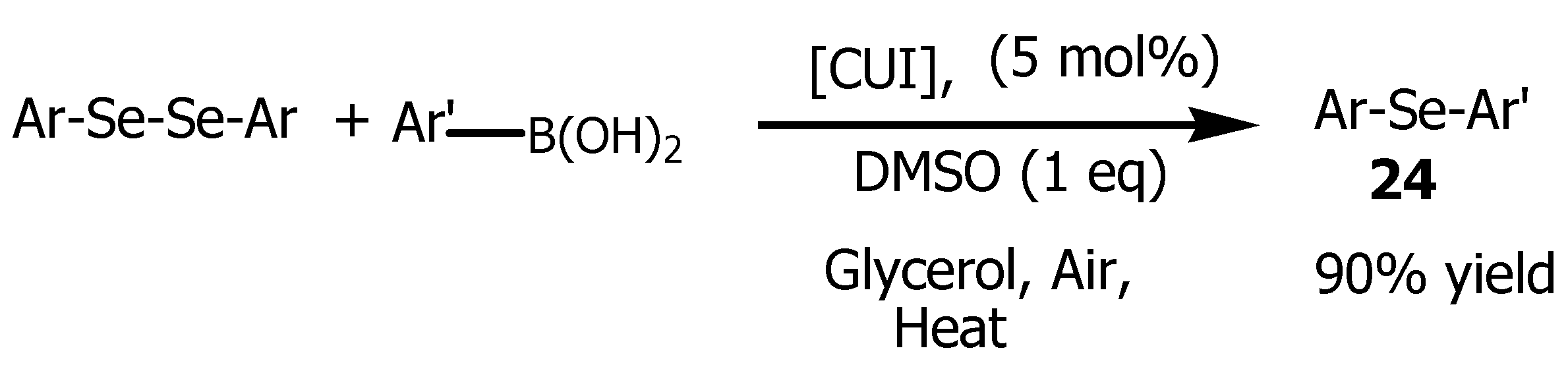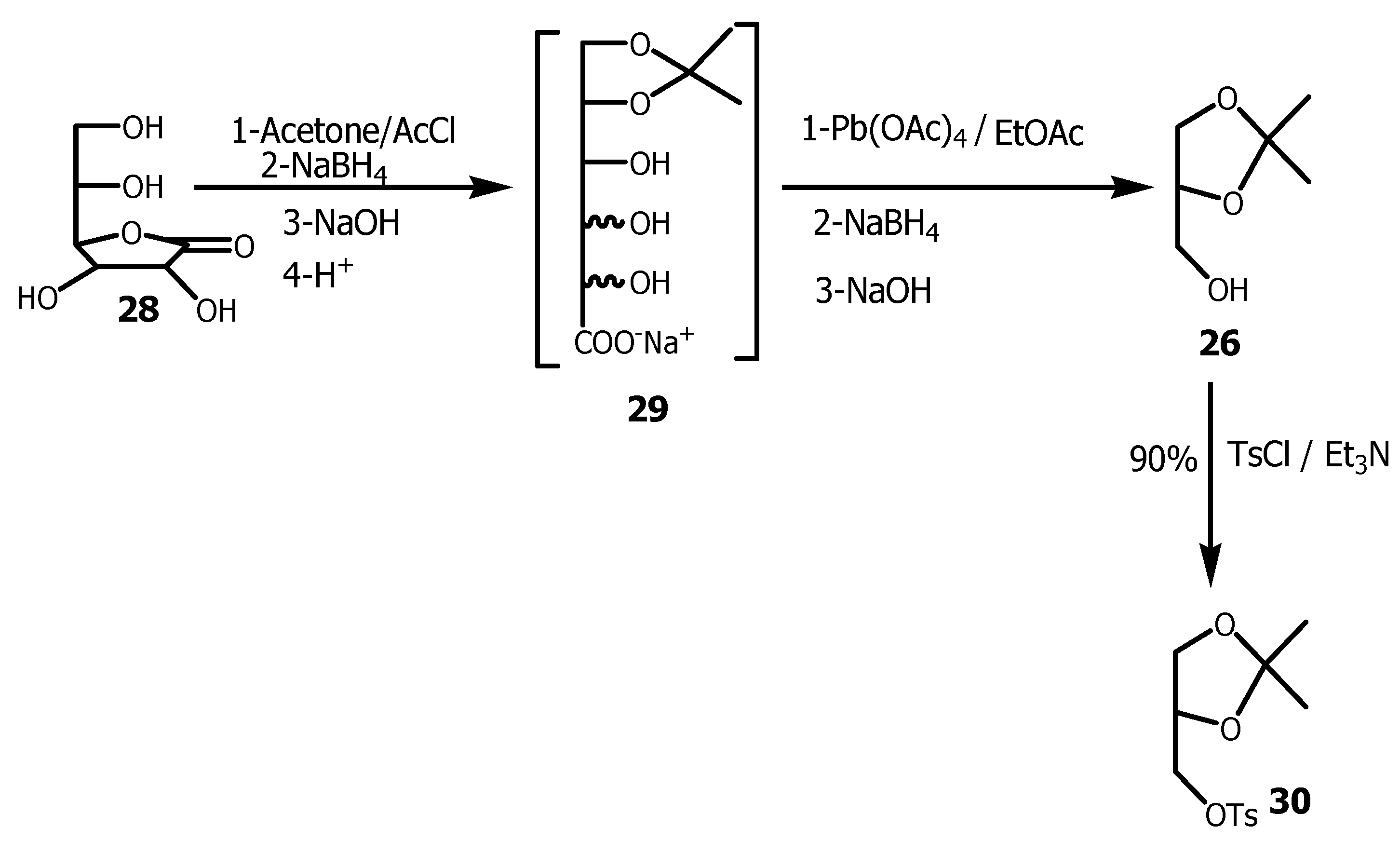1. Introduction
The use of fossil resources on the petrochemical processes for the production of fine chemical, fuels, and polymers is a matter of great concern due to the climate change. Therefore, several correctives measures were implemented in order to reduce the environmental pollution while satisfying the energy need and the production of chemicals [
1]. Bio-diesel is a green solvent compatible with diesel engines due to its properties such as biodegradability, lower emission and reduced toxicity [
2,
3,
4,
5].
The major by-product in biodiesel industry is glycerol, which is produced via trans-esterification reaction, while the focus it is still on ecologically friendly catalytic reactions in which waste (glycerol) is turned into commercial products [
6]. Currently, the abundance of glycerol formed during biodiesel production as a waste provides a vast low-cost feedstock. In the other hand, in the current climate change in which a major emphasis is being placed on the design of green processes, solvents are a key factor from both an economic and environmental point of view. Therefore, researchers have shown a growing interest in using glycerol as a sustainable solvent for the preparation of complex molecules, as well as an undeniable raw material for the synthesis of potential drugs.
Glycerol as a raw material has versatile applications due to its distinct combination of physio-chemical properties and typical elemental analysis. It is easy to handle and compatible with other substances. It is nontoxic to human health, viscous and stable under different conditions [
7,
8]. This molecule with low molecular weight has wide applications in cosmetics, foods, polymer, paint, automotive, pulp and paper, plastic and pharmaceutical industries [
9,
10]. However, the increasing over production of glycerol [
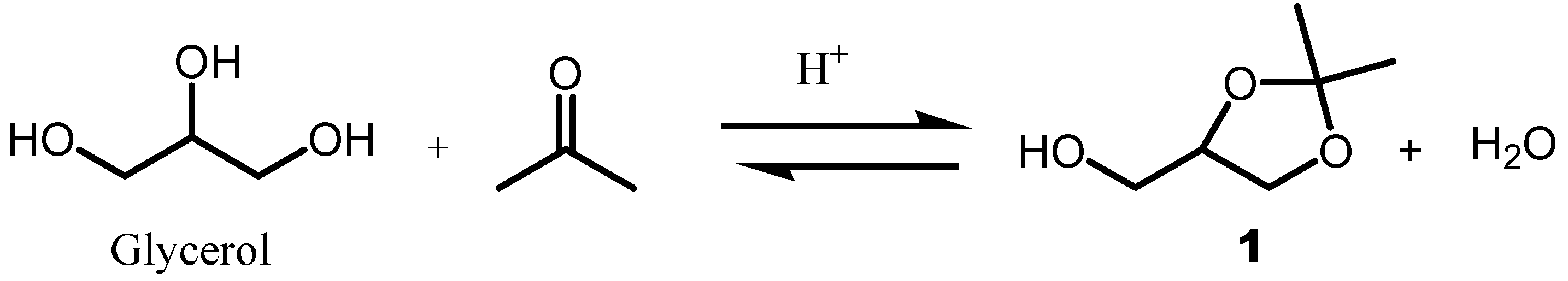
11] can turn to uncontrolled waste with the disposal and affect the economics of the biodiesel industries. One of the route to valorize glycerol involves its condensation with acetone in the presence of acid as catalyst to provide Solketal
1 (2,2-dimethyl-1,3-dioxolane-4-methanol) [
12,
13,
14,
15,
16] (
Scheme 1).
(
S)-Solketal was used as a chiral precursor in the synthesis of medicinally active unnatural products, including the hypotensive
β-adrenergic blockers, aryl-oxypropanolamines [
17], while (
R)-Solketal was used as a building block in the synthesis of Alkylglycerols and analogues found in the Greenland shark liver oil (SLO) mixture (
Centrophorus squamosus), which displayed anti-tumor and anti-metastatic activities on a model of grafted tumor in mice (3LL cells), as well as the ability to inhibit the endothelial cell migration [
18,
19,
20,
21]. In the other hand, tosylsolketal was used as a starting material for the synthesis of several biologically active compounds [
22]. Solketal was reported to control the emissions, to enhance the cold flow properties and decreases the gum formation [
23]. It was also used as a plasticizer and versatile solvent in the polymer industry [
24]
This review focuses on glycerol as a green solvent for the synthesis of complex molecules, and its conversion to Epichlorydrin, Glycidol, Solketal and Tosyl solketal as an efficient recycling process to avoid wastes, and key intermediates which were in turn used as building blocks in the preparation of glycerol derivatives, described as an undeniable new source of potentials drugs.
2. Synthetic Approaches with Glycerol as a Green Solvent
Solvents are used in most of organic reactions as a contact surface between reactants or reagents. In some cases, they can determine the chemical mechanism and the transition states of the intermediates and target molecules, as well as the recycling or disposal strategies. It was reported that a green solvent should possess certain characteristics such as low toxicity, low flammability, biodegradability, functional group compatibility, low volatility organic compounds (VOC) emission, cheap, easy to handle and recyclable with a limited environmental impact coming from the consuming of these solvents in chemical production [
25,
26,
27]. Conventional solvents such as halogenated, petroleum-based where suggested to be replaced by green solvent such as water, ethyl acetate and glycerol [
28].
