1. Introduction
Candidiasis is a notable fungal infection caused by opportunistic yeasts belonging to the genus
Candida. These fungi are commonly found in the environment and as part of the normal flora of various animal species. Under certain conditions, however,
Candida species can overgrow and lead to infection, resulting in significant health issues. Among the various animals affected by candidiasis,
Choloepus species (two-toed sloths) have garnered attention due to their unique physiology and the increasing number of cases reported, particularly in captive environments [
1,
2,
3,
4].
Choloepus species, comprising
Choloepus hoffmanni and
Choloepus didactylus are arboreal mammals native to Central and South America. These sloths are characterized by their slow metabolism, specialized diet, and distinctive behaviors, all of which contribute to their unique health care needs. The rise in popularity of sloths in captivity, whether in zoos, rescue centers, or as exotic pets, has led to increased scrutiny of their health and well-being. Among the health challenges faced by captive sloths, candidiasis has emerged as a significant concern [
1,
4].
Candidiasis in
Choloepus sp. can manifest in various forms, including oral thrush, cutaneous lesions, and gastrointestinal infections. These infections can compromise the overall health of the sloths, leading to weight loss, discomfort, and secondary infections. Factors such as stress, poor nutrition, compromised immune function, and inadequate husbandry practices are often implicated as predisposing factors for the development of candidiasis in these animals [
5,
6].
The aim of this review is to provide a comprehensive overview of candidiasis in Choloepus sp., encompassing the etiology, epidemiology, pathogenesis, clinical manifestations, diagnosis, treatment, and prevention of this infection. By synthesizing current knowledge and identifying gaps in research, this review seeks to enhance our understanding of candidiasis in two-toed sloths and improve strategies for their care and management in both captive and wild settings. Through a detailed exploration of this disease, we aim to contribute to the growing body of veterinary literature focused on the health and conservation of these unique and captivating animals.
2. Candida
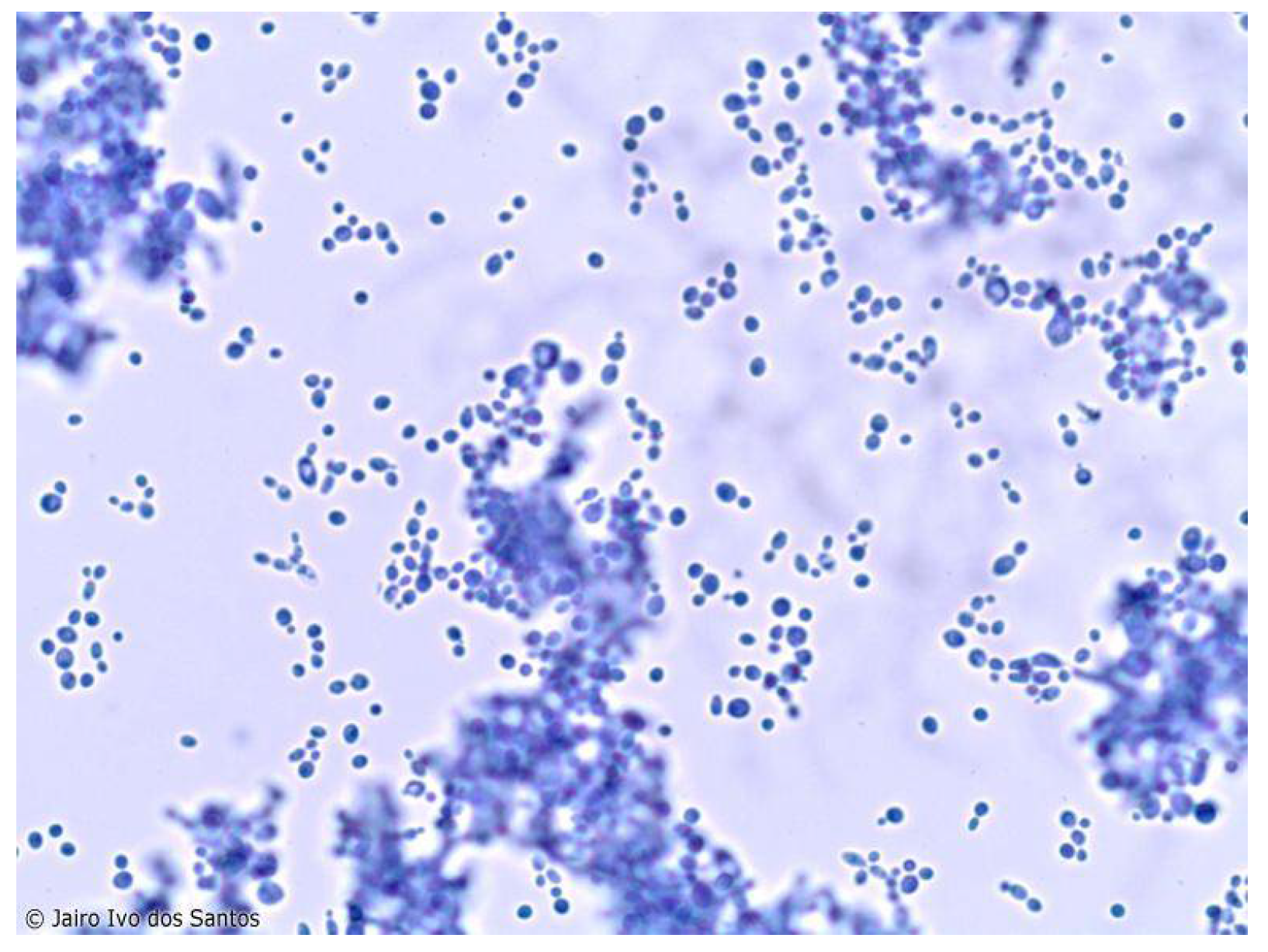
The genus
Candida sp. is composed of eukaryotic yeasts that lack photosynthetic pigments, are asexual and dimorphic, and are covered by a cell wall rich in ergosterol and chitin, using carbon dioxide absorbed from the environment as their main energy source. The cell wall of
Candida species is rich in chitin and ergosterol, which are key to maintaining their structural integrity and resistance to environmental stress.
Candida species primarily reproduce asexually through budding, although some species can also undergo sexual reproduction. The dimorphic nature of these fungi allows them to switch between yeast and filamentous forms, enhancing their ability to invade host tissues and evade the immune response [
1,
2,
7] (
Figure 1).
Candida species are facultative anaerobes capable of utilizing various carbon sources, including glucose and, uniquely, carbon dioxide absorbed from the environment. This metabolic flexibility allows them to thrive in diverse environments, from mucosal surfaces to the bloodstream.They are considered commensal fungi that naturally inhabit the mucosal microbiota of animals and humans, with more than 200 species already described [
1,
2,
3,
4]. However, there are increasing reports of infections by
Candida sp. linked to predisposing factors such as age, indiscriminate use of medications, and immunosuppression of individuals, with the most affected anatomical sites being the integument, urinary, gastrointestinal, and reproductive tracts [
7].
The main species considered zoonotic and associated with infection in domestic and wild animals are
Candida albicans,
C. tropicalis,
C. parapsilosis, and
C. guilliermondii, with the first responsible for 60% of isolations in humans due to disruptions in the parasite-host balance facilitating the adherence and multiplication of the agents. Consequently, these yeasts have become an important public health problem due to the high resistance of the agents to conventional treatments and difficulties in isolation and diagnosis [
3,
4,
8]. The distribution of Candida species shows regional variations. For instance,
C. albicans is universally prevalent, while
C. tropicalis is more common in tropical and subtropical regions.
C. glabrata and
C. krusei have been increasingly reported in Europe and North America, reflecting changes in antifungal resistance patterns [
8].
