Submitted:
28 June 2024
Posted:
01 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
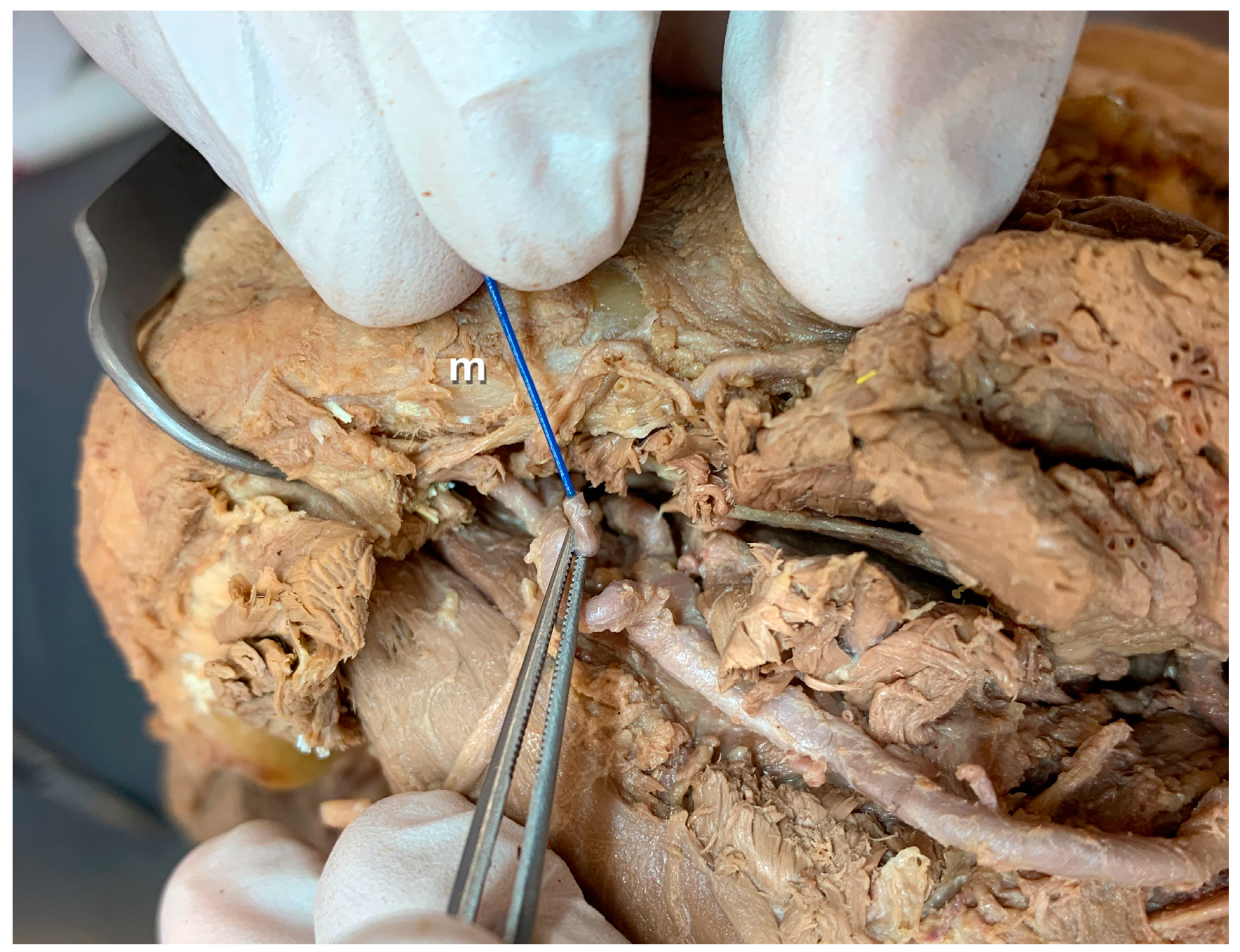
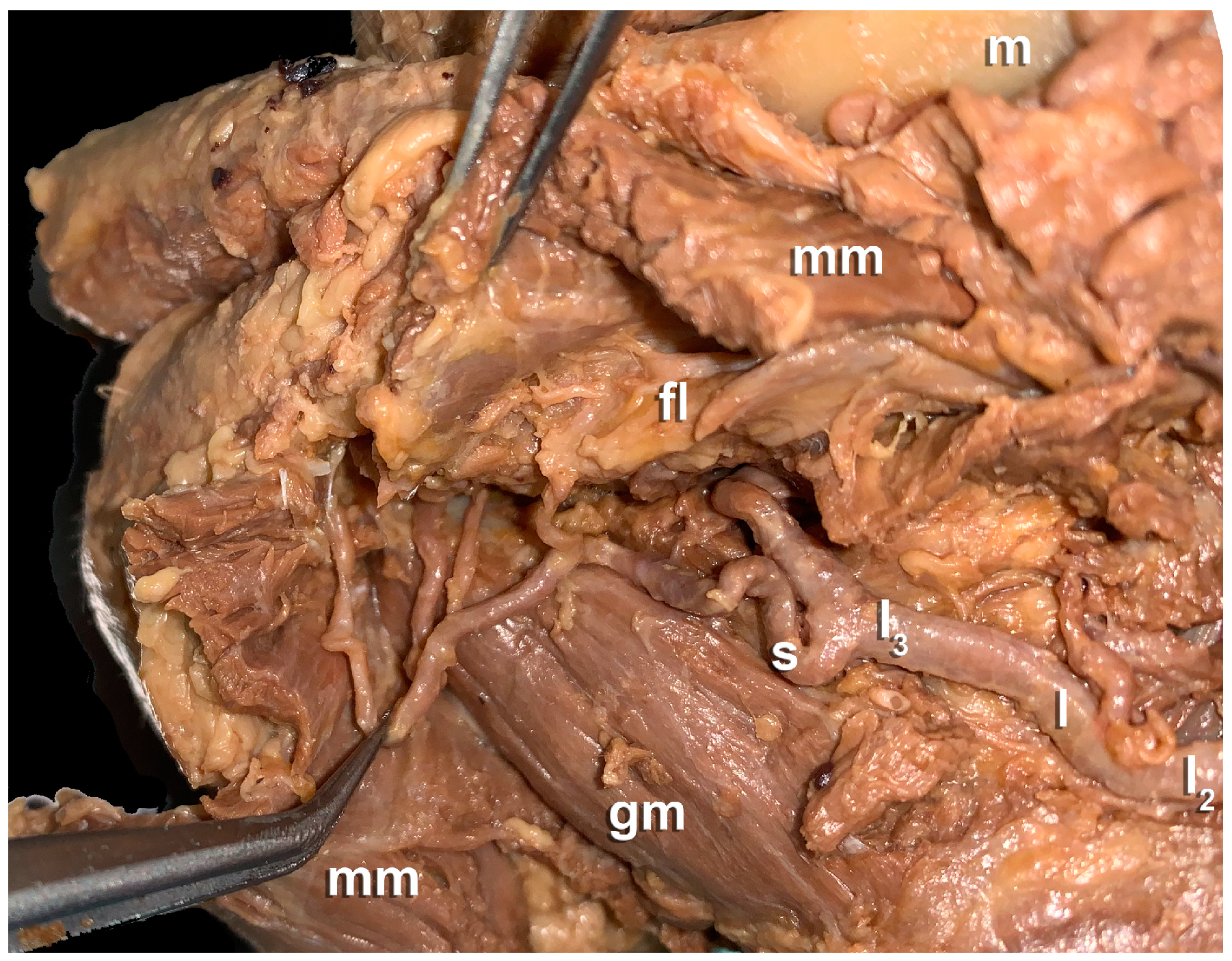
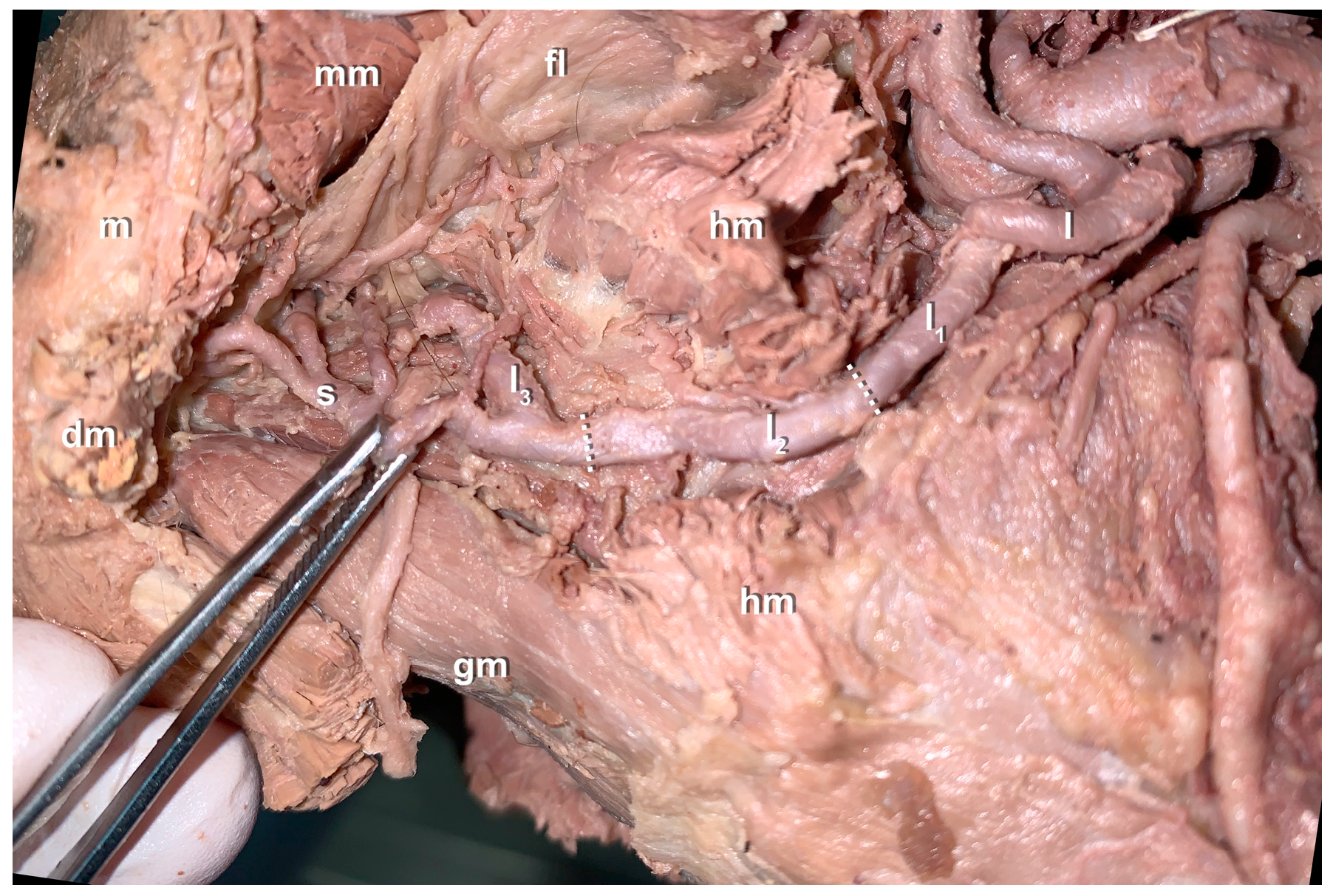
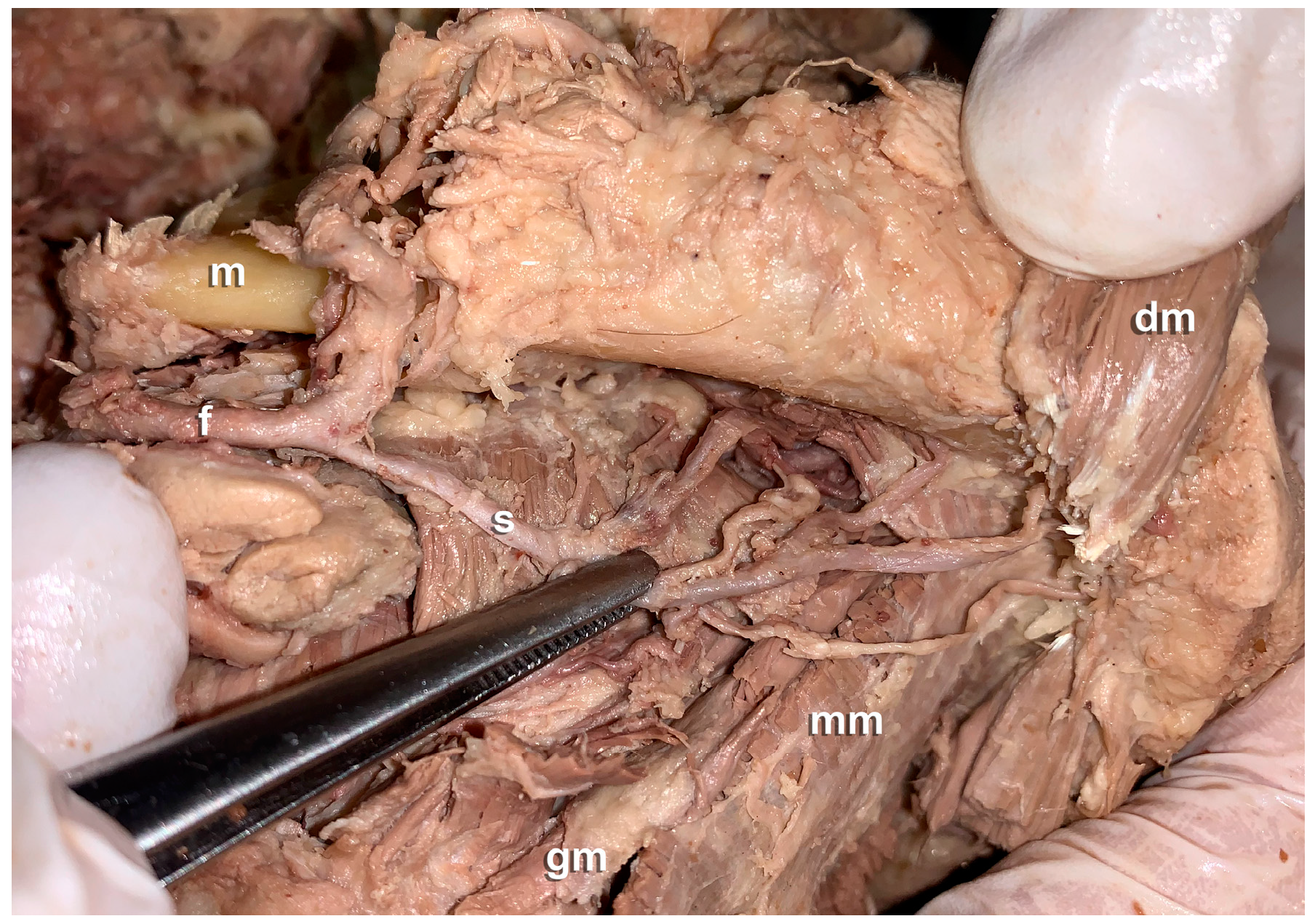
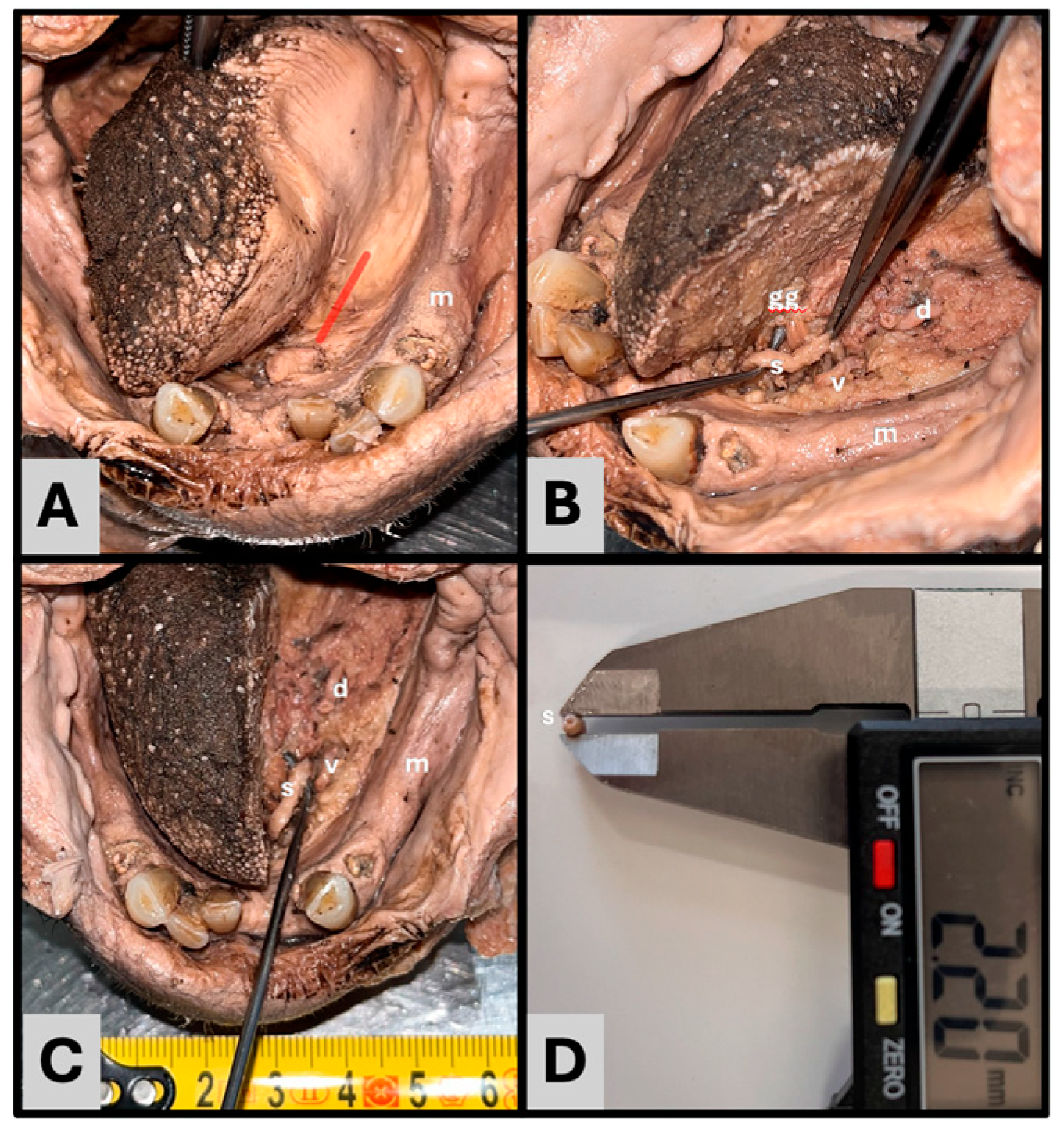
3. Results
- Anatomical findings:
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandtner, C.; Bürger, H.; Hachleitner, J.; Gaggl, A. The intraoral