Submitted:
06 June 2024
Posted:
07 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Therapeutic Intervention
2.3. Functional and Clinical Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jangnin, R.; Ritruangroj, W.; Kittisupkajorn, S.; Sukeiam, P.; Inchai, J.; Maneeton, B.; Maneetorn, N.; Chaiard, J.; Theerakittikul, T. Long-COVID Prevalence and Its Association with Health Outcomes in the Post-Vaccine and Antiviral-Availability Era. J. Clin. Med. 2024, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Estebanez-Pérez, M.J.; Pastora-Bernal, J.M.; Martín-Valero, R. The Effectiveness of a Four-Week Digital Physiotherapy Intervention to Improve Functional Capacity and Adherence to Intervention in Patients with Long COVID-19. Int. J. Environ. Res. Public Health 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. Organization, W.H. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020; World Health Organization: Geneva PP - Geneva.
- Sugiyama, A.; Takafuta, T.; Sato, T.; Kitahara, Y.; Yoshinaga, Y.; Abe, K.; Chanroth, C.; Ataa, A.G.; Phyo, Z.; Kurisu, A.; et al. Natural course of post-COVID symptoms in adults and children. Sci. Rep. 2024, 14, 3884. [Google Scholar] [CrossRef] [PubMed]
- Kayaaslan, B.; Eser, F.; Kalem, A.K.; Kaya, G.; Kaplan, B.; Kacar, D.; Hasanoglu, I.; Coskun, B.; Guner, R. Post-COVID syndrome: A single-center questionnaire study on 1007 participants recovered from COVID-19. J. Med. Virol. 2021, 93, 6566–6574. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Paniagua, J.; Díaz-Arribas, M.J.; Valera-Calero, J.A.; Gallardo-Vidal, M.I.; Fernández-De-las-Peñas, C.; López-De-Uralde-Villanueva, I.; del Corral, T.; Plaza-Manzano, G. A tele-health primary care rehabilitation program improves self-perceived exertion in COVID-19 survivors experiencing Post-COVID fatigue and dyspnea: A quasi-experimental study. PLoS One 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Pavli, A.; Theodoridou, M.; Maltezou, H.C. Post-COVID Syndrome: Incidence, Clinical Spectrum, and Challenges for Primary Healthcare Professionals. Arch. Med. Res. 2021, 52, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Agergaard, J.; Leth, S.; Pedersen, T.H.; Harbo, T.; Blicher, J.U.; Karlsson, P.; Østergaard, L.; Andersen, H.; Tankisi, H. Myopathic changes in patients with long-term fatigue after COVID-19. Clin. Neurophysiol. 2021, 132, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Tirelli, U.; Taibi, R.; Chirumbolo, S. Post COVID syndrome: a new challenge for medicine. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4422–4425. [Google Scholar] [CrossRef]
- Quaranta, V.N.; Portacci, A.; Dragonieri, S.; Locorotondo, C.; Buonamico, E.; Diaferia, F.; Iorillo, I.; Quaranta, S.; Carpagnano, G.E. The Predictors of Long COVID in Southeastern Italy. J. Clin. Med. 2023, 12. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Cerfoglio, S.; Capodaglio, P.; Rossi, P.; Verme, F.; Boldini, G.; Cvetkova, V.; Ruggeri, G.; Galli, M.; Cimolin, V. Tele-Rehabilitation Interventions for Motor Symptoms in COVID-19 Patients: A Narrative Review. Bioeng. (Basel, Switzerland) 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.J.; Lin, C.W.; Hsiao, M.Y.; Wang, T.G.; Liang, H.W. Long COVID and rehabilitation. J. Formos. Med. Assoc. 2024, 123, S61–S69. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Wei, R.; Wu, Y.; Zeng, R.; Luo, D.; Ma, Y.; Zhang, L.; Huang, W.; Zeng, H.; Leung, F.W.; et al. Long-term risks of respiratory diseases in patients infected with SARS-CoV-2: a longitudinal, population-based cohort study. EClinicalMedicine 2024, 69, 102500. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.J.; Baldwin, M.M.; Daynes, E.; Evans, R.A.; Greening, N.J.; Jenkins, R.G.; Lone, N.I.; McAuley, H.; Mehta, P.; Newman, J.; et al. Respiratory sequelae of COVID-19: pulmonary and extrapulmonary origins, and approaches to clinical care and rehabilitation. Lancet. Respir. Med. 2023, 11, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.T.; Dolezal, B.A.; Cooper, C.B. Pulmonary Rehabilitation for Chronic Obstructive Pulmonary Disease: Highly Effective but Often Overlooked. Tuberc. Respir. Dis. (Seoul). 2020, 83, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Zampogna, E.; Paneroni, M.; Cherubino, F.; Pignatti, P.; Rudi, M.; Casu, G.; Vitacca, M.; Spanevello, A.; Visca, D. Effectiveness of a Pulmonary Rehabilitation Program on Persistent Asthma Stratified for Severity. Respir. Care 2019, 64, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Cameron-Tucker, H.L.; Wood-Baker, R.; Owen, C.; Joseph, L.; Walters, E.H. Chronic disease self-management and exercise in COPD as pulmonary rehabilitation: a randomized controlled trial. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 513–523. [Google Scholar] [CrossRef]
- Kerti, M.; Balogh, Z.; Kelemen, K.; Varga, J.T. The relationship between exercise capacity and different functional markers in pulmonary rehabilitation for COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 717–724. [Google Scholar] [CrossRef]
- Gonzalez-Gerez, J.J.; Saavedra-Hernandez, M.; Anarte-Lazo, E.; Bernal-Utrera, C.; Perez-Ale, M.; Rodriguez-Blanco, C. Short-Term Effects of a Respiratory Telerehabilitation Program in Confined COVID-19 Patients in the Acute Phase: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18. [Google Scholar] [CrossRef]
- Bures, M.; Neumannova, K.; Blazek, P.; Klima, M.; Schvach, H.; Nema, J.; Kopecky, M.; Dygryn, J.; Koblizek, V. A Sensor Network Utilizing Consumer Wearables for Telerehabilitation of Post-acute COVID-19 Patients. IEEE Internet Things J. 2022, PP, 1. [Google Scholar] [CrossRef]
- Bezuidenhout, L.; Joseph, C.; Thurston, C.; Rhoda, A.; English, C.; Conradsson, D.M. Telerehabilitation during the COVID-19 pandemic in Sweden: a survey of use and perceptions among physiotherapists treating people with neurological diseases or older adults. BMC Health Serv. Res. 2022, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, H.; Louvaris, Z.; Frei, A.; Rabinovich, R.A.; de Jong, C.; Gimeno-Santos, E.; Loeckx, M.; Buttery, S.C.; Rubio, N.; Van der Molen, T.; et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax 2017, 72, 415–423. [Google Scholar] [CrossRef]
- Holland, A.E.; Hill, C.J.; Rochford, P.; Fiore, J.; Berlowitz, D.J.; McDonald, C.F. Telerehabilitation for people with chronic obstructive pulmonary disease: feasibility of a simple, real time model of supervised exercise training. J. Telemed. Telecare 2013, 19, 222–226. [Google Scholar] [CrossRef] [PubMed]
- M. K., S.; T., J.; E.Y.L., W.; W.M., R.; N.G., J.; G.F., M. Using Telehealth technology to deliver pulmonary rehabilitation to patients with chronic obstructive pulmonary disease. Can. Respir. J. 2011, 18, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Martínez, M.Á.; López-Liria, R.; Martínez-Cal, J.; Benzo-Iglesias, M.J.; Torres-Álamo, L.; Rocamora-Pérez, P. Telerehabilitation, A Viable Option in Patients with Persistent Post-COVID Syndrome: A Systematic Review. Healthc. (Basel, Switzerland) 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Zampolini, M.; Hermens, H.J.; Ilsbroukx, S. Tele-rehabilitation : present and future. 2008.