The prevalence of
Candida infections varies globally, with higher rates observed in hospital settings due to invasive procedures and the use of broad-spectrum antibiotics. In the United States and Europe,
Candida species are a leading cause of nosocomial infections, particularly in immunocompromised patients. In developing countries, the burden of
Candida infections is also significant, often exacerbated by limited access to healthcare and diagnostic facilities [
3,
4,
9].
Antifungal resistance is a growing concern worldwide.
C. glabrata and
C. krusei exhibit high levels of resistance to azole antifungals, complicating treatment strategies. The emergence of multidrug-resistant strains, particularly in hospital settings, underscores the need for ongoing surveillance and the development of new antifungal agents [
1,
10].
Candida species are versatile and adaptive pathogens with significant clinical and public health implications. Understanding their microbiological characteristics, species diversity, and global occurrence is crucial for developing effective prevention and treatment strategies. Continued research and surveillance are essential to address the challenges posed by antifungal resistance and to improve outcomes for individuals affected by
Candida infections.This review highlights the importance of a comprehensive approach to studying
Candida, integrating microbiological insights with epidemiological data to inform better clinical practices and public health policies [
2,
3,
4,
10].
3. Epidemiology
Regarding wild animals, the significant increase in emerging fungal diseases has affected the survival of certain populations, favoring the extinction process of more susceptible species and the greater spread of zoonoses. Therefore, investing in efficient diagnostic techniques also becomes essential for the maintenance of individuals both in the wild and in captivity, in addition to favoring the understanding of diseases that are still poorly reported in terms of clinical and health aspects [
2,
3,
4].
The presence of fungi with high zoonotic potential has already been described in this superorder, requiring attention due to their potential capacity to develop diseases in humans, such as Paracoccidioides brasiliensis, detected in organ samples like the liver and spleen of armadillos (
Dasypus novemcinctus) examined in the Tucuruí region, Pará, Brazil. This data is extremely important for understanding the ecology of fungi due to the high consumption of the meat and viscera of this species by native communities [
11].
Candida sp. has been described in bears, dolphins, wild felines, primates, birds, and xenarthrans as one of the main pathogenic fungi of importance in captivity, mainly affecting the gastrointestinal tract of young animals. The primary mode of transmission is direct contact or contact with surfaces containing contaminated feces, which underscores the importance of quarantines, use of personal protection when handling materials and animals, and thorough cleaning of enclosures and utensils due to the high zoonotic potential of the agents [
12,
13].
Candidiasis is also frequently associated with morbidity and mortality in birds due to impairments in the normal intestinal microbiota. Generally, these individuals can maintain yeasts in non-pathogenic quantities in the gastrointestinal tract, but imbalances associated with stress, indiscriminate use of antibiotics, and nutritional deficiencies favor the propagation and dissemination of
Candida, which becomes pathogenic, causing serious systemic damage to individuals that can lead to death [
2,
3,
4,
12,
13].
4. Pathogenis
The pathogenesis of candidiasis in
Choloepus species (two-toed sloths) is multifactorial, involving interactions between the host's immune system, the virulence of Candida species, and environmental conditions. In healthy sloths,
Candida species are typically commensal organisms residing in the mucosal surfaces of the gastrointestinal tract, skin, and other areas without causing disease. However, when the host's immune defenses are compromised, these fungi can become opportunistic pathogens [
2,
3,
4,
14].
Several factors can predispose
Choloepus sp. to candidiasis, including stress, poor nutrition, concurrent infections, and immunosuppressive conditions. Stress, which can arise from environmental changes, captivity, or handling, can weaken the immune response, allowing
Candida species to overgrow. Nutritional deficiencies, particularly in vitamins and minerals, can also impair the immune system, making sloths more susceptible to infections [
3,
4,
14,
15].
Candida species exhibit several virulence factors that enhance their pathogenicity. These include the ability to switch between yeast and hyphal forms, secretion of hydrolytic enzymes like proteases and phospholipases, and the formation of biofilms. The yeast-to-hyphal transition is crucial for tissue invasion and immune evasion, while hydrolytic enzymes facilitate the breakdown of host tissues and promote dissemination. Biofilm formation on mucosal surfaces and medical devices further complicates treatment by providing a protective environment against antifungal agents and the host immune system [
3,
4,
16,
17].
Once
Candida invades the host tissues, it triggers an inflammatory response. The fungi adhere to epithelial cells, invade the mucosal barrier, and proliferate, causing localized or systemic infections. In
Choloepus sp., candidiasis can affect various organs, leading to different clinical manifestations depending on the site of infection[
3,
4,
18,
19].
5. Clinical Manifestations
The clinical manifestations of candidiasis in
Choloepus sp. are diverse and depend on the site and severity of the infection. In many cases, the disease presents with nonspecific symptoms, making diagnosis challenging without laboratory confirmation. Common clinical signs include weight loss, lethargy, and poor coat condition, which may be indicative of systemic infection or underlying health issues [
2,
3,
4,
14,
15].
Oral candidiasis, or thrush, is one of the more recognizable forms of the disease, presenting as white, curd-like plaques on the mucous membranes of the mouth and tongue. Affected sloths may exhibit difficulty eating, drooling, and oral discomfort. Cutaneous candidiasis can present as red, inflamed skin lesions, often in areas subjected to moisture and friction, such as skin folds and the perineal area [
7,
12].
Gastrointestinal candidiasis is particularly concerning in young sloths, where it can lead to severe digestive disturbances. Symptoms include diarrhea, bloating, and anorexia. In severe cases, the infection can spread to the bloodstream, leading to systemic candidiasis or candidemia. This disseminated form of the disease can affect multiple organs, including the liver, spleen, kidneys, and lungs, and is often fatal if not promptly treated [
13].
Respiratory candidiasis, though less common, can occur in immunocompromised individuals or those with underlying respiratory conditions. Signs include nasal discharge, coughing, and difficulty breathing. The presence of
Candida species in the respiratory tract can exacerbate pre-existing conditions and lead to secondary bacterial infections [
3,
4,
14].
The varied clinical presentations of candidiasis in
Choloepus sp. necessitate a thorough clinical examination and the use of diagnostic tools such as culture, histopathology, and molecular techniques to confirm the presence of
Candida and determine the appropriate treatment. Early detection and intervention are critical to managing the infection and improving the prognosis for affected sloths [
3,
4,
14,
20].
Most wild species exhibit symptoms including inappetence, emesis, diarrhea, skin lesions, and lethargy. Diagnosis is similar to that performed in small animals, making the correct identification of the agent crucial. Different
Candida species affect these individuals, facilitating specific treatment and avoiding the development of drug resistance and the death of very sensitive animals [
3,
4,
14,
15,
19,
20].
6. Diagnostic and Tratament
Diagnosing candidiasis in
Choloepus species (two-toed sloths) requires a combination of clinical observation, laboratory tests, and advanced diagnostic techniques. Given the nonspecific nature of clinical symptoms, accurate identification of
Candida infections is essential for effective treatment and management [
21].The diagnostic process begins with a thorough clinical examination, noting any signs such as oral plaques, cutaneous lesions, gastrointestinal disturbances, or respiratory symptoms. Since these signs are not unique to candidiasis, further diagnostic tests are necessary to confirm the presence of
Candida species [
22].
One of the primary diagnostic tools is the culture of samples from affected tissues or bodily fluids. Samples can be taken from oral swabs, skin scrapings, fecal matter, or blood, depending on the suspected site of infection. These samples are cultured on Sabouraud dextrose agar or chromogenic media to encourage the growth of
Candida colonies. Positive cultures are followed by microscopic examination to identify the characteristic budding yeast cells and pseudohyphae, which are indicative of
Candida species [
21,
22].
