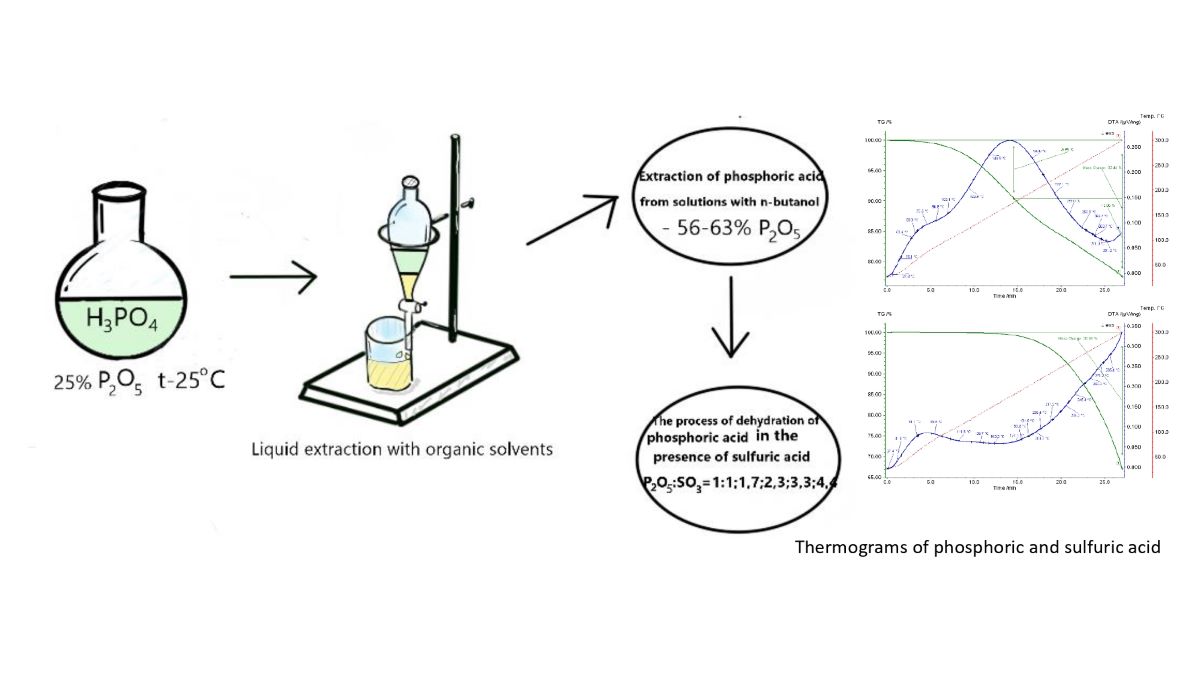
1. Introduction
Mineral fertilizer companies in Central Asia and Kazakhstan use phosphorites of Karatau phosphate deposit as phosphate raw material [
1,
2,
3].
Phosphoric acid is the basis for concentrated phosphate fertilizer technology [
4,
5]. The cheapest method of its production is currently the sulphuric acid method of phosphate raw material decomposition. Application of this method to Karatau phosphorite gives acid with relatively low content of P
2O
5 (18-20%), contaminated with impurities (magnesium, iron, aluminum salts, etc.). The quantity and ratio of impurities are determined by the quality of phosphate raw materials and the method of extraction phosphoric acid production. The total content of impurities in the acid is up to 15 mas. %. The main of them are fluoride ions, sulfate ions, iron, aluminum, calcium, magnesium, sodium, potassium cations. Fluoride compounds are present in phosphoric acid in the form of hydrofluoric acid, silicofluoric acid and in the form of their complex compounds with phosphoric acid, aluminum, iron [
6,
7,
8,
9,
10].
Attempts to increase the concentration of phosphoric acid by improving the technology or by evaporation do not lead to positive results: with increasing the concentration of acid increases its viscosity, violates the filtration mode, and during evaporation precipitation occurs [
11,
12].
At present there are new trends in the development of wet-process phosphoric acid production, associated with modernization and development of new and more advanced methods of purification, for example, with organic solvents (liquid extraction method) [
13,
14,
15,
16]. The main attention in the development of liquid extraction purification methods is paid to the selection of an organic extractant with a high distribution factor. Relatively few works are devoted to the issue of phosphoric acid extraction by solvents. N-butanol, isobutanol and isoamyl alcohol are widely used for the purification of wet-process phosphoric acid from metal cations and fluorine [
17,
18].
By decomposition of phosphate raw materials with concentrated sulfuric acid, followed by heating of the mixture and extraction with organic solvents, it is possible to obtain phosphoric acid with high yield of P
2O
5 [
19,
20,
21].
Dehydration of pure phosphoric acid has been studied in detail in a wide temperature range [
22,
23,
24]. It was shown that the composition of dehydration products depends on the amount of P
2O
5.
Dehydration of wet-process phosphoric acid [
25,
26,
27] in the presence of impurities (R
2O
3, F) proceeds differently than pure; polymorphs appear at lower temperatures and their content is higher than in the products of dehydration of pure phosphoric acid at the same total content of Р
2О
5. The impurities generally increase the viscosity of the acid, and the presence of aluminum ions leads to the formation of aluminum tripolyphosphate. No data on the dehydration of phosphoric acid in the presence of sulfuric acid have been found. There is only an indication in the patent references that H
2SO
3 in small amounts in the dehydration of phosphoric acid leads to the formation of ultrasulfophosphoric acid [
28].
Thus, research aimed at finding optimal and effective methods of purification of technical phosphoric acid is an urgent problem. The purpose of the present work is to obtain concentrated phosphoric acid from technical phosphoric acid by liquid extraction with organic solvents. In the work also the influence of the amount of sulfuric acid in the mixture on the process of dehydration of phosphoric acid is studied.
2. Research Objects and Methods
The object of the study was wet-process phosphoric acid for production of mineral fertilizers, obtained by dihydrate method from Karatau phosphorite (
Table 1). Concentration of phosphoric acid is 25% by P
2O
5, temperature - 25
0C.
To increase the concentration of phosphoric acid and its purification, the method of liquid extraction with organic solvents was used [
29,
30,
31,
32]. Phosphoric acid is washed out of the solvent with water according to the counterflow principle. Since technical acid contains free sulfuric acid, various magnesium, iron and aluminum salts, as well as fluorine (SiF
6) as impurities, the distribution of pure phosphoric acid between water and n-butyl, isoamyl alcohol and tributyl phosphate was studied in advance, depending on the temperature, concentration of phosphoric acid and concentration of compounds contained in technical phosphoric acid as H
2SO
4, MgSO
4, Mg(H
2PO
4) impurities.
