1. Introduction
In medico-legal entomology, the duration of insect colonization on human or animal remains at a crime scene provides critical insights for accurately estimating the postmortem interval (PMI). When conditions facilitate insect access to a carcass, insects typically begin colonization during the initial stages of decomposition. This process allows for the precise estimation of the Time of Colonization (TOC) or the minimum Postmortem Interval (mPMI) [
1,
2]. Medico-legal entomologists estimate the mPMI by analyzing larval growth duration as an indicator of the time since death [
3,
4,
5] or by examining the succession patterns of insects during different stages of decomposition [
6,
7]. Additionally, they strive to understand the decomposition process through changes in the weight and physical characteristics of the remains [
8,
9].
The blowfly (Diptera: Calliphoridae) plays a significant role as a primary decomposer of carcasses post-mortem, contributing to the ecological processes of decay [
10,
11,
12,
13]. Research into the community composition and succession of blowflies can provide valuable insights into the timing and environmental conditions of colonization events, enhancing the accuracy of forensic analyses [
14,
15]. Blowfly species diversity and distribution are influenced by various environmental factors such as temperature, habitat, and geographical location, which in turn affect their behavior and colonization patterns [
16,
17,
18,
19]. The behavior of blowflies, including their approach patterns to carcasses, is another critical aspect of medico-legal entomology. Seasonal variations and environmental conditions significantly influence these behaviors, affecting the timing and nature of colonization [
20,
21,
22].
Localized studies have shown that regional variations significantly impact blowfly population dynamics, highlighting the importance of region-specific data in medico-legal entomology [
15,
23]. Detailed studies on the ecological and biological aspects of blowfly activity can provide supplementary evidence in forensic cases, offering insights into the circumstances surrounding the discovery of remains [
24]. This is particularly relevant in regions like South Korea, where distinct seasonal and geographical factors can influence insect activity. While global databases like the Global Biodiversity Information Facility (GBIF) and the Korean Natural History Research Information System (NARIS) provide valuable biodiversity data, they often lack the regional and seasonal specificity needed for forensic applications [
14,
18]. Therefore, there is a pressing need for comprehensive, quantitative data on blowfly species diversity and distribution across well-defined regions and time frames to effectively support forensic investigations.
Globally, forensic research into the distribution and diversity of blowfly species is currently being conducted, with significant contributions from various countries including the United States [
15,
19,
23], Canada [
25,
26], Colombia [
27], Argentina [
28], Switzerland [
29,
30], Germany[
17,
31], Spain [
32], and Japan [
33]. Despite these extensive efforts, research focusing on the forensically significant blowfly species in South Korea remains sparse, with a notable lack of comprehensive regional coverage. Thus, detailed investigations into the blowfly populations across various South Korean regions are required. In this study, we aim to explore the spatio-temporal distribution of forensically relevant blowflies in Gyeongsangnam-do, South Korea. Given the known impact of environmental factors such as temperature, habitat, and geographical location on blowfly species distribution and diversity, this research posits the hypothesis that community composition, species abundance, and diversity will differ across different regions, seasons, and habitats.
2. Materials and Methods
2.1. Site Description
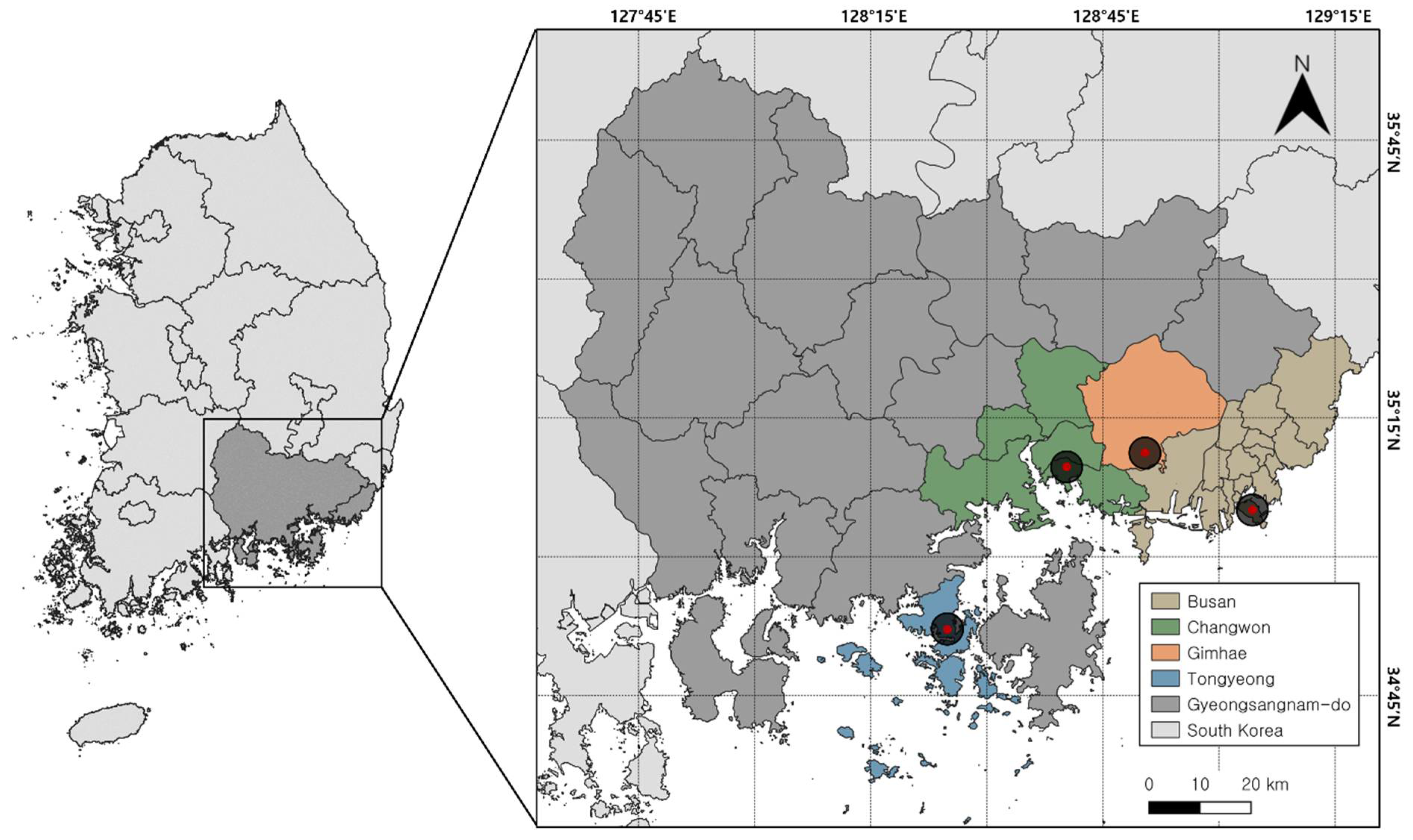
This study encompasses a comprehensive survey conducted over the span of one year within four distinct regions of Gyeongsangnam-do, South Korea: Busan, Changwon, Gimhae, and Tongyeong (
Figure 1). Each region was selected based on its unique geographical characteristics, as delineated below:
Busan: This region is characterized by its mountainous terrain, is bordered by the sea, and includes islands along the eastern and southern coasts, conferring a diverse ecosystem.
Changwon: Distinguished by a mountainous valley landscape, Changwon offers a unique combination of natural topographical features.
Gimhae: This region is notable for its proximity to the South Sea and several rivers, providing a distinctive aquatic and terrestrial interface.
Tongyeong: Tongyeong is a peninsula known for its ria coast and its landscape is marked by intricate coastlines and varied maritime habitats.
