Submitted:
27 June 2024
Posted:
29 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Assessment of the Toxicological Properties of Quinacridones
3.1. Comparison of Quinacridones and Biogenic Acridone
3.2. Applications of Quinacridones.
3.3. Quinacridone Particles: Application as a Gliding Layer
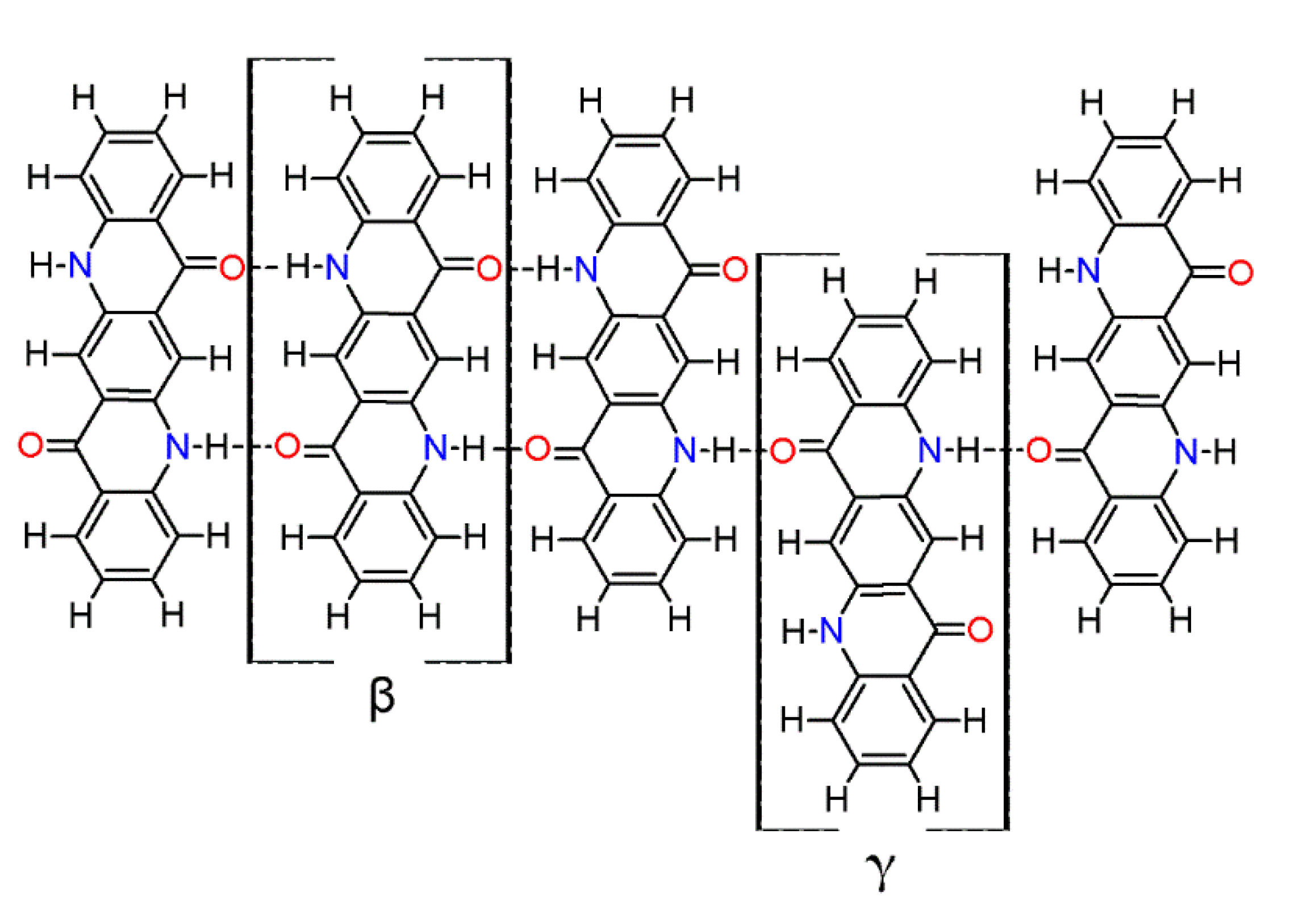
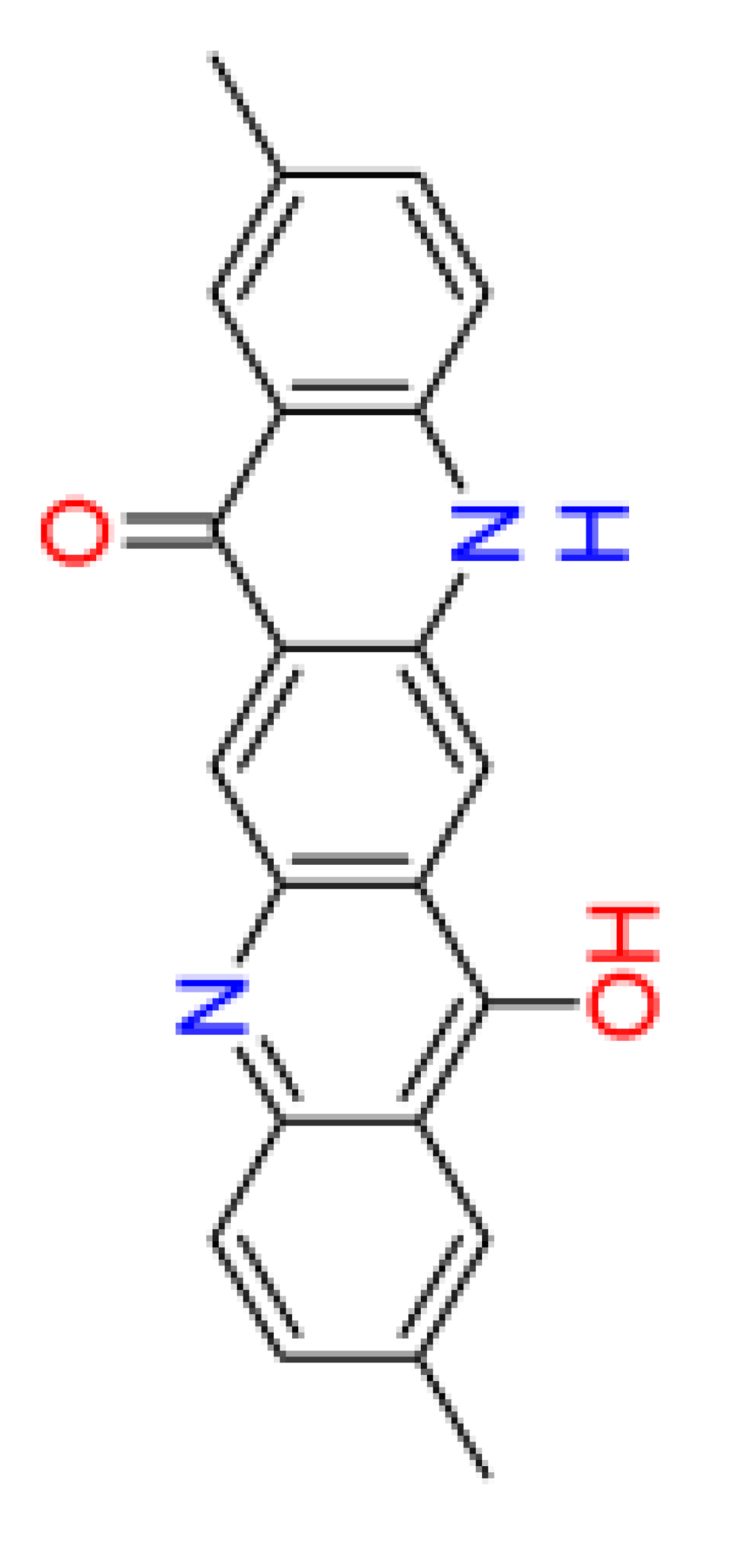
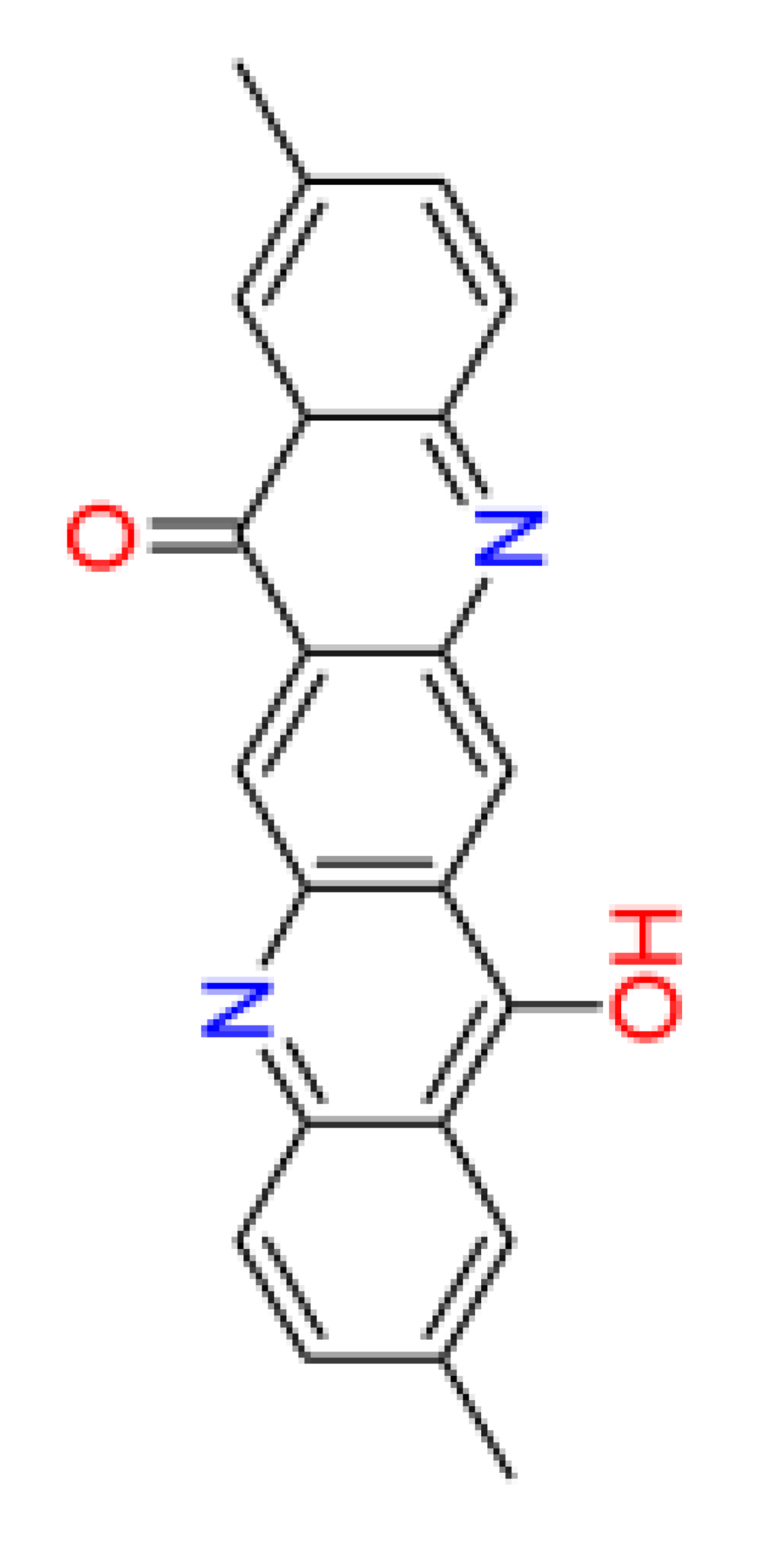
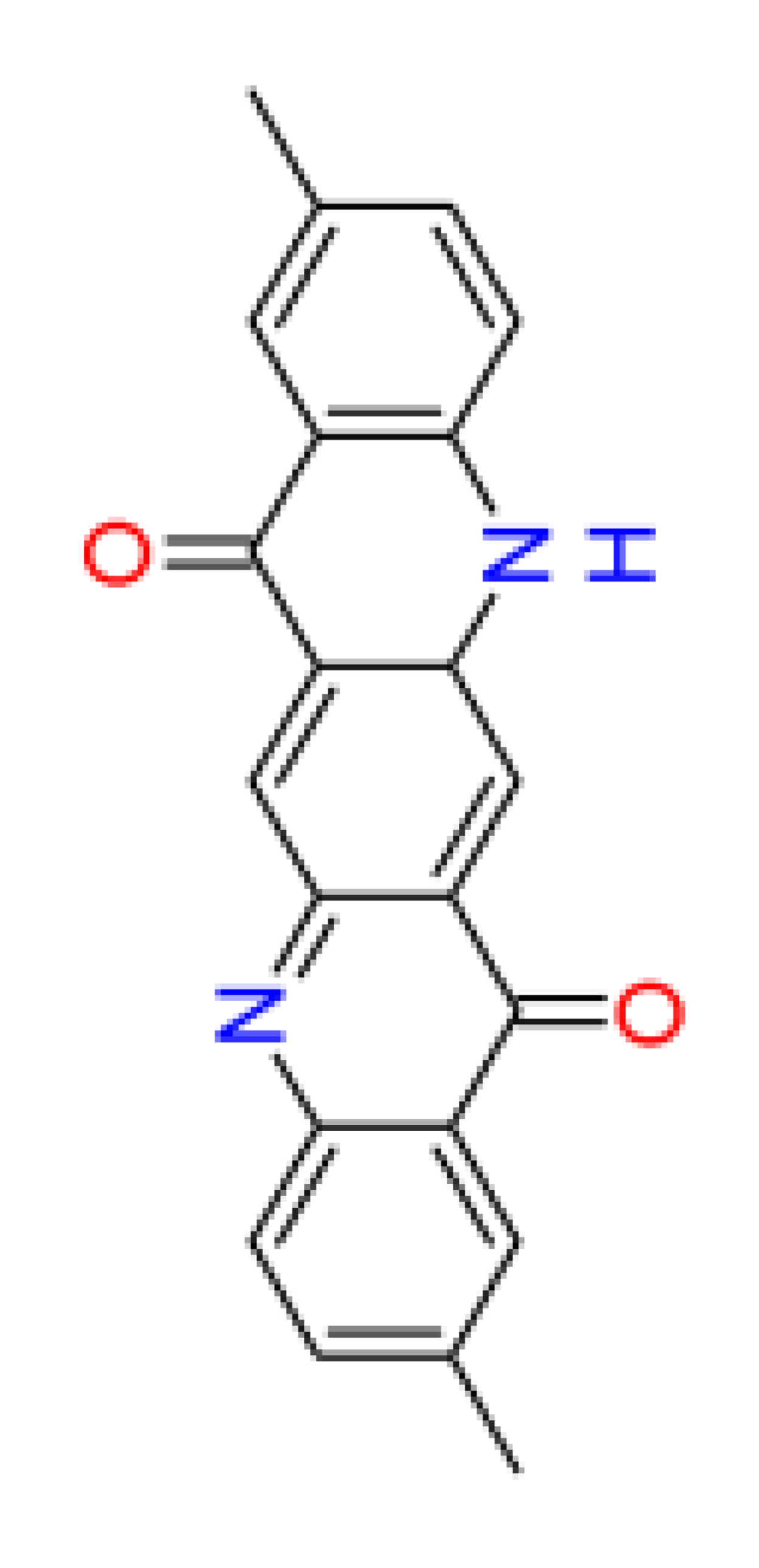
3.4. QSAR data of DQA Tautomers
3.5. Experimental Data
- density: 1.452 g/cm³ (20 °C)
- water solubility: 5.6 µg/L (24 °C)
- log(Kow): 2.2
- short-term toxicity to fish: LC50 (96 h) > 100 mg/L nominal concentration
- long-term toxicity to freshwater fish NOEC: 10 mg/L
- Bioconcentration Factor (BCF) [63]: 5
- Daphnia magna chronic NOEC [63]: 1.5 mg/L
4. Characterization of DQA Particles and Performance as a Gliding Layer
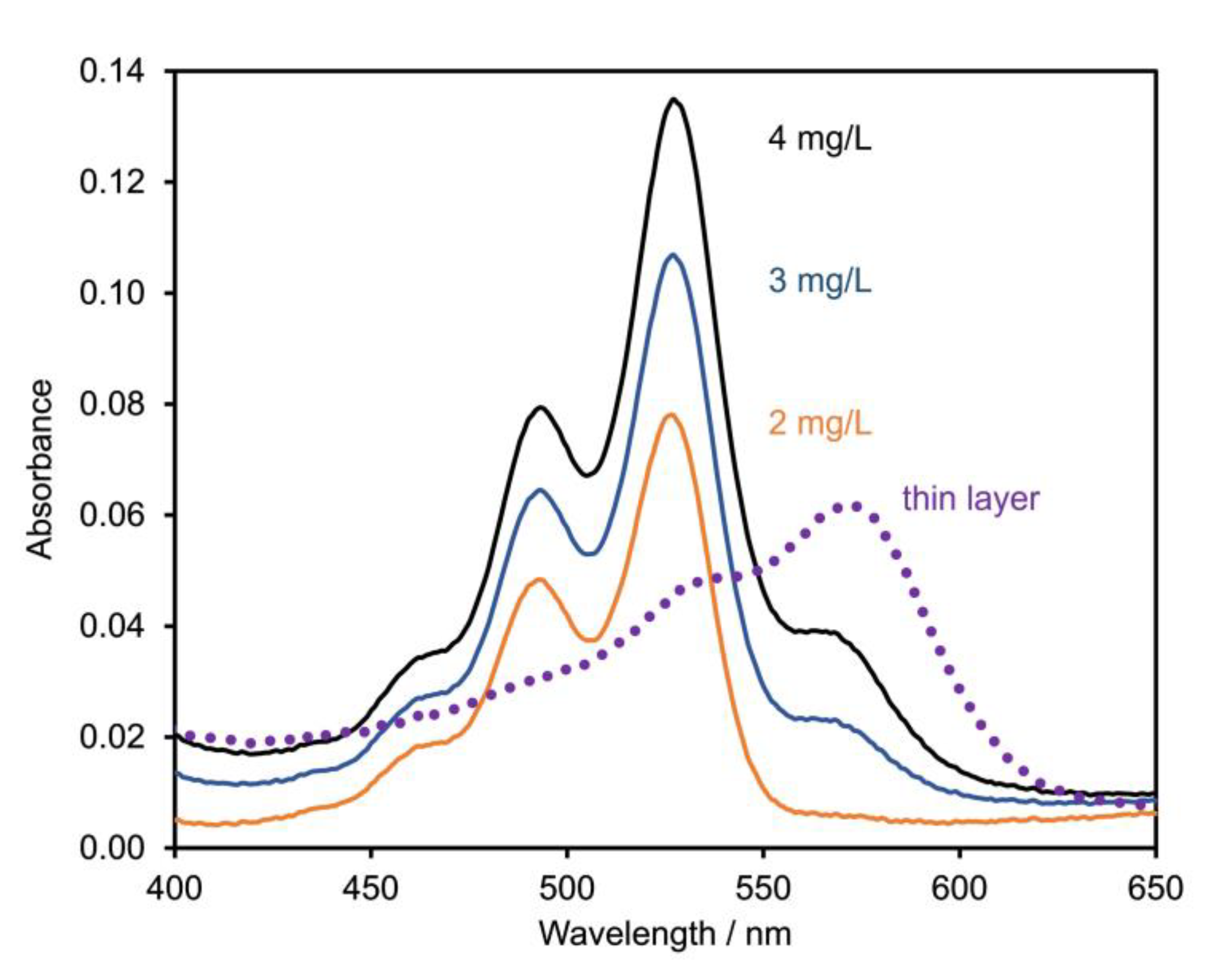
4.1. Purity and Electronic Absorption Spectra
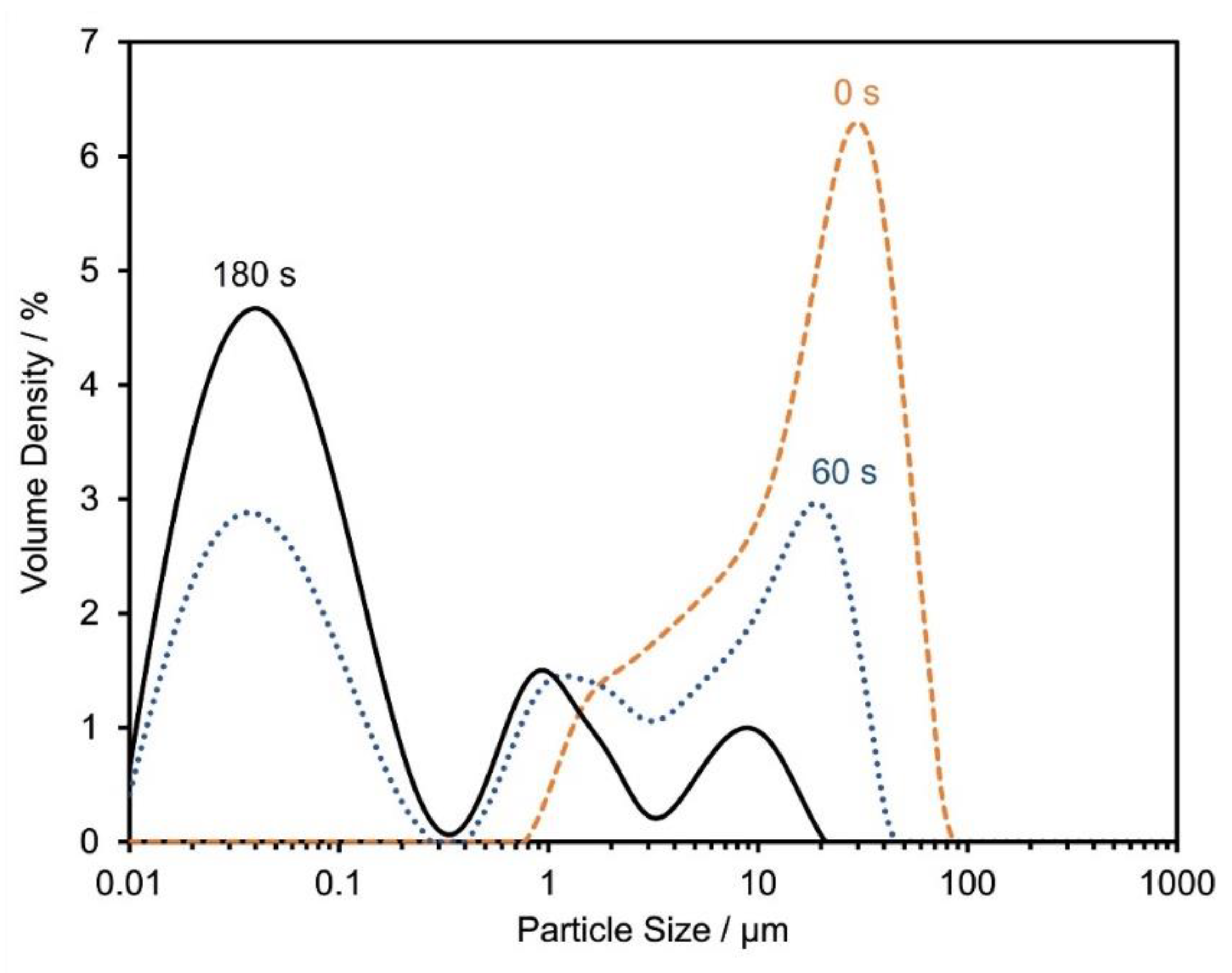
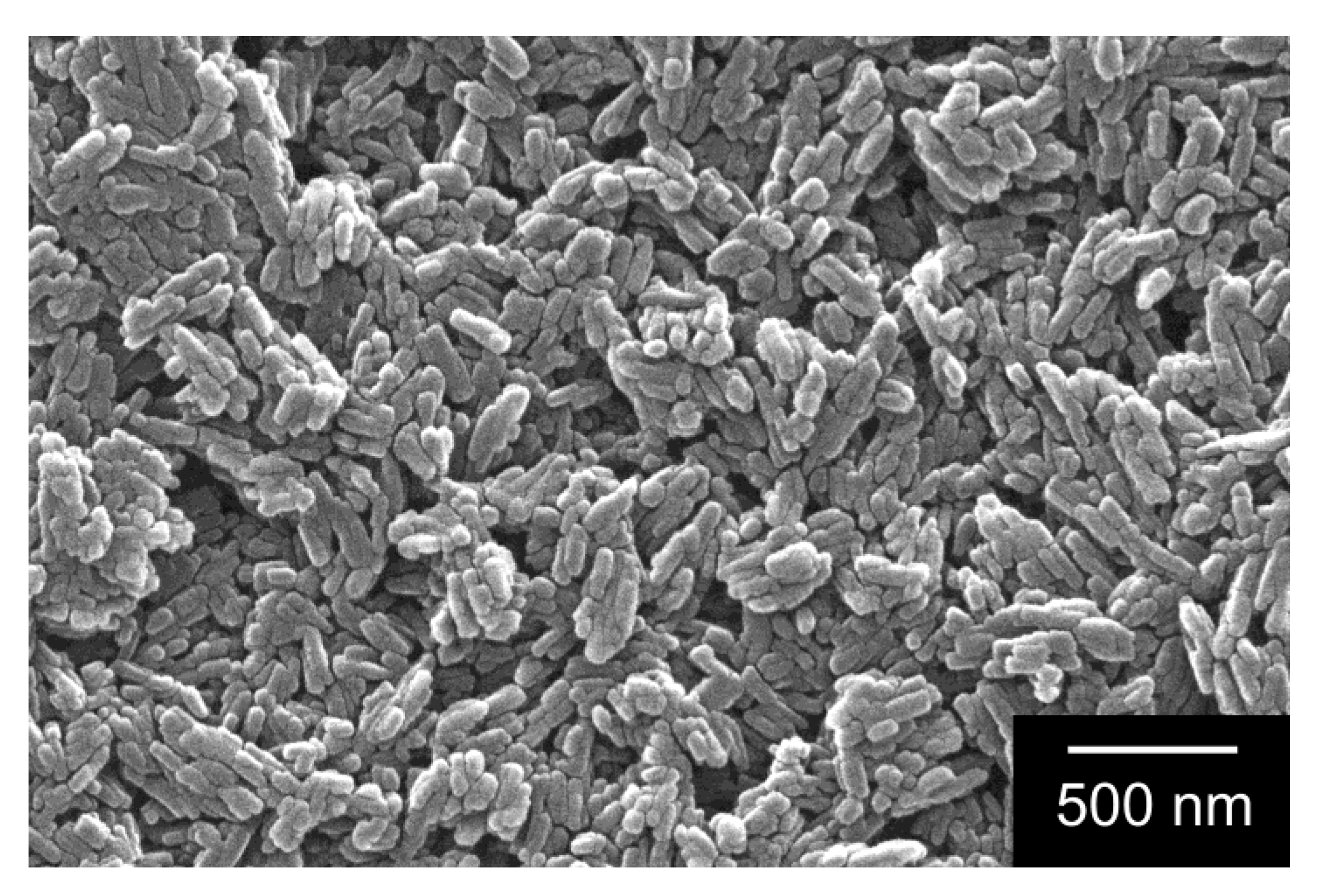
4.2. Particle Size and Morphology
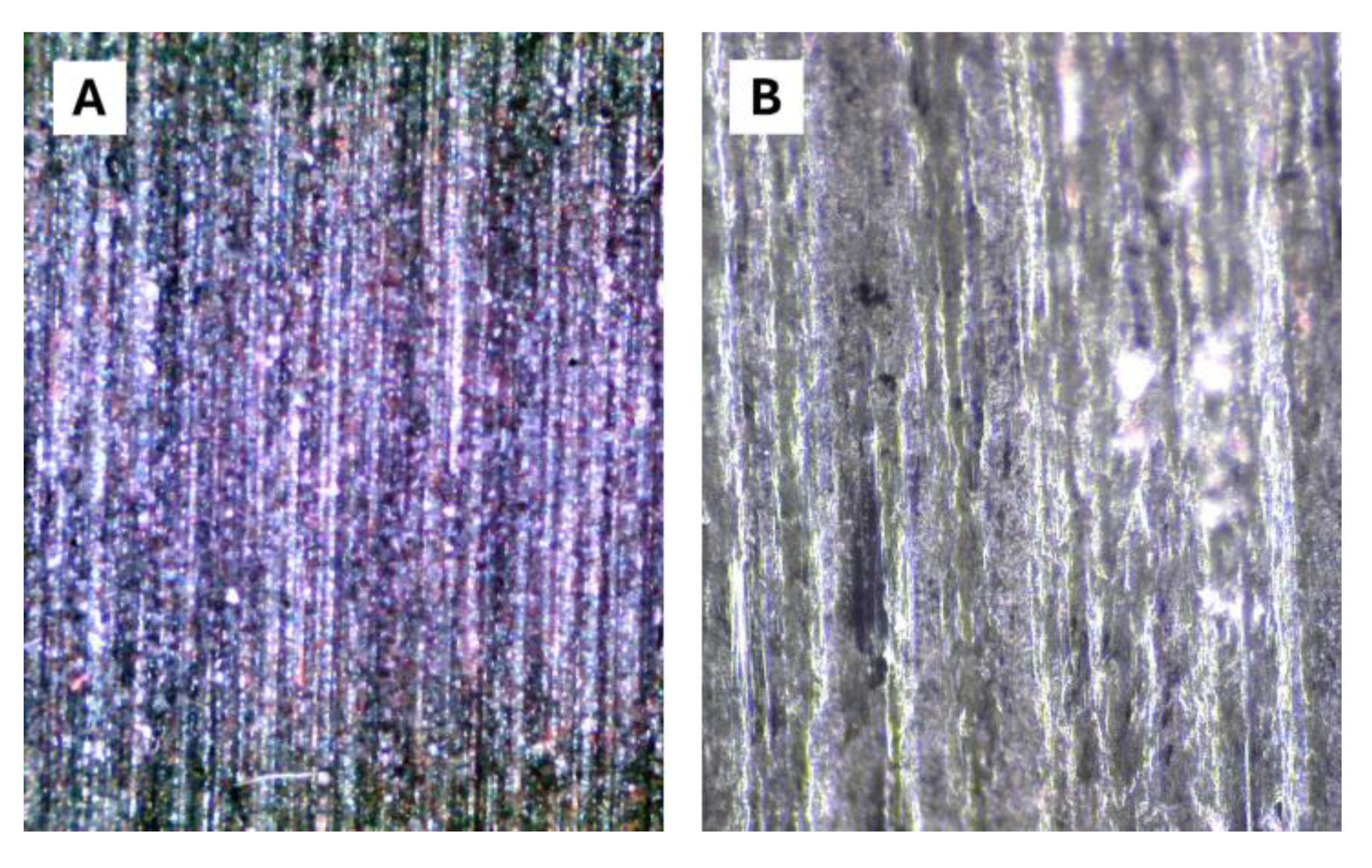
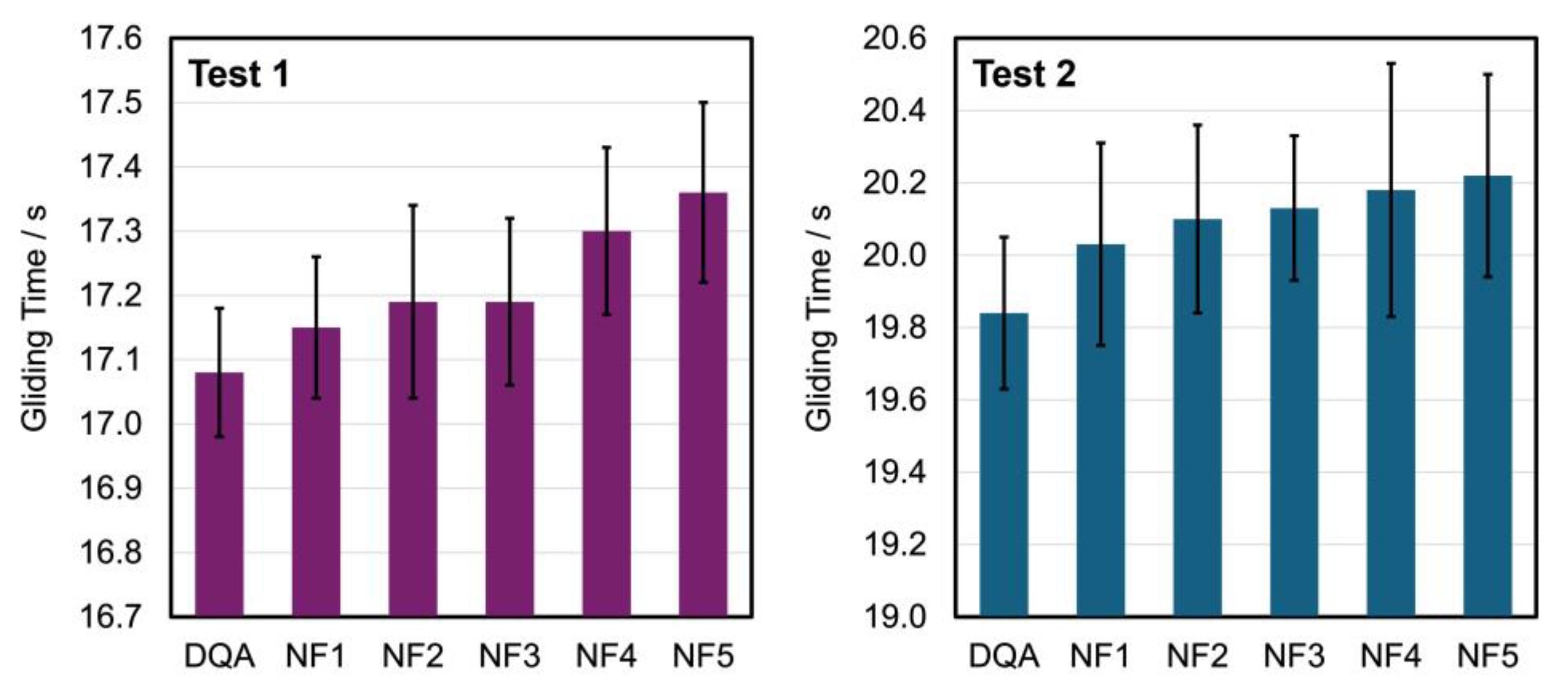
