Submitted:
03 July 2024
Posted:
04 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
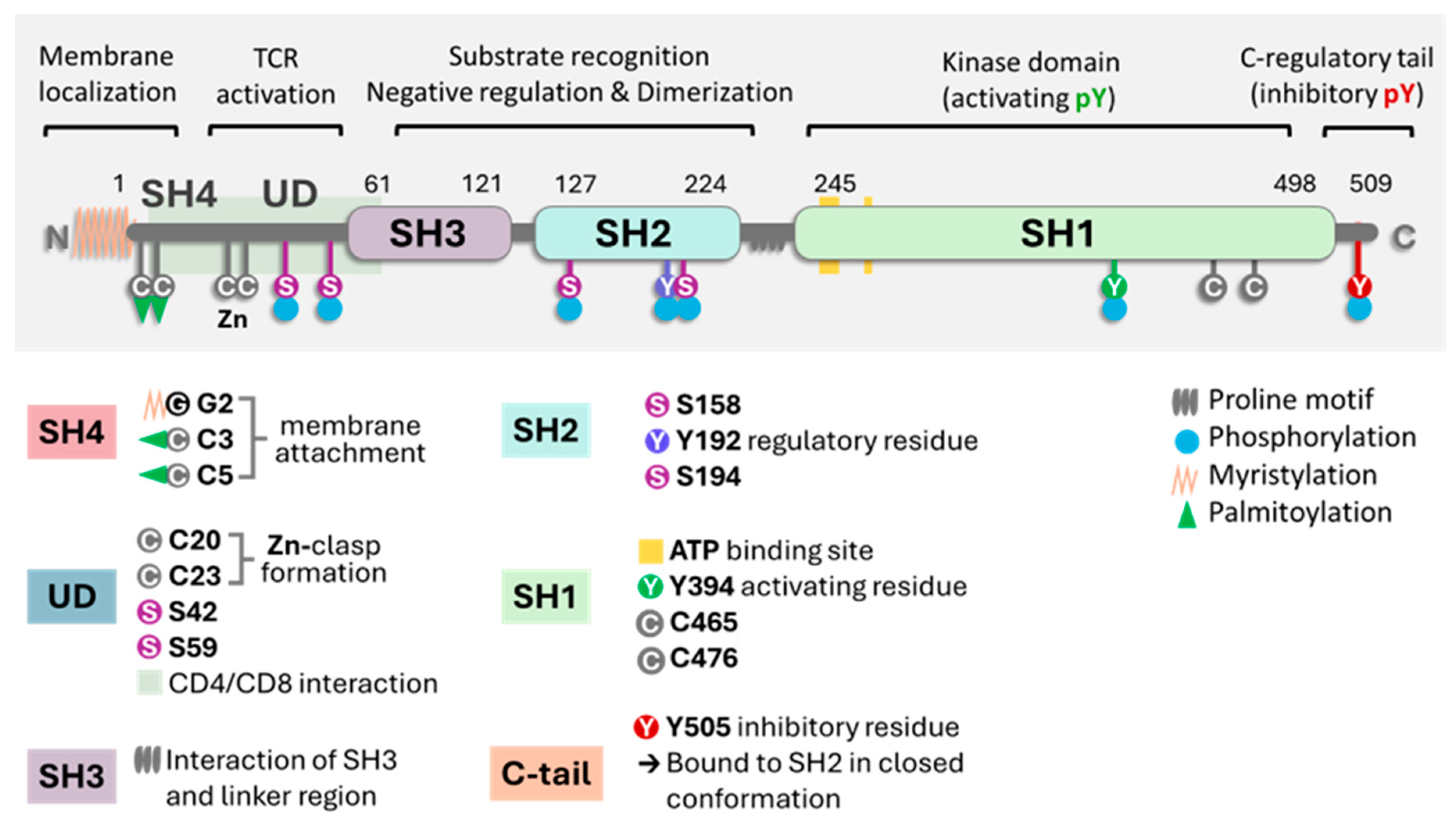
2. Key Biochemical Elements of Enzyme Activity
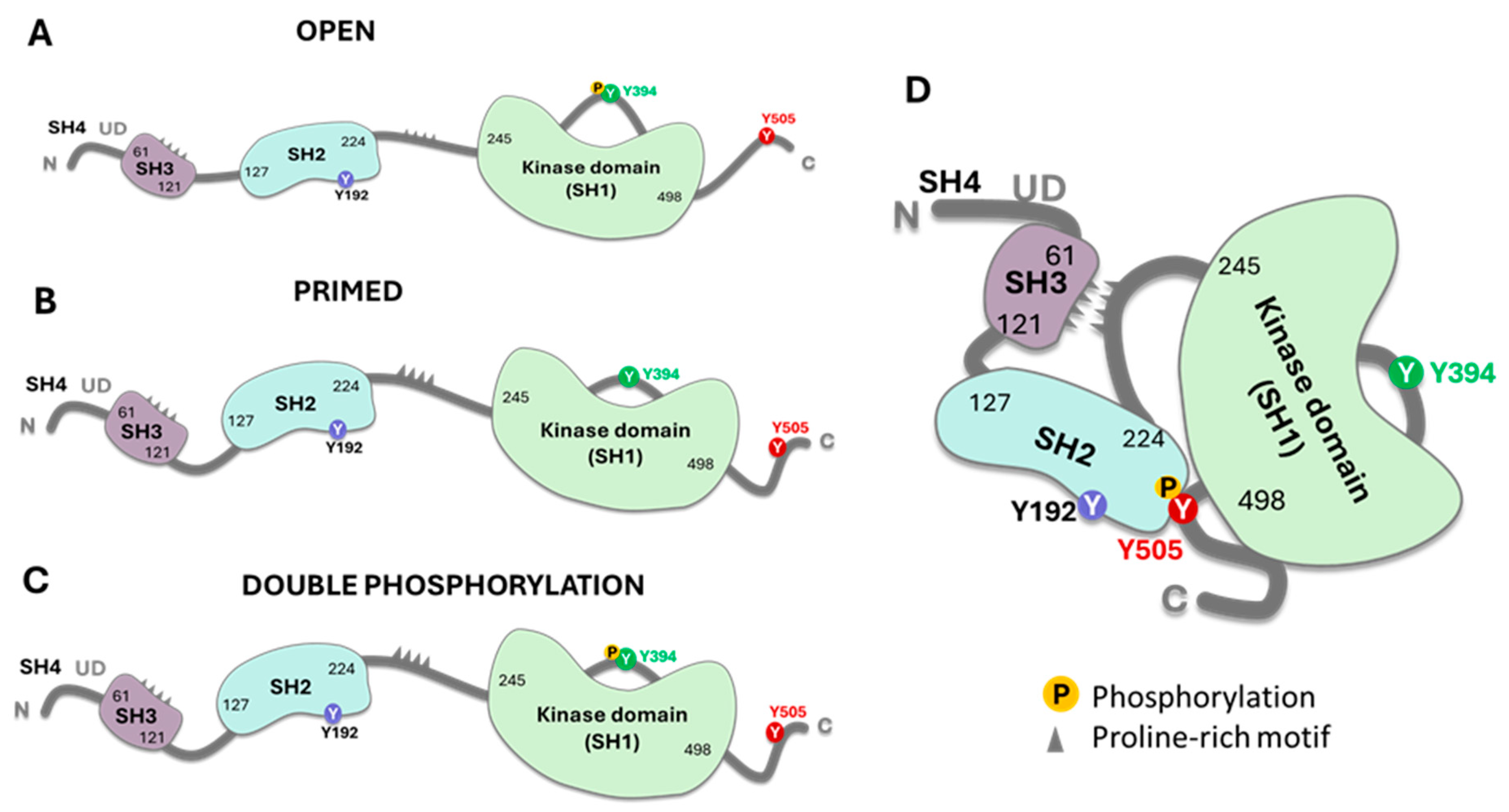
