Submitted:
30 June 2024
Posted:
02 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Biotic Threats: Carrot Diseases
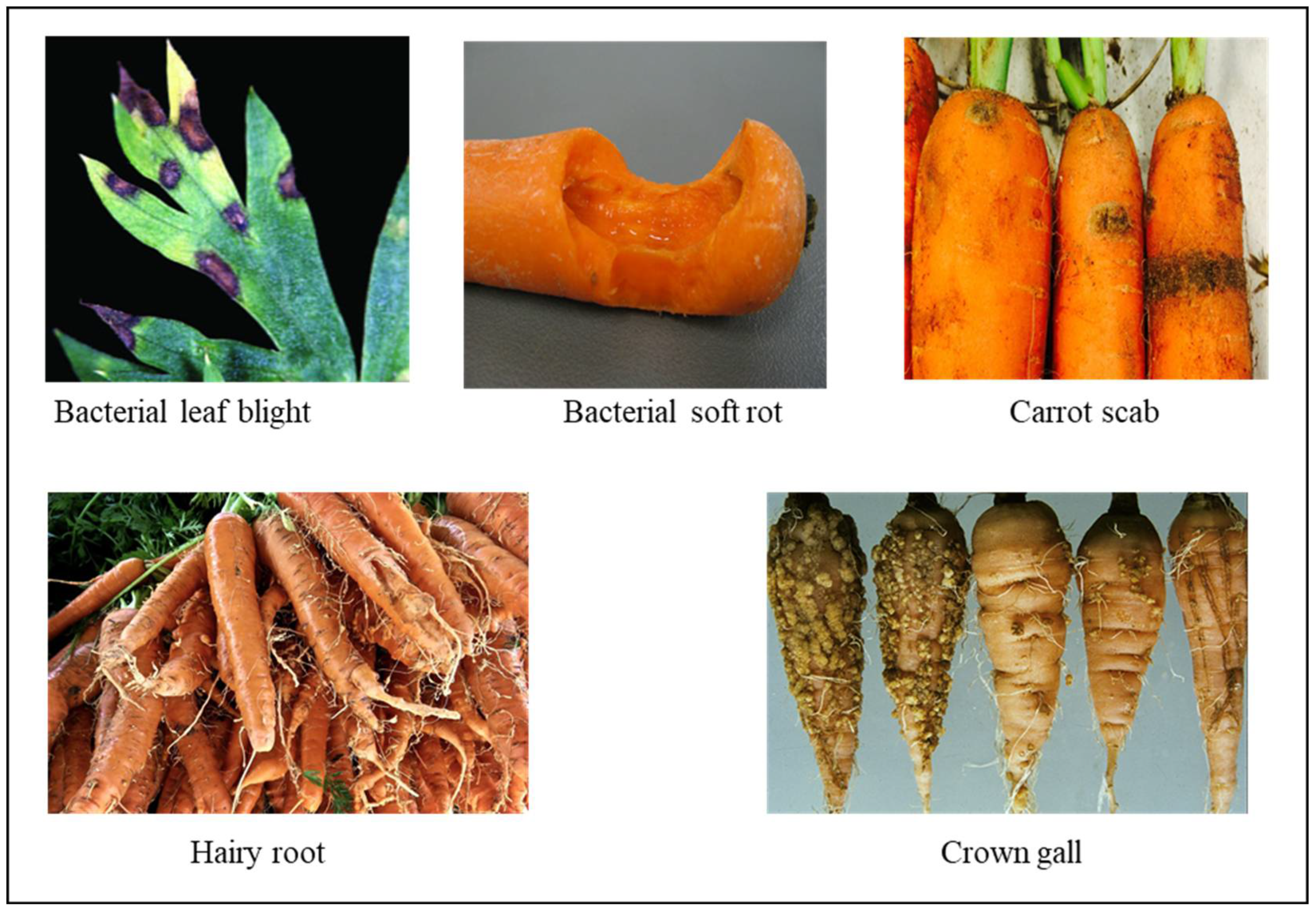
2.1. Bacterial Diseases
2.1.1. Bacterial Leaf Blight
2.1.2. Bacterial soft rot
2.1.3. Hairy Root
2.1.4. Crown Gall
2.1.5. Scab
3. Fungal and Oomycete Diseases
3.1. Alternaria Leaf Blights (ALB)
3.2. Black Root Rot (Black Mold)
3.3. Black Rot
3.4. Crown Rot (Rhizoctonia Canker)
3.5. Ring Rot Disease (Pythium Root Dieback )
3.6. Cavity Spot
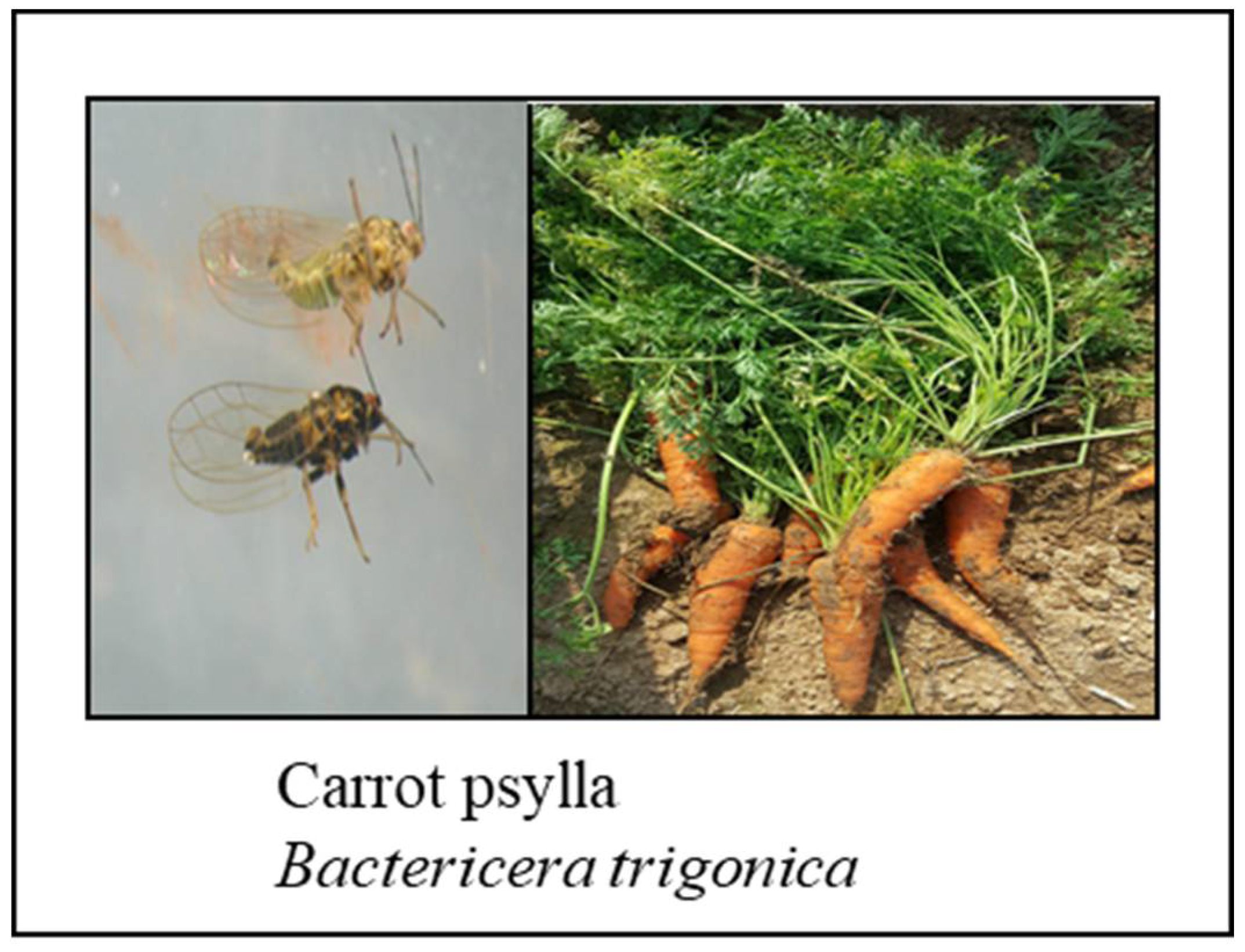
4. Insects
Carrot Psyllids – Candidatus Liberibacter Solanacearum
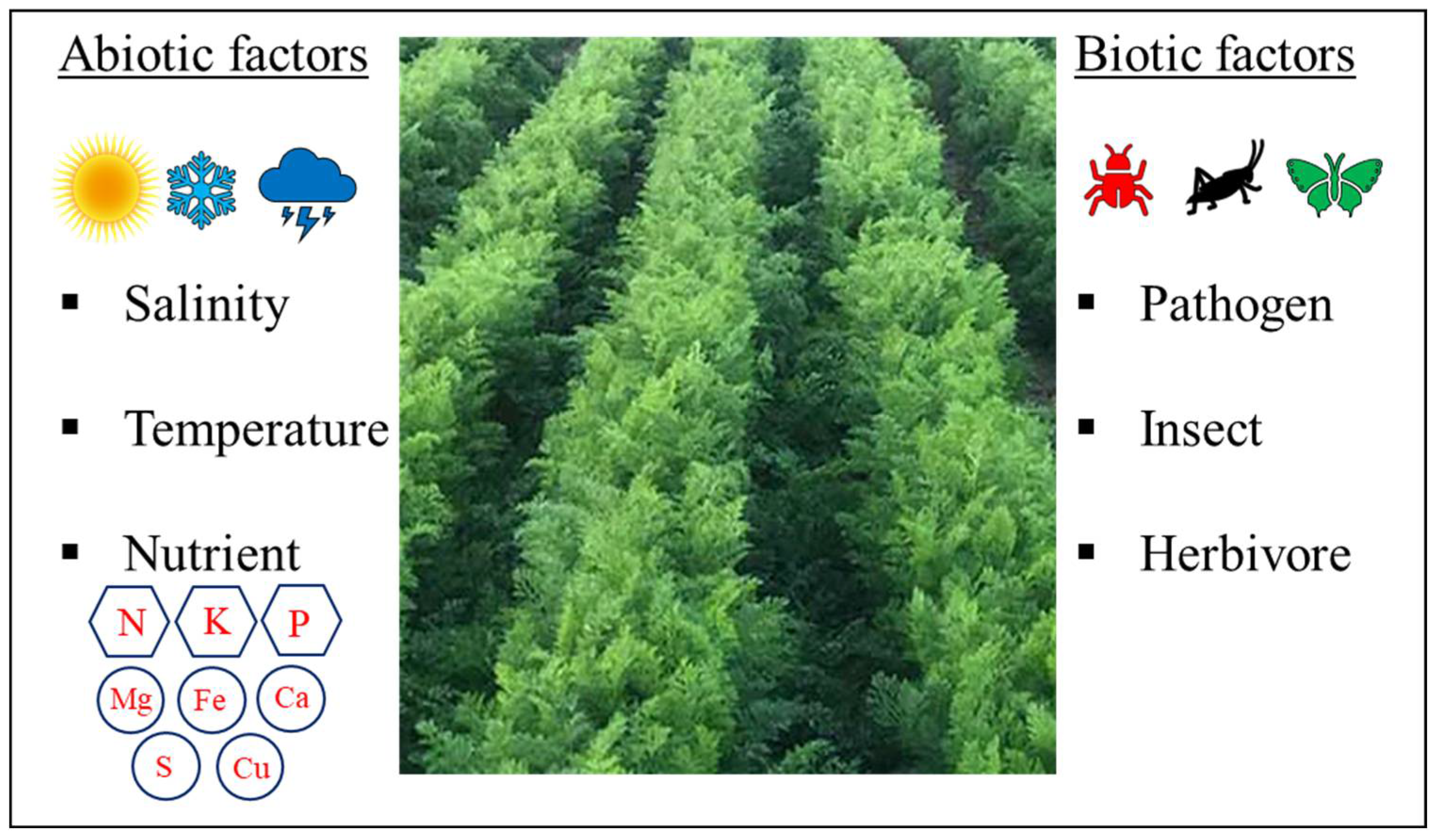
5. Influence of Abiotic Stresses on Carrot
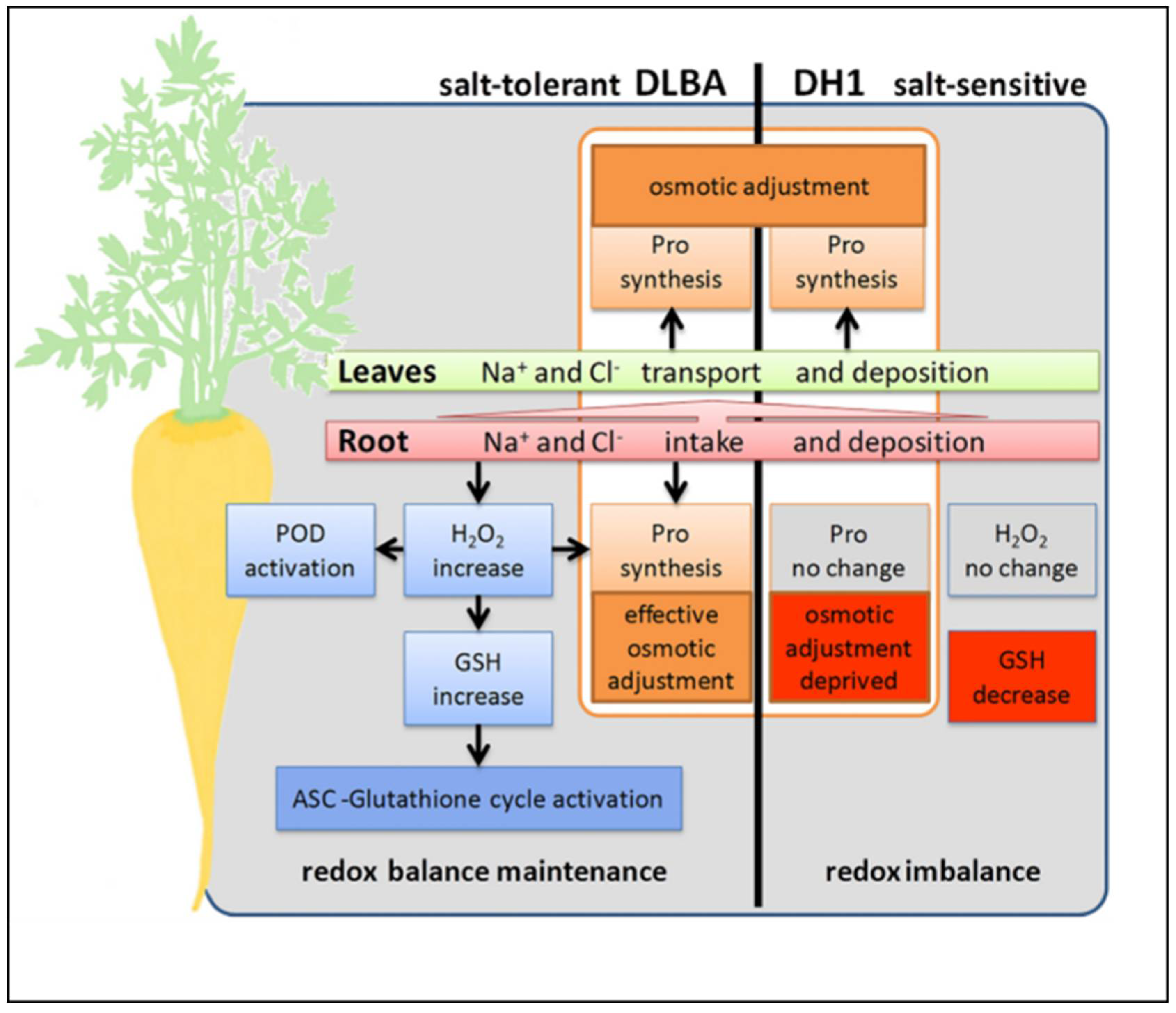
5.1. Salinity Stress
5.2. High Temperature
5.3. Drought Stress
6. Carrot Breeding: Genetic Resources and Genomic Selection
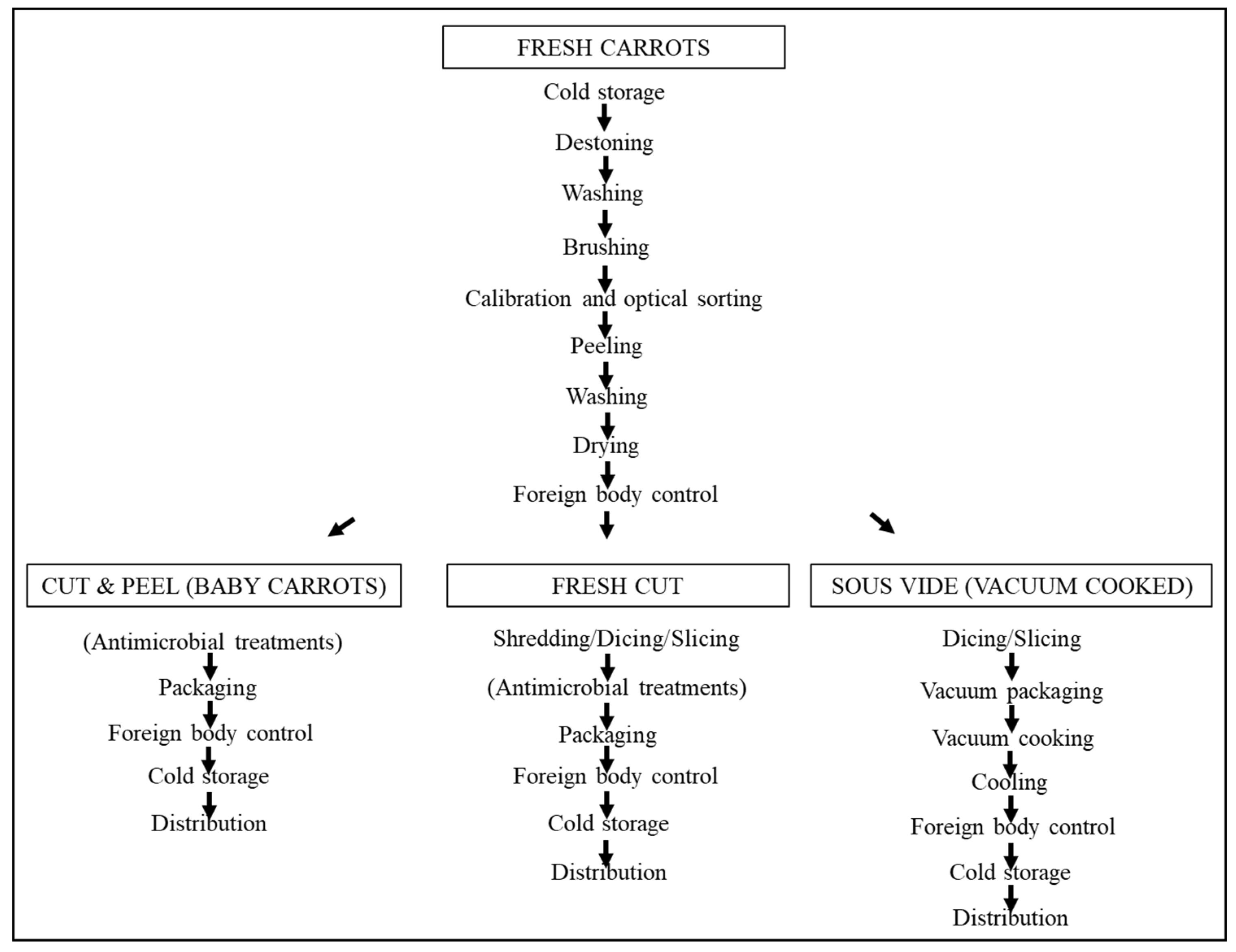
7. Post-Harvest Physiology
8. Carrots as a Perishable Food
9. Microbial Ecology of Carrots
9.1. Spoilage
9.2. Shelf Life Extension
| TREATMENT | EFFECT ON MICROORGANISMS | EFFECTS ON CARROTS CHEMICAL AND PHYSICAL PARAMETERS | REFERENCE |
|---|---|---|---|
| Modified atmosphere packaging (MAP) | Growth control of the psychrotrophic population, inhibition of yeast and molds. | Vitamin C preservation, a slight reduction of β-carotene, and minerals content decrease during storage. Negative effect on texture, preservation of color, and quality indexes. |
[106,107] |
