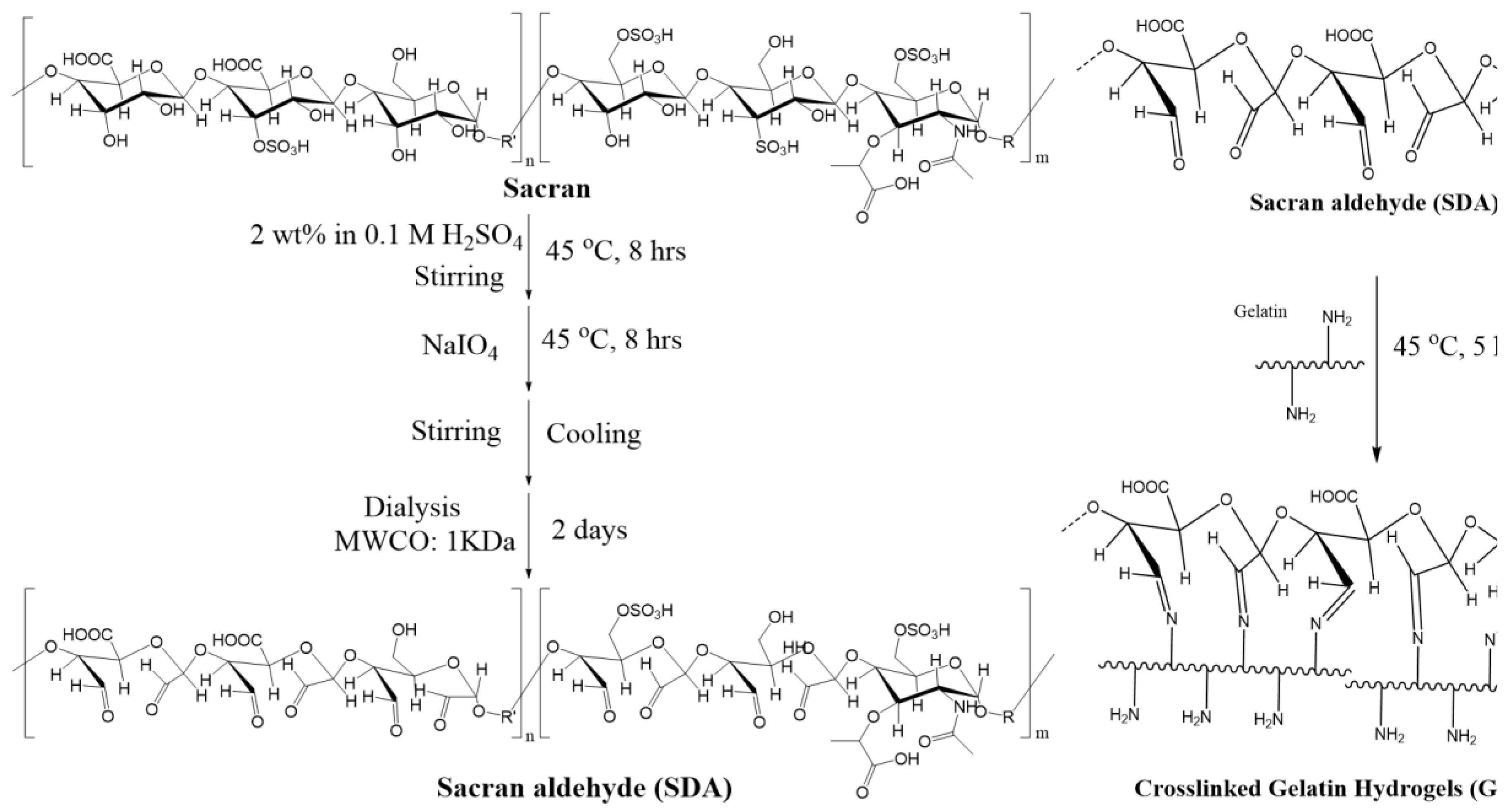
Sacran (1g) was dissolved in 0.1 M H2SO4 solution (50 mL) while continuously stirring at 40 ℃ for 4 h to obtain a homogeneous solution. Sodium periodate (4.2 mmol) was added to it and the solution was stirred again at 40 ℃ for 4 h. The solution was cooled to room temperature and kept on stirring at 80 rpm overnight. The product obtained was then transferred into a cellulose dialysis membrane (MWCO: 1KDa) and dialyzed in distilled water, changing the water every 12 h over 2 days. Cellulose membrane offers good chemical resistance, low protein binding makes it ideal candidate for various laboratory dialysis applications. The MWCO 1KDa are chosen on the basis of molecular weight of the product to be dialyzed. This has to be lower than the molecular weight of the product. The dialyzed solution was freeze-dried yielding 85% of the product. Sacran aldehyde molecular weight was calculated to be 6.8 x 106 g/mol from the SEC-MALLS technique.