Histopathological examination of biopsied tissues provides additional confirmation. Tissue samples are stained with periodic acid-Schiff (PAS) or Gomori methenamine silver (GMS) stains, which highlight the fungal elements within the tissue. This method helps to visualize the extent of tissue invasion and inflammation, providing insights into the severity of the infection [
22].
Molecular diagnostic techniques, such as polymerase chain reaction (PCR), offer high sensitivity and specificity for detecting
Candida DNA. PCR assays can identify and differentiate between various
Candida species, which is crucial for tailoring treatment plans. These methods are particularly useful in cases where traditional culture methods may be inconclusive or when rapid diagnosis is required [
21,
23].
Serological tests can also aid in diagnosing candidiasis by detecting antibodies or antigens associated with
Candida infections. However, these tests are less commonly used in veterinary practice and may have limited specificity due to cross-reactivity with other fungal pathogens [
3,
4,
21,
22,
23,
24].
Effective treatment of candidiasis in
Choloepus sp. involves a combination of antifungal therapy, supportive care, and environmental management. The choice of antifungal agent and treatment duration depends on the severity and location of the infection, as well as the specific
Candida species involved [
14,
25].
Azole antifungals, such as fluconazole and itraconazole, are commonly used to treat candidiasis in sloths. These drugs inhibit ergosterol synthesis, disrupting fungal cell membrane integrity. For systemic infections, fluconazole is often preferred due to its excellent oral bioavailability and ability to penetrate various tissues. Itraconazole may be used for refractory cases or when a broader spectrum of antifungal activity is needed [
14,
26,
27,
28,
29,
30].
Echinocandins, such as caspofungin, are another class of antifungals that inhibit the synthesis of β-(1,3)-D-glucan, an essential component of the fungal cell wall. These agents are particularly effective against
Candida species that exhibit resistance to azoles. However, their use in veterinary medicine is less common due to cost and availability.For topical infections, nystatin or clotrimazole can be applied directly to affected areas. These antifungals are effective for treating oral thrush and cutaneous lesions by disrupting the fungal cell membrane. Supportive care plays a crucial role in the treatment of candidiasis. Ensuring proper nutrition, hydration, and stress reduction is essential for the recovery of affected sloths. Providing a balanced diet rich in essential nutrients can help bolster the immune system and promote healing. In cases of gastrointestinal candidiasis, probiotics may be administered to restore healthy gut flora and prevent further fungal overgrowth. Environmental management is critical to prevent the recurrence of candidiasis. This includes maintaining clean and dry living conditions, reducing environmental stressors, and implementing proper sanitation protocols. Regular cleaning and disinfection of enclosures, feeding utensils, and water sources can minimize the risk of reinfection [
14,
25,
26,
27,
28,
29,
30].
Quarantine measures should be in place for newly acquired or symptomatic animals to prevent the spread of infection. Additionally, the use of personal protective equipment (PPE) by caregivers can reduce the risk of zoonotic transmission and protect both the animals and humans involved in their care.Regular monitoring and follow-up are essential to assess the effectiveness of the treatment and make necessary adjustments. Re-evaluation through clinical examination and laboratory tests ensures that the infection is under control and helps in early detection of any potential relapses.Combining these diagnostic tools and therapeutic protocols provides a comprehensive approach to managing candidiasis in
Choloepus sp., improving outcomes and ensuring the health and well-being of these unique animals [
14,
25].
7. Prevention and Control
Effective prevention and control strategies for candidiasis in
Choloepus species (two-toed sloths) are crucial to maintaining the health of these animals, especially in captive environments. These strategies involve a combination of environmental management, nutritional support, stress reduction, and routine health monitoring [
31].
Maintaining a clean and hygienic environment is fundamental to preventing candidiasis. Enclosures should be kept clean and dry to minimize the growth and spread of fungal spores. Regular cleaning protocols should include the thorough disinfection of surfaces, feeding utensils, and water sources using antifungal agents that are safe for use around animals. Proper ventilation within enclosures helps reduce humidity levels, which is important as
Candida species thrive in moist conditions. Additionally, bedding materials should be regularly changed and kept dry to prevent fungal colonization [
1,
2,
3,
4].
Providing a balanced and nutritionally adequate diet is essential for the prevention of candidiasis. Sloths require a diet rich in vitamins, minerals, and other essential nutrients to support a robust immune system. Nutritional deficiencies can compromise the immune response, making sloths more susceptible to infections. Diets should be tailored to meet the specific needs of
Choloepus sp., ensuring they receive adequate fiber, protein, and micronutrients. Supplementation with probiotics can also be beneficial in maintaining a healthy gut microbiota, which plays a role in preventing fungal overgrowth [
1,
2,
3,
4].
Stress is a significant predisposing factor for candidiasis in sloths. Stressful conditions, such as frequent handling, inadequate social interactions, and sudden changes in the environment, can weaken the immune system and increase susceptibility to infections. To mitigate stress, enclosures should mimic the natural habitat as closely as possible, providing ample climbing structures, foliage, and hiding spots. Caregivers should minimize handling and ensure that interactions with the sloths are calm and gentle. Additionally, efforts should be made to maintain a stable and predictable environment, avoiding sudden changes that could stress the animals [
31].
Regular health monitoring and early detection are key components of candidiasis prevention. Routine veterinary check-ups, including physical examinations and laboratory tests, can help identify early signs of infection before they become severe. Monitoring weight, appetite, and behavior can provide valuable insights into the health status of the sloths. Any deviations from normal should prompt further investigation, including cultures and molecular diagnostics, to rule out candidiasis or other infections [
1,
2,
3,
4].
Implementing quarantine protocols for new or symptomatic animals is essential to prevent the spread of candidiasis within captive populations. Newly acquired sloths should be quarantined for a period sufficient to monitor for signs of infection and to perform necessary health screenings. Symptomatic animals should be isolated from the healthy population to prevent cross-contamination. Caregivers should use personal protective equipment (PPE) when handling quarantined or isolated animals to reduce the risk of zoonotic transmission and contamination [
31].
Educating caregivers and staff about the importance of hygiene, stress reduction, and proper animal handling techniques is vital for the prevention and control of candidiasis. Training programs should cover the identification of early signs of infection, the implementation of cleaning and disinfection protocols, and the proper use of PPE. Informed and vigilant caregivers are better equipped to maintain a healthy environment and respond promptly to potential health issues [
31].
By integrating these prevention and control measures, the incidence of candidiasis in
Choloepus sp. can be significantly reduced. These strategies not only improve the overall health and well-being of captive sloths but also contribute to the broader efforts in wildlife conservation and management [
14,
31].
8. Choloepus sp.
The two-toed sloths of the genus
Choloepus belong to the superorder Xenarthra, order Pilosa, and family Megalonychidae. There are two species,
Choloepus didactylus and
Choloepus hoffmanni, which are mainly differentiated by the lighter color of the neck fur in
C. hoffmanni [
15,
32] (
Figure 3).
These are placental mammals known for their lethargic habits and low-energy diet consisting of leaves and fruits. Two-toed sloths can reach up to 86 cm in length and 8.4 kg in weight when fully grown. They are primarily distributed in the tropical forests from Venezuela to Peru and throughout the Brazilian Amazon [
6,
33,
34].
Due to intense habitat fragmentation, the presence of sloths, armadillos, and anteaters in captivity is increasing due to various human-associated problems such as road accidents, electric shocks, and burns. In captivity, these species become more susceptible to developing infectious diseases, either due to cross-contamination, the presence of comorbidities that make the animal more vulnerable, or close contact with pathogens in the hospital environment [
14,
35].