4.3. Performance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlson, G.L.; Tupper, S. Ski Wax Use Contributes to Environmental Contamination by Per- and Polyfluoroalkyl Substances. Chemosphere 2020, 261, 128078. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.; Costa, L.C.A.; Soares Rondan, F.; Matic, E.; Foster Mesko, M.; Kindness, A.; Feldmann, J. Per and Polyfluoroalkylated Substances (PFAS) Target and EOF Analyses in Ski Wax, Snowmelts, and Soil from Skiing Areas. Environ. Sci. Process. Impacts 2023, 25, 1926–1936. [Google Scholar] [CrossRef]
- Crawford, K.A.; Hartmann, N. Respiratory Exposure to Highly Fluorinated Chemicals via Application of Ski Wax and Related Health Effects. Curr. Environ. Health Rep. 2024, 11, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Bützer, P.; Brühwiler, D.; Bützer, M.R.; Al-Godari, N.; Cadalbert, M.; Giger, M.; Schär, S. Indigo—A New Tribological Substance Class for Non-Toxic and Ecological Gliding Surfaces on Ice, Snow, and Water. Materials 2022, 15, 883. [Google Scholar] [CrossRef] [PubMed]
- Niementowski, S. Ueber das Chinacridin. Berichte Dtsch. Chem. Ges. 1896, 29, 76–83. [Google Scholar] [CrossRef]
- Ullmann, F.; Maag, R. Ueber Chinacridon. Berichte Dtsch. Chem. Ges. 1906, 39, 1693–1696. [Google Scholar] [CrossRef]
