Submitted:
06 July 2024
Posted:
08 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
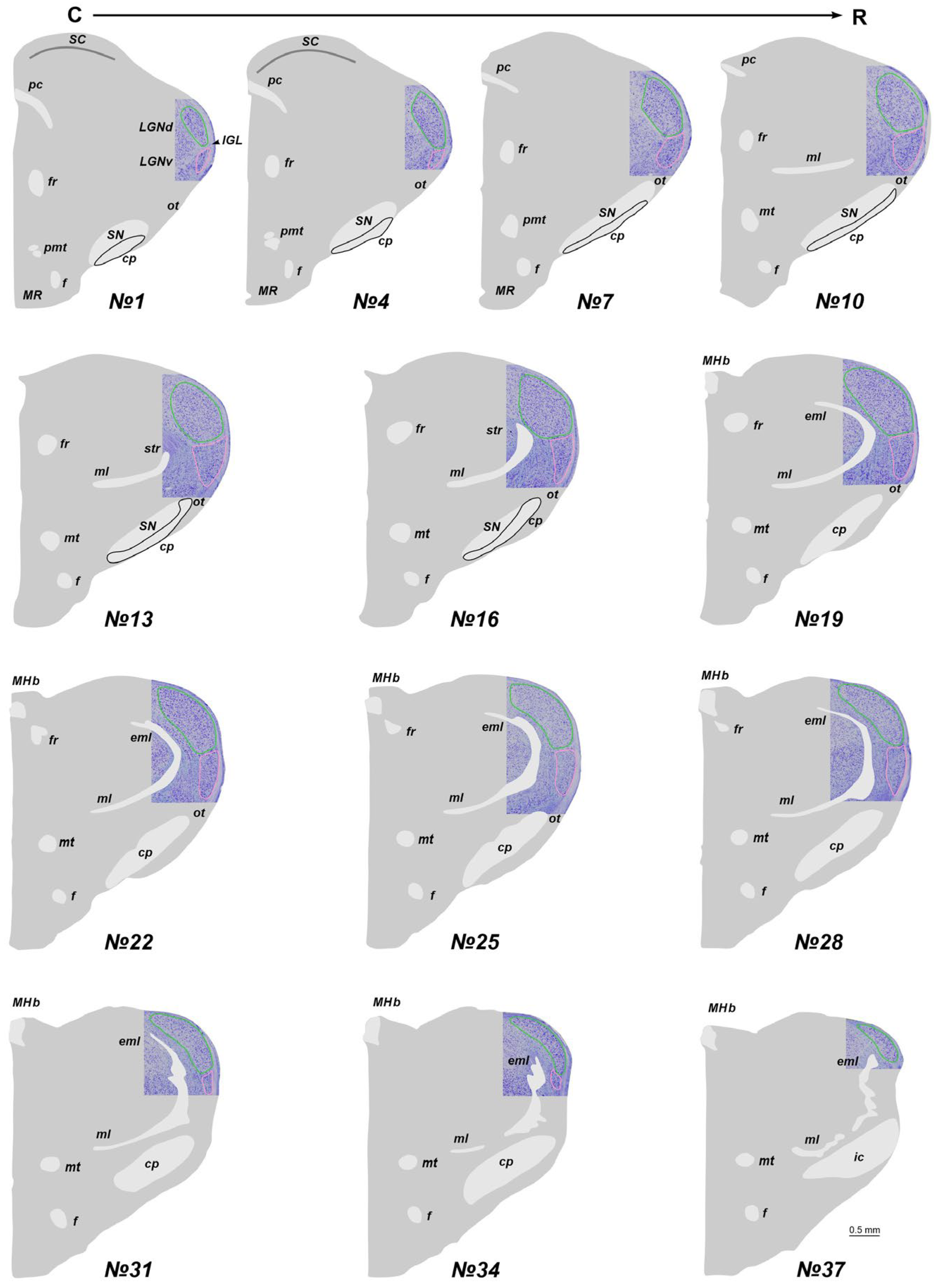
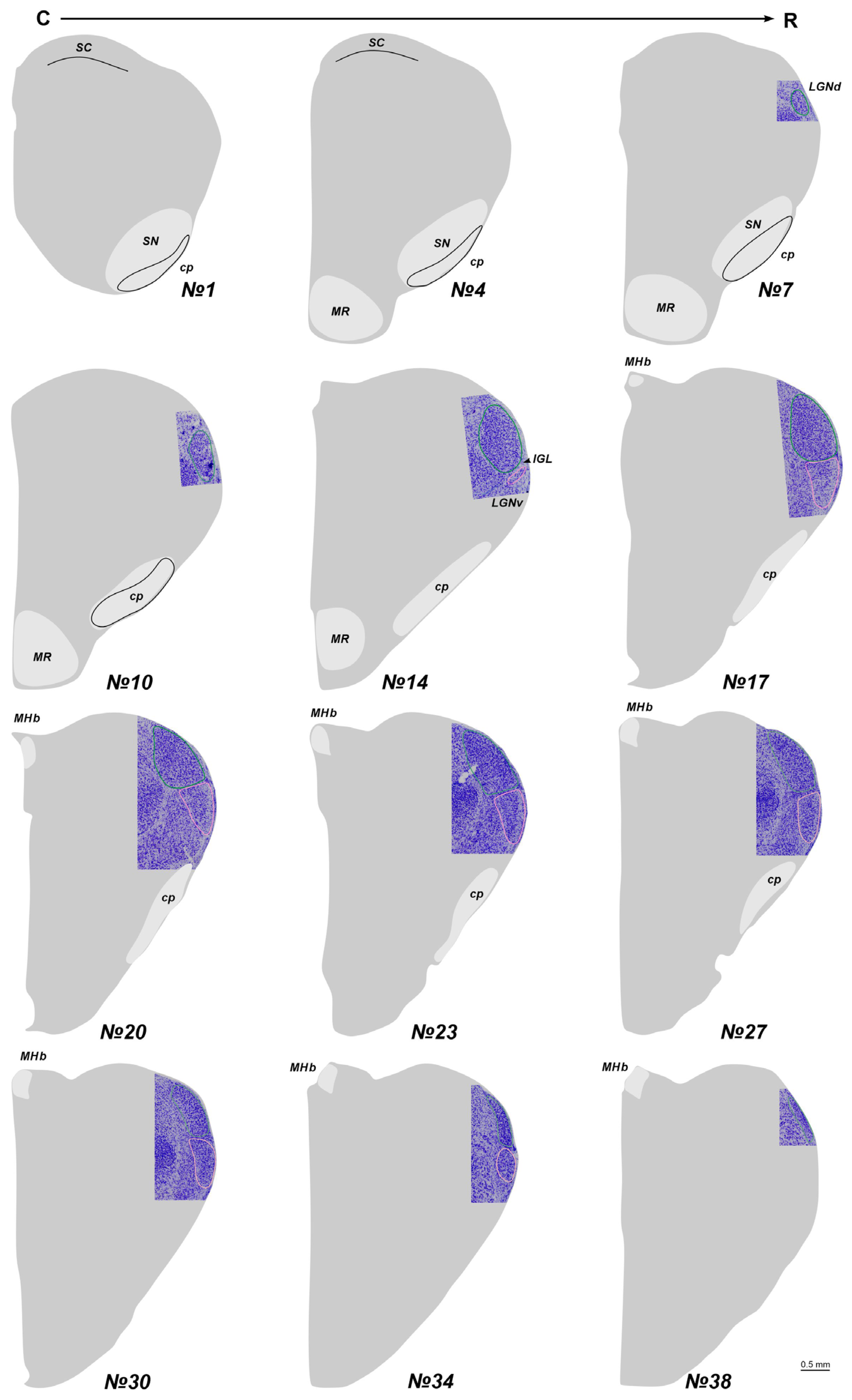
2.1. The Location of the LGN in Stereotaxic Levels
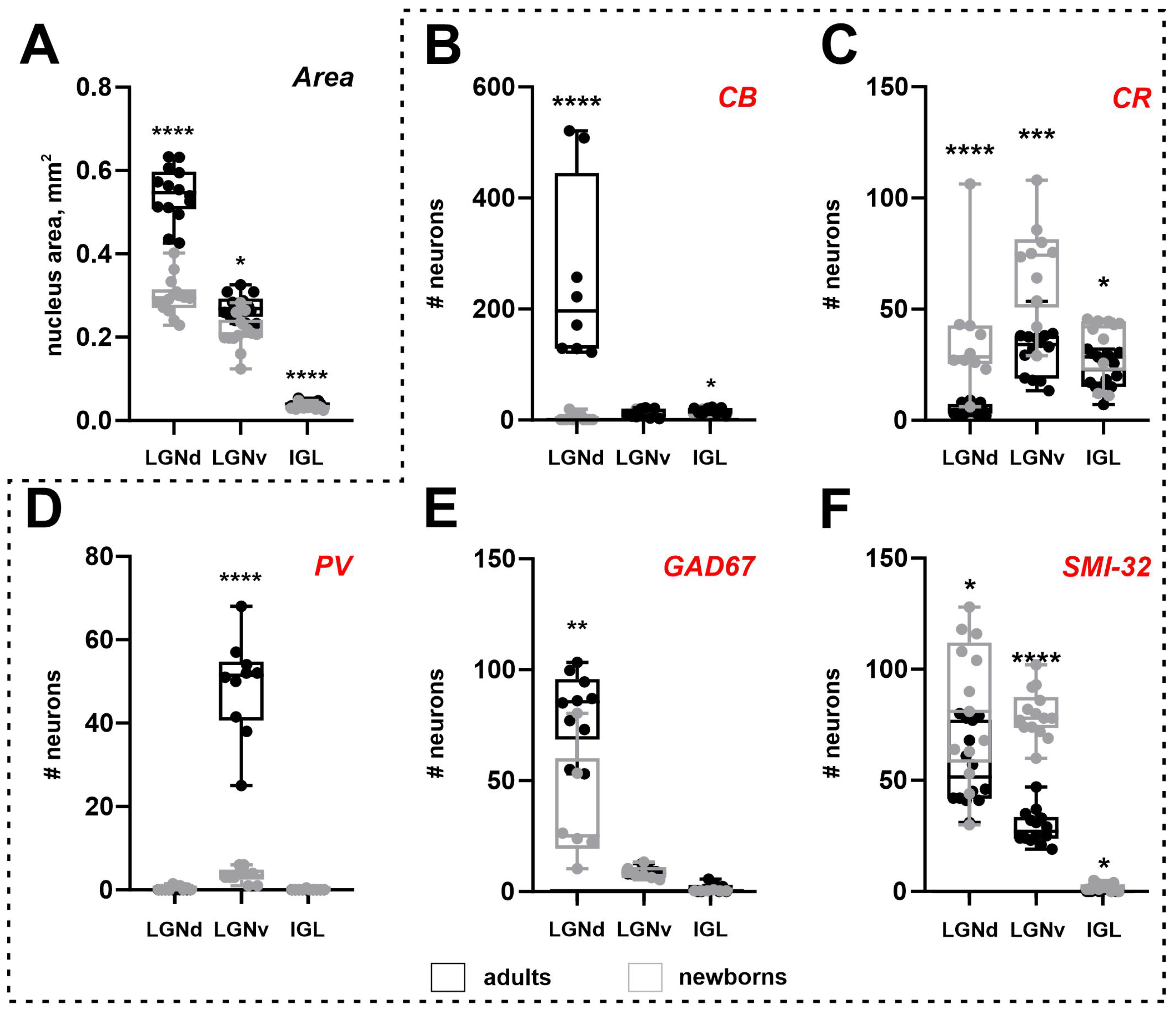
2.2. Area of the Lateral Geniculate Complex
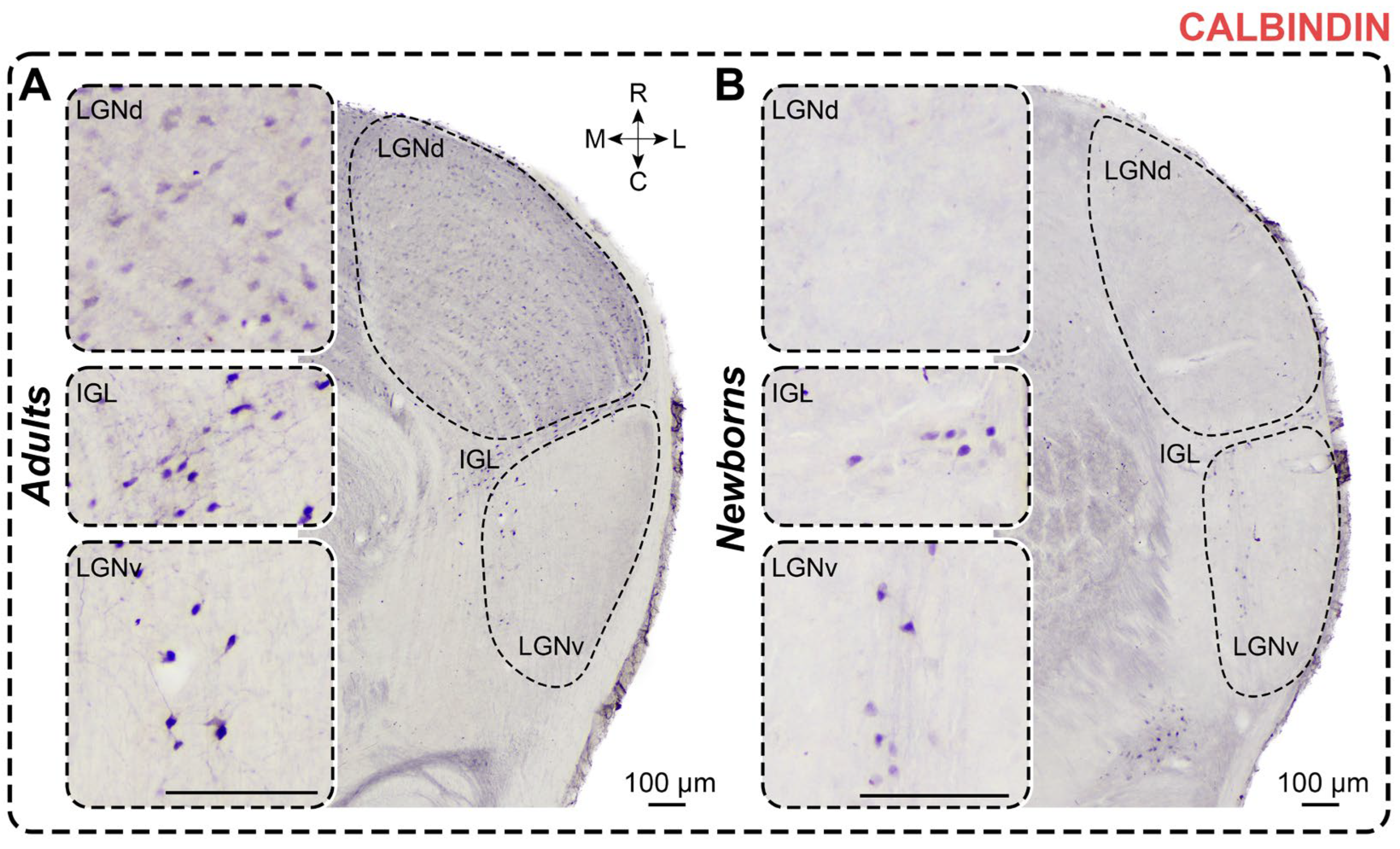
2.3. Calbindin Staining
2.3.1. Adults
2.3.2. Newborns
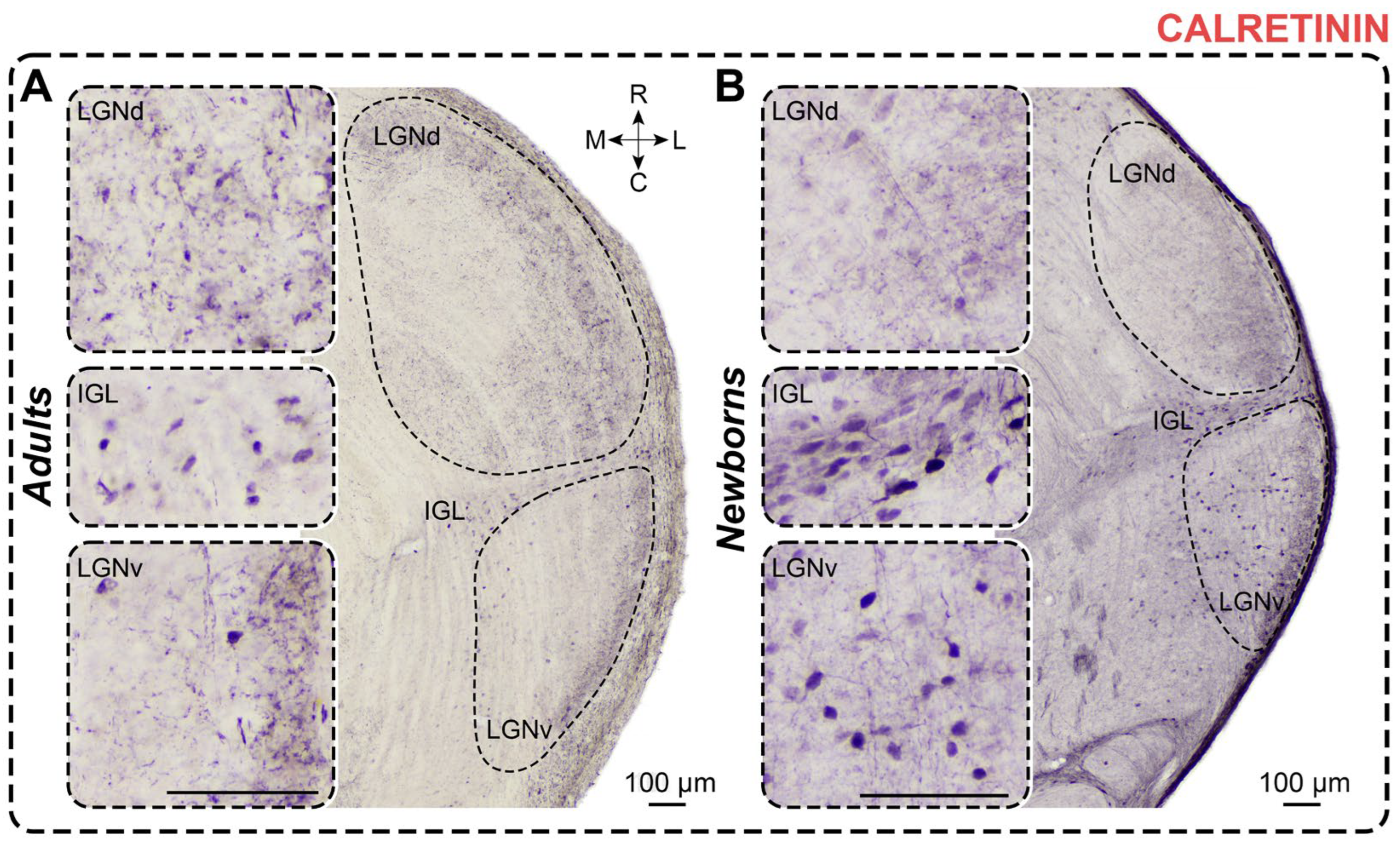
2.4. Calretinin Staining
2.4.1. Adults
2.4.2. Newborns
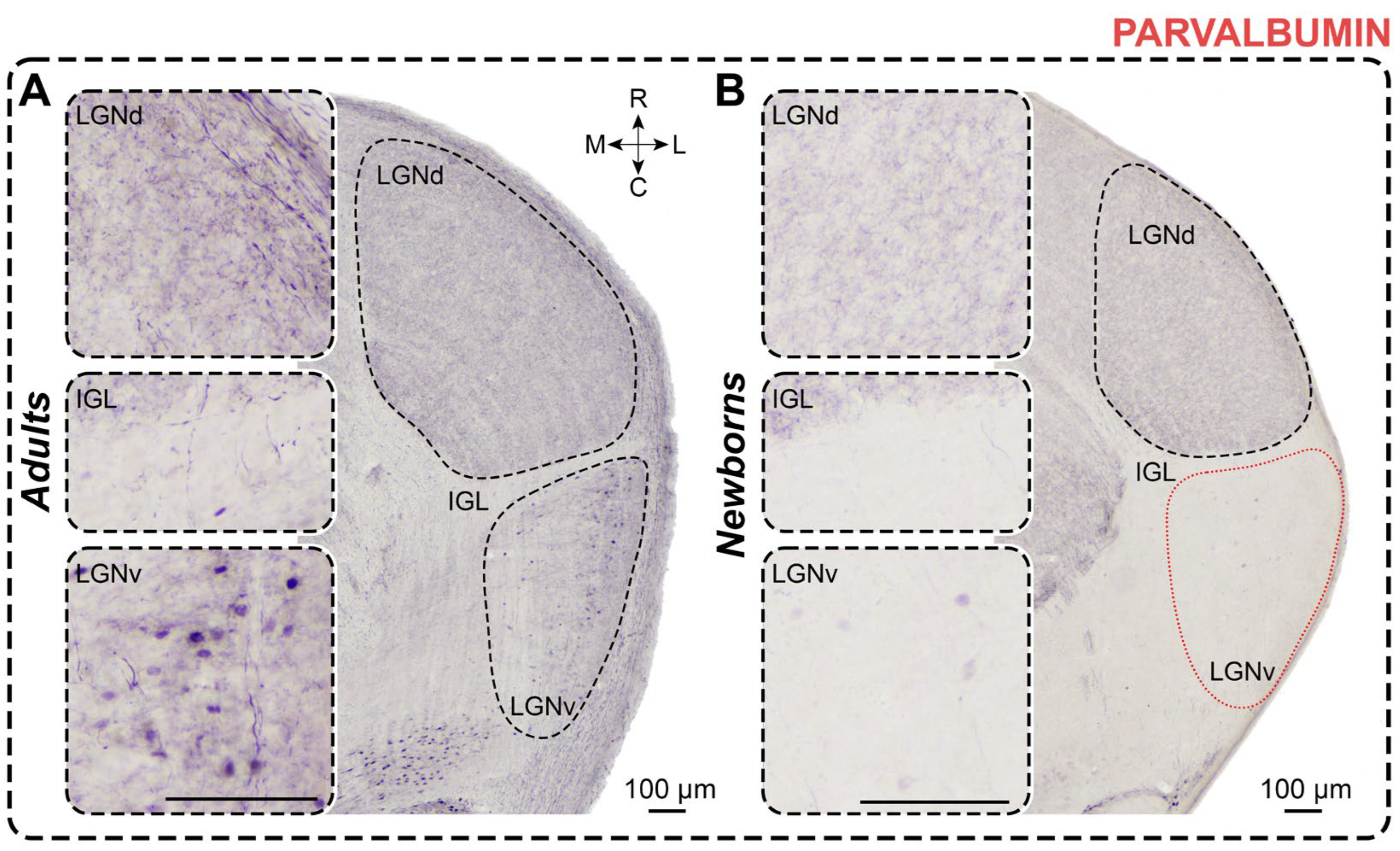
2.5. Parvalbumin Staining
2.5.1. Adults
