Submitted:
01 July 2024
Posted:
02 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plasmids
2.2. Construction of Plasmids Encoding Myc-Tagged pig TRIF Protein
2.3. Cells Culture
2.4. Western Blotting
2.5. IFN-β Luciferase Reporter Assay
2.6. TRIF Degradation Assay
2.7. Measurement of IFN-Stimulated Gene Expression
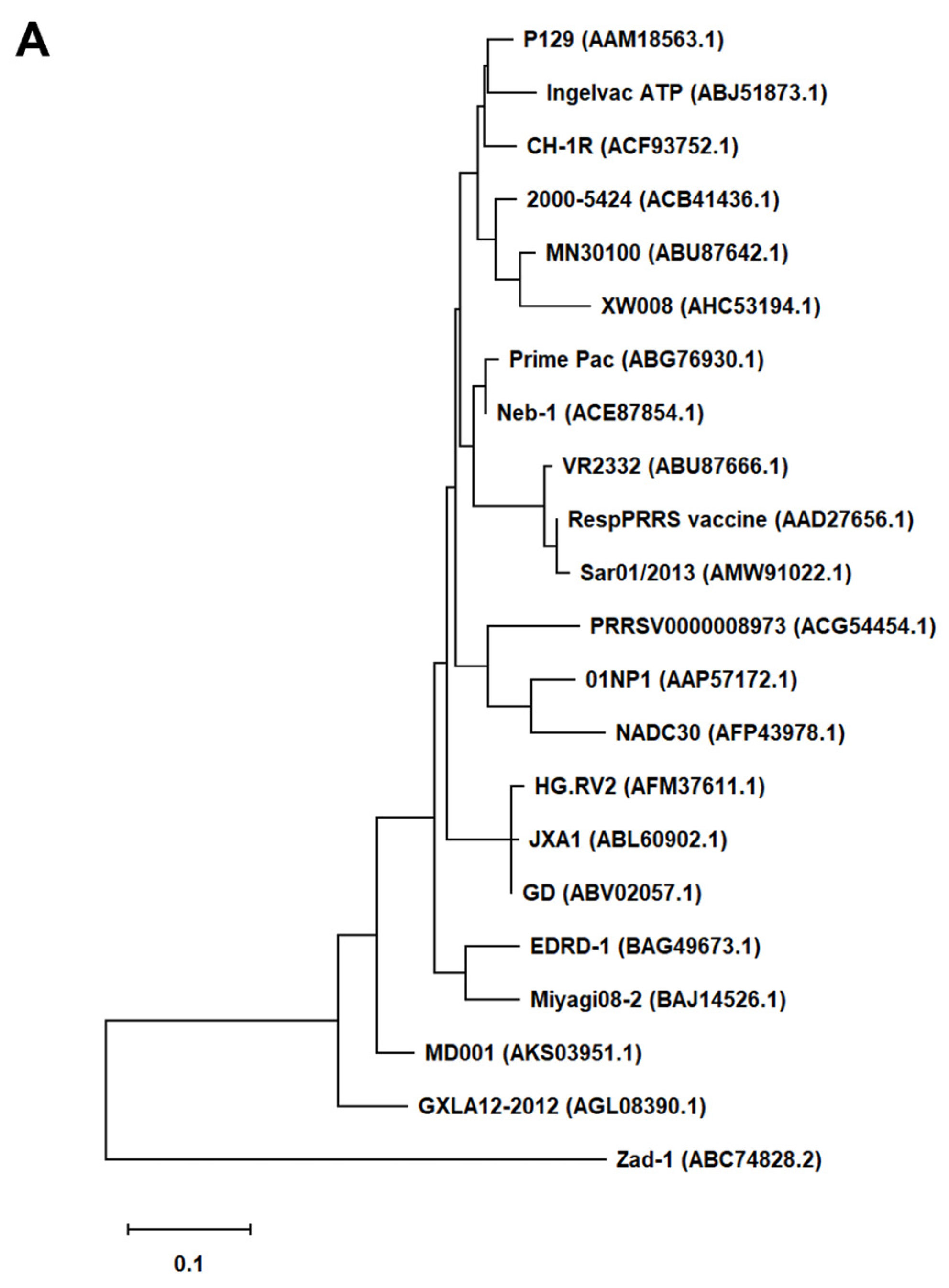
2.8. Alignment of GP5 Amino Acids and Phylogenetic Analysis
2.9. Statistical Analysis
3. Results
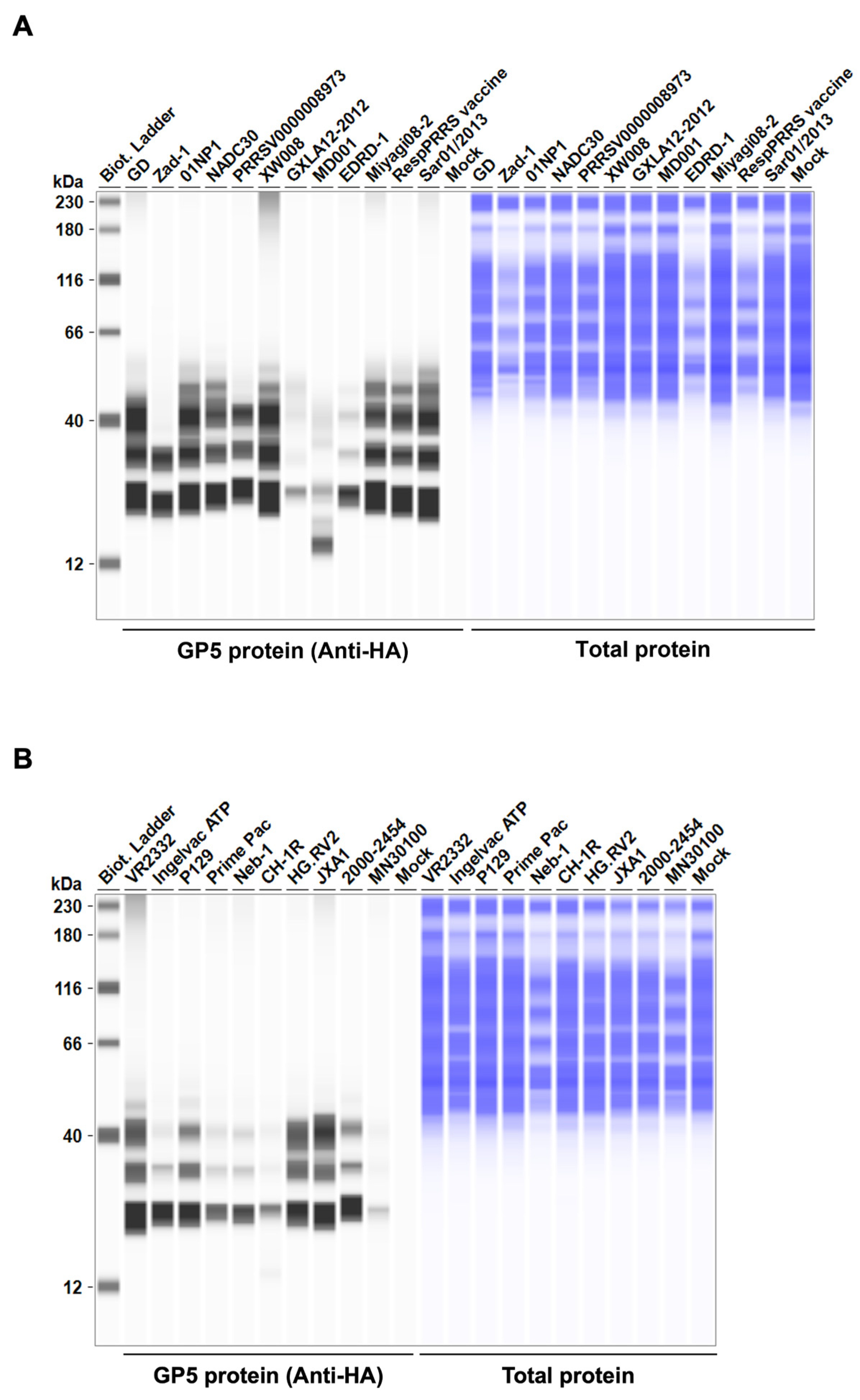
3.1. Genetic Characteristic of Arterivirus GP5 Proteins
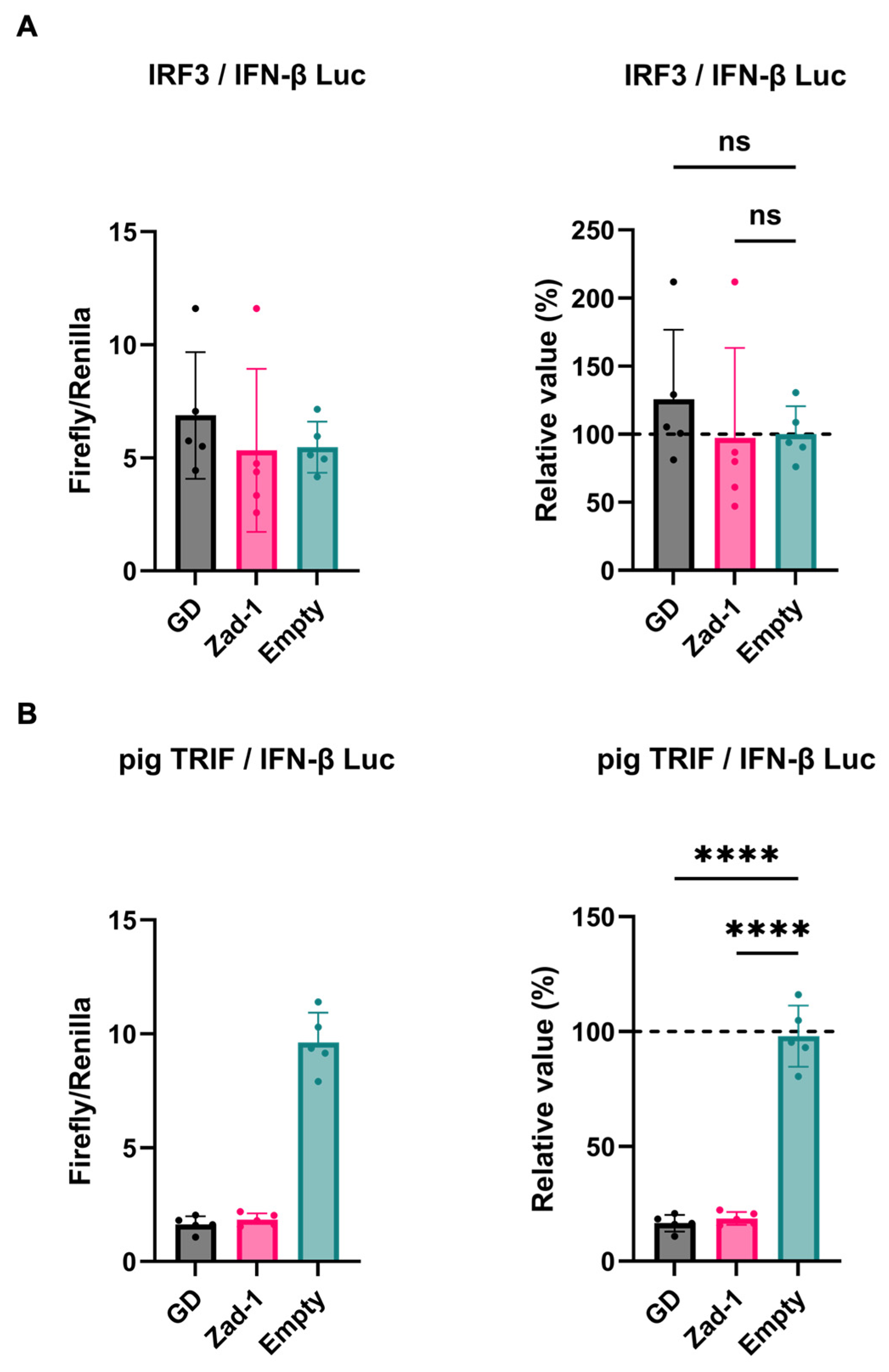
3.2. GP5 Proteins from both PRRSV-2 (GD) and PRRSV-1 (Zad-1) Inhibit TRIF-Mediated IFN-β Signaling
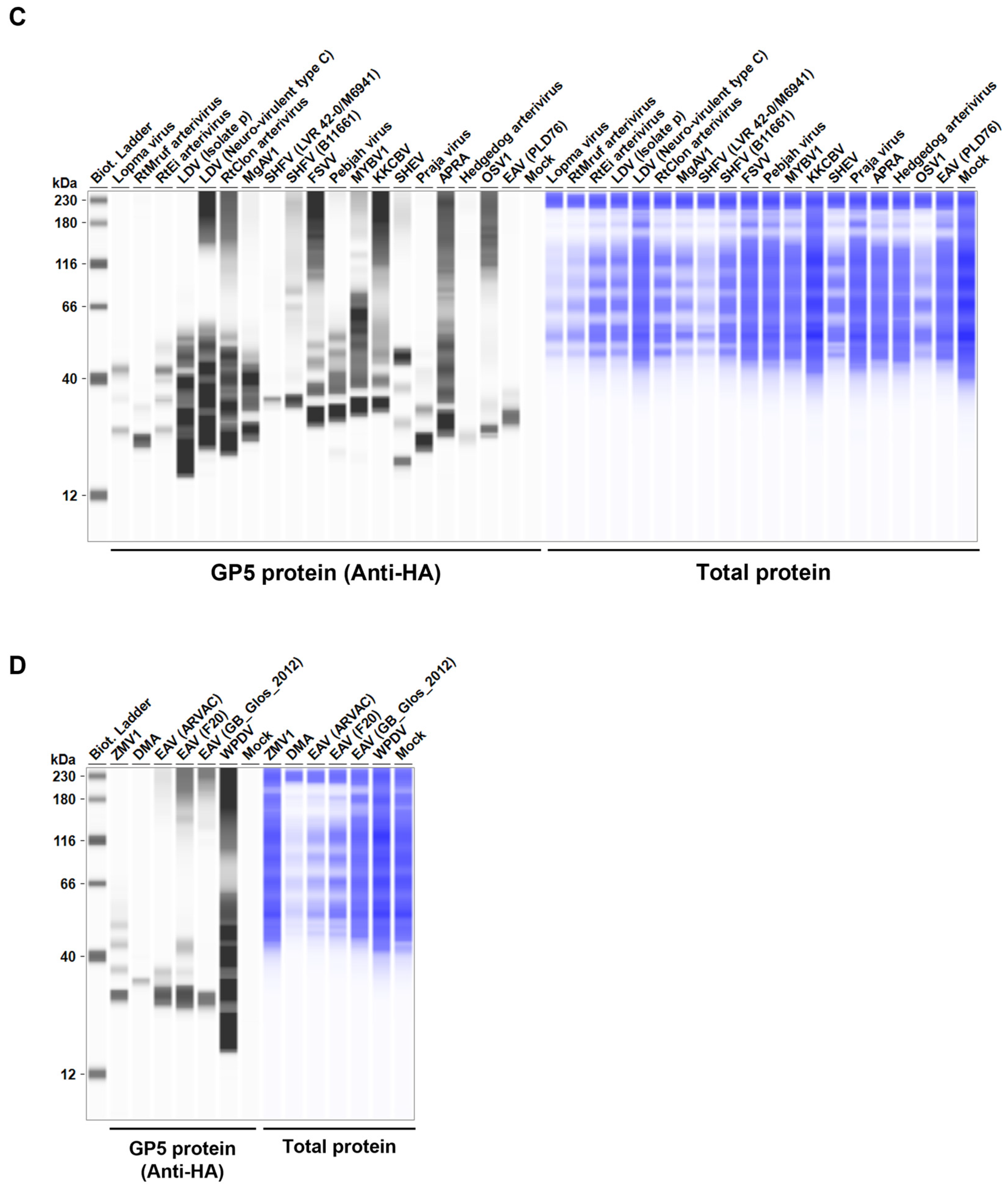
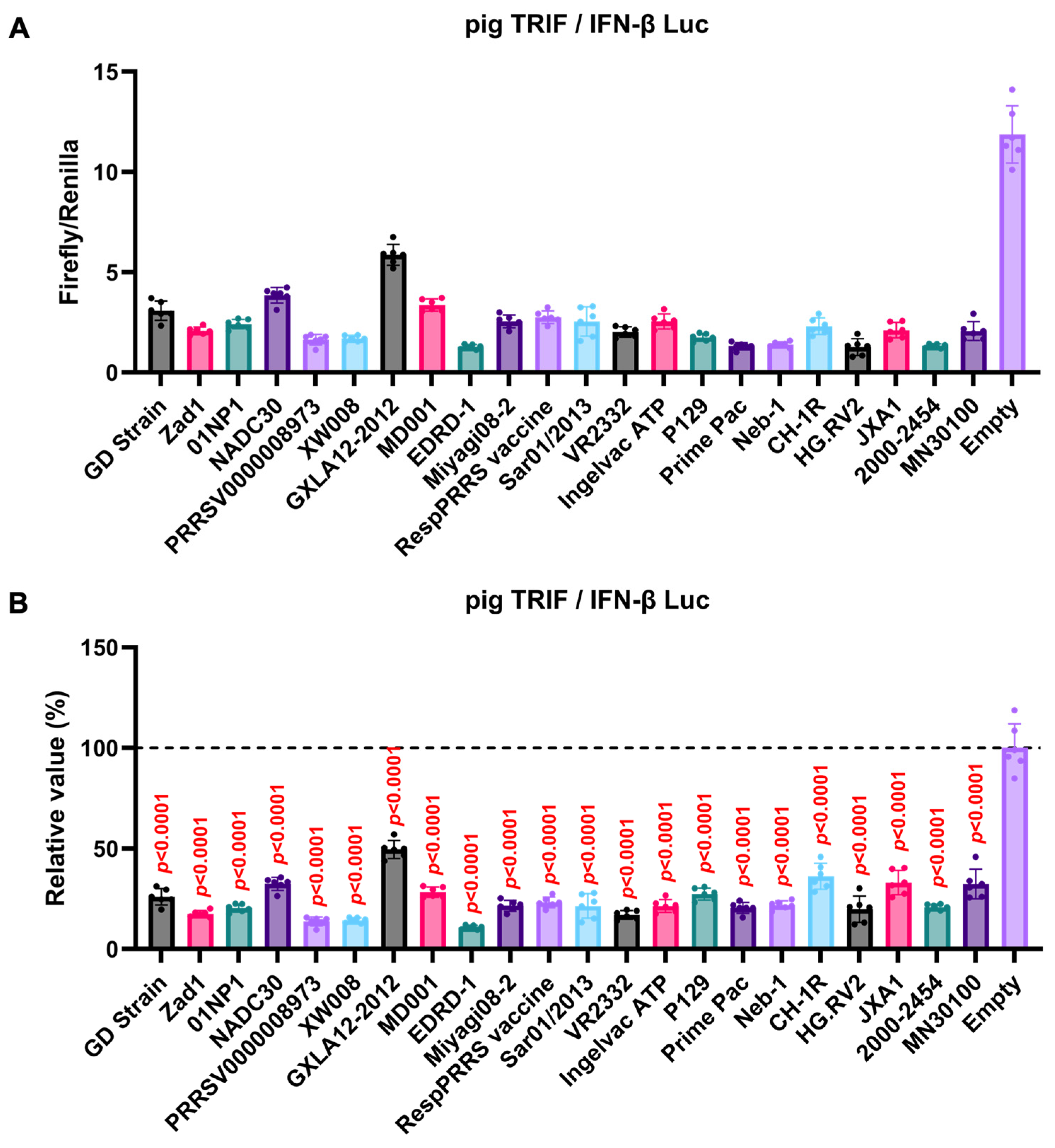
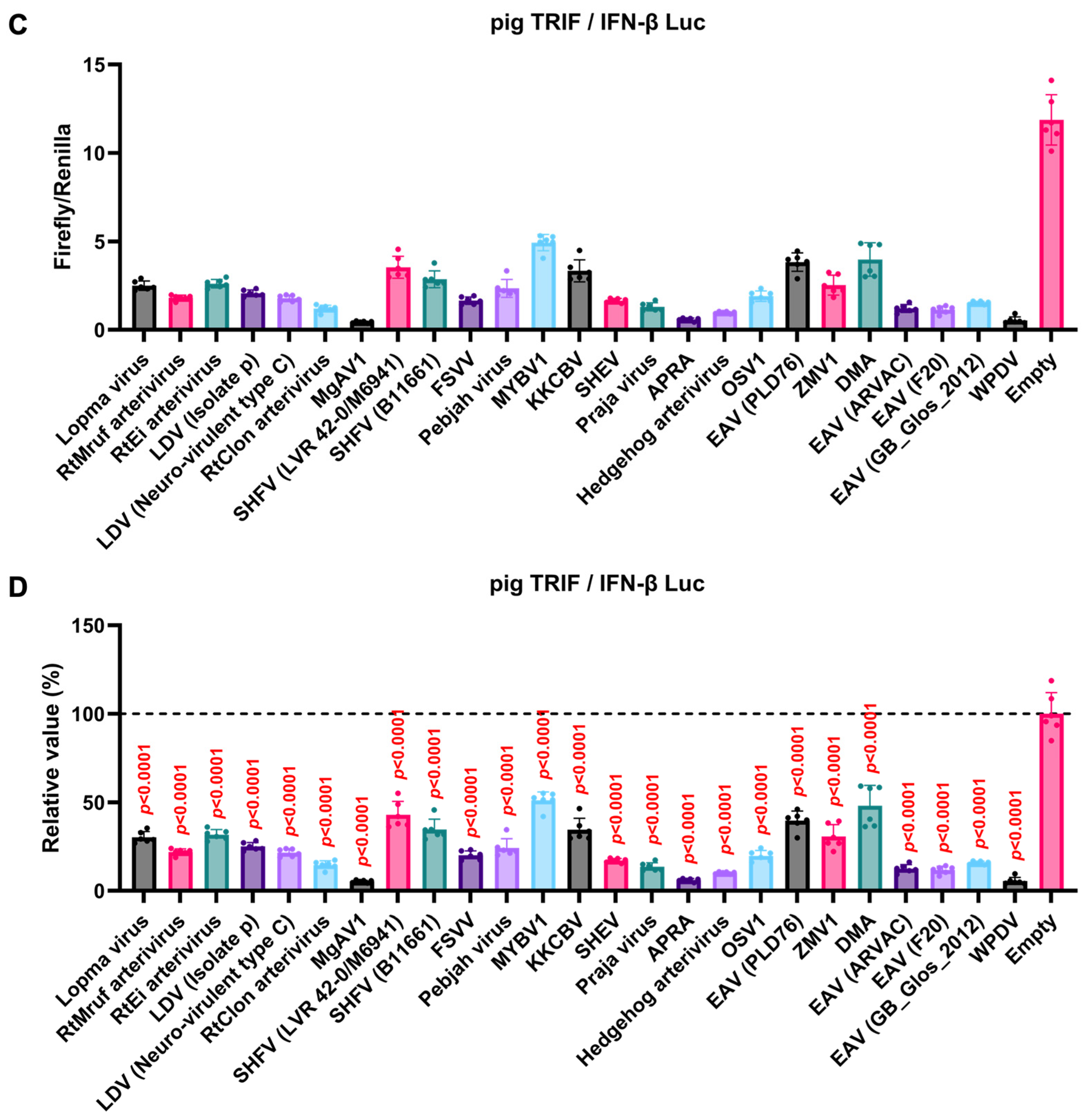
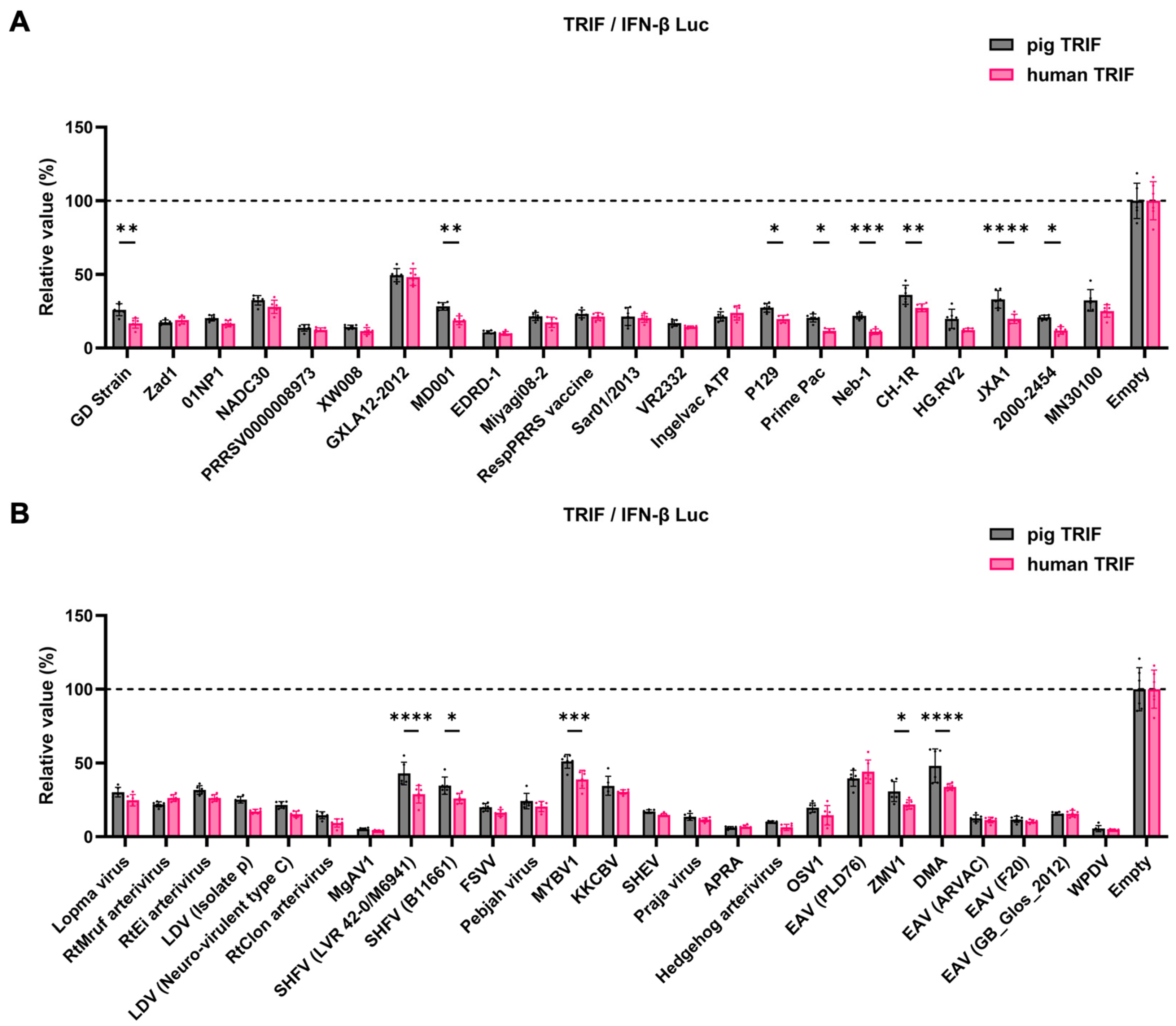
3.3. GP5 Proteins of Diverse Arteriviruses Inhibit pig TRIF-Mediated IFN-β Signaling
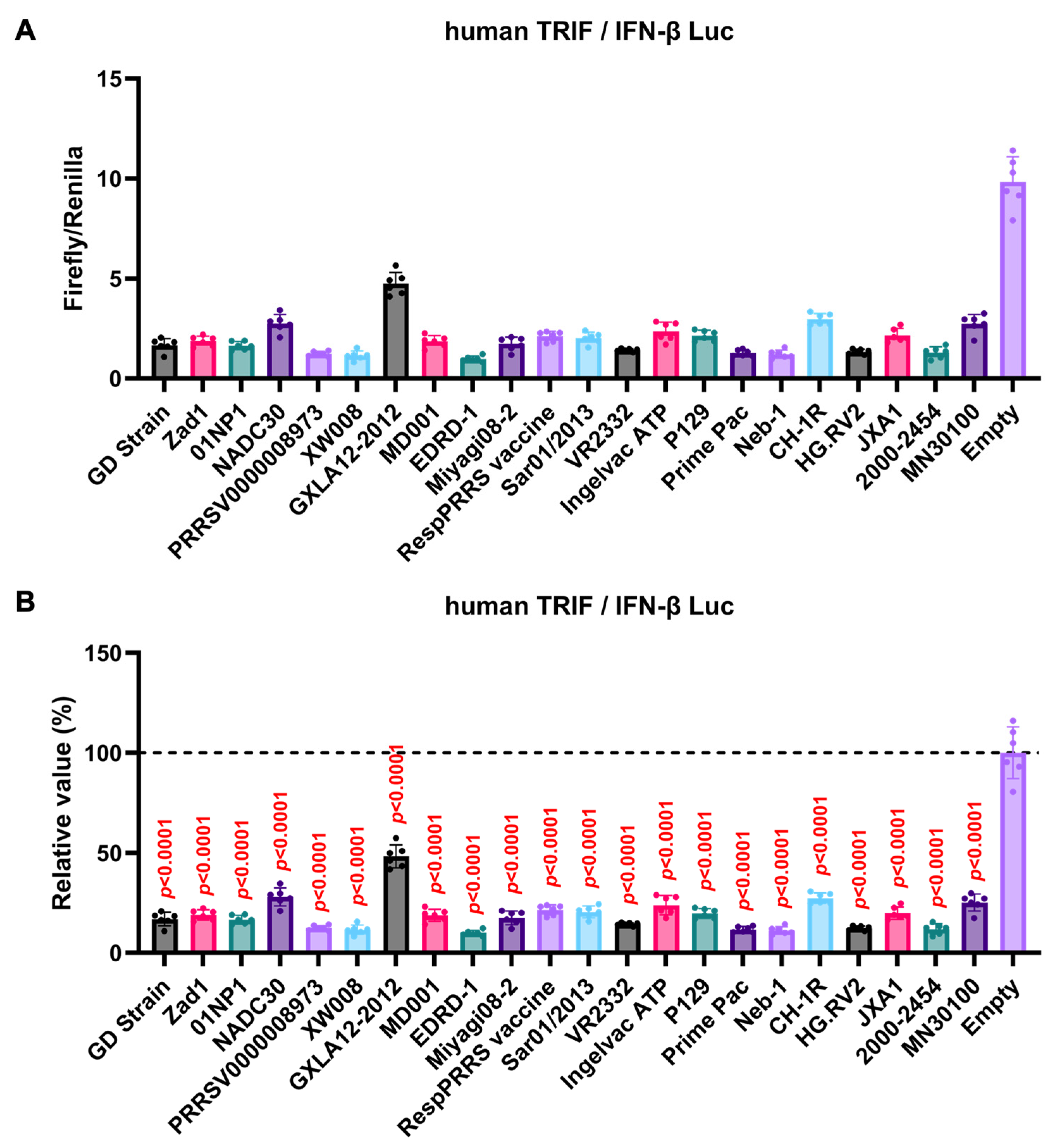
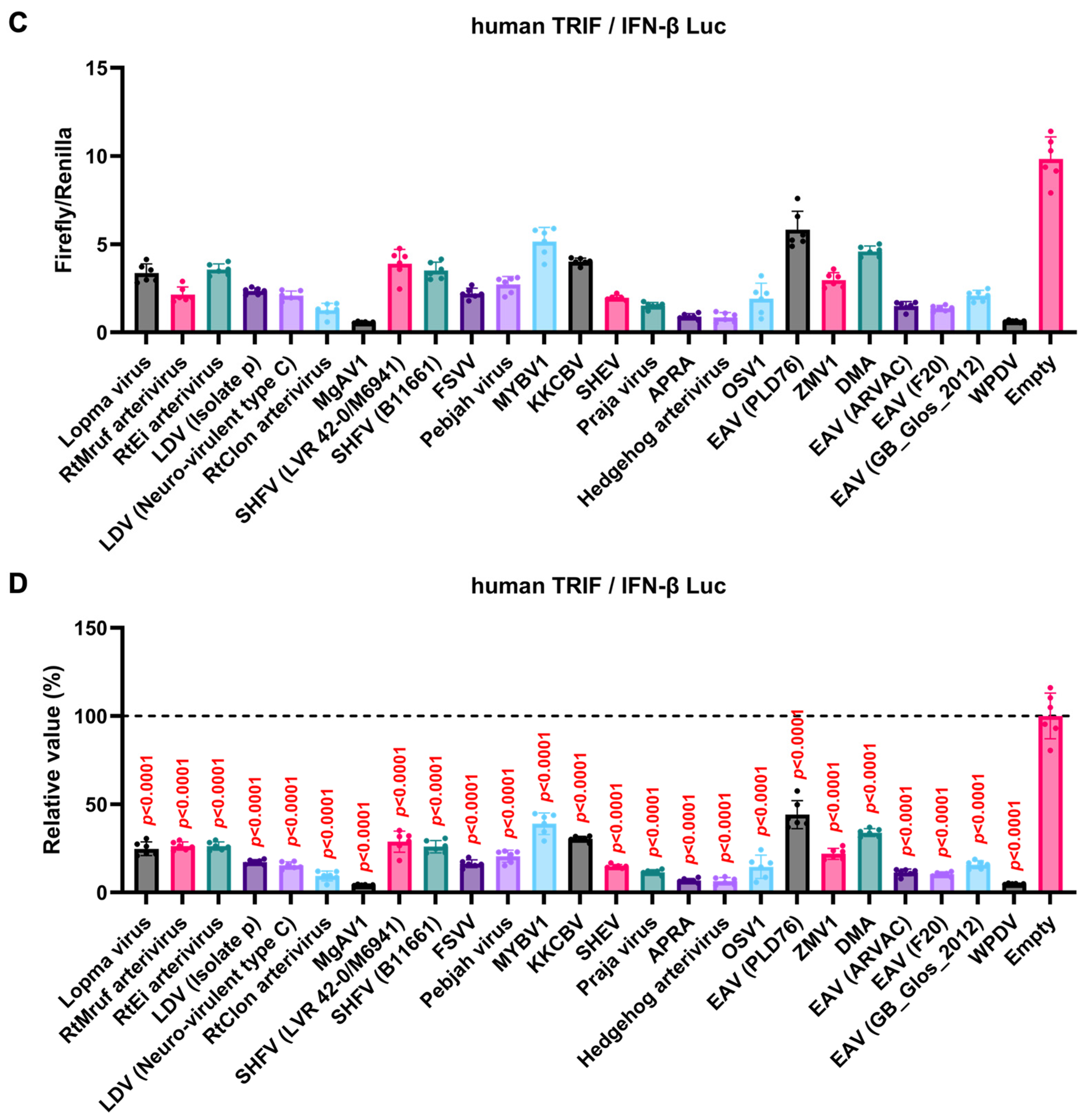
3.4. GP5 Proteins of Diverse Arteriviruses Inhibit the Human TRIF-Mediated IFN-β Signaling Pathway
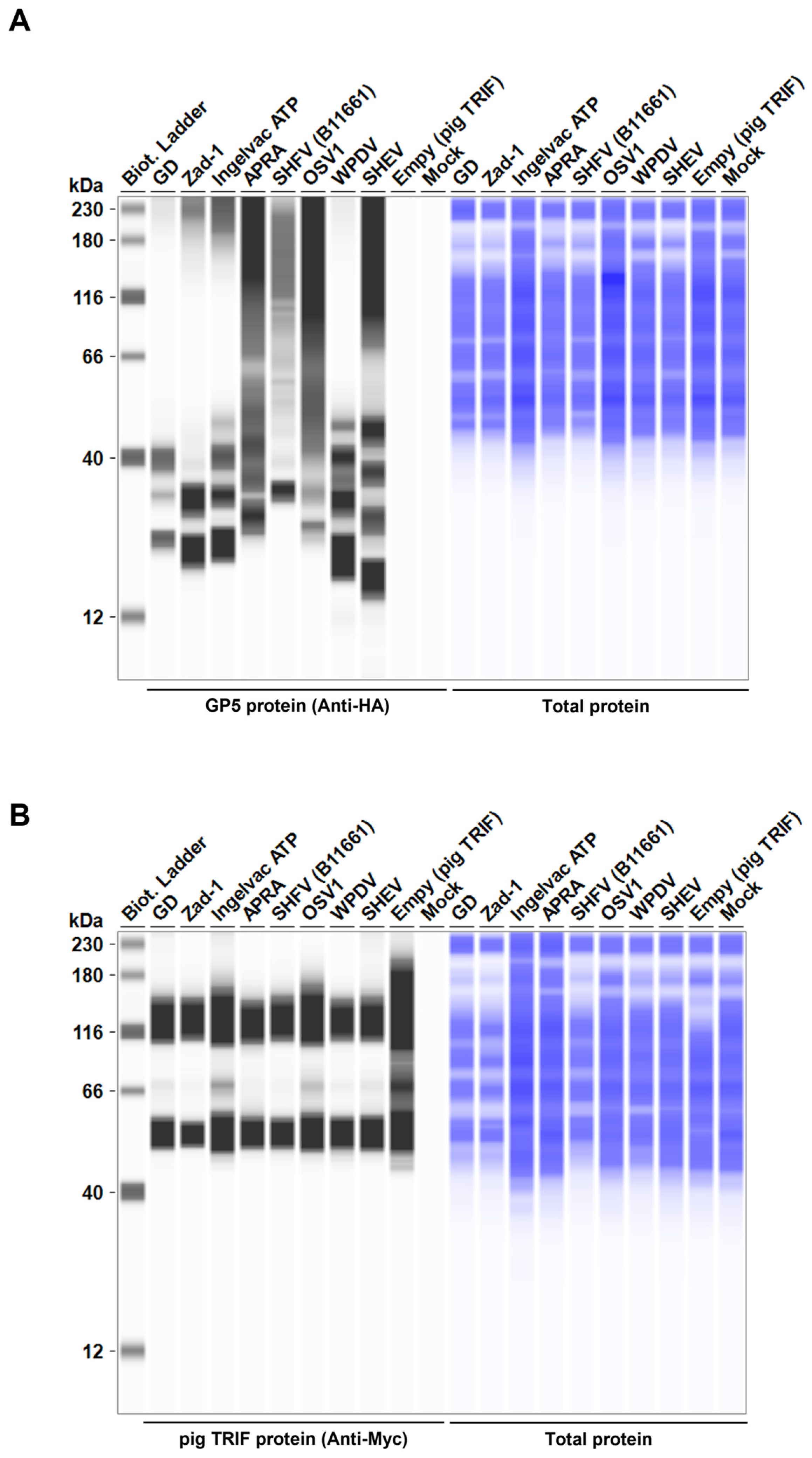
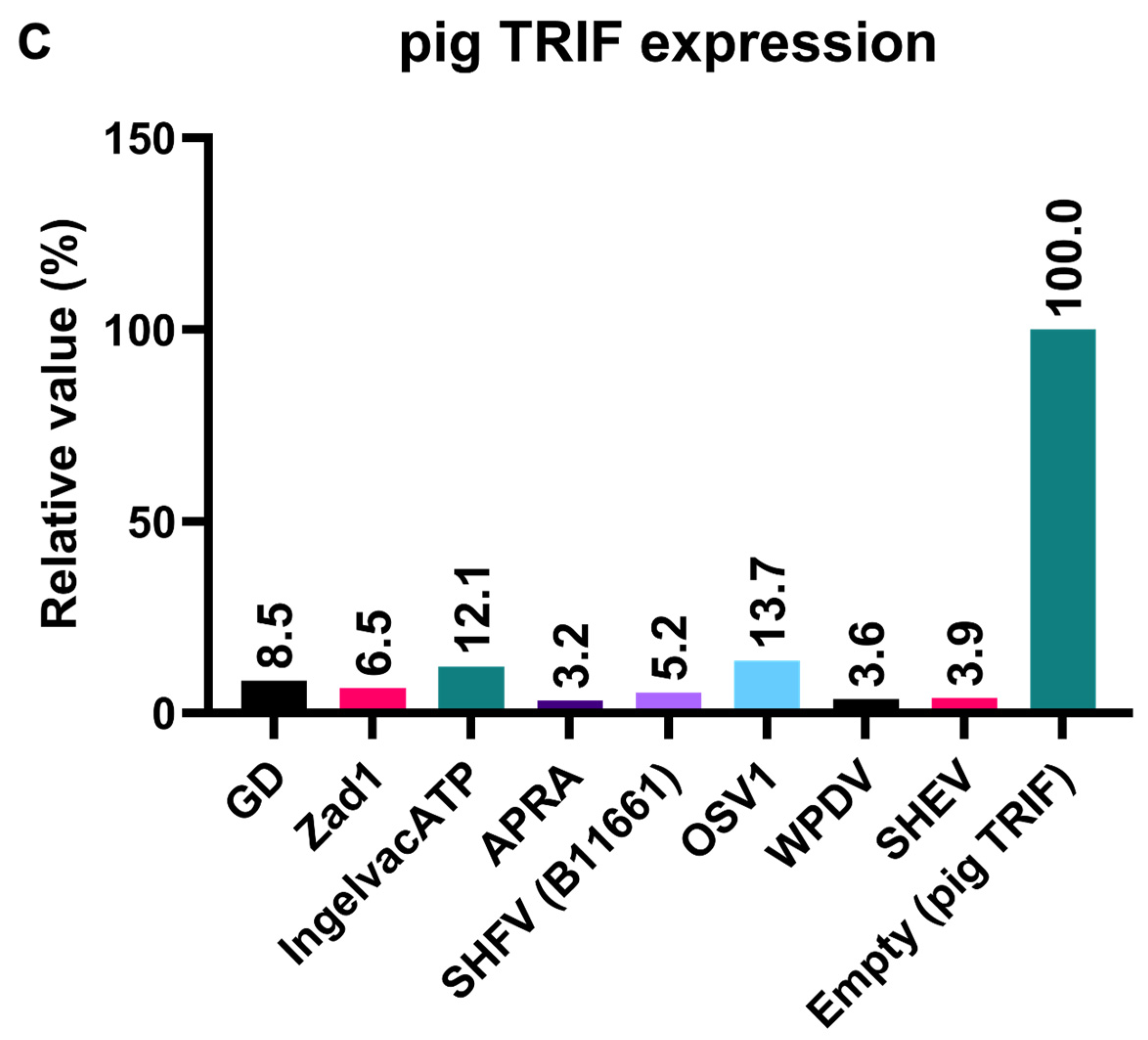
3.5. GP5 Proteins of Various Arteriviruses Have a Conserved Degradation Activity for TRIF
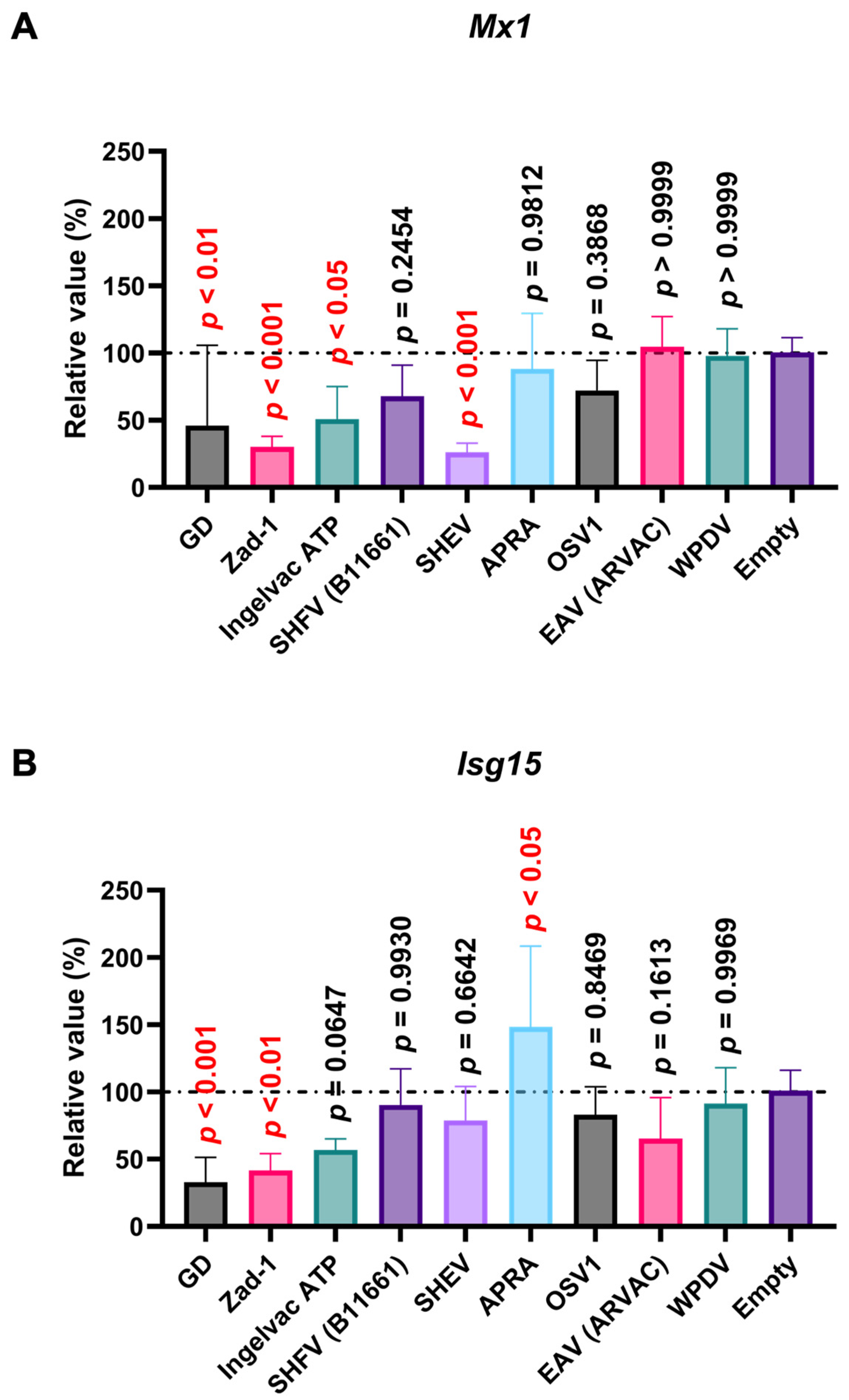
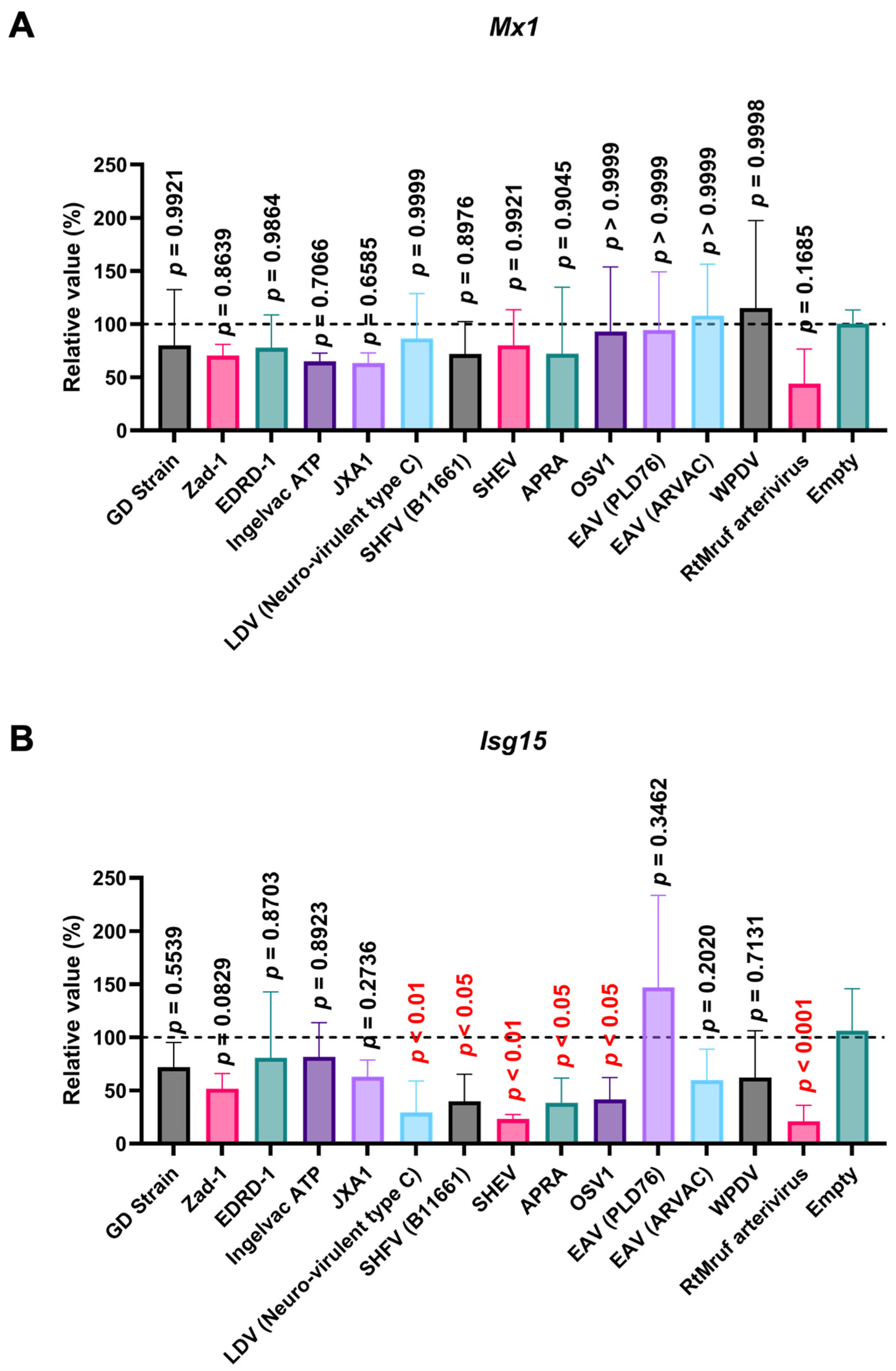
3.6. Arterivirus GP5 Proteins Differently Inhibit Poly (I:C) and IFN-β Triggered ISG Induction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanmechelen, B.; Vergote, V.; Laenen, L.; Koundouno, F.R.; Bore, J.A.; Wada, J.; Kuhn, J.H.; Carroll, M.W.; Maes, P. Expanding the Arterivirus Host Spectrum: Olivier’s Shrew Virus 1, A Novel Arterivirus Discovered in African Giant Shrews. Sci Rep 2018, 8, 11171. [CrossRef]