- Liebermann, H. Über die Bildung von Chinakridonen aus p-Di-arylamino-terephtalsäuren. 6. Mitteilung über Umwandlungsprodukte des Succinylobernsteinsäureesters. Justus Liebigs Ann. Chem. 1935, 518, 245–259. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Wang, Y. Quinacridone-Based π-Conjugated Electronic Materials. J. Mater. Chem. C 2016, 4, 9918–9936. [Google Scholar] [CrossRef]
- Dickson, R.; Mancini, E.; Garg, N.; Woodley, J.M.; Gernaey, K.V.; Pinelo, M.; Liu, J.; Mansouri, S.S. Sustainable Bio-Succinic Acid Production: Superstructure Optimization, Techno-Economic, and Lifecycle Assessment. Energy Environ. Sci. 2021, 14, 3542–3558. [Google Scholar] [CrossRef]
- Nagime, P.V.; Upaichit, A.; Cheirsilp, B.; Boonsawang, P. Bio-Succinic Acid Production from Palm Oil Mill Effluent Using Enterococcus Gallinarum with Sequential Purification of Biogas. Fermentation 2023, 9, 369. [Google Scholar] [CrossRef]
- Irimia-Vladu, M.; Kanbur, Y.; Camaioni, F.; Coppola, M.E.; Yumusak, C.; Irimia, C.V.; Vlad, A.; Operamolla, A.; Farinola, G.M.; Suranna, G.P.; et al. Stability of Selected Hydrogen Bonded Semiconductors in Organic Electronic Devices. Chem. Mater. 2019, 31, 6315–6346. [Google Scholar] [CrossRef] [PubMed]
- Potts, G.D.; Jones, W.; Bullock, J.F.; Andrews, S.J.; Maginn, S.J. The Crystal Structure of Quinacridone: An Archetypal Pigment. J. Chem. Soc. Chem. Commun. 1994, 2565–2566. [Google Scholar] [CrossRef]