2.2.2. Preparation of Gelatine Solution
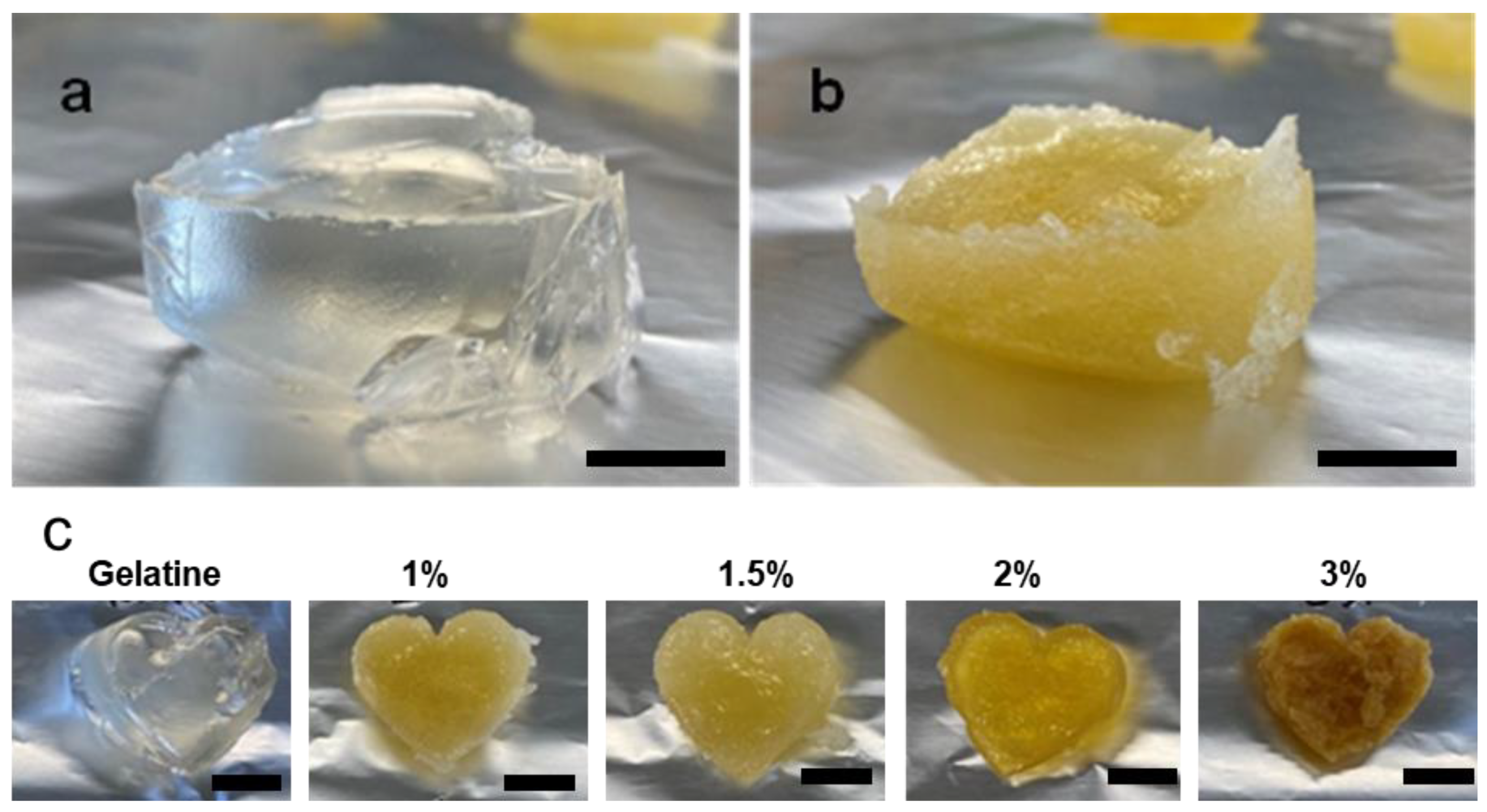
Throughout this study, the aqueous solution was used to create hydrogels. To make the solution, gelatine granules (5g) were dispersed in Milli-Q water (50 mL) and gently stirred for 20 minutes at 60 °C. The clear gelatine solution was stirred for another 5 minutes at 40 °C. As shown in
Figure 2a, pure gelatin forms a clear hydrogel.
2.2.3. Syntheses of Cross-Linked Hydrogels
The gelatine solution was slowly added to freeze-dried sacran aldehyde with continuous stirring overnight to obtain sacran aldehyde cross-linked gelatine hydrogels. Four hydrogel samples of gelatine cross-linked with sacran aldehyde were prepared as shown in
Scheme 1.
Sacran aldehyde cross-linked gelatine hydrogels samples of 1, 1.5, 2, 3 wt. % were prepared by adding 100, 150, 200, and 300 mg of sacran aldehyde respectively to glass bottles containing 10 mL of gelatine solution (10 w/v%). The mixture was then stirred at 40 °C for 4 h to complete the cross-linking reaction. The air bubbles formed during stirring were removed using centrifugation at 8000 rpm for 1 h. The resulting mixture was slowly poured into a silicon mold avoiding the formation of air bubbles to give it a particular shape as shown in
Figure 2b. The gels obtained became dense and a darker shade as the ratio of the crosslinker was increased (
Figure 2c). All the gels were kept in the dry environment under controlled humidity (RH 30%, 25 °C) until further characterization.
2.2.4. Measurement of Mechanical Properties of Hydrogels
The mechanical properties of the pure gelatine and cross-linked GSDA samples were investigated by employing them in compression testing. Pure gelatine and cross-linked G-SDA samples were kept in a humidity-controlled (relative humidity 30%, temperature 25 °C) environment for 2 days before subjecting to mechanical properties analysis. A total of six samples were subjected to mechanical testing. A compressing probe was set up on an Instron 3365 machine using a 5 kN load cell with a crosshead speed of 1 mm/min. The gels were cut into 5 mm x 5 mm x 5 mm of cubic geometry for compression measurements. The measurement was repeated three times to calculate the error involved.
2.2.5. Measurement of Swelling Degree of Hydrogels
The swelling properties of the cross-linked hydrogels were studied by incubating the gels in Phosphate Buffer Saline (PBS pH 7) at room temperature. The gels were slightly blotted and weighed every 30 min till the gels started dissolving. The weight of the swelled gels was then compared with the weight of dried gels to evaluate the swelling ratio of hydrogels. The gel samples were weighed and the swelling degree, q, was estimated as a weight ratio using the following equation,
2.2.6. Scanning Electron Microscopy
The dried gel membranes were coated with Au using a magnetron sputtering system (MSP-IS, Vacuum Device) and observed under a desktop scanning electron microscope (Hitachi, TM3030plus) with an acceleration voltage of 15 kV using standard scanning mode.
2.3. Characterization of Pure Sacran, Sacran Aldehyde and Gelatine
2.3.1. Molecular Weight Estimation Using SEC-MALLS
The prepared sacran solution was kept at 4 °C prior to use. The absolute molecular weight (
Mw) of sacran was determined using size exclusion chromatography combined with multi-angle static light scattering (SEC-MALLS) to be > 10
7 g/mol. [
19] Sacran has negative surface charges because of the presence of carboxylate anions and sulfate anions. The absolute molecular weight (
Mw) of Sacran aldehyde was measured to be between 10
6 - 10
7 g/mol.