- Brinton, M.A.; Gulyaeva, A.A.; Balasuriya, U.B.R.; Dunowska, M.; Faaberg, K.S.; Goldberg, T.; Leung, F.C.C.; Nauwynck, H.J.; Snijder, E.J.; Stadejek, T.; et al. ICTV Virus Taxonomy Profile: Arteriviridae 2021. Journal of General Virology 2021, 102. [CrossRef]
- Zhang, M.; Li, X.; Deng, Z.; Chen, Z.; Liu, Y.; Gao, Y.; Wu, W.; Chen, Z. Structural Biology of the Arterivirus Nsp11 Endoribonucleases. J Virol 2016, 91, e01309-16. [CrossRef]
- Guo, R.; Yan, X.; Li, Y.; Cui, J.; Misra, S.; Firth, A.E.; Snijder, E.J.; Fang, Y. A Swine Arterivirus Deubiquitinase Stabilizes Two Major Envelope Proteins and Promotes Production of Viral Progeny. PLOS Pathogens 2021, 17, e1009403. [CrossRef]
- Brinton, M.A.; Snijder, E.J. Arteriviruses. In Encyclopedia of Virology (Third Edition); Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, 2008; pp. 176–186 ISBN 978-0-12-374410-4.
- Wu, F.; Peng, K.; Tian, J.; Xu, X.; Zhou, E.; Chen, H. Immune Response to Fc Tagged GP5 Glycoproteins of Porcine Reproductive and Respiratory Syndrome Virus. Viral Immunology 2014, 27, 343–349. [CrossRef]
- Ammann, C.G.; Messer, R.J.; Peterson, K.E.; Hasenkrug, K.J. Lactate Dehydrogenase-Elevating Virus Induces Systemic Lymphocyte Activation via TLR7-Dependent IFNα Responses by Plasmacytoid Dendritic Cells. PLOS ONE 2009, 4, e6105. [CrossRef]
- Balasuriya, U.B.R.; Go, Y.Y.; MacLachlan, N.J. Equine Arteritis Virus. Veterinary Microbiology 2013, 167, 93–122. [CrossRef]