| Dipping/Coatings based on natural polymers (alginate, casein, chitosan, etc.) | Growth control of Specific Spoilage Organisms, Enterobacteriaceae, Pseudomonadaceae | Reduction of flavonoids and phenolic acids accumulation, bitterness reduction; moisture loss prevention, the anti-browning effect, color retention, and differences in antioxidant potential depending on the treatment. |
[109,110] |
| Coatings + MAP | Load reduction and growth control of yeast and molds, coliforms, and Pseudomonas spp. | Moisture loss prevention, respiration increase, prolonged firmness, prevention of surface whitening, color and texture retention. | [111] |
| Ozonation /Ozonated water | Inhibition of Escherichia coli O157:H7, STEC E. coli, Salmonella enterica and Listeria monocytogenes; Pectobacterium carotovorum. Fungistatic effect on B. cinerea and S. sclerotiorum |
Delay of carrots thickening, maintenance of pH, dose-dependent oxidative damages: pigment disruption, color change, increased respiratory rate, dehydration, and electrolyte loss. |
[112,113,114,115,116] |
| Ozone + UV-C rays | Reduction of total mesophilic population and coliforms. No effect on yeast and molds. | Not reported. | [117] |
| Ozone + MAP | Inhibition of microorganisms on the product surface. Reduction of total mesophilic population. | Reduction in total phenolics, enzyme activity, respiration, and ethylene rate, retention of total carotenoids and ascorbic acid, color maintenance | [114] |