Diseases affecting captive sloths vary according to several factors such as the animal's age and clinical condition upon arrival, management, nutrition, and whether it has had contact with other individuals and potential anthropogenic actions (road accidents, burns from fires or electric shocks) [
16]. Most problems are observed within the first six months of captivity, especially in young or highly debilitated animals, and are related to digestive disorders, including dental alterations and nutritional deficiencies, followed by respiratory problems and traumatic injuries [
17,
18].
Gastrointestinal tract diseases are among the leading causes of death in captive sloths, partly due to the particularities associated with this system, such as the presence of multiple fermentation chambers adapted to a highly fibrous and low-calorie diet, dietary specifics that are difficult to replicate in captivity, or high sensitivity to dietary variability [
19]. The presence of food-borne pathogens or those in the hospital environment and contact with other animals also promotes greater spread of commensal agents, primarily from the gastrointestinal and integumentary systems, associated with capture and daily handling stress and comorbidities, increasing the possibility of secondary infections [
36].
Among infectious diseases, notable are enteritis caused by
Escherichia coli,
Salmonella sp. and
Aeromonas sp. [
17], and vesicular stomatitis caused by viruses and fungi of the genus
Candida sp. and
Histoplasma sp. [
20]. The main symptoms include diarrhea, mucus in the feces, loss of appetite, tympanism, and colic, which can lead to death due to severe dysbiosis and associated pain [
37].
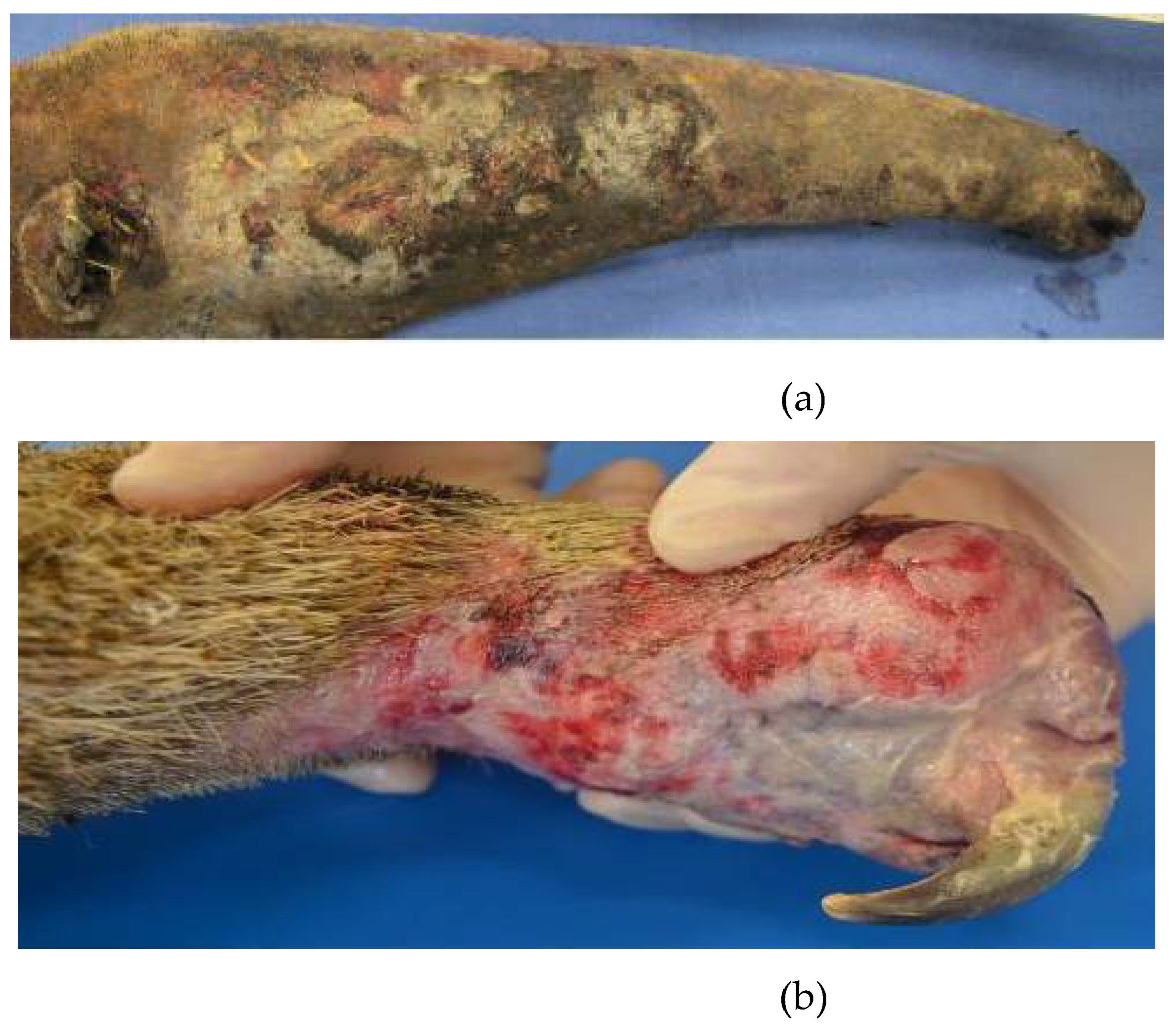
The presence of trauma and burns also predisposes to alterations in the normal microbiota of the integument and pathogens, similar to what occurs in humans, further associated with various temperature and humidity conditions of the enclosures in most institutions that keep these animals, which is not very suitable for the species' particularities [
38]. Small carbon pigment foci and abundant fungal structures resembling
Candida sp. have already been identified on the skin of anteaters injured by electric shocks (
Figure 4), alterations still poorly described in the literature [
14].
The main prophylactic methods are associated with food hygiene and the appropriate use of diets according to the biological demands of the individuals, as well as early diagnosis in the presence of any behavioral change or symptom. However, keeping Xenarthrans, especially sloths, in captivity remains a challenge, mainly due to the difficulty in replicating the biological and nutritional demands that the species present when taken from the wild [
14,
39,
40].
In the presence of clinical signs indicative of disease and potential diagnosis, targeted treatment of the causative agent and other comorbidities affecting immune response is essential for the animal's recovery, linked to sanitary, nutritional, and overall environmental management, ensuring the maintenance of the species in captivity [
16,
25].
8.2. Candidiasis in Sloths
Isolations of fungal and bacterial agents in xenarthrans are still scarce, but with the advancement of urbanization and the increased presence of these individuals in captivity, knowledge about these agents, especially those of zoonotic nature, becomes essential [
40].
Regarding the fungal agents found in sloths, most are primarily of digestive and/or integumentary origin. The first isolation and identification of
Microsporum canis, a dermatophyte fungus characteristically causing proliferative alopecia, was described in a sloth (
Bradypus tridactylus) received at the Emílio Goeldi Museum in Belém, PA, Brazil [
41].