To investigate the difference in species composition and abundance of blowflies based on habitat types within the study regions, the trap deployment sites were classified into two types: urban and forest. Urban habitats were defined by densely populated areas featuring man-made structures, such as roadsides or residential areas. Conversely, the forest habitats were characterized by sparsely populated areas, typically located at the base of mountains or within wooded terrains, devoid of man-made structures. The precise location of each trap was documented using GPS coordinates to ensure accuracy in data collection. The spatial distribution of the selected regions ranged from a minimum distance of approximately 15.3 km to a maximum distance of approximately 64.4 km, highlighting the study's geographical breadth.
2.2. Sampling Procedure
Bait traps, recognized for their efficacy in capturing the broad characteristics of seasonal and regional variations in blowfly populations, were employed as the primary collection method [
34]. The survey was conducted twice a month in each region from May 2022 to May 2023. For each sampling event, a total of 10 bait traps were deployed: five in the urban habitats and five in the forest habitats. These traps were placed for a duration of 48 h, with an inter-trap distance of approximately 20-50 m to ensure spatial independence. Thus, a total of 960 traps were deployed over the study period.
The design of the bait trap consisted of a conical structure equipped with a hook at the apex, suspended 1.5–2 m above ground level. This elevation strategy was adopted to restrict access to ants and other non-target ground insects [
34]. As an attractant, thawed fresh small mouse carcasses weighing between 20-30 g were employed. These carcasses were brought in vacuum packed at room temperature before use in the traps.
Given the observed decrease in fly activity during periods of rainfall [
11,
17,
24], efforts were made to schedule trap deployment during dry periods to avoid dilution of the attractant's scent by rainwater, which may impede blowfly attraction. Upon collection, the blowflies were euthanized using insecticide and preserved in 70% ethanol for subsequent analysis. Morphological identification of all adult specimens at the species level was conducted using the taxonomic keys provided by Ji [
35] and Kanō and Shinonaga [
36].
2.3. Data Analysis
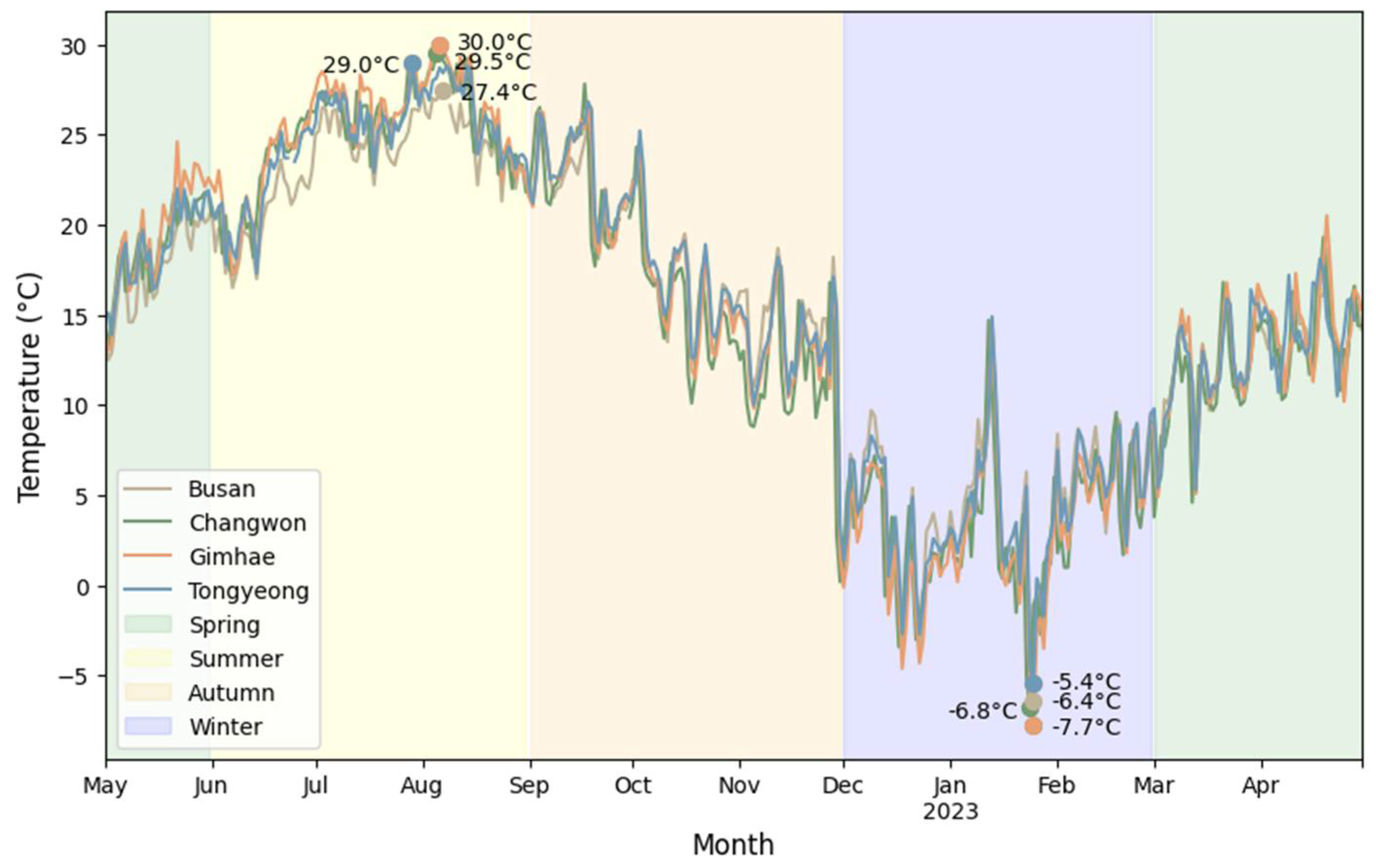
Blowfly community analysis was segmented by region, season, and habitat, with seasonal divisions according to the climatic patterns of Gyeongsangnam-do, South Korea, as delineated by the official weather website for spring (March to May), summer (June to August), autumn (September to November), and winter (December to February) (
Figure 2.) [
37]. To elucidate the diversity of blowfly species in each season and region, metrics such as relative abundance, species diversity determined via the Shannon-Wiener index (H'), and species richness calculated through the Margalef index (R) were computed.
To reveal differences in temperature across regions and seasons, a one-way analysis of variance (ANOVA) was performed, followed by a Tukey's HSD test as a post-hoc test. The average daily temperature was obtained from the Korea Meteorological Administration and was calculated from weather stations up to 10 km from the survey site.
One-way ANOVA was employed to identify regional and seasonal disparities in species diversity and richness. Statistically significant differences were analyzed using post-hoc analysis with Tukey’s HSD test. Furthermore, the Kruskal-Wallis test was utilized to evaluate differences in relative abundance across seasons for the nine species that constituted more than 1% of the total community captured using the traps. Subsequent to significant Kruskal-Wallis test results, the Bonferroni correction was used to adjust the P-values obtained from Dunn's test. Winter was excluded from the statistical analysis due to the zero variance of the data.
The temporal dynamics of species abundance were visualized through a heatmap generated via two-way hierarchical clustering analysis, employing the unweighted pair group method with arithmetic mean (UPGMA) algorithm and Bray-Curtis similarity. Each month throughout the survey period was plotted along the X-axis, while the nine predominant blowfly species were presented along the Y-axis.
Species diversity in different habitats was assessed using the Shannon-Wiener index (H') and Margalef index (R), employing the same procedures as previously mentioned. Habitat preferences of the dominant species, representing more than 1% of the total community, were examined using a chi-square test of the trap data acquired throughout the study. To provide a more comprehensive view of the distribution patterns of blowflies, the relative abundances of environmental parameters (region and season, habitat) were analyzed using principal component analysis (PCA).