- Zhang, Z.; Li, Z.; Li, H.; Yang, S.; Ren, F.; Bian, T.; Sun, L.; Zhou, B.; Zhou, L.; Qu, X. The Economic Impact of Porcine Reproductive and Respiratory Syndrome Outbreak in Four Chinese Farms: Based on Cost and Revenue Analysis. Front. Vet. Sci. 2022, 9. [CrossRef]
- Lauck, M.; Alkhovsky, S.V.; Bào, Y.; Bailey, A.L.; Shevtsova, Z.V.; Shchetinin, A.M.; Vishnevskaya, T.V.; Lackemeyer, M.G.; Postnikova, E.; Mazur, S.; et al. Historical Outbreaks of Simian Hemorrhagic Fever in Captive Macaques Were Caused by Distinct Arteriviruses. Journal of Virology 2015, 89, 8082–8087. [CrossRef]
- Dunowska, M.; Biggs, P.J.; Zheng, T.; Perrott, M.R. Identification of a Novel Nidovirus Associated with a Neurological Disease of the Australian Brushtail Possum (Trichosurus Vulpecula). Veterinary Microbiology 2012, 156, 418–424. [CrossRef]
- Anderson, G.W.; Rowland, R.R.; Palmer, G.A.; Even, C.; Plagemann, P.G. Lactate Dehydrogenase-Elevating Virus Replication Persists in Liver, Spleen, Lymph Node, and Testis Tissues and Results in Accumulation of Viral RNA in Germinal Centers, Concomitant with Polyclonal Activation of B Cells. J Virol 1995, 69, 5177–5185. [CrossRef]
- Allende, R.; Laegreid, W.W.; Kutish, G.F.; Galeota, J.A.; Wills, R.W.; Osorio, F.A. Porcine Reproductive and Respiratory Syndrome Virus: Description of Persistence in Individual Pigs upon Experimental Infection. J Virol 2000, 74, 10834–10837.
- Vatter, H.A.; Donaldson, E.F.; Huynh, J.; Rawlings, S.; Manoharan, M.; Legasse, A.; Planer, S.; Dickerson, M.F.; Lewis, A.D.; Colgin, L.M.A.; et al. A Simian Hemorrhagic Fever Virus Isolate from Persistently Infected Baboons Efficiently Induces Hemorrhagic Fever Disease in Japanese Macaques. Virology 2015, 474, 186–198. [CrossRef]