Glycerol is a sweet-tasting, clear, colorless, odorless and viscous liquid. It is a polar protic solvent with a dielectric constant of 42.5 (at 25
◦C), which is intermediate between that of water (78.5) and an ionic liquid such as 1-buyl-3-methylimidazolium hexafluorophosphate ([BMIm]PF6, 11.4) [
29]. Its low-cost, non-toxicity, high boiling point (290.8°C), negligible vapor pressure (<1 mmHg at 293 K), highest solubility for organic and inorganic compounds, low miscibility with other organic solvents such as ethers and alkanes [
30] have made glycerol a green solvent in the synthesis of pharmaceutically active ingredients or potential drugs in which the toxicity and residual solvents are attentively monitored.
2.1. Glycerol as a Viable Solvent in the Synthesis of Bis (Aryl) Ketones and Aryl Compounds
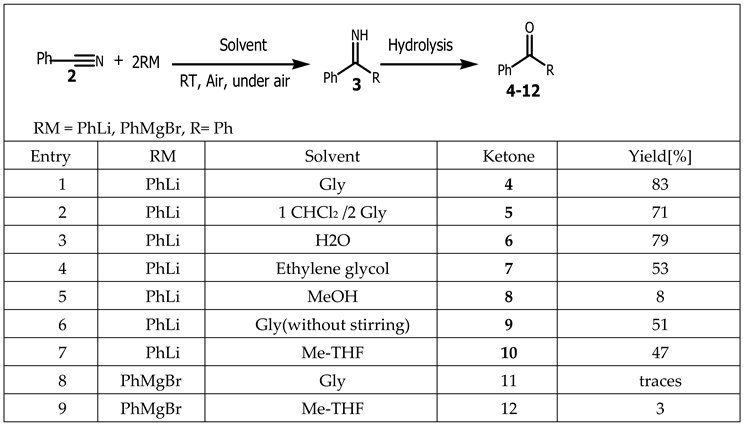
Organometallic compounds are generally prepared by Grignard reagent or lithium acetylides to yield carbon-lithium bonds, which are key intermediates in the synthesis of bioactives compounds. These reactions are carried out through nucleophilic addition or substitution. Due to the high reactivity associated with the Lithium-Carbon bond, constraining protocoles such as inert atmospheres, moisture and oxygen-free organic solvents, low temperatures are used in order to synthesize organolithium compounds [
31]. Thus, performing organolithium chemistry under anhydrous conditions and oxygen free without the need of moistureless organic solvents, it is one of the resulting challenge for researchers working in this field. Therefore, glycerol (Gly), owing exceptional physicochemical properties (high boiling point and polarity, low toxicity and flammability) was used as a green solvent to achieve the synthesis of unknown bis (aryl) ketone
4-
12 (
Table 1) [
32] under air and ambiant temperature. In the meanwhile, the most remarkable aspect of this reaction is that, the use of inert-atmosphere Schlenk techniques or low temperatures that is standard reaction conditions for manipulating organolithium reagents was not required.
Furthermore, once Gly was replaced by others green solvents such as water, dichlomethylene, 2-methyl tetrahydrofuran (Me-THF) or methanol, the yield of these reactions decreased. When phenyllithium or phenylmagnesium bromide (RM) was added to benzonitrile
2 in the presence of Gly and without stirring, the yield was reduced from 83% to 51% (entry 6) compared to water, where the absence of stirring can completely affect the reaction [
33].
Rodriguez-Alvarez et al. showed that Gly can function as an environmentally friendly reaction medium for the ultrafast and chemoselective addition of aryllithium reagents to nitriles like water under air and ambient temperature, which for almost a century was not mentioned in organometallic Chemistry [
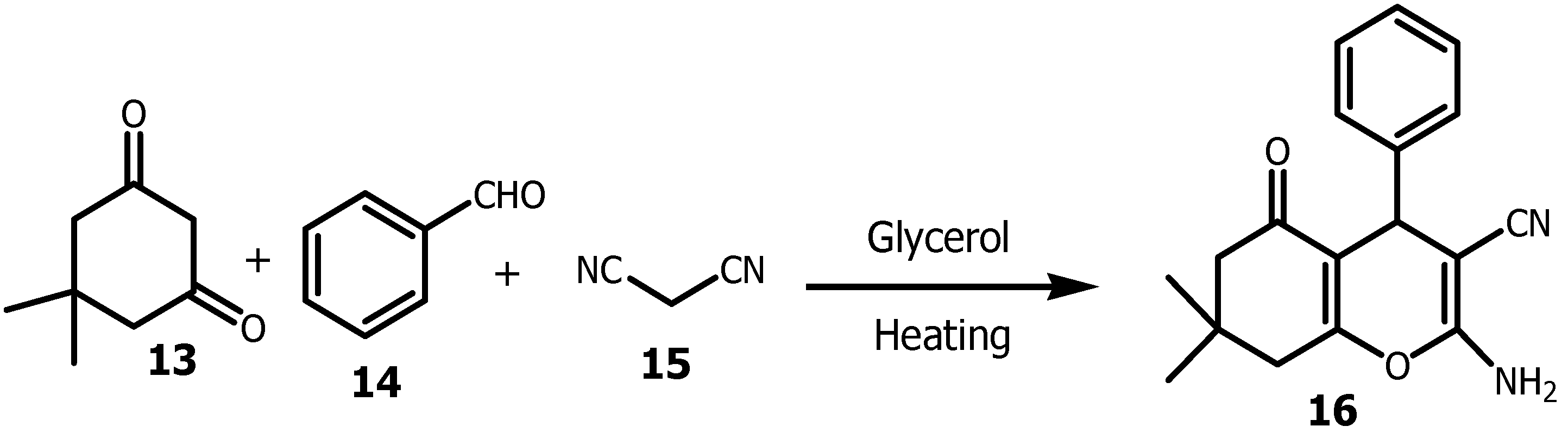
32]. Cost-effectiveness of glycerol made it also a good green solvent for the synthesis of 4H-pyrans
16 with catalyst-free (
Scheme 2). It was reported that, when Gly was replaced by water in this cyclization reaction (one-pot and three-component strategy), the yield of the reaction decreased down to 70% [
34].
α,β-Unsaturated carbonyl compounds are important intermediates in organic synthesis. The general structure is (O=CR)−C
α=C
β-R. In these compounds, the carbonyl group is conjugated with an alkene (unsaturated). A variety of well-established methodologies make use of
α,β-unsaturated carbonyl compounds to construct diverse building blocks used to prepare bio-active compounds including pharmaceuticals, precursors for materials flavors, fragrances, or optically important molecules [
36,
37,
38,
39,
40,
41]. Thus, the synthesis of
α,β -unsaturated carbonyl compounds remains an actual interesting task in the development of improved synthetic methodologies. In this context, the reaction of a nucleophilic addition on the
β position of
α,β-unsaturated carbonyl compound was carried out in different green solvents such as Gly, DMF, toluene and DMSO. Furthermore, it was reported that an aza-Michael reaction between
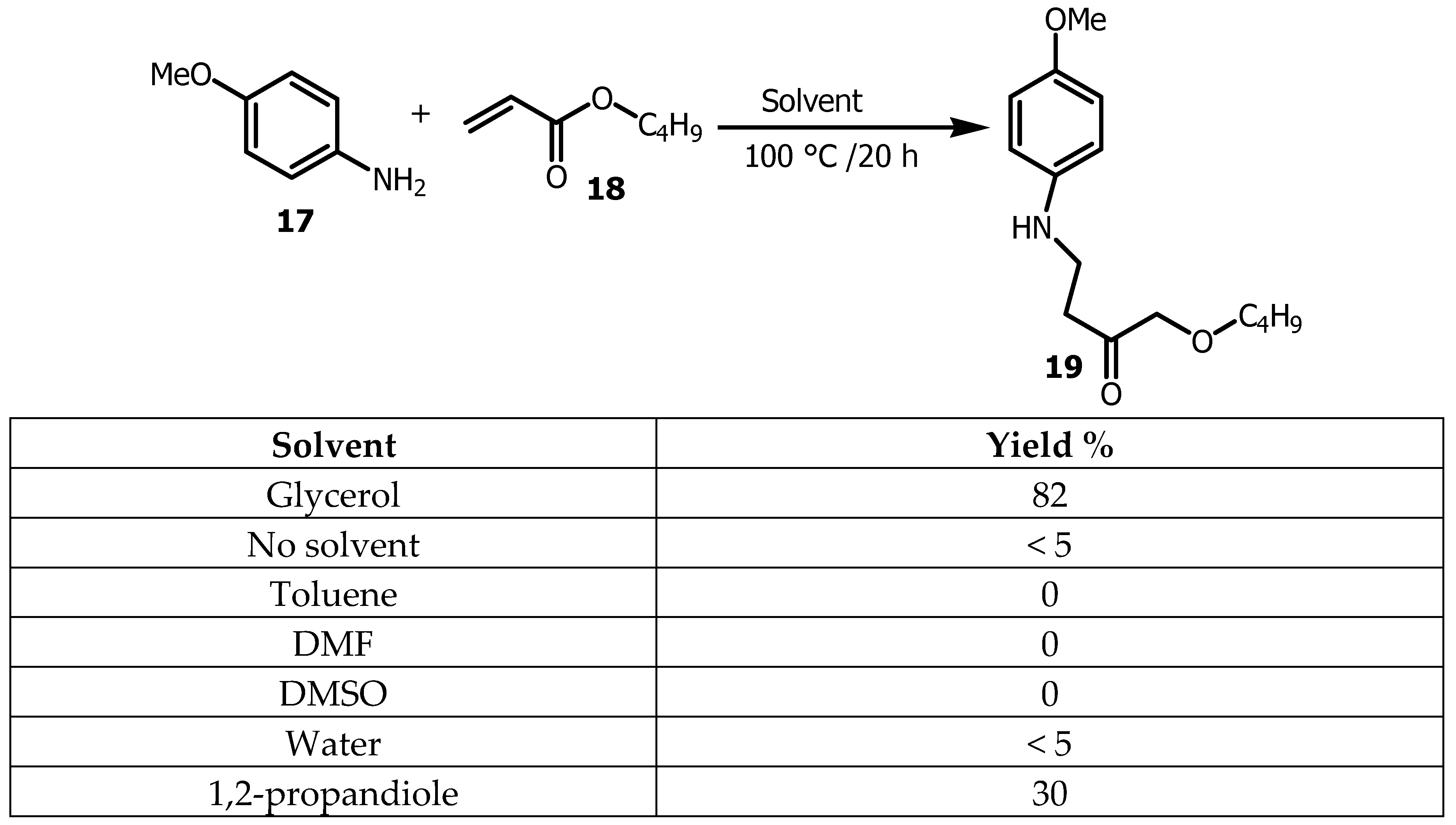
p-anisidine
17 and n-butyl acrylate
18 (
Scheme 3) was successfully proceeded under catalyst free conditions with 82% yield when Gly was used as a solvent, while 81% yield was obtained in technical grade glycerol, 30% in 1,2-propanediol, 5% in water and no desired product
19 was observed in toluene, DMSO, DMF or DCE [
35].
