Submitted:
08 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. A Brief History of Food Fortification
3. Global Large-Scale Food Fortification
4. Micronutrient Deficiencies
5. The “Big Four” Micronutrients
5.1. Iodine
5.2. Vitamin A
5.3. Zinc
5.4. Iron
6. The Link Poverty, Food Insecurity, and Malnutrition
6.1. Mother-Child Malnutrition in SSA
7. Alternatives to Food Fortification
8. Some Challenges in Sub-Saharan Africa
8.1. The Global Supply and Use od Premix
9. Fortification Strategies
10. Other Strategies or Vehicles for Fortification
11. Policy Implications
12. Prediction of Excessive Intake of Micronutrients
13. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giller, K.E.; Delaune, T.; Silva, J.V.; Descheemaeker, K.; van de Ven, G.; Schut, A.G.T.; van Wijk, M.; Hammond, J.; Hochman, Z.; Taulya, G.; et al. The Future of Farming: Who Will Produce Our Food? Food Secur. 2021, 13, 1073–1099. [Google Scholar] [CrossRef]
- International Labour Office The End to Poverty Initiative The ILO and the 2030 Agenda The End to Poverty Initiative: The ILO and the 2030 Agenda; Geneva, 2016.
- Struik, P.C.; Kuyper, T.W. Sustainable Intensification in Agriculture: The Richer Shade of Green. A Review. Agron. Sustain. Dev. 2017, 37, 1–15. [Google Scholar]
- Wijerathna-Yapa, A.; Pathirana, R. Sustainable Agro-Food Systems for Addressing Climate Change and Food Security. Agriculture 2022, 12, 1554. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Tapsell, L.C. Food, Not Nutrients, Is the Fundamental Unit in Nutrition. Nutr. Rev. 2007, 65, 439–450. [Google Scholar] [PubMed]
- Olabisi, M.; Obekpa, H.O.; Liverpool-Tasie, S. Is Growing Your Own Food Necessary for Dietary Diversity? Evidence from Nigeria. Food Policy 2021, 104, 102144. [Google Scholar] [CrossRef]
- Ritchie, H.; Spooner, F.; Roser, M. Clean Water. Available online: https://ourworldindata.org/clean-water (accessed on 21 January 2024).
- Emenike, C.P.; Tenebe, I.T.; Omole, D.O.; Ngene, B.U.; Oniemayin, B.I.; Maxwell, O.; Onoka, B.I. Accessing Safe Drinking Water in Sub-Saharan Africa: Issues and Challenges in South–West Nigeria. Sustain. Cities Soc. 2017, 30, 263–272. [Google Scholar] [CrossRef]
- Workman, C.L.; Stoler, J.; Harris, A.; Ercumen, A.; Kearns, J.; Mapunda, K.M. Food, Water, and Sanitation Insecurities: Complex Linkages and Implications for Achieving WASH Security. Glob. Public Health 2022, 17, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, B.; Paulavets, K.; Barreto-Gomez, L.; Echeverri, L.G.; Pachauri, S.; Boza-Kiss, B.; Zimm, C.; Rogelj, J.; Creutzig, F.; Ürge-Vorsatz, D.; et al. Pandemic, War, and Global Energy Transitions. Energies 2022, 15, 6114. [Google Scholar] [CrossRef]
- Wudil, A.H.; Usman, M.; Rosak-Szyrocka, J.; Pilař, L.; Boye, M. Reversing Years for Global Food Security: A Review of the Food Security Situation in Sub-Saharan Africa (SSA). Int. J. Environ. Res. Public Health 2022, 19, 14836. [Google Scholar] [CrossRef] [PubMed]
- OFID Zero Hunger by 2030: The Not-so-Impossible Dream; Viena, Austria, 2016.
- Rother, B.; Sosa, S.; Kim, D.; Kohler, L.; Pierre, G.; Kato, N.; Debbich, M.; Castrovillari, C.; Sharifzoda, K.; Heuvelen, E. Van; et al. Tackling the Global Food Crisis: Impact, Policy Response, and the Role of the IMF; Washington, DC, 2022. 2022. [Google Scholar]
- FAO, IFAD, UNICEF, W. and W. The State of Food Security and Nutrition in the World 2023; FAO: Rome, 2023.
- Kesari, A.; Noel, J.Y. Nutritional Assessment. StatPearls 2023, 1–14. [Google Scholar] [CrossRef]
- Odjo, S.; Traoré, F.; Zaki, C. Africa Agriculture Trade Monitor 2023; Kigali and Washington, DC, 2023.
- IFC Food Fortification. Global Agribusiness; Washington, DC, 2023.
- WHO Food Fortification. Available online: https://www.who.int/health-topics/food-fortification#tab=tab_1 (accessed on 21 January 2024).
- Leung, A.M.; Braverman, L.E.; Pearce, E.N. History of U. S. Iodine Fortification and Supplementation. Nutrients 2012, 4, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; De Tullio, A.; Triggiani, D.; Zupo, R.; Giagulli, V.A.; De Pergola, G.; Piazzolla, G.; Guastamacchia, E.; Sabbà, C.; Triggiani, V. Iodine Deficiency and Iodine Prophylaxis: An Overview and Update. Nutrients 2023, 15, 1004. [Google Scholar] [CrossRef] [PubMed]
- Katsidzira, L.; Gangaidzo, I.; Thomson, S.; Rusakaniko, S.; Matenga, J.; Ramesar, R. The Shifting Epidemiology of Colorectal Cancer in Sub-Saharan Africa. Lancet Gastroenterol. Hepatol. 2017, 2, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Maiyoh, G.K.; Tuei, V.C. Rising Cancer Incidence and Role of the Evolving Diet in Kenya. Nutr. Cancer 2019, 71, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Bigman, G.; Otieno, L.; Adebamowo, S.N.; Adebamowo, C. Dietary Intake and Cancer in Sub-Saharan Africa: A Critical Review of Epidemiological Studies. Nutr. Cancer 2022, 74, 2803–2814. [Google Scholar] [CrossRef] [PubMed]
- Valicente, V.M.; Peng, C.H.; Pacheco, K.N.; Lin, L.; Kielb, E.I.; Dawoodani, E.; Abdollahi, A.; Mattes, R.D. Ultraprocessed Foods and Obesity Risk: A Critical Review of Reported Mechanisms. Adv. Nutr. 2023, 14, 718–738. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Spence, N.D.; Holmes, M.D.; Barnett, J.B. Fiber Consumption and Breast Cancer Incidence: A Systematic Review and Meta-Analysis of Prospective Studies. Cancer 2020, 126, 3061–3075. [Google Scholar] [CrossRef]
- Zahangir, M.S.; Hasan, M.M.; Richardson, A.; Tabassum, S. Malnutrition and Non-Communicable Diseases among Bangladeshi Women: An Urban–Rural Comparison. Nutr. Diabetes 2017, 7, e250–e250. [Google Scholar] [CrossRef] [PubMed]
- Ofori, K.F.; Antoniello, S.; English, M.M.; Aryee, A.N.A. Improving Nutrition through Biofortification–A Systematic Review. Front. Nutr. 2022, 9, 1043655. [Google Scholar] [CrossRef] [PubMed]
- Avnee; Sood, S. ; Chaudhary, D.R.; Jhorar, P.; Rana, R.S. Biofortification: An Approach to Eradicate Micronutrient Deficiency. Front. Nutr. 2023, 10, 1233070.
- Chaudhary, V.; Saraswathy, K.; Sarwal, R. Dietary Diversity as a Sustainable Approach towards Micronutrient Deficiencies in India. Indian J. Med. Res. 2022, 156, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Kurpad, A. V.; Ghosh, S.; Thomas, T.; Bandyopadhyay, S.; Goswami, R.; Gupta, A.; Gupta, P.; John, A.T.; Kapil, U.; Kulkarni, B.; et al. Perspective: When the Cure Might Become the Malady: The Layering of Multiple Interventions with Mandatory Micronutrient Fortification of Foods in India. Am. J. Clin. Nutr. 2021, 114, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Bechoff, A.; de Bruyn, J.; Alpha, A.; Wieringa, F.; Greffeuille, V. Exploring the Complementarity of Fortification and Dietary Diversification to Combat Micronutrient Deficiencies: A Scoping Review. Curr. Dev. Nutr. 2023, 7. [Google Scholar] [CrossRef]
- Das, J.K.; Salam, R.A.; Mahmood, S. Bin; Moin, A.; Kumar, R.; Mukhtar, K.; Lassi, Z.S.; Bhutta, Z.A. Food Fortification with Multiple Micronutrients: Impact on Health Outcomes in General Population. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.M.; Rai, M.; Morando, D.W. Anemia Screening. StatPearls 2023.