Experiments on the distribution of phosphoric acid between water and organic solvents were carried out in a water thermostat, the temperature in which was regulated with an accuracy of ±0.50C. Equal volumes of the aqueous and organic phases were placed in dividing funnels supported on a disk in the thermostat. Stirring was carried out until equilibrium was established (15 min. in these experiments). After stirring the funnels were left in the thermostat until complete separation of the phases. To determine the acid concentration, samples were taken from the organic and aqueous phases, diluted with water and titrated with 0.05n. NaOH in the presence of phenolphthalein. In the presence of sulfuric acid, the titration was carried out using two indicators - methyl alcohol and phenolphthalein. The distribution factor was determined as the ratio of equilibrium concentrations in the organic and aqueous phases, respectively.
In the next series of experiments, the influence of magnesium salts and sulfuric acid, present in technical phosphoric acid as impurities, on the distribution factor of phosphoric acid was inquired. The magnesium sulfate and monophosphate were used, the concentration of which varied from 0.5 to 2.5% in terms of MgO. The amount of sulfuric acid varied from 0.30 to 6.19% SO3.
Since technical phosphoric acid obtained from Karatau phosphorites always contains free sulfuric acid along with magnesium salts, experiments were conducted to find out the influence of sulfuric acid concentration on the distribution factor of phosphoric acid in the system H3PO4-MgSO4-H2SO4-H2O-n-butanol, isoamyl alcohol, tributyl phosphate.
In the next series of experiments, the extraction of technical phosphoric acid obtained by sulfuric acid decomposition of Karatau phosphorites according to the dihydrate method with n-butyl alcohol was studied.
Phosphoric acid was treated with a given amount of alcohol in the ratio of organic solvent: acid equal to 4:1 and 3:1 three- or four times until almost complete extraction of phosphoric acid, the extract was sent for treatment of a new portion of phosphoric acid.
The obtained extracts were subjected to azeotropic distillation to extract the solvent. Water was added from a stoichiometric calculation for the formation of an azeotrope with n-butanol. The distillation temperature was maintained between 45-640C and pressure 410-420 mm Hg. Fluoric acids, partially passing into the organic phase, complicate the process of distillation of the solvent (stoppers of the distillation apparatus are clogged and corroded).
To remove this disadvantage, K
2SO
4, MgSO
4, Na
2SO
4 were added to technical phosphoric acid with the expectation of binding fluorine in the form of hard-soluble salts K
2SiF
6, Na
2SiF
6 and MgF
6 [
33].
The process of dehydration of phosphoric acid in the presence of sulfuric acid was investigated in wide intervals of ratios and temperatures. Mixtures of sulfuric and phosphoric acids were prepared in the following molar ratios: P
2O
5:SO
3 = 1:1; 1.7; 2.3; 3.3; 4.4. The experiments were carried out in platinum crucibles. The initial mixtures were kept in the desiccator. The content of P
2O
5 and SO
3 in the samples was determined by standard gravimetric methods. Hydrolysis of polyforms was prevented by addition of cold alkali, and the qualitative composition of products was determined by paper chromatography [
34].
Thermograms of the initial mixtures were taken on the SDTQ600 thermal analyzer. The device allows simultaneous recording of changes in sample mass (thermogravimetric analysis) and processes accompanied by heat release or absorption (differential scanning calorimetry/differential thermal analysis [
35].
Heating of the mixtures from 100 to 3000C was carried out in an oven for 4 h. The temperature was maintained with an accuracy of ±50C. Acid mixtures after heating had consistency from syrupy to solidifying into a solid mass.
3. Results and Discussions
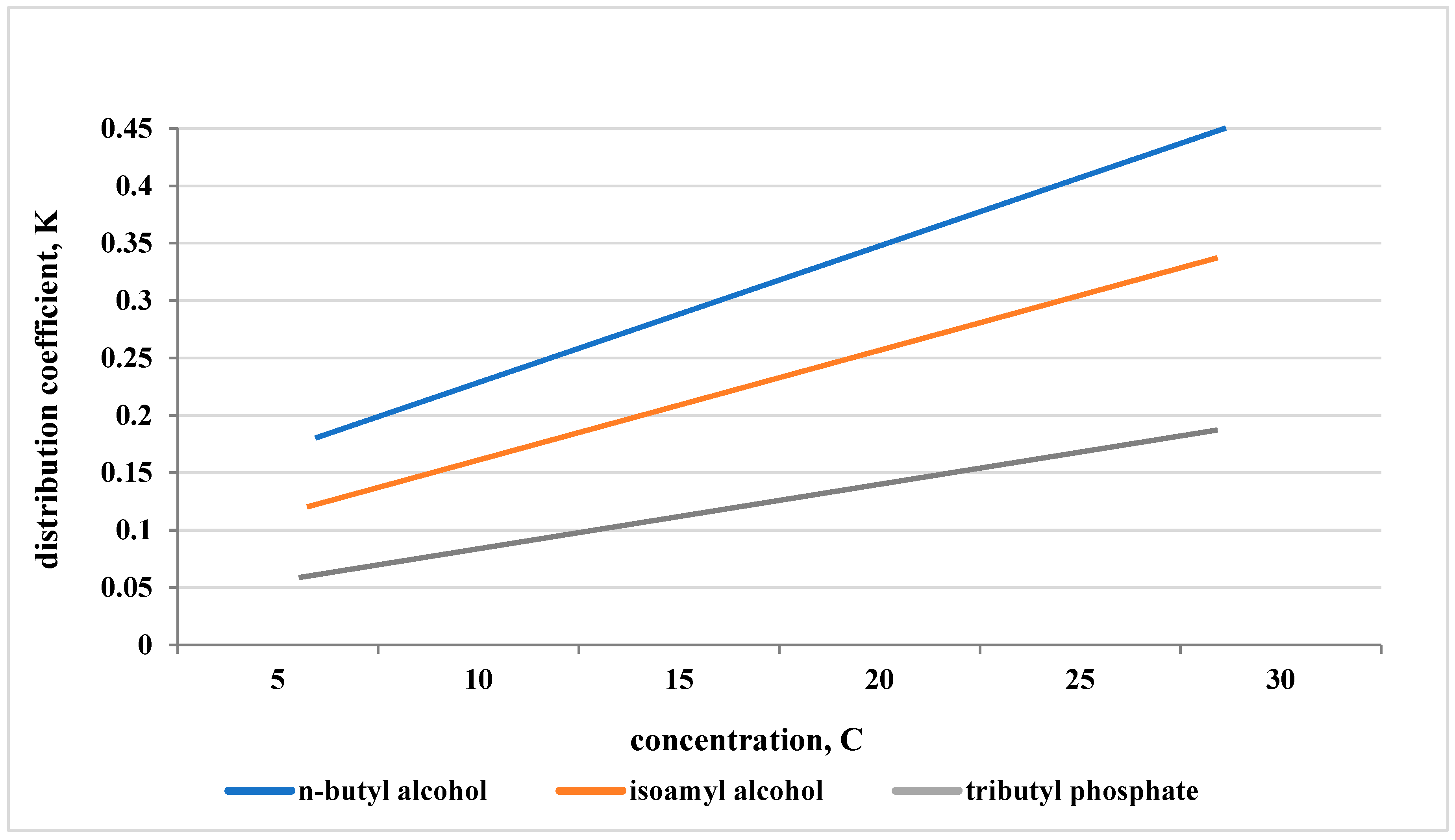
The results on the distribution of phosphoric acid as a function of initial concentration are shown in
Figure 1.