2.3.2. Characterization of Cross-Linked Hydrogels
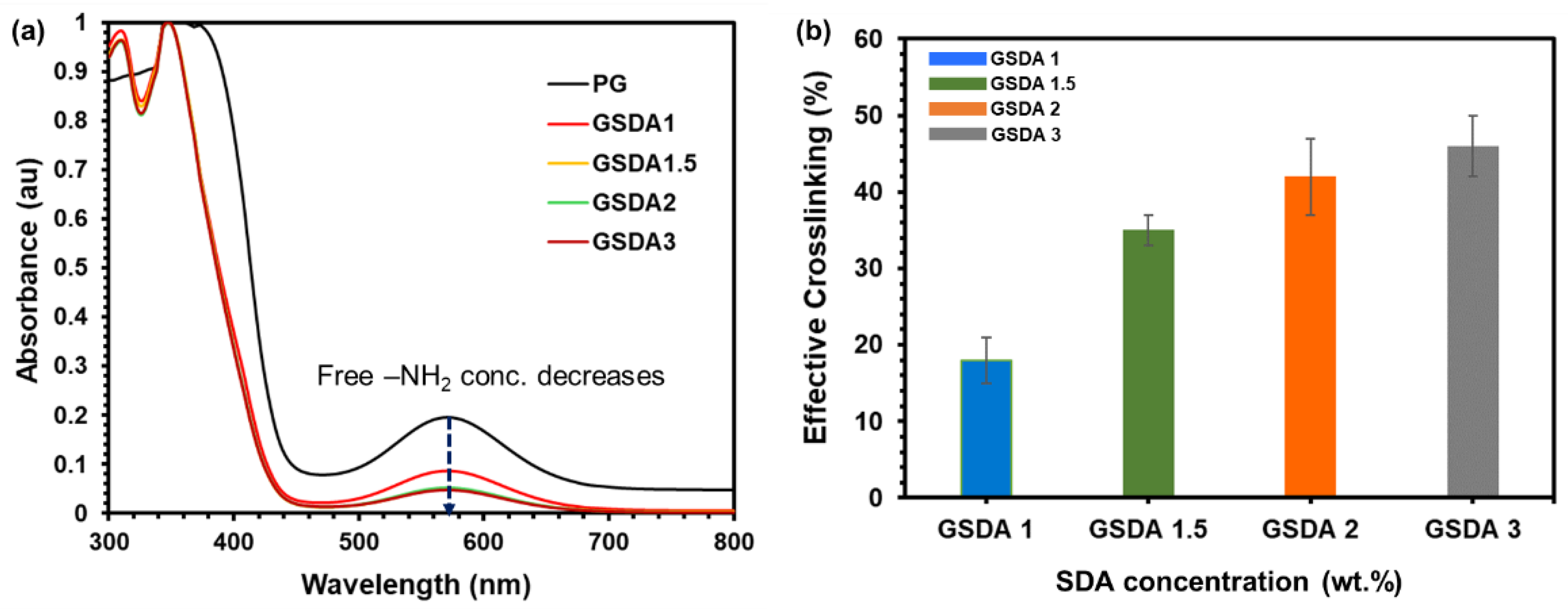
Ninhydrin (2,2-dihydroxy-1,3-indanedione) assay was conducted to determine the degree of cross-linking of the hydrogels. The lyophilized gels dissolved in 1 mL of distilled water were treated with 1 mL Ninhydrin solution (1.5% w/v in ethanol). The mixture was heated for 1 h at 80 ℃. After cooling down the mixture color change was observed from orange to violet representing effective cross-linking of the gelatine hydrogels and the optical absorbance was recorded using a UV-visible spectrometer (UV-1800, Shimadzu Corp., Kyoto, Japan) at wavelength 570 nm against a blank solution without gels.
Fourier Transform Infrared (FT-IR) spectra were recorded with PerkinElmer Spectrum One spectrometer between 4000 and 500 cm-1 to confirm the formation of sacran-aldehyde.
3. Results and Discussion
3.1. Formation of Sacran Aldehyde
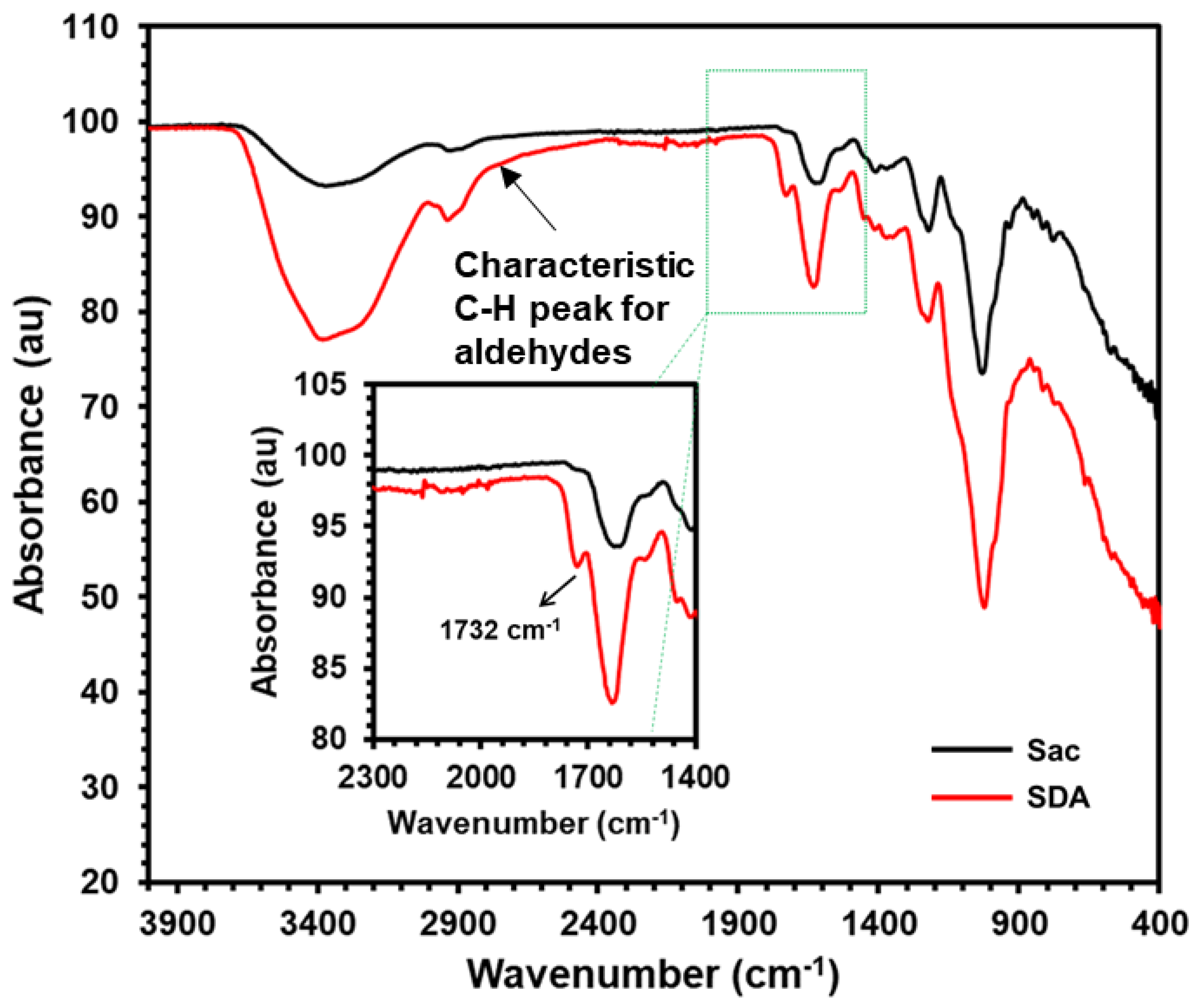
Sacran exhibits distinctive bands at 3328 cm
−1, CH
2 stretching vibration at 2925 cm
−1, polysaccharide (1→4) glycosidic bond stretching vibration at 1144 cm
−1, and C-O stretching vibrations at around 1007 cm
−1. In SDA, a new peak at 1732 cm
−1 reveals the presence of C=O stretching vibration in comparison to unmodified Sacran (
Figure 1 inset). Periodate can oxidize α-1,4-linked and α-1,6-linked anhydroglucoside units, forming either dialdehyde or aldehyde groups based on the chemical pathway, as reported by Bruneel et al. (1993). Also, slight shoulder at 2726 cm
-1 for C-H stretching for aldehyde confirmed the formation of aldehyde functional groups. Additionally, a slightly red-shifted OH signal at 3328 cm
−1 suggests that aldehyde or dialdehyde groups were introduced into the sacran chain through oxidation.
To improve the properties of gelatine hydrogels, sacran, a cyanobacterial polysaccharide, was employed. This was achieved by oxidizing sacran in the presence of sodium periodate, converting its hydroxyl groups to aldehyde.[
31] To verify the successful formation of sacran aldehyde, the samples were tested using Fourier Transform Infrared (FT-IR) spectroscopy. A distinct peak at 1732 cm
-1 wavelength (
Figure 1), indicating the oxidation of the hydroxyl groups and the formation of sacran aldehyde was observed. Importantly, in the spectra of pure sacran, no peak was present at this specific wavelength, confirming the effectiveness of the oxidation process. Cross-linking occurred between these –CHO groups from sacran aldehyde (SDA) and the –NH
2 groups present in the lysine and hydroxylysine residues of gelatine (as illustrated in
Scheme 1).
3.2. Cross-Linking of Hydrogels