For all statistical tests, the Shapiro-Wilk test was applied to assess data normality, and a P-value threshold of <0.05 was considered significant. Data analysis was performed in SPSS (version 23.0) [
38] and Python (version 3.12.0) along with the pandas (version 2.1.3), numpy (version 1.26.1), scipy (version 1.11.3), matplotlib (version 3.8.1), and seaborn (version 0.13.0) libraries.
3. Results
3.1. Seasonal and Regional Variations in Blowfly Abundances and Diversity
Throughout the investigation period, a total of 3,478 adult blowflies, encompassing 14 species across six genera were collected (
Table 1). Notably, no specimens were gathered during the winter season.
Lucilia porphyrina emerged as the most prevalent species, with 1,291 individuals accounting for 37.1% of the total collection, followed by
Chrysomya pinguis (956 individuals representing 27.5%),
Lucilia sericata (263 individuals, 7.6%), and
Lucilia illustris (247 individuals, 7.1%).
3.1.1. Analysis of Temperature Differences
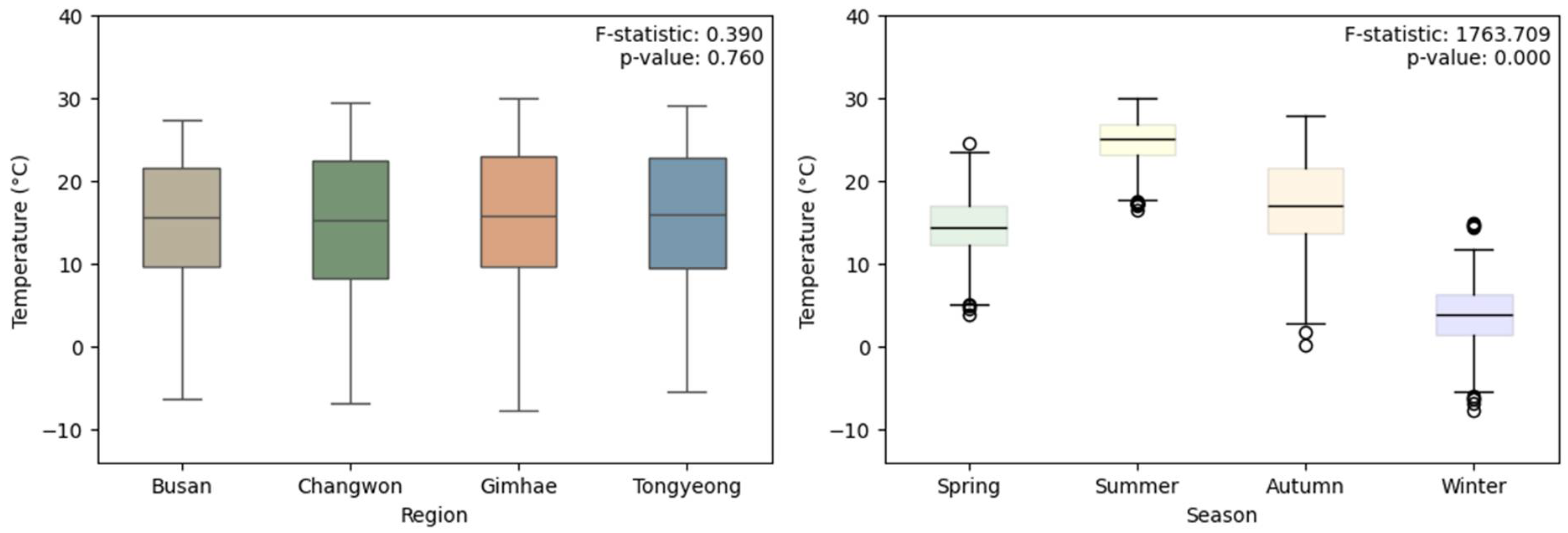
The analysis of temperature differences between regions and seasons showed that there was no significant difference in the mean temperature between regions (F=0.390, p=0.760), but there was a significant difference in the mean temperature between seasons (F=1763.709, P<0.001); moreover, a significant difference in the mean temperature between different seasons was found in all four seasons (P<0.001) (
Figure 3.).
3.1.2. Analysis of Blowfly Species Relative Abundance
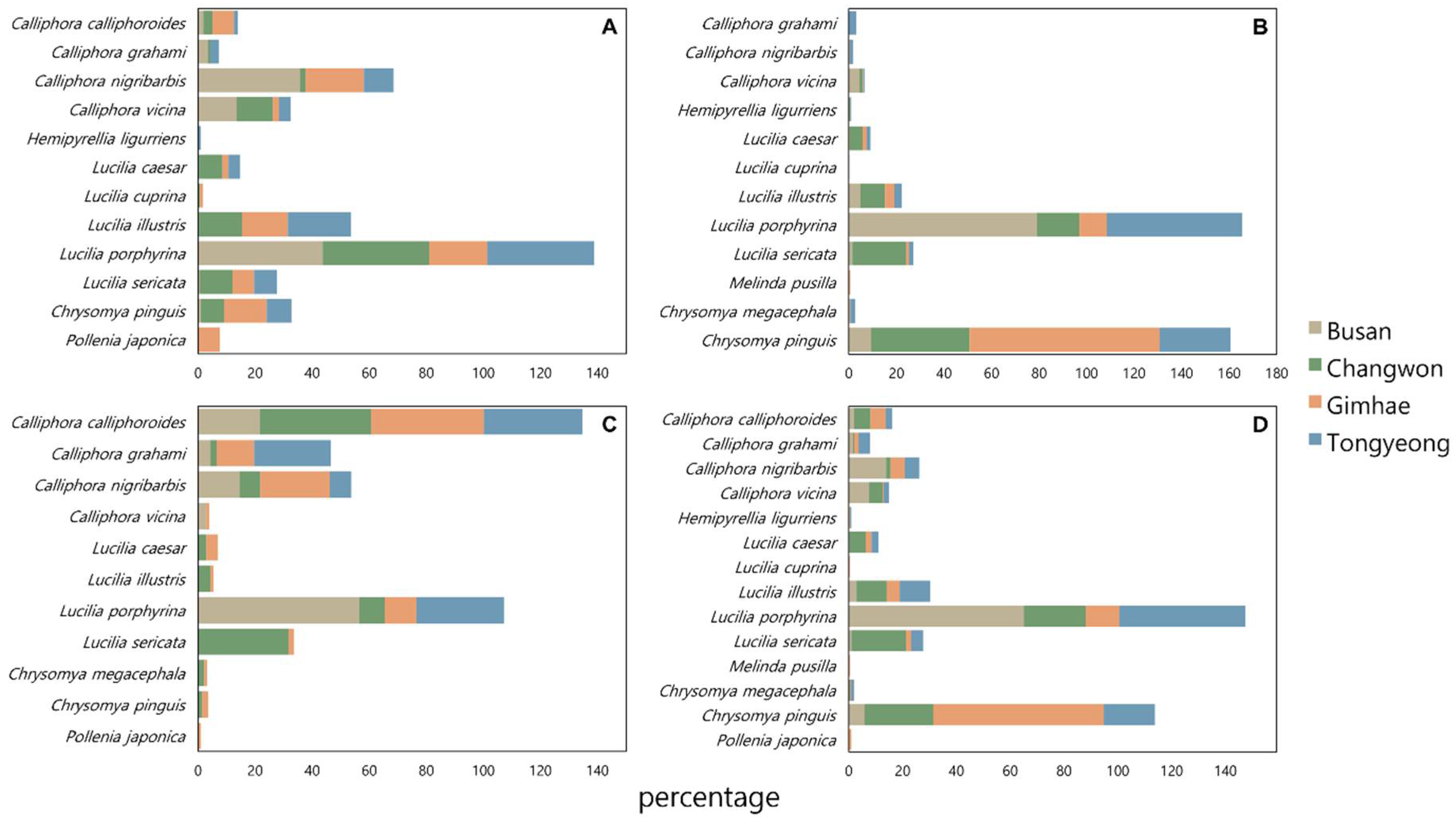
Analysis of the relative abundance of blowfly species across different regions and seasons revealed significant seasonal variations in species distribution (
Figure 4). In the spring,
Calliphora nigribarbis and
L. porphyrina exhibited the highest abundance in Gimhae, constituting 20.4 % of all blowflies, whereas only
L. porphyrina was predominantly abundant in Busan, Changwon, and Tongyeong, with proportions of 43.7%, 37.4%, and 37.4%, respectively. During the summer months,
Ch. pinguis exhibited the highest abundance in Gimhae and Changwon at 80.0% and 41.5%, respectively, while
L. porphyrina was most prevalent in Busan and Tongyeong, accounting for 79.0% and 56.0%, respectively. In autumn,
Calliphora calliphoroides was notably abundant in Gimhae, Changwon, and Tongyeong at 39.4%, 39.0%, and 34.6%, respectively, with