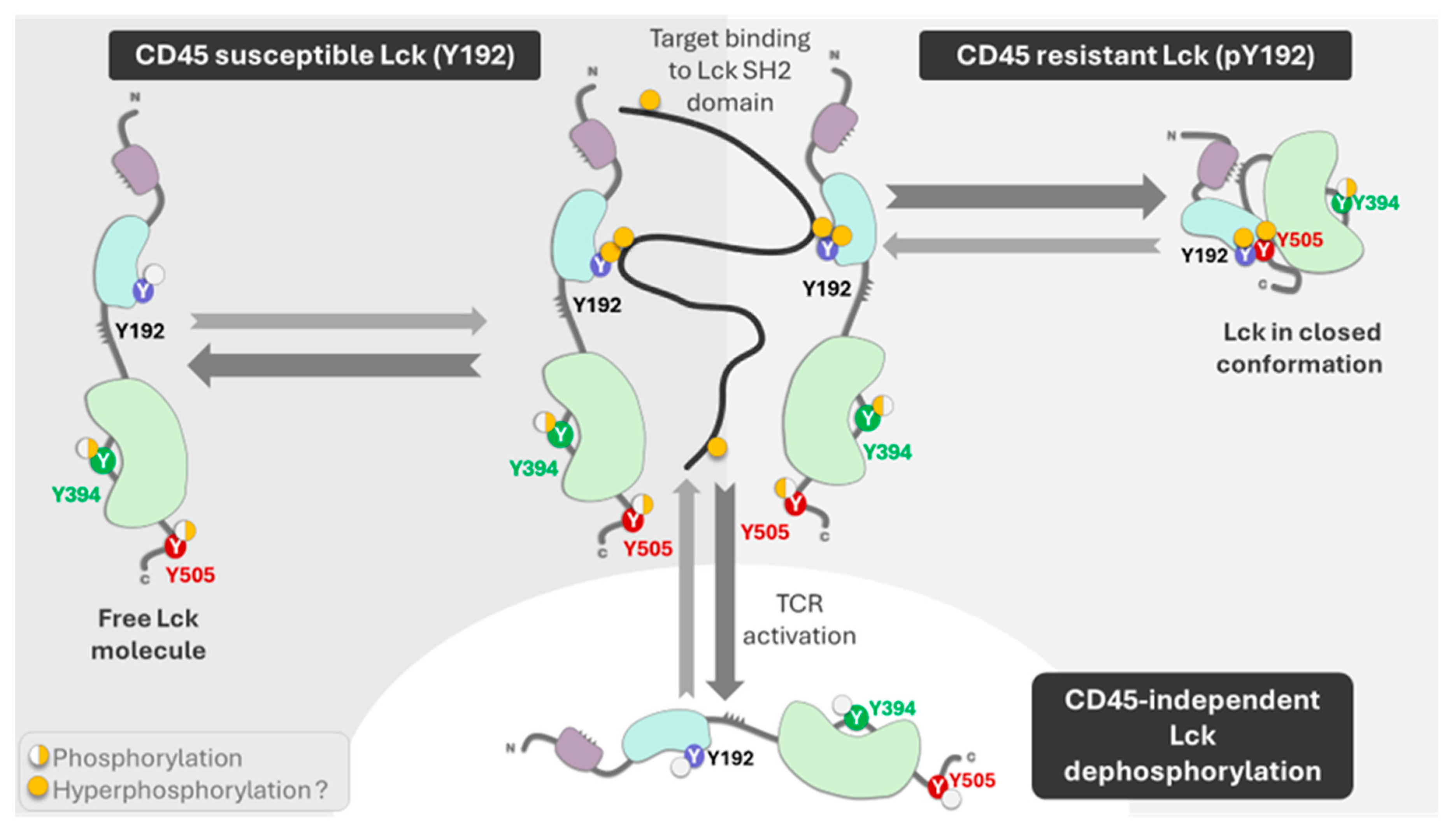
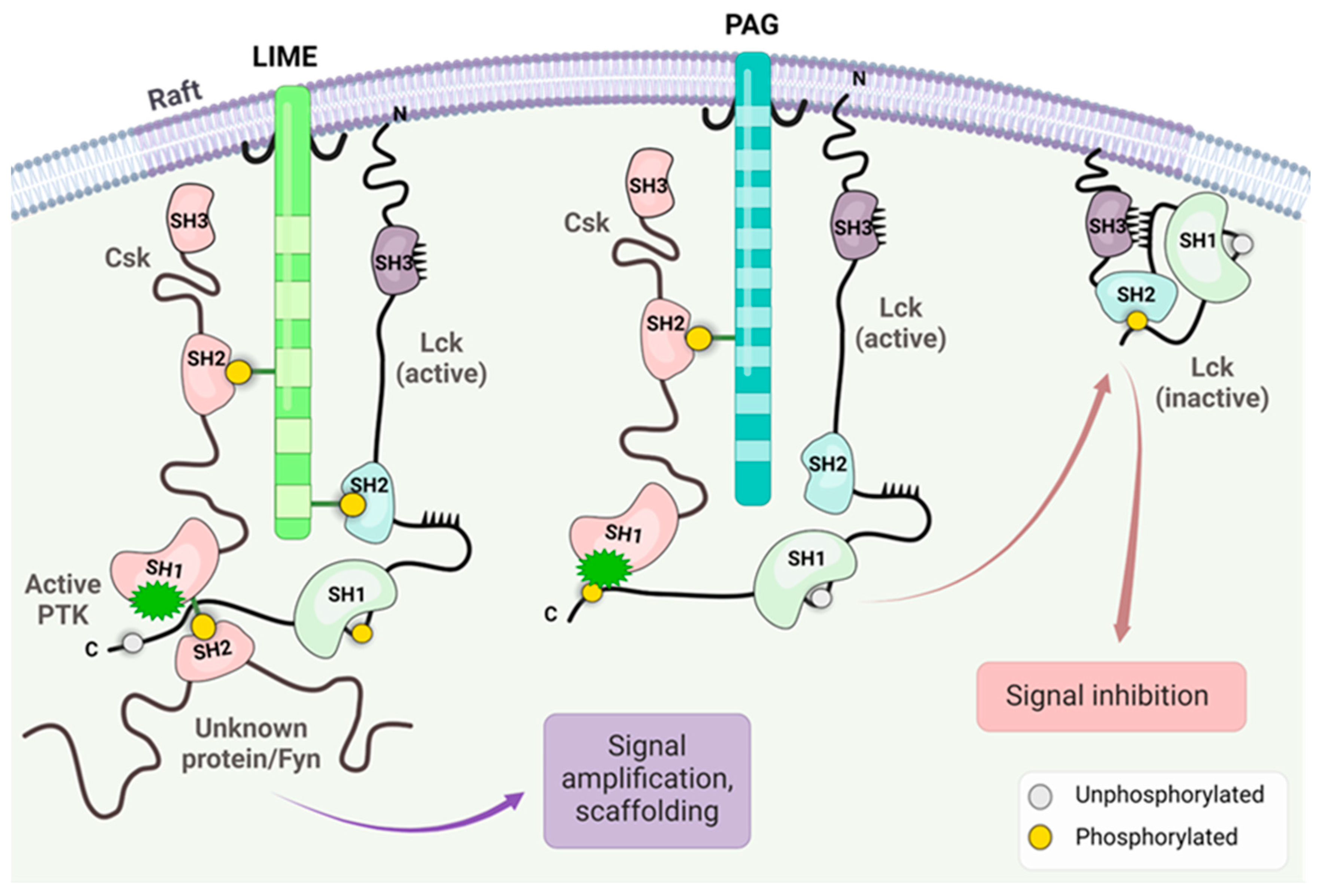
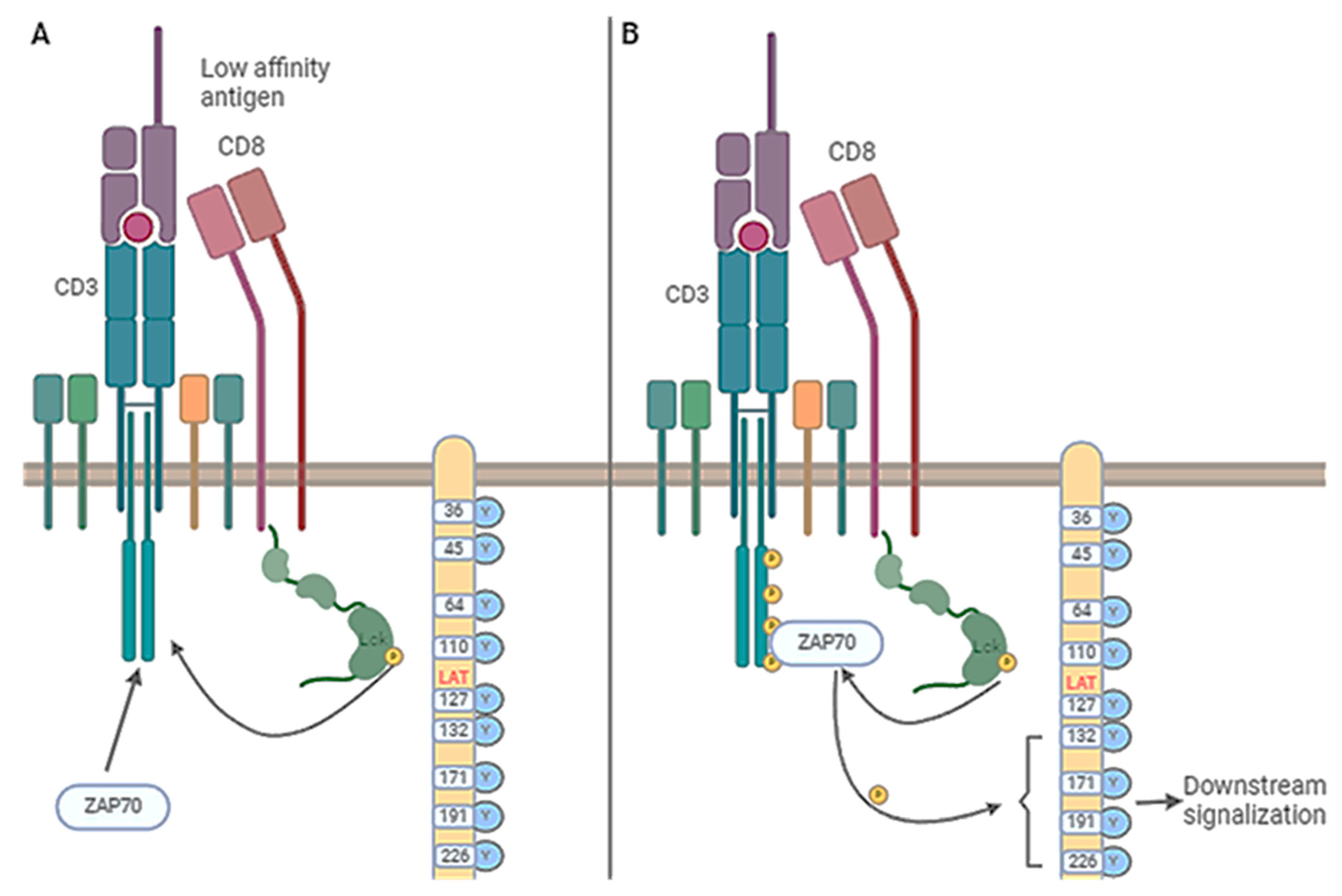
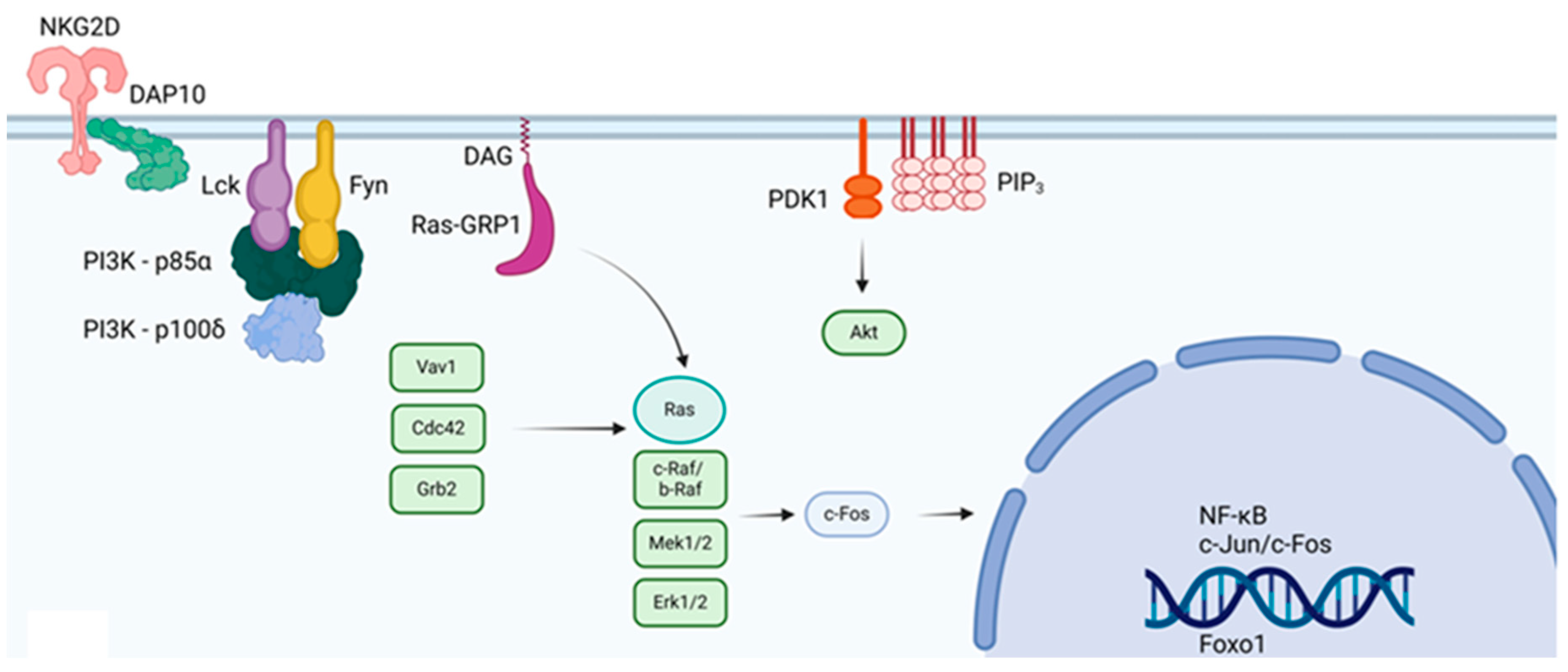
3. CAR T Cells and Lck Signaling
4. Lck Genetic Analysis