- Duggal, M.; Sesikeran, B.; Arlappa, N.; Nair, S.; Shekhar, V.; Sabharwal, V. Large-Scale Staple Food Fortification as a Complementary Strategy to Address Vitamin and Mineral Vulnerabilities in India: A Critical Review. Indian J. Public Health 2022, 66, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Pike, A. A Brief History of Food Fortification in the U.S. Available online: https://foodinsight.org/is-food-fortification-necessary-a-historical-perspective/ (accessed on 19 June 2024).
- Health Canada Addition of Vitamins and Minerals to Foods. Proposed Policy Recommendations; Ottawa, Canada.
- Institute of Medicine Dietary Reference Intakes: Guiding Principles for Nutrition Labeling and, Fortification; National Academies Press: Washington, D.C. Institute of Medicine Dietary Reference Intakes: Guiding Principles for Nutrition Labeling and Fortification; National Academies Press: Washington, D.C., 2003; ISBN 978-0-309-09143-5.
- Chen, P.J.; Antonelli, M. Conceptual Models of Food Choice: Influential Factors Related to Foods, Individual Differences, and Society. Foods 2020, 9, 1898. [Google Scholar] [CrossRef] [PubMed]
- Hoogendoorn, A.; Luthringer, C.; Parvanta, I.; Garrett, G.S. Food Fortification Global Mapping Study 2016; Technical assistance for strengthening capacities in food fortification, Publications Office, 2016.
- European Union Regulation (EU) No 609/2013 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/eli/reg/2013/609/oj (accessed on 19 June 2024).
- UNICEF Landscape Analysis of Large-Scale Fortification of Oil and Wheat Flour in Some West and Central African Countries: Status, Challenges and Opportunities for the Future; 2022.
- ASHA Do India’s Food Safety Regulator (FSSAI) and Indian Citizens Need Saving From (Foreign & Indian) Private Players Behind Food Fortification Initiatives? – A Report on the Objectionable Conflict of Interest That Pervades India’s Food Fortification Public Po; New Delhi, India, 2023.
- Bromage, S.; Gonchigsumlaa, E.; Traeger, M.; Magsar, B.; Wang, Q.; Bater, J.; Li, H.; Ganmaa, D. Awareness and Attitudes Regarding Industrial Food Fortification in Mongolia and Harbin. Nutrients 2019, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Bater, J.; Bromage, S.; Jambal, T.; Tsendjav, E.; Lkhagvasuren, E.; Jutmann, Y.; Martineau, A.R.; Ganmaa, D. Prevalence and Determinants of Vitamin D Deficiency in 9595 Mongolian Schoolchildren: A Cross-Sectional Study. Nutrients 2021, 13, 4175. [Google Scholar] [CrossRef] [PubMed]
- Menal-Puey, S.; Marques-Lopes, I. Regulatory Framework of Fortified Foods and Dietary Supplements for Athletes: An Interpretive Approach. Nutrients 2021, 13, 3858. [Google Scholar] [CrossRef] [PubMed]
- Global Fortification Data Exchange Country Fortification Dashboard. Available online: https://fortificationdata.org/list-of-countries-for-the-food-fortification-dashboard/ (accessed on 21 January 2024).
- OECD/EU Development Assistance and Conditionality: Challenges in Design and Options for More Effective Assistance; Washington, DC, 2017.
- Han, X.; Ding, S.; Lu, J.; Li, Y. Global, Regional, and National Burdens of Common Micronutrient Deficiencies from 1990 to 2019: A Secondary Trend Analysis Based on the Global Burden of Disease 2019 Study. eClinicalMedicine 2022, 44. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.H.; Bell, V. The Imprecision of Micronutrient Requirement Values: The Example of Vitamin D. J. Food Sci. 2024, 89, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Shortt, C.; Hasselwander, O.; Meynier, A.; Nauta, A.; Fernández, E.N.; Putz, P.; Rowland, I.; Swann, J.; Türk, J.; Vermeiren, J.; et al. Systematic Review of the Effects of the Intestinal Microbiota on Selected Nutrients and Non-Nutrients. Eur. J. Nutr. 2017, 57, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Hermes Sales, C.; de Mello Fontanelli, M.; Macedo Rogero, M.; Mori Sarti, F.; Fisberg, R.M. Dietary Inadequacies Overestimate the Blood Deficiencies of Magnesium, Zinc, and Vitamins A, C, E, and D among Residents of Sao Paulo. Clin. Nutr. ESPEN 2023, 53, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Wynants, M.; Kelly, C.; Mtei, K.; Munishi, L.; Patrick, A.; Rabinovich, A.; Nasseri, M.; Gilvear, D.; Roberts, N.; Boeckx, P.; et al. Drivers of Increased Soil Erosion in East Africa’s Agro-Pastoral Systems: Changing Interactions between the Social, Economic and Natural Domains. Reg. Environ. Chang. 2019, 19, 1909–1921. [Google Scholar] [CrossRef]
- Xie, H.; Huang, Y.; Chen, Q.; Zhang, Y.; Wu, Q. Prospects for Agricultural Sustainable Intensification: A Review of Research. Land 2019, 8, 157. [Google Scholar] [CrossRef]
- Oberč, B.P.; Arroyo Schnell, A. Approaches to Sustainable Agriculture: Exploring the Pathways towards the Future of Farming; IUCN, International Union for Conservation of Nature: Brussels, Belgium, 2020. ISBN 978-2-8317-2057-9.
- Sustainable Food Systems for Food Security. Need for Combination of Local and Global Approaches; Thomas, A., Alpha, A., Barczak, A., Zakhia-Rozis, N., Eds.; éditions Quae: Versailles, 2022. ISBN 978-2-7592-3576-6.
- Gernand, A.D.; Aguree, S.; Pobee, R.; Colecraft, E.K.; Murray-Kolb, L.E. Concurrent Micronutrient Deficiencies Are Low and Micronutrient Status Is Not Related to Common Health Indicators in Ghanaian Women Expecting to Become Pregnant. Curr. Dev. Nutr. 2019, 3. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.A.; Trilok-Kumar, G.; McIntosh, D.; Haszard, J.J.; Harper, M.J.; Reid, M.; Erhardt, J.; Bailey, K.; Gibson, R.S. Multiple Micronutrient Status and Predictors of Anemia in Young Children Aged 12-23 Months Living in New Delhi, India. PLoS One 2019, 14, e0209564. [Google Scholar] [CrossRef]
- WHO and FAO Guidelines on Food Fortification with Micronutrients; Allen, L., Benoist, B. de, Dary, O., Hurrell, R., Eds.; World Health Organization: Geneva, Switzerland, 2006; ISBN 92 4 159401 2.
- Saha, S.; Roy, A. Whole Grain Rice Fortification as a Solution to Micronutrient Deficiency: Technologies and Need for More Viable Alternatives. Food Chem. 2020, 326, 127049. [Google Scholar] [CrossRef]
- Swanepoel, E.; Havemann-Nel, L.; Rothman, M.; Laubscher, R.; Matsungo, T.M.; Smuts, C.M.; Faber, M. Contribution of Commercial Infant Products and Fortified Staple Foods to Nutrient Intake at Ages 6, 12, and 18 Months in a Cohort of Children from a Low Socio-Economic Community in South Africa. Matern. Child Nutr. 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines on Food Fortification with Micronutrients; Geneva, Switzerland, 2006.
