Submitted:
11 June 2024
Posted:
12 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Lines
2.3. Cell Proliferation Assasy
2.4. Western Blot Analysis
2.5. Annexin V Staining Assay
2.6. Human Tumor Xenografts in Nude Mice
2.7. Statistics
3. Results
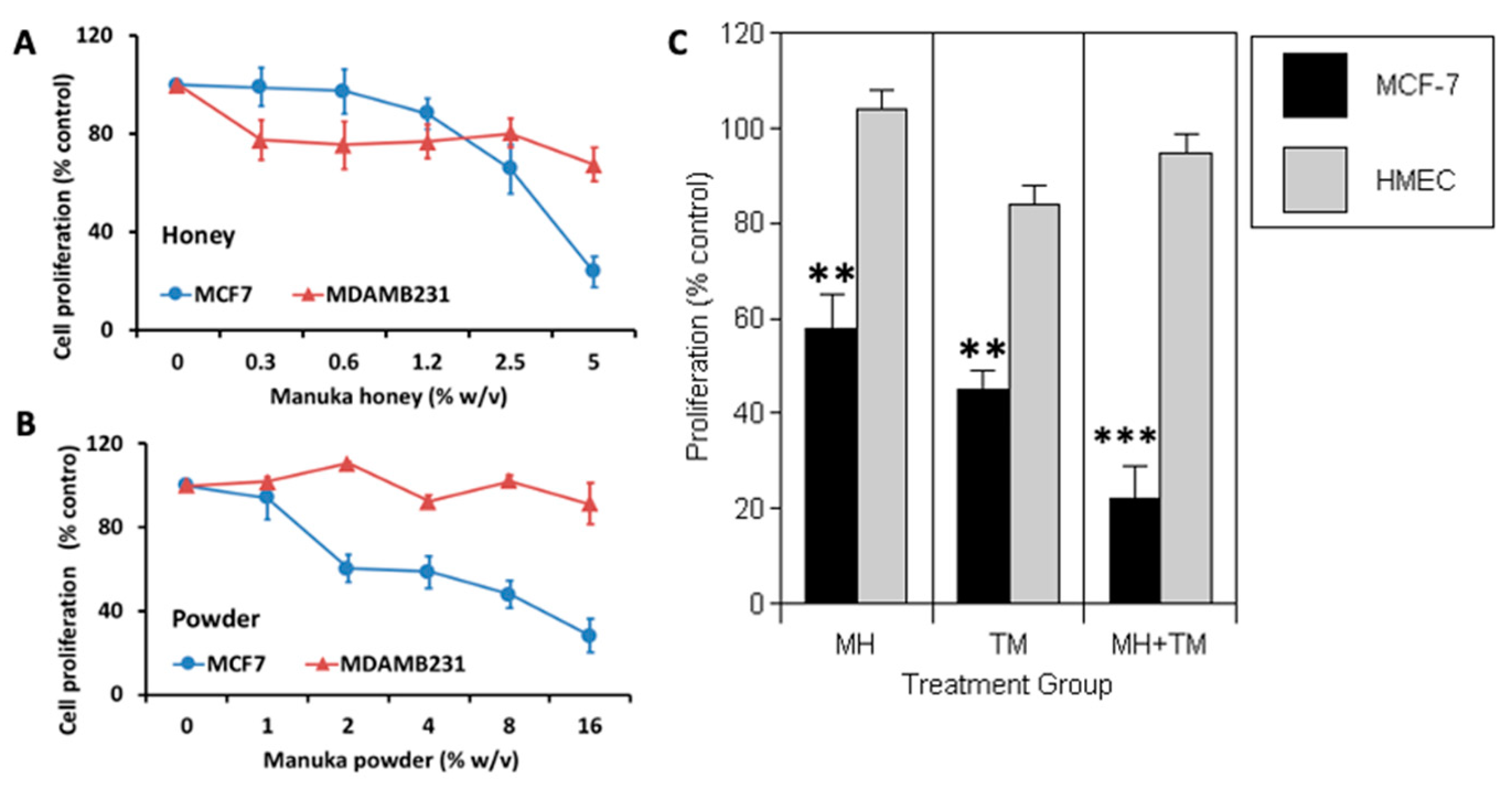
3.1. Manuka Honey Reduces the Proliferation of Human MCF-7 Breast Cancer Cells In Vitro
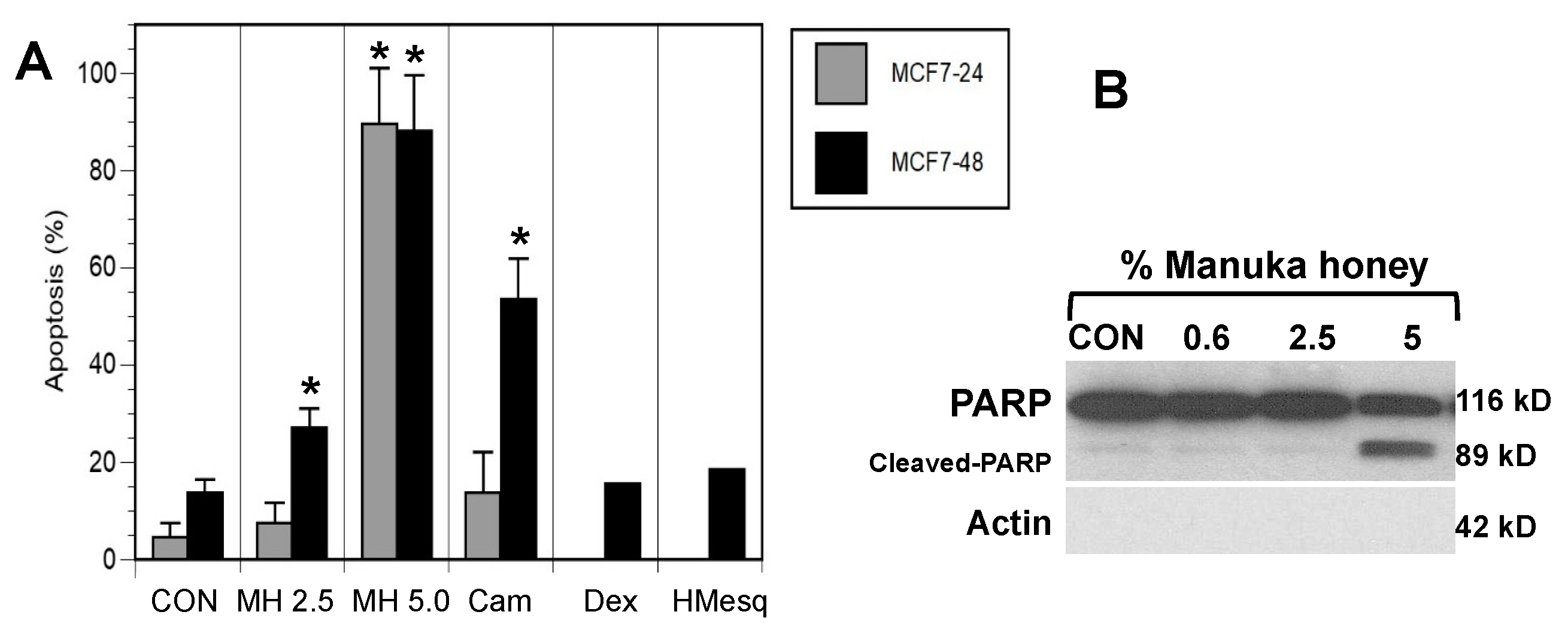
3.2. MH Induces Apoptosis in MCF-7 Breast Cancer Cells In Vitro
3.3. Manuka Honey Induces PARP-Cleavage Leading to Tumor Cell Apoptosis
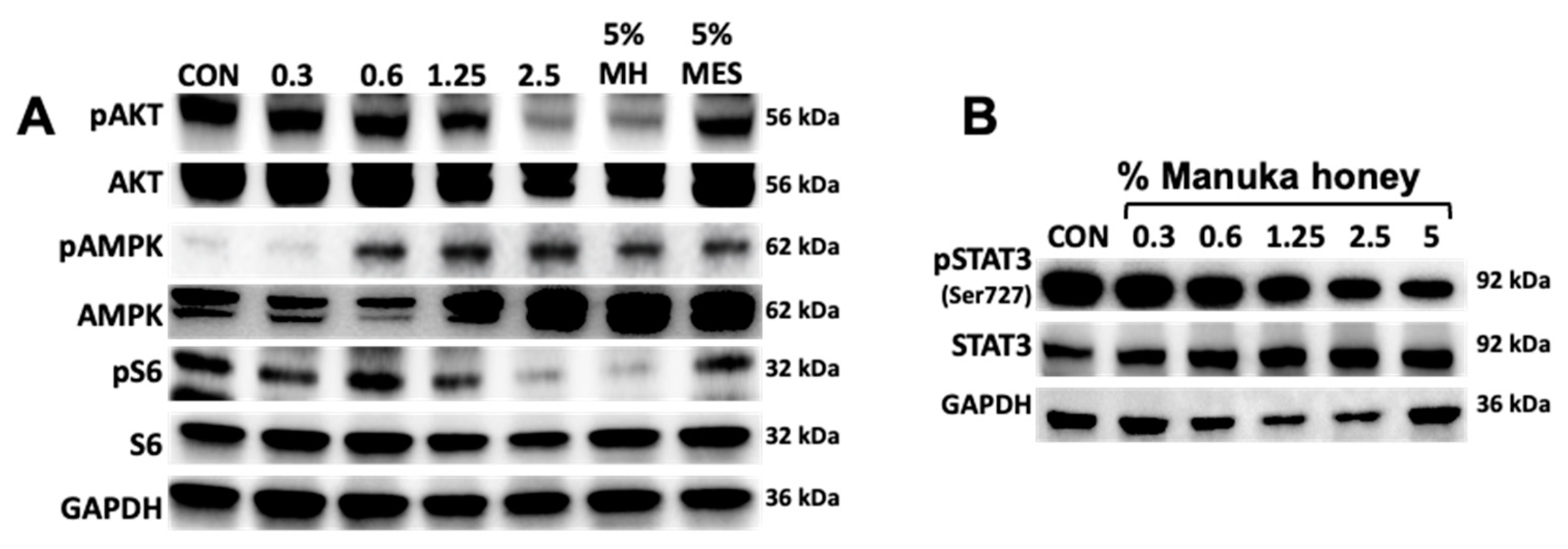
3.4. Manuka Honey Activates AMPK and Inhibits Downstream PI3K/AKT/mTOR Signaling.
3.5. Manuka Honey Inhibits STAT-3 Phosphorylation
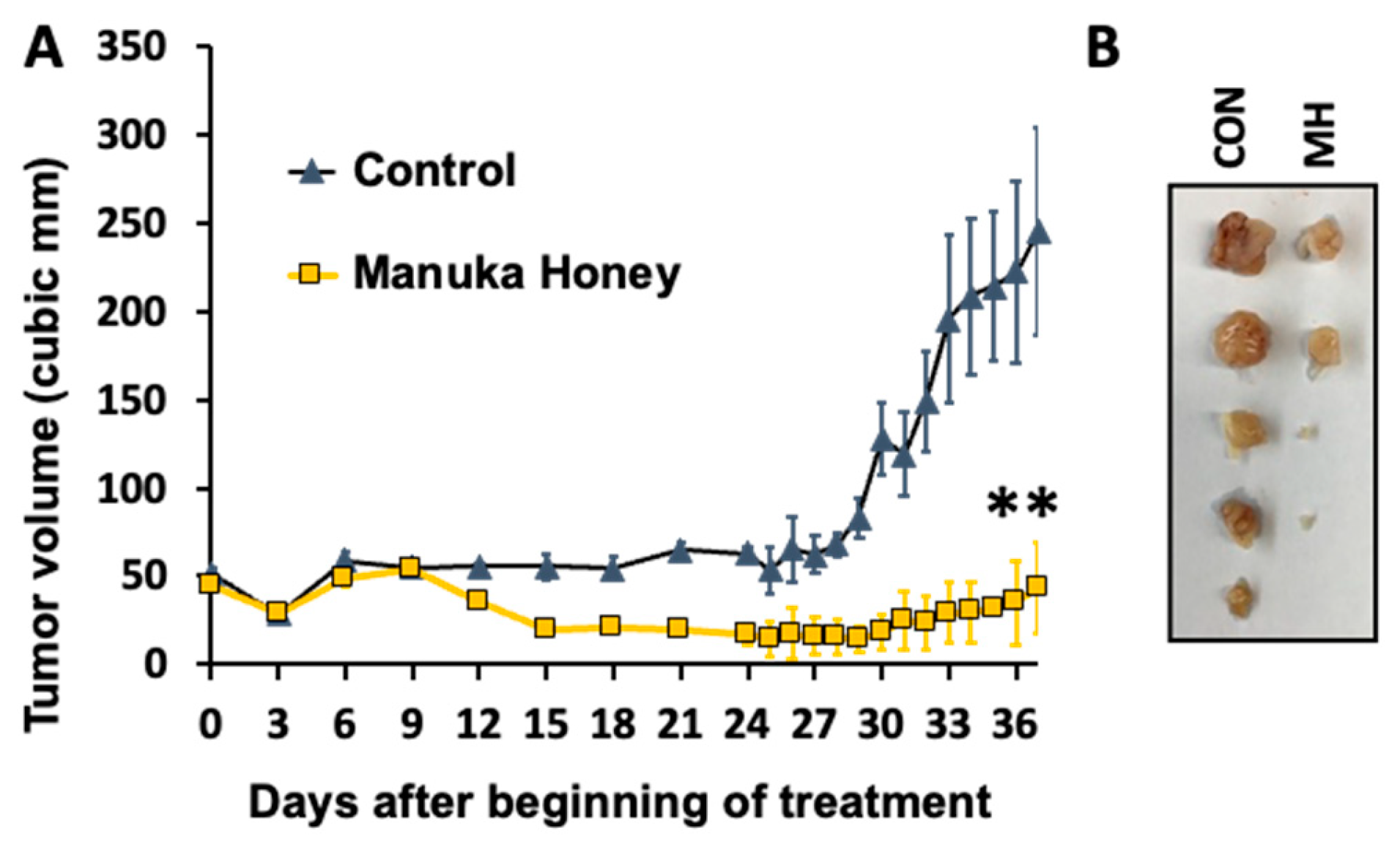
3.6. Antitumor Activity of Manuka Honey in Human Breast Cancer Xenografts In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hurvitz, S.A.; Pietras, R.J. Rational management of endocrine resistance in breast cancer: a comprehensive review of estrogen receptor biology, treatment options, and future directions. Cancer 2008, 113, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- King, W.J.; DeSombre, E.R.; Jensen, E.V.; Greene, G.L. Comparison of immunocytochemical and steroid-binding assays for estrogen receptor in human breast tumors. Cancer Res. 1985, 45, 293–304. [Google Scholar] [PubMed]
- Pietras, R.J.; Márquez-Garbán, D.C. Membrane-Associated Estrogen Receptor Signaling Pathways in Human Cancers. Clin. Cancer Res. 2007, 13, 4672–4676. [Google Scholar] [CrossRef] [PubMed]
- Chumsri, S.; Howes, T.; Bao, T.; Sabnis, G.; Brodie, A. Aromatase, aromatase inhibitors, and breast cancer. J Steroid Biochem Mol Biol 2011, 125, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C. Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast cancer. Br. J. Pharmacol. 2006, 147(Suppl 1), S269–S276. [Google Scholar] [CrossRef] [PubMed]
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010; 363: 1938-1948.
- Langford, V.; Gray, J.; Foulkes, B.; Bray, P.; McEwan, M.J. Application of Selected Ion Flow Tube-Mass Spectrometry to the Characterization of Monofloral New Zealand Honeys. J. Agric. Food Chem. 2012, 60, 6806–6815. [Google Scholar] [CrossRef] [PubMed]
- Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed 2011; 1: 154-160.
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009, 344, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaegel, S.; Gruner, M.; Wang, P.-N.; Boettcher, A.; Koelling-Speer, I.; Speer, K. Classification and Characterization of Manuka Honeys Based on Phenolic Compounds and Methylglyoxal. J. Agric. Food Chem. 2012, 60, 7229–7237. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Datta, N.; A Tomás-Barberán, F.; Ferreres, F.; Martos, I.; Singanusong, R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003, 81, 159–168. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Othman, N.H. Oral Administration of Tualang and Manuka Honeys Modulates Breast Cancer Progression in Sprague-Dawley Rats Model. Evidence-Based Complement. Altern. Med. 2017, 2017, 5904361. [Google Scholar] [CrossRef] [PubMed]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ahmad, A.H. Enhancement of BDNF Concentration and Restoration of the Hypothalamic-Pituitary-Adrenal Axis Accompany Reduced Depressive-Like Behaviour in Stressed Ovariectomised Rats Treated with Either Tualang Honey or Estrogen. ScientificWorldJournal 2014, 2014, 310821. [Google Scholar] [CrossRef] [PubMed]
- Bardy, J.; Slevin, N.J.; Mais, K.L.; Molassiotis, A. A systematic review of honey uses and its potential value within oncology care. J. Clin. Nurs. 2008, 17, 2604–2623. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cabezudo, M.J.; El-Kharrag, R.; Torab, F.; Bashir, G.; George, J.A.; El-Taji, H.; Al-Ramadi, B.K. Intravenous Administration of Manuka Honey Inhibits Tumor Growth and Improves Host Survival When Used in Combination with Chemotherapy in a Melanoma Mouse Model. PLOS ONE 2013, 8, e55993. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.C. Potential of Honey in the Treatment of Wounds and Burns. Am. J. Clin. Dermatol. 2001, 2, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G. Manuka honey improved wound healing in patients with sloughy venous leg ulcers. Evid Based Med 2009, 14, 148–148. [Google Scholar] [CrossRef] [PubMed]
- Jull AB, Rodgers A, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev 2008: CD005083.