- Carossino, M.; Dini, P.; Kalbfleisch, T.S.; Loynachan, A.T.; Canisso, I.F.; Cook, R.F.; Timoney, P.J.; Balasuriya, U.B.R. Equine Arteritis Virus Long-Term Persistence Is Orchestrated by CD8+ T Lymphocyte Transcription Factors, Inhibitory Receptors, and the CXCL16/CXCR6 Axis. PLOS Pathogens 2019, 15, e1007950. [CrossRef]
- Jian, Z.; Ma, R.; Zhu, L.; Deng, H.; Li, F.; Zhao, J.; Deng, L.; Lai, S.; Sun, X.; Tang, H.; et al. Evasion of Interferon-Mediated Immune Response by Arteriviruses. Front. Immunol. 2022, 13, 963923. [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu Rev Immunol 2014, 32, 513–545. [CrossRef]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Sig Transduct Target Ther 2021, 6, 1–24. [CrossRef]
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat Immunol 2010, 11, 373–384. [CrossRef]
- Singh, H.; Koury, J.; Kaul, M. Innate Immune Sensing of Viruses and Its Consequences for the Central Nervous System. Viruses 2021, 13, 170. [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I Interferons in Infectious Disease. Nat Rev Immunol 2015, 15, 87–103. [CrossRef]
- Perng, Y.-C.; Lenschow, D.J. ISG15 in Antiviral Immunity and Beyond. Nat Rev Microbiol 2018, 16, 423–439. [CrossRef]
- Lerolle, S.; Freitas, N.; Cosset, F.-L.; Legros, V. Host Cell Restriction Factors of Bunyaviruses and Viral Countermeasures. Viruses 2021, 13, 784. [CrossRef]
- Huang, C.; Zhang, Q.; Guo, X.; Yu, Z.; Xu, A.-T.; Tang, J.; Feng, W. Porcine Reproductive and Respiratory Syndrome Virus Nonstructural Protein 4 Antagonizes Beta Interferon Expression by Targeting the NF-κB Essential Modulator. Journal of Virology 2014, 88, 10934–10945. [CrossRef]