anastomosing technique in reconstructive surgery of the face–A consecutive case series of 70 patients. Journal of Cranio-Maxillofacial Surgery 2015, 43, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Gaggl, A.; Bürger, H.; Virnik, S.; Chiari, F. An intraoral anastomosing technique for microvascular bone flaps in alveolar ridge reconstruction: first clinical results. International journal of oral and maxillofacial surgery 2009, 38, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Nkenke, E.; Agaimy, A.; von Wilmowsky, C.; Eitner, S. Mandibular reconstruction using intraoral microvascular anastomosis following removal of an ameloblastoma. Journal of Oral and Maxillofacial Surgery 2013, 71, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, J.; Lv, M.M.; Wang, L.; Gupta, A.; Shen, Y. Expanded Transoral Microvascular Mandibular Reconstruction: A Scar-Free Approach. J Oral Maxillofac Surg 2022, 80, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Shao, Z.; Jia, J.; Liu, B.; Bu, L.-L. Analysis of intraoral microvascular anastomosis in maxillofacial defects reconstruction. Journal of Cranio-maxillo-facial Surgery: Official Publication of the European Association for Cranio-maxillo-facial Surgery, 2023, S1010-5182 (1023) 00008. [Google Scholar]
- Kämmerer, P.W.; Tavakoli, M.; Gaggl, A.; Maranzano, M. Intraoral Microvascular Anastomosis in Immediate Free Flap Reconstruction for Midfacial Tumor Defects: A Retrospective Multicenter Study. Journal of Clinical Medicine 2023, 12, 7064. [Google Scholar] [CrossRef] [PubMed]
- Lippert, H.; Pabst, R. Arterial variations in man: classification and frequency; Springer Verlag: 1985.
- von Lanz, T.; Wachsmuth, W. Praktische Anatomie: ein Lehr-und Hilfsbuch der anatomischen Grundlagen ärztlichen Handelns; Springer-Verlag: 2013.