As shown in
Figure 1, the more initial phosphoric acid concentration (from 5.38 to 25.61% P
2O
5), the more distribution factor (K=C
org./C
aqe.) at 25°C (from 0.178 to 0.436 for n-butyl alcohol, from 0.06 to 0.183 for isoamyl alcohol, and from 0.120 to 0.320 for tributyl phosphate).
It was found out that when the concentration of sulfuric acid changes from 0.30 to 6.19%, the distribution factor of phosphoric acid increases for all solvents used by 1.3-1.7 times. The distribution factor of sulfuric acid strongly depends on its initial concentration, increasing for n-butyl alcohol from 0.196 to 0.646, for isoamyl alcohol - from 0.077 to 0.240.
During the extraction of phosphoric and mixture of phosphoric and sulfuric acids, an increase in the equilibrium volume of the organic layer is observed for both alcohols and tributyl phosphate.
An increase in temperature from 25 to 60°C has almost no effect on the distribution factor.
The presence of magnesium sulfate in phosphoric acid in the amount of up to 1.05% MgO also hardly does not affect the distribution factor of phosphoric acid, and the presence of magnesium monophosphate reduces it almost twice.
The results of studies to determine the effect of sulfuric acid concentration on the phosphoric acid distribution factor in the H
3PO
4-MgSO
4-H
2SO
4-H
2O-n-butanol, isoamyl alcohol, tributyl phosphate system are summarized in
Table 2. From a comparison of the data in
Table 2 and
Table 3, it can be seen that in the combined presence of magnesium sulfate and sulfuric acid affect the phosphoric acid distribution factor as well as taken separately.
It follows from the experiments that the distribution factors of phosphoric acid strongly depend on the initial concentration of phosphoric acid in the presence of sulfuric acid. Phosphoric acid is extracted better with n-butanol. At the phosphoric acid concentration of 25% P2O5 in the presence of free sulfuric acid 3.57% SO3, the distribution factor reaches 0.500.
The waste aqueous phase was a gelatinous precipitate consisting of impurities that pass insignificantly into the organic phase: mainly phosphates and sulfates of iron, aluminum and magnesium. The extraction results showed that there is a limiting concentration of P2O5 in n-Butanol corresponding to about 4.5-5%, above which phosphoric acid does not transfer to butanol. At recycling leaching three times in the ratio 3:1 the consumption of phosphoric acid in relation to the whole phosphorus pentoxide is 72-78 %.
After azeotropic volatilization of the solvent, the concentration of phosphoric acid reached 48-55% by P2O5. The solvent recovery in laboratory experiments was 86-90%.
To eliminate this disadvantage, K2SO4, MgSO4, Na2SO4 were added to the technical phosphoric acid with the expectation of binding fluorine in the form of hard soluble salts K2SiF6, Na2SiF6 and MgF6. The results on extraction of such mixtures show that the additions of potassium and sodium sulfates bind fluorine into insoluble compounds, which prevents its transfer to the organic phase. In addition, in the presence of these sulfates increases the recovery factor of phosphoric acid, which is associated with the salting effect of sulfuric acid formed by the exchange reaction of potassium and sodium sulfates with fluorine-containing acids. Thus, the recovery factor of phosphorus pentaoxide in the organic phase without additives is 70-71%, and with potassium and sodium sulfate additives - 81-83%, while the content of free sulfuric acid in phosphoric acid increases to 4-5%.
The phosphoric acid obtained after volatilization of the solvent contains a high amount of phosphorus pentaoxide: up to 56-63%. The remaining aqueous phase after neutralization of free acid with ammonia or calcium oxide (calcium carbonate) and drying can be used as a fertilizer, since P2O5 (20-25%) contained in it is completely soluble in ammonium citrate.
Thus, it is shown that extraction of phosphoric acid from solutions obtained at sulfuric acid decomposition of Karatau phosphorites with n-butanol on the principle of counterflow with subsequent azeotropic volatilization of solvent allows to obtain highly concentrated (56-63% of P2O5) phosphoric acid.
With increasing temperature, the total weight loss increases for all mixtures almost equally. With increasing the amount of SO3 in the mixture, the total loss decreases, but insignificantly, reaching 12.33% for mixture V, 9.87-10.27% for mixtures II-IV. From the data of chemical analysis it follows that in the temperature range 100-2500C transition of orthoform to pyroform is not observed, starting from 3000C in all mixtures along with orthoform appears pyroform, the amount of which with increasing SO3 content in the mixture decreases. Thus, for mixture I at 40.96% SO3 pyroform is 10.60%; mixture IV at 57.94% SO3 contains 0.87% pyroform.
The content of SO3 in the mixtures depending on the heating temperature in the range from 100 to 2500C almost does not change, only at 3000C a slight loss of SO3 is observed. In the specified temperature range there is no loss of P2O5.
Thus, as shown by chemical analysis of initial mixtures and heating products, dehydration of phosphoric acid in the presence of sulfuric acid occurs differently than pure. If at dehydration of orthophosphoric acid [
36], starting from 220
0C and above, there is a loss of P
2O
5 and the formation of polyphosphoric acids, at the same temperature for phosphoric acid in the presence of sulfuric acid at the above content of SO
3 loss of P
2O
5 is not observed, and at a temperature of 300
0C appears only pyroform, the content of which decreases with increasing the amount of SO
3.