Four different gelatine hydrogels cross-linked with sacran-dialdehyde were synthesized. The preparation process involved the gradual addition of gelatine solution to freeze-dried SDA under continuous stirring. The slow addition and continuous stirring allowed for effective mixing and reaction between gelatine and sacran-dialdehyde, ensuring the formation of covalent bonds along with the subsequent establishment of a three-dimensional network structure. Moreover, the cross-linking density could be manipulated by adjusting the concentration of sacran-dialdehyde in the gelatine solution. As a result, hydrogels with varying degrees of cross-linking were obtained, each exhibiting distinct properties.
Figure 2.
a) Pure Gelatine; b) Cross-linked gelatine (the color change represents effective cross-linking) c) Gelatine hydrogels cross-linked with different amounts of sacran-dialdehyde. The scale bar in all images is 1 cm.
Figure 2.
a) Pure Gelatine; b) Cross-linked gelatine (the color change represents effective cross-linking) c) Gelatine hydrogels cross-linked with different amounts of sacran-dialdehyde. The scale bar in all images is 1 cm.
3.3. Effective Cross-Linking
To quantitatively assess the effective cross-linking in gelatine hydrogels, the Ninhydrin test was utilized, which measures the presence of unreacted free amines. By employing the following equation, and from the results of UV-Vis spectroscopy of all the samples (as shown in
Figure 3a), the extent of cross-linking within the hydrogel samples was evaluated
A ninhydrin assay was used to measure the percentage of free amino groups in gelatin samples, which can be converted to the degree of crosslinking by comparing to uncrosslinked gelatin. The content of free amino in the sample was directly proportional to the absorbance of the solution after being heated with ninhydrin. The degree of cross-linking exhibited a progressive trend, rising from 18% to 46% as the amount of oxidized sacran was elevated from GSDA 1 to GSDA 3 (as depicted in
Figure 3b). As the content of aldehyde groups increased, more sites became available for the amino groups present in the gelatine molecules to react. Consequently, a greater number of covalent bonds were formed, leading to a denser and more interconnected network within the gelatine hydrogels [
32].
The Ninhydrin assay provided valuable insights into the quantitative assessment of cross-linking efficiency, offering a reliable method to determine the impact of varying cross-linker concentrations on the resulting gelatine hydrogel structures[
33]. This information is crucial for tailoring the mechanical properties and stability of hydrogels to suit specific biomedical applications, where controlled and tunable cross-linking densities are of significant importance[
34]. The cross linking density (
ρ) is calculated using following equation.
where R and T are the gas constant and absolute temperature, respectively. The results are summarized in
Table 1. NH
2 content (
N) was calculated [
35] using the following equation and UV-Vis spectra.