| Chlorine dioxide | Reduction of mesophilic and psychrotrophic population, including Lactic Acid Bacteria. Scarce effect on yeast that determined the shelf life. | Moisture loss prevention, white discoloration prevention, slight pH reduction, and maintenance of sensory attributes. | [118] |
| High pressure | Inactivation of vegetative cells. | Maintenance of texture, red color, and carotenoid content, as well as dry matter reduction. Increase of free and bound phenolics, increase of antioxidant content. |
[119,120] |
| UV-C treatment | Variable inhibition of microbial growth, depending on the wavelength. Reduction of Sclerotinia sclerotorium load. |
Maintenance of aroma, color, nutritional, and physical-chemical characteristics. | [121,122] |
| Gamma irradiation | Limited effect because of the legal restrictions in the doses applicable. | Maintenance of quality attributes. | [123] |
| Irradiation + active coating | Reduction of total mesophilic population and yeast and mold count. | Improvement of mechanical and water vapor barrier characteristics of the coating, maintenance of weight, firmness, and color. | [123] |
| Nisin + plant extracts +irradiation | Reduction of total mesophilic population, yeast and molds, and Listeria monocytogenes count. | Maintenance of weight, firmness, and color. | [124] |
| Different Essential oils | Reduction of Sclerotinia sclerotiorum growth. | Increase in enzymes (polyphenol oxidase, peroxidases, chitinases etc.) content, inducers of resistance against the molds. | [125] |