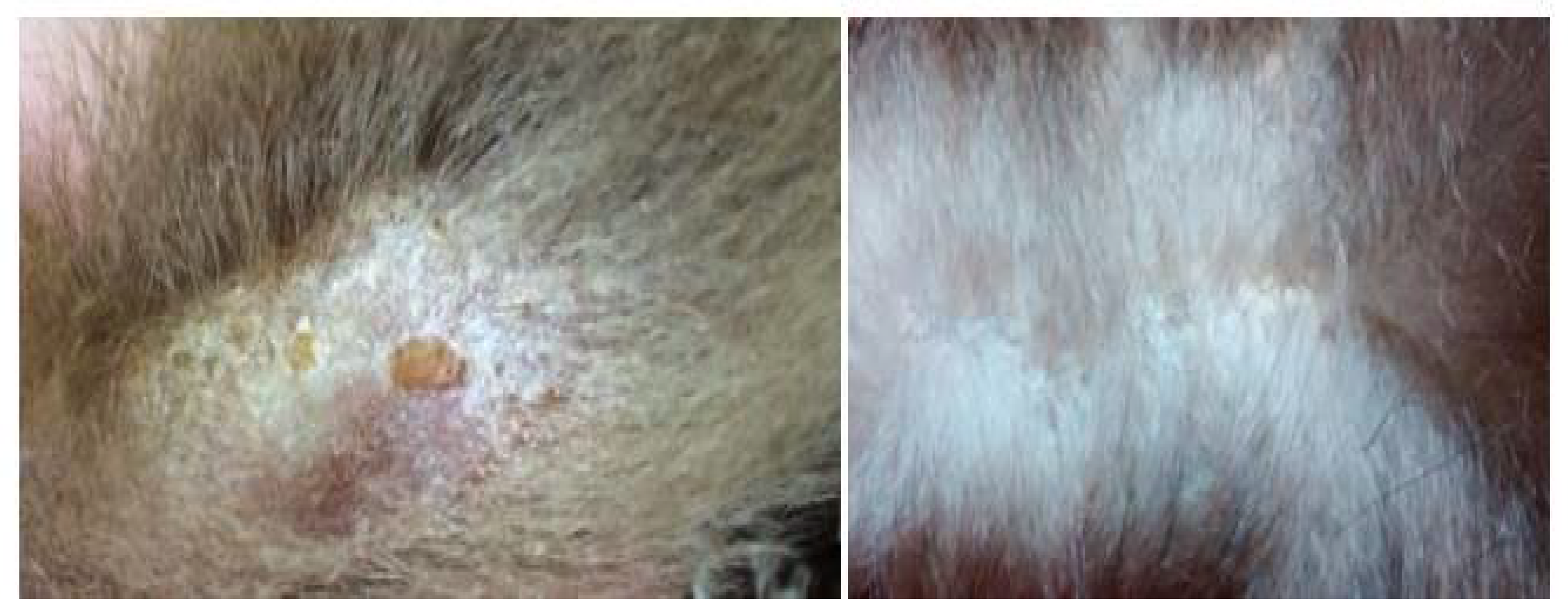
Similarly, the same fungus and
Microsporum gypseum were isolated for the first time in three specimens of the common sloth (
Bradypus variegatus) from the state of Pernambuco, which also exhibited areas of alopecia and crusts adhered to the fur (
Figure 5). In this case, the diagnosis was made from samples collected from the hair and crusts subjected to direct microscopic examination with 30% KOH and culture on Mycosel Agar. Direct examination revealed arthrospores in the hairs, and seven days after culture, colonies suggestive of the genus
Microsporum were observed, confirmed by the observation of macroconidia structures [
42].
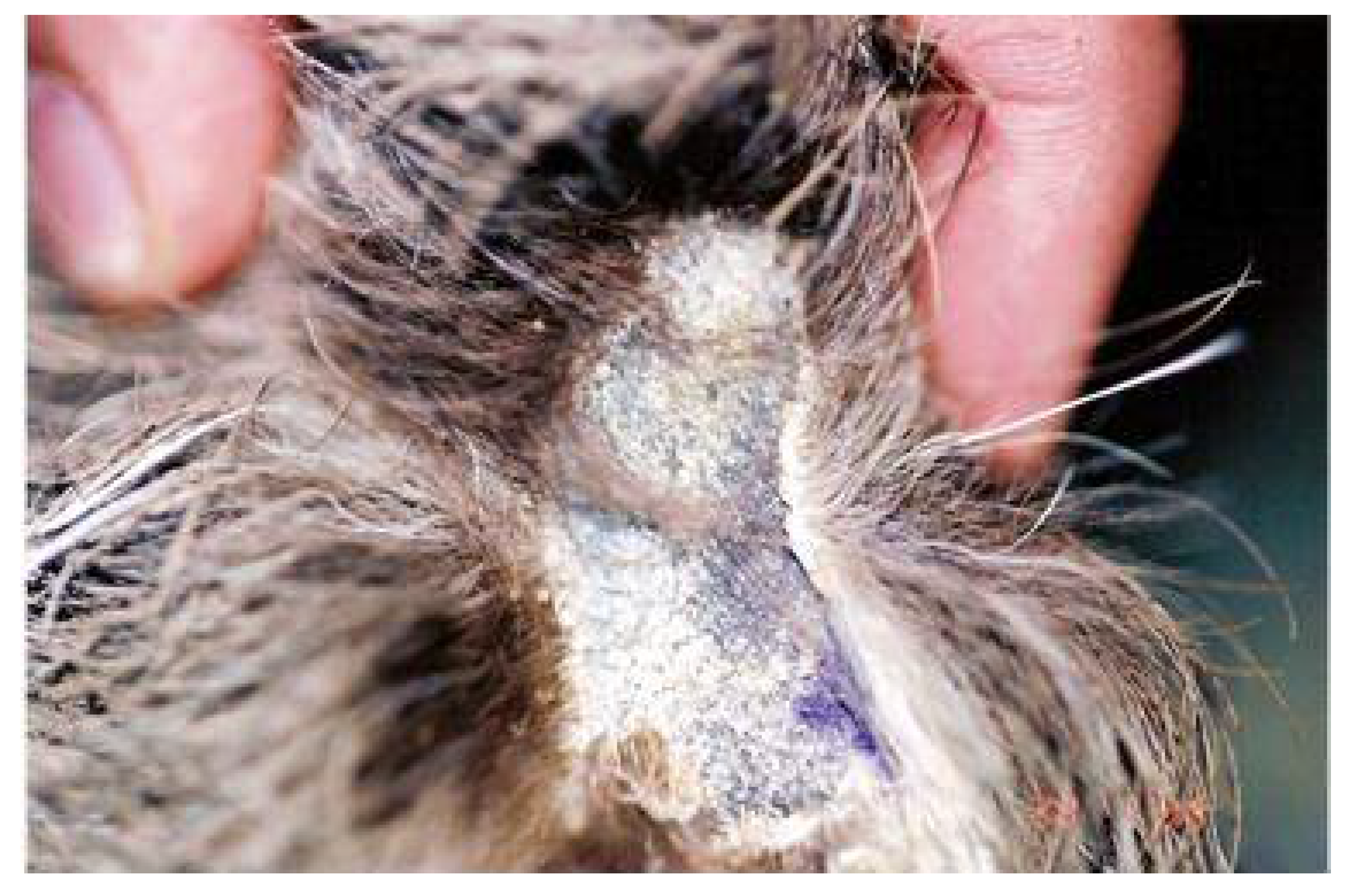
Regarding the genus
Choloepus sp., there are reports of the occurrence of
Malassezia sp. (
Figure 6) and
Microsporum gypseum [
16] as causes of integumentary and auricular alterations such as alopecia, pruritus, and crust formation, and
Paracoccidioides brasiliensis [
43],
Histoplasma capsulatum [
37], and
Candida sp. [
20] as causes of gastrointestinal alterations (gastric ulcers, mucosal desquamation, dysbiosis).