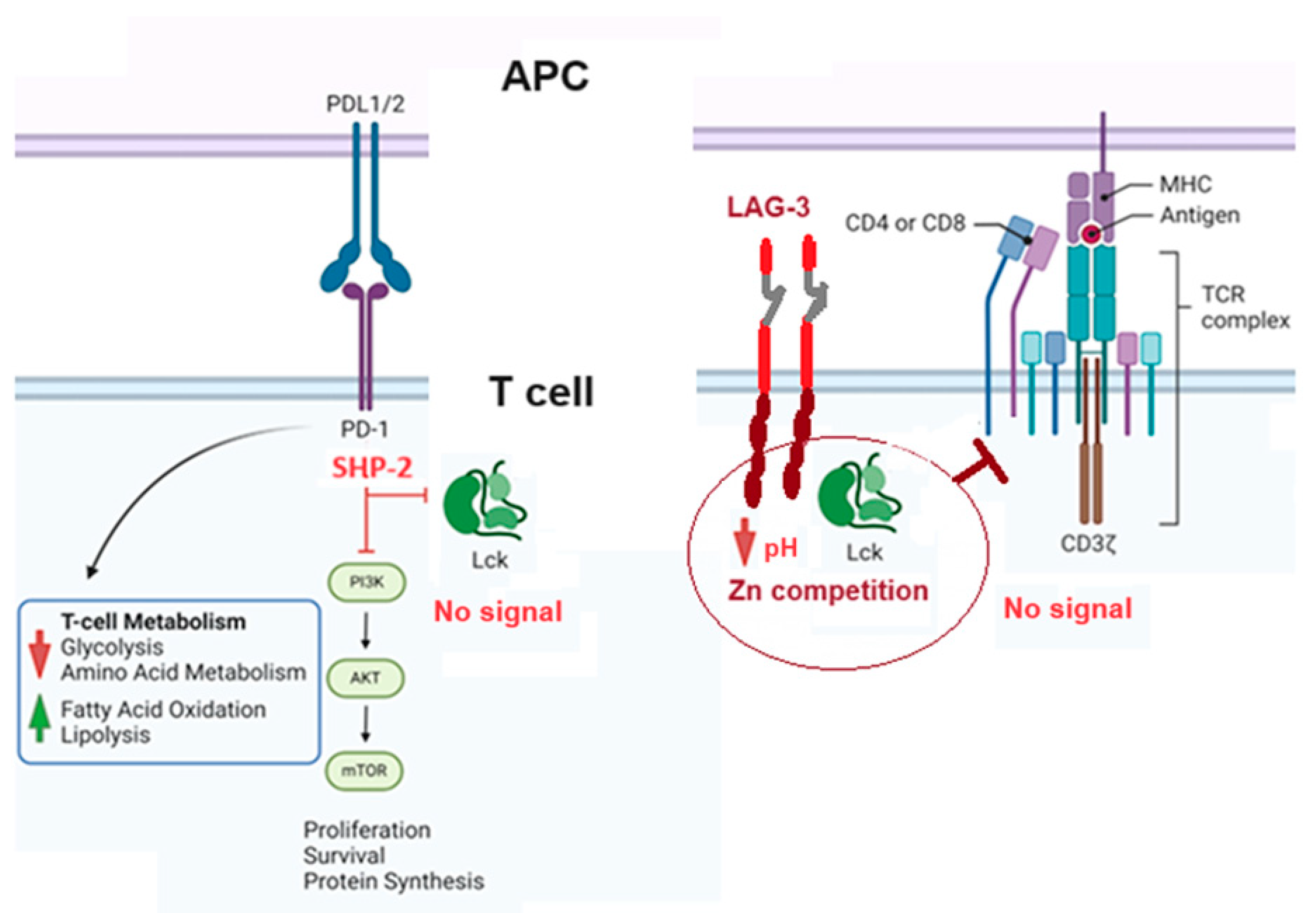
5. Pharmacological Modulation of Lck Activity
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- P06239 · LCK_human https://www.uniprot.org/uniprotkb/P06239/entry. Assessed June 9, 2024.