2.5.2. Newborns
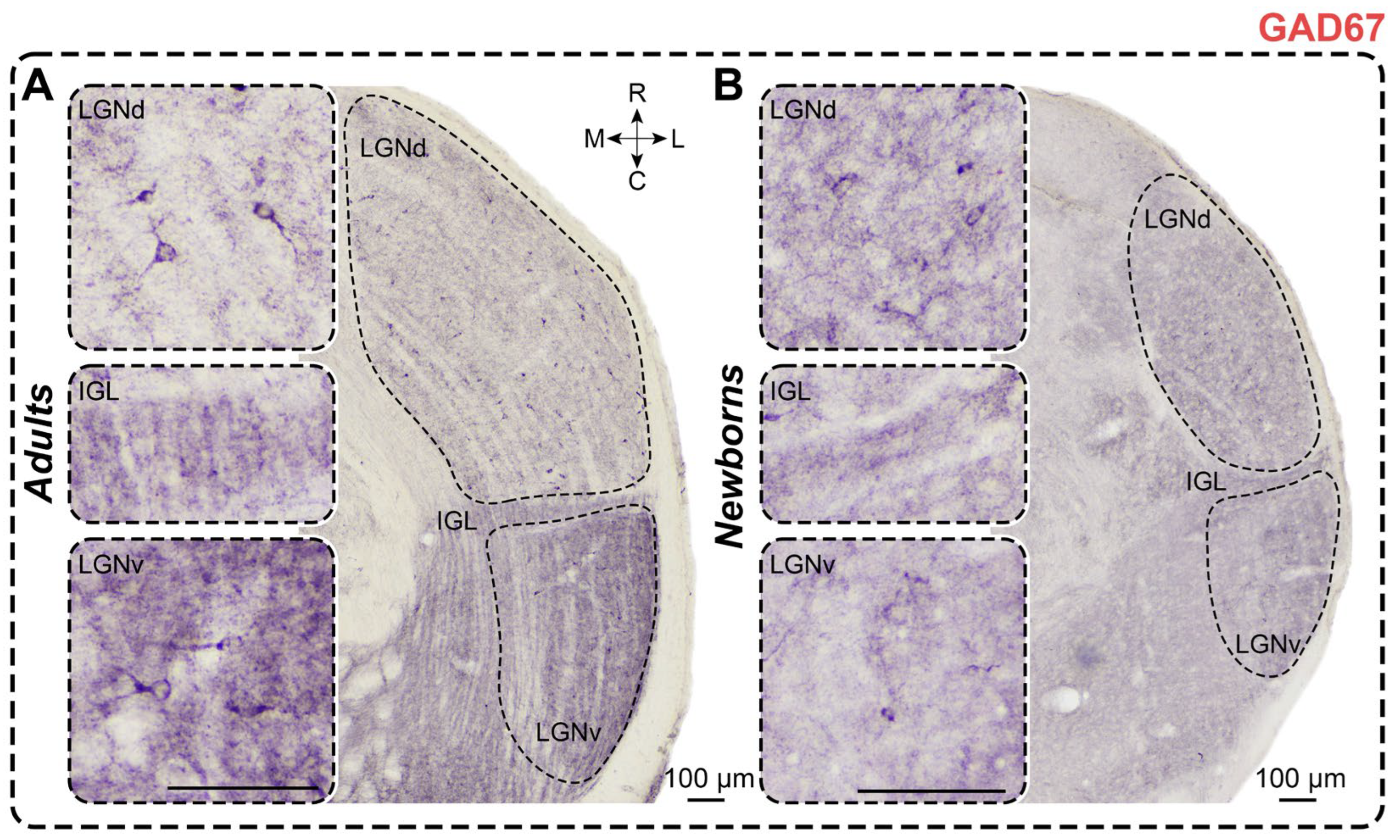
2.6. Glutamic Acid Decarboxylase Staining
2.6.1. Adults
2.6.2. Newborns
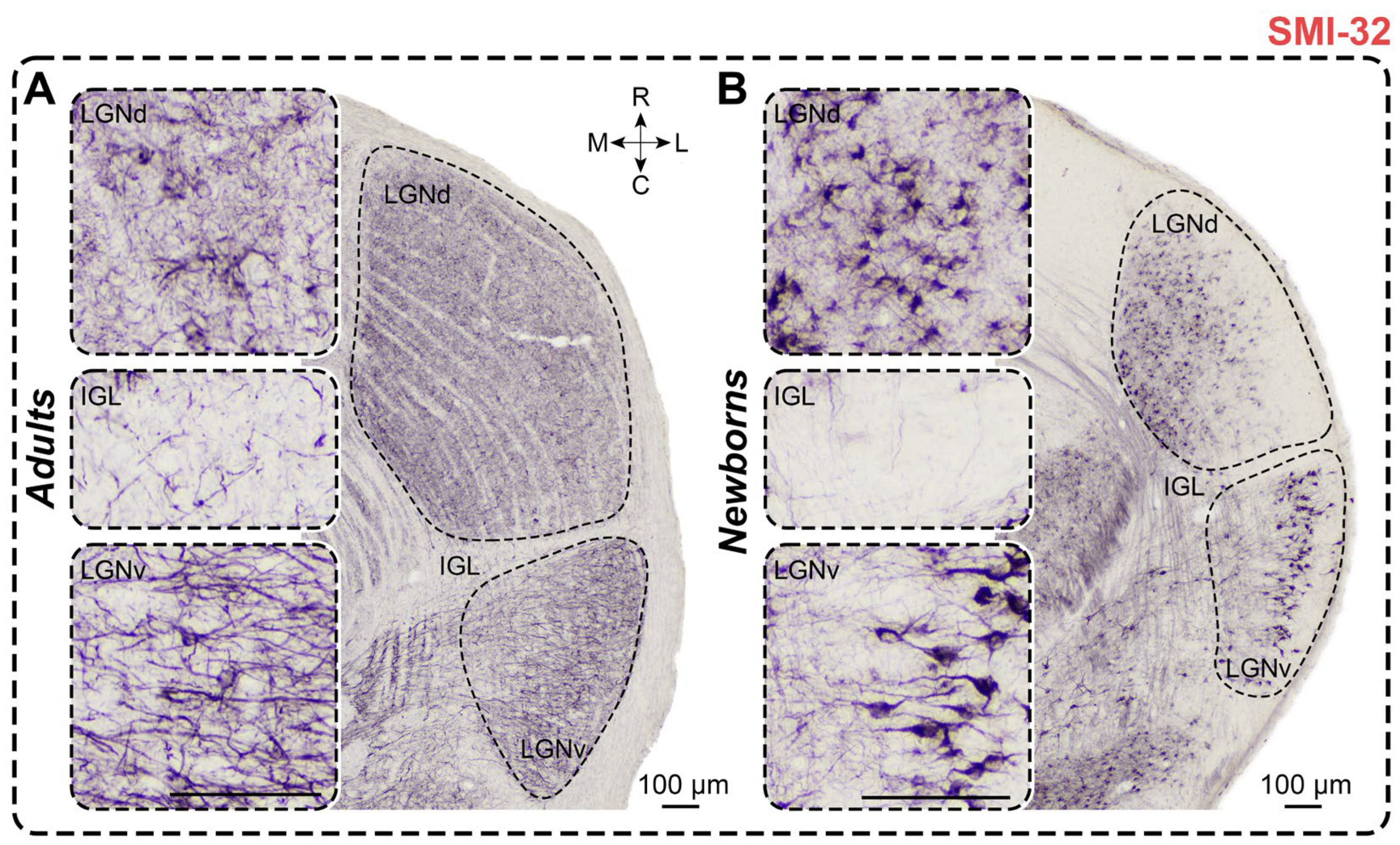
2.7. Non-Phosphorylated Heavy-Chain Neurofilament Staining
2.7.1. Adults
2.7.2. Newborns
2.8. Comparison of Different Staining
3. Discussion
3.1. Morphometry of the Lateral Geniculate Complex
3.2. Structural Division of the Lateral Geniculate Complex in Rodents
3.3. Calbindin Staining
3.4. Calretinin Staining
3.5. Parvalbumin Staining
3.6. GAD Staining
3.7. SMI-32 Staining
4. Materials and Methods