L. porphyrina maintaining the highest abundance in Busan at 56.5%.
3.1.3. Analysis of Species Diversity
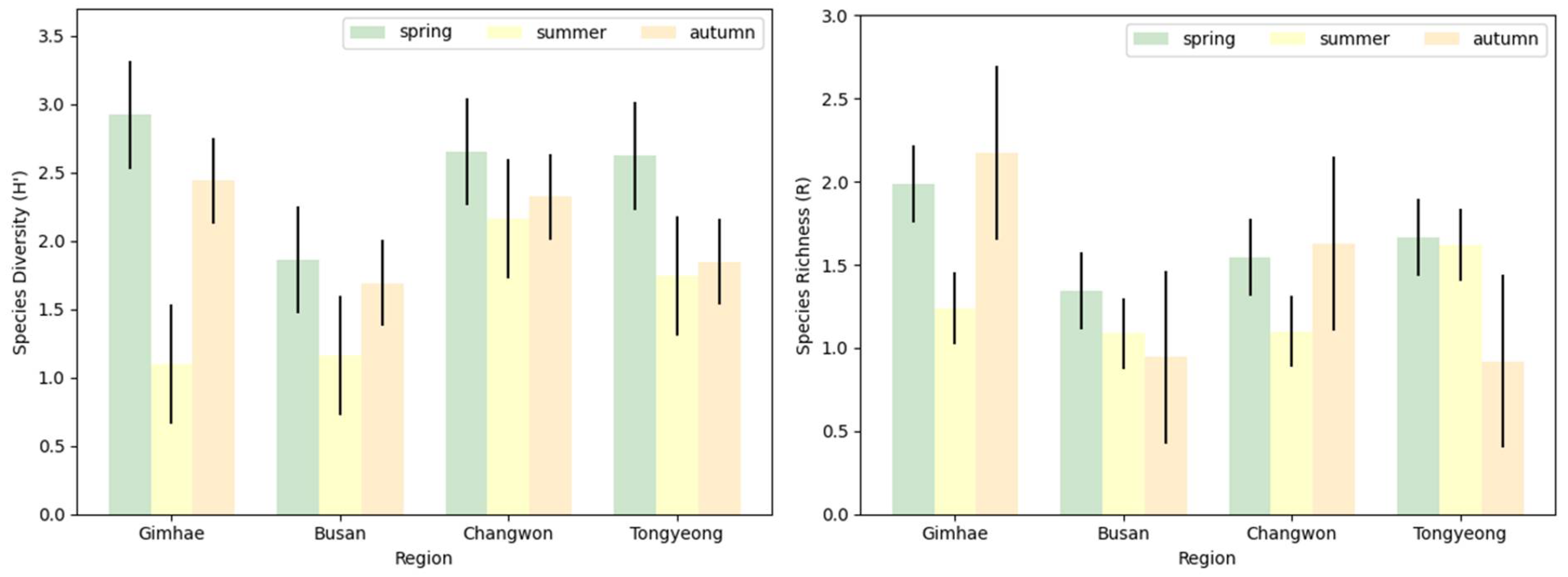
Although, statistical analysis of species diversity indicated no significant differences between regions (F=1.055, P=0.420), a notable variation between seasons (F=4.805, P=0.038) was observed (
Figure 5). Species diversity peaked in spring and was lowest in summer. Species diversity in autumn did not significantly differ from that in spring (P=0.382) or summer (P=0.258); however, there was a significant disparity in species diversity between spring and summer (P=0.031). Species richness showed no significant variation among regions (F=1.705, P=0.243) or seasons (F=0.851, P=0.459).
3.2. Seasonal Differences in Blowfly Abundance
The Kruskal-Wallis test was applied to examine the seasonal relative abundances of the nine predominant captured blowfly species that constituted more than 1% of the total community (
Table 2). The results indicated significant seasonal variations in the abundances of four species. The abundance of
C. calliphoroides,
Calliphora grahami and
Ch. pinguis exhibited significant seasonal differences between summer and autumn (P=0.004, P=0.043 and P=0.013, respectively). The abundances of
C. nigribarbis displayed notable seasonal differences between spring and summer (P=0.042).
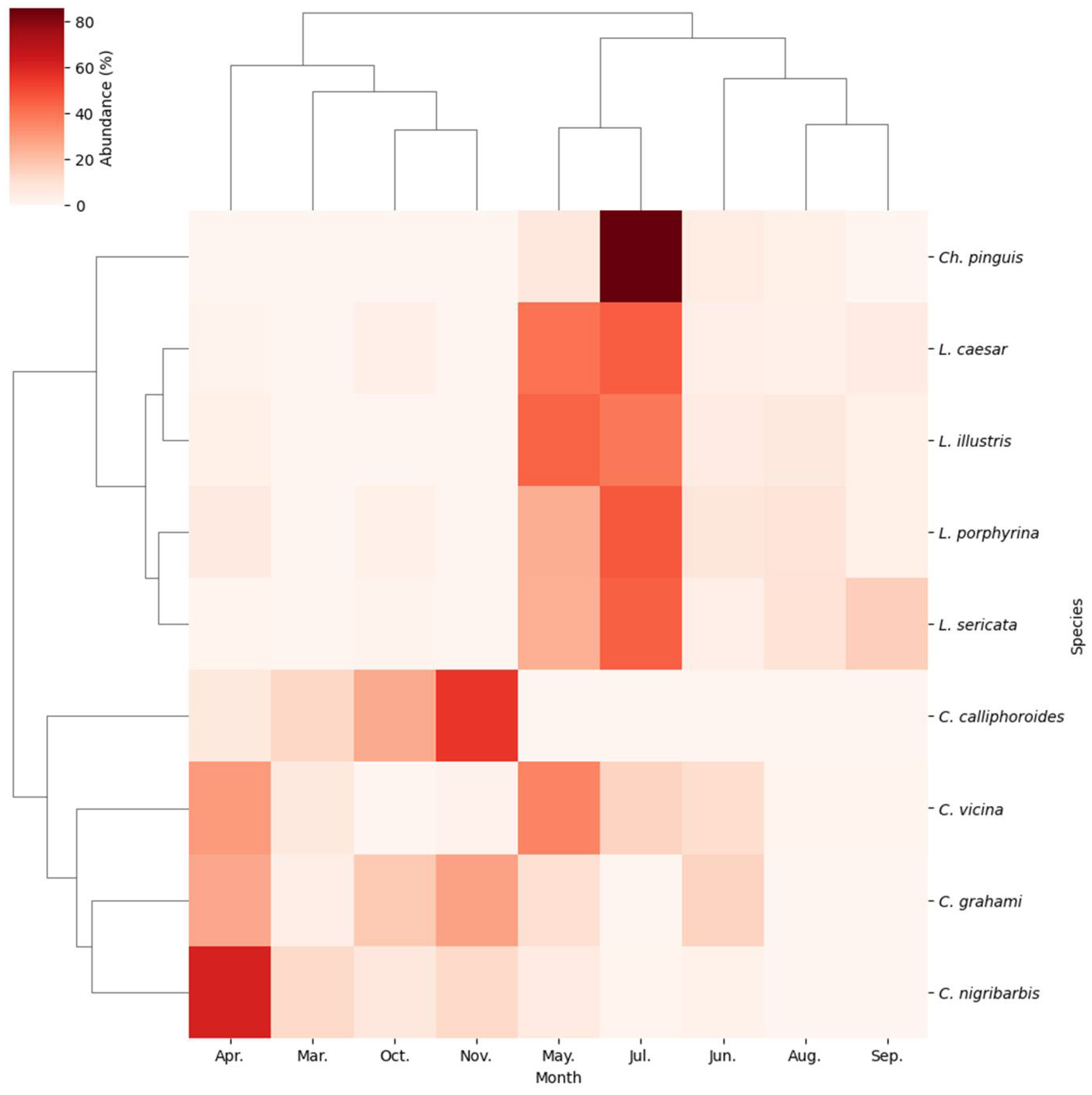
Two-way hierarchical clustering and heatmap analyses of the community composition of blowflies revealed distinct monthly correlations among the nine species (
Figure 6). At the genus level, the clustering resulted in a discernible separation into three groups: one comprising four
Calliphora species, one
Chrysomya, and four
Lucilia. Monthly clustering identified that March, April, October, and November, typically the early spring and late autumn months, formed distinct subgroups, while species in May through September clustered into another subgroup, indicating seasonal influences on community composition.