- Paulus, E.F.; Leusen, F.J.J.; Schmidt, M.U. Crystal Structures of Quinacridones. CrystEngComm 2007, 9, 131–143. [Google Scholar] [CrossRef]
- Scherwitzl, B.; Lassnig, R.; Truger, M.; Resel, R.; Leising, G.; Winkler, A. Adsorption, Desorption, and Film Formation of Quinacridone and Its Thermal Cracking Product Indigo on Clean and Carbon-Covered Silicon Dioxide Surfaces. J. Chem. Phys. 2016, 145, 094702. [Google Scholar] [CrossRef]
- Panina, N. Crystal Structure and Morphology Prediction of Organic Pigments. PhD Thesis, Radboud University Nijmegen: Netherlands, 2009.
- Jones, F.; Okui, N.; Patterson, D. The Thermal Stability of Linear Trans-Quinacridone Pigments. J. Soc. Dye. Colour. 1975, 91, 361–365. [Google Scholar] [CrossRef]
- Karlöf, L.; Smevold, T.; Tretterud, O.B.; Zupan, M. Swix Test Protocol for Testing of Glide Products, Technical Note # 3-2007. 2007.
- NIST Chemistry WebBook, 9(10H)-Acridinone. Available online: https://webbook.nist.gov/cgi/cbook.cgi?InChI=FZEYVTFCMJSGMP-UHFFFAOYSA-N (accessed on 10 April 2024).
- Pubchem, NIH. Thamnosma Montana. Available online: https://pubchem.ncbi.nlm.nih.gov/taxonomy/Thamnosma-montana (accessed on 10 April 2024).
