Submitted:
17 June 2024
Posted:
18 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
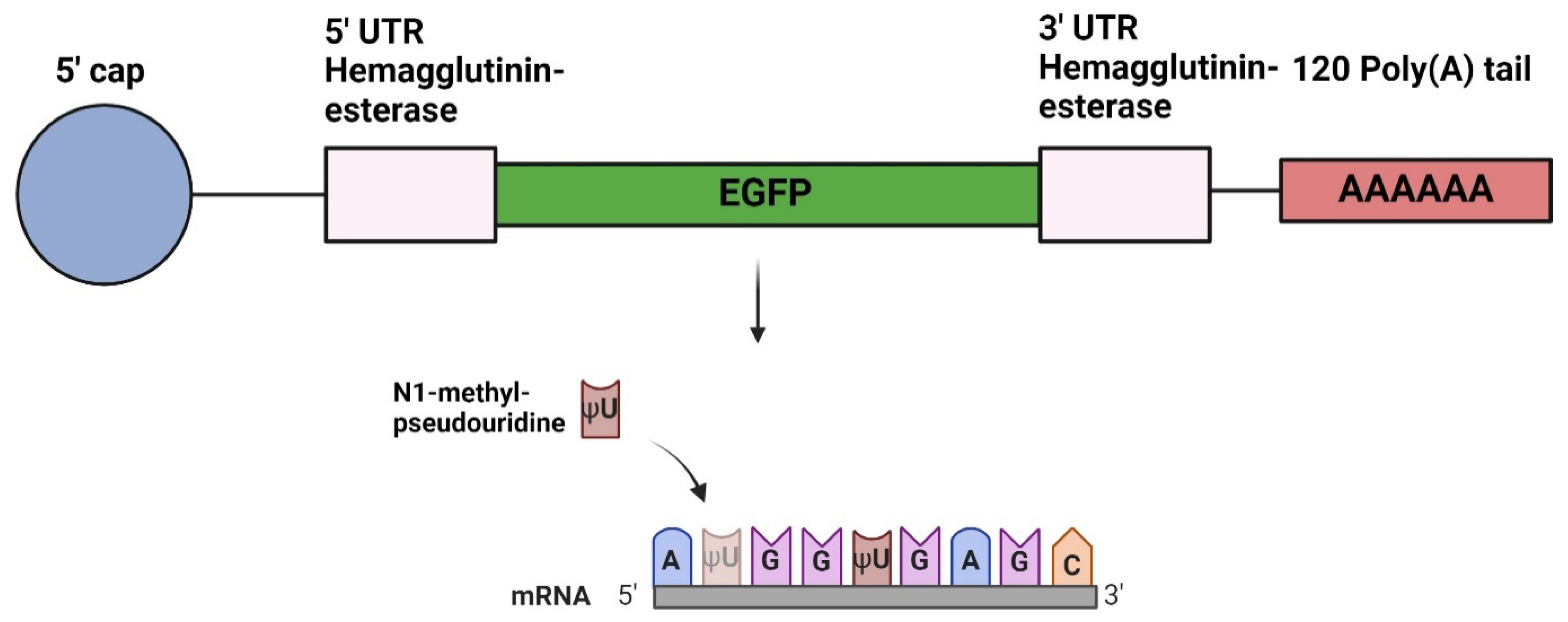
Design of DNA Plasmids
In Vitro Transcription of mRNA
mRNA-LNP Formulation
mRNA-LNP Characterization
In Vitro Expression
In Vitro Cytotoxicity of mRNA-LNPs and mRNA-Lipofectamine
Injection of mRNA-LNPs in Salmon
Immunohistochemistry
3. Results
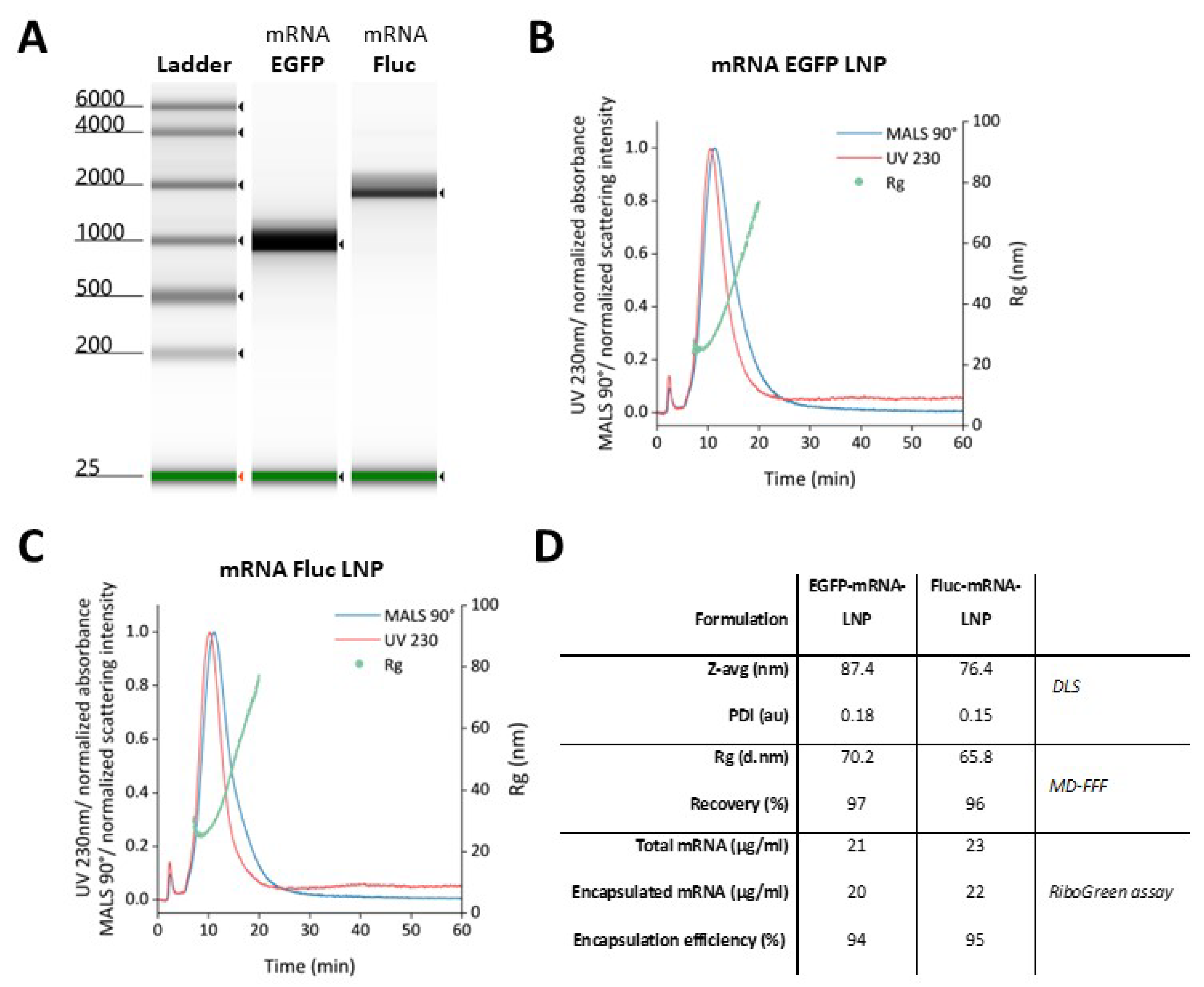