- Velez, M.; Lugo-Agudelo, L.H.; Patiño Lugo, D.F.; Glenton, C.; Posada, A.M.; Mesa Franco, L.F.; Negrini, S.; Kiekens, C.; Spir Brunal, M.A.; Roberg, A.S.B.; et al. Factors that influence the provision of home-based rehabilitation services for people needing rehabilitation: a qualitative evidence synthesis. Cochrane Database Syst. Rev. 2023, 2023. [Google Scholar] [CrossRef]
- Włodarczyk, O.M.; Barinow-Wojewódzki, A. The impact of resistance respiratory muscle training with a SpiroTiger(®) device on lung function, exercise performance, and health-related quality of life in respiratory diseases. Kardiochirurgia i torakochirurgia Pol. = Polish J. cardio-thoracic Surg. 2015, 12, 386–390. [Google Scholar] [CrossRef]
- Hyzy, M.; Bond, R.; Mulvenna, M.; Bai, L.; Dix, A.; Leigh, S.; Hunt, S. System Usability Scale Benchmarking for Digital Health Apps: Meta-analysis. JMIR Mhealth Uhealth 2022, 10, e37290. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Rasekaba, T.; Lee, A.L.; Naughton, M.T.; Williams, T.J.; Holland, A.E. The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern. Med. J. 2009, 39, 495–501. [Google Scholar] [CrossRef]
- Cimolin, V.; Gobbi, M.; Buratto, C.; Ferraro, S.; Fumagalli, A.; Galli, M.; Capodaglio, P. A Comparative Analysis of Shoes Designed for Subjects with Obesity Using a Single Inertial Sensor: Preliminary Results. Sensors 2022, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Godinho, C.; Domingos, J.; Cunha, G.; Santos, A.T.; Fernandes, R.M.; Abreu, D.; Gonçalves, N.; Matthews, H.; Isaacs, T.; Duffen, J.; et al. A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson’s disease. J. Neuroeng. Rehabil. 2016, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Storm, F.A.; Cesareo, A.; Reni, G.; Biffi, E. Wearable inertial sensors to assess gait during the 6-minute walk test: A systematic review. Sensors (Switzerland) 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Mandaresu, S.; Pilloni, G.; Porta, M.; Coghe, G.; Marrosu, M.G.; Cocco, E. Smoothness of gait detects early alterations of walking in persons with multiple sclerosis without disability. Gait Posture 2017, 58, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Schifino, G.; Cimolin, V.; Pau, M.; da Cunha, M.J.; Leban, B.; Porta, M.; Galli, M.; Souza Pagnussat, A. Functional Electrical Stimulation for Foot Drop in Post-Stroke People: Quantitative Effects on Step-to-Step Symmetry of Gait Using a Wearable Inertial Sensor. Sensors (Basel). 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Cimolin, V.; Cau, N.; Malchiodi Albedi, G.; Aspesi, V.; Merenda, V.; Galli, M.; Capodaglio, P. Do wearable sensors add meaningful information to the Timed Up and Go test? A study on obese women. J. Electromyogr. Kinesiol. 2019, 44, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [CrossRef]
- Sartori, R.; Barbi, E.; Poli, F.; Ronfani, L.; Marchetti, F.; Amaddeo, A.; Ventura, A. Respiratory training with a specific device in cystic fibrosis: A prospective study. J. Cyst. Fibros. 2008, 7, 313–319. [Google Scholar] [CrossRef]
- Resnik, L.; Borgia, M. Reliability of outcome measures for people with lower-limb amputations: distinguishing true change from statistical error. Phys. Ther. 2011, 91, 555–565. [Google Scholar] [CrossRef]