- Liyanage, C.; Hettiarachchi, M. Food Fortification. Ceylon Med. J. 2011, 56, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Shubham, K.; Anukiruthika, T.; Dutta, S.; Kashyap, A. V.; Moses, J.A.; Anandharamakrishnan, C. Iron Deficiency Anemia: A Comprehensive Review on Iron Absorption, Bioavailability and Emerging Food Fortification Approaches. Trends Food Sci. Technol. 2020, 99, 58–75. [Google Scholar] [CrossRef]
- Wells, J.C.K.; Marphatia, A.A.; Amable, G.; Siervo, M.; Friis, H.; Miranda, J.J.; Haisma, H.H.; Raubenheimer, D. The Future of Human Malnutrition: Rebalancing Agency for Better Nutritional Health. Global. Health 2021, 17, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Chadare, F.J.; Idohou, R.; Nago, E.; Affonfere, M.; Agossadou, J.; Fassinou, T.K.; Kénou, C.; Honfo, S.; Azokpota, P.; Linnemann, A.R.; et al. Conventional and Food-to-Food Fortification: An Appraisal of Past Practices and Lessons Learned. Food Sci. Nutr. 2019, 7, 2781–2795. [Google Scholar] [CrossRef] [PubMed]
- Damms-Machado, A.; Weser, G.; Bischoff, S.C. Micronutrient Deficiency in Obese Subjects Undergoing Low Calorie Diet. Nutr. J. 2012, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- OECD Regulatory Governance of Large-Scale Food Fortification: Literature Review Theoretical and Empirical Foundations for Developing a Measurement Framework; 2024.
- Akombi, B.J.; Agho, K.E.; Merom, D.; Renzaho, A.M.; Hall, J.J. Child Malnutrition in Sub-Saharan Africa: A Meta-Analysis of Demographic and Health Surveys (2006-2016). PLoS One 2017, 12, e0177338. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; De Sanctis, V.; Alaaraj, N.; Ahmed, S.; Alyafei, F.; Hamed, N.; Soliman, N. Early and Long-Term Consequences of Nutritional Stunting: From Childhood to Adulthood. Acta Biomed. Atenei Parm. 2021, 92, 11346–11346. [Google Scholar] [CrossRef]
- Mordor Intelligence Africa Vitamin Fortified and Mineral Enriched Food & Beverage Market Size & Share Analysis - Growth Trends & Forecasts (2024 - 2029). Available online: https://www.mordorintelligence.com/industry-reports/africa-vitamin-fortified-and-mineral-enriched-food-beverage-market (accessed on 15 January 2024).
- Liria-Domínguez, R.; Penny, M.; Kroon, P.A.; Burgos, G.; Dainty, J.; Zeder, C.; Zimmermann, M.B.; King, J.; Mithen, R.; Boy, E.; et al. Biofortified Yellow-Fleshed Potatoes Provide More Absorbable Zinc than a Commonly Consumed Variety: A Randomized Trial Using Stable Isotopes in Women in the Peruvian Highlands. J. Nutr. 2023, 153, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Bell, V.; Barros, A.B.; Fernandes, T.H. Food Fortification in Sub Saharan Africa: Science or Business? In Food and Nutrition Security in Africa; Fernandes, T.H., Ferrão, J., Facknath, S., Eds.; Mozambique: Alcance Ed., 2020 ISBN 978-989-8934-05-5.
- Reardon, T.; Tschirley, D.; Liverpool-Tasie, L.S.O.; Awokuse, T.; Fanzo, J.; Minten, B.; Vos, R.; Dolislager, M.; Sauer, C.; Dhar, R.; et al. The Processed Food Revolution in African Food Systems and the Double Burden of Malnutrition. Glob. Food Sec. 2021, 28, 100466. [Google Scholar] [CrossRef] [PubMed]
- Naiken, L. FAO Methodology for Estimating the Prevalence of Undernourishment. Available online: https://www.fao.org/3/Y4249E/y4249e06.htm (accessed on 10 January 2024).
- Fanzo, J. The Nutrition Challenge in Sub-Saharan Africa; Rome, 2012.
- Mukanu, M.M.; Mchiza, Z.J.R.; Delobelle, P.; Thow, A.M. Nutrition Policy Reforms to Address the Double Burden of Malnutrition in Zambia: A Prospective Policy Analysis. Health Policy Plan. 2023, 38, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Clapp, J.; Moseley, W.G.; Burlingame, B.; Termine, P. Viewpoint: The Case for a Six-Dimensional Food Security Framework. Food Policy 2022, 106, 102164. [Google Scholar] [CrossRef]
- DGA Dietary Guidelines for Americans, 2020-2025. Available online: https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials (accessed on 3 January 2024).
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN Micronutrient Guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)—Zinc Review. J. Nutr. 2016, 146, 858S–885S. [Google Scholar] [CrossRef]
- Fischer, P.W.F.; L’Abbé, M. Iodine in Iodized Table Salt and in Sea Salt. Can. Inst. Food Sci. Technol. J. 1980, 13, 103–104. [Google Scholar] [CrossRef]
- Hombali, A.S.; Solon, J.A.; Venkatesh, B.T.; Nair, N.S.; Peña-Rosas, J.P. Fortification of Staple Foods with Vitamin a for Vitamin a Deficiency. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, D.; Fabian, E.; Elmadfa, I. Vitamin A Content (Retinol and Retinyl Esters) in Livers of Different Animals. Food Chem. 2006, 98, 704–710. [Google Scholar] [CrossRef]
- Blaner, W.S. Vitamin A and Provitamin A Carotenoids. In Present Knowledge in Nutrition; Academic Press, 2020; pp. 73–91.
- National Institutes of Health Vitamin A and Carotenoids. Available online: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/ (accessed on 19 January 2024).
- Scientific Committee for Animal Nutrition Report of the Scientific Committee for Animal Nutrition on the Risk Of Hypervitaminosis A; 1992.
- Neela, S.; Fanta, S.W. Review on Nutritional Composition of Orange-Fleshed Sweet Potato and Its Role in Management of Vitamin A Deficiency. Food Sci. Nutr. 2019, 7, 1920–1945. [Google Scholar] [CrossRef] [PubMed]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef] [PubMed]
- Szabó, É.; Csölle, I.; Felső, R.; Kuellenberg de Gaudry, D.; Nyakundi, P.N.; Ibrahim, K.; Metzendorf, M.I.; Ferenci, T.; Lohner, S. Benefits and Harms of Edible Vegetable Oils and Fats Fortified with Vitamins A and D as a Public Health Intervention in the General Population: A Systematic Review of Interventions. Nutrients 2023, 15, 5135. [Google Scholar] [CrossRef] [PubMed]
- Gebregziabher, B.S.; Gebremeskel, H.; Debesa, B.; Ayalneh, D.; Mitiku, T.; Wendwessen, T.; Habtemariam, E.; Nur, S.; Getachew, T. Carotenoids: Dietary Sources, Health Functions, Biofortification, Marketing Trend and Affecting Factors – A Review. J. Agric. Food Res. 2023, 14, 100834. [Google Scholar] [CrossRef]
- Zambou, N.F.; Mbiapo, T.F.; Lando, G.; Tchana, K.A.; Gouado, I. [Effect of Onchocerca Volvulus Infestation on Plasma Vitamin A Concentration in School Children in a Rural Region of Cameroon]. Sante 1999, 9, 151–155. [Google Scholar] [PubMed]
- Gupta, S.; Brazier, A.K.M.; Lowe, N.M. Zinc Deficiency in Low- and Middle-Income Countries: Prevalence and Approaches for Mitigation. J. Hum. Nutr. Diet. 2020, 33, 624–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, M.; Mucci, L.A.; Giovannucci, E.L. Zinc Supplement Use and Risk of Aggressive Prostate Cancer: A 30-Year Follow-up Study. Eur. J. Epidemiol. 2022, 37, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Grüngreiff, K.; Gottstein, T.; Reinhold, D. Zinc Deficiency—An Independent Risk Factor in the Pathogenesis of Haemorrhagic Stroke? Nutrients 2020, 12, 3548. [Google Scholar] [CrossRef] [PubMed]
- Hanif, N.; Anwer, F. Chronic Iron Deficiency. StatPearls 2023.