Production of mRNA and LNP Encapsulation:
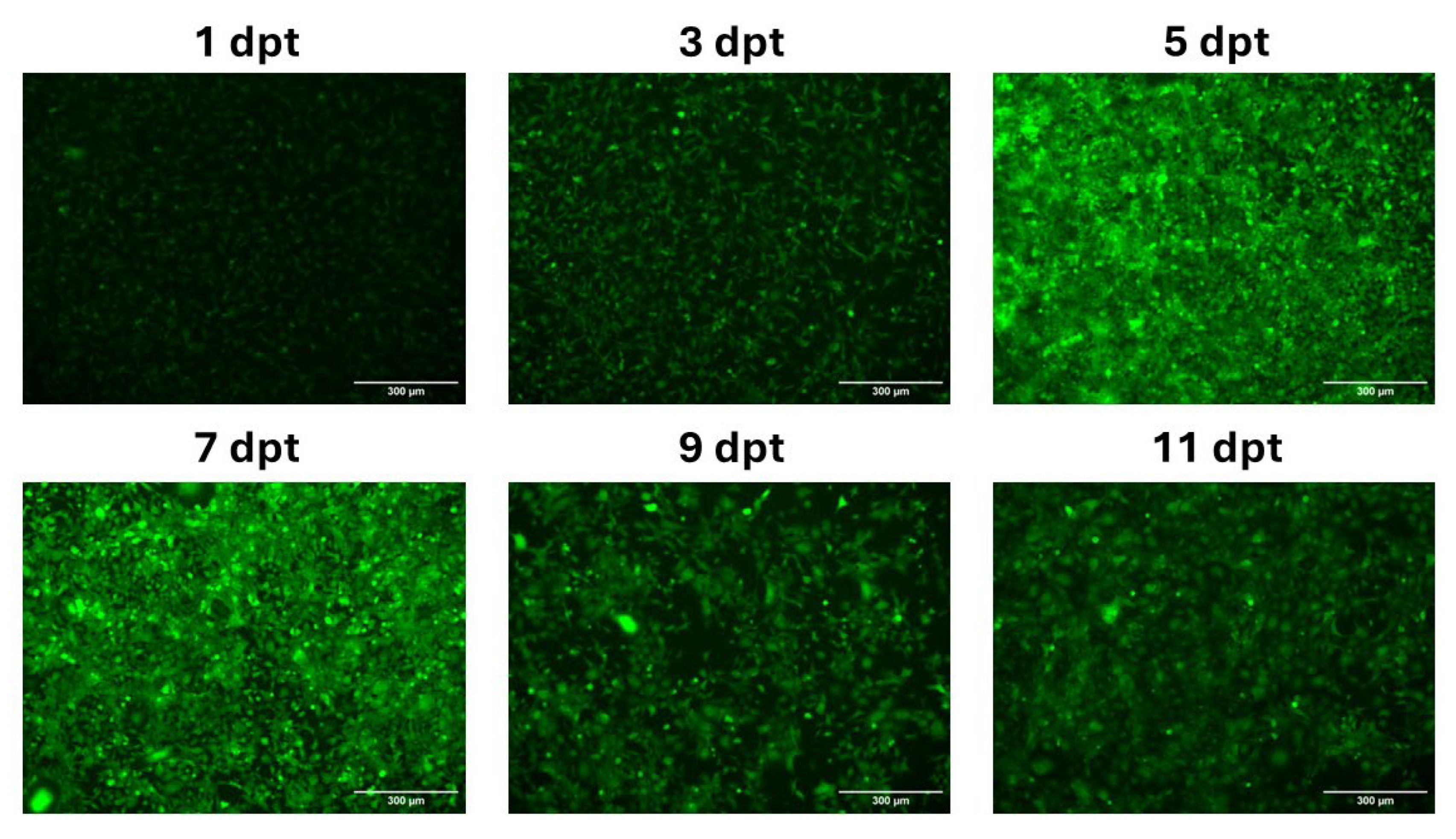
In Vitro Expression of mRNA-LNPs in CHH-1 Cells
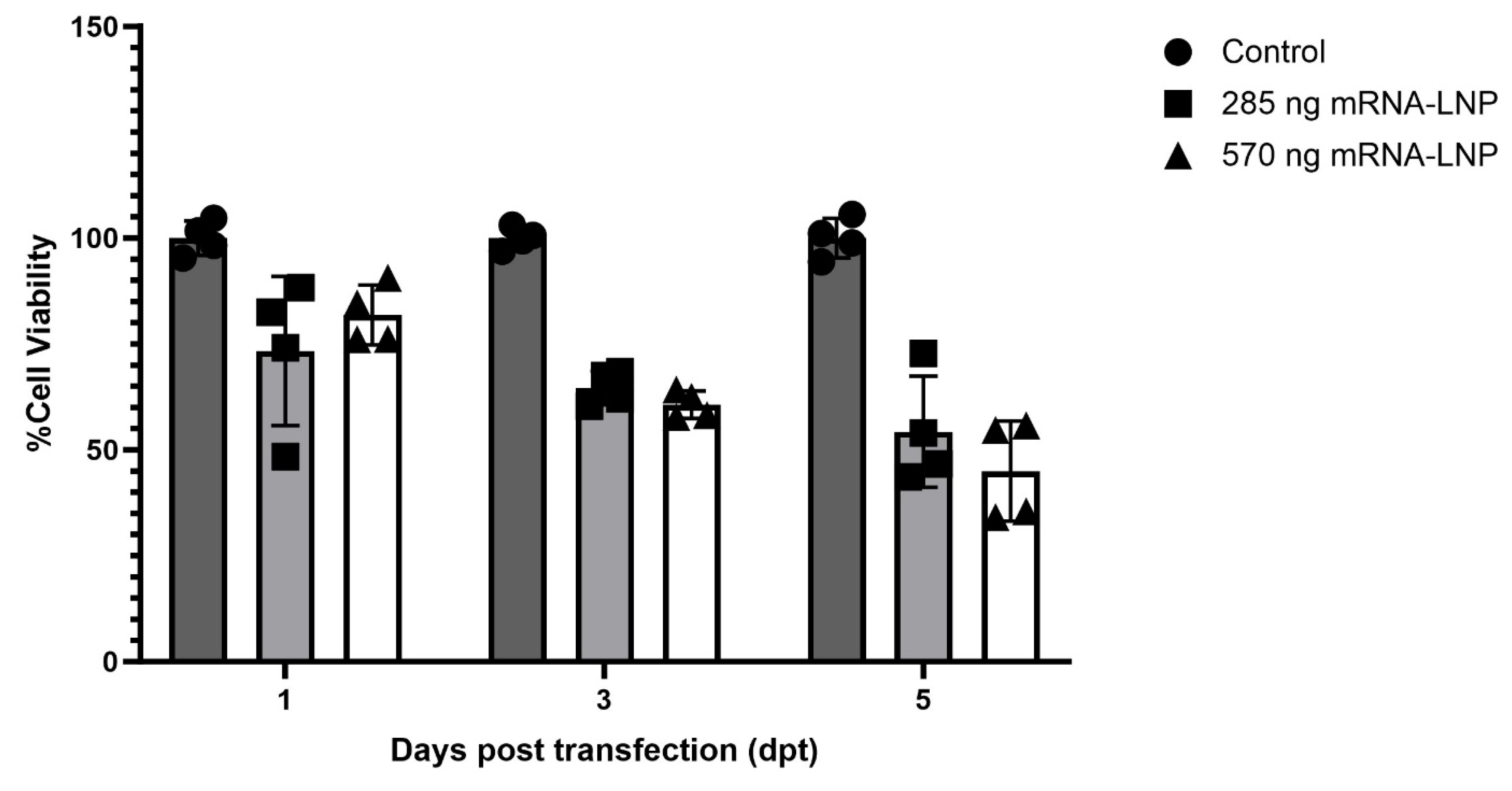
Evaluation of mRNA-LNP Cytotoxicity
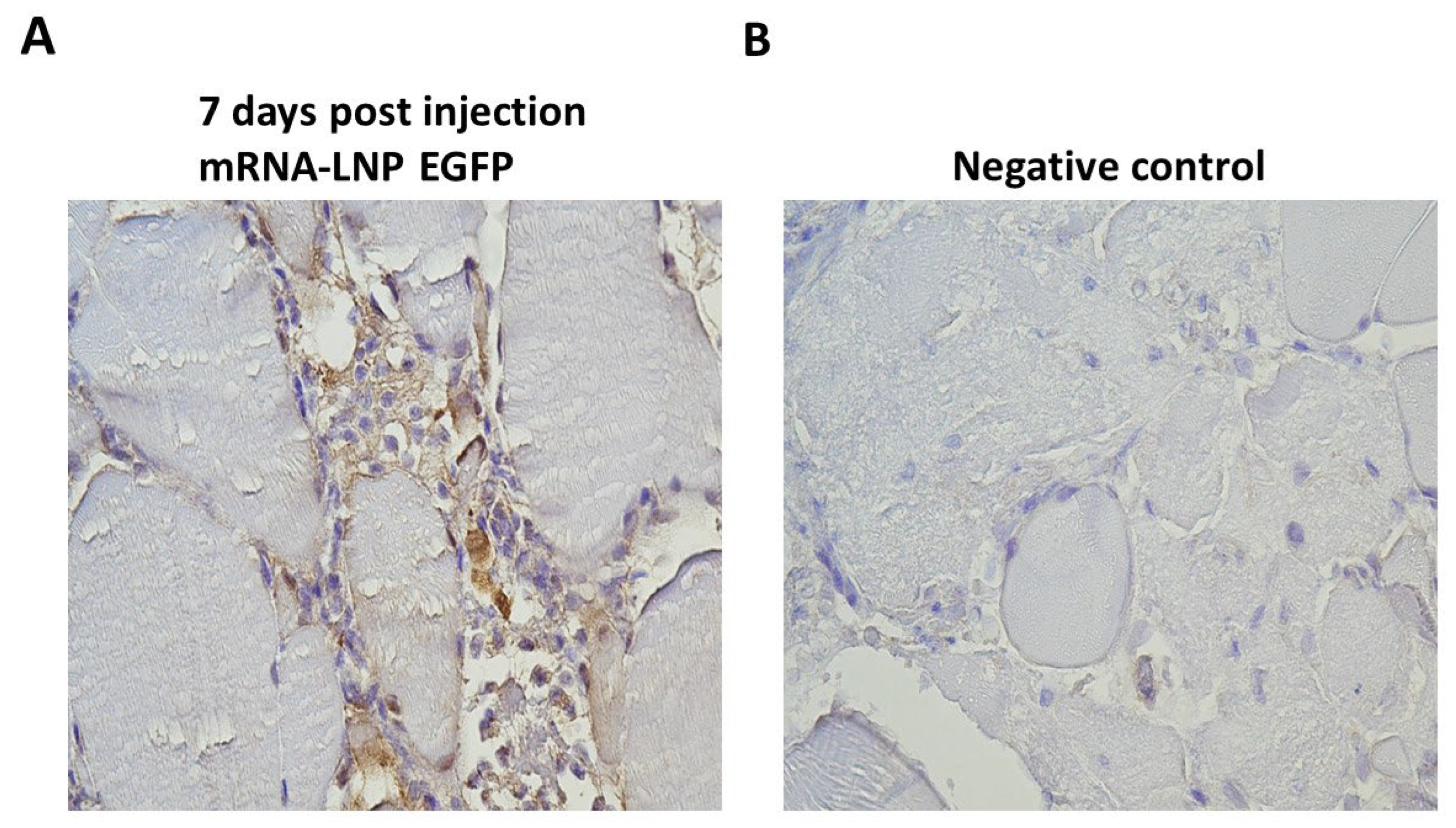
In Vivo Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Snieszko, S.F. A bacterial disease of carp in central Europe. The Progressive Fish-Culturist 1940, 7, 12–15. [Google Scholar] [CrossRef]
- Gudding, R.; Van Muiswinkel, W.B. A history of fish vaccination: Science-based disease prevention in aquaculture. Fish Shellfish Immunol 2013, 35, 1683–1688. [Google Scholar] [CrossRef]
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish Shellfish Immunol 2013, 35, 1759–1768. [Google Scholar] [CrossRef]
- Midtlyng, P.J.; Reitan, L.J.; Lillehaug, A.; Ramstad, A. Protection, immune responses and side effects in atlantic salmon (salmo salarl.) vaccinated against furunculosis by different procedures. Fish & Shellfish Immunology 1996, 6, 599–613. [Google Scholar]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A review of fish vaccine development strategies: Conventional methods and modern biotechnological approaches. Microorganisms 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Hølvold, L.B.; Myhr, A.I.; Dalmo, R.A. Strategies and hurdles using DNA vaccines to fish. Veterinary Research 2014, 45, 21. [Google Scholar] [CrossRef] [PubMed]
- Lu, S. Immunogenicity of DNA vaccines in humans: It takes two to tango. Hum Vaccin 2008, 4, 449–452. [Google Scholar] [CrossRef]
- Khan, F.H. The elements of immunology. Pearson Education India: 2009.