- Nakajima, K.; Tagaya, A.; Otonari-Yamamoto, M.; Seki, K.; Araki, K.; Sano, T.; Okano, T.; Nakamura, M. Composition of the blood supply in the sublingual and submandibular spaces and its relationship to the lateral lingual foramen of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol 2014, 117, e32–38. [Google Scholar] [CrossRef] [PubMed]
- Shangkuan, H.; Xinghai, W.; Zengxing, W.; Shizhen, Z.; Shiying, J.; Yishi, C. Anatomic bases of tongue flaps. Surg Radiol Anat 1998, 20, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Sumida, K.; Yamashita, K.; Baba, O.; Kitamura, S. Gross anatomical classification of the courses of the human lingual artery. Surg Radiol Anat 2017, 39, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Masui, T.; Seki, S.; Sumida, K.; Yamashita, K.; Kitamura, S. Gross anatomical classification of the courses of the human sublingual artery. Anat Sci Int 2016, 91, 97–105. [Google Scholar] [CrossRef]
- Bavitz, J.B.; Harn, S.D.; Homze, E.J. Arterial supply to the floor of the mouth and lingual gingiva. Oral Surg Oral Med Oral Pathol 1994, 77, 232–235. [Google Scholar] [CrossRef]
- Platzer, W.; Putz, R.; Poisel, S. Ein neues Konservierungs-und Aufbewahrungssystem für anatomisches Material. Cells Tissues Organs 1978, 102, 60–67. [Google Scholar] [CrossRef]
- Danesh-Meyer, H.V.; Savino, P.J.; Bilyk, J.R.; Eagle, R.C.; Sergott, R.C. Shrinkage: fact or fiction? Archives of Ophthalmology 2001, 119, 1217–1217. [Google Scholar] [PubMed]
- Sosin, M.; Sinada, G.G.; Rodriguez, E.D.; Dorafshar, A.H. Intraoral microvascular anastomosis of an iliac free flap for maxillary fibrous dysplasia. Journal of Oral and Maxillofacial Surgery 2015, 73, 2068–e2061. [Google Scholar] [CrossRef] [PubMed]
- Landes, C.; Cornea, P.; Teiler, A.; Ballon, A.; Sader, R. Intraoral anastomosis of a prelaminated radial forearm flap in reconstruction of a large persistent cleft palate. Microsurgery 2014, 34, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, Y.; Tanaka, R.; Hayashi, T.; Koga, T.; Takagi, R.; Ohshima, H. Variation in arterial supply to the floor of the mouth and assessment of relative hemorrhage risk in implant surgery. Clinical Oral Implants Research 2013, 24, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Konschake, M.; Brenner, E. “Mors auxilium vitae”—Causes of death of body donors in an Austrian anatomical department. Annals of Anatomy-Anatomischer Anzeiger 2014, 196, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Loukas, M.; Kinsella Jr, C.R.; Kapos, T.; Tubbs, R.S.; Ramachandra, S. Anatomical variation in arterial supply of the mandible with special regard to implant placement. International journal of oral and maxillofacial surgery 2008, 37, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Harii, K. Microvascular surgery in plastic surgery: Free-tissue transfer. Microsurgery 1979, 1, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.W. Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study. Plastic and reconstructive surgery 2010, 126, 752–758. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, T.H.; Kim, N.G.; Lee, K.S.; Han, K.H.; Son, D.G.; Kim, J.H.; Lee, S.-I.; Kang, D. The replantation of an amputated tongue by supermicrosurgery. Journal of plastic, reconstructive & aesthetic surgery 2007, 60, 1152–1155. [Google Scholar]
- Liu, H.-L. Microvascular anastomosis of submillimeter vessels—a training model in rats. Journal of hand and microsurgery 2013, 5, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Riederer, B.M.; Bolt, S.; Brenner, E.; Bueno-López, J.L.; Circulescu, A.R.; Davies, D.; Caro, R.d.; Gerrits, P.; McHanwell, S.; Pais, D. The legal and ethical framework governing body donation in Europe–1st update on current practice. 2012.
- McHanwell, S.; Brenner, E.; Chirculescu, A.R.; Drukker, J.; van Mameren, H.; Mazzotti, G.; Pais, D.; Paulsen, F.; Plaisant, O.; Caillaud, M.M. The legal and ethical framework governing Body Donation in Europe-A review of current practice and recommendations for good practice. European Journal of Anatomy 2020, 12, 1–24. [Google Scholar]
| Straight length | Stretched length | |
| Original segment | 21.9 ± 6.6 (7-30) | 39 ± 13.3 (20-70) |
| Segment within the hyoglossal muscle | 20.4 ± 5.0 (11-30) | 25.2 ± 7.8 (11-42) |
| Ascending segment of the deep lingual artery | 18.9 ± 5.2 (13-34) | 23.1 ± 5.5 (13-39) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