- Chen, J.; Wang, D.; Sun, Z.; Gao, L.; Zhu, X.; Guo, J.; Xu, S.; Fang, L.; Li, K.; Xiao, S. Arterivirus Nsp4 Antagonizes Interferon Beta Production by Proteolytically Cleaving NEMO at Multiple Sites. Journal of Virology 2019, 93, 10.1128/jvi.00385-19. [CrossRef]
- Wang, T.-Y.; Sun, M.-X.; Zhang, H.-L.; Wang, G.; Zhan, G.; Tian, Z.-J.; Cai, X.-H.; Su, C.; Tang, Y.-D. Evasion of Antiviral Innate Immunity by Porcine Reproductive and Respiratory Syndrome Virus. Front. Microbiol. 2021, 12. [CrossRef]
- Sun, Z.; Li, Y.; Ransburgh, R.; Snijder, E.J.; Fang, Y. Nonstructural Protein 2 of Porcine Reproductive and Respiratory Syndrome Virus Inhibits the Antiviral Function of Interferon-Stimulated Gene 15. J Virol 2012, 86, 3839–3850. [CrossRef]
- Hitoshi, N.; Ken-ichi, Y.; Jun-ichi, M. Efficient Selection for High-Expression Transfectants with a Novel Eukaryotic Vector. Gene 1991, 108, 193–199. [CrossRef]
- Gentili, M.; Kowal, J.; Tkach, M.; Satoh, T.; Lahaye, X.; Conrad, C.; Boyron, M.; Lombard, B.; Durand, S.; Kroemer, G.; et al. Transmission of Innate Immune Signaling by Packaging of cGAMP in Viral Particles. Science 2015, 349, 1232–1236. [CrossRef]
- Choonnasard, A.; Shofa, M.; Okabayashi, T.; Saito, A. Conserved Functions of Orthohepadnavirus X Proteins to Inhibit Type-I Interferon Signaling. International Journal of Molecular Sciences 2024, 25, 3753. [CrossRef]