- Yuen, H.-W.; Gossman, W.G. Iron Toxicity. StatPearls 2023.
- Miniero, R.; Talarico, V.; Galati, M.C.; Laura; Giancotti; Saracco, P.; Raiola, G.; Miniero, R.; Talarico, V.; Galati, M.C.; et al. Iron Deficiency and Iron Deficiency Anemia in Children. In Public Health and Nutrition in Developing Countries Part-I; IntechOpen, 2018; pp. 638–662 ISBN 978-1-78985-444-2.
- Singh, U.; Praharaj, C.S.; Singh, S.S.; Singh, N.P. Biofortification: Introduction, Approaches, Limitations, and Challenges. In Biofortification of Food Crops; Springer India, 2016; pp. 3–18 ISBN 9788132227168.
- Coelho, R.C.; Barsotti, R.C.F.; Maltez, H.F.; Lopes Júnior, C.A.; Barbosa, H. de S. Expanding Information on the Bioaccessibility and Bioavailability of Iron and Zinc in Biofortified Cowpea Seeds. Food Chem. 2021, 347, 129027. [CrossRef]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 301899. [Google Scholar] [CrossRef] [PubMed]
- Ohanenye, I.C.; Emenike, C.U.; Mensi, A.; Medina-Godoy, S.; Jin, J.; Ahmed, T.; Sun, X.; Udenigwe, C.C. Food Fortification Technologies: Influence on Iron, Zinc and Vitamin A Bioavailability and Potential Implications on Micronutrient Deficiency in Sub-Saharan Africa. Sci. African 2021, 11, e00667. [Google Scholar] [CrossRef]
- Kruger, J. Potential of Food-to-Food Fortification with Cowpea Leaves and Orange-Fleshed Sweet Potato, in Combination with Conventional Fortification, to Improve the Cellular Uptake of Iron and Zinc from Ready-to-Eat Maize Porridges. Food Sci. Nutr. 2020, 8, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Adetola, O.Y.; Kruger, J.; White, Z.; Taylor, J.R.N. Comparison between Food-to-Food Fortification of Pearl Millet Porridge with Moringa Leaves and Baobab Fruit and with Adding Ascorbic and Citric Acid on Iron, Zinc and Other Mineral Bioaccessibility. LWT 2019, 106, 92–97. [Google Scholar] [CrossRef]
- Adetola, O.Y.; Kruger, J.; Ferruzzi, M.G.; Hamaker, B.R.; Taylor, J.R.N. Potential of Moringa Leaf and Baobab Fruit Food-to-Food Fortification of Wholegrain Maize Porridge to Improve Iron and Zinc Bioaccessibility. Int. J. Food Sci. Nutr. 2022, 73, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Lubaale, J.; Taylor, J.R.N.; Emmambux, M.N.; Duodu, K.G. Extrusion Cooking of Food-to-Food Fortified Wholegrain Sorghum-Based Porridges Enhances Caco-2 Ferritin Formation. Cereal Chem. 2023, 100, 371–383. [Google Scholar] [CrossRef]
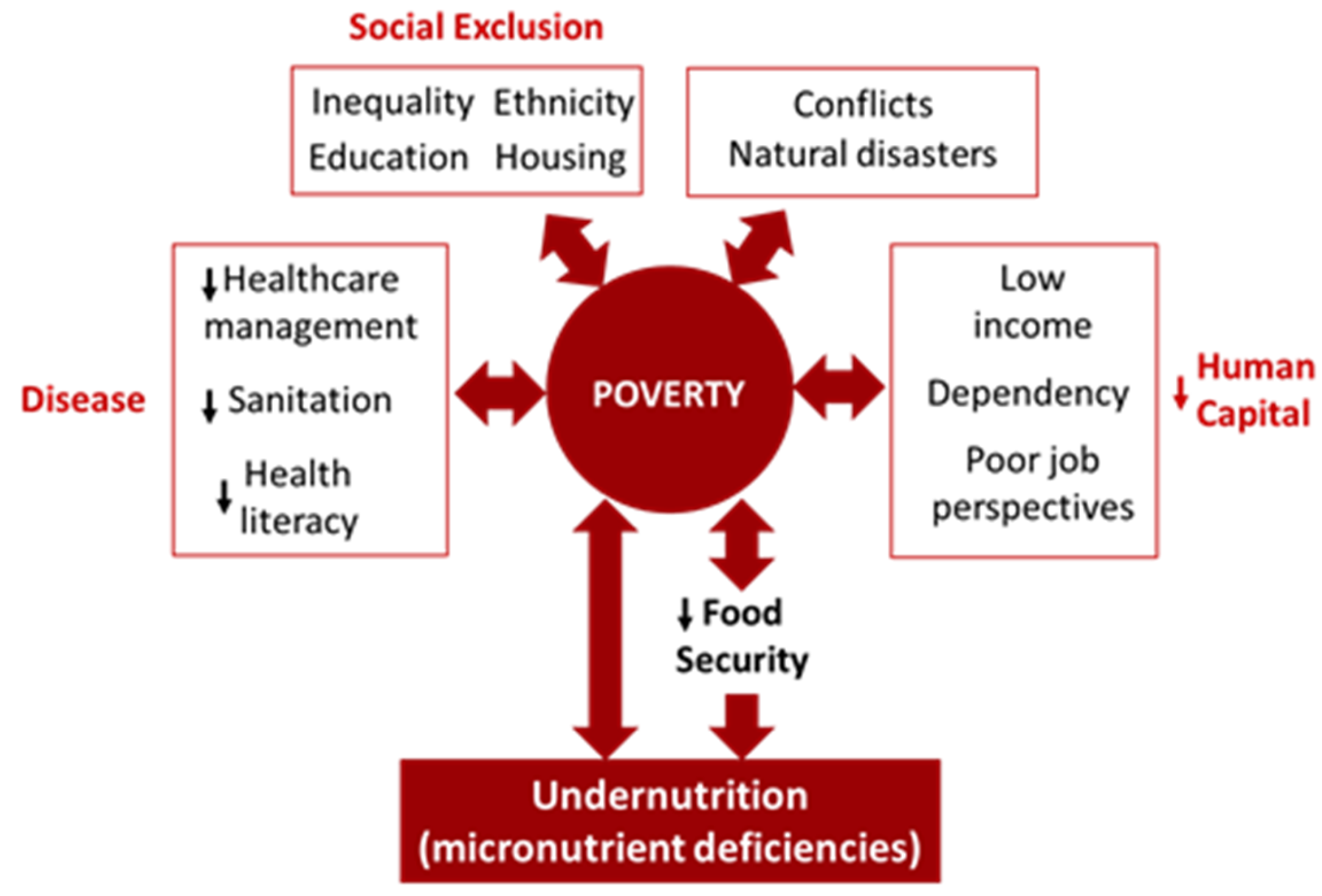
- Siddiqui, F.; Salam, R.A.; Lassi, Z.S.; Das, J.K. The Intertwined Relationship Between Malnutrition and Poverty. Front. Public Heal. 2020, 8, 525026. [Google Scholar] [CrossRef] [PubMed]
- United Nations (UN) Extreme Poverty in Developing Countries Inextricably Linked to Global Food Insecurity Crisis, Senior Officials Tell Second Committee.
- FAO Food Systems Resilience-Building for East and Southern Africa Enters New Phase. Available online: https://www.fao.org/support-to-investment/news/detail/en/c/1655420/ (accessed on 3 January 2024).