- Serfas, M. S.; Tyner, A. L. BRK, SRM, FRK, and SRC42A form a distinct family of intracellular SRC-Like tyrosine kinases. Oncology Research 2003, 13(6), 409–419. [CrossRef]
- Parsons, S.J.; Parsons, J.T. Src family kinases, key regulators of signal transduction. Oncogene. 2004 Oct 18;23(48):7906-9. [CrossRef]
- Palacios, E. H.; Weiss, A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene, 2004;23(48), 7990 8000. [CrossRef]
- Levin, S. E.; Weiss, A. Non-Receptor Tyrosine Kinases in T Cell Antigen Receptor Function. Handbook of Cell Signaling (Second Edition), 2009; Chapter 68. pp 507-516. Editor Bradshaw R.A.; Dennis, E.A. Academic Press. [CrossRef]
- Bommhardt, U.; Schraven, B.; Simeoni, L. Beyond TCR Signaling: Emerging Functions of Lck in Cancer and Immunotherapy. Int J Mol Sci. 2019;20(14):3500. [CrossRef]
- Filipp, D.; Ballek, O.; Manning, J. Lck, Membrane Microdomains, and TCR Triggering Machinery: Defining the New Rules of Engagement. Front Immunol. 2012 Jun 12;3:155. [CrossRef]
- Shah, K.; Al-Haidari, A.; Sun, J.; Kazi, J.U. T cell receptor (TCR) signaling in health and disease. Signal Transduct Target Ther. 2021 Dec 13;6(1):412. [CrossRef]
- Porciello, N.; Cipria, D.; Mai, G.; Lanz, A.L.; Milanetti, E.; Grottesi, A.; Howie, D.; Cobbold, S.P.; Schermelleh, L.; He, H.T.; D'Abramo, M.; Destainville, N.; Acuto, O,; Nika, K. Role of the membrane anchor in the regulation of Lck activity. J Biol Chem. 2022 Dec;298(12):102663. [CrossRef]
- Kocyła, A.M.; Czogalla, A.; Wessels, I.; Rink, L.; Krężel, A. A combined biochemical and cellular approach reveals Zn2+-dependent hetero- and homodimeric CD4 and Lck assemblies in T cells. Structure. 2024 Mar 7;32(3):292-303.e7. [CrossRef]
- Chen, Y.; Li, Y.; Wu, L. Protein S-palmitoylation modification: implications in tumor and tumor immune microenvironment. Front Immunol. 2024 Feb 13;15:1337478. [CrossRef]
- Mariuzza, R.A.; Shahid, S.; Karade, S.S. The immune checkpoint receptor LAG3: Structure, function, and target for cancer immunotherapy. J Biol Chem. 2024 May;300(5):107241. [CrossRef]
- Nika, K.; Soldani, C.; Salek, M.; Paster, W.; Gray, A.; Etzensperger, R.; Fugger, L.; Polzella, P.; Cerundolo, V.; Dushek, O.; Höfer, T.; Viola, A.; Acuto, O. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010 Jun 25;32(6):766-77. [CrossRef]
- Fernández-Aguilar, L.M.; Vico-Barranco, I.; Arbulo-Echevarria, M.M.; Aguado, E. A Story of Kinases and Adaptors: The Role of Lck, ZAP-70 and LAT in Switch Panel Governing T-Cell Development and Activation. Biology (Basel). 2023 Aug 24;12(9):1163. [CrossRef]
- Bozso, S.J.; Kang, J.J.H.; Nagendran, J. The role of competing mechanisms on Lck regulation. Immunol Res. 2020 Oct;68(5):289-295. [CrossRef]
- Kesavan, K.P.; Isaacson, C.C.; Ashendel, C.L.; Geahlen, R.L.; Harrison, M.L. Characterization of the in vivo sites of serine phosphorylation on Lck identifying serine 59 as a site of mitotic phosphorylation. J Biol Chem. 2002 Apr 26;277(17):14666-73. [CrossRef]
- Wu, J.; Li, G.; Li, L.; Li, D.; Dong, Z.; Jiang, P. Asparagine enhances LCK signalling to potentiate CD8+ T-cell activation and anti-tumour responses. Nature Cell Biology, 2021; 23(1), 75–86. [CrossRef]
- Wang, Q.; Zhu, T.; Miao, N.; Qu, Y.; Wang, Z.; Chao, Y.; Wang, J.; Wu, W.; Xu, X.; Xu, C.; Li, X.; Wang, F. Disulfiram bolsters T-cell anti-tumor immunity through direct activation of LCK-mediated TCR signaling. EMBO Journal, 2022: 41(16). [CrossRef]
- Rheinländer, A.; Schraven, B.; Bommhardt, U. CD45 in human physiology and clinical medicine. Immunol Lett. 2018 Apr;196:22-32. [CrossRef]
- Inderberg, E.M.; Mensali, N.; Oksvold, M.P.; Fallang. L.E.; Fåne, A.; Skorstad, G.; Stenvik, G.E.; Progida, C.; Bakke, O.; Kvalheim, G.; Myklebust, J.H.; Wälchli, S. Human c-SRC kinase (CSK) overexpression makes T cells dummy. Cancer Immunol Immunother. 2018 Apr;67(4):525-536. [CrossRef]
- Zhu, S.; Wang, H.; Ranjan K, Zhang D. Regulation, targets and functions of CSK. Front Cell Dev Biol. 2023 Jun 16;11:1206539. [CrossRef]
- Hui, E., & Vale, R. D. (2014). In vitro membrane reconstitution of the T cell receptor proximal signaling network. Nature Structural & Molecular Biology, ,1(2), 133. [CrossRef]
- Kästle, M., Merten, C., Hartig, R.; Kaehne, T.; Liaunardy-Jopeace, A.; Woessner, N. M.; Schamel, W. W. A.; James, J. R., Minguet, S., Simeoni, L., Schraven, B. Tyrosine 192 within the SH2 domain of the Src-protein tyrosine kinase p56Lck regulates T-cell activation independently of Lck/CD45 interactions. Cell Comm Signal, 2020: 18(1). [CrossRef]
- Courtney, A. H.; Amacher, J.; Kadlecek, T. A.; Mollenauer, M.; Au-Yeung, B. B.; Kuriyan, J., Weiss, A. A Phosphosite within the SH2 Domain of Lck Regulates Its Activation by CD45. Molecular Cell, 2017; 67(3), 498-511.e6. [CrossRef]
- Kästle, M.; Merten, C.; Hartig, R.; Plaza-Sirvent, C.; Schmitz, I.; Bommhardt, U.; Schraven, B.; Simeoni, L. Type of PaperY192 within the SH2 Domain of Lck Regulates TCR Signaling Downstream of PLC-γ1 and Thymic Selection. Int J Mol Sci. 2022 Jun 30;23(13):7271. [CrossRef]
- Brian, B. F., 4th; Sjaastad, F. V.; Freedman, T. S. SH3-domain mutations selectively disrupt Csk homodimerization or PTPN22 binding. Scientific reports, 2022; 12(1), 5875. [CrossRef]
- Okada, M. Regulation of the SRC family kinases by Csk. Int J Mol Sci, 2012; 8(10), 1385–1397. [CrossRef]