- Zhu, J.; Smith, K.; Hsieh, P.N.; Mburu, Y.K.; Chattopadhyay, S.; Sen, G.C.; Sarkar, S.N. High-Throughput Screening for TLR3–IFN Regulatory Factor 3 Signaling Pathway Modulators Identifies Several Antipsychotic Drugs as TLR Inhibitors. The Journal of Immunology 2010, 184, 5768–5776. [CrossRef]
- Kasza, L.; Shadduck, J.A.; Christofinis, G.J. Establishment, Viral Susceptibility and Biological Characteristics of a Swine Kidney Cell Line SK-6. Research in Veterinary Science 1972, 13, 46–53. [CrossRef]
- Shofa, M.; Saito, A. Generation of Porcine PK-15 Cells Lacking the Ifnar1 or Stat2 Gene to Optimize the Efficiency of Viral Isolation. PLOS ONE 2023, 18, e0289863. [CrossRef]
- Vats, A.; Gautam, D.; Maharana, J.; Singh Chera, J.; Kumar, S.; Rout, P.K.; Werling, D.; De, S. Poly I:C Stimulation in-Vitro as a Marker for an Antiviral Response in Different Cell Types Generated from Buffalo (Bubalus Bubalis). Molecular Immunology 2020, 121, 136–143. [CrossRef]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential Roles of MDA5 and RIG-I Helicases in the Recognition of RNA Viruses. Nature 2006, 441, 101–105. [CrossRef]
- Zhixuan, X.; Xiaoyun, N.; Yabing, S.; Danping, S.; Fei, W.; Rui-ai, C.; Dongsheng, H. Evolution of Porcine Reproductive and Respiratory Syndrome Virus GP5 and GP3 Genes under swIFN-β Immune Pressure and Interferon Regulatory Factor-3 Activation Suppressed by GP5. Research in Veterinary Science 2015, 101, 175–179. [CrossRef]
- Han, M.; Kim, C.Y.; Rowland, R.R.R.; Fang, Y.; Kim, D.; Yoo, D. Biogenesis of Non-Structural Protein 1 (Nsp1) and Nsp1-Mediated Type I Interferon Modulation in Arteriviruses. Virology 2014, 458–459, 136–150. [CrossRef]