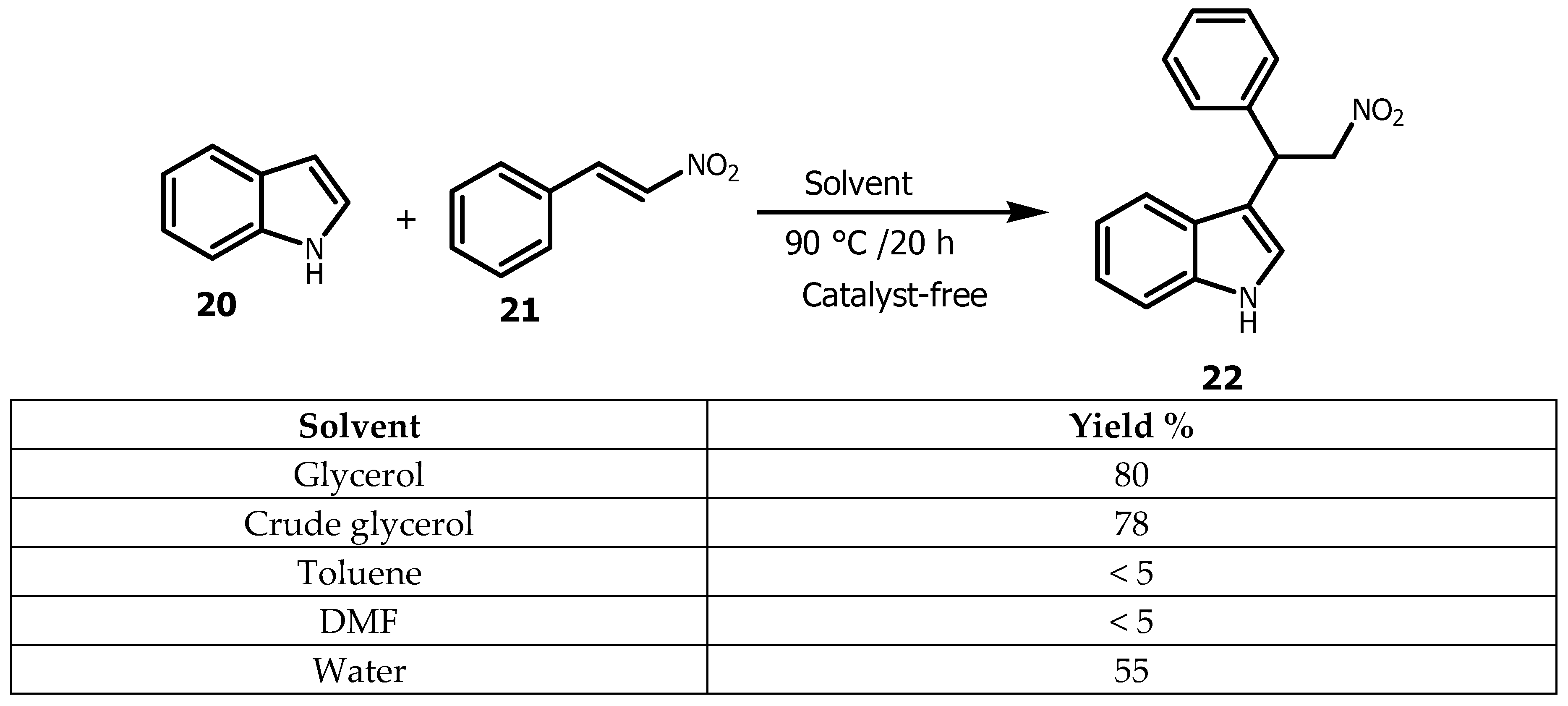
A comparable phenomena was observed in the Michael addition when reacting indole
20 with nitostyrene
21 (
Scheme 4), in which only glycerol was found to be capable of providing the desired product
22 in 80% yield under catalyst-free conditions. The desired product was also isolated in liquid-liquid phase extraction with ethyl acetate allowing the recycling of glycerol.
These experimental data accumulated so far displayed the importance of using glycerol as a green solvent free catalyst in organic reactions, thus simplifying the work-up procedure and consequently increasing the cost-effectiveness in the synthetic methodology.
2.2. Glycerol as a Suitable Solvent in the Synthesis of Metallic Complexes
Metallic complexes have attracted huge interest in fields such as nanomaterials, optics, catalysis, molecular framework materials and biomedicine, due to their optical and electrochemical properties, and high specific surface areas [
42,
43,
44]. Key to all these applications is the dependence of complex composition and structure on the metal ion, and its oxidation state. Glycerol was used as a green solvent in the synthesis of organometallic and coordination compounds due to its properties (viscosity, solubility and lower temperature), while some green solvent with high boiling point are often unsuitable for the synthesis of metal complexes [
45]. It was also reported that the free hydroxyl group of Gly hindered the formation of C-H bond involved in cyclometallation reactions [
46]. Homoleptic Ir
III complexes containing 2-arylpyridine-based ligands (L) of the general formula [Ir(k
2-
N,
C-L)
3]
23 have been efficiently prepared in glycerol at high temperature (
Scheme 5).
Following the synthesis of homoleptic Ir
III complexes, some metallic complexes other than iridium derivatives have been synthesized ( mono- and polymetallic Ru
II complexes) containing conjugated N-donor ligands, bimetallic cyclometallated Rh
III complexes, and monometallic Zn
II and Cd
II complexes in glycerol, which have also found applications in different fields due to their optical or electrochemical properties [
47,
48,
49,
50].
Palladium and copper-based catalytic systems are the most used in catalysis reaction when Gly is used as solvent to form C-C and C-heteroatom bonds. The N-arylation of primary and secondary amines with aryl halides under basic conditions in glycerol were catalyzed by CU
II and CU
I to provide better yield than when DMF and DMSO were used as solvents [
51]. Glycerol also provided an efficient catalyst immobilization allowing the catalytic phase to keep its properties and to be recycled up to six times. It was also reported that diaryl-diselenides cross-coupling reaction with aryl boronic acids in glycerol yielded corresponding diaryl-selenides
24 (
Scheme 6) when DMSO was added as an additive.
Cyclometallated complexes of transition metals synthesized in glycerol had shown many applications due to their properties. Gly also displays enormous potential for both molecular and colloidal-based catalysts in metal-mediated reactions. Glycerol accelerates reactions, immobilizes the catalyst mainly in the case of nanoparticles systems, allowing the recycling of the catalytic phase and provides metal-free target compounds [
45].
3. Glycerol as a key Synthetic Intermediate
Nowadays, special attention is focused on the environmental health due to the climate change in which any chemical waste can be transformed into useful products. Glycerol is an industrial waste, and one of the trends is the comprehensive recycling of glycerol into valuable compounds for new applications, because it is produced in large amount as a by-product of biodiesel production, which is equivalent to approximately 10 wt% of the total biodiesel manufactured. Therefore, Gly was transformed into useful keys synthetic intermediate such as Epichlorydrin, Glycidol, Solketal or Tosyl solketal as its new recycling process.
3.1. Conversion of Glycerol to Solketal
Acetalization reaction has gained potential interest in applications for the better use of excess glycerol produced from the biodiesel process. Solketal can be synthesized from renewable resources such as glycerol and acetone (
Scheme 1). It is extracted from biomass and was reported to be a suitable approach for various applications such as fuel additives and in medicine industries. Gly was reacted with acetone in the presence of acid as a catalyst to provide a five membered heterocycle named Solketal (1,2-
O-isopropylidene-glycerol)
2 almost exclusively, [
52] along with the corresponding by-product 1,3-
O-isopropylidenc derivative formed only in trace amount. Solketal can also be obtained from
D and
L-Serine,
D-mannitol, (
L)-ascorbic acid as well as (
S)-and (
R)-methyl benzylamine.
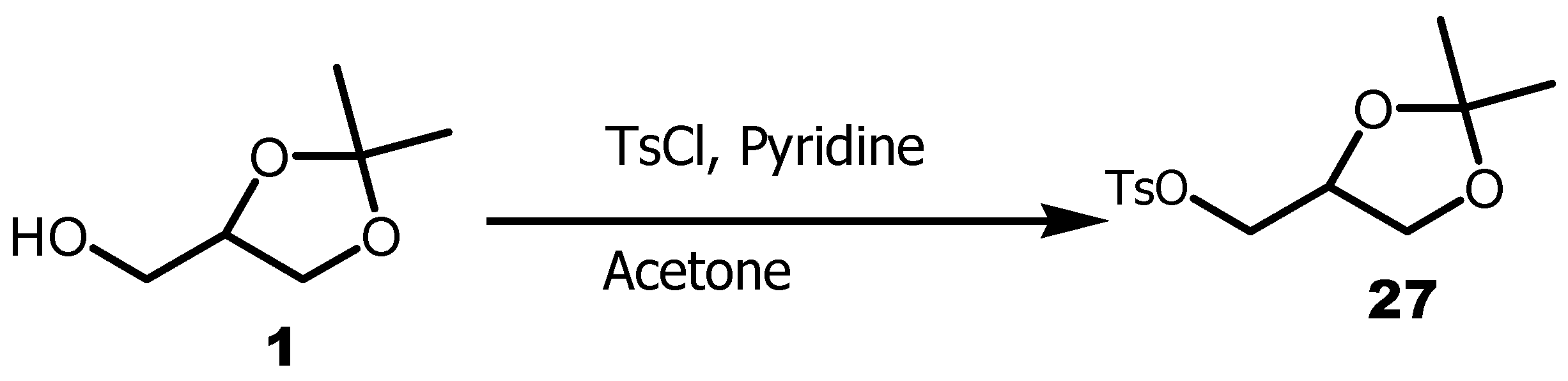
3.2. Conversion of Glycerol to Tosylsolketal
Glycerol has been used in a great number of common applications in cosmetics, pharmaceuticals, food industries and as a platform for bio-based polymers. Tosyl solketal
27 it is another key intermediate of glycerol. This compound was widely used in the preparation of bioactive lipopeptides, spiro heterocycles, pheromones, glyco-glycerolipids [
53]. Tosyl-isopropylideneglycerol
27 was obtained by reacting compound
1 with
p-toluenesulfonyl chloride in the presence of pyridine in acetone (
Scheme 7).