- Michael, J.P. Quinoline, Quinazoline and Acridone Alkaloids. Nat. Prod. Rep. 2003, 20, 476–493. [Google Scholar] [CrossRef]
- Wu, T.-S. Alkaloids and Coumarins of Citrus Grandis. Phytochemistry 1988, 27, 3717–3718. [Google Scholar] [CrossRef]
- Pubchem, NIH. Acridone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2015 (accessed on 31 May 2024).
- European Chemicals Agency; 5,12-dihydroquino[2,3-b]acridine-7,14-dione. Available online: https://echa.europa.eu/de/registration-dossier/-/registered-dossier/14755/4/9.
- European Chemicals Agency; 5,12-dihydro-2,9-dimethylquino[2,3-b]acridine-7,14-dione. Available online: https://echa.europa.eu/de/registration-dossier/-/registered-dossier/15097/6/2/1.
- Pubchem, NIH. 2,9-Dimethylquinacridone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/70423 (accessed on 31 May 2024).
- Sander, T. OSIRIS Property Explorer 2017.
- IMPPAT: Indian Medicinal Plants, Phytochemistry And Therapeutics. Available online: https://cb.imsc.res.in/imppat/druglikeproperties/IMPHY005654 (accessed on 10 April 2024).
- Ecological Structure Activity Relationships (ECOSAR) Predictive Model, v2.0, U.S. Environmental Protection Agency. 2017.
- Virtual Models for Property Evaluation of Chemicals within a Global Architecture (VEGA) v1.2.0; Istituto Di Ricerche Farmacologiche Mario Negri, Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS): Milan, Italy, 2021.
- Manabe, K.; Kusabayashi, S.; Yokoyama, M. Long-Life Organic Solar Cell Fabrication Using Quinacridone Pigment. Chem. Lett. 1987, 16, 609–612. [Google Scholar] [CrossRef]
- Tomida, M.; Kusabayashi, S.; Yokoyama, M. Organic Solar Cell Fabrication Using Quinacridone Pigments. Chem. Lett. 1984, 13, 1305–1308. [Google Scholar] [CrossRef]
- Dunst, S.; Karner, E.; Coppola, M.E.; Trimmel, G.; Irimia-Vladu, M. Comparison of the Solution and Vacuum-Processed Quinacridones in Homojunction Photovoltaics. Monatshefte Für Chem. - Chem. Mon. 2017, 148, 863–870. [Google Scholar] [CrossRef]
- Yang, P.; Ma, L.; Bi, S.; Xi, X.; Huang, T.; Liu, R.; Su, Y.; Wu, D. Superior Anodic Lithium Storage Behavior of Organic Pigment 2,9-Dimethylquinacridone. Chem. Eng. J. 2020, 394, 124924. [Google Scholar] [CrossRef]
- Yumusak, C.; Sariciftci, N.S.; Irimia-Vladu, M. Purity of Organic Semiconductors as a Key Factor for the Performance of Organic Electronic Devices. Mater. Chem. Front. 2020, 4, 3678–3689. [Google Scholar] [CrossRef]
- ECHA - Allowed Colorants: Annex IV, Regulation 1223/2009/EC on Cosmetic Products. Available online: https://echa.europa.eu/de/cosmetics-colorant (accessed on 27 April 2024).
- Serup, J.; Hutton Carlsen, K.; Dommershausen, N.; Sepehri, M.; Hesse, B.; Seim, C.; Luch, A.; Schreiver, I. Identification of Pigments Related to Allergic Tattoo Reactions in 104 Human Skin Biopsies. Contact Dermatitis 2020, 82, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hauri, U.; Hohl, C. Photostability and Breakdown Products of Pigments Currently Used in Tattoo Inks. In Current Problems in Dermatology; Serup, J., Kluger, N., Bäumler, W., Eds.; S. Karger AG, 2015; Vol. 48, pp. 164–169 ISBN 978-3-318-02776-1.
- Stratmann, H.; Hellmund, M.; Veith, U.; End, N.; Teubner, W. Indicators for Lack of Systemic Availability of Organic Pigments. Regul. Toxicol. Pharmacol. 2020, 115, 104719. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.E. Quinacridone Pigments. In High Performance Pigments; Smith, H.M., Ed.; Wiley, 2001 ISBN 978-3-527-30204-8.
- Chamberlain, T.R. Quinacridone Pigments. In High Performance Pigments; Faulkner, E.B., Schwartz, R.J., Eds.; Wiley, 2009 ISBN 978-3-527-31405-8.
- Huang, Z.; Sun, H.; Zhang, H.; Wang, Y.; Li, F. π–π interaction of quinacridone derivatives. J. Comput. Chem. 2011, 32, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Wang, J.; Sun, H.; Liu, Y.; Mu, Z.; Li, F.; Jiang, S.; Zhang, J.; Zhang, H.; Wang, Y.; et al. Supramolecular Structures and Assembly and Luminescent Properties of Quinacridone Derivatives. J. Phys. Chem. B 2005, 109, 8008–8016. [Google Scholar] [CrossRef] [PubMed]
- Trixler, F.; Markert, T.; Lackinger, M.; Jamitzky, F.; Heckl, W.M. Supramolecular Self-Assembly Initiated by Solid–Solid Wetting. Chem. – Eur. J. 2007, 13, 7785–7790. [Google Scholar] [CrossRef] [PubMed]
- Federal Institute for Occupational Safety and Health: Activities with Nanomaterials – Technical Rule for Hazardous Substances (TRGS 527), January 2020.
- GESTIS DNEL List: Hazardous substance information system of the German Social Accident Insurance. Available online: https://www.dguv.de/ifa/gestis/gestis-dnel-liste/index.jsp (accessed on 26 April 2024).
- WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; WHO European Centre for Environment and Health: Bonn, Germany, 2021; ISBN 978-92-4-003422-8.
- Chesman, A.S.R.; Liepa, A.J. Some Products from C=O Condensations of Quinacridones. Aust. J. Chem. 2021, 74, 111. [Google Scholar] [CrossRef]
- Analog Identification Methodology (AIM), U.S. EPA, Risk Assessment Division, v1.01. 2013.
- EPA, CompTox Chemicals Dashboard v2.2, C.I. Pigment Red 122, 980-26-7. Available online: https://comptox.epa.gov/dashboard/chemical/similar-molecules/DTXSID2052655 (accessed on 26 April 2024).
- Guevara-Vela, J.M.; Gallegos, M.; Valentín-Rodríguez, M.A.; Costales, A.; Rocha-Rinza, T.; Pendás, Á.M. On the Relationship between Hydrogen Bond Strength and the Formation Energy in Resonance-Assisted Hydrogen Bonds. Molecules 2021, 26, 4196. [Google Scholar] [CrossRef] [PubMed]
- Brühwiler, D.; Bützer, P.; Bützer, M.R. QSAR Data of 2,9-Dimethylquinacridone Tautomers [Data Set]. Zenodo. [CrossRef]
- ACD/ChemSketch, v2.5, Advanced Chemistry Development, Inc. 2015.
- Exposure Assessment Tools and Models. Estimation Program Interface (EPI) v4.1.1; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2019.
- National Toxicology Program (NTP), OPERA: Open Structure Activity Relationship App, U.S. Department of Health and Human Services: Washington, DC, USA, 2021.
- Schreiver, I. Tattoo Pigments: Biodistribution and Toxicity of Corresponding Laser Induced Decomposition Products, PhD Thesis, Freie Universität Berlin, 2018.
- Grosjean, D.; Salmon, L.G.; Cass, G.R. Fading of Organic Artists’ Colorants by Atmospheric Nitric Acid: Reaction Products and Mechanisms. Environ. Sci. Technol. 1992, 26, 952–959. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhou, J.; Wu, S.; Wei, Y.; Chang, A.; Liu, X.; Shangguan, D. DNA Interaction, Cellular Localization and Cytotoxicity of Quinacridone Derivatives. Dyes Pigments 2015, 121, 328–335. [Google Scholar] [CrossRef]
- Gaudron, S.; Ferrier-Le Bouëdec, M.; Franck, F.; D’Incan, M. Azo Pigments and Quinacridones Induce Delayed Hypersensitivity in Red Tattoos. Contact Dermatitis 2015, 72, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Greve, B.; Chytry, R.; Raulin, C. Contact Dermatitis from Red Tattoo Pigment (Quinacridone) with Secondary Spread. Contact Dermatitis 2003, 49, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Pubchem, NIH, ChemIDplus. Cinquasia Red. Available online: https://chem.nlm.nih.gov/chemidplus/rn/1047-16-1 (accessed on 26 April 2024).
- Pubchem, NIH, ChemIDplus. C.I. Pigment Red 122. Available online: https://chem.nlm.nih.gov/chemidplus/rn/980-26-7 (accessed on 26 April 2024).
- National Institute of Technology and Evolution, Tokio, Chemical Evaluation and Research Organization, Combined Repeated Dose and Reproductive/Developmental Toxicity Screening Test of Quino[2,3-b]Acridine-7,14-Dione, 5,12-Dihydro-2,9-Dimethyl- by Oral Administration in Rats. Available online: https://www.nite.go.jp, 45summary_980267_422.pdf (accessed on 13 June 2022).
- Federal Office for the Environment: Swiss Eco-Factors 2021 according to the Ecological Scarcity Method. Available online: https://www.bafu.admin.ch/bafu/de/home/themen/thema-wirtschaft-und-konsum/wirtschaft-und-konsum--publikationen/publikationen-wirtschaft-und-konsum/oekofaktoren-schweiz.html (accessed on 21 April 2023).
- U.S. Environmental Protection Agency EPA’s Safer Choice Standard 2015.