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B. , et al. Efficacy and safety of the mrna-1273 sars-cov-2 vaccine. N Engl J Med 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C. , et al. Safety and efficacy of the bnt162b2 mrna covid-19 vaccine. N Engl J Med 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Houseley, J.; Tollervey, D. The many pathways of rna degradation. Cell 2009, 136, 763–776. [Google Scholar] [CrossRef]
- Scheel, B.; Braedel, S.; Probst, J.; Carralot, J.P.; Wagner, H.; Schild, H.; Jung, G.; Rammensee, H.G.; Pascolo, S. Immunostimulating capacities of stabilized rna molecules. Eur J Immunol 2004, 34, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Ni, H.; Capodici, J.; Lamphier, M.; Weissman, D. Mrna is an endogenous ligand for toll-like receptor 3. J Biol Chem 2004, 279, 12542–12550. [Google Scholar] [CrossRef] [PubMed]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded rna via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef] [PubMed]
- Pichlmair, A.; Schulz, O.; Tan, C.P.; Näslund, T.I.; Liljeström, P.; Weber, F.; Reis e Sousa, C. Rig-i-mediated antiviral responses to single-stranded rna bearing 5’’-phosphates. Science 2006, 314, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mrna yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of rna recognition by toll-like receptors: The impact of nucleoside modification and the evolutionary origin of rna. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Holtkamp, S.; Kreiter, S.; Selmi, A.; Simon, P.; Koslowski, M.; Huber, C.; Türeci, O.; Sahin, U. Modification of antigen-encoding rna increases stability, translational efficacy, and t-cell stimulatory capacity of dendritic cells. Blood 2006, 108, 4009–4017. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and challenges in the delivery of mrna-based vaccines. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef]
- Dowdy, S.F. Overcoming cellular barriers for rna therapeutics. Nat Biotechnol 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Chen, S.; Cullis, P.R.; van der Meel, R. Lipid nanoparticle technology for clinical translation of sirna therapeutics. Acc Chem Res 2019, 52, 2435–2444. [Google Scholar] [CrossRef]
- Jeeva, S.; Kim, K.H.; Shin, C.H.; Wang, B.Z.; Kang, S.M. An update on mrna-based viral vaccines. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Schlich, M.; Palomba, R.; Costabile, G.; Mizrahy, S.; Pannuzzo, M.; Peer, D.; Decuzzi, P. Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng Transl Med 2021, 6, e10213. [Google Scholar] [CrossRef] [PubMed]
- Fenton, O.S.; Kauffman, K.J.; McClellan, R.L.; Appel, E.A.; Dorkin, J.R.; Tibbitt, M.W.; Heartlein, M.W.; DeRosa, F.; Langer, R.; Anderson, D.G. Bioinspired alkenyl amino alcohol ionizable lipid materials for highly potent in vivo mrna delivery. Adv Mater 2016, 28, 2939–2943. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mrna delivery. Nat Rev Mater 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E. , et al. Nucleoside-modified mrna vaccines induce potent t follicular helper and germinal center b cell responses. J Exp Med 2018, 215, 1571–1588. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R.; Nita-Lazar, M.; Giomarelli, B.; Ahmed, H.; Du, S.; Cammarata, M.; Parrinello, N.; Bianchet, M.A.; Amzel, L.M. Structural and functional diversity of the lectin repertoire in teleost fish: Relevance to innate and adaptive immunity. Dev Comp Immunol 2011, 35, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Yoder, J.A.; Litman, G.W. The phylogenetic origins of natural killer receptors and recognition: Relationships, possibilities, and realities. Immunogenetics 2011, 63, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, J.O.; Zarkadis, I.K.; Lambris, J.D. Complement diversity: A mechanism for generating immune diversity? Immunol Today 1998, 19, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Sousa de Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem Soc Rev 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Shiomi, A.; Nagao, K.; Kasai, H.; Hara, Y.; Umeda, M. Changes in the physicochemical properties of fish cell membranes during cellular senescence. Bioscience, Biotechnology, and Biochemistry 2020, 84, 583–593. [Google Scholar] [CrossRef]