In other words, a reversible covalent crosslinking bond was formed between the amino group of gelatin and the aldehyde group of SDA via the Schiff base reaction. Second, the hydroxyl groups of SDA and the amino groups on the surface of the gelatin formed a hydrogen bond that produced physical crosslinks. The dynamic SDA hydrogel network was effectively created by double crosslinking using both chemical and physical methods (
Scheme 1). The SDA hydrogel synthesis method has the advantages of moderate and fast reaction conditions, a simpler and more effective process, and the avoidance of the requirement for additional crosslinking agents or initiators [
36].
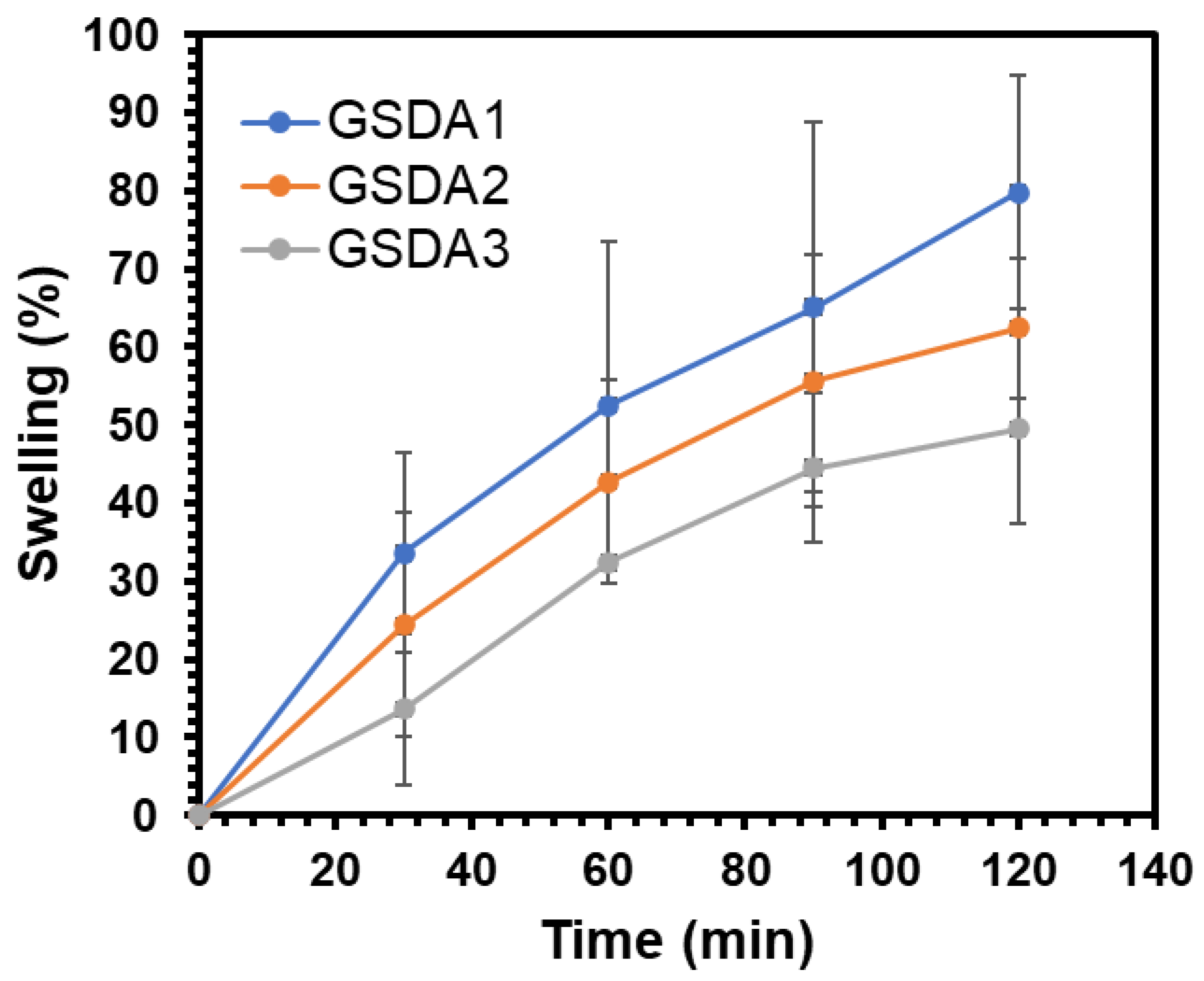
3.4. Degree of Swelling
The swelling degree (
q) of the hydrogels was determined using the following equation,
The experiment revealed that all the gels reached equilibrium swelling within 120 minutes. As depicted in
Figure 4, and
Table 2 there was a decrease in the swelling percentage as the content of oxidized sacran cross-linker increased, indicating a more effective cross-linking in the gelatine hydrogels [
37].
3.5. Mechanical Properties
The enhancement of oxidized aldehyde groups for effective cross-linking in gel formation brings about notable improvements in the rigidity and stability of the resulting gels. These findings are depicted in
Figure 5. The stress-strain curve shows that the incorporation and systematic increase in GSDA weight% resulted in increase in stress with same mechanical strain as shown in
Figure 5a. The mechanical strength of the gels correlates directly with the degree of cross-linking. This increase in mechanical strength makes the gels capable of withstanding higher applied pressures, particularly evident in the 50% compression tests. Cross-linking plays a crucial role in the mechanical properties of hydrogels, affecting their overall performance [
34]. By introducing oxidized aldehyde groups through SDA, the gelatine matrix undergoes a structural transformation, leading to the formation of a robust and rigid network. This strengthened network is characterized by enhanced interactions between the polymer chains, resulting in a cohesive and stable gel structure. The resulting tensile strength with incorporated GSDA samples with varying wt.% are shown in
Figure 5b and
Table 2.
The newly formed bonds act as bridges, connecting neighbouring gelatine molecules and reinforcing the gels’ overall structure. During compression testing, the ability of the gels to resist deformation is significantly influenced by their cross-linking density. Hydrogels with higher cross-linking exhibit increased resistance to compression forces due to the enhanced intermolecular interactions. This property is valuable, such as in tissue engineering scaffolds, where the ability to withstand mechanical stresses is essential for the successful integration and support of cells.