- Wijesinghe, M.; Weatherall, M.; Perrin, K.; Beasley, R. Honey in the treatment of burns: a systematic review and meta-analysis of its efficacy. N Z Med J. 2009, 122, 47–60. [Google Scholar] [PubMed]
- Ghashm, A.A.; Othman, N.H.; Khattak, M.N.; Ismail, N.M.; Saini, R. Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complement. Altern. Med. 2010, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Mandal, M. Antiproliferative Effects of Honey and of Its Polyphenols: A Review. J. Biomed. Biotechnol. 2009, 2009, 830616. [Google Scholar] [CrossRef]
- Swellam, T.; Miyanaga, N.; Onozawa, M.; Hattori, K.; Kawai, K.; Shimazui, T.; Akaza, H. Antineoplastic activity of honey in an experimental bladder cancer implantation model: In vivo and in vitro studies. Int. J. Urol. 2003, 10, 213–219. [Google Scholar] [CrossRef]
- Ahmed, S.; Othman, N.H. Honey as a Potential Natural Anticancer Agent: A Review of Its Mechanisms. Evidence-Based Complement. Altern. Med. 2013, 2013, 829070. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Effects of Honey and Its Mechanisms of Action on the Development and Progression of Cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef]
- Yaacob, N.S.; Ismail, N.F. Comparison of cytotoxicity and genotoxicity of 4-hydroxytamoxifen in combination with Tualang honey in MCF-7 and MCF-10A cells. BMC Complement. Altern. Med. 2014, 14, 106–106. [Google Scholar] [CrossRef]
- Fauzi, A.N.; Norazmi, M.N.; Yaacob, N.S. Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines. Food Chem. Toxicol. 2011, 49, 871–878. [Google Scholar] [CrossRef]
- Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front Neuroendocrinol 2010; 31: 400-419.
- Márquez, D.C.; Chen, H.-W.; Curran, E.M.; Welshons, W.V.; Pietras, R.J. Estrogen receptors in membrane lipid rafts and signal transduction in breast cancer. Mol. Cell. Endocrinol. 2006, 246, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Garbán, D.C.; Deng, G.; Comin-Anduix, B.; Garcia, A.J.; Xing, Y.; Chen, H.-W.; Cheung-Lau, G.; Hamilton, N.; Jung, M.E.; Pietras, R.J. Antiestrogens in combination with immune checkpoint inhibitors in breast cancer immunotherapy. J. Steroid Biochem. Mol. Biol. 2019, 193, 105415–105415. [Google Scholar] [CrossRef]
- Márquez-Garbán, D.C.; Chen, H.-W.; Fishbein, M.C.; Goodglick, L.; Pietras, R.J. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 2007, 72, 135–143. [Google Scholar] [CrossRef]
- Weinberg, O.K.; Marquez-Garban, D.C.; Fishbein, M.C.; Goodglick, L.; Garban, H.J.; Dubinett, S.M.; Pietras, R.J. Aromatase Inhibitors in Human Lung Cancer Therapy. Cancer Res. 2005, 65, 11287–11291. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, R.G. Phytoestrogens and breast cancer. Am. J. Clin. Nutr. 2004, 79, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Tsiapara, A.V.; Jaakkola, M.; Chinou, I.; Graikou, K.; Tolonen, T.; Virtanen, V.; Moutsatsou, P. Bioactivity of Greek honey extracts on breast cancer (MCF-7), prostate cancer (PC-3) and endometrial cancer (Ishikawa) cells: Profile analysis of extracts. Food Chem. 2009, 116, 702–708. [Google Scholar] [CrossRef]
- Aryappalli, P.; Shabbiri, K.; Masad, R.J.; Al-Marri, R.H.; Haneefa, S.M.; Mohamed, Y.A.; Arafat, K.; Attoub, S.; Cabral-Marques, O.; Ramadi, K.B.; et al. Inhibition of Tyrosine-Phosphorylated STAT3 in Human Breast and Lung Cancer Cells by Manuka Honey is Mediated by Selective Antagonism of the IL-6 Receptor. Int. J. Mol. Sci. 2019, 20, 4340. [Google Scholar] [CrossRef] [PubMed]
- Keerthana, C.K.; Rayginia, T.P.; Shifana, S.C.; Anto, N.P.; Kalimuthu, K.; Isakov, N.; Anto, R.J. The role of AMPK in cancer metabolism and its impact on the immunomodulation of the tumor microenvironment. Front. Immunol. 2023, 14, 1114582. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez MJ, Perrone MC, Riggio M, Palafox M, Salinas V, Elia A et al. Targeting mTOR to overcome resistance to hormone and CDK4/6 inhibitors in ER-positive breast cancer models. Sci Rep 2023; 13: 2710.