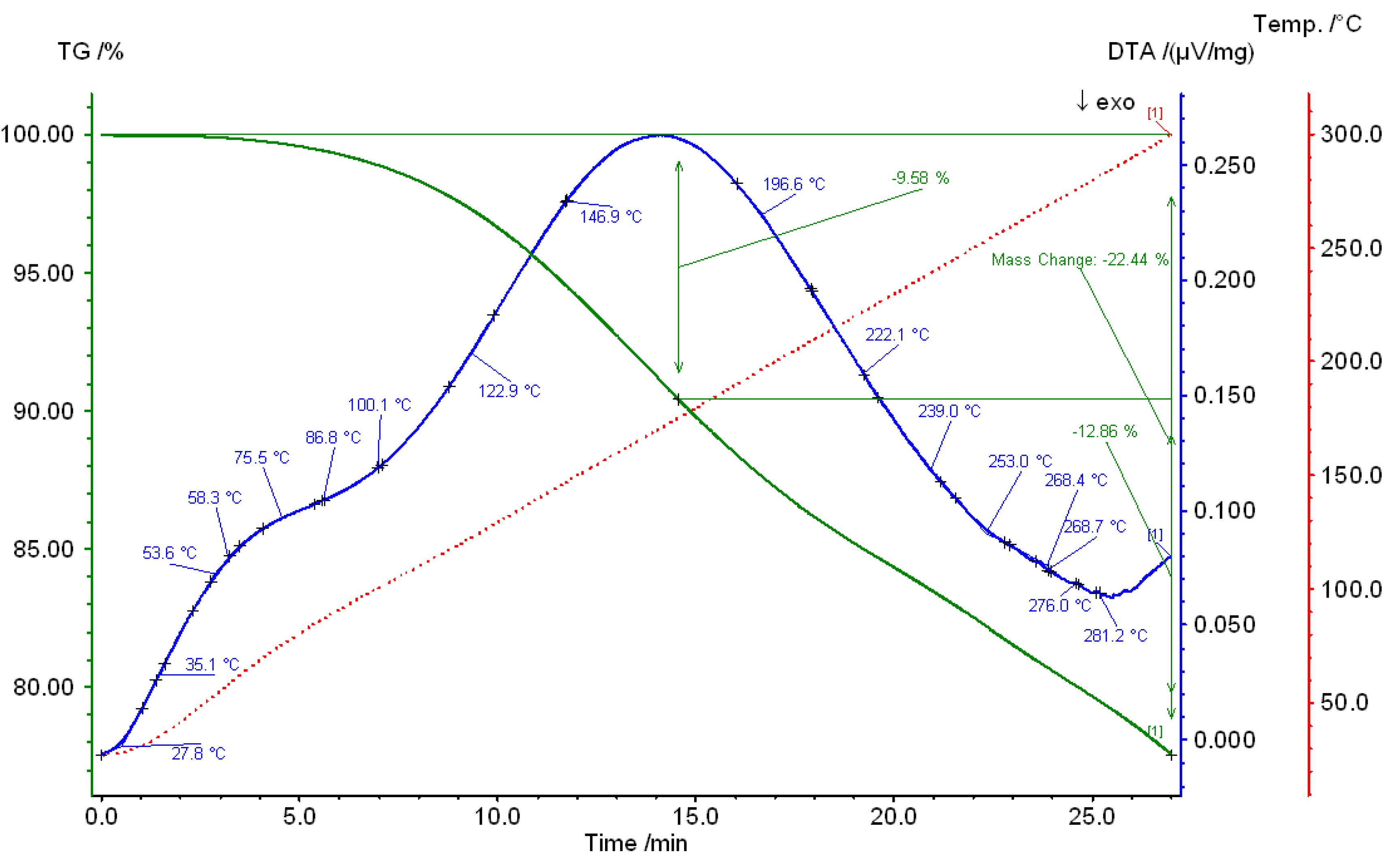
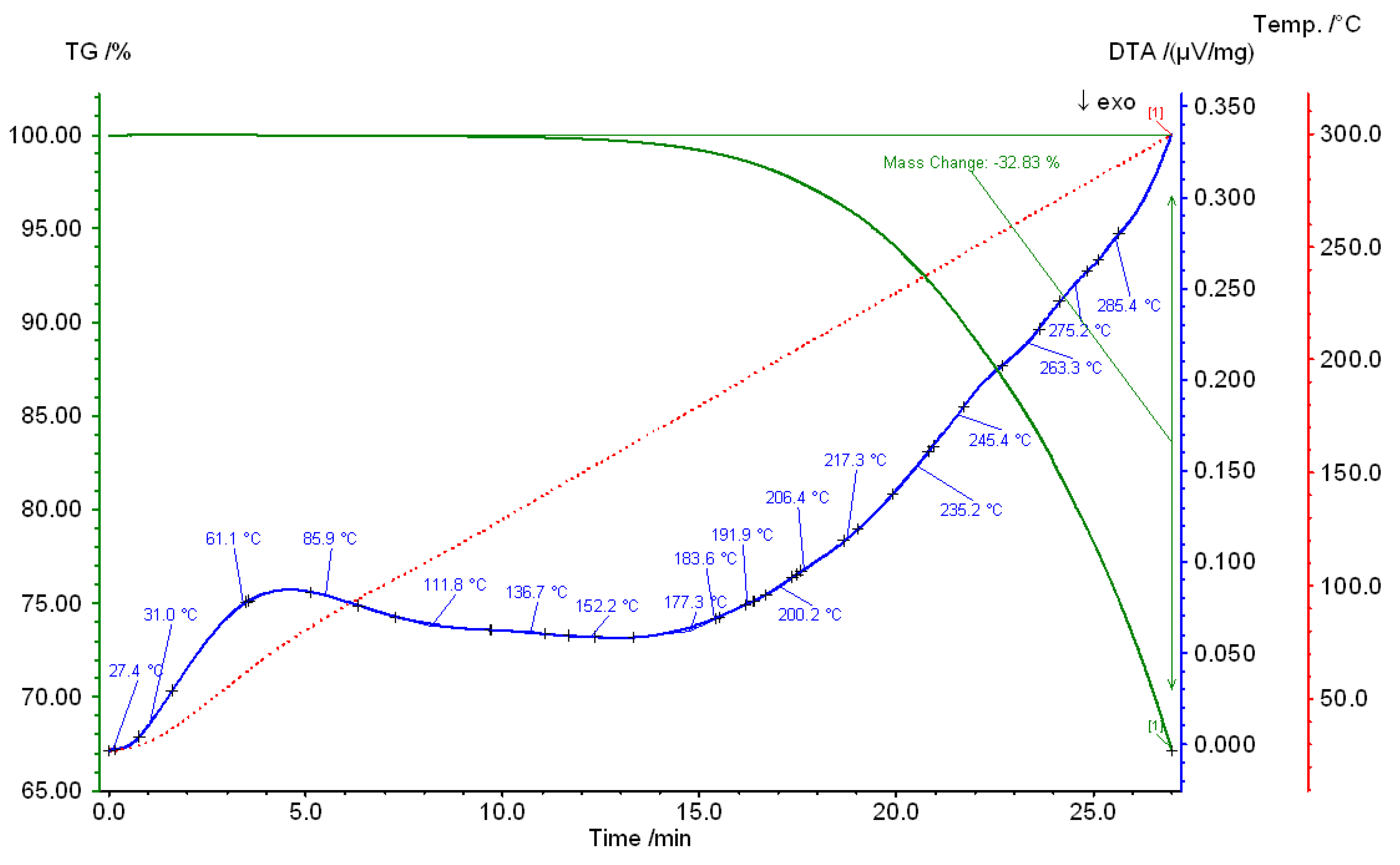
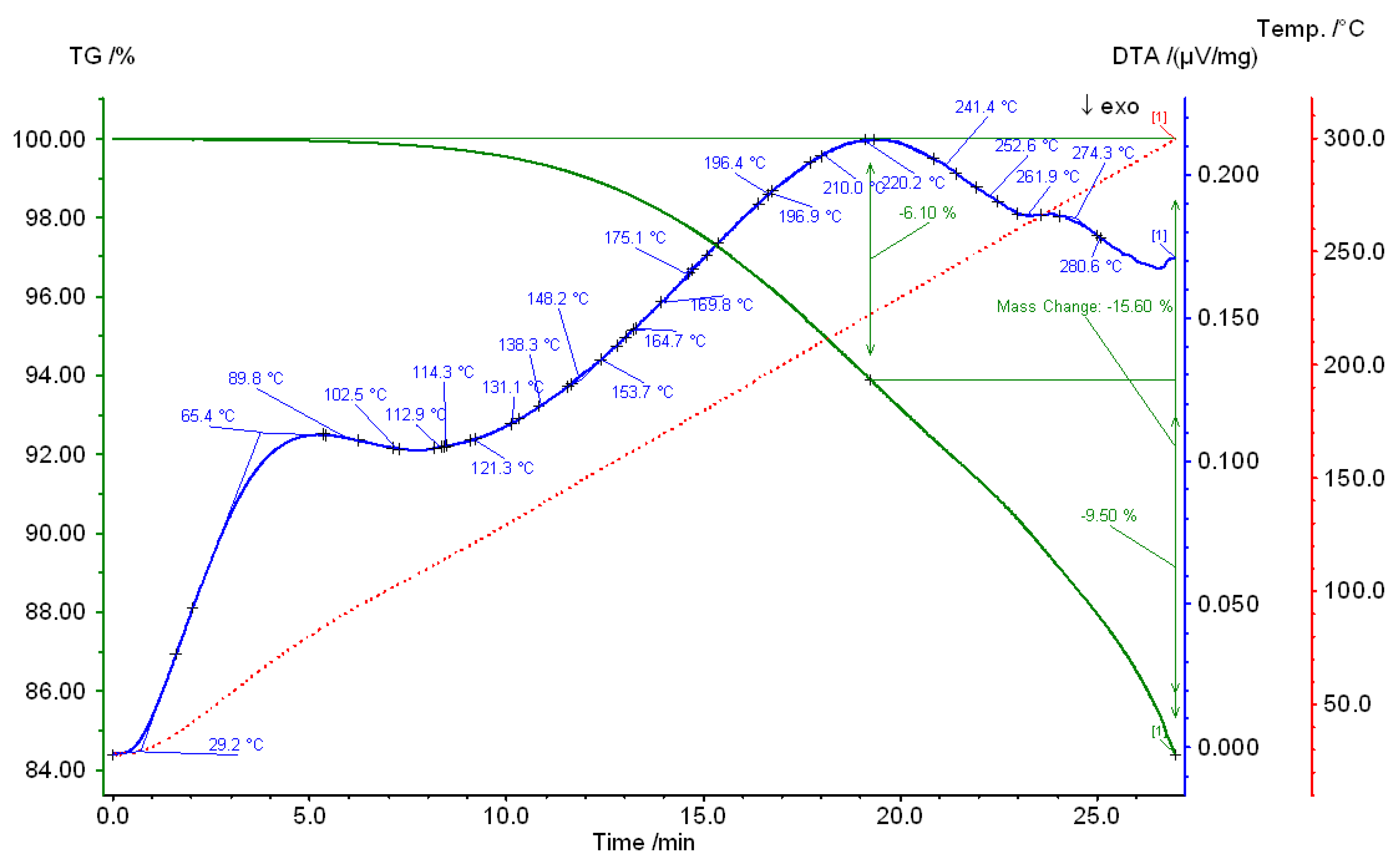
Heating of pure phosphoric and sulfuric acids is accompanied by significant endothermic effects, shifting towards higher temperatures with increasing acid concentration [