- Hur, E.M.; Son, M.; Lee, O.H.; Choi, Y.B.; Park, C.; Lee, H.; Yun, Y. LIME, a novel transmembrane adaptor protein, associates with p56lck and mediates T cell activation. J Exp Med. 2003 Nov 17;198(10):1463-73. [CrossRef]
- Ventimiglia, L.N.; Alonso, M.A. The role of membrane rafts in Lck transport, regulation and signalling in T-cells. Biochem J. 2013 Sep 1;454(2):169-79. [CrossRef]
- Strazza, M.; Azoulay-Alfaguter, I.; Peled, M.; Adam, K.; Mor, A. Transmembrane adaptor protein PAG is a mediator of PD-1 inhibitory signaling in human T cells. Communications Biol, 2021: 4. [CrossRef]
- Schultz, A.; Schnurra, M.; El-Bizri, A.; Woessner, N. M.; Hartmann, S. E.; Hartig, R.; Minguet, S.; Schraven, B.; Simeoni, L. A Cysteine Residue within the Kinase Domain of Zap70 Regulates Lck Activity and Proximal TCR Signaling. Cells, 2022; 11(17), 2723. [CrossRef]
- Feng, S.; Cheng, X.; Zhang, L.; Lu, X.; Chaudhary, S.; Teng, R.; Frederickson, C.; Champion, M.M.; Zhao, R.; Cheng, L.; Gong, Y.; Deng, H.; Lu, X. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc Natl Acad Sci U S A. 2018 Oct 2;115(40):10094-10099. [CrossRef]
- Mohanasundaram, K.A.; Haworth, N.L.; Grover, M.P.; Crowley, T.M.; Goscinski, A.; Wouters, M.A. Potential role of glutathione in evolution of thiol-based redox signaling sites in proteins. Front Pharmacol. 2015 Mar 10;6:1. [CrossRef]
- Nakamura, K.; Hori, T.; Yodoi, J. Alternative binding of p56lck and phosphatidylinositol 3-kinase in T cells by sulfhydryl oxidation: implication of aberrant signaling due to oxidative stress in T lymphocytes. Mol Immunol. 1996 Jul;33(10):855-65. [CrossRef]
- Lasser, S. A.; Ozbay Kurt, F. G.; Arkhypov, I.; Utikal, J.; Umansky, V. Myeloid-derived suppressor cells in cancer and cancer therapy. Nature reviews. Clinical oncology, 2024; 21(2), 147–164. [CrossRef]
- Rudd, C.E. How the Discovery of the CD4/CD8-p56lck Complexes Changed Immunology and Immunotherapy. Front Cell Dev Biol. 2021 Mar 15;9:626095. [CrossRef]
- Liang, Y.; Ye, L. Bound to be perfect: Lck and T cell co-receptors. Nat Immunol. 2023 Jan;24(1):5-7. [CrossRef]
- Horkova, V.; Drobek, A.; Mueller, D.; Gubser, C.; Niederlova, V.; Wyss, L.; King, C.G.; Zehn, D.; Stepanek, O. Dynamics of the Coreceptor-LCK Interactions during T Cell Development Shape the Self-Reactivity of Peripheral CD4 and CD8 T Cells. Cell Rep. 2020 Feb 4;30(5):1504-1514.e7. [CrossRef]
- Qin, Z.; Hou, P.; Lin, H.; Chen, M.; Wang, R.; Xu, T. Inhibition of Lck/Fyn kinase activity promotes the differentiation of induced Treg cells through AKT/mTOR pathway. Int Immunopharmacol. 2024 Jun 15;134:112237. [CrossRef]
- Le Page, A,; Dupuis, G.; Larbi, A.; Witkowski, J.M., Fülöp T. Signal transduction changes in CD4+ and CD8+ T cell subpopulations with aging. Exp Gerontol. 2018 May; 105:128-139. [CrossRef]
- Tedeschi, V.; Paldino, G.; Kunkl, M.; Paroli, M.; Sorrentino, R.; Tuosto, L.; Fiorillo, M.T. CD8+ T Cell Senescence: Lights and Shadows in Viral Infections, Autoimmune Disorders and Cancer. Int J Mol Sci. 2022;23(6):3374. [CrossRef]
- Vieira Braga, F.A.; Hertoghs, K.M.; van Lier, R.A.; van Gisbergen, K.P. Molecular characterization of HCMV-specific immune responses: Parallels between CD8(+) T cells, CD4(+) T cells, and NK cells. Eur J Immunol. 2015 Sep;45(9):2433-45. [CrossRef]
- Esensten, J.H.; Helou, Y.A.; Chopra, G.; Weiss, A.; Bluestone, J.A. CD28 Costimulation: From Mechanism to Therapy. Immunity. 2016 May 17;44(5):973-88. [CrossRef]
- Paprckova, D.; Niederlova, V.; Moudra, A.; Drobek, A.; Pribikova, M.; Janusova, S.; Schober, K.; Neuwirth, A.; Michalik, J.; Huranova, M.; Horkova, V.; Cesnekova, M.; Simova, M.; Prochazka, J.; Balounova, J.; Busch, D.H.; Sedlacek, R.; Schwarzer, M.; Stepanek, O. Self-reactivity of CD8 T-cell clones determines their differentiation status rather than their responsiveness in infections. Front Immunol. 2022 Oct 6;13:1009198. [CrossRef]
- Huang, L.; Zhu, P.; Xia, P.; Fan, Z. WASH has a critical role in NK cell cytotoxicity through Lck-mediated phosphorylation. Cell Death Dis 2016; 7, e2301. [CrossRef]
- Moore, E.K.; Strazza, M.; Mor, A. Combination Approaches to Target PD-1 Signaling in Cancer. Front Immunol. 2022 Jul 14;13:927265. [CrossRef]
- Chiang, G. G.; Sefton, B. M. Specific Dephosphorylation of the Lck Tyrosine Protein Kinase at Tyr-394 by the SHP-1 Protein-tyrosine Phosphatase. J Biol Chem, 2001; 276(25), 23173-23178. [CrossRef]
- Poirier, A.; Ormonde, J. V. S.; Aubry, I.; Abidin, B. M.; Feng, C.; Martínez-Córdova, Z.; Hincapie, A. M.; Wu, C.; Pérez-Quintero, L. A.; Wang, C.; Gingras, A.; Madrenas, J.; Tremblay, M. L. The induction of SHP-1 degradation by TAOK3 ensures the responsiveness of T cells to TCR stimulation. Science Signaling, 2024; 17(817). [CrossRef]
- Celis-Gutierrez, J.; Blattmann, P.; Zhai, Y.; Jarmuzynski, N.; Ruminski, K.; Grégoire, C.; Ounoughene, Y.; Di Fiore, F.; Aebersold, R.; Roncagalli, R.; Gstaiger, M.; Malissen, B. (). Quantitative interactomics in primary T cells provides a rationale for concomitant PD-1 and BTLA coinhibitor blockade in cancer immunotherapy. Cell Reports, 2019; 27(11), 3315-3330.e7. [CrossRef]
- Li, K.; Yuan, Z.; Lyu, J.; Ahn, E.; Davis, S.J.; Ahmed, R.; Zhu, C. PD-1 suppresses TCR-CD8 cooperativity during T-cell antigen recognition. Nat Commun. 2021 May 12;12(1):2746. [CrossRef]
- Wang, R.; He, S.; Long, J.; Wang, Y.; Jiang, X.; Chen, M.; Wang, J. Emerging therapeutic frontiers in cancer: insights into posttranslational modifications of PD-1/PD-L1 and regulatory pathways. Exp Hematol Oncol. 2024 Apr 23;13(1):46. [CrossRef]