Racemic form of Solketal was first reported by Fischer in 1895 and was also prepared from glycerol many times [
54]. (
R)-Solketal is a precursor to alkylglycerol synthesis that naturally occurs in the S configuration. While (S)-Solketal is usually prepared from
D-mannitol, it is not the same for
R enantiomer, which access is much challenging. Several chiral precursors have been described to lead to (
R)-Tosylsolketal
30 such as
L-ascorbic acid (vitamin C),
L-serine and
L-tartaric acid, but the use of (
L)-ascorbic acid as a starting material was put forward compared to others.
The saturated diol function of ascorbic acid
28 it is easily protected as acetonide by dissolving ascorbic acid in excess acetone containing a catalytic amount of acetyl chloride, which in turn crystallized directly from the reaction in 80-85% yields [
55]. The powder obtained was treated with sodium borohydride followed by sodium hydroxide and the product obtained was then acidified to pH 7 to provide
29, that was then oxidized in the presence of lead tetraacetate to afford
30. Due to
30 instability, it was immediately reduced with an excess of sodium borohydride and basified to provide
26 in 50-60%, which in turn reacted with
p-toluene sulfonyl chloride in the presence of triethylamine to provide
30 in 90% yield (
Scheme 8) [
56].
Following this first synthesis, others less efficient methods for the preparation of 26 from inexpensive naturally occurring materials were also investigated.
3.3. Conversion of glycerol to Epichlorohydrin
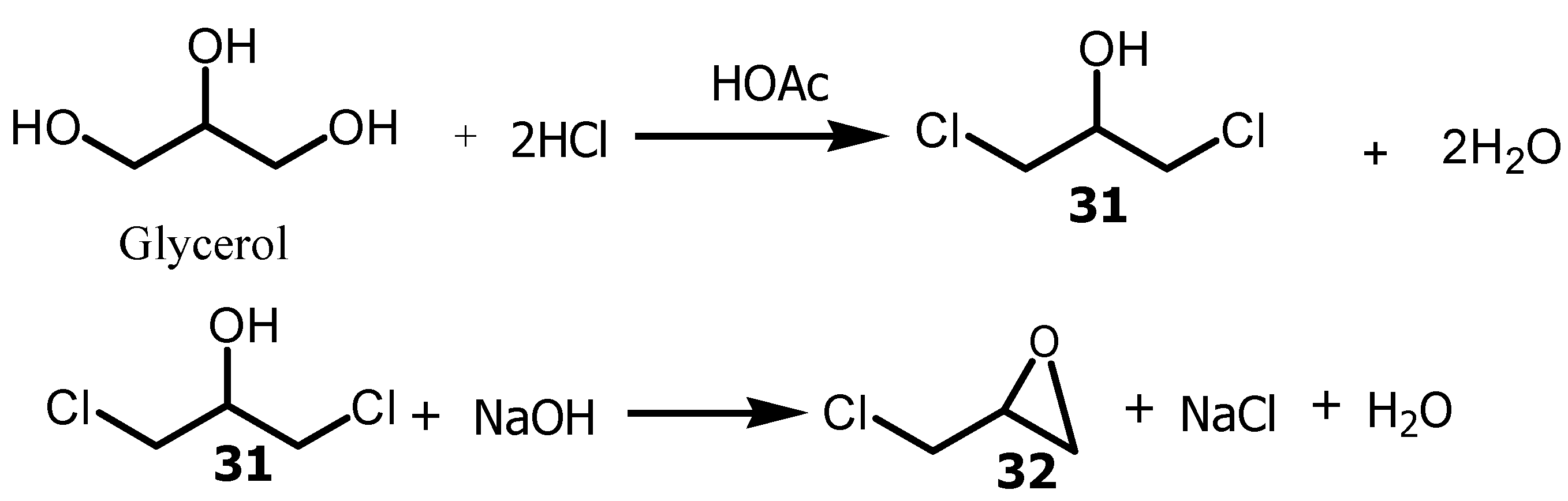
Epichlorohydrin
32 is used in the production of epoxy resins, adhesives, elastomers, rubbers and plastics, paints, cellulose and pesticide formulation [
57]. Epichlorohydrin was obtained when glycerol was reacted with hydrogen chloride in the presence of carboxylic acid as a catalyst to yield the intermediate
31, which was then treated with a base to provide
32 (
Scheme 9).
3.4. Conversion of Glycerol to (R)-Glycidol
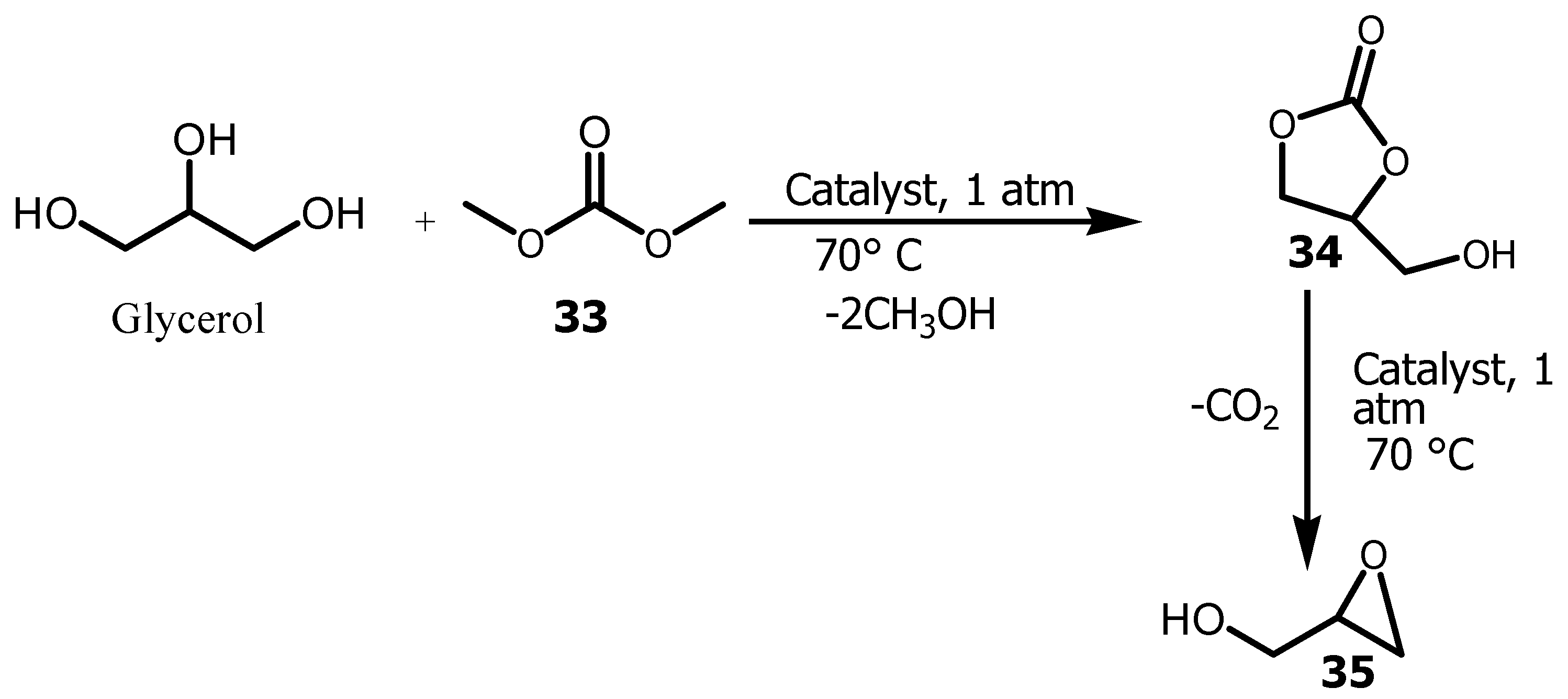
Oxiranes are a group of compounds used in organic synthesis as a building block because of their ring opening in the presence of nucleophilic group, reducing agents or lewis acid to form a new carbon-carbon bond. Several methods were also established to provide optically active epoxide in one spot step or two steps [
58]. Glycidol
35 is one of the most important glycerol derivative generally used in the pharmaceutical industry, perfumes, paints and detergents. It was also used in the preparation of compounds such as glycidyl ethers, polyglycerols and polyurethanes [
59]. Glycidol (GL) was prepared either via epoxidation of allyl alcohol, or of 3-chloro-1, 2-propanediol with bases [
59,
60,
61]. But most of these reactions were challenged by multistep synthesis, the product cost and petrochmical derived raw materials, which impacting the environment from the viewpoint of sustainability. Therefore
35 was synthesized in one pot via the transesterification of Glycerol with Dimethyl carbonate (DMC)
33 using nanoparticulate KNO
3/Al
2O
3 as a solid base catalyst in almost 64% yield (
Scheme 10) [
62]
The rise of asymmetric synthesis is mainly due to the determining role of the absolute configuration of chiral compounds which provide different physiological or pharmacological properties. Indeed, the activity of these products essentially depends on their recognition by the specific chiral receptors which have different chemical behaviors with respect to the two enantiomers [
63]. The difference in biological activity linked to the absolute configuration can be illustrated by the case of propranolol where the two enantiomers are used for different therapeutic purposes. The
S antipode is a
β-blocker involved in the treatment of heart disease, while the
R configuration compound is used for contraceptive purposes. Enantiomeric purity is therefore essential for the clinical use of this molecule. Access to enantiomeric enriched molecules can currently be achieved in three ways: Creation from prochiral precursors, use of chiral pool materials, or disconnection of racemates. The hydrolytic kinetic resolution (HKR) of terminal epoxides catalyzed by chiral (salen) cobalt (III) complex affords both recovered unreacted epoxide and 1,2-diol products in highly enantioenriched form. Consequently, the HKR provides general access to useful, highly enantioenriched chiral building blocks (enantiomers terminal oxiranes and 1,2-diols). The reaction was reported to afford a higher enantioselectivity (generally ≥ 99%) within an enantiomeric ratio 50:50 of relative rates of the two enantiomers [
64]. The HKR also provided practical access to a series of enantioenriched 1-halo-2,3-propane diol derivatives. Therefore, Epichlorohydrin
32 underwent ring opening to afford 1-chloro-2,3-propanediol
37 in 95% ee and 40% yield catalyzed by chiral (salen)Co
III complex (1.OAc), which in the presence of a base provided (
R)-glycidol
38 (
Scheme 11).
Therefore, the development of efficient syntheses of enantiomerically pure chiral synthons is the subject of intense current study. Compound 38 was used as a starting material in the preparation of optically bioactive compounds (l-blockers centrally-acting antihypertensives, antiglaucoma agents, antitussive drug, alkylglycerols and glycerophospholipids).