4.1. Subjects
4.2. Perfusion and Histological Slices Preparing
4.3. Immunohistochemistry
4.4. Image Processing
4.5. Statistics
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matias Santos, D.; Rita, A.M.; Casanellas, I.; Brito Ova, A.; Araújo, I.M.; Power, D.; Tiscornia, G. Ear Wound Regeneration in the African Spiny Mouse Acomys Cahirinus. Regeneration 2016, 3, 52–61. [CrossRef]
- Maden, M.; Brant, J.O.; Rubiano, A.; Sandoval, A.G.W.; Simmons, C.; Mitchell, R.; Collin-Hooper, H.; Jacobson, J.; Omairi, S.; Patel, K. Perfect Chronic Skeletal Muscle Regeneration in Adult Spiny Mice, Acomys Cahirinus. Sci. Rep. 2018, 8, 8920. [CrossRef]
- Jiang, T.-X.; Harn, H.I.-C.; Ou, K.-L.; Lei, M.; Chuong, C.-M. Comparative Regenerative Biology of Spiny (Acomys Cahirinus) and Laboratory (Mus Musculus) Mouse Skin. Exp. Dermatol. 2019, 28, 442–449. [CrossRef]
- Sandoval, A.G.W.; Maden, M. Regeneration in the Spiny Mouse, Acomys, a New Mammalian Model. Curr. Opin. Genet. Dev. 2020, 64, 31–36. [CrossRef]
- Fluckiger, E.; Operschall, P. Die Funktionelle Reife Der Neurohypophyse Bei Neonaten Nestfl Uchtern Und Nesthockern. Rev Suisse Zool. 1962, 69, 297–301.