- Arnot, J.A.; Gobas, F.A. A Review of Bioconcentration Factor (BCF) and Bioaccumulation Factor (BAF) Assessments for Organic Chemicals in Aquatic Organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Toropov, A.A.; Toropova, A.P.; Pizzo, F.; Lombardo, A.; Gadaleta, D.; Benfenati, E. CORAL: Model for No Observed Adverse Effect Level (NOAEL). Mol. Divers. 2015, 19, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Dai, W. Aryl Hydrocarbon Receptor: Its Roles in Physiology. Biochem. Pharmacol. 2021, 185, 114428. [Google Scholar] [CrossRef] [PubMed]
- Mosa, F.E.S.; El-Kadi, A.O.S.; Barakat, K. Targeting the Aryl Hydrocarbon Receptor (AhR): A Review of the In-Silico Screening Approaches to Identify AhR Modulators. In High-Throughput Screening for Drug Discovery; Saxena, S.K., Ed.; IntechOpen, 2022 ISBN 978-1-83962-947-1.
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl Hydrocarbon Receptor and Intestinal Immunity. Mucosal Immunol. 2018, 11, 1024–1038. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Jin, U.; Park, H.; Chapkin, R.S.; Jayaraman, A. Aryl Hydrocarbon Receptor (AHR) Ligands as Selective AHR Modulators (SAhRMs). Int. J. Mol. Sci. 2020, 21, 6654. [Google Scholar] [CrossRef] [PubMed]
- Torti, M.F.; Giovannoni, F.; Quintana, F.J.; García, C.C. The Aryl Hydrocarbon Receptor as a Modulator of Anti-Viral Immunity. Front. Immunol. 2021, 12, 624293. [Google Scholar] [CrossRef] [PubMed]
- Barroso, A.; Mahler, J.V.; Fonseca-Castro, P.H.; Quintana, F.J. The Aryl Hydrocarbon Receptor and the Gut–Brain Axis. Cell. Mol. Immunol. 2021, 18, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, C.F.; Bolden, A.L.; Liroff, R.A.; Rochester, J.R.; Vandenbergh, J.G. Twenty-Five Years of Endocrine Disruption Science: Remembering Theo Colborn. Environ. Health Perspect. 2016, 124. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental Endocrine-Disrupting Chemical Exposure: Role in Non-Communicable Diseases. Front. Public Health 2020, 8, 553850. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of Graphene-Family Nanoparticles: A General Review of the Origins and Mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Chortarea, S.; Kuru, O.C.; Netkueakul, W.; Pelin, M.; Keshavan, S.; Song, Z.; Ma, B.; Gómes, J.; Abalos, E.V.; de Luna, L.A.V. Hazard Assessment of Abraded Thermoplastic Composites Reinforced with Reduced Graphene Oxide. J. Hazard. Mater. 2022, 435, 129053. [Google Scholar] [CrossRef] [PubMed]
- Kuempel, E.D.; Sorahan, T. Carbon Black; Identification of Research Needs to Resolve the Carcinogenicity of High-Priority IARC Carcinogens: Views and Expert Opinions of an IARC/NORA Expert Group Meeting. Lyon, France: 30 June – 2 July 2009; International Agency for Research on Cancer; ISBN 978-92-832-2449-5.