4. Synthesis of Glycerol Derivatives
The development of organic processes based on the use of glycerol as a safe organic building block is strongly limited by the physicochemical properties of glycerol (strong hydrophilicity, three unprotected hydroxy groups). Thus Gly was first converted into either Solketal, Glycidol or Tosylsolketal and then used as a building block in the synthesis of bioactives molecules.
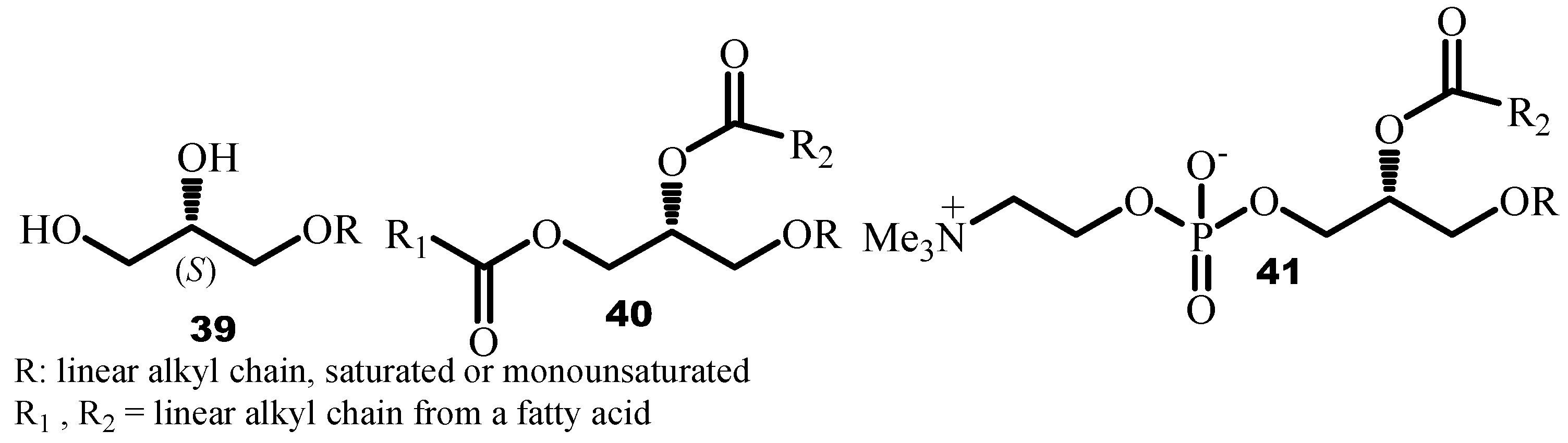
4.1. Synthesis of 1-O-Alkylglycerol from Solketal
Shark liver oil (SLO) mixture was used in folk medicine in Scandinavian countries and in Japan, for its fortifying or healing properties. Its widespread use particularly in Europe, is now experiencing growing interest. Chemical studies carried out on the shark liver oil mixture have made it possible to characterize a certain type of etherlipids present in abundance named alkylglycerols (AKGs)
39, in diacylated form: alkyldiacylglycerols (AKDAGs)
40 and phospholipids
41 [
65]. It was also established that the alkyl chain of a 1-
O-alkylglycerol was bound to the glycerol backbone at the
sn-1 position, thus leading to an
S configuration at the asymmetric carbon [
66] (
Figure 1).
1-
O-alkylglycerols (AKGs)
39 are compounds derived from glycerol where one of the primary alcohol functions of glycerol is etherified by an alkyl chain. These compounds are present in many marine natural sources (sharks, rays, stars of sea, chimera, molluscs), but also in cattle and in humans. In the latter, AKGs were found in the liver, spleen, bone marrow, erythrocytes and in breast milk. It is particularly in the liver oil mixture of certain sharks that they are much more abundant and where they can represent more than 50% by weight of this oil. Beneficial effects of the SLO mixture on health were recognized in the traditional medicine of northern countries involved in fishing such as Japan, Norway and Iceland [
67].
In these countries, the ancestral use of the SLO mixture was empirically as strengthening and wound healing medication. Later in the 20th century, beneficial effects on health of the SLO mixture were attributed to ether-linked glycerols known as 1-
O-alkylglycerols (AKGs). Experimental studies were performed during the last century, aiming to demonstrate whether AKGs from the SLO mixture had biological properties and beneficial effects. Indeed, several studies did observe interesting effects such as hematopoiesis stimulation, lowering radiotherapy-induced injuries, reducing tumor growth, anti-microbial, antigiogenic, and improving vaccination efficiency [
68,
69,
70,
71,
72,
73,
74,
75].
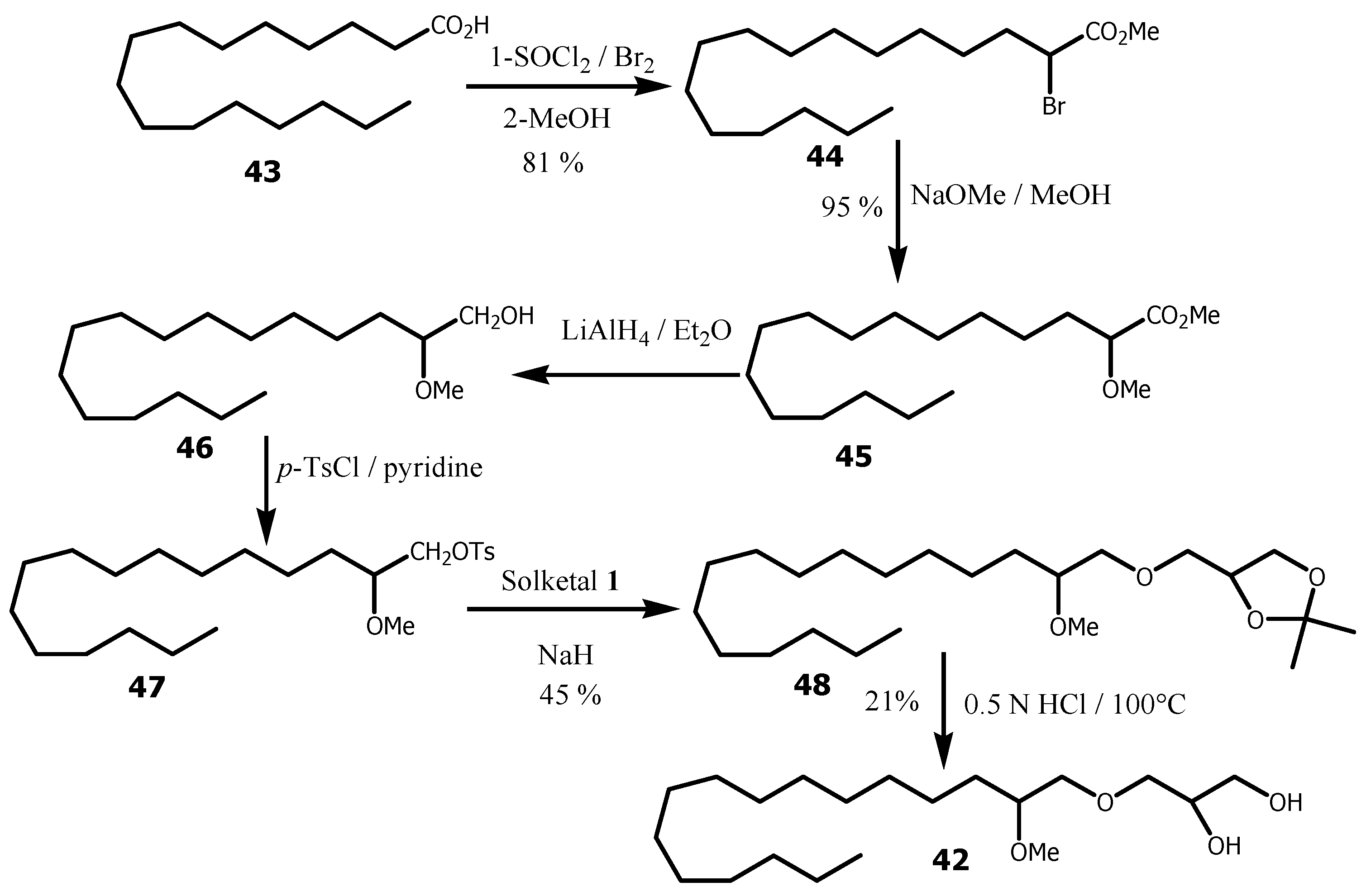
In the SLO mixture from Greenland, small amounts (2-4%) of AKGs were also identified, possessing an additional methoxy group (methyl glyceryl ethers: MGE) at the 2 position in the alkyl chain and varying from C-14 to C-22 length alkyl chain [
19]. It was further reported that a natural 2-methoxy alkyl glycerol ether owned the 2’R, 2S configuration, when the NMR spectra and optical rotation value of each synthesized stereoisomer of 1-O-(2’-methoxyhexadecyl)glycerol where compared. MGEs isolated from the Greenland SLO mixture were able to inhibit tumour growth and metastasis formation, and to stimulate the immunoreactivity in mice [
75]. MGE
42 was first synthesized as a mixture of stereoisomers in racemic form (
Scheme 12) [
76]. Palmitic acid
43 was converted to acid chloride by refluxing with thionyle chloride followed by bromine in methanol to provide methyl 2-bromohexadecanoate
44, that was in turn treated with sodium methoxide in methanol to yield methyl 2-methoxylhexadecanoate
45. Reduction of
45 with lithium aluminium hydride in ethyl ether afforded 2-methoxyhexadecanol
46, which upon tosylation provided
46, followed by its alkylation with solketal
1 in the presence of sodium hydride to provide
47. Acetonide
47 cleavage in acidic conditions provided
42 in 21% yield.
4.2. Synthesis of Glycerol Derivatives from Glycidol
Glycidol is an organic compound that contains both epoxide and alcohol fonctional group. Being bifunctional, provides it with a variety of industrial uses. This compound is a slightly viscous liquid and slightly unstable. It is not often encountered in pure form.
The development of novel, selective and efficient chemical pathways towards application of glycerol-derived products remains a key scientific and industrial challenge. Very recently, because of this unique molecule structure, glycidol received a special attention as a valuable product in many applications such as the production of monomer and semi-product in the synthesis of surface-active agents. One of the most important applications of glycidol is the synthesis of analgesic and antiviral drugs, where the latter is the active compound fighting with the human immunodeficiency virus (HIV) [
77,
78]. Several syntheses of molecules have been reported for racemic glycidol
35 (
Scheme 13). For example, heating
35 with CH
2FCN in the presence of tetraethylammonium bromide gives oxazoline
49 [
79]. Its reaction with phenyl isocyanate under similar conditions gives oxazolidinone
50 [
80]. Treating also an aldehydes with
35 provide dioxolane
51. Finally, esterification of
35 provided the α
-toluenesulfonylacetate which when reacting with dicyclocarbodiimide and followed by lithium diisopropylamide resulted in ring closure, forming the lactone
52 [
77].