3.3. Habitat Preferences and Environmental Influences on Blowfly Species Distribution
The investigation into species diversity and richness across different habitats revealed that, in urban habitats, species diversity was 2.895, but was 2.019 in forest habitats, indicating a higher diversity in urban environments. Conversely, species richness presented similar values between habitats (1.153 in urban habitats and 1.027 in forest settings). These results suggest a distinction in habitat suitability for various blowfly species.
Further analysis identified specific habitat preferences among the blowfly species (
Table 3). Five species demonstrated a preference towards urban habitats, with
C. calliphoroides and
L. sericata showing a significantly higher preference than that of other species (P<0.001). Additionally,
Calliphora vicina favored urban settings, albeit without statistical significance. Conversely, three species exhibited a preference for forest habitats, with
L. porphyrina and
Ch. pinguis displaying a marked inclination towards these environments (P<0.001).
Calliphora nigribarbis did not exhibit a clear preference for either habitat type.
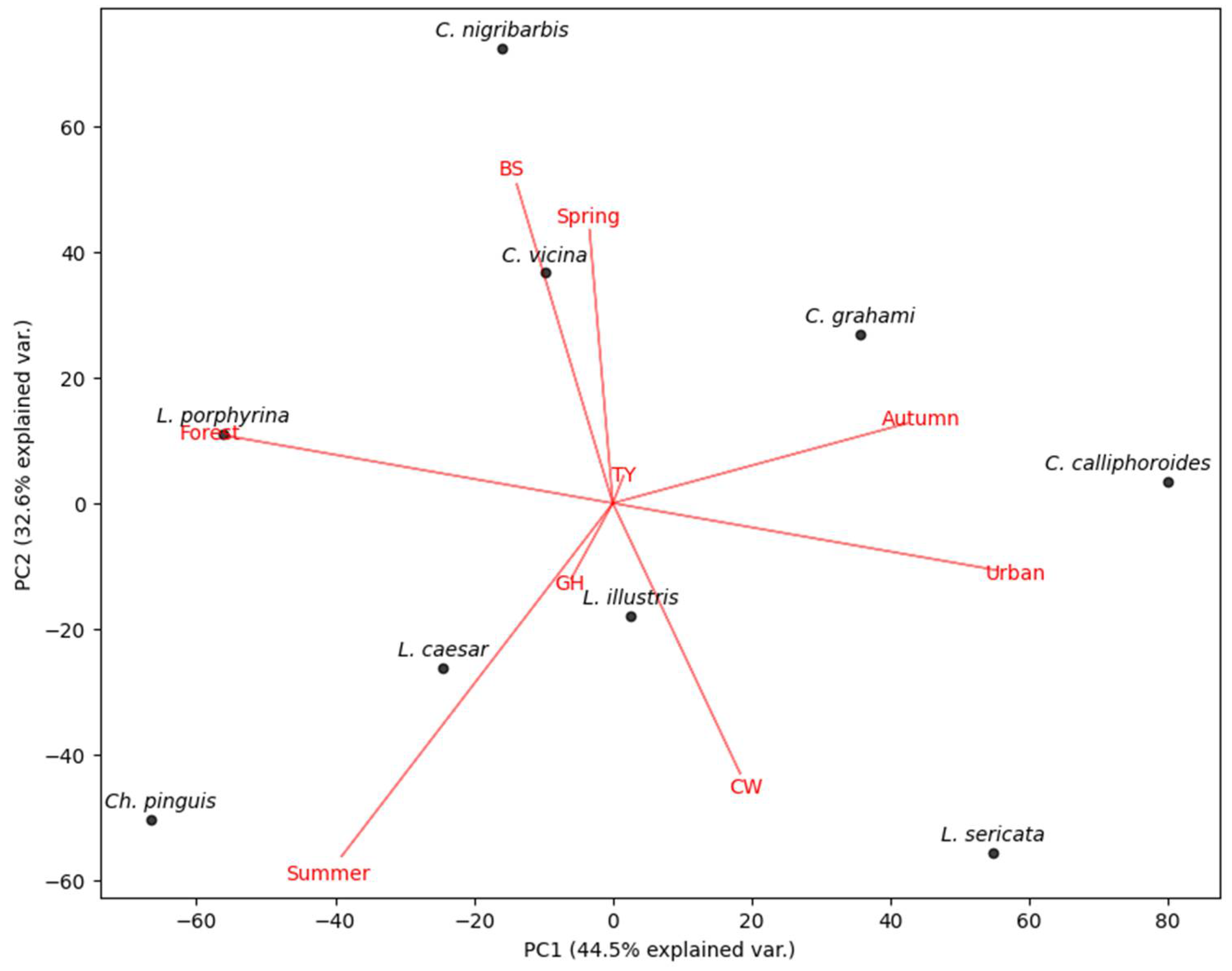
The distribution of blowflies is clearly reflected in the PCA, which is related to environmental influences such as season and habitat (
Figure 7). The PCA (PC1 and PC2 accounting for 77.1% of the variation) showed that spring and autumn contrasted clearly with summer. Species belonging to the genus Calliphora were found to be associated with spring and autumn, while
Ch. pinguis,
Lucilia caesar, and
L. illustris were generally associated with summer. The vectors for Gimhae and Tongyeong were found to have no significant explanatory power compared to the other vectors.
4. Discussion
This research presents the first comprehensive analysis of blowfly communities within the Gyeongsangnam-do region. This study successfully identified 14 distinct blowfly species, with L. porphyrina (37.1%), Ch. pinguis (27.5%), L. sericata (7.6%), and L. illustris (7.1%) emerging as the predominant species. Notably, the composition of the blowfly community did not exhibit significant variations between different sites within the region. However, seasonal distinctions were apparent, with community composition differing between every season apart from winter. This seasonal variation in community composition underscores the influence of climatic factors on blowfly activity and diversity. Regarding climatic factors, no significant differences were found because of analysis of variance on the annual average daily temperature of the four study regions. This suggests that the cause affecting the distribution of blowflies in the region is not the difference in daily average temperature. However, significant differences in average temperature between seasons were confirmed. This supports the findings of this study that the seasonal division of the four regions was appropriately differentiated according to daily average temperature, and that the distribution of blowflies is more influenced by seasonal factors governing different environmental conditions.