- Chyuan, I.T.; Liao, H.J.; Tan, T.H.; Chuang, H.C.; Chu, Y.C.; Pan, M.H.; Wu, C.S.; Chu, C.L.; Sheu, B.C.; Hsu, P.N. Association of TRAIL receptor with phosphatase SHP-1 enables repressing T cell receptor signaling and T cell activation through inactivating Lck. J Biomed Sci. 2024 Mar 27;31(1):33. [CrossRef]
- Graydon, C.G.; Mohideen, S.; Fowke, K.R. LAG3's Enigmatic Mechanism of Action. Front Immunol. 2021 Jan 8;11:615317. [CrossRef]
- Guy, C.; Mitrea, D.M.; Chou, P.C.; Temirov, J.; Vignali, K.M.; Liu, X.; Zhang, H.; Kriwacki, R.; Bruchez, M.P.; Watkins, S.C.; Workman, C.J.; Vignali, D.A.A. LAG3 associates with TCR-CD3 complexes and suppresses signaling by driving co-receptor-Lck dissociation. Nat Immunol. 2022 May;23(5):757-767. [CrossRef]
- Luke, J.J.; Patel, M.R.; Blumenschein, G.R. Hamilton, E.; Chmielowski, B.; et al. The PD-1- and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: a phase 1 trial. Nat Med 2023; 29, 2814–2824 (). [CrossRef]
- Binder, C.; Cvetkovski, F.; Sellberg, F.; Berg, S.; Paternina Visbal, H.; Sachs, D.H.; Berglund, E.; Berglund, D. CD2 Immunobiology. Front Immunol. 2020 Jun 9;11:1090. [CrossRef]
- Nunes, R.J.; Castro, M.A.; Gonçalves, C.M.; Bamberger, M.; Pereira, C.F.; Bismuth, G.; Carmo, A.M. Protein interactions between CD2 and Lck are required for the lipid raft distribution of CD2. J Immunol. 2008 Jan 15;180(2):988-97. [CrossRef]
- Burgueño-Bucio, E.; Mier-Aguilar, C.A.; Soldevila, G. The multiple faces of CD5. J Leukoc Biol. 2019 May;105(5):891-904. [CrossRef]
- Baaten, B.J.; Li, C.R.; Bradley, L.M. Multifaceted regulation of T cells by CD44. Commun Integr Biol. 2010 Nov;3(6):508-12. [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front Cell Dev Biol. 2017 Mar 7;5:18. [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol. 2018 May 10;11(1):64. [CrossRef]
- Duan, H.; Jing, L.; Jiang, X.; Ma, Y.; Wang, D.; Xiang, J.; Chen, X.; Wu, Z.; Yan, H.; Jia, J.; Liu, Z.; Feng, J.; Zhu, M.; Yan, X. CD146 bound to LCK promotes T cell receptor signaling and antitumor immune responses in mice. J Clin Invest. 2021 Nov 1;131(21):e148568. [CrossRef]
- Raychaudhuri, S.K.; Abria, C.; Raychaudhuri, S.P. Phenotype and pathological significance of MCAM+ (CD146+) T cell subset in psoriatic arthritis. Mol Biol Rep. 2021 Oct;48(10):6787-6796. [CrossRef]
- Al-Harbi, N.O.; Ahmad, S.F.; Almutairi, M.; Alanazi, A.Z.; Ibrahim, K.E.; Alqarni, S.A.; Alqahtani, F.; Alhazzani, K.; Alharbi, M.; Alasmari, F.; Nadeem, A. Lck signaling inhibition causes improvement in clinical features of psoriatic inflammation through reduction in inflammatory cytokines in CD4+ T cells in imiquimod mouse model. Cell Immunol. 2022 Jun;376:104531. [CrossRef]
- McArdel, S.L.; Terhorst, C.; Sharpe, A.H. Roles of CD48 in regulating immunity and tolerance. Clin Immunol. 2016 Mar;164:10-20. [CrossRef]
- Li, B.; Lu, Y.; Zhong, M.C.; Qian, J., Li, R.; Davidson, D.; Tang, Z.; Zhu, K.; Argenty, J.; de Peredo, A.G.; Malissen, B.; Roncagalli, R.; Veillette, A. Cis interactions between CD2 and its ligands on T cells are required for T cell activation. Sci Immunol. 2022 Aug 5; 7(74):eabn6373. [CrossRef]
- Bharti, R.; Dey, G.; Lin, F.; Lathia, J.; Reizes, O. CD55 in cancer: Complementing functions in a non-canonical manner. Cancer Lett. 2022 ;ec 28;551:215935. [CrossRef]
- Saygin, C.; Wiechert, A.; Rao, V.S.; Alluri, R.; Connor, E.; Thiagarajan, P.S.; Hale, J.S., et al.. CD55 regulates self-renewal and cisplatin resistance in endometrioid tumors. J Exp Med. 2017 Sep 4;214(9):2715-2732. [CrossRef]
- Giustiniani, J.; Bensussan, A.; Marie-Cardine, A. Identification and characterization of a transmembrane isoform of CD160 (CD160-TM), a unique activating receptor selectively expressed upon human NK cell activation. J Immunol. 2009 Jan 1;182(1):63-71. [CrossRef]
- Oumeslakht, L.; Aziz, A.I.; Bensussan, A.; Ben Mkaddem, S. CD160 receptor in CLL: Current state and future avenues. Front Immunol. 2022 Nov 7;13:1028013. [CrossRef]
- Zhan, F.; He, L.; Yu, Y.; Chen, Q., Guo, Y., Wang, L. A multimodal radiomic machine learning approach to predict the LCK expression and clinical prognosis in high-grade serous ovarian cancer. Sci Rep 2023; 13, 16397 (). [CrossRef]
- Wang, F.; Zheng, A.; Zhang, D.; Zou, T.; Xiao, M.; et al. Molecular profiling of core immune-escape genes highlights LCK as an immune-related prognostic biomarker in melanoma. Front Immunol. 2022 Oct 20;13:1024931. [CrossRef]
- Weiße, J.; Rosemann, J.; Müller, L.; Kappler, M.; Eckert, A. W.; Glaß, M., et al. Identification of lymphocyte cell-specific protein-tyrosine kinase (LCK) as a driver for invasion and migration of oral cancer by tumor heterogeneity exploitation. Mol Cancer 2021; 20, 88 . [CrossRef]
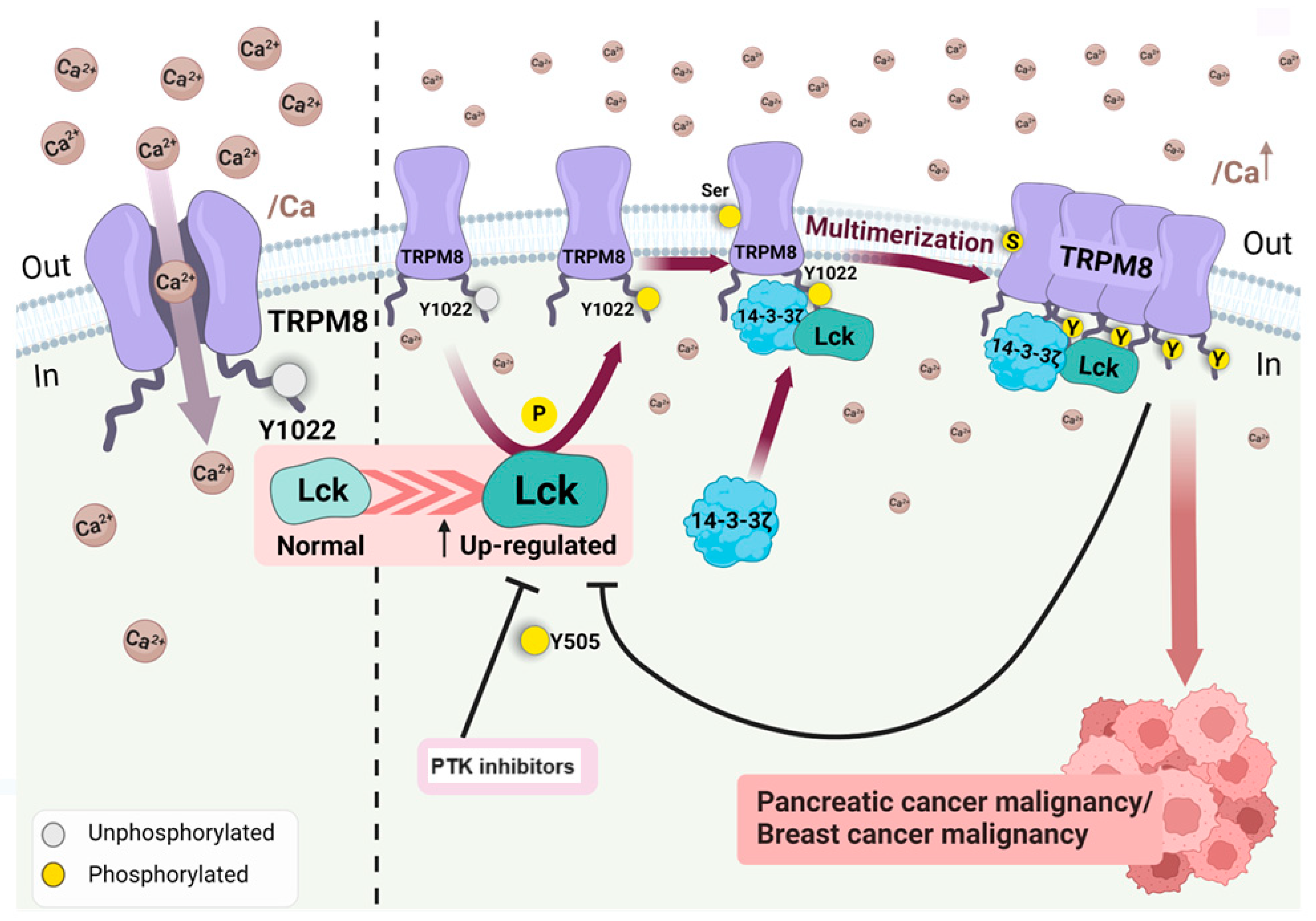
- Huang, Y.; Li, S.; Liu, Q; et al. The LCK-14-3-3ζ-TRPM8 axis regulates TRPM8 function/assembly and promotes pancreatic cancer malignancy. Cell Death Dis 2022; 13, 524. [CrossRef]
- Honikel, M.M.; Olejniczak, S.H. Co-Stimulatory Receptor Signaling in CAR-T Cells. Biomolecules. 2022 Sep 15;12(9):1303. [CrossRef]
- Curio, S.; Jonsson, G.; Marinović, S. A summary of current NKG2D-based CAR clinical trials. Immunother Adv. 2021 Aug 13;1(1):ltab018. [CrossRef]
- Czaplicka, A.; Lachota, M.; Pączek, L.; Zagożdżon, R.; Kaleta, B. Chimeric Antigen Receptor T Cell Therapy for Pancreatic Cancer: A Review of Current Evidence. Cells. 2024 Jan 4;13(1):101. [CrossRef]