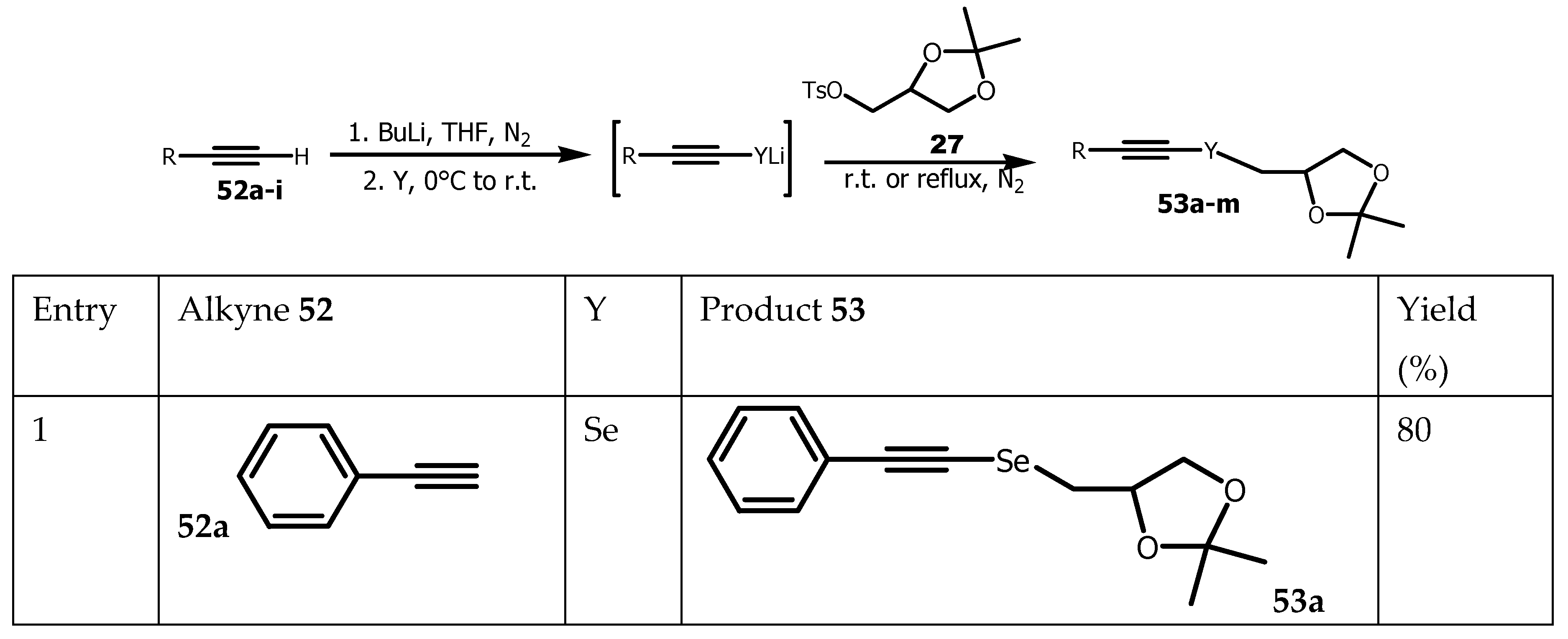
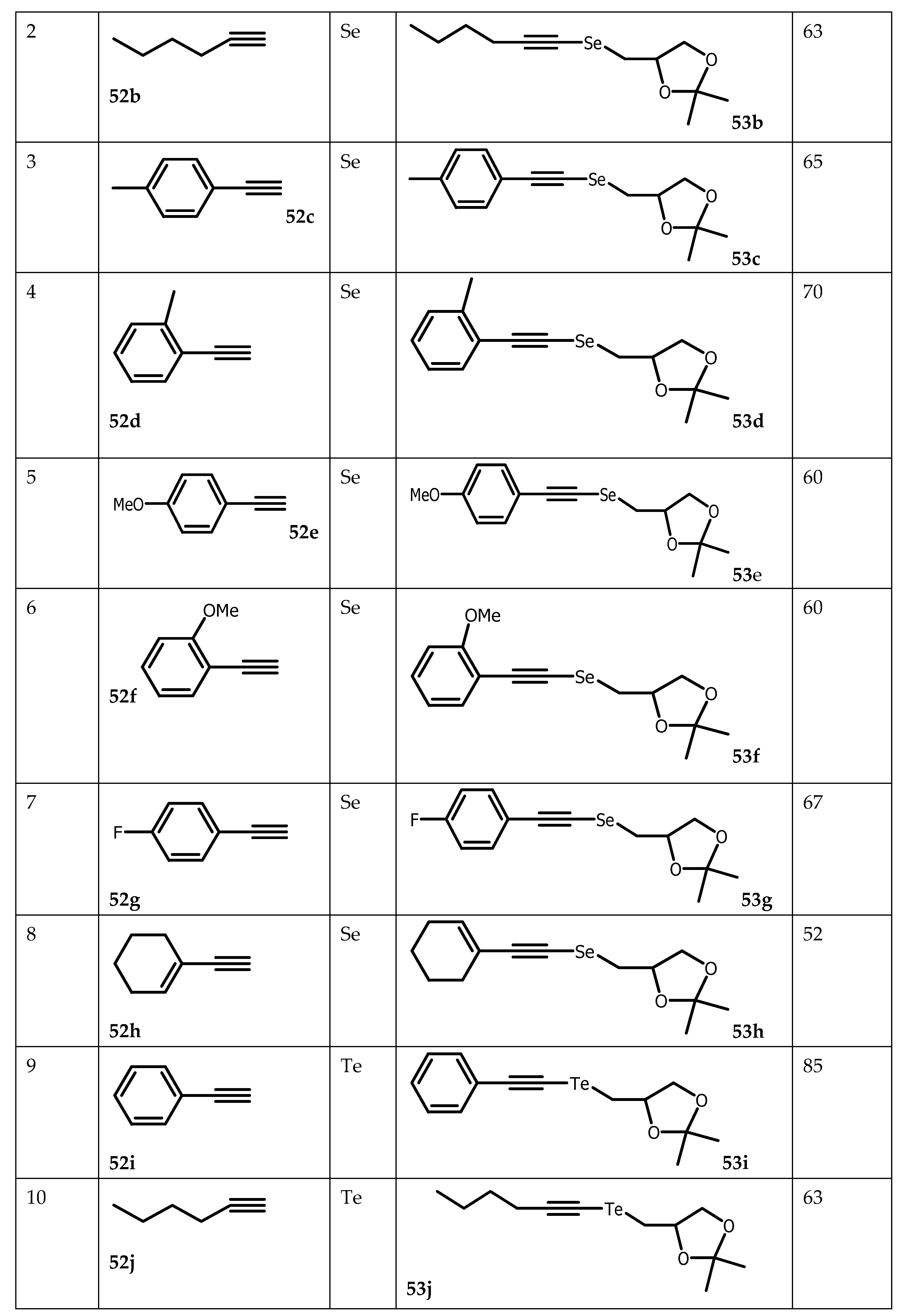
4.3. Synthesis of Organochalcogen from TosylSolketal
Organochalcogen compounds have attracted the interest of a multitude of studies to design potential therapeutic agents. For instance, organoselanyl and organotellanyl alkynes have become extensively studied due to their pharmacological and biological activities [
81,
82,
83] and their use as starting material in organic synthesis [
84,
85]. Organotellanyl alkynes for example exhibited antidepressive-like activity [
86], while alkyne-derived organotellanyl alkenes showed in vitro antioxidant activity with slight toxicity [
87,
88]. On the other hand, tosyl solketal
27 was used as a building block in the synthesis of several biologically active compounds. Chalcogenyl alkynes were selectively prepared from the reaction of glycerol-derived dichalcogenides with terminal alkynes in the presence of NaBH
4, using ethanol as the solvent. However, reaction times were in the range of 5 to 26 h and the scope of the reaction was limited to organoselanyl alkynes, and only the synthesis of one organotellanyl alkyne in 55% yield was reported [
89]. To address these limitations, terminal alkyne
52 were first reacted with
n-butyl lithium to provide lithium alkynylchalcogenolate (Se and Te), which in turn were reacted with tosyl solketal
27 to yield organoselanyl
53a-h and organotellanyl alkynes
53i-m (
Scheme 12).
5. Conclusion
This review summarizes the chemistry of glycerol including some of its keys synthetic intermediates and derivatives, as the use of fossil resources for the petrochemical processes is a matter of great concern due to the climate change and the environmental pollution. Therefore, Glycerol was used as a green solvent for the preparation of 4H-pyrans, bis (aryl) ketones and aryl compounds, metallic complexes, as well as an undeniable raw material for the synthesis of potential drugs with eco-friendly processes.
It was shown that Glycerol possesses most characteristics of a green solvent: low toxicity, low flammability, biodegradability, functional group compatibility, low volatility organic compounds (VOC) emission, cheap, easy to handle and recyclable with a limited environmental impact. Nevertheless, the abundance of glycerol formed during biodiesel production as a waste provides a vast low-cost feedstock that can turn to uncontrolled waste with the disposal, and affect the economics of the biodiesel industries. Therefore, Glycerol was converted into Epichlorydrin, Glycidol, Solketal and Tosylsolketal as an efficient recycling process and useful keys synthetic intermediates, which were in turn used in the synthesis of glycerol derivatives such as Alkylglycerols, Organochalcogen and others molecules of potential beneficial effects. It was also noticed that Glycerol remains a green solvent of choice when compared to others green solvents in the synthesis of pharmaceutically active ingredients, where the toxicity and residual solvents are attentively monitored.
References
- Li L, Korányi, TI, Sels, BF and Pescarmona, PP. Highly-efficient conversion of glycerol to solketal over heterogeneous Lewis acid catalysts Green Chem., 2012, 14, 1611-1619. [CrossRef]
- Coombs, J and Hall, K. Chemicals and polymers from biomass Renewable Energy, 1998, 15, 1-4. [CrossRef]
- Corma, A, Iborra S and Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev., 2007, 107, 2411–2502. [CrossRef]
- Lichtenthaler FW and Mondel, S. Perspectives in the use of low molecular weight carbohydrates as organic raw materials Pure Appl. Chem. 1997, 69, 1853–1866. [CrossRef]
- Gallezot, P. Catalytic routes from renewables to fine chemicals Catal. Today, 2007, 121, 76–91. [CrossRef]
- Liang, Y, et Cui, Y, Trushenski, J, Blackburn JW.Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour Technol 2010, 101(19): P. 7581-7586.