- Shih, Y.-H.; Chen, C.-C.; Kuo, Y.-H.; Fuh, L.-J.; Lan, W.-C.; Wang, T.-H.; Chiu, K.-C.; Nguyen, T.-H.V.; Hsia, S.-M.; Shieh, T.-M. Caffeic Acid Phenethyl Ester and Caffeamide Derivatives Suppress Oral Squamous Cell Carcinoma Cells. Int. J. Mol. Sci. 2023, 24, 9819. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.-J.; Zhang, A.-P.; Qin, W.; Zheng, L.; Zhu, Y.-F.; Chen, X. AMP-activated protein kinase (AMPK) activation is involved in chrysin-induced growth inhibition and apoptosis in cultured A549 lung cancer cells. Biochem. Biophys. Res. Commun. 2012, 423, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Pavlíková, N. Caffeic Acid and Diseases—Mechanisms of Action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef]
- Gong, H.; Tai, H.; Huang, N.; Xiao, P.; Mo, C.; Wang, X.; Han, X.; Zhou, J.; Chen, H.; Tang, X.; et al. Nrf2-SHP Cascade-Mediated STAT3 Inactivation Contributes to AMPK-Driven Protection Against Endotoxic Inflammation. Front. Immunol. 2020, 11, 414. [Google Scholar] [CrossRef]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998; 139: 4252-4263.
- Al-Waili, N.S. Natural Honey Lowers Plasma Glucose, C-Reactive Protein, Homocysteine, and Blood Lipids in Healthy, Diabetic, and Hyperlipidemic Subjects: Comparison with Dextrose and Sucrose. J. Med. Food 2004, 7, 100–107. [Google Scholar] [CrossRef]
- Fourkala, E.-O.; Zaikin, A.; Burnell, M.; Gentry-Maharaj, A.; Ford, J.; Gunu, R.; Soromani, C.; Hasenbrink, G.; Jacobs, I.; Dawnay, A.; et al. Association of serum sex steroid receptor bioactivity and sex steroid hormones with breast cancer risk in postmenopausal women. Endocrine-Related Cancer 2011, 19, 137–147. [Google Scholar] [CrossRef]
- Chiang, E.-P.I.; Tsai, S.-Y.; Kuo, Y.-H.; Pai, M.-H.; Chiu, H.-L.; Rodriguez, R.L.; Tang, F.-Y. Caffeic Acid Derivatives Inhibit the Growth of Colon Cancer: Involvement of the PI3-K/Akt and AMPK Signaling Pathways. PLOS ONE 2014, 9, e99631. [Google Scholar] [CrossRef]
- Liang, L.; Zhao, L.; Yu, B.; Hu, H.; He, X.; Zhang, Y. Caffeic acid phenethyl ester reverses doxorubicin resistance in breast cancer cells via lipid metabolism regulation at least partly by suppressing the Akt/mTOR/SREBP1 pathway. Kaohsiung J. Med Sci. 2023, 39, 605–615. [Google Scholar] [CrossRef]
- Zhu, X.; Li, R.; Wang, C.; Zhou, S.; Fan, Y.; Ma, S.; Gao, D.; Gai, N.; Yang, J. Pinocembrin Inhibits the Proliferation and Metastasis of Breast Cancer via Suppression of the PI3K/AKT Signaling Pathway. Front. Oncol. 2021, 11, 661184. [Google Scholar] [CrossRef] [PubMed]
- Arnst, J. When Taxol met tubulin. J Biol Chem 2020; 295: 13994-13995.
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med Princ. Pr. 2016, 25(Suppl 2), 41–59. [Google Scholar] [CrossRef] [PubMed]
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 1971; 93: 2325-2327.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