| Coriandrum sativum EO | Reduction of Salmonella enterica growth. | Maintenance of sensory traits of the product, as well as color stability. | [100] |
| Thyme EO | Reduction of Escherichia coli O157:H7 count. | Not reported. | [113] |
| Thyme EO + ClO2 + ozonated water | Effective reduction of Escherichia coli O157:H7 count. | Not reported. | [113] |
| Microencapsulated Chitosan + thyme EO | Reduction and control of mesophilic, psychrophilic, yeast, and mold populations during time. | Increase of total phenolics content (TPC) and antioxidant capacity. | [126] |
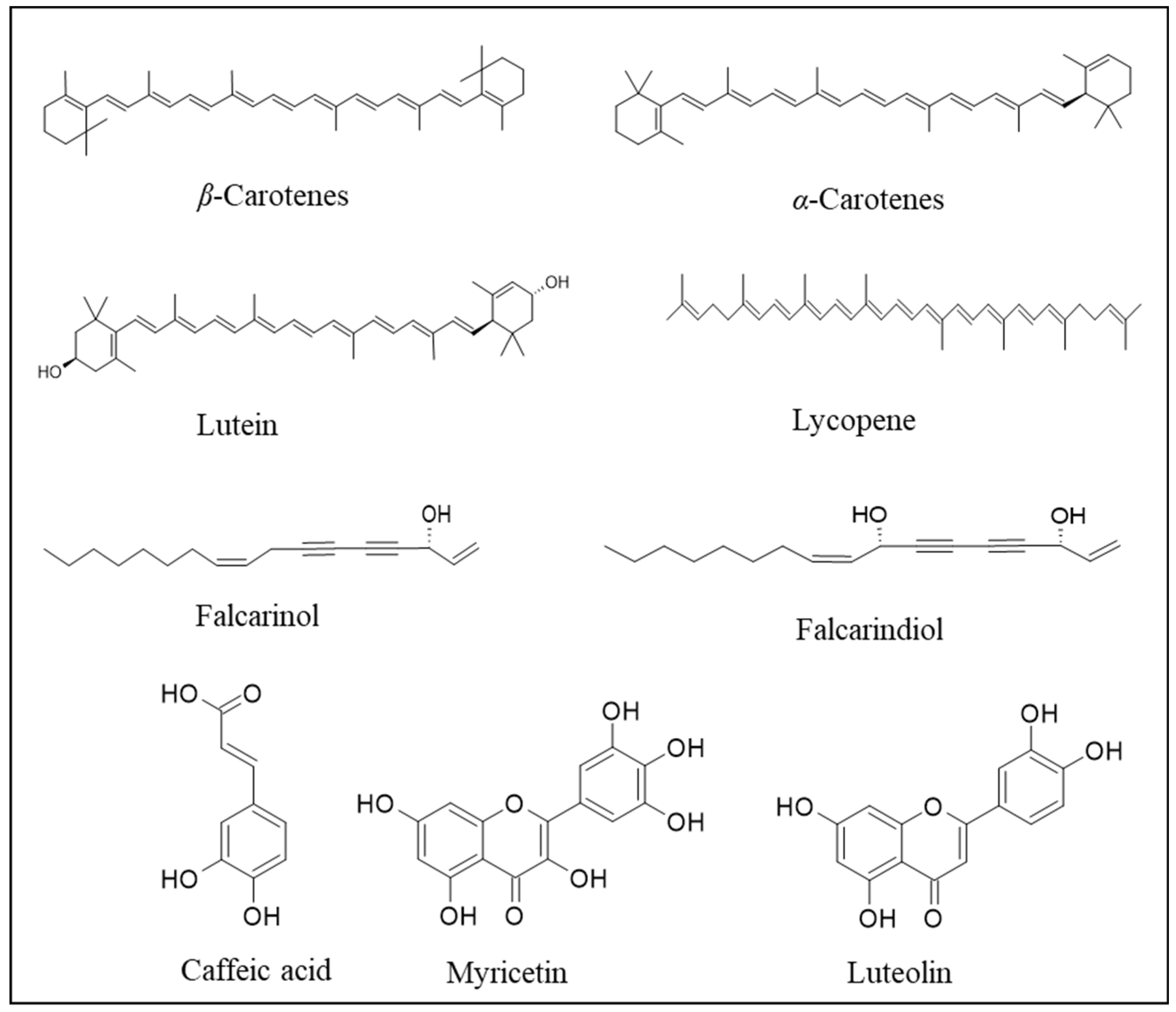
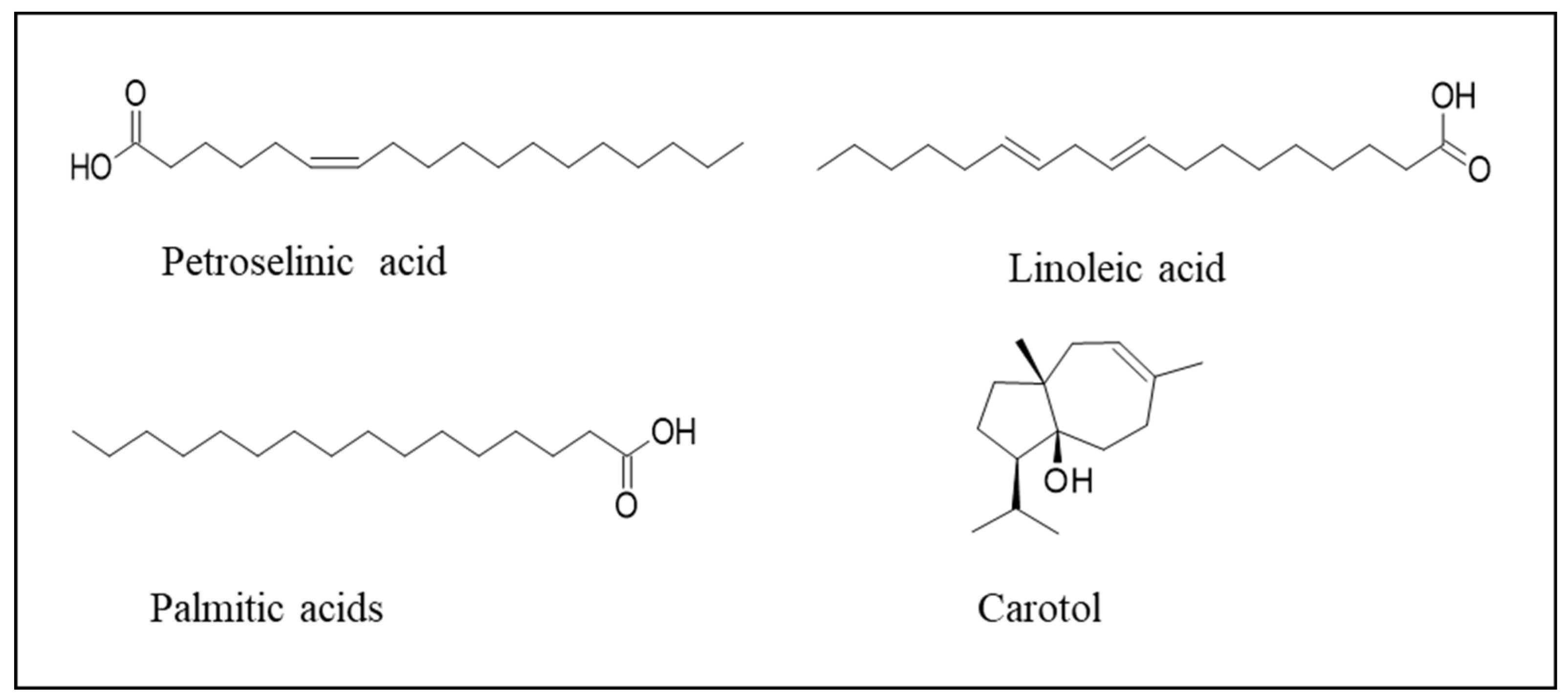
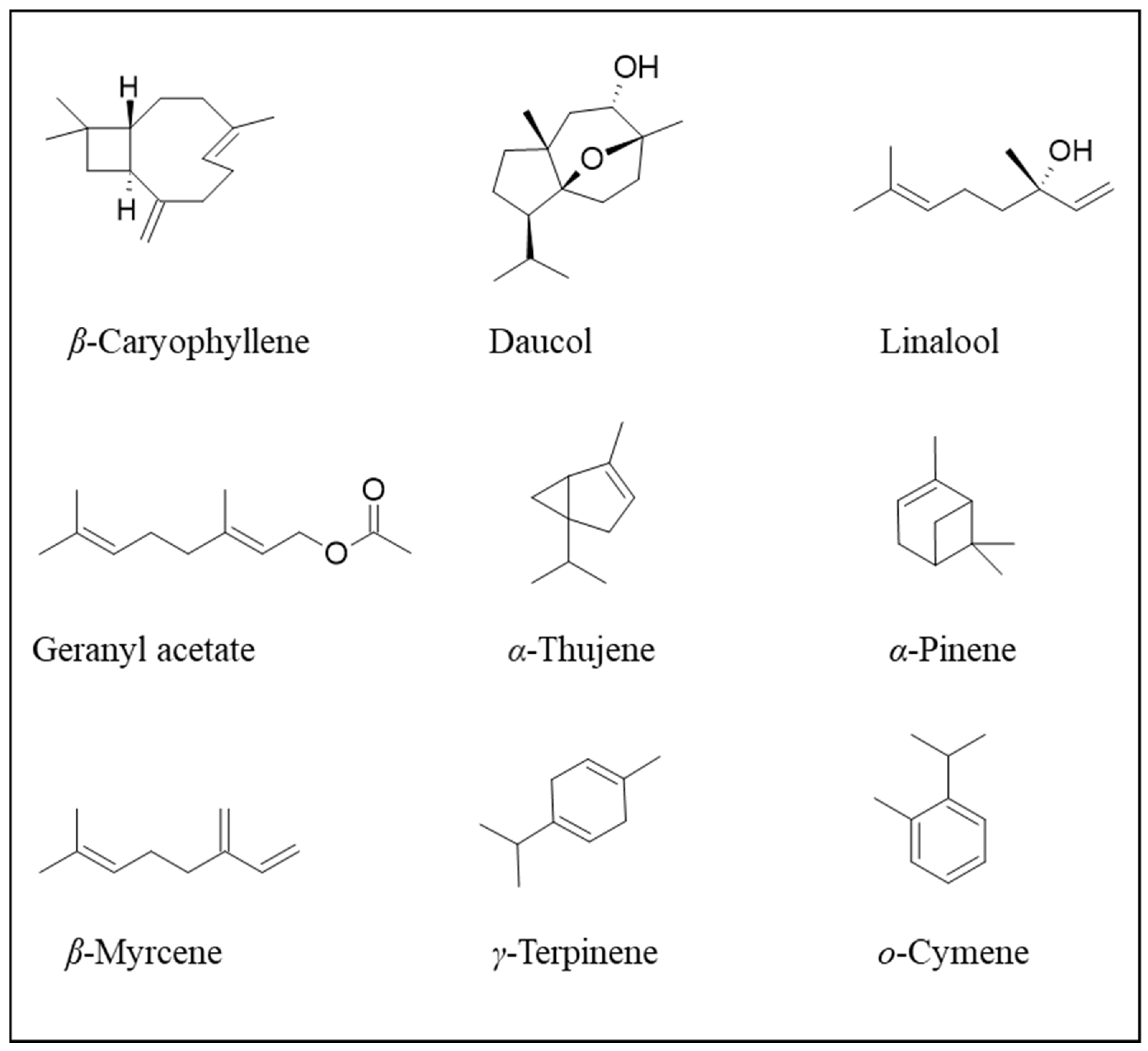
10. Bioactive Compounds Obtained from Carrots and Potential Applications
Future Prospects
References
- McCormick, N. RNA-mediated virus resistance for carrot (Daucus carota var. sativum) and celery (Apium graveolens var. dulce). Ph.D. Thesis, University of Melbourne 2006.
- Que, F.; Hou, X.-L.; Wang, G.-L.; Xu, Z.-S.; Tan, G.-F.; Li, T.; Wang, Y.-H.; Khadr, A.; Xiong, A.-S. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic. Res. 2019, 6. [Google Scholar] [CrossRef]
- Hui, Y.H.; Chen, F.; Nollet, L.M.; Guiné, R.P.; Martín-Belloso, O.; Mínguez-Mosquera, M.I.; Paliyath, G.; Pessoa, F.L.; Le Quéré, J.-L.; Sidhu, J.S. Handbook of fruit and vegetable flavors; Wiley Online Library: 2010; Volume 64.