- Deng, Y.; Kumar, A.; Xie, K.; Schaaf, K.; Scifo, E.; Morsy, S.; Li, T.; Ehninger, A.; Bano, D.; Ehninger, D. Targeting senescent cells with NKG2D-CAR T cells. Cell Death Discov. 2024 May 4;10(1):217. [CrossRef]
- Acharya, S.; Basar, R.; Daher, M.; Rafei, H.; Li, P.; Uprety, N, et al.. CD28 costimulation augments CAR signaling in NK cells via the LCK/CD3Z/ZAP70 signaling axis. Cancer Discov. 2024 Jun 20. [CrossRef]
- Wu, L.; Brzostek, J.; Sakthi Vale, P.D.; Wei, Q.; Koh, C.K.T.; et al.. CD28-CAR-T cell activation through FYN kinase signaling rather than LCK enhances therapeutic performance. Cell Rep Med. 2023 Feb 21;4(2):100917.. [CrossRef]
- Zhang, J.; Jiang, Z.; Zhang, X.; Yang, Z.; Wang, J.; Chen, J.; et al. THEMIS is a substrate and allosteric activator of SHP1, playing dual roles during T cell development. Nat Struct Mol Biol. 2024 Jan;31(1):54-67. [CrossRef]
- Goldsmith, M.A.; Weiss A. Isolation and characterization of a T-lymphocyte somatic mutant with altered signal transduction by the antigen receptor. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6879-83. [CrossRef]
- Oh-Hori, N.; Koga, Y.; Yoshida, H.; Morita, M.; Kimura, G.; Nomoto, K. Human T-cell leukemia virus type-I-infected T-cell lines scarcely produce p56lck, whether or not they express lck mRNA. Int J Cancer. 1990 Aug 15;46(2):315-9. [CrossRef]
- Hauck, F.; Randriamampita, C.; Martin, E.; Gerart, S.; Lambert, N.; Lim, A.; Soulier, J.; Maciorowski, Z.; Touzot, F.; Moshous, D.; et al. Primary T-Cell Immunodeficiency with Immunodysregulation Caused by Autosomal Recessive LCK Deficiency. J Allergy Clin Immunol 2012, 130, 1144-1152.e11. [CrossRef]
- Lanz, A.-L.; Erdem, S.; Ozcan, A.; Ceylaner, G.; Cansever, M.; Ceylaner, S.; Conca, R.; Magg, T.; Acuto, O.; Latour, S.; et al. A Novel Biallelic LCK Variant Resulting in Profound T-Cell Immune Deficiency and Review of the Literature. J Clin Immunol 2023, 44, 1. [CrossRef]
- Lui, V.G.; Hoenig, M.; Cabrera-Martinez, B.; Baxter, R.M.; Garcia-Perez, J.E., et al.. A partial human LCK defect causes a T cell immunodeficiency with intestinal inflammation. J Exp Med. 2024 Jan 1;221(1):e20230927. [CrossRef]
- Lanz, A.L.; Erdem, S.; Ozcan, A.; Ceylaner, G.; et al. Novel Biallelic LCK Variant Resulting in Profound T-Cell Immune Deficiency and Review of the Literature. J Clin Immunol. 2023 Dec 15;44(1):1. [CrossRef]
- Hulme, J.S.; Barratt, B.J.; Twells, R.C.J.; Cooper, J.D.; Lowe, C.E.; Howson, J.M.M.; et al. Association Analysis of the Lymphocyte-Specific Protein Tyrosine Kinase (LCK) Gene in Type 1 Diabetes. Diabetes 2004, 53, 2479–2482. [CrossRef]
- Zhu, Q.; Wang, J.; Zhang, L.; Bian, W.; Lin, M.; Xu, X.; Zhou, X. LCK Rs10914542-G Allele Associates with Type 1 Diabetes in Children via T Cell Hyporesponsiveness. Pediatr Res 2019, 86, 311–315. [CrossRef]
- Han, M.; Li, Y.; Guo, Y.; Zhu, W.; Jiang, J. Integrative and Comprehensive Pan-Cancer Analysis of Lymphocyte-Specific Protein Tyrosine Kinase in Human Tumors. Int J Mol Sci. 2022 Nov 13;23(22):13998. [CrossRef]
- Bai, F.; Jin, Y.; Zhang, P.; Chen, H.; Fu, Y.; Zhang, M.; Weng, Z.; Wu K. Bioinformatic profiling of prognosis-related genes in the breast cancer immune microenvironment. Aging (Albany NY). 2019 Nov 12;11(21):9328-9347. [CrossRef]
- Elkamhawy, A.; Ali, E.M.H.; Lee, K. New horizons in drug discovery of lymphocyte-specific protein tyrosine kinase (Lck) inhibitors: a decade review (2011-2021) focussing on structure-activity relationship (SAR) and docking insights. J Enzyme Inhib Med Chem. 2021 Dec;36(1):1574-1602. [CrossRef]
- Roskoski, R Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2024 update. Pharmacol Res. 2024 Feb;200:107059. [CrossRef]
- Musumeci, F.; Schenone, S. Unlocking Potential and Limits of Kinase Inhibitors: The Highway to Enhanced Cancer Targeted Therapy. Pharmaceutics. 2024 May 7;16(5):625. [CrossRef]
- Bommhardt, U.; Schraven, B.; Simeoni, L. Beyond TCR Signaling: Emerging Functions of Lck in Cancer and Immunotherapy. Int J Mol Sci. 2019 Jul 16;20(14):3500. [CrossRef]
- Zhang, J.; Wu, Y.J.; Hu, X.X.; Wei, W. New insights into the Lck-NF-κB signaling pathway. Front Cell Dev Biol. 2023 Feb 24;11:1120747. [CrossRef]
- Cheng, Y.; Ji, C.; Xu, J.; Chen, R.; Guo, Y.; Bian, Q.; Shen, Z.; Zhang, B. LCK-SafeScreen-Model: An Advanced Ensemble Machine Learning Approach for Estimating the Binding Affinity between Compounds and LCK Target. Molecules. 2023 Nov 1;28(21):7382. [CrossRef]
- Schindler, C.G.; Armbrust, T.; Gunawan, B.; Langer, C.; Füzesi, L.; Ramadori, G. Gastrointestinal stromal tumor (GIST) -- single center experience of prolonged treatment with imatinib. Z Gastroenterol. 2005 Mar;43(3):267-73. [CrossRef]
- Schlemmer, M.; Bauer, S.; Schütte, R.; Hartmann, J.T.; Bokemeyer, C.; Hosius, C, Reichardt, P. Activity and side effects of imatinib in patients with gastrointestinal stromal tumors: data from a German multicenter trial. Eur J Med Res. 2011 May 12;16(5):206-12. [CrossRef]
- Lam, T.J.R.; Udonwa, S.A.; Masuda, Y.; Yeo, M.H.X.; Farid Bin Harunal Ras, M., Goh, B.K.P. A systematic review and meta-analysis of neoa;djuvant imatinib use in locally advanced and metastatic gastrointestinal stromal tumors. World J Surg. 2024 May 17. [CrossRef]
- Karim, N.A.; Ullah, A.; Wang, H.; Shoukier, M.; Pulliam, S.; Khaled, A.; Patel, N.; Morris, J.C. A Phase I Study of the Non-Receptor Kinase Inhibitor Bosutinib in Combination with Pemetrexed in Patients with Selected Metastatic Solid Tumors. Curr Oncol. 2022 Dec 3;29(12):9461-9473. [CrossRef]