- Enengl, S.; Enengl, C.; Pluczyk, S.; Glowacki, E.D.; Lapkowski, M.; Ehrenfreund, E.; Neugebauer, H.; Sariciftci, N.S. Spectroscopic Characterization of Charge Carriers of the Organic Semiconductor Quinacridone Compared with Pentacene during Redox Reactions. J. Mater. Chem. C 2016, 4, 10265–10278. [Google Scholar] [CrossRef]
- Głowacki, E.D.; Leonat, L.; Irimia-Vladu, M.; Schwödiauer, R.; Ullah, M.; Sitter, H.; Bauer, S.; Sariciftci, N.S. Intermolecular Hydrogen-Bonded Organic Semiconductors—Quinacridone versus Pentacene. Appl. Phys. Lett. 2012, 101, 023305. [Google Scholar] [CrossRef]
- De Feyter, S.; Gesquière, A.; De Schryver, F.C.; Keller, U.; Müllen, K. Aggregation Properties of Soluble Quinacridones in Two and Three Dimensions. Chem. Mater. 2002, 14, 989–997. [Google Scholar] [CrossRef]
- Meyer, G.; Matthäi, M.; Auge, J.; Lindow, H. Crystallisation Processes and Hardness of Paraffin Waxes Characterised by DSC, Ultrasonic, X-Ray and Needle Penetration Measurements. SOFW - Int. J. Appl. Sci. 2005, 8, 51–58. [Google Scholar]
- He, Z.; Zhuo, Y.; Zhang, Z.; He, J. Design of Icephobic Surfaces by Lowering Ice Adhesion Strength: A Mini Review. Coatings 2021, 11, 1343. [Google Scholar] [CrossRef]
- Bützer, P.; Bützer, M.R. Is a Sliding Layer Formed When Gliding on Ice or Snow? A Chronological Overview – as Time-Specific Knowledge. Part I. Gliding 2022, 22–36. [Google Scholar]
- Bützer, P.; Bützer, M.R. Is a Sliding Layer Formed When Gliding on Ice or Snow? A Chronological Overview – as Time-Specific Knowledge. Part II. Gliding 2023, 10–42. [Google Scholar]
- Li, J.; Zhang, M.; Ge, Y.; Wen, Y.; Luo, J.; Yin, D.; Wang, C.; Wang, C. Emission Characteristics of Tyre Wear Particles from Light-Duty Vehicles. Atmosphere 2023, 14, 724. [Google Scholar] [CrossRef]
 |
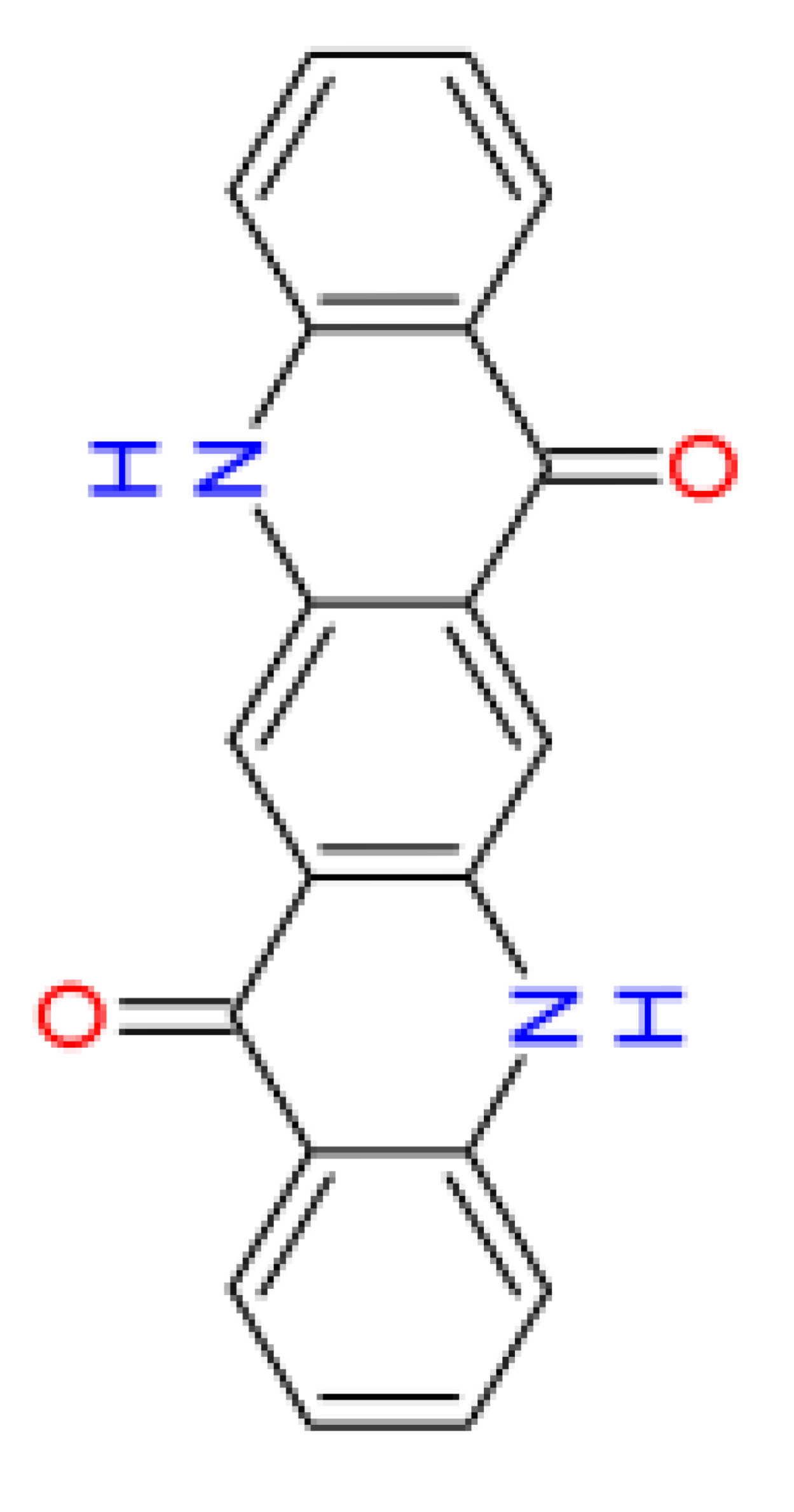 |
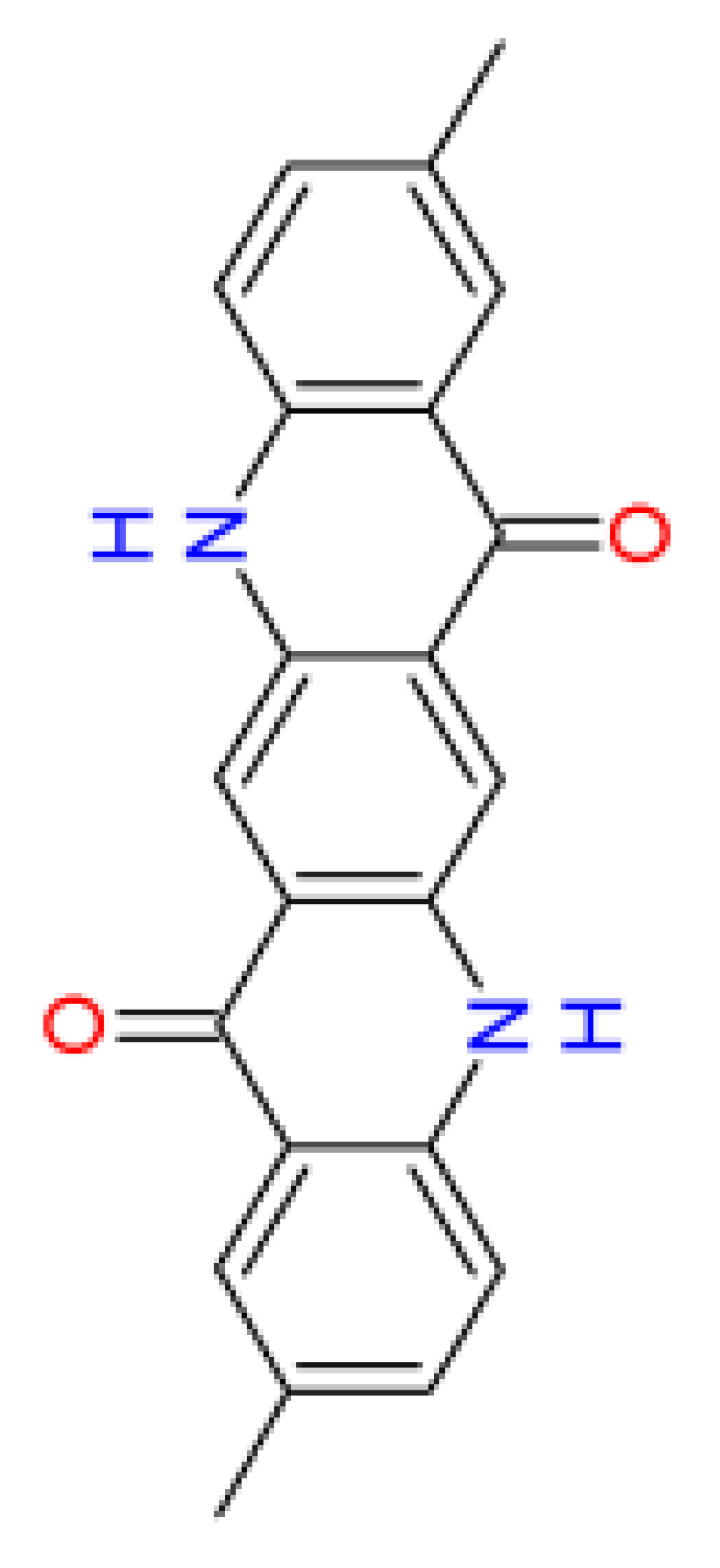 |
|
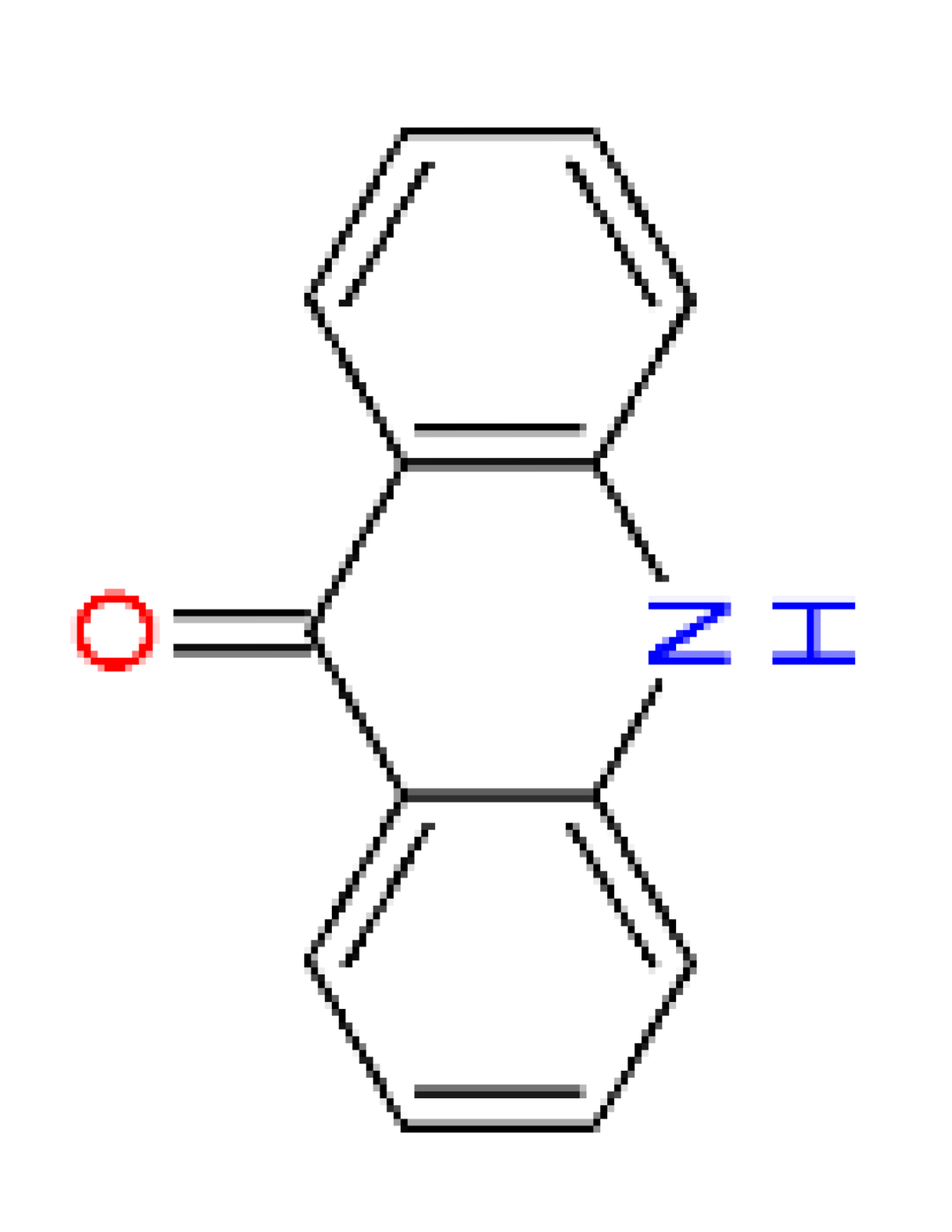
| Name | acridone [18] | quinacridone | 2,9-dimethyl- quinacridone |
| Abbreviation | – | QA | DQA |
| CAS No. | 578-95-0 | 1047-16-1 | 980-26-7 |
| Origin | biogenic [19,20,21] |
synthetic | synthetic |
| Water solubility [mg/L] (24 °C) | 0.0047 [22] | 0.0103 [23] | 0.0056 [24] |
| log(Kow) gm7 | 2.6 | 3.1 | 4.0 |
| Bioconcentration factor (BCF) gm7 | 20.5 | 14.8 | 26.7 |
| log(Koc) gm3 | 3.3 | 5.0 | 5.1 |
| Green algae EC50 (96 h) [mg/L] a | 112 | 293 | 55.8 |
| Algae chronic (NOEC) [mg/L] b | 0.057 | 0.027 | 0.025 |
| DM acute EC50 [mg/L] b,gm2 | 0.35 | 0.55 | 0.56 |
| DM LC50 (48 h) [mg/L] b,gm2 | 0.97 | 1.33 | 1.12 |
| DM chronic (NOEC) [mg/L] b | 0.42 | 1.27 | 1.10 |
| Fish acute LC50 [mg/L] b | 10 – 100 | 1 – 10 | 1 – 10 |
| Fish chronic (NOEC) [mg/L] b | 0.071 | 0.039 | 0.028 |
| Sludge EC50 [mg/L] b | 18.0 | 26.3 | 28.9 |
| Earthworm LC50 (14 d) [mg/L] a | 366 | 630 | 529 |
| Bee toxicity [µg/bee] b | >100 | >100 | >100 |