- Escriba, M, Eras, J, Duran, M, Simon, S, Cristina Butchosa, C, Villorbina, G, Balcells, M, Canela, R. From glycerol to chlorohydrin esters using a solvent-free system. Microwave irradiation versus conventional heating. Tetrahedron, 2009, 65, 10370–103. [CrossRef]
- Luo, X, Ge, X, Cui, S, Li, Y. Value-added processing of crude glycerol into chemicals and polymersBioresour Technol, 2016, 215, 144-154. [CrossRef]
- Pérez-Barrado, E, Pujol, MC, Aguiló, M, Llorca, J, Cesteros, Y, Díaz, F, Pallarès, J, Marsal, LF, Salagre, P. Influence of acid–base properties of calcined MgAl and CaAl layered double hydroxides on the catalytic glycerol etherification to short-chain polyglycerols. Elselvier Sciences Direct, 2015. 264, 547-556.
- Wang, Z, Zhuge, J, Fang, H, Prior, BA. Glycerol production by microbial fermentation: A review Biotechnology Advances, 2001, 19, 201-223. [CrossRef]
- Len, C, Luque, R. Continuous flow transformations of glycerol to valuable products: An overview. Sustain. Chem. Process. 2014, 2, 1–10. [CrossRef]
- García, E, Laca, M, Pérez,E, Garrido, A and Peinado,JN. New Class of Acetal Derived from Glycerin as a Biodiesel Fuel Component Energy Fuels, 2008, 22, 4274–4280. [CrossRef]
- Garcia, JI, Garcia-Marin, H, Pires, E. Glycerol based solvents: Synthesis, properties and applications. Green Chem., 2014,16, 1007-1033. [CrossRef]
- Costa, ICR, Itabaiana, I, Flores, MC, Lourenço, AC, Leite, SGF, Miranda, LS, Leal, ICR, Souza, RO. Biocatalyzed Acetins Production under Continuous-Flow Conditions: Valorization of Glycerol Derived from Biodiesel Industry. J. of Flow Chemistry , 2013, 3 41-45. [CrossRef]
- Krief, A, Provins,L and Froidbise, A.Diastereoselective synthesis of dimethyl cyclopropane-1,1-dicarboxylates from a γ-alkoxy-alkylidene malonate and sulfur and phosphorus ylides.Tetrahedron Letters,1998, 39, 1437–1440. [CrossRef]
- Ray, PC and Roberts, SM.Overcoming intrinsic diastereoselection using polyleucine as a chiral epoxidation catalyst. Tetrahedron Letters. 1999, 40, 1779–1782. [CrossRef]
- Michael, EJ and Teresa, JS. Total synthesis of (R)-glycerol acetonide and the antiepileptic and hypotensive drug (-)-.gamma.-amino-.beta.-hydroxybutyric acid (GABOB): Use of vitamin C as a chiral starting material.Journal of American ChemialSociety. 1980, 102, 6304-6311. [CrossRef]
- Deniau, AL, Mosset, P, Le Bot, D, Legrand, AB. Which alkylglycerols from shark liver oil have anti-tumour activities? Biochimie, 2011, 93, 1-3. [CrossRef]
- Momha, R, Kuete, V, Pagès, JM, Pegnyemb, DE, Mosset, P. Synthesis and Biological Evaluation of Four New Ricinoleic Acid-Derived 1-O-alkylglycerols.Marine Drugs,2020, 18(2), 113. [CrossRef]
- Momha, R., Pegnyemb, DE, Mosset, P.Synthesis of (Z)-(2′ R)-1-O-(2′-methoxynonadec-10′-enyl)-sn-glycerol, a new analog of bioactive ether lipids.Tetrahedron2012, 68, 2973-2983. [CrossRef]
- Momha, R. Synthesis and therapeutic effects of Alkylglycerols, LAMBERT Academic Publishing, 2020, pp 1-56. ISBN: 978-3-330-02986-6.
- Oh, K, Yamada, K, Asami,T, Yoshizawa, Y. Synthesis of novel brassinosteroid biosynthesis inhibitors based on the ketoconazole scaffold.Bioorganic and Medicinal Chemistry Letters. 2012, 22, 1625-1628. [CrossRef]
-
Callam, CS; et al. Computational analysis of the potential energy surfaces of glycerol in the gas and aqueous phases: Effects of level of theory, basis set, and solvation on strongly intramolecularly hydrogen-bonded systems.Journal of American Chemical Society. 2001, 123 (47), 11743-11754; 23. Callam, CS; et al. Computational analysis of the potential energy surfaces of glycerol in the gas and aqueous phases: Effects of level of theory, basis set, and solvation on strongly intramolecularly hydrogen-bonded systems.Journal of American Chemical Society. 2001, 123 (47), 11743-11754. [CrossRef]
- Da Silva, GP, De Lima, CJB,and Contiero,JJCT. Production and productivity of 1, 3-propanediol from glycerol by Klebsiella pneumoniae GLC29. Catalysis Today. 2015, 257, 259-266. [CrossRef]
- Simo MO, Li CJ Green. Chemistry oriented organic synthesis in water. Green Chemistry, 2012, 41, 1415-1427. [CrossRef]
- Capello, C, Fisher,U and Kungerb, uhler.“What Is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents”. Green Chemistry.2007, 9, 927–934. [CrossRef]
- Leal-Duaso, A, Perez,P, Mayoral, JA, Pires, E and Garcia JI. Glycerol as a source of designer solvents:physicochemical properties of low melting mixtures containing glycerol ethers and ammonium salts. Phys. Chem. Chem. Phys., 2017, 19, 28302-28312. [CrossRef]
- Yin Len Kua, Suyin Gan, Andrew Morris, Hoon Kiat Ng. Ethyl lactate as a potential green solvent to extract hydrophilic (polar) and lipophilic (non-polar) phytonutrients simultaneously from fruit and vegetable by-products. Sustainable Chemistry and Pharmacy 2016, 4, 21-31. [CrossRef]
- Gu, Y and François J. Glycerol as a sustainable solvent for green chemistry.Green Chemistry. 2010, 12, P. 1127–1138. [CrossRef]
- Garcia, JI, Garcia-Marin, H, Mayoral, JA, P. Perez, P. Quantitative structure–property relationships prediction of some physico-chemical properties of glycerol based solvents. Green Chem. 2013, 15, 2283 –2293. [CrossRef]
- Capriati, V, Perna, FM, Salomone, A The Great Beauty” of organolithium chemistry: A land still worth exploring. Dalton Transactions. 2014, 43, 14204-14210. [CrossRef]
- Maria J, Rodriguez-Alvarez, Joaquin Garcia-lvarez, Marina Uzelac, Michael Fairley, Charles T. O’Hara, and Eva Hevia. Introducing Glycerol as a Sustainable Solvent to Organolithium Chemistry: Ultrafast Chemoselective Addition of Aryllithium Reagents to Nitriles under Air and at Ambient Temperature. Chemistry. A European Journal. 2018, 24, 1720–1725. [CrossRef]
- Butler, RN, Coyne, AG. Organic synthesis reactions on-water at the organic–liquid water interface Organic and Biomolecular Chemistry.2016, 14, 9945-9960. [CrossRef]
- Safaei, HR, Shekouhy, M, Rahmanpur, S, Shirinfeshan, A. Glycerol as a biodegradable and reusable promoting medium for the catalyst-free one pot three component synthesis of 4H-pyrans. Green Chemistry.2012, 14, 1696-1704. [CrossRef]
- Gu Y, Barrault J, Jerome F. Glycerol. as an efficient promoting medium for organic reactions. Advanced Synthesis and catalysis.2008, 350, 2007-2012. [CrossRef]
- Choi, JW, Jang, BK, Cho, NC, Park, JH, Yeon, SK, Ju, EJ, Lee, YS, Han, G, Pae, AN, Kim, DJ, and Park, KD.Synthesis of Amide and Ester Derivatives of Cinnamic Acid and Its Analogs: Evaluation of Their Free Radical Scavenging and Monoamine Oxidase and Cholinesterase Inhibitory Activities.Chemical and Pharmaceutical Bulletin, 2017, 65(11), 1020-1027. [CrossRef]
- Green,O, Smith, NA, Ellis, AB and Burstyn, JN.AgBF4-Impregnated Poly(vinyl phenyl ketone): An Ethylene Sensing Film. Journal of American Chemical Society.2004, 126 (19), 5952–5953. [CrossRef]
- Bianco,A, Cavarischia, C and Guiso, M. The Heck Coupling Reaction Using Aryl Vinyl Ketones: Synthesis of Flavonoids.European Journal of Organic Chemistry. 2004, 13, 2894 – 2898. [CrossRef]
- Schroeder, M, Mathys, M, Ehrensperger, N and Buchel, M. γ-Unsaturated Aldehydes as Potential Lilial Replacers. Chem. Biodiversity, 2014, 11, 1651–1673.
- Hu, L, Lu, X and Deng, L. Catalytic Enantioselective Peroxidation of α,β-Unsaturated Aldehydes for the Asymmetric Synthesis of Biologically Important Chiral Endoperoxides. Journal of American Chemical Society.2015, 137(26), 8400–8403. [CrossRef]
- Breuer, M, Ditrich, K, Habicher,T, Hauer, B, Kesseler, M, Sturmer, R and Zelinski, T. Industrial Methods for the Production of Optically Active Intermediates. Angew. Chem., Int. Ed., 2004, 43, 788–824. [CrossRef]
- Liu, L, Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chemical Reviews. 2018, 118(10), 4981−5079. [CrossRef]
- Roat-Malone, RM. Bioinorganic Chemistry: A Short Course. Wiley: Hoboken, NJ, 2007.