Regarding
Candida sp., the agent was isolated from a two-toed sloth (
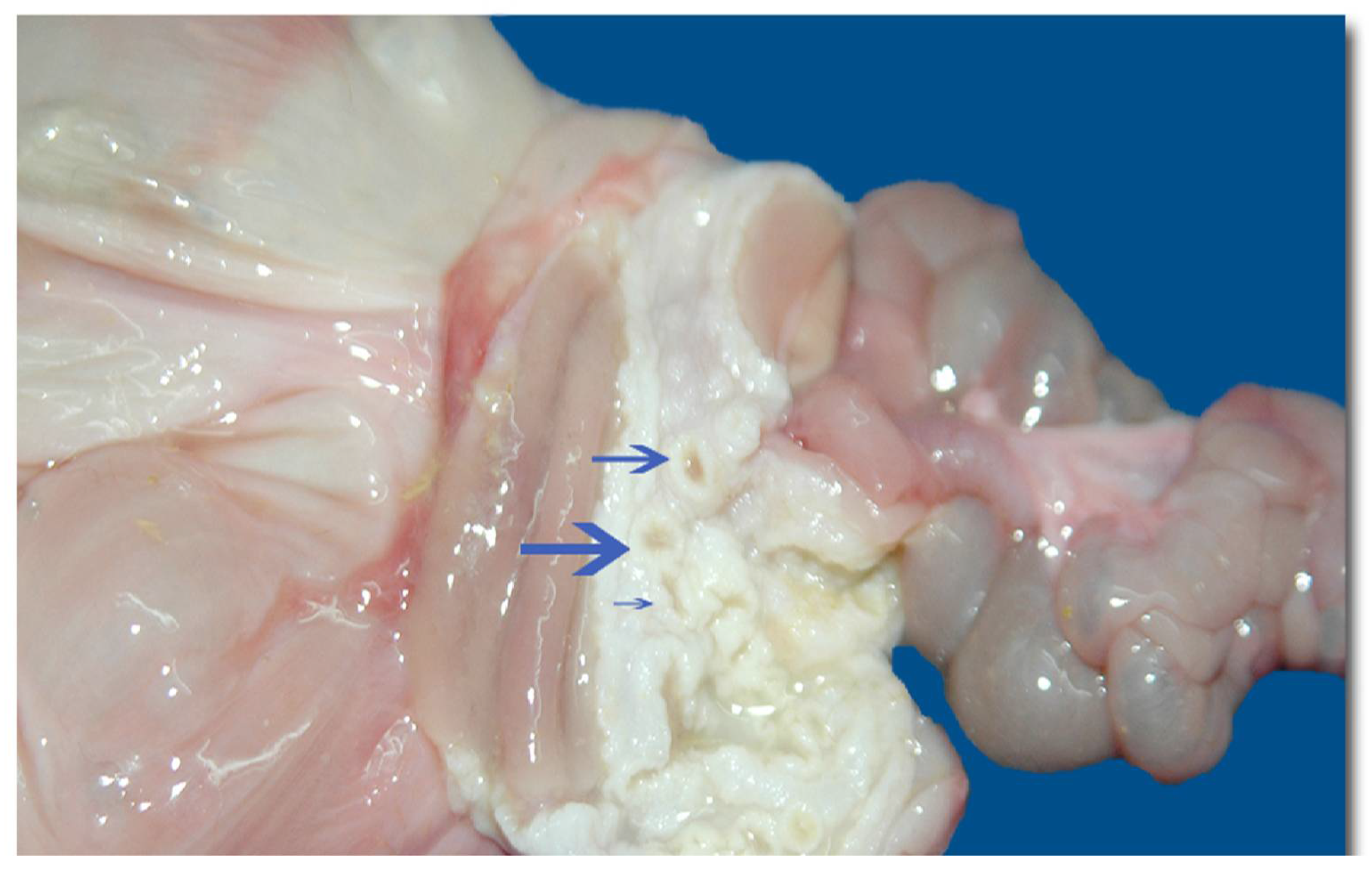
C. hoffmanni) with a history of bronchopneumonia due to gastric reflux, following ineffective treatments. Necropsy of the individual revealed a dilated distal rectum (
Figure 7), gastric mucosa with multiple crater-like nodular formations, and pulmonary alterations indicative of necrosis [
20].
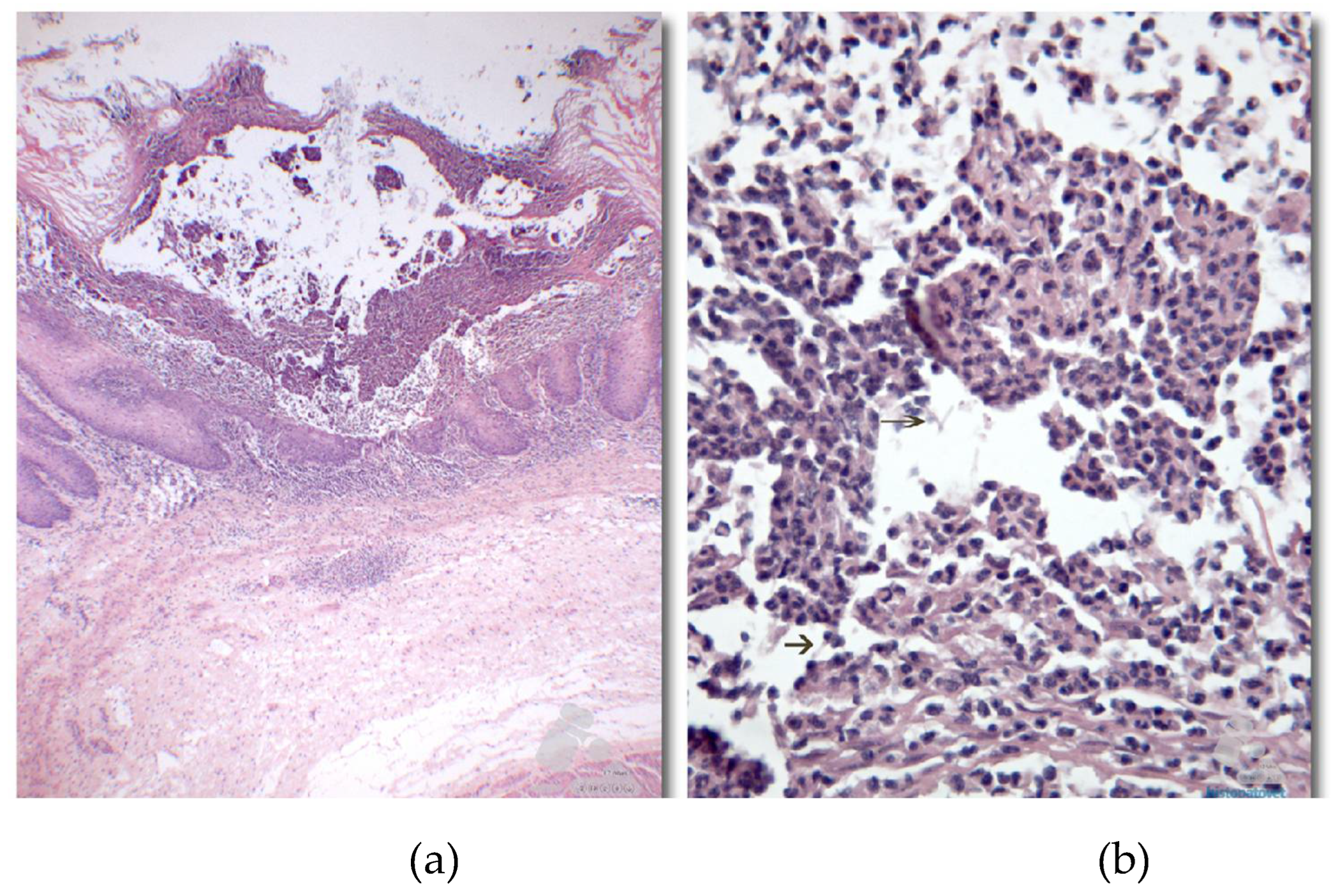
Histopathologically, squamous epithelium in the pre-pyloric region exhibited multiple areas of necrosis affecting the muscularis mucosae, along with cellular debris diagnosed as yeast and hyphae within the epithelium (
Figure 8), confirming the diagnosis of multifocal necrotic fungal gastritis caused by
Candida sp., which led to the animal's death following bronchoaspiration [
20].