- Deplanque, G.; Demarchi, M.; Hebbar, M.; Flynn, P.; Melichar, B.; Atkins, J.; Nowara, E.; Moyé, L. et al. A randomized, placebo-controlled phase III trial of masitinib plus gemcitabine in the treatment of advanced pancreatic cancer. Ann Oncol. 2015 Jun;26(6):1194-1200. [CrossRef]
- Adenis, A.; Blay, J.Y.; Bui;Nguyen, B.; Bouché, O.; Bertucci, F.; et al.. Masitinib in advanced gastrointestinal stromal tumor (GIST) after failure of imatinib: a randomized controlled open-label trial. Ann Oncol. 2014 Sep;25(9):1762-1769. [CrossRef]
- Le Cesne, A.; Blay, J.Y.; Bui, B.N.; Bouché, O.; Adenis, A. et al. Phase II study of oral masitinib mesilate in imatinib-naïve patients with locally advanced or metastatic gastro-intestinal stromal tumour (GIST). Eur J Cancer. 2010 May;46(8):1344-51. [CrossRef]
- Larkin, J.; Marais, R.; Porta, N.; Gonzalez de Castro, D.; Parsons, L.; Messiou, C.; et al. Nilotinib in KIT-driven advanced melanoma: Results from the phase II single-arm NICAM trial. Cell Rep Med. 2024 Mar 19;5(3):101435. [CrossRef]
- Mishra R. Oral tumor heterogeneity, its implications for patient monitoring and designing anti-cancer strategies. Pathology, research and practice, 2024; 253, 154953. [CrossRef]
- Barnwal, A.; Das, S.; Bhattacharyya, J. Repurposing Ponatinib as a PD-L1 Inhibitor Revealed by Drug Repurposing Screening and Validation by In Vitro and In Vivo Experiments. ACS Pharmacol Transl Sci. 2023 Jan 12;6(2):281-289. [CrossRef]
- Li, L.; Cui, Y.; Shen, J.; Dobson, H.; Sun, G. Evidence for activated Lck protein tyrosine kinase as the driver of proliferation in acute myeloid leukemia cell, CTV-1. Leuk Res. 2019 Mar;78:12-20. [CrossRef]
- Zepecki, J.P.; Snyder, K.M.; Moreno, M.M.; Fajardo, E.; Fiser, A.; Ness, J.; Sarkar, A.; Toms, S.A.; Tapinos, N. Regulation of human glioma cell migration, tumor growth, and stemness gene expression using a Lck targeted inhibitor. Oncogene. 2019 Mar;38(10):1734-1750. [CrossRef]
- Harada, D.; Isozaki, H.; Kozuki, T.; Yokoyama, T.; Yoshioka, H.; Bessho, A. et al. Crizotinib for recurring non-small-cell lung cancer with EML4-ALK fusion genes previously treated with alectinib: A phase II trial. Thorac Cancer. 2021 Mar;12(5):643-649. [CrossRef]
- Camidge, D.R.; Bang, Y.J.; Kwak, E.L.; Iafrate, A.J.; Varella-Garcia, M. , et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012 Oct;13(10):1011-9. [CrossRef]
- Cruz, B.D.; Barbosa, M.M.; Torres, L.L.; Azevedo, P.S.; Silva, V.E.A.; Godman, B.; Alvares-Teodoro, J. Crizotinib Versus Conventional Chemotherapy in First-Line Treatment for ALK-Positive Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Oncol Ther. 2021 Dec;9(2):505-524. [CrossRef]
- Tanaka, A.; Nishikawa, H.; Noguchi, S.; Sugiyama, D.; Morikawa, H.; Takeuchi, Y. et al. Tyrosine kinase inhibitor imatinib augments tumor immunity by depleting effector regulatory T cells. J Exp Med. 2020 Feb 3;217(2):e20191009. [CrossRef]
- Burchat, A.; Borhani, D.W.; Calderwood, D.J.; Hirst, G.C.; Li, B.; Stachlewitz, R.F. Discovery of A-770041, a src-family selective orally active lck inhibitor that prevents organ allograft rejection. Bioorg Med Chem Lett. 2006 Jan 1;16(1):118-22. [CrossRef]
- Kumar Singh, P.; Kashyap, A.; Silakari, O. Exploration of the therapeutic aspects of Lck: A kinase target in inflammatory mediated pathological conditions. Biomed Pharmacother. 2018 Dec;108:1565-1571. [CrossRef]
- Kagawa, K.; Sato, S.; Koyama, K.; Imakura, T.; Murakami, K.; Yamashita, Y.; Naito, N.; Ogawa, H.; Kawano, H.; Nishioka, Y. The lymphocyte-specific protein tyrosine kinase-specific inhibitor A-770041 attenuates lung fibrosis via the suppression of TGF-β production in regulatory T-cells. PLoS One. 2022 Oct 27;17(10):e0275987. [CrossRef]
- Alqarni, S.A.; Bineid, A.; Ahmad, S.F.; Al-Harbi, N.O.; Alqahtani, F.; Ibrahim, K.E.; Ali, N.; Nadeem, A. Blockade of Tyrosine Kinase, LCK Leads to Reduction in Airway Inflammation through Regulation of Pulmonary Th2/Treg Balance and Oxidative Stress in Cockroach Extract-Induced Mouse Model of Allergic Asthma. Metabolites. 2022 Aug 25;12(9):793. [CrossRef]
- Carter, N.M.; Pomerantz, J.L. Calcineurin inhibitors target Lck activation in graft-versus-host disease. J Clin Invest. 2021 Jun 1;131(11):e149934. [CrossRef]
- Olivieri, A.; Mancini, G.; Olivieri, J.; Marinelli Busilacchi, E.; Cimminiello, M. et al. Nilotinib in steroid-refractory cGVHD: prospective parallel evaluation of response, according to NIH criteria and exploratory response criteria (GITMO criteria). Bone Marrow Transplant. 2020 Nov;55(11):2077-2086. [CrossRef]
- Srour, M.; Alsuliman, T.; Labreuche, J.; Bulabois, C.E.; Chevallier, P. et al. Nilotinib efficacy and safety as salvage treatment following imatinib intolerance and/or inefficacy in steroid refractory chronic graft-versus-host-disease (SR-cGVHD): a prospective, multicenter, phase II study on behalf of the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC). Bone Marrow Transplant. 2023 Apr;58(4):401-406. [CrossRef]
- Lin, C.T.; Hsueh, P.R.; Wu, S.J.; Yao, M.; Ko, B.S.; Li, C.C.; Hsu, C.A.; Tang, J.L.; Tien, H.F. Repurposing Nilotinib for Cytomegalovirus Infection Prophylaxis after Allogeneic Hematopoietic Stem Cell Transplantation: A Single-Arm, Phase II Trial. Biol Blood Marrow Transplant. 2018 Nov;24(11):2310-2315. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