37]. The results of thermographic analysis of pure phosphoric and sulfuric acids and their mixtures for 4 hours are presented in
Figure 2,
Figure 3,
Figure 4, Figure 5 and Figure 6.
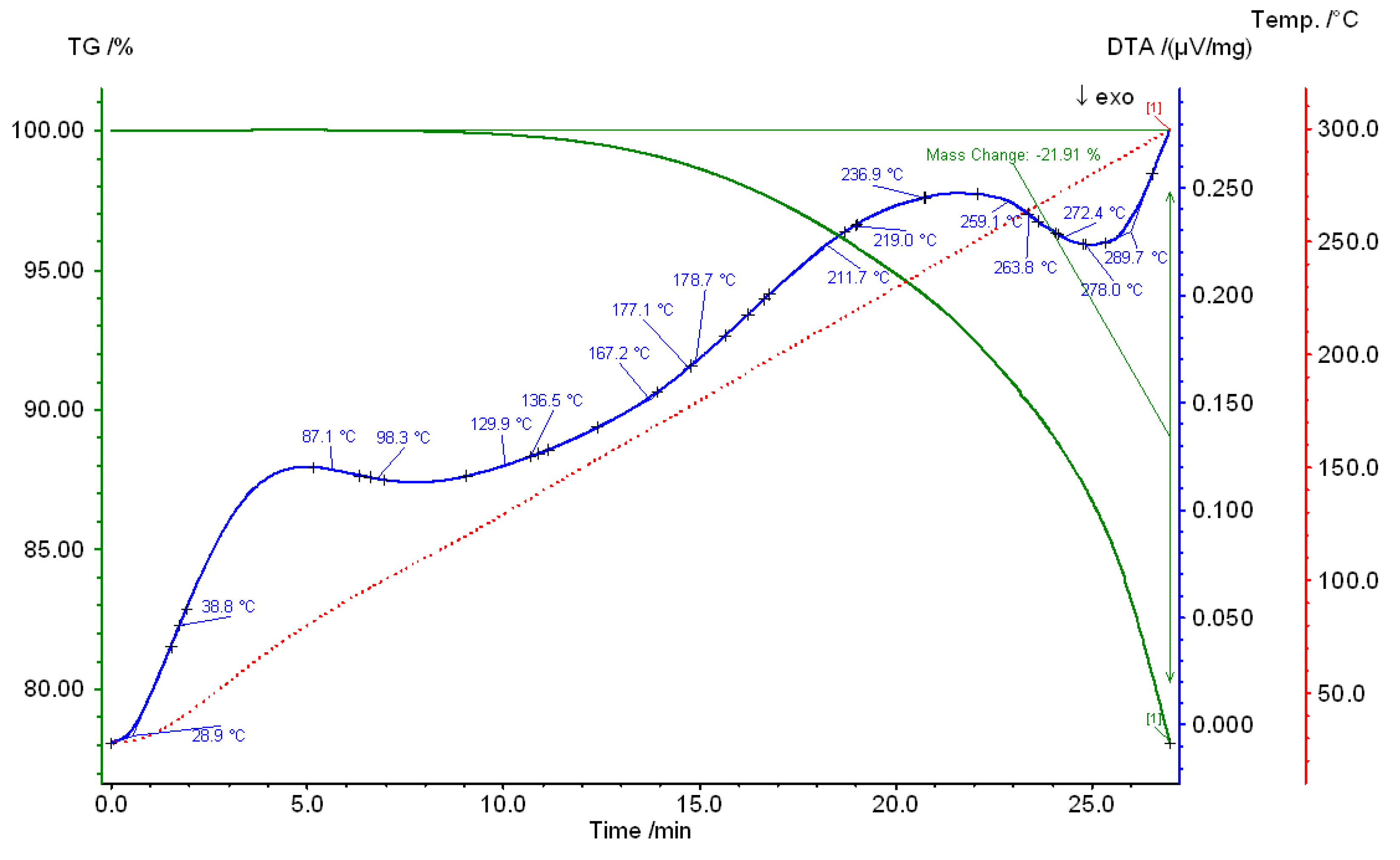
Figure 2.