- Ruggeri Laderchi, C.; Saith, R.; Stewart, F. Does It Matter That We Do Not Agree on the Definition of Poverty? A Comparison of Four Approaches. Oxford Dev. Stud. 2003, 31, 243–274. [Google Scholar] [CrossRef]
- Dieterich, C.M.; Felice, J.P.; O’Sullivan, E.; Rasmussen, K.M. Breastfeeding and Health Outcomes for the Mother-Infant Dyad. Pediatr. Clin. North Am. 2013, 60, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Dewi, N.U.; Mahmudiono, T. Effectiveness of Food Fortification in Improving Nutritional Status of Mothers and Children in Indonesia. Int. J. Environ. Res. Public Health 2021, 18, 2133. [Google Scholar] [CrossRef] [PubMed]
- Okeyo, D.O. Impact of Food Fortification on Child Growth and Development during Complementary Feeding. Ann. Nutr. Metab. 2018, 73, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Mphasha, M.H.; Makwela, M.S.; Muleka, N.; Maanaso, B.; Phoku, M.M. Breastfeeding and Complementary Feeding Practices among Caregivers at Seshego Zone 4 Clinic in Limpopo Province, South Africa. Children 2023, 10, 986. [Google Scholar] [CrossRef] [PubMed]
- UNICEF-WHO-WB Joint Child Malnutrition Estimates 2023. Available online: https://datatopics.worldbank.org/child-malnutrition/ (accessed on 4 January 2024).
- Colecraf, E.K.; Otoo, G.E. Methods and Metrics for Food Security and Nutrition Outcome Indicators; African Economic Research Consortium, 2022.
- Abebe, Z.; Haki, G.D.; Baye, K. Simulated Effects of Home Fortification of Complementary Foods with Micronutrient Powders on Risk of Inadequate and Excessive Intakes in West Gojjam, Ethiopia. Matern. Child Nutr. 2018, 14. [Google Scholar] [CrossRef]
- Suchdev, P.S.; Jefferds, M.E.D.; Ota, E.; da Silva Lopes, K.; De-Regil, L.M. Home Fortification of Foods with Multiple Micronutrient Powders for Health and Nutrition in Children under Two Years of Age. Cochrane Database Syst. Rev. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Masuke, R.; Msuya, S.E.; Mahande, J.M.; Diarz, E.J.; Stray-Pedersen, B.; Jahanpour, O.; Mgongo, M. Effect of Inappropriate Complementary Feeding Practices on the Nutritional Status of Children Aged 6-24 Months in Urban Moshi, Northern Tanzania: Cohort Study. PLoS One 2021, 16, e0250562. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, S.; Khan, F.-.; Begum, A.; Ali, B.B.; Akram, Z.; Ariff, M.; Jamshed, S.; Khan, F.; Begum, A.; Ali, B.B.; et al. Frequency of Low Birth Weight and Its Relationship With Maternal Nutritional and Dietary Factors: A Cross-Sectional Study. Cureus 2020, 12. [CrossRef]
- Khatun, W.; Rasheed, S.; Alam, A.; Huda, T.M.; Dibley, M.J. Assessing the Intergenerational Linkage between Short Maternal Stature and Under-Five Stunting and Wasting in Bangladesh. Nutrients 2019, 11, 1818. [Google Scholar] [CrossRef] [PubMed]
- Akinola, R.; Pereira, L.M.; Mabhaudhi, T.; de Bruin, F.M.; Rusch, L. A Review of Indigenous Food Crops in Africa and the Implications for More Sustainable and Healthy Food Systems. Sustainability 2020, 12, 3493. [Google Scholar] [CrossRef] [PubMed]
- Kamgain, T.; Kwazi Zuma, A.D.; Mbhenyane, M.; Knowledge, X.; Kesa, H.; Kamgain, A.D.T.; Kwazi Zuma, M.; Mbhenyane, X. Knowledge, Perception and Consumption of Indigenous Foods in Gauteng Region, South Africa. Int. J. Environ. Res. Public Health 2023, 20, 6961. [Google Scholar] [CrossRef] [PubMed]
- Prado, E.L.; Arnold, C.D.; Wessells, K.R.; Stewart, C.P.; Abbeddou, S.; Adu-Afarwuah, S.; Arnold, B.F.; Ashorn, U.; Ashorn, P.; Becquey, E.; et al. Small-Quantity Lipid-Based Nutrient Supplements for Children Age 6–24 Months: A Systematic Review and Individual Participant Data Meta-Analysis of Effects on Developmental Outcomes and Effect Modifiers. Am. J. Clin. Nutr. 2021, 114, 43S–67S. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.P.; Vosti, S.A.; Arnold, C.D.; Engle-Stone, R.; Prado, E.L.; Stewart, C.P.; Wessells, K.R.; Dewey, K.G. The Cost-Effectiveness of Small-Quantity Lipid-Based Nutrient Supplements for Prevention of Child Death and Malnutrition and Promotion of Healthy Development: Modelling Results for Uganda. Public Health Nutr. 2023, 26, 2083–2095. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Arnold, C.D.; Wessells, K.R.; Stewart, C.P. Lipid-Based Nutrient Supplements for Prevention of Child Undernutrition: When Less May Be More. Am. J. Clin. Nutr. 2023, 118, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- UNICEF Small Supplements for the Prevention of Malnutrition in Early Childhood (Small Quantity Lipid-Based Nutrient Supplements). Brief Guidance Note 2023.
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One Health, Fermented Foods, and Gut Microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- FAO Unleashing the Potential of Millets; FAO: Rome, Italy. 2023; ISBN 978-92-5-138105-2.
- Mahajan, S.; Hausladen, C.I.; Argota Sánchez-Vaquerizo, J.; Korecki, M.; Helbing, D. Participatory Resilience: Surviving, Recovering and Improving Together. Sustain. Cities Soc. 2022, 83, 103942. [Google Scholar] [CrossRef]
- Children’s Work in African Agriculture. The Harmful and the Harmless.; Sumber, J., Wheeler, R.S.-, Eds.; Bristol University Press.: Bristol, 2023; ISBN 978-1-5292-2607-2. [Google Scholar]
- Un-Nutrition Transforming Nutrition. UN-Nutrition J. 2022, 1. Un-Nutrition Transforming Nutrition. UN-Nutrition J. 2022, 1. [CrossRef]
- Method, A.; Tulchinsky, T.H. Commentary: Food Fortification: African Countries Can Make More Progress. Adv. Food Technol. Nutr. Sci. - Open J. 2015, SE, S22–S28. [CrossRef]
- Mokoena, O.P.; Ntuli, T.S.; Ramarumo, T.; Seeletse, S.M. Sustainability of Rural Small-Scale Farmers Using a Thematic Content-Fed Analytic Hierarchy Process. Sustainability 2023, 15, 11983. [Google Scholar] [CrossRef]
- Sirdey, N.; Alpha, A. Food Fortification and Domestic Small-Scale Food Chains’ Actors - The Case of Burkina Faso; CIRAD-ES-UMR MOISA, 2020.
- Ebata, A.; Thorpe, J.; Islam, A.; Sultana, S.; Mbuya, M.N.N. Understanding Drivers of Private-Sector Compliance to Large-Scale Food Fortification: A Case Study on Edible Oil Value Chains in Bangladesh. Food Policy 2021, 104, 102127. [Google Scholar] [CrossRef] [PubMed]
- Luthringer, C.L.; Rowe, L.A.; Vossenaar, M.; Garrett, G.S. Regulatory Monitoring of Fortified Foods: Identifying Barriers and Good Practices. Glob. Heal. Sci. Pract. 2015, 3, 446–461. [Google Scholar] [CrossRef] [PubMed]
- Malézieux, E.; Verger, E.O.; Avallone, S.; Alpha, A.; Ngigi, P.B.; Lourme-Ruiz, A.; Bazile, D.; Bricas, N.; Ehret, I.; Martin-Prevel, Y.; et al. Biofortification versus Diversification to Fight Micronutrient Deficiencies: An Interdisciplinary Review. Food Secur. 2023, 1–15. [Google Scholar] [CrossRef]
- Varzakas, T.; Smaoui, S. Global Food Security and Sustainability Issues: The Road to 2030 from Nutrition and Sustainable Healthy Diets to Food Systems Change. Foods 2024, 13, 306. [Google Scholar] [CrossRef] [PubMed]
- Haysom, G. Integrating Food Sensitive Planning and Urban Design into Urban Governance Actions. Urban Forum 2021, 32, 289–310. [Google Scholar] [CrossRef]
- Ju, P.; Anser, M.K.; Osabohien, R.; Ochuba, O.; Ahuru, R.R.; Ashraf, J. Trade Openness, Foreign Direct Investment and Sustainable Agriculture in Africa. Probl. Ekorozwoju 2022, 17, 246–255. [Google Scholar] [CrossRef]
- Ahwireng, A.K. Small Pelagic Fish for Food: Governance and Performance of Small Pelagic Fish Value Chains for Food Security and Nutrition in Ghana, Amsterdam Institute for Social Science Research (AISSR), 2022.