We analyzed the distribution of blowflies by considering both species diversity and species richness to better understand their response to ecological and environmental changes in the study area. In all regions, species diversity decreased during the summer, which may be due to the dominance of L. porphyrina and Ch. pinguis, which accounted for a significant proportion of the total individuals collected during this period. Such a high prevalence of these two species in the summer suggests they have a possible competitive advantage or preference for the environmental conditions present during this season.
4.1. Ecological Niches and Seasonal Distribution of Forensic Blowflies
Our findings reveal a distinct season-dependent prevalence of the genus
Calliphora, which was more abundant in spring and autumn than in summer. Specifically,
C. calliphoroides (also known as
Triceratopyga calliphoroides) was absent during summer and winter, and its highest relative abundance occurred in autumn. This pattern underscores its pronounced seasonal preference. The genus
Calliphora is typically found colonizing remains in significant numbers during the cool season, such as autumn, winter, and spring, with the exact timing varying by geographic location [
16,
19,
26,
39]. In Korea,
Calliphora species have been frequently observed on carcasses in spring and autumn [
7]. Forensic autopsy data obtained in Korea corroborated our findings, with species such as
C. calliphoroides,
C. grahami,
C. nigribarbis, and
C. vicina predominantly identified in these seasons [
40], highlighting their forensic significance in determining the mPMI.
The genus
Lucilia was present in spring, summer, and autumn, with the most abundant during the summer.
Lucilia porphyrina, the dominant species throughout Gyeongsangnam-do, exhibited limited abundance in metropolitan autopsies [
40]. Notably,
L. porphyrina thrives in Southeast Asia and tropical and subtropical mountainous regions with higher altitudes [
41], suggesting that geographical and latitudinal differences may influence its distribution. Other
Lucilia species mirrored the seasonal pattern of
L. porphyrina, with
L. sericata being particularly prevalent in domestic autopsies, underscoring its forensic importance in these cases [
40].
Within the genus
Chrysomya, both
Ch. pinguis and
Ch. megacephala were collected but their abundances varied significantly.
Chrysomya pinguis emerged as the second most abundant species, exhibiting a strong association with summer. Although
Ch. megacephala is globally recognized as forensically significant [
42], it constituted only 0.4% of the total population in this study.
Chrysomya pinguis has a notable presence across various Asian countries [
43,
44,
45] and is a key forensic species in Korea that is frequently found on carcasses and in autopsies across spring, summer, and autumn [
6,
7,
40,
46], which is further supported by its high abundance in this study. The differential abundances of
Ch. pinguis and
Ch. megacephala may result from ecological niche differentiation, with potential interspecific competition for limited resources [
36,
45,
47]. This competition might lead to
Ch. megacephala being ecologically displaced by
Ch. pinguis. Alternatively, Yang & Shiao [
45] proposed that temperature differentiation might influence the ecological niches of these species, but our study did not obtain constant abundance data to fully elucidate seasonal distribution differences only for those species.
4.2. Ecological Niches and Habitat Preferences of Forensic Blowflies
In this study, species within the
Calliphora genus exhibited a general preference for urban habitats.
Calliphora calliphoroides and
C. grahami demonstrated a clear bias toward urban environments, while
C. vicina was more frequently found in urban habitats, albeit without statistical significance. Despite
C. vicina's known association with urban habitats, regional and environmental variations may influence its lifecycle dynamics [
48,
49,
50]. Altitude has been documented to affect blowflies' seasonal distribution and oviposition behavior [
33,
51]. Although no significant habitat preference was noted for
C. nigribarbis in this study, previous research indicates its tendency to migrate to higher altitudes during summer for breeding, returning to lower altitudes in autumn [
33,
52]. In addition, Kimura [
53] discussed the migration patterns of
C. nigribarbis and
C. grahami in the context of univoltine life cycles, distinguishing between upward and downward migration, emphasizing the possibility of diapause-associated migration of low altitude individuals. In particular, only some of the high-altitude individuals were moving at low altitudes, therefore whether this migration can be classified as dispersion remains controversial. The similarities between migration and dispersion complicates identifying a clear definition, underscoring the need for further research on these species' elevation-based life cycles and migration behaviors.
The
Lucilia genus displayed varied habitat preferences among species.
Lucilia caesar and
L. porphyrina showed a predilection for forested habitats, while
L. sericata and
L. illustris favored urban settings.
Lucilia porphyrina's migration from highlands to lowlands for hibernation, followed by returning to highlands for oviposition in summer [
41], may explain its forest-biased habitat preference, as its lifecycle is predominantly spent in woodlands during summer. Here,
L. sericata exhibited a strong preference for urban habitats and is known as an urban species with human affinities; it has been found in many autopsies in domestic indoor spaces [
40,
48,
54,
55].
Lucilia caesar and
L. illustris are known to be morphologically similar sister species [
56,
57]; the habitat distribution in this study showed conflicting results.
Lucilia caesar is generally known to be abundant in shady and sparsely populated forested habitats, where its ecology is influenced by low temperature and high humidity factors [
58,
59,
60]. These habitat differences may be related to niche partitioning. As carcasses are a limited and transient resource, carrion ecosystems are characterized by intense resource competition [
60,
61]. Therefore, it is possible that the two species have diverged to adapt to different habitats to minimize direct competition.
Chrysomya pinguis exhibited a preference for forested habitats, aligning with observations of its prevalence on corpses in less densely populated regions [
55] and its abundance in mountainous areas during summer [
52]. This species likely dominates high-land or forest habitats in summer, with potential spring and autumn migrations to low-land areas, possibly related to the diapause-associated migration behaviors described for
C. nigribarbis and
C. grahami [
53,
62].
4.3. Ecological Insights and Forensic Dynamics of Blowflies
This year-long investigation in Gyeongsangnam-do, South Korea, yielded fundamental forensic entomological data, laying the groundwork for enhancing forensic case studies in this region. The species catalogued in this research have been observed in both domestic animal carcasses and human autopsies, affirming their forensic relevance within South Korea [
6,
7,
40]. The local blowfly data obtained by this study underscores the necessity for a comprehensive nationwide blowfly distribution dataset for refining forensic insect estimation techniques in future investigations.
This study was limited to blowfly colonization during the early stages of carcass decomposition because the bait used to replace the odor of the decomposing carcass was only allowed to colonize for two days after the onset of decomposition. Despite this limitation, our findings support the hypothesis that different blowfly species exhibit distinct habitat preferences, as well as specific seasonal and geographical distribution patterns. These observed patterns are instrumental for forensic entomologists to determine the geographical origin of insects found on a corpse, assessing potential corpse movement, and deducing the location of the crime. Understanding the ecological niches and behavior of blowfly species can significantly enhance the utility of forensic entomology in criminal investigations. By recognizing species-specific habitat preferences and their seasonal and geographical distribution, forensic entomologists can draw inferences about the environmental conditions surrounding a crime scene [
49]. This information can help identify the likely locations where a body may have been moved from or to, providing critical evidence in crime scene reconstruction [
18].
The data from this study also highlights the importance of localized research in building a robust forensic framework. The specific blowfly species identified, and their patterns of colonization offer valuable insights into the regional biodiversity and ecological dynamics. Detailed regional studies are crucial for developing accurate forensic models that can be applied to various scenarios, ultimately improving the precision of forensic analyses. Beyond immediate forensic applications, the ecological information gained from this study contributes to a broader understanding of blowfly ecology and their role in the decomposition process. The observed habitat preferences and distribution patterns can inform future studies on blowfly behavior and their interactions with the environment, which is vital for both forensic science and ecological research.