Thermogram of pure phosphoric acid.
Figure 2.
Thermogram of pure phosphoric acid.
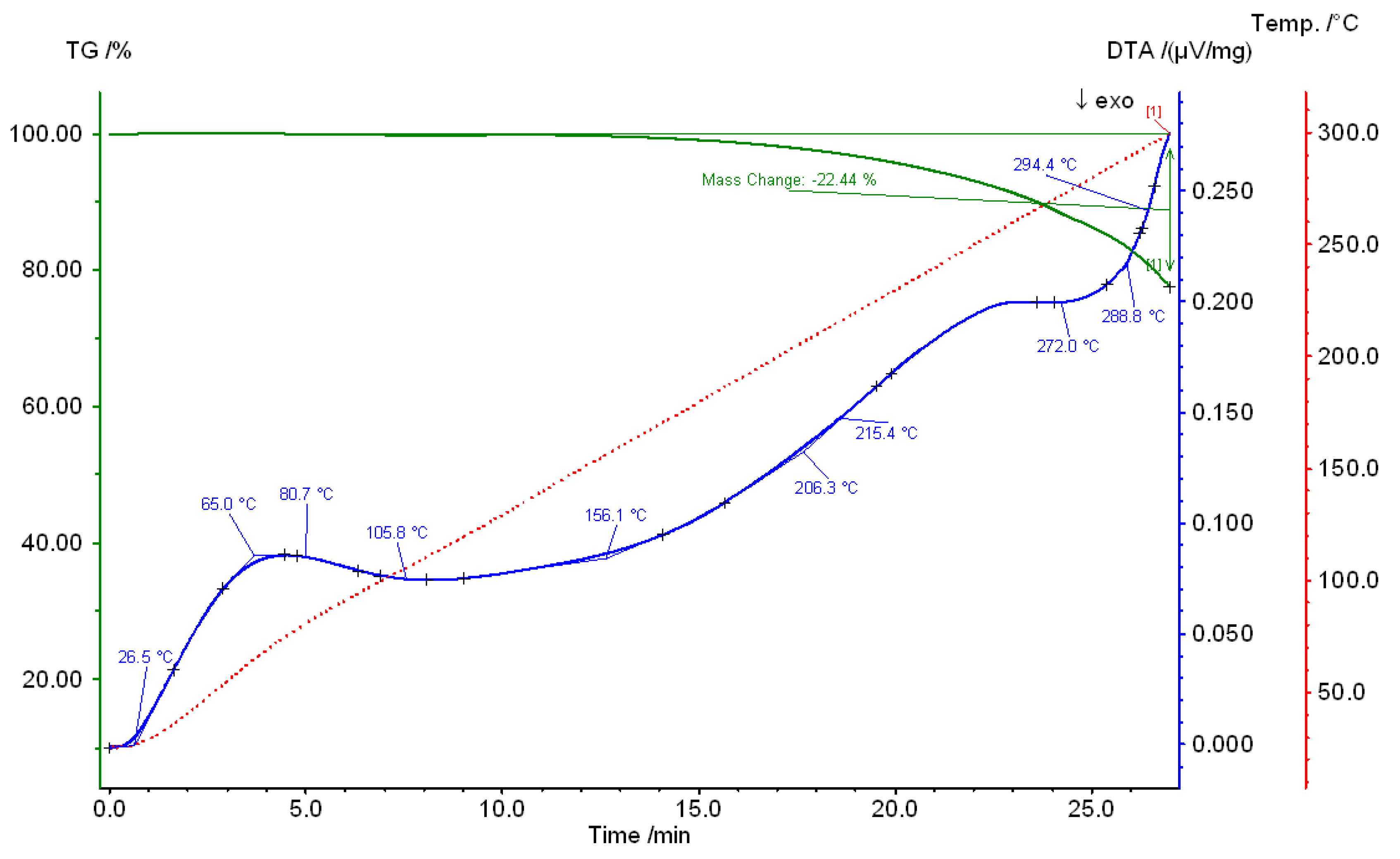
Figure 3.
Thermogram of pure sulfuric acid.
Figure 3.
Thermogram of pure sulfuric acid.
Figure 4.
Thermogram of a mixture of phosphoric and sulfuric acid in the ratio P2O5:SO3 = 1:1.
Figure 4.
Thermogram of a mixture of phosphoric and sulfuric acid in the ratio P2O5:SO3 = 1:1.
Figure 5.
Thermogram of a mixture of phosphoric and sulfuric acid in the ratio P2O5:SO3 = 1: 2,3.
Figure 5.
Thermogram of a mixture of phosphoric and sulfuric acid in the ratio P2O5:SO3 = 1: 2,3.
Figure 5.
Thermogram of a mixture of phosphoric and sulfuric acid in the ratio P2O5:SO3 = 1: 4.4.
Figure 5.
Thermogram of a mixture of phosphoric and sulfuric acid in the ratio P2O5:SO3 = 1: 4.4.
Thermograms of all studied mixtures are characterized by two endothermic effects corresponding, apparently, to the boiling points of phosphoric and sulfuric acids. Thus, the boiling point of 100% H3PO4 is 2610C; that of 98.3% H2SO4 is 3380C. It should be noted that the effect corresponding to the boiling point of phosphoric acid shifts toward higher temperatures with increasing sulfuric acid in the mixture. For example, for mixture I (36.79% SO3) this effect is noted at 2400C, and for mixture IV (55.90% SO3) - 2800C. The same can be said about the character of the effect corresponding to the boiling point of sulfuric acid, which shifts towards higher temperatures with increasing P2O5 in the mixture: for mixture I (38.34% P2O5) - 3750C, and for mixture IV (22.20% P2O5) - 3500C.
In the next series of experiments, the dehydration of phosphoric acid in the presence of sulfuric acid with ratios P2O5 : SO3 = 1:1 : 1.7 as a function of heating duration at 2500C was carried out.
The results of experiments showed that the dehydration of phosphoric acid is affected by the duration of heating. Thus, at 4 hours heating phosphoric acid is only in orthoform, at 10 hours heating the SO3 content increases. Finally, at 150-160 hours heating, high-molecular polyforms appear, with the amount of P2O5 total 78.84-80.00% and SO3 2.35-2.30%. In these dehydrated products at long storage (1-2 months) in the desiccator over P2O5 precipitates in the form of thin, long, crossed into druse needles. Acids of composition 78,84% P2O5 and 2,30% SO3 are crystallized completely under mechanical exposure with a glass rod.