- Brunjes, P.C. A Stereological Study of Neocortical Maturation in the Precocial Mouse, Acomys Cahirinus. Brain Res. 1985, 351, 279–287. [CrossRef]
- Felch, D.L.; Van Hooser, S.D. Molecular Compartmentalization of Lateral Geniculate Nucleus in the Gray Squirrel (Sciurus Carolinensis). Front. Neuroanat. 2012, 6, 12. [CrossRef]
- Fiuza, F.P.; Queiroz, J.P.G.; Aquino, A.C.Q.; Câmara, D.A.; Brandão, L.E.M.; Lima, R.H.; Cavalcanti, J.R.L.P.; Engelberth, R.C.G.J.; Cavalcante, J.S. Aging Alters Daily and Regional Calretinin Neuronal Expression in the Rat Non-Image Forming Visual Thalamus. Front. Aging Neurosci. 2021, 13, 613305. [CrossRef]
- Demeulemeester, H.; Arckens, L.; Vandesande, F.; Orban, G.A.; Heizmann, C.W.; Pochet, R. Calcium Binding Proteins as Molecular Markers for Cat Geniculate Neurons. Exp. Brain Res. 1991, 83, 513–520. [CrossRef]
- Arckens, L.; Rosier, A.; Heizmann, C.W.; Orban, G.A.; Vandesande, F. Partial Colocalization of the GABAA Receptor with Parvalbumin and Calbindin D-28K in Neurons of the Visual Cortex and the Dorsal Lateral Geniculate Nucleus of the Cat. J. Chem. Neuroanat. 1994, 8, 1–10. [CrossRef]
- Yan, Y.H.; Winarto, A.; Mansjoer, I.; Hendrickson, A. Parvalbumin, Calbindin, and Calretinin Mark Distinct Pathways during Development of Monkey Dorsal Lateral Geniculate Nucleus. J. Neurobiol. 1996, 31, 189–209. [CrossRef]
- Goodchild, A.K.; Martin, P.R. The Distribution of Calcium-Binding Proteins in the Lateral Geniculate Nucleus and Visual Cortex of a New World Monkey, the Marmoset, Callithrix Jacchus. Vis. Neurosci. 1998, 15, 625–642. [CrossRef]
- Erlander, M.G.; Tobin, A.J. The Structural and Functional Heterogeneity of Glutamic Acid Decarboxylase: A Review. Neurochem. Res. 1991, 16, 215–226. [CrossRef]
- Lee, S.-E.; Lee, Y.; Lee, G.H. The Regulation of Glutamic Acid Decarboxylases in GABA Neurotransmission in the Brain. Arch. Pharm. Res. 2019, 42, 1031–1039. [CrossRef]
- Sternberger, L.A.; Sternberger, N.H. Monoclonal Antibodies Distinguish Phosphorylated and Nonphosphorylated Forms of Neurofilaments in Situ. Proc. Natl. Acad. Sci. 1983, 80, 6126–6130. [CrossRef]
- Bickford, M.E.; Guido, W.; Godwin, D.W. Neurofilament Proteins in Y-Cells of the Cat Lateral Geniculate Nucleus: Normal Expression and Alteration with Visual Deprivation. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 6549–6557. [CrossRef]
- Bourne, J.A.; Rosa, M.G.P. Neurofilament Protein Expression in the Geniculostriate Pathway of a New World Monkey (Callithrix Jacchus). Exp. Brain Res. 2003, 150, 19–24. [CrossRef]
- Gutierrez, C.; Yaun, A.; Cusick, C.G. Neurochemical Subdivisions of the Inferior Pulvinar in Macaque Monkeys. J. Comp. Neurol. 1995, 363, 545–562. [CrossRef]
- Greenberg, G. Depth Perception in Mongolian Gerbils (Meriones Unguiculatus) and Spiny Mice (Acomys Russatus and A. Cahirinus). J. Comp. Psychol. Wash. DC 1983 1986, 100, 81–84.
- Goldman, M.; Skolnick, A.J.; Hernandez, T.P.; Tobach, E. Distance Perception in the Spiny Mouse Acomys Cahirinus: Vertical Jumping. Percept. Mot. Skills 1992, 75, 883–895. [CrossRef]
- Chevret, P.; Denys, C.; Jaeger, J.J.; Michaux, J.; Catzeflis, F.M. Molecular Evidence That the Spiny Mouse (Acomys) Is More Closely Related to Gerbils (Gerbillinae) than to True Mice (Murinae). Proc. Natl. Acad. Sci. U. S. A. 1993, 90, 3433–3436. [CrossRef]
- Gustavsen, C.R.; Kvicerova, J.; Dickinson, H.; Heller, R.S. Acomys, the Closest Relatives to Gerbils, Do Express Pdx-1 Protein and Have Similar Islet Morphology to Gerbils. Islets 2009, 1, 191–197. [CrossRef]
- Satorre, J.; Cano, J.; Reinoso-Suárez, F. Quantitative Cellular Changes during Postnatal Development of the Rat Dorsal Lateral Geniculate Nucleus. Anat. Embryol. (Berl.) 1986, 174, 321–327. [CrossRef]
- Rübsamen, R.; Gutowski, M.; Langkau, J.; Dörrscheidt, G.J. Growth of Central Nervous System Auditory and Visual Nuclei in the Postnatal Gerbil (Meriones Unguiculatus). J. Comp. Neurol. 1994, 346, 289–305. [CrossRef]
- Heumann, D.; Rabinowicz, T. Postnatal Development of the Dorsal Lateral Geniculate Nucleus in the Normal and Enucleated Albino Mouse. Exp. Brain Res. 1980, 38, 75–85. [CrossRef]
- Gonzalez, D.; Satriotomo, I.; Miki, T.; Lee, K.-Y.; Yokoyama, T.; Touge, T.; Matsumoto, Y.; Li, H.-P.; Kuriyama, S.; Takeuchi, Y. Effects of Monocular Enucleation on Calbindin-D 28k and c-Fos Expression in the Lateral Geniculate Nucleus in Rats. Okajimas Folia Anat. Jpn. 2005, 82, 9–18. [CrossRef]
- Li, H.; Zhou, Q.; Chen, Y.; Hu, H.; Gao, L.; Takahata, T. Three-Dimensional Topography of Eye-Specific Domains in the Lateral Geniculate Nucleus of Pigmented and Albino Rats. Cereb. Cortex N. Y. N 1991 2023, 33, 9599–9615. [CrossRef]
- Grubb, M.S.; Thompson, I.D. Biochemical and Anatomical Subdivision of the Dorsal Lateral Geniculate Nucleus in Normal Mice and in Mice Lacking the Beta2 Subunit of the Nicotinic Acetylcholine Receptor. Vision Res. 2004, 44, 3365–3376. [CrossRef]
- Horowitz, S.S.; Blanchard, J.H.; Morin, L.P. Intergeniculate Leaflet and Ventral Lateral Geniculate Nucleus Afferent Connections: An Anatomical Substrate for Functional Input from the Vestibulo-Visuomotor System. J. Comp. Neurol. 2004, 474, 227–245. [CrossRef]
- Radtke-Schuller, S.; Schuller, G.; Angenstein, F.; Grosser, O.S.; Goldschmidt, J.; Budinger, E. Brain Atlas of the Mongolian Gerbil (Meriones Unguiculatus) in CT/MRI-Aided Stereotaxic Coordinates. Brain Struct. Funct. 2016, 221, 1–272. [CrossRef]
- Resende, N.R.; Soares Filho, P.L.; Peixoto, P.P.A.; Silva, A.M.; Silva, S.F.; Soares, J.G.; do Nascimento, E.S.; Cavalcante, J.C.; Cavalcante, J.S.; Costa, M.S.M.O. Nuclear Organization and Morphology of Cholinergic Neurons in the Brain of the Rock Cavy (Kerodon Rupestris) (Wied, 1820). J. Chem. Neuroanat. 2018, 94, 63–74. [CrossRef]
- Bhagwandin, A.; Gravett, N.; Bennett, N.C.; Manger, P.R. Distribution of Parvalbumin, Calbindin and Calretinin Containing Neurons and Terminal Networks in Relation to Sleep Associated Nuclei in the Brain of the Giant Zambian Mole-Rat (Fukomys Mechowii). J. Chem. Neuroanat. 2013, 52, 69–79. [CrossRef]
- Gravett, N.; Bhagwandin, A.; Fuxe, K.; Manger, P.R. Distribution of Orexin-A Immunoreactive Neurons and Their Terminal Networks in the Brain of the Rock Hyrax, Procavia Capensis. J. Chem. Neuroanat. 2011, 41, 86–96. [CrossRef]
- Giolli, R.A.; Creel, D.J. The Primary Optic Projections in Pigmented and Albino Guinea Pigs: An Experimental Degeneration Study. Brain Res. 1973, 55, 25–39. [CrossRef]