Similarly, gastrointestinal alterations have been identified that promote stomatitis, gastritis, constipation, and enteritis as the primary causes of the highest morbidity and mortality rates in adult sloths (between four and 15 years) of the genus
Choloepus sp. in North America, with the most severe cases involving stomatitis and ulcerative gastritis associated with
Candida sp., diagnosed through cytology and oral swabs of affected animals [
20].
Regarding therapeutics, there are mentions of using itraconazole or ketoconazole, both at a dose of 5 mg/kg, orally, for 4 to 6 weeks for the treatment of disseminated dermatophytosis, accompanied by chlorhexidine baths to control the infection. Miconazole can be used topically for fungal otitis, and terbinafine for the topical treatment of crusts present on the skin. All treatments require long periods of use of at least one to two weeks for real control of the infectious agent, emphasizing that it is important to associate supportive therapy with supplementation and treatment of concomitant diseases for greater success in controlling fungi. There are still no studies addressing other systemic therapies for the treatment of
Candida sp. in these animals, and it is frequently found in necropsies [
16].
There is a notable need for studies with greater focus on sloths and the occurrence of infectious alterations, as well as proper diagnosis and therapeutics, which directly impact these individuals in captivity, causing severe health damage that can even lead to premature death.
9. Conclusion
Candidiasis in Choloepus sp. represents a significant health concern, especially for individuals in captivity, where the prevalence of infectious diseases tends to increase due to various stressors and environmental factors. The unique biological and nutritional requirements of two-toed sloths make them particularly susceptible to gastrointestinal and integumentary infections, including those caused by Candida sp. The complexity of their digestive system, which includes multiple fermentation chambers adapted to a fibrous and low-calorie diet, poses challenges for maintaining their health in captivity.The occurrence of Candida infections in sloths has been associated with several clinical manifestations, including stomatitis, gastritis, constipation, and enteritis. These infections can lead to severe systemic issues and high mortality rates if not properly managed. The isolation of Candida sp. and other zoonotic fungal pathogens from sloths highlights the importance of comprehensive diagnostic approaches and the need for effective therapeutic protocols.Trea tment of Candida infections in sloths often involves the use of antifungal medications such as itraconazole and ketoconazole, as well as supportive care to address underlying comorbidities and stress factors. However, the lack of specific studies on systemic therapies for Candida in sloths underscores the need for further research to develop tailored treatment strategies. Preventive measures, including strict hygiene practices, appropriate dietary management, and early diagnosis of behavioral changes or symptoms, are crucial for minimizing the risk of infection. The increasing urbanization and habitat fragmentation necessitate greater attention to the health management of captive sloths to ensure their well-being and conservation. Overall, the study of candidiasis in Choloepus sp. reveals significant gaps in our understanding of the disease's pathogenesis, diagnosis, and treatment. Addressing these gaps through dedicated research and improved veterinary practices will be essential for enhancing the care and survival of these unique and vulnerable animals in captivity.
Author Contributions
Conceptualization, M.S.C.B. and F.M.S.; methodology, M.S.C.B. and F.M.S.; formal analysis, M.S.C.B. and F.M.S.; data curation, M.S.C.B. and F.M.S.; writing—original draft preparation, M.S.C.B. and F.M.S writing—review and editing, M.S.C.B. and F.M.S; supervision, F.M.S.; project administration, F.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FAPESPA (Fundação Amazônia de Amparo a Estudos e Pesquisas do Estado do Pará), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; finance code: 001), and PROPESP-UFPA (Pró-Reitoria de Pesquisa e Pós-Graduação da Universidade Federal do Pará) for paying the publication fee for this article via the Programa Institucional de Apoio à Pesquisa (PAPQ/2023-2024).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ciurea, C.N.; Kosovski, I.B.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and candidiasis—opportunism versus pathogenicity: a review of the virulence traits. Microorg. 2020, 8, 857. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Bosco, S.M.G.; de Hoog, S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensen, H.E.; Martel, A.; Mignon, B.; Pasmans, F.; Piecková, E.; Rodrigues, A.M.; Singh, K.; Vicente, V.A.; Wibbelt, G.; Wiederhold, N.P.; Guillot, J. Fungal infections in animals: a patchwork of different situations. Med. Mycol. Erratum in: Med Mycol. 2018 Nov 1;56(8):e4. 10.1093/mmy/myy028. 2018, 1, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Ugochukwu, I.C.I.; Aneke, C.I.; Sani, N.A.; Omeke, J.N.; Anyanwu, M.U.; Odigie, A.E.; Onoja, R.I.; Ocheja, O.B.; Ugochukwu, M.O.; Makanju, O.A.; Aneke, C.I. Important mycoses of wildlife: emphasis on etiology, epidemiology, diagnosis, and pathology—a review: part 1. Animals 2022, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Ugochukwu, I.C.I.; Luca, I.; Sani, N.A.; Omeke, J.N.; Anyanwu, M.U.; Odigie, A.E.; Onoja, R.I.; Ocheja, O.B.; Ugochukwu, M.O.; Makanju, O.A.; Aneke, C.I. Important Mycosis of Wildlife: Emphasis on Etiology, Epidemiology, Diagnosis, and Pathology—A Review: PART 2. Animals 2022, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Green, J.L. Intertooth variation of orthodentine microwear in armadillos (Cingulata) and tree sloths (Pilosa). J. Mammal 2009, 90, 768–778. [Google Scholar] [CrossRef]
- Martins, M.D.; Pinheiro, L.L.; Mesquista, E.Y.E.; Branco, E.; Lima, A.R. Anatomy and surgical approach to the humerus, radius and ulna of common sloth (Bradypus variegatus). Ciên. Rur. 2022, 52, e20210498. [Google Scholar] [CrossRef]
- Samaranayake, Y.H.; Samaranayake, L.P. Experimental oral candidiasis in animal models. Clin. Microbiol. Rev. 2001, 14, 398–429. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Rodrigues, T.J.S.; Castelo-Branco, D.S.C.M.; Teixeira, C.E.C.; Macedo, R.B.; Bandeira, S.P.; Pereira, A.L.; Monteiro, A.J.; Cordeiro, R.A.; Bandeira, T.J.P.G.; Moreira, J.L.B.; Sidrim, J.J.C.; Rocha, M.F.G. Antifungal susceptibility and virulence attributes of animal-derived isolates of Candida parapsilosis complex. J. Med. Microbiol. 2014, 63, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Santos, G.C.O.; Vasconcelos, C.C.; Lopes, A.J.O.; Cartágenes, M.D.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; Monteiro, C.A. Candida infections and therapeutic strategies: mechanisms of action for traditional and alternative agents. Front. Microbiol. 2018, 3, 1351. [Google Scholar] [CrossRef]
- Naiff, R.D.; Ferreira, L.C.L.; Barrett, T.V.; Naiff, M.F.; Arias, J.R. Enzootic paracoccidioidomycosis in armadillos (Dasypus novemcinctus) in the state of Pará. Rev. Inst. Med. Trop. S. P. 1986, 28, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.M. Animal models of oral candidiasis: a review. Oral Surg., Oral Med., Oral Pathol. 1994, 78, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.A.; Jr Wellehan, J.F.X.; Quesenberry, K. Gastrointestinal disease associated with non-albicans candida species in six birds. J. Avian. Med. Surg. 2019, 33, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.V.C.; Matos, P.C.M.; Soares, A.R.B.; Lopes, C.T.A.; Domingues, S.F.S. Casuistry of xenarthrans treated at the Veterinary Hospital of the Federal University of Pará, Brazilian Amazon. Pesq. Vet. Bras. 2023, 43, e07058. [Google Scholar] [CrossRef]
- Adam, P.J. Choloepus didactylus
. Mammal. Spec. 1999, 621, 1–8. [Google Scholar] [CrossRef]
- Oliger, C.D.; Nicolai, G.P. Manual de manejo, medicina y rehabilitación de perezosos. 1st ed. Fundación Huálamo:Valdivia, Chile, 2017, pp.162.