The total weight loss depends on the heating time and SO3 content of the mixtures. At 15 hours heating the weight loss is equal to 50.6%. With further heating up to 150-160 hours the value of weight loss changes very little and is only: 0.76%. With increasing duration of heating along with the loss of SO3 (95-97%) there is a loss of P2O5 (4,59-11,65%). When SO3 is removed from the mixture, the observed total losses are small and the condensation process is very slow as pure phosphoric acid. The heating temperature of the mixture was raised to 4000C to increase the dehydration duration. Experiments were carried out with mixtures with 1:1.7 and 1:4.4 ratios, which were heated until SO3 release ceased (3 hours). The results of chemical analysis show that SO3 is completely absent in the products, the total content of P2O5 is 86.88-87.41% and does not depend on the initial composition of the mixture. The content of polyforms in the products is equal to 83-89.3% of the total amount of phosphorus pentaoxide.
The dehydrated products indicate the presence of all condensed forms of phosphoric acid and correspond to those for the dehydration product of orthophosphoric acid.
Thus, the dehydration of phosphoric acid in the presence of sulfuric acid depends both on the temperature and duration of the process and on the amount of sulfuric acid in the mixture.
4. Conclusions
The results on the distribution of phosphoric acid depending on the initial concentration and temperature showed that with an increase in the initial concentration of phosphoric acid from 5.38 to 25.61% P2O5, the distribution coefficient at 25°C increases. An increase in temperature from 25 to 60°C has almost no effect on the distribution coefficient;
The distribution coefficient of sulfuric acid, depending on its initial concentration, increases for n-butyl and isoamyl alcohols;
The presence of magnesium sulfate in phosphoric acid in amounts up to 1.05% MgO has almost no effect on the distribution coefficient of phosphoric acid, and the presence of magnesium monophosphate reduces it almost twice;
Phosphoric acid is extracted better with n-butanol. As a result of azeotropic sublimation of the solvent, phosphoric acid is obtained relatively pure, with a high content of phosphorus pentaoxide (56-63%);
Dehydration of phosphoric acid in the presence of sulfuric acid has been studied depending on the amount of the latter in the mixture, on the temperature and duration of the dehydration process;
It is established that in the presence of sulfuric acid, when heated to a temperature of 2500C, no polyforms of phosphoric acid are formed and no loss of P2O5 and SO3 occurs. At 3000C, no polyform formation is observed, and its amount decreases with increasing SO3 in the mixture. At 4000C, there is complete removal of SO3
Prolonged heating (160-200 hours) of mixtures of phosphoric and sulfuric acids at 2500C leads to almost complete removal of SO3 (95-97%) and the formation of polyphosphoric acids.
Author Contributions
Conceptualization, S.O.A., S.O.A.*; methodology, S.O.A.*; software, S.O.A., A.S.B.; validation, S.O.A., S.O.A.*, M.Sh.S. L.M.K. and G.K.K.; formal analysis, S.O.A.*, A.S.B.; investigation, S.O.A., S.O.A.*, L.M.K., M.Sh.S. and G.K.K.; resources, S.O.A., S.O.A.* and A.S.B.; data curation, L.M.K., S.O.A.*, A.S.B.; writing-original draft preparation, S.O.A.* and S.O.A.; writing-review and editing, S.O.A.* and M.Sh.S., A.S.B.; visualization, G.K.K.; supervision, S.O.A. and S.O.A.*; project administration, S.O.A. and S.O.A.*. All authors have read and agreed to the published version of the manuscript.
Conflict of interests
The authors declare that there is no conflict of interest.
The research profile of the authors can be verified from their ORCID ids. given below:
References
- U. Ryszko, P. Rusek, D. Kołodyńska, Quality of Phosphate Rocks from Various Deposits Used in Wet Phosphoric Acid and P-Fertilizer Production, Materials, 16 (2023) 2. 793. [CrossRef]
- S.V. Vakal, E. Karpovich, H, Turgumbaeva, Investigation of the process of concentration of phosphoric acid obtained from Karatau phosphorite, Chemical Journal of Kazakhstan, 1 (2013) 104-109. [in Russian].
- B. M. Rakishev, The role and prospects of mineral resources in the development of the economy of Kazakhstan, News of the National Academy of Sciences of the Republic of Kazakhstan, Series of Geology and Technical Sciences, 2 (2016) 29-39. [in Russian].
- B. Numonov et al. Low-waste process of complex fertilizer based on sulphuric acid processing thermic calcinated phosphorite concentrate. Journal of Chemical Technology & Metallurgy, 4 (2020) 55. 4.
- D. Guelfi, A.P.P.Nunes, L.F.Sarkis, D.P. Oliveira, Innovative Phosphate Fertilizer Technologies to Improve Phosphorus Use Efficiency in Agriculture, Sustainability, 14 (2022) 14266. [CrossRef]
- M.A. de Boer, L. Wolzak, J.C. Slootweg, Phosphorus: Reserves, Production, and Applications. In: Ohtake, H., Tsuneda, S. (eds) Phosphorus Recovery and Recycling. Springer, Singapore, (2019). [CrossRef]
- S. Matta, K. Stephan, J. Stephan, R. Lteif, C. Goutaudier, J. Saab, Phosphoric acid production by attacking phosphate rock with recycled hexafluosilicic acid, International Journal of Mineral Processing, 161 (2017) 21-27, ISSN 0301-7516, . [CrossRef]
- Ibtissem Boumnijel, Hedi Ben Amor, Cheker Chtara, Effect of calcinated and activated perlite on improving efficiency of dihydrate process for phosphoric acid, International Journal of Mineral Processing, 125 (2013) 112-117. [CrossRef]
- M. Schorr et al. Phosphate ore processing for phosphoric acid production: classical and novel technology, Mineral Processing and Extractive Metallurgy, 119 (2010) 3, 125-129. [CrossRef]
- A. Mizane, A. Boumerah, N. Dadda, R. Rehamnia, S.Belhait, Obtaining the partially acidulated phosphate rocks by means of intermediate-grade phosphate and diluted phosphoric acid: Influence of some parameters, Polish Journal of Chemical Technology, 18 (2016) 39-43. [CrossRef]
- N.S. Awwad, Y.A. El-Nadi, M.M. Hamed, Successive processes for purification and extraction of phosphoric acid produced by wet process, Chemical Engineering and Processing: Process Intensification, 74 (2013) 69-74. [CrossRef]
- G. Kodirova, I. Shamshidinov, B. Sultonov, R. Najmiddinov, B. Mamurov, Investigation of the Process of Purification of Wet-Process Phosphoric Acid and Production of Concentrated Phosphoric Fertilizers Based on it. Chemical Science International Journal, 30(1) (2021) 1-10. [CrossRef]
- N.I. Khurramov, T.I. Nurmurodov, A.U. Erkaev, Investigation of the process of extraction phosphoric acid production from washed dried phosphate rock, Universum: technical sciences: electron. nauchn. zhurn. 2 (2021) 83. [in Russian]. [CrossRef]
- E.D. Pluzhnikova, R.R. Yakubova, M.M. Eskendirova, Study of sulfuric acid decomposition of phosphorite of Zhanatas deposit, Bulletin of Science of South Kazakhstan, 2 (2020) 134-138. [in Russian].