- Ulrich, D.; Nothnagel, T.; Schulz, H. Influence of cultivar and harvest year on the volatile profiles of leaves and roots of carrots (Daucus carota spp. sativus Hoffm.). J. Agric. Food Chem. 2015, 63, 3348–3356. [Google Scholar] [CrossRef] [PubMed]
- Boedo, C.; Benichou, S.; Berruyer, R.; Bersihand, S.; Dongo, A.; Simoneau, P.; Lecomte, M.; Briard, M.; Le Clerc, V.; Poupard, P. Evaluating aggressiveness and host range of Alternaria dauci in a controlled environment. Plant Pathol. 2012, 61, 63–75. [Google Scholar] [CrossRef]
- Boedo, C.; Berruyer, R.; Lecomte, M.; Bersihand, S.; Briard, M.; Le Clerc, V.; Simoneau, P.; Poupard, P. Evaluation of different methods for the characterization of carrot resistance to the alternaria leaf blight pathogen (Alternaria dauci) revealed two qualitatively different resistances. Plant Pathol. 2010, 59, 368–375. [Google Scholar] [CrossRef]
- Breton, D.; Béasse, C.; Montfort, F.; Villeneuve, F. Focus on the recent evolution of soil-borne diseases of carrot in France. In Proceedings of the Proc. 30th Intl. Carrot Conf; 2003. [Google Scholar]
- Béasse, C.; Bellalou, S. Evaluation of biocontrol solutions against Pythium of carrot in the field. In Proceedings of the II International Symposium on Carrot and Other Apiaceae 1264; 2018; pp. 263–268. [Google Scholar]
- Briard, M. Carrot biotic stresses: challenges and research priorities. In Proceedings of the II International Symposium on Carrot and Other Apiaceae 1264; 2018; pp. 113–122. [Google Scholar]
- Du Toft, L.; Crowe, F.; Derie, M.; Simmons, R.; Pelter, G. Bacterial blight of carrot seed crops in the Pacific Northwest. Phytopathology 2004, 94. [Google Scholar] [CrossRef]
- Myung, I.-S.; Yoon, M.-J.; Lee, J.-Y.; Kim, G.-D.; Lee, M.-H.; Hwang, E.-Y.; Shim, H. First report of bacterial leaf blight of carrot caused by Xanthomonas hortorum pv. carotae in Korea. Plant Dis. 2014, 98, 275–275. [Google Scholar] [CrossRef]
- Pruvost, O.; Boyer, C.; Robène-Soustrade, I.; Jouen, E.; Saison, A.; Hostachy, B.; Benimadhu, S. First report of Xanthomonas hortorum pv. carotae causing bacterial leaf blight of carrot in Mauritius. Plant Dis. 2010, 94, 1069–1069. [Google Scholar] [CrossRef]
- Lee, M.-H.; Hong, S.-J.; Park, D.S.; Ham, H.; Kong, H.G. Genomic analysis of the carrot bacterial blight pathogen Xanthomonas hortorum pv. carotae in Korea. Plant Pathol. J. 2023, 39, 409. [Google Scholar] [CrossRef]
- Chandrashekar, B.; Prasannakumar, M.; Puneeth, M.; Teli, K.; Priyanka, K.; Mahesh, H.; Desai, R.U. First report of bacterial soft rot of carrot caused by Klebsiella variicola in India. New Dis. Rep. 2018, 37, 21. [Google Scholar] [CrossRef]
- Parvin, S.M.R.; Taghavi, S.M.; Osdaghi, E. Field surveys indicate taxonomically diverse Pectobacterium species inducing soft rot of vegetables and annual crops in Iran. Plant Pathol. 2023, 72, 1260–1271. [Google Scholar] [CrossRef]
- Baranski, R. Genetic transformation of carrot (Daucus carota) and other Apiaceae species. Transgenic Plant J. 2008, 2, 18–31. [Google Scholar]
- Hayward, A.; Waterston, J. CMI descriptions of pathogenic fungi and bacteria. CMI descriptions of pathogenic fungi and bacteria 1965. [Google Scholar]
- Lippincott, J.A.; Lippincott, B.B. The genus Agrobacterium and plant tumorigenesis. Annu. Rev. Microbiol. 1975, 29, 377–405. [Google Scholar] [CrossRef] [PubMed]
- Fahy, P.C.; Persley, G.J. Plant bacterial diseases, a diagnostic guide; Academic Press: 1983.
- Janse, J. A Streptomyces species identified as the cause of carrot scab. Neth. J. Plant Pathol. 1988, 94, 303–306. [Google Scholar] [CrossRef]
- Goyer, C.; Vachon, J.; Beaulieu, C. Pathogenicity of Streptomyces scabies mutants altered in thaxtomin A production. Phytopathology 1998, 88, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Nunez, J.; Davis, R. Influence of gibberellic acid on carrot growth and severity of Alternaria leaf blight. Plant Dis. 2000, 84, 555–558. [Google Scholar] [CrossRef]
- Grogan, R.; Zink, F.w.; Kimble, K. Pathological anatomy of carrot root scab and some factors affecting its incidence and severity. Hilgardia 1961, 31, 53–68. [Google Scholar] [CrossRef]
- Hanson, L.; Lacy, M. Carrot scab caused by Streptomyces spp. in Michigan. Plant Dis. 1990, 74. [Google Scholar] [CrossRef]
- Clarke, C.R.; Kramer, C.G.; Kotha, R.R.; Luthria, D.L. The phytotoxin thaxtomin a is the primary virulence determinant for scab disease of beet, carrot, and radish caused by Streptomyces scabiei. Phytopathology 2022, 112, 2288–2295. [Google Scholar] [CrossRef]
- Le Clerc, V.; Aubert, C.; Cottet, V.; Yovanopoulos, C.; Piquet, M.; Suel, A.; Huet, S.; Koutouan, C.; Hamama, L.; Chalot, G. Breeding for carrot resistance to Alternaria dauci without compromising taste. Mol. Breed. 2019, 39, 1–15. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, D.; Ou, C.; Hao, W.; Zhang, Y.; He, Y.; Zhao, Z.; Zhuang, F. Draft genome sequence of carrot alternaria leaf blight pathogen Alternaria dauci. Plant Dis. 2023, 107, 2197–2200. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.J.; Pryor, B.M.; Davis, R.M. Alternaria diseases of carrot. Plant Dis. 2004, 88, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Vintal, H.; Ben-Noon, E.; Shlevin, E.; Yermiyahu, U.; Shtienberg, D.; Dinoor, A. Influence of rate of soil fertilization on Alternaria leaf blight (Alternaria dauci) in carrots. Phytoparasitica 1999, 27, 193–200. [Google Scholar] [CrossRef]
- Ben-Noon, E.; Shtienberg, D.; Shlevin, E.; Vintal, H.; Dinoor, A. Optimization of chemical suppression of Alternaria dauci, the causal agent of Alternaria leaf blight in carrots. Plant Dis. 2001, 85, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Kainulainen, P.; Tarhanen, J.; Tiilikkala, K.; Holopainen, J. Foliar and emission composition of essential oil in two carrot varieties. J. Agric. Food Chem. 1998, 46, 3780–3784. [Google Scholar] [CrossRef]
- Keilwagen, J.; Lehnert, H.; Berner, T.; Budahn, H.; Nothnagel, T.; Ulrich, D.; Dunemann, F. The terpene synthase gene family of carrot (Daucus carota L.): identification of QTLs and candidate genes associated with terpenoid volatile compounds. Front. Plant Sci. 2017, 8, 1930. [Google Scholar] [CrossRef] [PubMed]
- Koutouan, C.; Briard, M.; Baltenweck, R.; Claudel, P.; Halter, D.; Hugueney, P.; Hamama, L.; Suel, A.; Huet, S.; Bouvet Merlet, M.-H. Carrot resistance against Alternaria leaf blight: potential involvement of terpenes and flavonoids. In Proceedings of the II International Symposium on Carrot and Other Apiaceae 1264; 2018; pp. 191–198. [Google Scholar]
- Lecomte, M.; Berruyer, R.; Hamama, L.; Boedo, C.; Hudhomme, P.; Bersihand, S.; Arul, J.; N’guyen, G.; Gatto, J.; Guilet, D. Inhibitory effects of the carrot metabolites 6-methoxymellein and falcarindiol on development of the fungal leaf blight pathogen Alternaria dauci. Physiol. Mol. Plant Pathol. 2012, 80, 58–67. [Google Scholar] [CrossRef]
- McIlveen, W.; Edgington, L. Isolation of Thielaviopsis basicola from soil with umbelliferous root tissue as baits. Canad. J. Bot. 1972, 50, 1363–1366. [Google Scholar] [CrossRef]
- Yarwood, C. The occurrence of Chalara elegans. Mycol. 1981, 73, 524–530. [Google Scholar] [CrossRef]
- Punja, Z.; Chittaranjan, S.; Gaye, M. Development of black root rot caused by Chalara elegans on fresh market carrots. Can. J. Plant Pathol. 1992, 14, 299–309. [Google Scholar] [CrossRef]
- Boedo, C.; Le Clerc, V.; Briard, M.; Simoneau, P.; Chevalier, M.; Georgeault, S.; Poupard, P. Impact of carrot resistance on development of the Alternaria leaf blight pathogen (Alternaria dauci). Eur. J. Plant Pathol. 2008, 121, 55–66. [Google Scholar] [CrossRef]
- Benedict, W. Effect of soil temperature on the pathology of Alternaria radicina on carrots. Canad. J. Bot. 1977, 55, 1410–1418. [Google Scholar] [CrossRef]
- Hasan, M.; Jannat, R.; Briste, P.S.; Hossain, M.M.; Bhuiyan, M.K.A. Bio management of Crown Rot and Southern Blight of carrot by using Trichoderma Fortified compost. Egypt. J. Agric. Res. 2021, 99, 221–230. [Google Scholar]
- Davis, R.M. Carrot diseases and their management. In Diseases of Fruits and Vegetables Volume I: Diagnosis and Management; Springer: 2004; pp. 397–439.