- Diniz, L.S.; Oliveira, P.M. Clinical problems of sloths (Bradypus sp. and Choloepus sp.) in captivity. J. Zoo Wild. Med. 2009, 30, 76–80. [Google Scholar]
- Martínez, N.; Antelo, C.; Rumiz, D.I. Rehabilitación de perezosos (Bradypus variegatus) urbanos en reservas privadas aledañas a Santa Cruz de La Sierra: una iniciativa multipropósito de investigación, manejo y educación. Rev. Bol. Ecol. 2004, 16, 1–10. [Google Scholar]
- Goffart, M. Function and form in the sloth. 1st ed. Pergamon Press: Oxford, NY, EUA, 1971; pp 225.
- Patel, H.; Buchanan, F.; Chai, C. Candida-induced emphysematous gastritis in a multiple myeloma patient: conservative management with favorable outcome. Cureus 2021, 13, e18508. [Google Scholar] [CrossRef] [PubMed]
- Neppelenbroek, K.H.; Seó, R.S.; Urban, V.M.; Silva, S.; Dovigo, L.N.; Jorge, J.H.; Campanha, N.H. Identification of Candida species in the clinical laboratory: a review of conventional, commercial, and molecular techniques. Oral Dis. 2014, 20, 329–44. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Non-culture diagnostics for invasive candidiasis: promise and unintended consequences. J. Fungi 2018, 4, 27. [Google Scholar] [CrossRef]
- Marinho, S.A.; Teixeira, A.B.; Santos, O.S.; Cazanova, R,F.; Ferreira, C.A.; Cherubini, K.; de Oliveira, S.D. Identification of Candida spp. by phenotypic tests and PCR. Braz. J. Microbiol. 2010, 41, 286–94. [Google Scholar] [CrossRef] [PubMed]
- Yaman, G.; Akyar, I.; Can, S. Evaluation of the MALDI TOF-MS method for identification of Candida strains isolated from blood cultures. Diagn. Microbiol. Infect. Dis. 2012, 73, 65–7. [Google Scholar] [CrossRef] [PubMed]
- Cubas, Z.S.; Silva, J.C.R.; Catão Dias, J.L. Tratado de Animais Selvagens-Medicina Veterinária. 2nd ed. Roca: São Paulo, SP, Brazil, 2014; pp. 2512.
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Hector, R.F. An overview of antifungal drugs and their use for treatment of deep and superficial mycoses in animals. Clin. Techn. Small Anim. Pract. 2005, 20, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Rochette, F.; Engelen, M. Vanden Bossche, H. Antifungal agents of use in animal health--practical applications. J. Vet. Pharmacol. Ther. 2003, 26, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, C.; Leonardi, D. Candida infections, causes, targets, and resistance mechanisms: traditional and alternative antifungal agents. Biomed. Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef] [PubMed]
- Dedeaux, A.; Grooters, A.; Wakamatsu-Utsuki, N.; Taboada, J. Opportunistic fungal infections in small animals. J. Am. Anim. Hosp. Assoc. 2018, 54, 327–337. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, D. Zoonoses and Wildlife: One Health Approach. Animals 2022, 12, 480. [Google Scholar] [CrossRef] [PubMed]
- Miranda, F.R.; Rohe, F.; Vaz, S.M. Avaliação do risco de extinção de Choloepus didactylus (ILLIGER, 1811) no Brasil. Editora ICMBio: Brasília, DF, Brazil, 2015; pp.250.
- Reis, N.R.; Peracchi, A.L.; Pedro, W.A.; Lima, I.P. Mamíferos do Brasil, 1st ed.; UEL: Londrina, PR, Brazil, 2006; pp. 71–94. [Google Scholar]
- Superina, M.; Plese, T.; Barros, N.M.; Abba, A.M. The 2010 sloth red list assessment. Edentata 2010, 11, 115–135. [Google Scholar] [CrossRef]
- Chahud, A. Xenarthra provenientes de atividades de caça da comunidade Awá-Guajá do Estado do Maranhão: considerações taxonômicas e tafonômicas. Biota Amaz. 2022, 12, 11–15. [Google Scholar] [CrossRef]
- Carvalho, M.M.; Pieri, N.C.G.; Pereira, K.F.; Lima, F.C.; Carniatto, C.H.O.; Miglino, M.A.; Ricci, R.E.; Martins, D.S. Comparative characterization of the intestine of species from theXenarthra Order. Pesq. Vet. Bras. 2014, 34, 49–56. [Google Scholar] [CrossRef]
- Sybille, K.F.; Quandt, S.K.F.; Nesbit, J.W. Disseminated histoplasmosis in a two-toed sloth (Choloepus didactylus). J. Zoo. Wild. Med. 1992, 23, 369–373. [Google Scholar]
- Ballard, J.; Edelman, L.; Saffle, J.; Sheridan, R.; Kagan, R.; Bracco, D.; Cancio, L.; Cairns, B.; Baker, R.; Fillari, P.; Wibbenmeyer, L.; Voight, D.; Palmieri, T.; Greenhalgh, D.; Kemalyan, N.; Caruso, D. Multicenter trials group, american burn association. positive fungal cultures in burn patients: a multicenter review. J. Burn. Care Res. 2008, 29, p.213-21. [CrossRef]
- Dierenfeld, E.S.; Graffam, W.S. Manual de nutricion y dietas para animales silvestres en cautiverio (ejemplos para animales de america latina). Zcog, 1996, pp.111.
- Torres, A.A.A. Estudo observacional de afecções da superordem Xenarthra de vida livre e cativeiro no Brasil. Ph.D. Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2019. [Google Scholar]
- Silva, M.B.; Moreira, S.B.; Costa, P.F.; Justino, C.H.S.; et al. Isolamento de Microsporum canis em alopecia tonsurante de preguiça bentinho (Bradypus tridactylus). In: VI Congresso e XI Encontro da Associação Brasileira de Veterinários de Animais Selvagens, Vitória, ES, Brazil, p. 247, 2002.
- Xavier, G.A.A.; Silva, L.B.G.; Silva, D.R.; Peixoto, R.M.; Lino, G.C.; Mota, R.A. Dermatophytosis caused by Microsporum canis and Microsporum gypseum in free-living Bradypus variegatus (Schiz, 1825) in the state of Pernambuco, Brazil. Braz. J. Microb. 2008, 39, 508–510. [Google Scholar] [CrossRef]
- Trejo-Chávez, A.; Ramírez-Romero, R.; Ancer-Rodríguez, J.; Nervárez-Garza, A.M.; Rodríguez-Tovar, L.E. Disseminated paracoccidioidomycosis in a southern two-toed sloth (Choloepus didactylus). J. Comp. Pathol. 2011, 144, p–231. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).