- Vaidya, P.G. A Study of the Lateral Geniculate Body in the Diurnal Ground Squirrel, Citellus Tridecemlineatus Tridecemlineatus and in the Nocturnal Guinea Pig. Anat. Rec. 1963, 147, 499–505. [CrossRef]
- Niimi, K.; Kanaseki, T.; Takimoto, T. The Comparative Anatomy of the Ventral Nucleus of the Lateral Geniculate Body in Mammals. J. Comp. Neurol. 1963, 121, 313–323. [CrossRef]
- Dwarika, S.; Maseko, B.C.; Ihunwo, A.O.; Fuxe, K.; Manger, P.R. Distribution and Morphology of Putative Catecholaminergic and Serotonergic Neurons in the Brain of the Greater Canerat, Thryonomys Swinderianus. J. Chem. Neuroanat. 2008, 35, 108–122. [CrossRef]
- Bickford, M.E.; Zhou, N.; Krahe, T.E.; Govindaiah, G.; Guido, W. Retinal and Tectal “Driver-Like” Inputs Converge in the Shell of the Mouse Dorsal Lateral Geniculate Nucleus. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 10523–10534. [CrossRef]
- Okigawa, S.; Yamaguchi, M.; Ito, K.N.; Takeuchi, R.F.; Morimoto, N.; Osakada, F. Cell Type- and Layer-Specific Convergence in Core and Shell Neurons of the Dorsal Lateral Geniculate Nucleus. J. Comp. Neurol. 2021, 529, 2099–2124. [CrossRef]
- Huberman, A.D.; Wei, W.; Elstrott, J.; Stafford, B.K.; Feller, M.B.; Barres, B.A. Genetic Identification of an On-Off Direction-Selective Retinal Ganglion Cell Subtype Reveals a Layer-Specific Subcortical Map of Posterior Motion. Neuron 2009, 62, 327–334. [CrossRef]
- Kay, J.N.; De la Huerta, I.; Kim, I.-J.; Zhang, Y.; Yamagata, M.; Chu, M.W.; Meister, M.; Sanes, J.R. Retinal Ganglion Cells with Distinct Directional Preferences Differ in Molecular Identity, Structure, and Central Projections. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 7753–7762. [CrossRef]
- Marshel, J.H.; Kaye, A.P.; Nauhaus, I.; Callaway, E.M. Anterior-Posterior Direction Opponency in the Superficial Mouse Lateral Geniculate Nucleus. Neuron 2012, 76, 713–720. [CrossRef]
- Piscopo, D.M.; El-Danaf, R.N.; Huberman, A.D.; Niell, C.M. Diverse Visual Features Encoded in Mouse Lateral Geniculate Nucleus. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 4642–4656. [CrossRef]
- Cruz-Martín, A.; El-Danaf, R.N.; Osakada, F.; Sriram, B.; Dhande, O.S.; Nguyen, P.L.; Callaway, E.M.; Ghosh, A.; Huberman, A.D. A Dedicated Circuit Links Direction-Selective Retinal Ganglion Cells to the Primary Visual Cortex. Nature 2014, 507, 358–361. [CrossRef]
- Kerschensteiner, D.; Guido, W. Organization of the Dorsal Lateral Geniculate Nucleus in the Mouse. Vis. Neurosci. 2017, 34, E008. [CrossRef]
- Martersteck, E.M.; Hirokawa, K.E.; Evarts, M.; Bernard, A.; Duan, X.; Li, Y.; Ng, L.; Oh, S.W.; Ouellette, B.; Royall, J.J.; et al. Diverse Central Projection Patterns of Retinal Ganglion Cells. Cell Rep. 2017, 18, 2058–2072. [CrossRef]
- Krahe, T.E.; El-Danaf, R.N.; Dilger, E.K.; Henderson, S.C.; Guido, W. Morphologically Distinct Classes of Relay Cells Exhibit Regional Preferences in the Dorsal Lateral Geniculate Nucleus of the Mouse. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 17437–17448. [CrossRef]
- Huberman, A.D.; Manu, M.; Koch, S.M.; Susman, M.W.; Lutz, A.B.; Ullian, E.M.; Baccus, S.A.; Barres, B.A. Architecture and Activity-Mediated Refinement of Axonal Projections from a Mosaic of Genetically Identified Retinal Ganglion Cells. Neuron 2008, 59, 425–438. [CrossRef]
- Sabbagh, U.; Govindaiah, G.; Somaiya, R.D.; Ha, R.V.; Wei, J.C.; Guido, W.; Fox, M.A. Diverse GABAergic Neurons Organize into Subtype-Specific Sublaminae in the Ventral Lateral Geniculate Nucleus. J. Neurochem. 2021, 159, 479–497. [CrossRef]
- Moore, R.Y.; Card, J.P. Intergeniculate Leaflet: An Anatomically and Functionally Distinct Subdivision of the Lateral Geniculate Complex. J. Comp. Neurol. 1994, 344, 403–430. [CrossRef]
- Moore, R.Y.; Weis, R.; Moga, M.M. Efferent Projections of the Intergeniculate Leaflet and the Ventral Lateral Geniculate Nucleus in the Rat. J. Comp. Neurol. 2000, 420, 398–418. [CrossRef]
- Mikkelsen, J.D.; Cozzi, B.; Møller, M. Efferent Projections from the Lateral Geniculate Nucleus to the Pineal Complex of the Mongolian Gerbil (Meriones Unguiculatus). Cell Tissue Res. 1991, 264, 95–102. [CrossRef]
- Cant, N.B.; Benson, C.G. Multiple Topographically Organized Projections Connect the Central Nucleus of the Inferior Colliculus to the Ventral Division of the Medial Geniculate Nucleus in the Gerbil, Meriones Unguiculatus. J. Comp. Neurol. 2007, 503, 432–453. [CrossRef]
- Schmidt-Kastner, R.; Meller, D.; Eysel, U.T. Immunohistochemical Changes of Neuronal Calcium-Binding Proteins Parvalbumin and Calbindin-D-28k Following Unilateral Deafferentation in the Rat Visual System. Exp. Neurol. 1992, 117, 230–246. [CrossRef]
- Okoyama, S.; Moriizumi, T. Onset of Calbindin-D 28K and Parvalbumin Expression in the Lateral Geniculate Complex and Olivary Pretectal Nucleus during Postnatal Development of the Rat. Int. J. Dev. Neurosci. 2001, 19, 655–661. [CrossRef]
- Frassoni, C.; Bentivoglio, M.; Spreafico, R.; Sánchez, M.P.; Puelles, L.; Fairen, A. Postnatal Development of Calbindin and Parvalbumin Immunoreactivity in the Thalamus of the Rat. Brain Res. Dev. Brain Res. 1991, 58, 243–249. [CrossRef]
- Arai, M.; Arai, R.; Kani, K.; Jacobowitz, D.M. Immunohistochemical Localization of Calretinin in the Rat Lateral Geniculate Nucleus and Its Retino-Geniculate Projection. Brain Res. 1992, 596, 215–222. [CrossRef]
- Arai, R.; Winsky, L.; Arai, M.; Jacobowitz, D.M. Immunohistochemical Localization of Calretinin in the Rat Hindbrain. J. Comp. Neurol. 1991, 310, 21–44. [CrossRef]
- Winsky, L.; Montpied, P.; Arai, R.; Martin, B.M.; Jacobowitz, D.M. Calretinin Distribution in the Thalamus of the Rat: Immunohistochemical and in Situ Hybridization Histochemical Analyses. Neuroscience 1992, 50, 181–196. [CrossRef]
- Frassoni, C.; Arcelli, P.; Selvaggio, M.; Spreafico, R. Calretinin Immunoreactivity in the Developing Thalamus of the Rat: A Marker of Early Generated Thalamic Cells. Neuroscience 1998, 83, 1203–1214. [CrossRef]
- Merkulyeva, N.; Mikhalkin, А.; Kostareva, A.; Vavilova, T. Transient Neurochemical Features of the Perigeniculate Neurons during Early Postnatal Development of the Cat. J. Comp. Neurol. 2022, 530, 3193–3208. [CrossRef]
- Hada, Y.; Yamada, Y.; Imamura, K.; Mataga, N.; Watanabe, Y.; Yamamoto, M. Effects of Monocular Enucleation on Parvalbumin in Rat Visual System during Postnatal Development. Invest. Ophthalmol. Vis. Sci. 1999, 40, 2535–2545.