5. Conclusion
This investigation provides essential data on the spatio-temporal distribution of blowfly species in Gyeongsangnam-do, South Korea, emphasizing the need for comprehensive regional datasets. The findings highlight the significance of species diversity, habitat preferences, and ecological niches in forensic entomology, offering valuable insights for crime scene investigations and advancing the field's scientific foundation. Future research should focus on expanding the dataset to include various stages of decomposition and different environmental conditions to further refine forensic entomological methods and applications.
Author Contributions
H.S.O. Methodology, Validation, Investigation, Formal analysis, Writing - Original Draft, Writing - Review & Editing, Visualization. I.S.B. Methodology, Validation, Investigation. M.G.K. Methodology, Investigation. S.H.P. Conceptualization, Methodology, Validation, Writing - Reviewing & Editing, Supervision, Funding acquisition.
Funding
This research was supported by the Projects for Research and Development of Police Science and Technology under the Center for Research and Development of Police Science and Technology of the Korean National Police Agency (grant no. PR10-04-000-22).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
We would like to thank Yi-Re Kim who helped with the field research for this paper and Dr Jae-Bong Jung for his comments on the paper's methodology. This research was supported by the Projects for Research and Development of Police Science and Technology under the Center for Research and Development of Police Science and Technology of the Korean National Police Agency (grant no. PR10-04-000-22).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Catts, E.P.; Goff, M.L. Forensic entomology in criminal investigations. Annual review of Entomology. 1992, 37, 253–272. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Benbow, M.E.; Tarone, A.M.; Mohr, R.M. Basic research in evolution and ecology enhances forensics. Trends in Ecology & Evolution. 2011, 26, 53–55. [Google Scholar]
- Jung, J.B.; Park, H.D.; Yoon, M.H. Estimating Postmortem Interval of Human Cadavers Based on The Rearing Data of Maggots. Korean Journal of Scientific Criminal Investigation. 2014, 8, 157–163. [Google Scholar]
- Park, J.E.; Shin, S.E.; Park, S.H.; Jeong, S.J.; Park, S.H.; Moon, T.Y.; Lee, J.W. A Study for Estimating Growth Time of Calliphoridae Flies Using Statistical Models. Journal of Scientific Criminal Investigation 2019, 13, 89–94. [Google Scholar] [CrossRef]
- Shin, S.E.; Park, J.H.; Jeong, S.J.; Park, S.H. The growth model of forensically important Lucilia sericata (Meigen) (Diptera: Calliphoridae) in South Korea. Insects. 2021, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Moon, T.Y. Consistency of seasonal patterns of insect succession on pig cadavers at Yeongdo Island in Busan, Korea. Entomological Research. 2021, 51, 24–35. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, J.H.; Woo, D.H.; Ji, B.H.; Moon, T.Y. Insect diversity and succession patterns on pig cadavers in Changwon, South Korea. Entomological Research. 2022, 52, 241–250. [Google Scholar] [CrossRef]
- Oh, H.S.; Baek, I.S.; Kim, N.Y.; Park, S.H. Influence of carcass mass on decomposition rate: A medico-legal entomology perspective. Entomological Research. 2024, 54, e12705. [Google Scholar] [CrossRef]
- Park, S.H.; Baek, S.H.; Moon, T.Y. The impact of blowflies on pig cadaver decomposition on Yeongdo Island, Busan, South Korea. Entomological Research. 2021, 51, 578–584. [Google Scholar] [CrossRef]
- Campobasso, C.P.; Di Vella, G.; Introna, F. Factors affecting decomposition and Diptera colonization. Forensic science international. 2001, 120, 18–27. [Google Scholar] [CrossRef]
- Mann, R.W.; Bass, W.M.; Meadows, L. Time since death and decomposition of the human body: variables and observations in case and experimental field studies. Journal of forensic sciences. 1990, 35, 103–111. [Google Scholar] [CrossRef]
- Goff, M.L. Estimation of postmortem interval using arthropod development and successional patterns. Forensic Science Review. 1993, 5, 81–81. [Google Scholar]
- Amendt, J.; Krettek, R.; Zehner, R. Forensic entomology. Naturwissenschaften. 2004, 91, 51–65. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Mohr, R.; Benbow, M.E.; Tarone, A.M.; Vanlaerhoven, S. A roadmap for bridging basic and applied research in forensic entomology. Annual Review of entomology. 2011, 56, 401–421. [Google Scholar] [CrossRef]
- Pinto, J.; Magni, P.A.; O’Brien, R.C.; Dadour, I.R. Forensically relevant blow flies (Diptera: Calliphoridae) of Central Connecticut, USA. Forensic Science International. 2021, 327, 110940. [Google Scholar] [CrossRef]
- George, K.A.; Archer, M.S.; Toop, T. Abiotic environmental factors influencing blowfly colonisation patterns in the field. Forensic Science International. 2013, 229, 100–107. [Google Scholar] [CrossRef]
- Lutz, L.; Verhoff, M.A.; Amendt, J. Environmental factors influencing flight activity of forensically important female blow flies in Central Europe. International Journal of Legal Medicine. 2019, 133, 1267–1278. [Google Scholar] [CrossRef]
- Moon, T.Y.; Moon, G.J. Medicolegal Entomology. The Kosin Journal of Health Sciences. 1997, 7, 33–52. [Google Scholar]
- Weidner, L.M.; Jennings, D.E.; Tomberlin, J.K.; Hamilton, G.C. Seasonal and geographic variation in biodiversity of forensically important blow flies (Diptera: Calliphoridae) in New Jersey, USA. Journal of medical entomology. 2015, 52, 937–946. [Google Scholar] [CrossRef]
- Mohr, R.M.; Tomberlin, J.K. Environmental factors affecting early carcass attendance by four species of blow flies (Diptera: Calliphoridae) in Texas. Journal of medical entomology. 2014, 51, 702–708. [Google Scholar] [CrossRef]
- Jo, T.H. Seasonal Prevalence and Altitudinal Distribution of the Flies in Mt. Hallasan, Jejudo Island, Korea. Journal of the Environmental Sciences. 2010, 19, 491–507. [Google Scholar] [CrossRef]
- Jung, J.B.; Yoon, M.H. A Forensic Entomological Study Using Pig Carrions in Different Seasons and the Exposed Extent. Korean Police Studies Review. 2013, 12, 335–354. [Google Scholar]
- Brundage, A.; Bros, S.; Honda, J.Y. Seasonal and habitat abundance and distribution of some forensically important blow flies (Diptera: Calliphoridae) in Central California. Forensic Science International. 2011, 212, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.Y. Cadaver Entomofauna as Forensic Indicators. The Korean Journal of Legal Medicine. 1994, 18, 33–40. [Google Scholar]
- Langer, S.V.; Kyle, C.J.; Illes, M.; Larkin, S.; Beresford, D.V. Urban and rural spatial delineations in blow fly species (Diptera: Calliphoridae) across Canada: implications for forensic entomology. Journal of medical entomology. 2019, 56, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Poirier, L.M.; Anderson, G.S. The effect of season and urbanisation on Calliphoridae (Diptera) diversity in British Columbia, Canada, using baited traps. The Canadian Entomologist. 2023, 155, e24. [Google Scholar] [CrossRef]
- Alvarez Garcia, D.M.; Pérez-Hérazo, A.; Amat, E. Spatial and temporal variation of the Blowflies Community (Diptera: Calliphoridae) from an urban area in Northern South America. Journal of Medical Entomology. 2019, 56, 464–471. [Google Scholar] [CrossRef]
- Dufek, M.I.; Oscherov, E.B.; Damborsky, M.P.; Mulieri, P.R. Calliphoridae (Diptera) in human-transformed and wild habitats: diversity and seasonal fluctuations in the Humid Chaco Ecoregion of South America. Journal of Medical Entomology. 2019, 56, 725–736. [Google Scholar] [CrossRef]
- Feddern, N.; Amendt, J.; Schyma, C.; Jackowski, C.; Tschui, J. A preliminary study about the spatiotemporal distribution of forensically important blow flies (Diptera: Calliphoridae) in the area of Bern, Switzerland. Forensic science international. 2018, 289, 57–66. [Google Scholar] [CrossRef]
- Hodecek, J.; Jakubec, P. Spatio-temporal distribution and habitat preference of necrophagous Calliphoridae based on 160 real cases from Switzerland. International Journal of Legal Medicine. 2022, 136, 923–934. [Google Scholar] [CrossRef]
- Fremdt, H.; Amendt, J. Species composition of forensically important blow flies (Diptera: Calliphoridae) and flesh flies (Diptera: Sarcophagidae) through space and time. Forensic Science International. 2014, 236, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vega, D.; Baz, A. Sarcosaprophagous Diptera assemblages in natural habitats in central Spain: spatial and seasonal changes in composition. Medical and Veterinary Entomology. 2013, 27, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, M.; Matsushima, K.; Inoue, A. Seasonal and spatial distributions in relation to reproduction of blowflies (Diptera, Calliphoridae) in Hokkaido, Japan. Medical Entomology and Zoology. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Norris, K.R. The bionomics of blow flies. Annual review of Entomology. 1965, 10, 47–68. [Google Scholar] [CrossRef]
- Ji, B.H. Diagnostic Taxonomy and Forensic Implication of Necrophagous Flies Associated with Dead Animal. master’s dissertation, Dissertation for the Degree of master’s, Kosin University, Busan, 2023.