- Beura, L.K.; Sarkar, S.N.; Kwon, B.; Subramaniam, S.; Jones, C.; Pattnaik, A.K.; Osorio, F.A. Porcine Reproductive and Respiratory Syndrome Virus Nonstructural Protein 1beta Modulates Host Innate Immune Response by Antagonizing IRF3 Activation. J Virol 2010, 84, 1574–1584. [CrossRef]
- Li, H.; Zheng, Z.; Zhou, P.; Zhang, B.; Shi, Z.; Hu, Q.; Wang, H. The Cysteine Protease Domain of Porcine Reproductive and Respiratory Syndrome Virus Non-Structural Protein 2 Antagonizes Interferon Regulatory Factor 3 Activation. J Gen Virol 2010, 91, 2947–2958. [CrossRef]
- Sun, Y.; Ke, H.; Han, M.; Chen, N.; Fang, W.; Yoo, D. Nonstructural Protein 11 of Porcine Reproductive and Respiratory Syndrome Virus Suppresses Both MAVS and RIG-I Expression as One of the Mechanisms to Antagonize Type I Interferon Production. PLOS ONE 2016, 11, e0168314. [CrossRef]
- Shi, X.; Wang, L.; Li, X.; Zhang, G.; Guo, J.; Zhao, D.; Chai, S.; Deng, R. Endoribonuclease Activities of Porcine Reproductive and Respiratory Syndrome Virus Nsp11 Was Essential for Nsp11 to Inhibit IFN-β Induction. Mol Immunol 2011, 48, 1568–1572. [CrossRef]
- Align Results | Phylogenetic Tree | UniProt Available online: https://www.uniprot.org/align/clustalo-R20240618-065809-0031-11841362-p1m/percent-identity-matrix (accessed on 19 June 2024).
- Warren, C.J.; Yu, S.; Peters, D.K.; Barbachano-Guerrero, A.; Yang, Q.; Burris, B.L.; Worwa, G.; Huang, I.-C.; Wilkerson, G.K.; Goldberg, T.L.; et al. Primate Hemorrhagic Fever-Causing Arteriviruses Are Poised for Spillover to Humans. Cell 2022, 185, 3980-3991.e18. [CrossRef]
- Han, M.; Yoo, D. Modulation of Innate Immune Signaling by Nonstructural Protein 1 (Nsp1) in the Family Arteriviridae. Virus Research 2014, 194, 100. [CrossRef]