- Iitembu, J.A.; Mafwila, S.K.; Ndara, S.; Erasmus, V.N. Observed Fishery Regulatory Violations in Namibia and Their Possible Implications for the Sustainable Management of Fishery Resources. Reg. Stud. Mar. Sci. 2023, 63, 103004. [Google Scholar] [CrossRef]
- Voortman, R.L. Why the Green Revolution Failed in Sub-Saharan Africa. Rural 21 2013.
- Niazi, S.K. Handbook of Pharmaceutical Manufacturing Formulations; CRC Press, 2004.
- Dary, O. Establishing Safe and Potentially Efficacious Fortification Contents for Folic Acid and Vitamin B12. Food Nutr. Bull. 2008, 29. [Google Scholar] [CrossRef] [PubMed]
- Yetley, E.A.; Rader, J.I. Modeling the Level of Fortification and Post-Fortification Assessments: U. S. Experience. Nutr. Rev. 2004, 62, S50–S59. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.B.; Arnipalli, S.R.; Mehta, P.; Carrau, S.; Ziouzenkova, O. Iron Deficiency Anemia: Efficacy and Limitations of Nutritional and Comprehensive Mitigation Strategies. Nutrients 2022, 14, 2976. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.C.; Marx, L.M.; Raiff, M.E. Cartel Price Announcements: The Vitamins Industry ☆. Int. J. Ind. Organ. 2008, 26, 762–802. [Google Scholar] [CrossRef]
- Sight and Life Mauricio Müller Adade. Available online: https://sightandlife.org/about-us/our-team/mauricio-muller-adade (accessed on 21 January 2024).
- DSM Nutritional Products: Structural Adjustments in the Region of Basel as a Consequence of Acquisitions and a Strategic Re-Direction. Available online: https://www.dsm.com/anh/news/press-releases/2014/2014-01-21-dsm-nutritional-products-structural-adjustments-in-the-region-of-basel-as-a-consequence-of-acquisitions-and-a-strategic-re-direction.html# (accessed on 15 March 2024).
- Food Fortification Initiative Flour Millers Toolkit for Fortification. Available online: https://www.ffinetwork.org/implement_sub (accessed on 20 January 2024).
- The Lancet Unveiling the Predatory Tactics of the Formula Milk Industry. Lancet 2023, 401, 409. [CrossRef] [PubMed]
- EFSA Tolerable Upper Intake Levels for Vitamins and Minerals; 2006.
- Fortune Business Insights The Food Premix Market Is Projected to Grow from $6.69 Billion in 2022 to $10.70 Billion by 2029, at a CAGR of 6.93% during the Forecast Period, 2022-2029. Available online: https://www.fortunebusinessinsights.com/food-premix-market-102456 (accessed on 15 January 2024).
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional Risk Screening and Assessment. J. Clin. Med. 2019, 8, 1065. [Google Scholar] [CrossRef] [PubMed]
- Guinot, P.; Jallier, V.; Blasi, A.; Guyondet, C.; Van Ameringen, M. GAIN Premix Facility: An Innovative Approach for Improving Access to Quality Vitamin and Mineral Premix in Fortification Initiatives. Food Nutr. Bull. 2012, 33. [Google Scholar] [CrossRef] [PubMed]
- Noort, M.W.J.; Renzetti, S.; Linderhof, V.; du Rand, G.E.; Marx-Pienaar, N.J.M.M.; de Kock, H.L.; Magano, N.; Taylor, J.R.N. Towards Sustainable Shifts to Healthy Diets and Food Security in Sub-Saharan Africa with Climate-Resilient Crops in Bread-Type Products: A Food System Analysis. Foods 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- FAO, IFAD, UNICEF, W. and W. The State of Food Security and Nutrition in the World 2022; FAO, 2022.
- Manikas, I.; Ali, B.M.; Sundarakani, B. A Systematic Literature Review of Indicators Measuring Food Security. Agric. Food Secur. 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Unsal, F.; Farid, M.; Kemoe, L.; Fayad, D.; Spray, J.; Okou, C.; Salgado Baptista, D.M.; Lanci, L.; Muehlschlegel, T.; et al. Climate Change and Chronic Food Insecurity in Sub-Saharan Africa. Dep. Pap. 2022, 2022, 1. [Google Scholar] [CrossRef]
- Chea, N.; Tegene, Y.; Astatkie, A.; Spigt, M. Prevalence of Undernutrition among Pregnant Women and Its Differences across Relevant Subgroups in Rural Ethiopia: A Community-Based Cross-Sectional Study. J. Heal. Popul. Nutr. 2023, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Valente, F.L.S. The Corporate Capture of Food and Nutrition Governance: A Threat to Human Rights and Peoples’ Sovereignty. Right to Food Nutr. Watch 2015, 1, 15–22. [Google Scholar]
- Scheffler, C.; Hermanussen, M.; Soegianto, S.D.P.; Homalessy, A.V.; Touw, S.Y.; Angi, S.I.; Ariyani, Q.S.; Suryanto, T.; Matulessy, G.K.I.; Fransiskus, T.; et al. Stunting as a Synonym of Social Disadvantage and Poor Parental Education. Int. J. Environ. Res. Public Health 2021, 18, 1350. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E.; Shah, M.; Badireddy, M. Failure to Thrive. StatPearls 2023, 183–199. [Google Scholar] [CrossRef]
- Frumence, G.; Jin, Y.; Kasangala, A.A.; Mang’enya, M.A.; Bakar, S.; Ochieng, B. A Qualitative Exploration on Perceived Socio-Cultural Factors Contributing to Undernutrition Among Under-Fives in the Southern Highlands of Tanzania. Int. J. Public Health 2023, 68, 1605294. [Google Scholar] [CrossRef] [PubMed]
- Dary, O.; Hainsworth, M. The Food Fortification Formulator: Technical Determination of Fortification Levels and Standards for Mass Fortification; 2008.
- Tam, E.; Keats, E.C.; Rind, F.; Das, J.K.; Bhutta, Z.A. Micronutrient Supplementation and Fortification Interventions on Health and Development Outcomes among Children Under-Five in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- WHO and FAO Implementing Effective and Sustainable Food Fortification Programmes. In Guidelines on food fortification with micronutrients; Allen, L., Benoist, B. de, Dary, O., Hurrell, R., Eds.; WHO: Geneva, Switzerland, 2006.
- WHO WHO Guideline: Fortification of Maize Flour and Corn Meal with Vitamins and Minerals; WHO: Geneva, Switzerland, 2016. ISBN 9789241549936.
- Ohanenye, I.C.; Emenike, C.U.; Mensi, A.; Medina-Godoy, S.; Jin, J.; Ahmed, T.; Sun, X.; Udenigwe, C.C. Food Fortification Technologies: Influence on Iron, Zinc and Vitamin A Bioavailability and Potential Implications on Micronutrient Deficiency in Sub-Saharan Africa. Sci. African 2021, 11. [Google Scholar] [CrossRef]
- Lopes, S.O.; Abrantes, L.C.S.; Azevedo, F.M.; Morais, N. de S. de; Morais, D. de C.; Gonçalves, V.S.S.; Fontes, E.A.F.; Franceschini, S. do C.C.; Priore, S.E. Food Insecurity and Micronutrient Deficiency in Adults: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 1074. [Google Scholar]
- Ministry of Health and Family Welfare Food Safety and Standards (Fortification of Foods) Regulations, 2018. Gaz. India 2018, Part III-S.