- Le Clerc, V.; Briard, M.; Crops, R. Carrot disease management. Carrots and related Apiaceae crops 2020, 33, 115–129. [Google Scholar]
- Marcou, S.; Wikström, M.; Ragnarsson, S.; Persson, L.; Höfte, M. Occurrence and anastomosis grouping of Rhizoctonia spp. inducing black scurf and greyish-white felt-like mycelium on carrot in Sweden. J. Fungus 2021, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Bannai, T.; Misawa, T. First report of leaf blight and petiole rot of carrot caused by Rhizoctonia solani AG-1 IB. J. Gen. Plant Pathol. 2021, 87, 42–45. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Hashmi, A.; Khan, M.R.; Parveen, A. Management of bacteria Pectobacterium carotovorum, Xanthomonas campestris pv. carotae, and fungi Rhizoctonia solani, Fusarium solani and Alternaria dauci with silicon dioxide nanoparticles on carrot. Int. J. Veg. Sci. 2020, 26, 547–557. [Google Scholar] [CrossRef]
- Ahamad, L.; Siddiqui, Z.A.; Hashem, A.; Abd_Allah, E.F. Use of AM fungus Rhizophagus irregularis and silicon dioxide nanoparticles for the management of Meloidogyne incognita, Alternaria dauci and Rhizoctonia solani and growth of carrot. Arch. Phytopathol. Pflanzenschutz 2023, 56, 466–488. [Google Scholar] [CrossRef]
- Williamson-Benavides, B.A.; Dhingra, A. Understanding root rot disease in agricultural crops. Hortic. 2021, 7, 33. [Google Scholar] [CrossRef]
- Chaudhry, M.; Sidhu, J.; Nunez, J.; Gillard, J.; Francis, I. First report of strains within the Pythium spinosum species complex causing carrot cavity spot in California. Plant Dis. 2022, 106, 1534. [Google Scholar] [CrossRef]
- Van der Plaats-Niterink, A.J. Monograph of the genus Pythium. Studies in Mycology 1981. [Google Scholar]
- Howard, R.J.; Garland, J.A.; Seaman, W.L.; Grafius, E.J. Diseases and pests of vegetable crops in Canada. J. Econ. Entomol. 1996, 89, 1045–1045. [Google Scholar]
- Howard, R.; Pratt, R.; Williams, P. Pathogenicity to carrots of Pythium species from organic soils of North America. Phytopathology 1978, 68, 1293–1296. [Google Scholar] [CrossRef]
- Davis, R.; Nunez, J. Influence of crop rotation on the incidence of Pythium-and Rhizoctonia-induced carrot root dieback. Plant Dis. 1999, 83, 146–148. [Google Scholar] [CrossRef]
- Teet, S.E.; Hashim, N. Recent advances of application of optical imaging techniques for disease detection in fruits and vegetables: A review. Food Control 2023, 109849. [Google Scholar] [CrossRef]
- Gossen, B.D.; Carisse, O.; Kawchuk, L.M.; Van Der Heyden, H.; McDonald, M.R. Recent changes in fungicide use and the fungicide insensitivity of plant pathogens in Canada. Can. J. Plant Pathol. 2014, 36, 327–340. [Google Scholar] [CrossRef]
- Ghorbani, R.; Wilcockson, S.; Koocheki, A.; Leifert, C. Soil management for sustainable crop disease control: a review. Environ. Chem. Lett. 2008, 6, 149–162. [Google Scholar] [CrossRef]
- Wang, J.; Haapalainen, M.; Nissinen, A.I.; Pirhonen, M. Dual transcriptional profiling of carrot and ‘Candidatus Liberibacter solanacearum’at different stages of infection suggests complex host-pathogen interaction. Mol. Plant Microbe Interact. 2021, 34, 1281–1297. [Google Scholar] [CrossRef] [PubMed]
- Teresani, G.R.; Bertolini, E.; Alfaro-Fernández, A.; Martínez, C.; Tanaka, F.A.O.; Kitajima, E.W.; Roselló, M.; Sanjuán, S.; Ferrándiz, J.C.; López, M.M. Association of ‘Candidatus Liberibacter solanacearum’with a vegetative disorder of celery in Spain and development of a real-time PCR method for its detection. Phytopathology 2014, 104, 804–811. [Google Scholar] [CrossRef]
- Nissinen, A.; Haapalainen, M.; Jauhiainen, L.; Lindman, M.; Pirhonen, M. Different symptoms in carrots caused by male and female carrot psyllid feeding and infection by ‘Candidatus Liberibacter solanacearum’. Plant Pathol. 2014, 63, 812–820. [Google Scholar] [CrossRef]
- Nissinen, A.I.; Haapalainen, M.; Ojanen, H.; Pirhonen, M.; Jauhiainen, L. Spreading of Trioza apicalis and development of “Candidatus Liberibacter solanacearum” infection on carrot in the field conditions. Ann. Appl. Biol. 2021, 178, 39–50. [Google Scholar] [CrossRef]
- Keshet-Sitton, A.; Piasezky, A.; Assoline, N.; Dror, O.; Bahar, O. Effect of plant age, temperature, and vector load on ‘Candidatus Liberibacter solanacearum’in planta titer and shoot proliferation symptoms in carrot. Phytopathology 2022, 112, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Li, M.; Li, D.; Khan, S.-A.; Hepworth, S.R.; Wang, S.-M. Transcriptome analysis reveals regulatory framework for salt and osmotic tolerance in a succulent xerophyte. BMC Plant Biol. 2019, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vij, S.; Tyagi, A.K. Emerging trends in the functional genomics of the abiotic stress response in crop plants. Plant Biotechnol. J. 2007, 5, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.H.; Pieckenstain, F.L.; Szymanski, J.; Erban, A.; Bromke, M.; Hannah, M.A.; Kraemer, U.; Kopka, J.; Udvardi, M.K. Comparative functional genomics of salt stress in related model and cultivated plants identifies and overcomes limitations to translational genomics. PloS one 2011, 6, e17094. [Google Scholar] [CrossRef] [PubMed]
- Maynard, D.; Hochmuth, G. Knott’s Handbook for Vegetable growers. 582p. 1997. [Google Scholar]
- Kamińska, I.; Lukasiewicz, A.; Klimek-Chodacka, M.; Długosz-Grochowska, O.; Rutkowska, J.; Szymonik, K.; Baranski, R. Antioxidative and osmoprotecting mechanisms in carrot plants tolerant to soil salinity. Sci. Rep. 2022, 12, 7266. [Google Scholar] [CrossRef]
- Maas, E.V.; Grattan, S. Crop yields as affected by salinity. Agricultural Drainage 1999, 38, 55–108. [Google Scholar]
- Kasiri, M.R.; Hassandokht, M.R.; Kashi, A.; Shahi-Gharahlar, A. Evaluation of genetic diversity in Iranian yellow carrot accessions (Daucus carota var. sativus), an exposed to extinction rooty vegetable, using morphological characters. IJACS 2013, 6, 151–156. [Google Scholar]
- Kwolek, K.; Klimek-Chodacka, M.; Macko-Podgórni, A.; Grzebelus, D. Determination of genomic regions associated with carrot response to salinity stress. In Proceedings of the II International Symposium on Carrot and Other Apiaceae 1264; 2018; pp. 205–210. [Google Scholar]
- Simpson, K.; Fuentes, P.; Quiroz-Iturra, L.F.; Flores-Ortiz, C.; Contreras, R.; Handford, M.; Stange, C. Unraveling the induction of phytoene synthase 2 expression by salt stress and abscisic acid in Daucus carota. J. Exp. Bot. 2018, 69, 4113–4126. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, W.M.; Vieira, J.V.; Silva, G.O.; Reitsma, K.R.; Cantliffe, D.J. Carrot seed germination at high temperature: effect of genotype and association with ethylene production. Hortic. Sci. 2008, 43, 1538–1543. [Google Scholar] [CrossRef]
- Simon, P.; Matthews, W.; Roberts, P. Evidence for simply inherited dominant resistance to Meloidogyne javanica in carrot. Theor. Appl. Genet. 2000, 100, 735–742. [Google Scholar] [CrossRef]
- Simon, P.W.; Freeman, R.E.; Vieira, J.V.; Boiteux, L.S.; Briard, M.; Nothnagel, T.; Michalik, B.; Kwon, Y.-S. Carrot. Vegetables II: Fabaceae, Liliaceae, Solanaceae, and Umbelliferae 2008, 327-357.