- Assavacheep, P.; Thanawongnuwech, R. Porcine Respiratory Disease Complex: Dynamics of Polymicrobial Infections and Management Strategies after the Introduction of the African Swine Fever. Frontiers in Veterinary Science 2022, 9. [CrossRef]
- Zhao, D.; Yang, B.; Yuan, X.; Shen, C.; Zhang, D.; Shi, X.; Zhang, T.; Cui, H.; Yang, J.; Chen, X.; et al. Advanced Research in Porcine Reproductive and Respiratory Syndrome Virus Co-Infection With Other Pathogens in Swine. Front Vet Sci 2021, 8, 699561. [CrossRef]
- Zhang, J.; Wang, P.; Xie, C.; Ha, Z.; Shi, N.; Zhang, H.; Li, Z.; Han, J.; Xie, Y.; Qiu, X.; et al. Synergistic Pathogenicity by Coinfection and Sequential Infection with NADC30-like PRRSV and PCV2 in Post-Weaned Pigs. Viruses 2022, 14. [CrossRef]
- Luo, Q.; Zheng, Y.; Zhang, H.; Yang, Z.; Sha, H.; Kong, W.; Zhao, M.; Wang, N. Research Progress on Glycoprotein 5 of Porcine Reproductive and Respiratory Syndrome Virus. Animals : an Open Access Journal from MDPI 2023, 13. [CrossRef]
- Butler, J.E.; Lager, K.M.; Golde, W.; Faaberg, K.S.; Sinkora, M.; Loving, C.; Zhang, Y.I. Porcine Reproductive and Respiratory Syndrome (PRRS): An Immune Dysregulatory Pandemic. Immunologic Research 2014, 59, 81. [CrossRef]
- Zheng, Q.; Chen, D.; Li, P.; Bi, Z.; Cao, R.; Zhou, B.; Chen, P. Co-Expressing GP5 and M Proteins under Different Promoters in Recombinant Modified Vaccinia Virus Ankara (rMVA)-Based Vaccine Vector Enhanced the Humoral and Cellular Immune Responses of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). Virus Genes 2007, 35, 585. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