- M. Daryani, N. Jodeiri, E. Fatehifar, Shahbazi, J. Optimization of operating conditions in purification of wet process phosphoric acid in a liquid-liquid extraction column, Chemical Engineering Communications, 209(8) (2021). 1082–1095. [CrossRef]
- B. Wang, Q. Zhou, C. Chen, H. Liu, L. Yang, Separation of phosphoric acid and magnesium from wet process phosphoric acid by solvent extraction. Canadian Metallurgical Quarterly, 62(4) (2022) 791–802. [CrossRef]
- S. Zhang, Y. Chen, T. Zhang, L. Lv, D. Zheng, B. Zhong, S. Tang, Separation of H3PO4 from HCl-wet-processing phosphate rocks leach liquor by TBP, Extraction equilibria and mechanism study, Separation and Purification Technology, 249 (2020) 117156, . [CrossRef]
- L. Xinxin, F. Wu, G. Qu, C. Jin, Y, Liu, L. Kuang, H. Li, X. Chen, Z. Wang, Y. Cheng, Application prospect of advanced oxidation technology in wet process phosphoric acid production, Journal of Environmental Chemical Engineering, 10 (2022) 6, 108868, . [CrossRef]
- Y. Jin et al., Extraction Kinetics of Phosphoric Acid from the Phosphoric Acid Calcium Chloride Solution by Tri-n-butyl Phosphate, Industrial & Engineering Chemistry Research, 54, 1 (2015) 108-116. [CrossRef]
- U.K. Alimov, A.M. Reimov, S.S. Namazov, et al. The insoluble part of phosphorus fertilizers, obtained by processing of phosphorites of central Kyzylkum with partially ammoniated extraction phosphoric acid, Russ J. Appl. Chem, 83, (2010). 545–552 . [CrossRef]
- M. Lassis, A. Mizane, N. Dadda, R. Rehamnia, Dissolution of Djebel Onk phosphate ore using sulfuric acid, Environmental Nanotechnology, Monitoring & Management, 4 (2015) 12-16, . [CrossRef]
- R. Gilmour, Phosphoric Acid. Purification, Uses, Technology and Econimics, CRC Press, USA. (2013). [CrossRef]
- A.S. Egorkin et al. Mathematical modeling of the half-hydrate stage of the dihydrate-semihydrate process of extraction phosphoric acid production from phosphate rock, Fundamental Research, 6 (2013) 33-37. [in Russian].
- Samir, Abu-Eishah, M. Nizar Abu-Jabal, Parametric study on the production of phosphoric acid by the dihydrate process, Chemical Engineering Journal, 81 (2001) 1–3, 231-250. [CrossRef]
- S. P. Kochetkov, N. N. Smirnov, A. P. Ilyin, V. M. Lembrikov, S.V. Khromov, Methods for activating the processes of dehydration and defluorination of extraction phosphoric acid. News of higher educational institutions, Chemistry and Chemical Technology, 50 (5) (2007) 41-47. [in Russian].
- R. Kijkowska, D. Pawlowska-Kozinska, Z. Kowalski, M. Jodko, Z. Wzorek, Wet-process phosphoric acid obtained from Kola apatite. Purification from sulphates, fluorine, and metals, Separation and Purification Technology, 28, 3 (2002) 197-205, . [CrossRef]
- H. Li, W. Ge, J. Zhang, R. M. Kasomo, J. Leng, X. Weng, Q. Chen, Q. Gao, S. Song, L. Xiao, C. Tian, Control foaming performance of phosphate rocks used for wet-process of phosphoric acid production by phosphoric acid, Hydrometallurgy, 195 (2020) 105364, . [CrossRef]
- Device and process used for restoring fluorine from smoke after phosphorus absorption by hydration in burning process in furnace for producing phosphoric acid. МПК B01D 53/68 №201514455.5 Hou Yonghe (CN), Wei Shifa (CN), Wei Chenjuan (CN)19.12.2017.
- Magda, A. et al., Liquid-liquid extraction technique for purification of Egyptian WET process phosphoric acid, Periodica Polytechnica Chemical Engineering, 54, 2 (2010.0), 57–62. [CrossRef]
- M.I. Amin, M.M. Ali, H.M. Kamal, A.M. Youssef, M.A. Akl, Recovery of high grade phosphoric acid from wet process acid by solvent extraction with aliphatic alcohols, Hydrometallurgy, 105, 1–2, (2010) 115-119, . [CrossRef]
- M. Chen, J. Li, Y. Jin, J. Luo, X. Zhu, D. Yu, Efficient solvent extraction of phosphoric acid with dibutyl sulfoxide, J. Chem. Technol. Biotechnol, 93 (2018) 467-475. [CrossRef]
- H. Ghanadzadeh, A. Ghanadzadeh, Z. Aghajani, S. Abbasnejad, S. Shekarsaraee, (Liquid+liquid) equilibria in ternary aqueous mixtures of phosphoric acid with organic solvents at T=298.2K, The Journal of Chemical Thermodynamics, 42, 6 (2010), 695-699, . [CrossRef]
- M.S. Mokhort et al. Investigation of the peculiarities of decofluorination of extraction phosphoric acid. Neftegazokhimiya, Proceedings of the V International Scientific and Technical Forum on Chemical Technologies and Oil and Gas Processing, Minsk, BGTU, (2022) 64-68. [in Russian].
- 34. M. Basha, Chromatography, In: Analytical Techniques in Biochemistry, Springer Protocols Handbooks. Humana, New York, NY. (2022). [CrossRef]
- C. Schick, D. Lexa, L. Leibowitz, Differential scanning calorimetry and differential thermal analysis, Characterization of materials. John Wiley & Sons Inc, New York, (2012) 483-495. [CrossRef]
- А. Cheremysinova, I. Sknar, Y. Kozlov, O. Sverdlikovska, O. Sigunov, Study of thermal dehydration of sodium orthophosphate monosubstituted. Sigunov, Study of thermal dehydration of sodium orthophosphate monosubstituted. East European Journal of Advanced Technology, 3 (6) (2017) 60-66. [in Russian]. [CrossRef]
- A.N. Strashko Thermal analysis: methodological instructions for laboratory works on the course "Physico-chemical methods of analysis" for students of IV year, studying in the direction 240501 "Chemical technology of materials of modern energy", Tomsk Polytechnic University, (2014) 16. [in Russian].
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).