- Areavibes. https://www.areavibes.com/bakersfield-ca/weather/ 2018.
- Reid, J.; Gillespie, R. Yield and quality responses of carrots (Daucus carota L.) to water deficits. N. Z. J. Crop Hortic. Sci. 2017, 45, 299–312. [Google Scholar] [CrossRef]
- Lada, R.; Stiles, A.; Pettipas, C. Physiological mechanism and genotypic variation in drought tolerance of processing carrots. Hort. Sci. 2004, 39, 855C–855. [Google Scholar] [CrossRef]
- Vorosmarty, C.J.; Green, P.; Salisbury, J.; Lammers, R.B. Global water resources: vulnerability from climate change and population growth. Science 2000, 289, 284–288. [Google Scholar] [CrossRef]
- Gouda, A.C.; Ndjiondjop, M.N.; Djedatin, G.L.; Warburton, M.L.; Goungoulou, A.; Kpeki, S.B.; N’Diaye, A.; Semagn, K. Comparisons of sampling methods for assessing intra-and inter-accession genetic diversity in three rice species using genotyping by sequencing. Sci. Rep. 2020, 10, 13995. [Google Scholar] [CrossRef] [PubMed]
- Coleman-Jensen; Alisha, C.G.; Singh., A. Household Food Security in the United States in 2013. ERR-173, U.S. Department of Agriculture, Economic Research Service, 2014.
- Simon, P.; Navazio, J.; Colley, M.; McCluskey, C.; Zystro, J.; Hoagland, L.; Roberts, P.; Du Toit, L.; Waters, T.; Silva, E. The CIOA (carrot improvement for organic agriculture) project: location, cropping system and genetic background influence carrot performance including top height and flavour. Acta Hortic. 2017, 1–8. [Google Scholar] [CrossRef]
- Turner, S.D.; Maurizio, P.L.; Valdar, W.; Yandell, B.S.; Simon, P.W. Dissecting the genetic architecture of shoot growth in carrot (Daucus carota L.) using a diallel mating design. G3: Genes, Genomes, Genetics 2018, 8, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Corak, K.E.; Genger, R.K.; Simon, P.W.; Dawson, J.C. Comparison of genotypic and phenotypic selection of breeding parents in a carrot (Daucus carota) germplasm collection. Crop Sci. 2023, 63, 1998–2011. [Google Scholar] [CrossRef]
- Corak, K.; Ellison, S.; Simon, P.; Spooner, D.; Dawson, J. Comparison of representative and custom methods of generating core subsets of a carrot germplasm collection. Crop Sci. 2019, 59, 1107–1121. [Google Scholar] [CrossRef]
- Riaz, N.; Yousaf, Z.; Yasmin, Z.; Munawar, M.; Younas, A.; Rashid, M.; Aftab, A.; Shamsheer, B.; Yasin, H.; Najeebullah, M. Development of carrot nutraceutical products as an alternative supplement for the prevention of nutritional diseases. Front. Nutr. 2022, 8, 787351. [Google Scholar] [CrossRef]
- Le Clerc, V.; Marques, S.; Suel, A.; Huet, S.; Hamama, L.; Voisine, L.; Auperpin, E.; Jourdan, M.; Barrot, L.; Prieur, R. QTL mapping of carrot resistance to leaf blight with connected populations: stability across years and consequences for breeding. Theor. Appl. Genet. 2015, 128, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Le Clerc, V.; Pawelec, A.; Birolleau-Touchard, C.; Suel, A.; Briard, M. Genetic architecture of factors underlying partial resistance to Alternaria leaf blight in carrot. Theor. Appl. Genet. 2009, 118, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Aguayo, E.; Gómez, P.A.; Artés, F.; Artés-Hernández, F. UV-C and hyperoxia abiotic stresses to improve healthiness of carrots: Study of combined effects. JFST 2016, 53, 3465–3476. [Google Scholar] [CrossRef]
- Chubey, B.; Nylund, R. Surface browning of carrots. Can. J. Plant Sci. 1969, 49, 421–426. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agr. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Saltveit, M.E.; Krochta, J.M. Mechanism of surface white discoloration of peeled (minimally processed) carrots during storage. J. Food Sci. 1995, 60, 320–323. [Google Scholar] [CrossRef]
- Alegria, C.; Pinheiro, J.; Duthoit, M.; Gonçalves, E.M.; Moldão-Martins, M.; Abreu, M. Fresh-cut carrot (cv. Nantes) quality as affected by abiotic stress (heat shock and UV-C irradiation) pre-treatments. WT - Food Sci. Technol. 2012, 48, 197–203. [Google Scholar] [CrossRef]
- Maesano, G.; Hamam, M.; Pecorino, B.; Pappalardo, G.; D'Amico, M.; Chinnici, G. Trends in consumers’ preferences towards fresh-cut vegetables during the Covid-19 pandemic. Economia agro-alimentare, 2022. [Google Scholar]
- Paparella, A.; Purgatorio, C.; Chaves-López, C.; Rossi, C.; Serio, A. The multifaceted relationship between the COVID-19 pandemic and the food system. Foods 2022, 11, 2816. [Google Scholar] [CrossRef]
- Raffo, A.; Paoletti, F. Fresh-cut vegetables processing: environmental sustainability and food safety issues in a comprehensive perspective. Front. Sustain. Food Syst. 2022, 5, 681459. [Google Scholar] [CrossRef]
- Holton, A.E.; Love, B.; Mackert, M. Exchanging health for commercialization: The news media’s mediation of the baby carrots campaign. Cases in Public Health Communication & Marketing. 2011, 5, 2–25. [Google Scholar]
- Määttä, J.; Lehto, M.; Kuisma, R.; Kymäläinen, H.-R.; Mäki, M. Microbiological quality of fresh-cut carrots and process waters. J. Food Prot. 2013, 76, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Dharmarha, V.; Pulido, N.; Boyer, R.R.; Pruden, A.; Strawn, L.K.; Ponder, M.A. Effect of post-harvest interventions on surficial carrot bacterial community dynamics, pathogen survival, and antibiotic resistance. Int. J. Food Microbiol. 2019, 291, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Kangas, S.; Takkinen, J.; Hakkinen, M.; Nakari, U.-M.; Johansson, T.; Henttonen, H.; Virtaluoto, L.; Siitonen, A.; Ollgren, J.; Kuusi, M. Yersinia pseudotuberculosis O: 1 traced to raw carrots, Finland. Emerg. Infect. Dis. 2008, 14, 1959. [Google Scholar] [CrossRef] [PubMed]
- Rimhanen-Finne, R.; Niskanen, T.; Hallanvuo, S.; Makary, P.; Haukka, K.; Pajunen, S.; Siitonen, A.; Ristolainen, R.; Pöyry, H.; Ollgren, J. Yersinia pseudotuberculosis causing a large outbreak associated with carrots in Finland, 2006. Epidemiol. Infect. 2009, 137, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Rossi, C.; Palmieri, S.; Maggio, F.; Chaves-López, C.; Lo Sterzo, C.; Paparella, A.; De Medici, D.; Ricci, A.; Serio, A. Salmonella enterica control in stick carrots through incorporation of coriander seeds essential oil in sustainable washing treatments. Front. Sustain. Food Syst. 2020, 4, 14. [Google Scholar] [CrossRef]
- Kahala, M.; Blasco, L.; Joutsjoki, V. Molecular characterization of spoilage bacteria as a means to observe the microbiological quality of carrot. J. Food Prot. 2012, 75, 523–532. [Google Scholar] [CrossRef] [PubMed]
- CREA. Consiglio per la Ricerca in Agricoltura e l’Analisi dell’Economia Agraria https://www.alimentinutrizione.it/tabelle-nutrizionali/005150 2019.