| LD50 (rat, oral) [mg/kg] b; (experimental rat [mg/kg]) [25] |
2402 | 3352 (>20) |
2633 (>23) |
| Endocrine disruptor activity b | inactive | inactive | inactive |
| Total body elimination half-life [h] b | 5.2 | 8.4 | 10.8 |
| NOAEL [mg/kg bw] | 4.9 | 6.3 | 10.9 |
| Druglikeness [26,27] | 0.78 | 0.79 | – 1.03 |
| Drug-Score [26] | 0.39 | 0.14 | 0.08 |
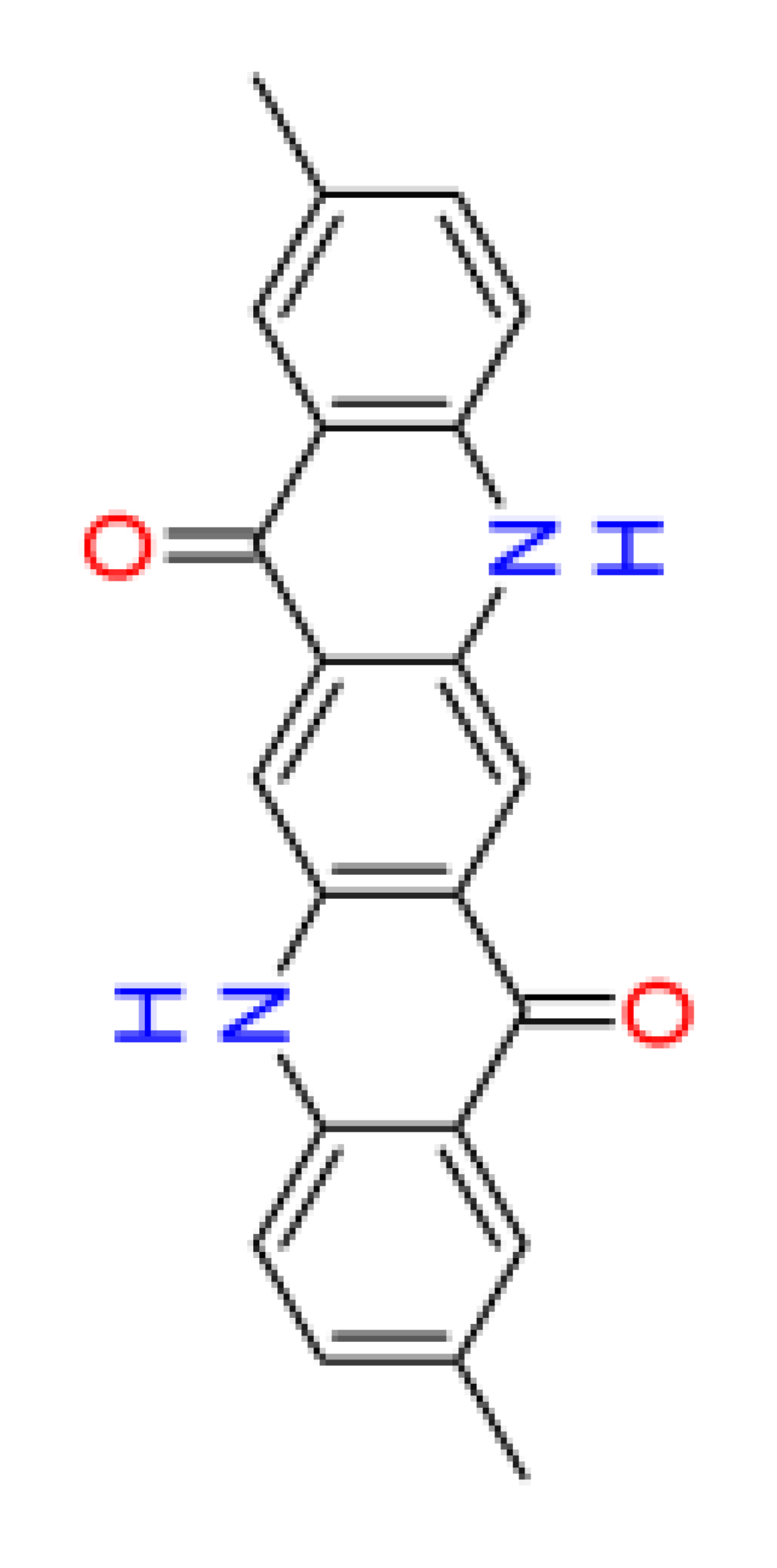 |
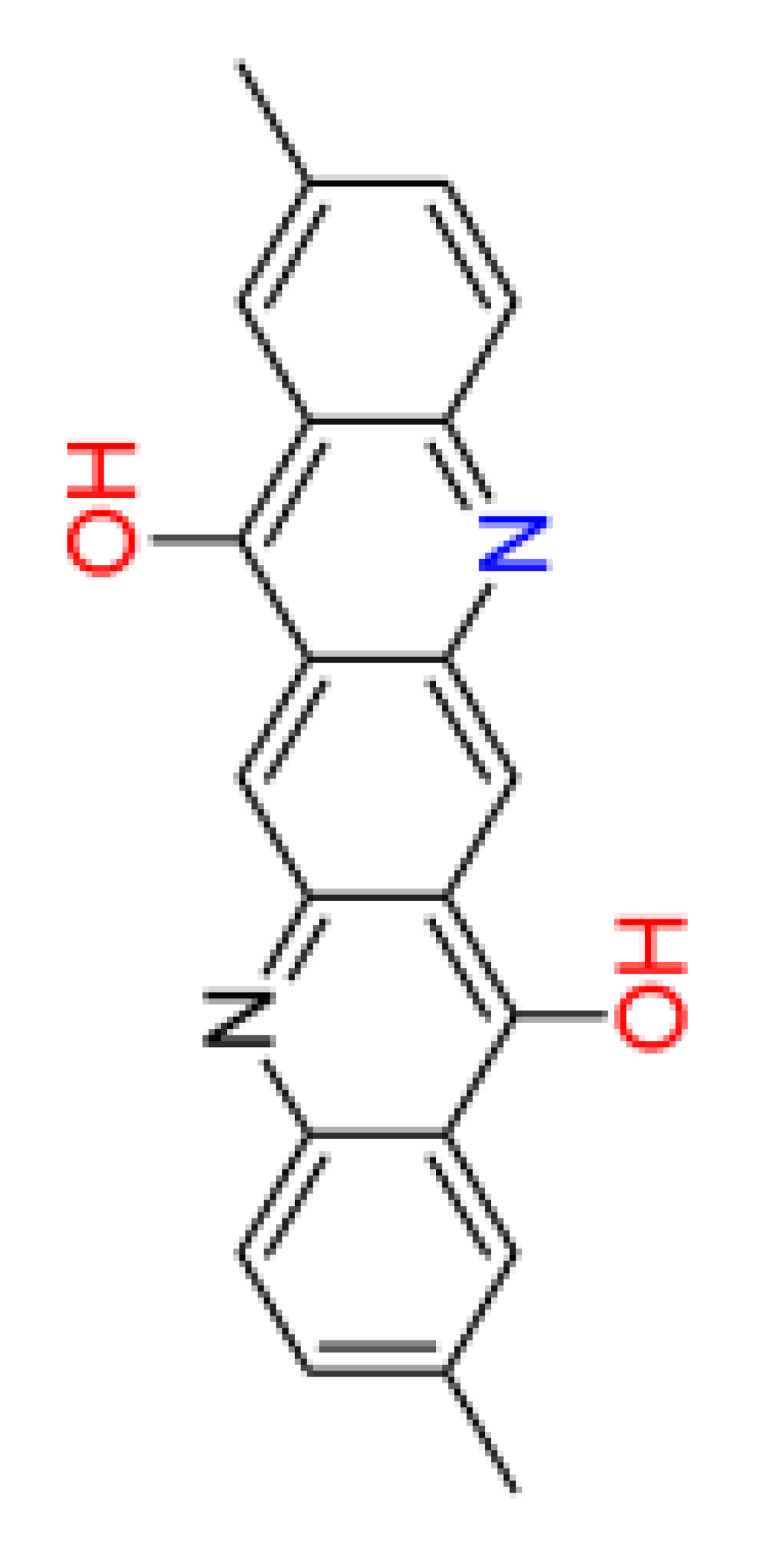 |
 |
 |
 |
|
| Number of aromatic atoms d | 12 | 22 | 20 | 14 | 12 |
| sp3sp2 hybridization ratio d | 0.136 | 0.0909 | 0.0909 | 0.136 | 0.136 |
| Density [g/cm3] a | 1.31 ± 0.06 | 1.41 ± 0.06 | 1.36 ± 0.06 | 1.38 ± 0.1 | 1.38 ± 0.1 |
| Molar volume [cm3] a | 260.3 ± 3.0 | 241.7 ± 3.0 | 251.0 ± 3.0 | 246.3 ± 7.0 | 246.3 ± 7.0 |
| Polarizability [10–24 cm3] a | 38.82 ± 0.5 | 42.50 ± 0.5 | 40.66 ± 0.5 | 39.10 ± 0.5 | 39.10 ± 0.5 |
| Surface tension [dyne/cm] a | 54.3 ± 3.0 | 77.0 ± 3.0 | 65.7 ± 3.0 | 53.3 ± 7.0 | 53.3 ± 7.0 |
| Total polar surface area e | 58.2 | 66.2 | 62.2 | 62.0 | 58.3 |
| pKa d | 6.4 | 5.1 | 8.3 | 5.4 | 6.4 |
| log(Kow) b | 4.1 | 4.8 | 3.6 | 4.7 | 4.1 |
| log(BCF) d | 1.33 | 1.76 | 1.53 | 1.69 | 1.33 |
| Water solubility [mg/L] b | 2.2 | 0.046 | 0.44 | 0.056 | 2.2 |
| NOAEL [mg/kg bw] c | 20.6 | 26.8 | 11.0 | 28.3 | 20.6 |
| Acute toxicity (LD50) [mg/kg bw] d | 2244 | 2747 | 3170 | 623 | 2244 |
| Persistence (sediment) [days] c | 229.1 | 70.8 | 49.0 | 229.1 | 229.1 |
| Persistence (soil) [days] c | 33.9 | 22.9 | 4.9 | 33.9 | 33.9 |
| Persistence (water) [days] c | 26.3 | 4.2 | 3.9 | 22.4 | 26.3 |
| Sewage treatment plant total removal b | 34.83 % | 69.53 % | 16.34 % | 65.05 % | 34.83 % |
| Sludge (EC50) [mg/L] c | 31.13 | 23.92 | 32.01 | 35.09 | 31.13 |
| Estrogen Receptor-mediated effect c | NON active (all tautomers) | ||||
| Estrogen Receptor activity, binding d,f | 0 (all tautomers) | ||||
| Estrogen Receptor activity, agonist d,f | 0 (all tautomers) | ||||
| Estrogen Receptor activity, antagonist d,f | 0 (all tautomers) | ||||
| Androgen Receptor-mediated effect c | NON active (all tautomers) | ||||
| Androgen Receptor activity, binding d,f | 1 (all tautomers) | ||||
| Androgen Receptor activity, agonist d,f | 0 (all tautomers) | ||||
| Androgen Receptor activity, antagonist d,f | 1 (all tautomers) | ||||
| Thyroid Receptor alpha effect c | Inactive (all tautomers) | ||||
| Thyroid Receptor beta effect c | Inactive (all tautomers) | ||||
| Endocrine disruptor activity screening c | Inactive (all tautomers) | ||||
| Conditions | Test 1 | Test 2 |
|---|---|---|
| Location | Hoch-Ybrig, Switzerland | St. Moritz, Switzerland |
| Date | January 8, 2021 | January 20, 2021 |
| Snow temperature [°C] | –8.5 | –7 |
| Air temperature [°C] | –8 | –2 |
| Air humidity [%] | 83 | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