- Kanō, R.; S. Shinonaga. Fauna Japonica: Calliphoridae (Insecta: Diptera). Biogeographical Society of Japan and National Science Museum, Tokyo, Japan, 1968.
- Korea Meteorological Administration: Climate characteristics by region in Korea. Available online: www.weather.go.kr (accessed on 13 May 2024).
- IBM SPSS Inc (2016). SPSS statistics for Windows. IBM Corp. Released 2012.
- Matoba, K.; Terazawa, K. Estimation of the time of death of decomposed or skeletonized bodies found outdoors in cold season in Sapporo city, located in the northern district of Japan. Legal Medicine. 2008, 10, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.E. Taxonomic and ecological study on necrophagous insects and its forensic application. Doctoral dissertation, Dissertation for the Degree of Doctor, Kangwon National University, 2019.
- Suenaga, O.; Kurahashi, H. Life cycle of an Oriental blow fly, Lucilia porphyrina (Walker) in Nagasaki, Western Japan. 熱帯医学 Tropical medicine. 1995, 37, 99–107. [Google Scholar]
- Sukontason, K.; Piangjai, S.; Siriwattanarungsee, S.; Sukontason, K.L. Morphology and developmental rate of blowflies Chrysomya megacephala and Chrysomya rufifacies in Thailand: application in forensic entomology. Parasitology Research. 2008, 102, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Klong-Klaew, T.; Ngoen-Klan, R.; Moophayak, K.; Sukontason, K.; Irvine, K.N.; Tomberlin, J.K.; Somboon, P.; Chareonviriyaphap, T.; Kurahashi, H.; Sukontason, K.L. Predicting geographic distribution of forensically significant blow flies of subfamily Chrysomyinae (Diptera: Calliphoridae) in Northern Thailand. Insects. 2018, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.D.; SINGH, M.M.; Suzuki, K.; Miura, M.; Kurahashi, H. Male eye dimorphism and synanthropy in Chrysomya pinguis (Walker): Diptera: Calliphoridae. Medical entomology and zoology. 1994, 45, 299–302. [Google Scholar] [CrossRef]
- Yang, S.T.; Shiao, S.F. Temperature adaptation in Chrysomya megacephala and Chrysomya pinguis, two blow fly species of forensic significance. Entomologia Experimentalis et Applicata. 2014, 152, 100–107. [Google Scholar] [CrossRef]
- Shin, S.E.; Jang, M.S.; Park, J.H.; Park, S.H. A forensic entomology case estimating the minimum postmortem interval using the distribution of fly pupae in fallow ground and maggots with freezing injury. The Korean Journal of Laboratory Medicine. 2015, 39, 17–21. [Google Scholar] [CrossRef]
- Harvey, M.L.; Gaudieri, S.; Villet, M.H.; Dadour, I.R. A global study of forensically significant calliphorids: implications for identification. Forensic science international. 2008, 177, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.S. The use of insects in death investigations: an analysis of cases in British Columbia over a five year period. Canadian Society of Forensic Science Journal. 1995, 28, 277–292. [Google Scholar] [CrossRef]
- Boudreau, D.R.; Hammami, N.; Moreau, G. Environmental and evolutionary factors favouring the coexistence of sarcosaprophagous Calliphoridae species competing for animal necromass. Ecological Entomology. 2021, 46, 1293–1300. [Google Scholar] [CrossRef]
- Reiter, C. Zum wachstumsverhalten der maden der blauen schmeißfliege Calliphora vicina. Zeitschrift für Rechtsmedizin. 1984, 91, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, H.; Kawai, S.; Shudo, C.; Wada, Y. The life history of Calliphora nigribarbis Vollenhoven in Mt. Hachijo-Fuji, Hachijo Island. Japanese Journal of Sanitary Zoology. 1994, 45, 327–327. [Google Scholar]
- Shinonaga, S. The altitudinal distribution of flies on Mt. Fuji in summer season. Medical Entomology and Zoology. 1965, 16, 263–269. [Google Scholar] [CrossRef]
- Kimura, M.T. Altitudinal migration of insects. Entomological Science. 2021, 24, 35–47. [Google Scholar] [CrossRef]
- TACHIBANA, S.I.; Numata, H. Seasonal prevalence of blowflies and flesh flies in Osaka City. Entomological science. 2006, 9, 341–345. [Google Scholar] [CrossRef]
- Toukairin, Y.; Arai, T.; Hoshi, T.; Trejo, J.A.O.; Nogami, M. The geographical distribution of fly larvae on corpses in Saitama Prefecture in Japan during the summer season. Legal Medicine. 2017, 24, 75–77. [Google Scholar] [CrossRef]
- Boehme, P.; Amendt, J.; Zehner, R. The use of COI barcodes for molecular identification of forensically important fly species in Germany. Parasitology research. 2012, 110, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Sonet, G.; Jordaens, K.; Braet, Y.; Desmyter, S. Why is the molecular identification of the forensically important blowfly species Lucilia caesar and L. illustris (family Calliphoridae) so problematic? Forensic Science International. 2012, 223, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Morris, O.S.; Titchener, R.N. Blowfly species composition in sheep myiasis in Scotland. Medical and veterinary entomology. 1997, 11, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Turner, B.D. Spatial and temporal variability of necrophagous Diptera from urban to rural areas. Medical and veterinary entomology. 2005, 19, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Arias-Robledo, G.; Stevens, J.R.; Wall, R. Spatial and temporal habitat partitioning by calliphorid blowflies. Medical and Veterinary Entomology. 2019, 33, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, I.; Wall, R. Population dynamics of the sheep blowfly Lucilia sericata: seasonal patterns and implications for control. Journal of applied ecology. 2002, 39, 493–501. [Google Scholar] [CrossRef]
- Sukontason, K.L.; Sanit, S.; Limsopatham, K.; Wannasan, A.; Somboon, P.; Sukontason, K. Chrysomya pinguis (Walker)(Diptera: Calliphoridae), blow fly of forensic importance: A review of bionomics and forensic entomology appraisal. Acta Tropica. 2022, 232, 106506. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).