- Farrar, J.; Nunez, J.; Davis, R. Influence of soil saturation and temperature on Erwinia chrysanthemi soft rot of carrot. Plant Dis. 2000, 84, 665–668. [Google Scholar] [CrossRef]
- Barry-Ryan, C.; Pacussi, J.; O'beirne, D. Quality of shredded carrots as affected by packaging film and storage temperature. J. Food Sci. 2000, 65, 726–730. [Google Scholar] [CrossRef]
- Towner, D.; Beraha, L. Plant disease reporter--april 1976 357 core-rot: A bacterial disease of carrots. Plant Dis. Rep. 1976, 60, 357. [Google Scholar]
- Pilon, L.; Oetterer, M.; Gallo, C.R.; Spoto, M.H. Shelf life of minimally processed carrot and green pepper. Trends Food Sci. Technol 2006, 26, 150–158. [Google Scholar] [CrossRef]
- Ayhan, Z.; Eştürk, O.; Taş, E. Effect of modified atmosphere packaging on the quality and shelf life of minimally processed carrots. Turk. J. Agric. For. 2008, 32, 57–64. [Google Scholar]
- Cisneros-Zevallos, L.; SALTVEIT, M.E.; KROCHTA, J.M. Hygroscopic coatings control surface white discoloration of peeled (minimally processed) carrots during storage. J. Food Sci. 1997, 62, 363–366. [Google Scholar] [CrossRef]
- Ranjitha, K.; Rao, D.S.; Shivashankara, K.; Oberoi, H.S.; Roy, T.K.; Bharathamma, H. Shelf-life extension and quality retention in fresh-cut carrots coated with pectin. Innov. Food Sci. Emerg. Technol. 2017, 42, 91–100. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Packaging strategies to prolong the shelf life of fresh carrots (Daucus carota L.). Innov. Food Sci. Emerg. Technol. 2012, 13, 215–220. [Google Scholar] [CrossRef]
- Leceta, I.; Molinaro, S.; Guerrero, P.; Kerry, J.; De la Caba, K. Quality attributes of map packaged ready-to-eat baby carrots by using chitosan-based coatings. Postharvest Biol. Technol. 2015, 100, 142–150. [Google Scholar] [CrossRef]
- de Souza, L.P.; Faroni, L.R.D.A.; Heleno, F.F.; Cecon, P.R.; Gonçalves, T.D.C.; da Silva, G.J.; Prates, L.H.F. Effects of ozone treatment on postharvest carrot quality. Lwt 2018, 90, 53–60. [Google Scholar] [CrossRef]
- Singh, N.; Singh, R.; Bhunia, A.; Stroshine, R. Efficacy of chlorine dioxide, ozone, and thyme essential oil or a sequential washing in killing Escherichia coli O157: H7 on lettuce and baby carrots. LWT 2002, 35, 720–729. [Google Scholar] [CrossRef]
- Sarron, E.; Gadonna-Widehem, P.; Aussenac, T. Ozone treatments for preserving fresh vegetables quality: A critical review. Foods 2021, 10, 605. [Google Scholar] [CrossRef]
- Hassenberg, K.; Frohling, A.; Geyer, M.; Schluter, O.; Herppich, W. Ozonated wash water for inhibition of Pectobacterium carotovorum on carrots and the effect on the physiological behaviour of produce. Eur. J. Hortic. Sci. 2008, 73, 37. [Google Scholar]
- Liew, C.L.; Prange, R.K. Effect of ozone and storage temperature on postharvest diseases and physiology of carrots (Daucus carota L.). J. Am. Soc. Hortic. Sci. 1994, 119, 563–567. [Google Scholar] [CrossRef]
- Selma, M.V.; Allende, A.; Lopez-Galvez, F.; Conesa, M.A.; Gil, M.I. Disinfection potential of ozone, ultraviolet-C and their combination in wash water for the fresh-cut vegetable industry. Food Microbiol. 2008, 25, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, V.; Devlieghere, F.; Ragaert, P.; Debevere, J. Shelf-life extension of minimally processed carrots by gaseous chlorine dioxide. Int. J. Food Microbiol. 2007, 116, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, L.; Van der Plancken, I.; Grauwet, T.; Verlinde, P.; Matser, A.; Hendrickx, M.; Van Loey, A. Thermal versus high pressure processing of carrots: a comparative pilot-scale study on equivalent basis. Innov. Food Sci. Emerg. Technol. 2012, 15, 1–13. [Google Scholar] [CrossRef]
- Viacava, F.; Ramos-Parra, P.A.; Welti-Chanes, J.; Jacobo-Velázquez, D.A. High hydrostatic pressure processing of whole carrots: Effect of static and multi-pulsed mild intensity hydrostatic pressure treatments on bioactive compounds. Foods 2021, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, G.; Raja, P.; Abitha, M.; Ganapathy, S.; Rajkumar, P. Standardization of UV-C treatment, Ozonization and chlorination for reducing microbial growth in carrot under laboratory conditions. J. Pharmacogn. Phytochem. 2020, 9, 557–563. [Google Scholar] [CrossRef]
- Ojaghian, M.R.; Zhang, J.-Z.; Xie, G.-L.; Wang, Q.; Li, X.-L.; Guo, D.-P. Efficacy of UV-C radiation in inducing systemic acquired resistance against storage carrot rot caused by Sclerotinia sclerotiorum. Postharvest Biol. Technol. 2017, 130, 94–102. [Google Scholar] [CrossRef]
- Ben-Fadhel, Y.; Cingolani, M.C.; Li, L.; Chazot, G.; Salmieri, S.; Horak, C.; Lacroix, M. Effect of γ-irradiation and the use of combined treatments with edible bioactive coating on carrot preservation. Food Packaging and Shelf Life 2021, 28, 100635. [Google Scholar] [CrossRef]
- Ndoti-Nembe, A.; Vu, K.D.; Doucet, N.; Lacroix, M. Antimicrobial effects of essential oils, nisin, and irradiation treatments against Listeria monocytogenes on ready-to-eat carrots. J. Food Sci. 2015, 80, M795–M799. [Google Scholar] [CrossRef]
- Ojaghian, S.; Saremi, M.; Pashaei, S. Inhibitory Effect of Crude Extracts Derived from Aromatic Plants Against White Mold of Brassica juncea var. tumida. Pakistan Journal of Phytopathology 2019, 31, 35–46. [Google Scholar] [CrossRef]
- Viacava, G.E.; Cenci, M.P.; Ansorena, M.R. Effect of chitosan edible coatings incorporated with free or microencapsulated thyme essential oil on quality characteristics of fresh-cut carrot slices. Food Bioproc. Tech. 2022, 15, 768–784. [Google Scholar] [CrossRef]
- Fai, A.E.C.; de Souza, M.R.A.; de Barros, S.T.; Bruno, N.V.; Ferreira, M.S.L.; de Andrade Gonçalves, É.C.B. Development and evaluation of biodegradable films and coatings obtained from fruit and vegetable residues applied to fresh-cut carrot (Daucus carota L.). Postharvest Biol. Technol. 2016, 112, 194–204. [Google Scholar] [CrossRef]
- Pushkala, R.; Parvathy, K.; Srividya, N. Chitosan powder coating, a novel simple technique for enhancement of shelf life quality of carrot shreds stored in macro perforated LDPE packs. Innov. Food Sci Emerg. Technol. 2012, 16, 11–20. [Google Scholar] [CrossRef]
- Pedreschi, F.; Mariotti-Celis, M.S. Irradiation kills microbes: Can it do anything harmful to the food? In Genetically modified and irradiated food; Elsevier: 2020; pp. 233–242.
- Ojaghian, S.; Wang, L.; Xie, G.L.; Zhang, J.Z. Inhibitory efficacy of different essential oils against storage carrot rot with antifungal and resistance-inducing potential. J. Phytopathol. 2019, 167, 490–500. [Google Scholar] [CrossRef]
- Ergun, M.; Süslüoğlu, Z. Evaluating carrot as a functional food. Middle East J. Sci. 2018, 4, 113–119. [Google Scholar] [CrossRef]
- Šeregelj, V.; Vulić, J.; Ćetković, G.; Čanadanovć-Brunet, J.; Šaponjac, V.T.; Stajčić, S. Natural bioactive compounds in carrot waste for food applications and health benefits. Studies in Natural Products Chemistry 2020, 67, 307–344. [Google Scholar]
- Blando, F.; Marchello, S.; Maiorano, G.; Durante, M.; Signore, A.; Laus, M.N.; Soccio, M.; Mita, G. Bioactive compounds and antioxidant capacity in anthocyanin-rich carrots: A comparison between the black carrot and the Apulian landrace “Polignano” carrot. Plants 2021, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Chalchat, J.C. Chemical composition of carrot seeds (Daucus carota L.) cultivated in Turkey: characterization of the seed oil and essential oil. Grasas y aceites 2007, 58, 359–365. [Google Scholar]
- Sieniawska, E.; Świątek, Ł.; Rajtar, B.; Kozioł, E.; Polz-Dacewicz, M.; Skalicka-Woźniak, K. Carrot seed essential oil-Source of carotol and cytotoxicity study. Ind. Crops Prod. 2016, 92, 109–115. [Google Scholar] [CrossRef]
- Glišić, S.B.; Mišić, D.R.; Stamenić, M.D.; Zizovic, I.T.; Ašanin, R.M.; Skala, D.U. Supercritical carbon dioxide extraction of carrot fruit essential oil: Chemical composition and antimicrobial activity. Food Chem. 2007, 105, 346–352. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Cardoso, S.M.; Salgueiro, L. New claims for wild carrot (Daucus carota subsp. carota) essential oil. J. Evid. Based Complementary Altern. Med. 2016, 2016. [Google Scholar]
- Muturi, E.J.; Doll, K.; Ramirez, J.L.; Rooney, A.P. Bioactivity of wild carrot (Daucus carota, Apiaceae) essential oil against mosquito larvae. J. Med. Entomol. 2019, 56, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Radwan, M.M.; Wanas, A.S.; Khan, I.A. Repellent activity of carrot seed essential oil and its pure compound, carotol, against mosquitoes. J. Am. Mosq. Control Assoc. 2018, 34, 272–280. [Google Scholar] [CrossRef]
- Kataria, D. Carrot plant-a potential source of high value compounds and biological activities: a review. Proc. Indian Natn. Sci. Acad. 2016, 82, 1237–1248. [Google Scholar] [CrossRef]
- Bridge, J.; Starr, J.L. Plant nematodes of agricultural importance: a color handbook; Elsevier: 2007.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).