However, it is essential to strike a balance in the degree of cross-linking to avoid potential drawbacks. Excessive cross-linking can lead to a reduction in the hydrogel's water uptake capacity, hindering its ability to absorb and retain moisture [
38]. In certain biomedical applications, the hydrogels' ability to hold and release water or bioactive substances is crucial for their efficacy. Therefore, optimization of the cross-linking density becomes a critical aspect in tailoring the properties of the hydrogels to suit specific applications.
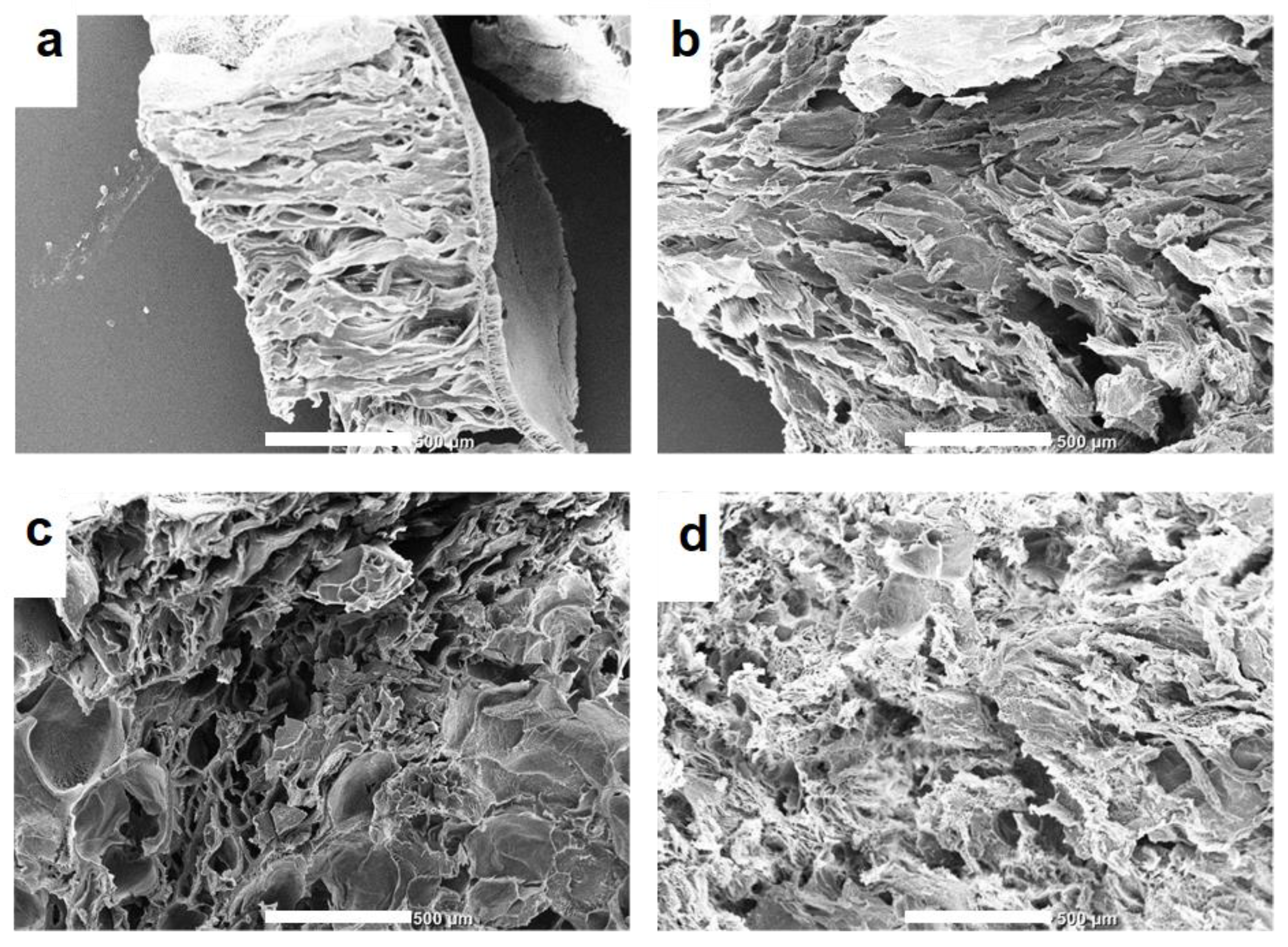
3.6. Morphology of Hydrogels
Samples of crosslinked hydrogels were freeze dried before subjecting them to the SEM analysis. Freeze drying kept the hydrogel structure of the samples intact [
39]. SEM images of the hydrogels (
Figure 6) reveal distinct differences in morphology between gelatine with and without oxidized polysaccharides. As cross-linking becomes more effective, the hydrogel's pore size decreases. On the other hand, pure gelatine exhibits a fibril-like morphology.
The SEM images clearly illustrate how the introduction of oxidized polysaccharide as a cross-linker impacts the overall structure of the gelatine hydrogels. With increased cross-linking, the gelatine chains are more tightly connected, leading to a reduction in the size of the pores within the gel matrix. This decrease in pore size indicates a more compact and homogeneous network of the hydrogel, which can influence its physical and mechanical properties [
40].
In contrast, the pure gelatine hydrogel exhibits a fibril-like appearance, characteristic of its native structure. Without the presence of the cross-linker, the gelatine chains remain relatively unconnected, resulting in a porous structure.
4. Conclusion
Despite gelatine hydrogels having numerous biomedical applications such as wound healing, adhesive, plasma expander, and drug delivery, their practical utility is limited due to poor stability at higher pressures and temperatures. This research aimed to address this limitation by synthesizing and characterizing gelatine hydrogels using a naturally occurring polysaccharide cross-linker called sacran, in the form of sacran aldehyde. By increasing the content of the aldehyde group for gelatine matrix preparation, effective cross-linking was achieved up to 45%, resulting in the formation of a rigid network within the gelatine hydrogels. This led to a reduction in the water uptake capacity of the hydrogels. Simultaneously, the degree of cross-linking was found to significantly enhance the mechanical stability of the gels, increasing it by 5 times compared to pure gelatine. The incorporation of sacran aldehyde as a cross-linker for gelatine opens up new possibilities and applications for these hydrogels in the field of biomedicine.