- Gonzalez, D.; Satriotomo, I.; Miki, T.; Lee, K.-Y.; Yokoyama, T.; Touge, T.; Matsumoto, Y.; Li, H.-P.; Kuriyama, S.; Takeuchi, Y. Changes of Parvalbumin Immunoreactive Neurons and GFAP Immunoreactive Astrocytes in the Rat Lateral Geniculate Nucleus Following Monocular Enucleation. Neurosci. Lett. 2006, 395, 149–154. [CrossRef]
- Arai, R.; Jacobowitz, D.M.; Deura, S. Distribution of Calretinin, Calbindin-D28k, and Parvalbumin in the Rat Thalamus. Brain Res. Bull. 1994, 33, 595–614. [CrossRef]
- Giolli, R.A.; Peterson, G.M.; Ribak, C.E.; McDonald, H.M.; Blanks, R.H.; Fallon, J.H. GABAergic Neurons Comprise a Major Cell Type in Rodent Visual Relay Nuclei: An Immunocytochemical Study of Pretectal and Accessory Optic Nuclei. Exp. Brain Res. 1985, 61, 194–203. [CrossRef]
- Benson, D.L.; Isackson, P.J.; Gall, C.M.; Jones, E.G. Contrasting Patterns in the Localization of Glutamic Acid Decarboxylase and Ca2+/Calmodulin Protein Kinase Gene Expression in the Rat Central Nervous System. Neuroscience 1992, 46, 825–849. [CrossRef]
- Hammer, S.; Carrillo, G.L.; Govindaiah, G.; Monavarfeshani, A.; Bircher, J.S.; Su, J.; Guido, W.; Fox, M.A. Nuclei-Specific Differences in Nerve Terminal Distribution, Morphology, and Development in Mouse Visual Thalamus. Neural Develop. 2014, 9, 16. [CrossRef]
- Langel, J.; Ikeno, T.; Yan, L.; Nunez, A.A.; Smale, L. Distributions of GABAergic and Glutamatergic Neurons in the Brains of a Diurnal and Nocturnal Rodent. Brain Res. 2018, 1700, 152–159. [CrossRef]
- Ohara, P.T.; Lieberman, A.R.; Hunt, S.P.; Wu, J.Y. Neural Elements Containing Glutamic Acid Decarboxylase (GAD) in the Dorsal Lateral Geniculate Nucleus of the Rat; Immunohistochemical Studies by Light and Electron Microscopy. Neuroscience 1983, 8, 189–211. [CrossRef]
- Gabbott, P.L.; Somogyi, J.; Stewart, M.G.; Hamori, J. GABA-Immunoreactive Neurons in the Rat Dorsal Lateral Geniculate Nucleus: Light Microscopical Observations. Brain Res. 1985, 346, 171–175. [CrossRef]
- Gabbott, P.L.; Somogyi, J.; Stewart, M.G.; Hámori, J. A Quantitative Investigation of the Neuronal Composition of the Rat Dorsal Lateral Geniculate Nucleus Using GABA-Immunocytochemistry. Neuroscience 1986, 19, 101–111. [CrossRef]
- De Biasi, S.; Amadeo, A.; Arcelli, P.; Frassoni, C.; Spreafico, R. Postnatal Development of GABA-Immunoreactive Terminals in the Reticular and Ventrobasal Nuclei of the Rat Thalamus: A Light and Electron Microscopic Study. Neuroscience 1997, 76, 503–515. [CrossRef]
- McDonald, J.K.; Speciale, S.G.; Parnavelas, J.G. The Development of Glutamic Acid Decarboxylase in the Visual Cortex and the Dorsal Lateral Geniculate Nucleus of the Rat. Brain Res. 1981, 217, 364–367. [CrossRef]
- Soares, J.G.M.; Rosado De Castro, P.H.; Fiorani, M.; Nascimento-Silva, S.; Gattass, R. Distribution of Neurofilament Proteins in the Lateral Geniculate Nucleus, Primary Visual Cortex, and Area MT of Adult Cebus Monkeys. J. Comp. Neurol. 2008, 508, 605–614. [CrossRef]
- Mikhalkin, A.; Nikitina, N.; Merkulyeva, N. Heterochrony of Postnatal Accumulation of Nonphosphorylated Heavy-Chain Neurofilament by Neurons of the Cat Dorsal Lateral Geniculate Nucleus. J. Comp. Neurol. 2021, 529, 1430–1441. [CrossRef]
- Coombs, J.; van der List, D.; Wang, G.-Y.; Chalupa, L.M. Morphological Properties of Mouse Retinal Ganglion Cells. Neuroscience 2006, 140, 123–136. [CrossRef]
- Bleckert, A.; Schwartz, G.W.; Turner, M.H.; Rieke, F.; Wong, R.O.L. Visual Space Is Represented by Nonmatching Topographies of Distinct Mouse Retinal Ganglion Cell Types. Curr. Biol. CB 2014, 24, 310–315. [CrossRef]
- Reinhard, K.; Li, C.; Do, Q.; Burke, E.G.; Heynderickx, S.; Farrow, K. A Projection Specific Logic to Sampling Visual Inputs in Mouse Superior Colliculus. eLife 2019, 8, e50697. [CrossRef]
- Kutcher, M.R.; Duffy, K.R. Cytoskeleton Alteration Correlates with Gross Structural Plasticity in the Cat Lateral Geniculate Nucleus. Vis. Neurosci. 2007, 24, 775–785. [CrossRef]
- Shkorbatova, P.Y.; Veshchitskii, A.A.; Mikhalkin, A.A.; Nikitina, N.I.; Belyaev, A.V.; Merkulyeva, N.S. Development of Acomys Cahirinus in the Laboratory Conditions. J. Evol. Biochem. Physiol. 60.
- Dickinson, H.; Walker, D. Managing a Colony of Spiny Mice (Acomys Cahirinus) for Perinatal Research. ANZCCART News 20.
- Allen Institute for Brain Science Allen Mouse Brain Atlas [Dataset]. Available Mousebrain-Map.org 2004.
- Paxinos, G.; Franklin, K.B.J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Academic Press, 2019; ISBN 978-0-12-816158-6.