- Renwick, A.G. Toxicology of Micronutrients: Adverse Effects and Uncertainty. J. Nutr. 2006, 136, 493S–501S. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.R.A.; Campos, R.F. de A.; Rocha, F.; Emmendoerfer, M.L.; Vidigal, M.C.T.R.; da Rocha, S.J.S.S.; Lucia, S.M. Della; Cabral, L.F.M.; de Carvalho, A.F.; Perrone, Í.T. Perceived Healthiness of Foods: A Systematic Review of Qualitative Studies. 2021, 4, 100056. [CrossRef]
- Regional Committee for Africa Progress Report on the Implementation of the Regional Strategy on Enhancing the Role of Traditional Medicine in Health Systems 2013–2023: Information Document; 2020.
- Adebisi, Y.A.; Nwogu, I.B.; Alaran, A.J.; Badmos, A.O.; Bamgboye, A.O.; Rufai, B.O.; Okonji, O.C.; Malik, M.O.; Teibo, J.O.; Abdalla, S.F.; et al. Revisiting the Issue of Access to Medicines in Africa: Challenges and Recommendations. Public Heal. Challenges 2022, 1, e9. [Google Scholar] [CrossRef]
- Giller, K.E.; Delaune, T.; Silva, J.V.; Descheemaeker, K.; van de Ven, G.; Schut, A.G.T.; van Wijk, M.; Hammond, J.; Hochman, Z.; Taulya, G.; et al. The Future of Farming: Who Will Produce Our Food? Food Secur. 2021, 13, 1073–1099. [Google Scholar] [CrossRef]
- Vilar-Compte, M.; Burrola-Méndez, S.; Lozano-Marrufo, A.; Ferré-Eguiluz, I.; Flores, D.; Gaitán-Rossi, P.; Teruel, G.; Pérez-Escamilla, R. Urban Poverty and Nutrition Challenges Associated with Accessibility to a Healthy Diet: A Global Systematic Literature Review. Int. J. Equity Health 2021, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Salas, S.; Gonzalez-Arias, M. Nutrition: Micronutrient Intake, Imbalances, and Interventions. StatPearls 2023.
- Grammatikaki, E.; Wollgast, J.; Caldeira, S. High Levels of Nutrients of Concern in Baby Foods Available in Europe That Contain Sugar-Contributing Ingredients or Are Ultra-Processed. Nutrients 2021, 13, 3105. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C. A Lawsuit Is Accusing Poland Spring of Selling “Ordinary Groundwater”. The Suit Highlights the Opaque, Controversial Business of Bottled Water. Available online: https://www.vox.com/the-goods/2019/4/3/18292549/poland-spring-water-nestle-lawsuit-spring-water (accessed on 15 January 2024).
- Australian Food News Nestle and Gerber Sued over Fluoride-Fortified Food Products. Available online: https://www.ausfoodnews.com.au/2011/10/12/nestle-and-gerber-sued-over-fluoride-fortified-food-products.html (accessed on 15 January 2024).
- Kujinga, P.; Galetti, V.; Onyango, E.; Jakab, V.; Buerkli, S.; Andang’O, P.; Brouwer, I.D.; Zimmermann, M.B.; Moretti, D. Effectiveness of Zinc-Fortified Water on Zinc Intake, Status and Morbidity in Kenyan Pre-School Children: A Randomised Controlled Trial. Public Health Nutr. 2018, 21, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- Nestlé Nigeria Corporate Communications and Public Affairs Nestlé Nigeria. Creating Shared Value Report 2013; Ilupeju, 2013.
- Coca-Cola Adds Two New Flavor Variants to Its Vitamin Water Range. Available online: https://www.foodbusinessafrica.com/coca-cola-adds-two-new-flavor-variants-to-its-vitamin-water-range/ (accessed on 15 January 2024).
- Yusufali, R.; Sunley, N.; de Hoop, M.; Panagides, D. Flour Fortification in South Africa: Post-Implementation Survey of Micronutrient Levels at Point of Retail. Food Nutr. Bull. 2012, 33. [Google Scholar] [CrossRef] [PubMed]
- Van Jaarsveld, P.J.; Faber, M.; Van Stuijvenberg, M.E. Vitamin A, Iron, and Zinc Content of Fortified Maize Meal and Bread at the Household Level in 4 Areas of South Africa. Food Nutr. Bull. 2015, 36, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Wojcicki, J.M.; Heyman, M.B. Malnutrition and the Role of the Soft Drink Industry in Improving Child Health in Sub-Saharan Africa. Pediatrics 2010, 126, e1617–e1621. [Google Scholar] [CrossRef] [PubMed]
- TechnoServe The Coca-Cola Company, TechnoServe and The Gates Foundation Partner to Boost Incomes of 50,000 Small-Scale Farmers in East Africa. Available online: https://www.technoserve.org/news/the-coca-cola-company-technoserve-and-the-gates-foundation-partner-to-boost/ (accessed on 15 January 2024).
- Council for Responsible Nutrition (CRN) Americans Do Not Get All the Nutrients They Need From Food. Available online: https://www.crnusa.org/resources/americans-do-not-get-all-nutrients-they-need-food (accessed on 16 January 2024).
- SADC SADC Minimum Standard for Food Fortification; Gaborone, Botswana, 2022.
- Wang, V.H.C.; Foster, V.; Yi, S.S. Are Recommended Dietary Patterns Equitable? Public Health Nutr. 2022, 25, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Aaron, G.J.; Friesen, V.M.; Jungjohann, S.; Garrett, G.S.; Neufeld, L.M.; Myatt, M. Coverage of Large-Scale Food Fortification of Edible Oil, Wheat Flour, and Maize Flour Varies Greatly by Vehicle and Country but Is Consistently Lower among the Most Vulnerable: Results from Coverage Surveys in 8 Countries. J. Nutr. 2017, 147, 984S–994S. [Google Scholar] [CrossRef] [PubMed]
- Theriault, V.; Kirimi, L.; Wineman, A.; Kinyumu, E.; Tschirley, D. Assessment of the Policy Enabling Environment for Large-Scale Food Fortification: A Novel Framework with an Application to Kenya. PLOS Glob. Public Heal. 2024, 4, e0003211. [Google Scholar] [CrossRef] [PubMed]
- Global Fortification Data Exchange Interactive Map. Available online: https://fortificationdata.org/interactive-map-fortification-legislation/ (accessed on 27 June 2024).
- Kroker-Lobos, M.F.; Mazariegos, M.; Guamuch, M.; Ramirez-Zea, M. Ultraprocessed Products as Food Fortification Alternatives: A Critical Appraisal from Latin America. Nutrients 2022, 14, 1413. [Google Scholar] [CrossRef] [PubMed]
- Horton, S. The Economics of Food Fortification. J. Nutr. 2006, 136, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Ekpa, O.; Palacios-Rojas, N.; Kruseman, G.; Fogliano, V.; Linnemann, A.R. Sub-Saharan African Maize-Based Foods - Processing Practices, Challenges and Opportunities. Food Rev. Int. 2019, 35, 609–639. [Google Scholar] [CrossRef]
- Yang, P.; Wang, H.; Zhu, M.; Ma, Y. Effects of Choline Chloride, Copper Sulfate and Zinc Oxide on Long-Term Stabilization of Microencapsulated Vitamins in Premixes for Weanling Piglets. Animals 2019, 9, 1154. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Vitolins, M.Z. Food Fortification and Supplement Use—Are There Health Implications? Crit. Rev. Food Sci. Nutr. 2016, 56, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Guallar, E.; Stranges, S.; Mulrow, C.; Appel, L.J.; Miller, E.R. Enough Is Enough: Stop Wasting Money on Vitamin and Mineral Supplements. Ann. Intern. Med. 2013, 159, 850–851. [Google Scholar] [CrossRef] [PubMed]
- Engle-Stone, R.; Vosti, S.A.; Luo, H.; Kagin, J.; Tarini, A.; Adams, K.P.; French, C.; Brown, K.H. Weighing the Risks of High Intakes of Selected Micronutrients Compared with the Risks of Deficiencies. Ann. N. Y. Acad. Sci. 2019, 1446, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Hamishehkar, H.; Ranjdoost, F.; Asgharian, P.; Mahmoodpoor, A.; Sanaie, S. Vitamins, Are They Safe? Adv. Pharm. Bull. 2016, 6, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Rogovik, A.L.; Vohra, S.; Goldman, R.D. Safety Considerations and Potential Interactions of Vitamins: Should Vitamins Be Considered Drugs? Ann. Pharmacother. 2010, 44, 311–324. [Google Scholar] [CrossRef] [PubMed]
- McEldrew, E.P.; Lopez, M.J.; Milstein, H. Vitamin A. In StatPearls; StatPearls Publishing, 2023.