- Lee, JS, Vlaisavljevich, B, Britt, DK, Brown, CM, Haranczyk, M, Neaton, JB, Smit, B, Long, JR, Queen, WL. Understanding Small-Molecule Interactions in Metal−Organic Frameworks: Coupling Experiment with Theory. Advanced Materials. 2015, 27(38), 5785-5796. [CrossRef]
- Chahdoura, F, Favier, I and Gomez, M. Glycerol as Suitable Solvent for Synthesis of Metallic Species and Catalysis.Chemistry A European Journal.2014, 20, 1–11. [CrossRef]
- Albrecht, M. Cyclometalation Using d-Block Transition Metals: Fundamental Aspects and Recent Trends. Chemistry Reviews. 2010, 110, 576 –623. [CrossRef]
- Kobayashi, K, Ishikubo, M, Kanaizuka, K, Kosuge, K, Masaoka, S, Sakai, K, Nozaki, K, Haga, MA. Proton-induced tuning of metal-metal communication in rack-type dinuclear Ru complexes containing benzimidazolyl moieties. Chemistry A European Journal. 2011, 17(25), 6954-6963. [CrossRef]
- Chen, PL, Chao, H, Xu, J, Wang, L, Li, H. Luminescence properties of a di-ruthenium(II) complex with an intramolecular hydrogen bond modulated by DNA and copper(II) ion. Transition Metal Chemistry, 2009, 34, 773– 778. [CrossRef]
- Sprouse,S, King, KA, Spellane, PJ, Watts, RJ. Photophysical effects of metal-carbon .sigma. bonds in ortho-metalated complexes of iridium(III) and rhodium(III). Journal Of The American Chemical Society, 1984, 106, 22, 6647-6653. [CrossRef]
- Yue, SM, Xu, HB, Ma, JF, Su, ZM, Zhang HJ, Design and syntheses of blue luminescent zinc(II) and cadmium(II) complexes with bidentate or tridentate pyridyl-imidazole ligands. Polyhedron, 2006, 25, 635-644. [CrossRef]
- Khatri, PK, Jain, SL. Glycerol ingrained copper: An efficient recyclable catalyst for the N-arylation of amines with aryl halides. Tetrahedron Lett. 2013, 54, 2740 –2743. [CrossRef]
- Gordon, JF, Chittenden. Some aspects of the reaction of glycerol with 2,2-dimethoxypropane. Carbohydrate research. 1983, 121, 316-323. [CrossRef]
- Patrick, C, Nobre, Elton L, Borges, Cristian M, Silva, Angela M, Casaril, Débora M, Martinez Eder J, Lenardão, Diego Alves, Lucielli Savegnago, Gelson Perin. Organochalcogen compounds from glycerol: Synthesis of new antioxidants.Bioorganic & Medecinal Chemistry. 2014, 22, 6242–6249. [CrossRef]
- Irvine, J, MacDonald, J, Soutar, C.Condensation of acetone and benzaldehyde with glycerol. Preparation of glycerol α-methyl ether. Journal of the Chemical Society. 1915, 107, 337. [CrossRef]
- Jackson, K& Jones, J. Synthesis of 3-hexuloses. Canadian Journal of Chemistry. 1969, 47, 2498-2501.
- Michael E, Jung and Teresa J, Shaw. Total Synthesis of (R)-Glycerol Acetonide and the Antiepileptic and Hypotensive Drug (-)-y-Amino-P-hydroxybutyric Acid (GABOB): Use of Vitamin C as a Chiral Starting Material. Journal of the American Chemical Society. 1980, 102(20), 6304–6311. [CrossRef]
- Kong PS, Kheireddine M, Mohd W, Wan A. Conversion of crude and pure glycerol into derivatives : A feasibility evaluation. Renewable and Sustainable Energy Reviews, 2016, 63, pp. 533–555. [CrossRef]
- Smith, AG. Synthetically Useful Reactions of Epoxides. Synthesis 1984, 8, 629-656. [CrossRef]
- Palomo, M, Segura,RL, Mateo, C, Terreni, M, Guisan, JM and Fernández-Lafuente, R. Tetrahedron Asymmetry:, 2005, 16, 869-874. [CrossRef]
- Hanson, RM. The synthetic methodology of nonracemic glycidol and related 2,3-epoxy alcohols Chem. Rev., 1991, 91, 437-475. [CrossRef]
- Pagliaro, M, Ciriminna, R, Kimura, H, Rossi, M and Pina, CD. From Glycerol to Value-Added Products Angew. Chem. Int. Ed. 2007, 46, 4434-4440. [CrossRef]
- Elrasheed, E, Wang,H, Imran, M, Hegazi, SEF, Hassan, M, Eldoma, MA, Hakami, J, Wani, WA, Chaudhary, AA. Nanocatalyst-Assisted Facile One-Pot Synthesis of Glycidol from Glycerol and Dimethyl Carbonate. ACS Omega 2022. [CrossRef]
- Crosby J. Synthesis of optically active compounds: A large scale perspective. Tetrahedron,1991 47, 4789-4846. [CrossRef]
- Schaus, SE, Brandes, BD.; Larrow, JF.; Tokunaga, M, Hansen, KB.; Gould, AE, Furrow, ME, Jacobsen, EN. Highly Selective Hydrolytic Kinetic Resolution of Terminal Epoxides Catalyzed by Chiral (salen)CoIII Complexes. Practical Synthesis of Enantioenriched Terminal Epoxides and 1,2-Diols. J. Am. Chem. Soc. 2002, 124, 1307-1315. [CrossRef]
- Bordier, CG, Sellier, N, Foucault, AP, Le Goffic, F. Purification and characterization of deep sea shark Centrophorus squamosus liver oil 1-O-alkylglycerol ether lipids. Lipids, 1996, 31, 521–528. [CrossRef]
- Baer, E.; Fisher, H. O. L. J. Biol. Chem.1941, 140, 397-410.
- Momha, R, Pegnyemb, DE, Mosset, P. Synthesis of halogenated 1-O-alkylglycerols from ricinoleic acid derivatives. Synthetic Communications, 2020, 50, 1656-1664. [CrossRef]
- Linman, JW, Long, MJ, Korst, DR, Bethell, FH. J. Lab. Clin. Med.1959, 54, 335–343.
- Brohult, A, Brohult, J, Brohult, S, Joelsson, I. Effect of alkoxyglycerols on the frequency of injuries following radiation therapy for carcinoma of the uterine cervix.Acta Obst. Gynecol. Scand.1977, 56, 441–448. PMID: 602713. [CrossRef]
- Momha, R, Bayiha, GBN, Pegnyemb, DE, Mosset, P. First total synthesis of two 1-O-alkylglycerols based-alkyne analogues of bioactive natural products.ChemistrySelect 2020, 5, 6678-6682. [CrossRef]
- Brohult, A, Brohult, J, Brohult, S. Regression of tumour growth after administration of Alkoxyglycerols. Acta Obstet. Gynecol. Scand.1978, 57, 79–83. [CrossRef]
- Momha, R, Le Bot, D, Mosset, P, Legrand, AB. Anti-Angiogenic and Cytotoxicity Effects of Selachyl Alcohol Analogues. Anticancer Agents in Medicinal Chemestry. 2022, 10, 1913-1920. [CrossRef]
- Ngwenya, BZ & Foster, DM. Enhancement of antibody production by lysophosphatidylcholine and alkylglycerol. Proceedings of the Society for Experimental Biology and Medicine. 1991, 196, 69-75. [CrossRef]
- Brohult, A, Brohult, J, Brohult, S. Effect of irradiation and alkoxyglycerol treatment on the formation of antibodies after Salmonella vaccination. Experientia, 1972, 28, 954–955. [CrossRef]
- Hallgren, B& Ställberg, G. Methoxy-substituted glycerol ethers isolated from Greenland shark liver oil. Acta Chem. Scand.1967, 21, 1519-1529.
- Hallgren, B.; Ställberg, G. Acta Chem. Scand. 1967, 21, 1519-1529.
- Hanson, RM. The synthetic methodology of nonracemic glycidol and related 2,3-epoxy alcohols. Chem. Rev. 1991, 91(4), 437–475. [CrossRef]
- Desai, SP, Taylor, MS. Diarylborinic Acid-Catalyzed Regioselective Ring Openings of Epoxy Alcohols with Pyrazoles, Imidazoles, Triazoles, and Other Nitrogen Heterocycles. Org. Lett., 2021, 23(18), 7049–7054. [CrossRef]
- Fotadar, U, Becu, C; Borremans, F. AM, Anteunis, M J0. Synthetic and conformational aspects of trimethylammonium-methyl substituted 2-oxazolines as potential cholinergics .Tetrahedron, 1978, 34, 3537-44. [CrossRef]
- Farrissey, WJ; Nashu, AMJ. The rearrangement of glycicyl N-phenylcarbamate. Heterocycl. Chem. 1970, 7, 331-333. [CrossRef]
- Barcellos, AM, Abenante, L, Sarro, MT, Leo, ID, Lenardão, EJ, Perin, G, Santi, C. New prospective for redox modulation mediated by organoselenium and organotellurium compounds. Current. Organic Chemistry.2017, 21, 1–18. [CrossRef]
- Mugesh, G, du Mont, WW, Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chemistry. Review. 2001, 101, 2125–2179. [CrossRef]
- Santoro, S, Azeredo, JB, Nascimento, V, Sancineto, L, Braga, AL, Santi, C. The green side of the moon:Ecofriendly aspects of organoselenium chemistry. RSC Advances.2014, 4, 31521–31535. [CrossRef]
- Mitamura, T, Ogawa, A. Palladium-catalyzed alkynylselenation of acetylenedicarboxylates leading to enyne selenides and application to synthesis of multisubstituted aryl selenides. Tetrahedron Letters, 2010, 51, 3538–3541. [CrossRef]
- Liu, CR., Yang, FL, Jin, YZ, Ma, XT, Cheng, DJ, Li, N, Tian, SK. Catalytic regioselective synthesis of structurally diverse indene derivatives from n-benzylic sulfonamides and disubstituted alkynes. Organic Letters, 2010, 12, 3832–3835. [CrossRef]
- Okoronkwo, AE, Godoi, B, Schumacher, RF, Neto, JSS, Luchese, C, Prigol, M, Nogueira, CW, Zeni, G.Csp3-tellurium copper cross-coupling: Synthesis of alkynyl tellurides a novel class of antidepressive-like compounds. Tetrahedron Lett. 2009, 50, 909–915. [CrossRef]
- Savegnago, L, Borges, VC, Alves, D, Jesse, CR, Rocha, JBT, Nogueira, CW. Evaluation of antioxidant activity and potential toxicity of 1-buthyltelurenyl-2-methylthioheptene. Life Sciences, 2006, 79, 1546–1552. [CrossRef]
- Ávila, DS, Gubert, P, Palma, A, Colle, D, Alves, D, Nogueira, CW, Rocha, JBT, Soares, FAA. An organotellurium compound with antioxidant activity against excitotoxic agents without neurotoxic effects in brain of rats. Brain Resaerch Bulletin 2008, 76, 114–123. [CrossRef]
- Soares, LK, Silva, RB, Peglow, TJ, Silva, MS, Jacob, RG, Alves, D, Perin, G. Selective synthesis of vinyl- or alkynyl chalcogenides from glycerol and their water-soluble derivatives. ChemistrySelect. 2016, 1, 2009–2013. [CrossRef]
- Lenardão, EJ, Borges, EL, Stach, G, Liane K, Alves, SD, Schumacher, RF, Bagnoli, L, Marini, F and Perin, G Glycerol as Precursor of Organoselanyl and Organotellanyl Alkynes. Molecules. 2017, 22, 391. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).