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [CrossRef]
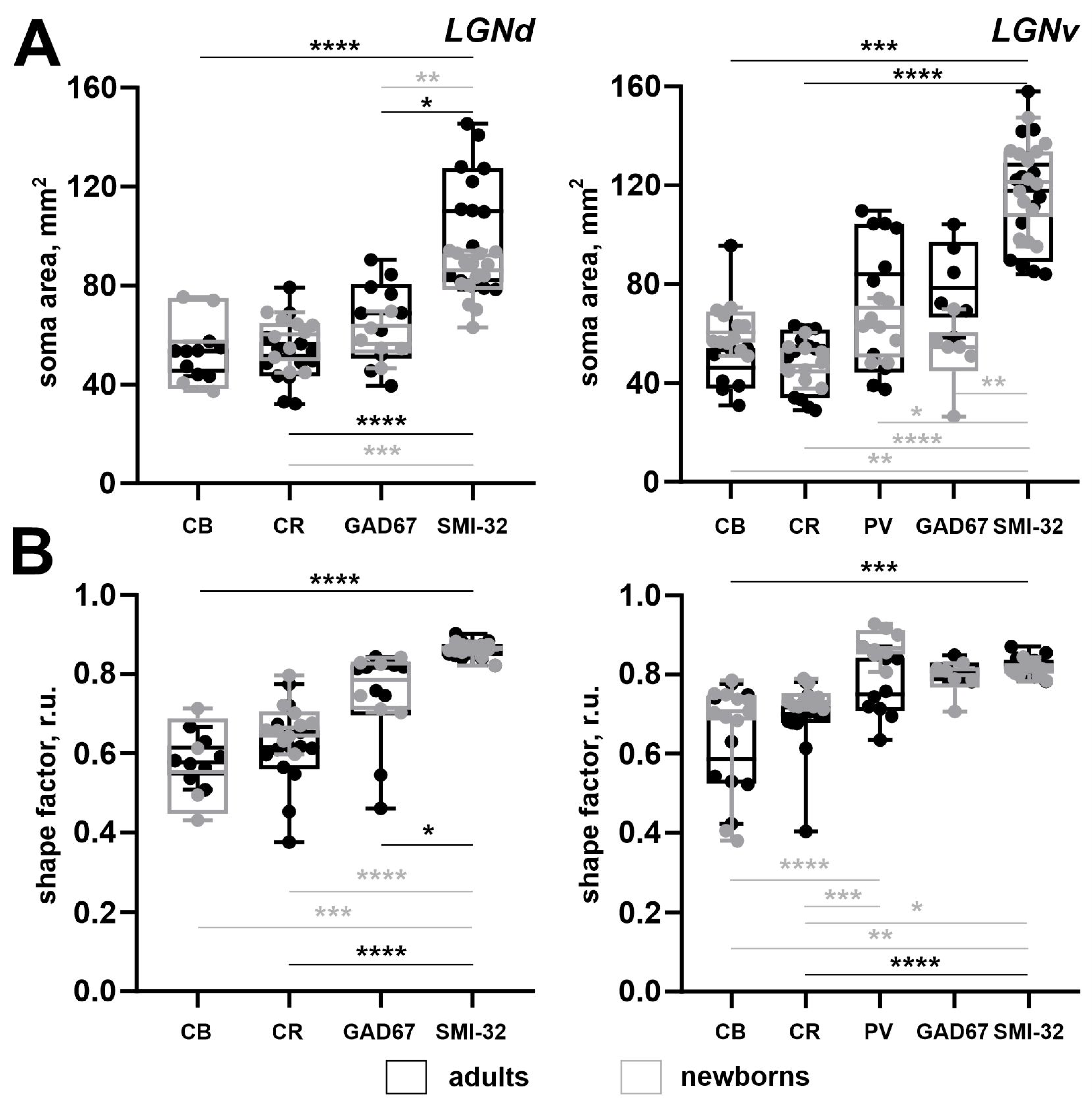
| Adults | Newborns | |||||
| LGNd | LGNv | IGL | LGNd | LGNv | IGL | |
| Soma area, µm2 | ||||||
| CB | 50.9 ± 5.3 | 50.5 ± 20.1 | 53.1 ± 11.0 | 56.6 ± 20.6 ns |
61.5 ± 6.9 * |
59.6 ± 7.6 ns |
| CR | 51.7 ± 11.6 | 49.9 ± 12.6 | – | 57.8 ± 8.7 ns |
48.6 ± 6.9 ns |
47.6 ± 4.9 ns |
| PV | – | 76.4 ± 29.7 | – | – | 61.8 ± 10.0 ns |
– |
| GAD67 | 66.8 ± 16.9 | 80.6 ± 17.1 | – | 57.5 ± 7.9 ns |
52.5 ± 14.2 ** |
– |
| SMI-32 | 106.7 ± 23.8 | 115.1 ± 23.1 | – | 83.7 ± 9.7 * |
120.8 ± 16.2 ns |
– |
| Maximal diameter, µm | ||||||
| CB | 11.8 ± 0.8 | 11.4 ± 2.0 | 12.6 ± 1.5 | 11.8 ± 2.4 ns |
12.3 ± 1.5 ns |
12.4 ± 0.9 ns |
| CR | 11.6 ± 1.9 | 11.2 ± 1.6 | 11.9 ± 1.7 | 11.6 ± 1.0 ns |
10.6 ± 0.8 ns |
11.0 ± 0.6 ns |
| PV | – | 12.9 ± 2.3 | – | – | 11.2 ± 1.2 ns |
– |
| GAD67 | 12.7 ± 1.1 | 13.7 ± 1.4 | – | 12.0 ± 1.1 ns |
11.2 ± 1.6 * |
– |
| SMI-32 | 14.7 ± 1.5 | 16.1 ± 1.7 | – | 13.2 ± 0.7 ** |
16.8 ± 1.3 ns |
– |
| Minimal diameter, µm | ||||||
| CB | 7.3 ± 0.2 | 7.3 ± 1.7 | 6.9 ± 0.9 | 7.9 ± 1.8 ns |
8.1 ± 0.9 ns |
7.7 ± 0.7 * |
| CR | 7.3 ± 0.9 | 7.2 ± 1.2 | 7.1 ± 1.2 | 8.0 ± 0.7 ns |
7.1 ± 0.5 ns |
6.7 ± 0.3 ns |
| PV | – | 8.3 ± 1.5 | – | – | 7.4 ± 0.6 ns |
– |
| GAD67 | 7.7 ± 0.9 | 8.2 ± 1.3 | – | 6.9 ± 0.5 * |
6.6 ± 0.9 * |
– |
| SMI-32 | 9.9 ± 1.1 | 10.0 ± 0.9 | – | 8.7 ± 0.6 * |
10.2 ± 0.7 ns |
– |
| Shape factor, r.u. | ||||||
| CB | 0.58 ± 0.05 | 0.61 ± 0.13 | 0.62 ± 0.05 | 0.56 ± 0.12 ns |
0.66 ± 0.14 ns |
0.65 ± 0.05 ns |
| CR | 0.60 ± 0.10 | 0.68 ± 0.09 | 0.67 ± 0.03 | 0.68 ± 0.05 * |
0.74 ± 0.03 ** |
0.70 ± 0.02 * |
| PV | – | 0.77 ± 0.08 | – | – | 0.87 ± 0.04 ** |
– |
| GAD67 | 0.75 ± 0.13 | 0.81 ± 0.03 | – | 0.78 ± 0.06 ns |
0.79 ± 0.04 ns |
– |
| SMI-32 | 0.86 ± 0.02 | 0.83 ± 0.02 | – | 0.87 ± 0.03 ns |
0.82 ± 0.02 ns |
– |
| Adults | Newborns | |||||
| LGNd | LGNv | IGL | LGNd | LGNv | IGL | |
| Number of neurons | ||||||
| CB | 257 ± 166 | 12 ± 8 | 17 ± 5 | 3 ± 6 **** |
12 ± 4 ns |
12 ± 4 * |
| CR | 5 ± 2 | 31 ± 11 | 22 ± 8 | 37 ± 27 **** |
69 ± 23 *** |
35 ± 14 * |
| PV | – | 49 ± 12 | – | – | 4 ± 2 **** |
– |
| GAD67 | 81 ± 17 | 9 ± 2 | 2 ± 2 | 36 ± 26 ** |
8 ± 3 ns |
0.1 ± 0.2 ns |
| SMI-32 | 56 ± 17 | 29 ± 8 | 0.8 ± 0.9 | 82 ± 31 * |
76 ± 8 **** |
2 ± 2 * |
| Cellular density | ||||||
| CB | 517 ± 394 | 40 ± 28 | – | 10 ± 17 **** |
43 ± 14 ns |
– |
| CR | 10 ± 5 | 99 ± 32 | – | 124 ± 81 **** |
349 ± 109 **** |
– |
| PV | – | 241 ± 74 | – | – | 1 ± 2 **** |
– |
| GAD67 | 154 ± 36 | 28 ± 11 | – | 118 ± 85 ns |
52 ± 16 ** |
– |
| SMI-32 | 107 ± 36 | 147 ± 40 | – | 330 ± 128 **** |
492 ± 90 **** |
– |
| Antibody | Host | Clonality | Dilution | Manufacturer | Lot | RRID |
|---|---|---|---|---|---|---|
| Calbindin | Mouse | Monoclonal | 1:10000 | Sigma-Aldrich | C9848 | AB_476894 |
| Calretinin | Rabbit | Polyclonal | 1:10000 | Sigma-Aldrich | AB5054 | AB_2068506 |
| Parvalbumin | Rabbit | Polyclonal | 1:5000 | Abcam | ab11427 | AB_298032 |
| GAD67 | Mouse | Monoclonal | 1:2000 | Sigma-Aldrich | MAB5406 | AB_2278725 |
| SMI-32 | Mouse | Monoclonal | 1:5000 | BioLegend | 801701 | AB_2564642 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