Author Contributions
Conceptualization, Maninder Singh, Maiko Okajima and Tatsuo Kaneko; Data curation, Maninder Singh and Gargi Joshi; Formal analysis, Alisha Debas and Gargi Joshi; Funding acquisition, Gargi Joshi and Tatsuo Kaneko; Investigation, Maninder Singh and Gargi Joshi; Methodology, Maninder Singh, Alisha Debas, Gargi Joshi, Robin Rajan, and Tatsuo Kaneko; Project administration, Tatsuo Kaneko; Supervision, Tatsuo Kaneko; Validation, Tatsuo Kaneko; Writing – original draft, Maninder Singh, Gargi Joshi, and Tatsuo Kaneko; Writing – review & editing, Maninder Singh, Maiko Okajima, Robin Rajan, Kazuaki Matsumura, and Tatsuo Kaneko.
Acknowledgments
Authors acknowledge the financial support provided by a Grant-in-aid,A-step (AS2915173U) of JST. GJ is grateful for the Research Fellowship from the Japan Society for the Promotion of Sciences (JSPS) and the JSPS KAKEHNI Grant number JP18J11881. The authors also acknowledge Green Science Materials Ltd.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. ☒ The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Tatsuo Kaneko reports financial support was provided by JST Adaptable and SeamLess Technology Transfer Program Through Target-driven R and D (A-STEP, AS2915173U). Gargi Joshi reports financial support was provided by Japan Society for the Promotion of Science (KAKEHNI, JP18J11881).
References
- P.K. Panda, K. Sadeghi, J. Seo, Recent advances in poly (vinyl alcohol)/natural polymer based films for food packaging applications: A review, Food Packag. Shelf Life. 33 (2022) 100904. [CrossRef]
- S. Laquerbe, J. Es Sayed, C. Lorthioir, C. Meyer, T. Narita, G. Ducouret, P. Perrin, N. Sanson, Supramolecular Crosslinked Hydrogels: Similarities and Differences with Chemically Crosslinked Hydrogels, Macromolecules. (2023). [CrossRef]
- R. Foudazi, R. Zowada, I. Manas-Zloczower, D.L. Feke, Porous Hydrogels: Present Challenges and Future Opportunities, Langmuir. 39 (2023) 2092–2111. [CrossRef]
- Z. Li, Z. Lin, Recent advances in polysaccharide-based hydrogels for synthesis and applications, Aggregate. 2 (2021) e21. [CrossRef]
- R. Zowada, R. Foudazi, Macroporous Hydrogels for Soil Water Retention in Arid and Semi-Arid Regions, RSC Appl. Polym. (2023). [CrossRef]
- J.-Y. Yu, S.E. Moon, J.H. Kim, S.M. Kang, Ultrasensitive and Highly Stretchable Multiple-Crosslinked Ionic Hydrogel Sensors with Long-Term Stability, Nano-Micro Lett. 15 (2023) 51. [CrossRef]
- G. Joshi, K. Okeyoshi, F. Adila Amat Yusof, T. Mitsumata, M.K. Okajima, T. Kaneko, Interfacial self-assembly of polysaccharide rods and platelets bridging over capillary lengths, J. Colloid Interface Sci. 591 (2021) 483–489. [CrossRef]
- G. Joshi, K. Okeyoshi, T. Mitsumata, T. Kaneko, Micro-deposition control of polysaccharides on evaporative air-LC interface to design quickly swelling hydrogels, J. Colloid Interface Sci. 546 (2019) 184–191. [CrossRef]
- G. Joshi, K. Okeyoshi, M.K. Okajima, T. Kaneko, Directional control of diffusion and swelling in megamolecular polysaccharide hydrogels, Soft Matter. 12 (2016) 5515–5518. [CrossRef]
- S. Mitura, A. Sionkowska, A. Jaiswal, Biopolymers for hydrogels in cosmetics: review, J. Mater. Sci. Mater. Med. 31 (2020) 50. [CrossRef]
- H.M. Nguyen, T.T. Ngoc Le, A.T. Nguyen, H.N. Thien Le, T.T. Pham, Biomedical materials for wound dressing: recent advances and applications, RSC Adv. 13 (2023) 5509–5528. [CrossRef]
- J. Tkaczewska, M. Wielgosz, P. Kulawik, M. Zajac, The effect of drying temperature on the properties of gelatin from carps (Cyprinus carpio) skin, Czech J. Food Sci. 37 (2019) 246–251. https://cjfs.agriculturejournals.cz/artkey/cjf-201904-0005.php.