- Degerud, E.M.; Manger, M.S.; Strand, T.A.; Dierkes, J. Bioavailability of Iron, Vitamin A, Zinc, and Folic Acid When Added to Condiments and Seasonings. Ann. N. Y. Acad. Sci. 2015, 1357, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Bangham, C.R.M. HTLV-1 Persistence and the Oncogenesis of Adult T-Cell Leukemia/Lymphoma. Blood 2023, 141, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Elechi, J.O.G.; Sirianni, R.; Conforti, F.L.; Cione, E.; Pellegrino, M. Food System Transformation and Gut Microbiota Transition: Evidence on Advancing Obesity, Cardiovascular Diseases, and Cancers—A Narrative Review. Foods 2023, 12, 2286. [Google Scholar] [CrossRef] [PubMed]
- SHI, J.; XIE, Y.; LI, Y.; REN, D.; ZHANG, Y.; SHAO, H.; LIU, Y.; WANG, X.; LI, Y. Effects of Food-Grade Iron(III) Oxide Nanoparticles on Cecal Digesta- and Mucosa-Associated Microbiota and Short-Chain Fatty Acids in Rats. Biosci. microbiota, food Heal. 2024, 43. [CrossRef]
- Bobrek, K.S.; Broersen, B.; Aburto, N.J.; Garg, A.; Serdula, M.; Beltrán Velázquez, F.; Wong, E.C.; Pachón, H. Most National, Mandatory Flour Fortification Standards Do Not Align with International Recommendations for Iron, Zinc, and Vitamin B12 Levels. Food Policy 2021, 99. [Google Scholar] [CrossRef]
- Schutter, O. de Report of the Special Rapporteur on the Right to Food, Olivier de Schutter. Final Report : The Transformative Potential of the Right to Food; United Nations, 2014.
- Archer, N.S.; Cochet-Broch, M.; Mihnea, M.; Garrido-Bañuelos, G.; Lopez-Sanchez, P.; Lundin, L.; Frank, D. Sodium Reduction in Bouillon: Targeting a Food Staple to Reduce Hypertension in Sub-Saharan Africa. Front. Nutr. 2022, 9, 746018. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.; Hurrell, R.F.; Cercamondi, C.I. Bouillon Cubes. In Food Fortification in a Globalized World; Mannar, M.G.V., Hurrell, R.F., Eds.; Academic Press, 2018; pp. 159–165.
- Nijman, C.A.J.; Zijp, I.M.; Sierksma, A.; Roodenburg, A.J.C.; Leenen, R.; van den Kerkhoff, C.; Weststrate, J.A.; Meijer, G.W. A Method to Improve the Nutritional Quality of Foods and Beverages Based on Dietary Recommendations. Eur. J. Clin. Nutr. 2007, 61, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Dötsch-Klerk, M.; Pmm Goossens, W.; Meijer, G.W.; Van Het Hof, K.H. Reducing Salt in Food; Setting Product-Specific Criteria Aiming at a Salt Intake of 5 g per Day. Eur. J. Clin. Nutr. 2015, 69, 799–804. [Google Scholar] [CrossRef]
- Vlassopoulos, A.; Masset, G.; Charles, V.R.; Hoover, C.; Chesneau-Guillemont, C.; Leroy, F.; Lehmann, U.; Spieldenner, J.; Tee, E.S.; Gibney, M.; et al. A Nutrient Profiling System for the (Re)Formulation of a Global Food and Beverage Portfolio. Eur. J. Nutr. 2017, 56, 1105–1122. [Google Scholar] [CrossRef] [PubMed]
- Vieux, F.; Privet, L.; Masset, G. Food- and Diet-Based Validations of a Nestlé Nutrient Profiling System for Reformulation in Two Nationally Representative Surveys. Br. J. Nutr. 2018, 120, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Fortified Whole Grain Alliance Rwanda. Available online: https://fwg-alliance.org/our-initiatives/rwanda/ (accessed on 30 June 2024).
- Taylor, J.R.N.; de Kock, H.L.; Makule, E.; Hamaker, B.R.; Milani, P. Reduction in Rancidity Development in Fortified Whole-Grain Maize Meal by Hot-Air Drying of the Grain. Cereal Chem. 2024, 101, 323–333. [Google Scholar] [CrossRef]
- Holmes, M.D.; Dalal, S.; Sewram, V.; Diamond, M.B.; Adebamowo, S.N.; Ajayi, I.O.; Adebamowo, C.; Chiwanga, F.S.; Njelekela, M.; Laurence, C.; et al. Consumption of Processed Food Dietary Patterns in Four African Populations. Public Health Nutr. 2018, 21, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- WHO Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 7 February 2024).
- Imamura, F.; Micha, R.; Khatibzadeh, S.; Fahimi, S.; Shi, P.; Powles, J.; Mozaffarian, D. Dietary Quality among Men and Women in 187 Countries in 1990 and 2010: A Systematic Assessment. Lancet Glob. Heal. 2015, 3, e132–e142. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.; Gavin-Smith, B.; Ferraboschi, C.; Kraemer, K. Food Fortification: The Advantages, Disadvantages and Lessons from Sight and Life Programs. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, D.C. 2001.
- Assuncąõ, M.C.; Santos, I.S.; Barros, A.J.; Gigante, D.P.; Victora, C.G. Flour Fortification with Iron Has No Impact on Anaemia in Urban Brazilian Children – Corrigendum. Public Health Nutr. 2013, 16, 188–188. [Google Scholar] [CrossRef]
- Engle-Stone, R.; Vosti, S.A.; Luo, H.; Kagin, J.; Tarini, A.; Adams, K.P.; French, C.; Brown, K.H. Weighing the Risks of High Intakes of Selected Micronutrients Compared with the Risks of Deficiencies. Ann. N. Y. Acad. Sci. 2019, 1446, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.F.M.; Mustar, R.D.N.G.; Suraiami, A.; Noh, M.F.M.; Mustar, R.D.N.G.; Suraiami, A. Vitamin A in Health and Disease. In Vitamin A; IntechOpen, 2019 ISBN 978-1-78923-946-1.
- Dary, O. Establishing Safe and Potentially Efficacious Fortification Contents for Folic Acid and Vitamin B12. Food Nutr. Bull. 2016, 29. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, K.; Trichkova-Kashamova, E.; Dimitrov, S. Applying a Mathematical Model for Calculating the Ideal Nutrition for Sheep. Mathematics 2024, 12, 1270. [Google Scholar] [CrossRef]


| Iodine | Iron | Vitamin A | Zinc | |
|---|---|---|---|---|
| Condiments | Salt | Sauces (soy, fish) | Sugar | |
| Cereals | Wheat flour, corn flour, pasta, rice | Cereal flours | Cereals | |
| Dairy products | Milk (powder&liquid) | Milk | ||
| Fat & Oils | Oil, margarine | |||
| Vegetables | Potatoes, beans |
| Food | RAE per serving (mcg) | Daily Value (%) |
|---|---|---|
| Animal liver 85g | 6600 | 731 |
| Orange flesh sweet potato 100g | 1600 | 156 |
| Spinach 60g | 570 | 64 |
| Pumpkin 130g | 490 | 54 |
| Papaya 100g | 756 | 32 |
| African Food | Iron (mg/100g FW) |
|---|---|
| Cowpea (Vigna unguiculata) | 4,7 |
| African spinach (Amaranthus caudatus) | 5,9 - 12,7 |
| Algae sirisiri (Sesuvium portulacastrum) | 33,5 |
| Alligator pepper (Aframomum melegueta) | 37,8 |
| Beef liver | 6,7 |
| Locust bean (Leucaena leucocephala) | 33,6 |
| Moringa (Moringa oleifera) leaves | 28 |
| Squash (Pumpkin cucurbita) leaves | 3,2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