- Thompson, K.D.; Henderson, R.J.; Tatner, M.F. A comparison of the lipid composition of peripheral blood cells and head kidney leucocytes of atlantic salmon (salmo salar l.). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 1995, 112, 83–92. [Google Scholar] [CrossRef]
- Hatit, M.Z.C.; Lokugamage, M.P.; Dobrowolski, C.N.; Paunovska, K.; Ni, H.; Zhao, K.; Vanover, D.; Beyersdorf, J.; Peck, H.E.; Loughrey, D. , et al. Species-dependent in vivo mrna delivery and cellular responses to nanoparticles. Nature Nanotechnology 2022, 17, 310–318. [Google Scholar] [CrossRef]
- Parot, J.; Mehn, D.; Jankevics, H.; Markova, N.; Carboni, M.; Olaisen, C.; Hoel, A.D.; Sigfúsdóttir, M.S.; Meier, F.; Drexel, R. , et al. Quality assessment of lnp-rna therapeutics with orthogonal analytical techniques. Journal of Controlled Release 2024, 367, 385–401. [Google Scholar] [CrossRef]
- Lannan, C.N.; Winton, J.R.; Fryer, J.L. Fish cell lines: Establishment and characterization of nine cell lines from salmonids. In Vitro 1984, 20, 671–676. [Google Scholar] [CrossRef]
- Linares-Fernández, S.; Moreno, J.; Lambert, E.; Mercier-Gouy, P.; Vachez, L.; Verrier, B.; Exposito, J.Y. Combining an optimized mrna template with a double purification process allows strong expression of in vitro transcribed mrna. Mol Ther Nucleic Acids 2021, 26, 945–956. [Google Scholar] [CrossRef]
- Yu, M.; Song, W.; Tian, F.; Dai, Z.; Zhu, Q.; Ahmad, E.; Guo, S.; Zhu, C.; Zhong, H.; Yuan, Y. , et al. Temperature- and rigidity-mediated rapid transport of lipid nanovesicles in hydrogels. Proc Natl Acad Sci U S A 2019, 116, 5362–5369. [Google Scholar] [CrossRef]
- Anderson, B.R.; Muramatsu, H.; Jha, B.K.; Silverman, R.H.; Weissman, D.; Karikó, K. Nucleoside modifications in rna limit activation of 2’-5’-oligoadenylate synthetase and increase resistance to cleavage by rnase l. Nucleic Acids Res 2011, 39, 9329–9338. [Google Scholar] [CrossRef]
- Andries, O.; Mc Cafferty, S.; De Smedt, S.C.; Weiss, R.; Sanders, N.N.; Kitada, T. N1-methylpseudouridine-incorporated mrna outperforms pseudouridine-incorporated mrna by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. Journal of Controlled Release 2015, 217, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Svitkin, Y.V.; Cheng, Y.M.; Chakraborty, T.; Presnyak, V.; John, M.; Sonenberg, N. N1-methyl-pseudouridine in mrna enhances translation through eif2α-dependent and independent mechanisms by increasing ribosome density. Nucleic Acids Res 2017, 45, 6023–6036. [Google Scholar] [CrossRef] [PubMed]
- Baiersdörfer, M.; Boros, G.; Muramatsu, H.; Mahiny, A.; Vlatkovic, I.; Sahin, U.; Karikó, K. A facile method for the removal of dsrna contaminant from in vitro-transcribed mrna. Mol Ther Nucleic Acids 2019, 15, 26–35. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Z.; Huang, C.; Qiu, M.; Song, D.; Li, Y.; Xu, Q. Lipid nanoparticle-mediated lymph node-targeting delivery of mrna cancer vaccine elicits robust cd8(+) t cell response. Proc Natl Acad Sci U S A 2022, 119, e2207841119. [Google Scholar] [CrossRef] [PubMed]
- Bowen, L.; von Biela, V.R.; McCormick, S.D.; Regish, A.M.; Waters, S.C.; Durbin-Johnson, B.; Britton, M.; Settles, M.L.; Donnelly, D.S.; Laske, S.M. , et al. Transcriptomic response to elevated water temperatures in adult migrating yukon river chinook salmon (oncorhynchus tshawytscha). Conserv Physiol 2020, 8, coaa084. [Google Scholar] [CrossRef] [PubMed]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P. , et al. Lipid nanoparticles enhance the efficacy of mrna and protein subunit vaccines by inducing robust t follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e2877. [Google Scholar] [CrossRef] [PubMed]
- Naderi Sohi, A.; Kiani, J.; Arefian, E.; Khosrojerdi, A.; Fekrirad, Z.; Ghaemi, S.; Zim, M.K.; Jalili, A.; Bostanshirin, N.; Soleimani, M. Development of an mrna-lnp vaccine against sars-cov-2: Evaluation of immune response in mouse and rhesus macaque. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Hassett, K.J.; Rajlic, I.L.; Bahl, K.; White, R.; Cowens, K.; Jacquinet, E.; Burke, K.E. Mrna vaccine trafficking and resulting protein expression after intramuscular administration. Mol Ther Nucleic Acids 2024, 35, 102083. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