- T.U. Rashid, S. Sharmeen, S. Biswas, T. Ahmed, A.K. Mallik, M. Shahruzzaman, M. Nurus Sakib, P. Haque, M.M. Rahman, Gelatin-Based Hydrogels BT - Cellulose-Based Superabsorbent Hydrogels, in: M.I.H. Mondal (Ed.), Springer International Publishing, Cham, 2018: pp. 1–41. [CrossRef]
- Ahmady, N.H. Abu Samah, A review: Gelatine as a bioadhesive material for medical and pharmaceutical applications, Int. J. Pharm. 608 (2021) 121037. [CrossRef]
- M.A. Ali, M. Singh, S. Zhang, D. Kaneko, M.K. Okajima, T. Kaneko, Metal-Assisted Injection Spinning of Ultra Strong Fibers from Megamolecular LC Polysaccharides, Polymers (Basel). 16 (2024). [CrossRef]
- M. Okajima-Kaneko, M. Ono, K. Kabata, T. Kaneko, Extraction of novel sulfated polysaccharides from Aphanothece sacrum (Sur.) Okada, and its spectroscopic characterization, Pure Appl. Chem. 79 (2007) 2039–2046. [CrossRef]
- M.K. Okajima, S. Sornkamnerd, T. Kaneko, Development of Functional Bionanocomposites Using Cyanobacterial Polysaccharides, Chem. Rec. 18 (2018) 1167–1177. [CrossRef]
- M.K. Okajima, T. Bamba, Y. Kaneso, K. Hirata, E. Fukusaki, S. Kajiyama, T. Kaneko, Supergiant Ampholytic Sugar Chains with Imbalanced Charge Ratio Form Saline Ultra-absorbent Hydrogels, Macromolecules. 41 (2008) 4061–4064. [CrossRef]
- M.K. Okajima, D. Kaneko, T. Mitsumata, T. Kaneko, J. Watanabe, Cyanobacteria That Produce Megamolecules with Efficient Self-Orientations, Macromolecules. 42 (2009) 3057–3062. [CrossRef]
- K. Takada, A. Komuro, M.A. Ali, M. Singh, M. Okajima, K. Matsumura, T. Kaneko, Cell-adhesive gels made of sacran/collagen complexes, Polym. J. 54 (2022) 581–589. [CrossRef]
- K. Budpud, K. Okeyoshi, M.K. Okajima, T. Kaneko, Cyanobacterial supra-polysaccharide: Self-similar hierarchy, diverse morphology, and application prospects of sacran fibers, Biopolymers. 113 (2022) e23522. [CrossRef]
- M. Singh, G. Joshi, H. Qiang, M.K. Okajima, T. Kaneko, Facile design of antibacterial sheets of sacran and nanocellulose, Carbohydr. Polym. Technol. Appl. 5 (2023) 100280. [CrossRef]
- M. Singh, G. JOSHI, H. Qiang, M.K. Okajima, T. Kaneko, Dataset for Sac/CNF-Ag nanocomposite for antibacterial properties, Mendeley Data, V3. (2023). [CrossRef]
- Z. Zhai, K.J. Edgar, Polysaccharide Aldehydes and Ketones: Synthesis and Reactivity, Biomacromolecules. (2024). [CrossRef]
- W. Hyon, S.-H. Hyon, K. Matsumura, Evaluation of the optimal dose for maximizing the anti-adhesion performance of a self-degradable dextran-based material, Carbohydr. Polym. Technol. Appl. 4 (2022) 100255. [CrossRef]
- P. Nonsuwan, K. Matsumura, Amino-Carrageenan@Polydopamine Microcomposites as Initiators for the Degradation of Hydrogel by near-Infrared Irradiation for Controlled Drug Release, ACS Appl. Polym. Mater. 1 (2019) 286–297. [CrossRef]
- K. Matsumura, R. Rajan, Oxidized Polysaccharides as Green and Sustainable Biomaterials, Curr. Org. Chem. 25 (2021) 1483–1496. [CrossRef]
- W. Hyon, S. Shibata, E. Ozaki, M. Fujimura, S.-H. Hyon, K. Matsumura, Elucidating the degradation mechanism of a self-degradable dextran-based medical adhesive, Carbohydr. Polym. 278 (2022) 118949. [CrossRef]
- S.-H. Hyon, N. Nakajima, H. Sugai, K. Matsumura, Low cytotoxic tissue adhesive based on oxidized dextran and epsilon-poly-l-lysine, J. Biomed. Mater. Res. Part A. 102 (2014) 2511–2520. [CrossRef]
- P. Nonsuwan, A. Matsugami, F. Hayashi, S.-H. Hyon, K. Matsumura, Controlling the degradation of an oxidized dextran-based hydrogel independent of the mechanical properties, Carbohydr. Polym. 204 (2019) 131–141. [CrossRef]
- S.F. Plappert, S. Quraishi, N. Pircher, K.S. Mikkonen, S. Veigel, K.M. Klinger, A. Potthast, T. Rosenau, F.W. Liebner, Transparent, Flexible, and Strong 2,3-Dialdehyde Cellulose Films with High Oxygen Barrier Properties, Biomacromolecules. 19 (2018) 2969–2978. [CrossRef]
- P. Heidarian, A.Z. Kouzani, A self-healing nanocomposite double network bacterial nanocellulose/gelatin hydrogel for three dimensional printing, Carbohydr. Polym. 313 (2023) 120879. [CrossRef]
- M. Friedman, Applications of the Ninhydrin Reaction for Analysis of Amino Acids, Peptides, and Proteins to Agricultural and Biomedical Sciences, J. Agric. Food Chem. 52 (2004) 385–406. [CrossRef]
- G. Joshi, K. Amornwachirabodee, M.K. Okajima, K. Okeyoshi, T. Kaneko, Oriented Polysaccharide Bigels from Interfacial Crosslinking, Chem. Lett. 49 (2020) 1484–1486. [CrossRef]
- R.N. Kale, A.N. Bajaj, Ultraviolet Spectrophotometric Method for Determination of Gelatin Crosslinking in the Presence of Amino Groups, J. Young Pharm. 2 (2010) 90–94. [CrossRef]
- P.K. Panda, K. Park, J. Seo, Development of poly (vinyl alcohol)/regenerated chitosan blend film with superior barrier, antioxidant, and antibacterial properties, Prog. Org. Coatings. 183 (2023) 107749. [CrossRef]
- Q. Xing, K. Yates, C. Vogt, Z. Qian, M.C. Frost, F. Zhao, Increasing Mechanical Strength of Gelatin Hydrogels by Divalent Metal Ion Removal, Sci. Rep. 4 (2014) 4706. [CrossRef]
- A.P.C. Almeida, J.N. Saraiva, G. Cavaco, R.P. Portela, C.R. Leal, R.G. Sobral, P.L. Almeida, Crosslinked bacterial cellulose hydrogels for biomedical applications, Eur. Polym. J. 177 (2022) 111438. [CrossRef]
- F. Mattea, Á. Martín, Supercritical drying of thermoresponsive gels based on N-isopropylacrylamide, J. Taiwan Inst. Chem. Eng. 110 (2020) 120–129. [CrossRef]
- N. Contessi Negrini, A. Angelova Volponi, P.T. Sharpe, A.D. Celiz, Tunable Cross-Linking and Adhesion of Gelatin Hydrogels via Bioorthogonal Click Chemistry, ACS Biomater. Sci. Eng. 7 (2021) 4330–4346. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).