- Rostagno, C.; Gensini, G.F. Six minute walk test: A simple and useful test to evaluate functional capacity in patients with heart failure. Intern. Emerg. Med. 2008, 3, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European respiratory society/American thoracic society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Lachant, D.J.; Light, A.; Offen, M.; Adams, J.; White, R.J. Heart rate monitoring improves clinical assessment during 6-min walk. Pulm. Circ. 2020, 10. [Google Scholar] [CrossRef]
- Dünnwald, T.; Kienast, R.; Niederseer, D.; Burtscher, M. The use of pulse oximetry in the assessment of acclimatization to high altitude. Sensors (Switzerland) 2021, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pires, I.M.; Villasana, M.V.; Sá, J.; Denysyuk, H.V.; Marques, D.L.; Morgado, J.F.; Albuquerque, C.; Zdravevski, E. Development Technologies for the Monitoring of Six-Minute Walk Test: A Systematic Review. Sensors 2022, 22, 1–29. [Google Scholar] [CrossRef]
- Ardestani, M.M.; Ferrigno, C.; Moazen, M.; Wimmer, M.A. From normal to fast walking: Impact of cadence and stride length on lower extremity joint moments. Gait Posture 2016, 46, 118–125. [Google Scholar] [CrossRef]
- Egerton, T.; Danoudis, M.; Huxham, F.; Iansek, R. Central gait control mechanisms and the stride length - cadence relationship. Gait Posture 2011, 34, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.P.; Kory, R.C.; Clarkson, B.H.; Sepic, S.B. Comparison of free and fast speed walking patterns of normal men. Am. J. Phys. Med. 1966, 45, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.; Wallace, C.; Stokic, D.S. Stride length-cadence relationship is disrupted in below-knee prosthesis users. Gait Posture 2013, 38, 883–887. [Google Scholar] [CrossRef]
- Abe, T.; DeHoyos, D.V.; Pollock, M.L.; Garzarella, L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur. J. Appl. Physiol. 2000, 81, 174–180. [Google Scholar] [CrossRef]
- Morgan, S.P.; Visovsky, C.; Thomas, B.; Klein, A.B. Respiratory Muscle Strength Training in Patients Post-COVID-19: A Systematic Review. Clin. Nurs. Res. 2024, 33, 60–69. [Google Scholar] [CrossRef] [PubMed]
- McNarry, M.A.; Berg, R.M.G.; Shelley, J.; Hudson, J.; Saynor, Z.L.; Duckers, J.; Lewis, K.; Davies, G.A.; Mackintosh, K.A. Inspiratory muscle training enhances recovery post-COVID-19: a randomised controlled trial. Eur. Respir. J. 2022, 60. [Google Scholar] [CrossRef]
- Grier, R.A.; Bangor, A.; Kortum, P.; Peres, S.C. The system usability scale: Beyond standard usability testing. Proc. Hum. Factors Ergon. Soc. 2013, 187–191. [Google Scholar] [CrossRef]
- Tiruneh, S.A.; Tesema, Z.T.; Azanaw, M.M.; Angaw, D.A. The effect of age on the incidence of COVID-19 complications: a systematic review and meta-analysis. Syst. Rev. 2021, 10, 80. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. an Off. J. Int. Assoc. Study Obes. 2020, 21, e13128. [Google Scholar] [CrossRef] [PubMed]
- Steenblock, C.; Hassanein, M.; Khan, E.G.; Yaman, M.; Kamel, M.; Barbir, M.; Lorke, D.E.; Everett, D.; Bejtullah, S.; Lohmann, T.; et al. Obesity and COVID-19: What are the Consequences? Horm. Metab. Res. 2022, 54, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Nour, T.Y.; Altintaş, K.H. Effect of the COVID-19 pandemic on obesity and it is risk factors: a systematic review. BMC Public Health 2023, 23, 1–24. [Google Scholar] [CrossRef]
- Dalbosco-Salas, M.; Torres-Castro, R.; Leyton, A.R.; Zapata, F.M.; Salazar, E.H.; Bastías, G.E.; Díaz, M.E.B.; Allers, K.T.; Fonseca, D.M.; Vilaró, J. Effectiveness of a primary care telerehabilitation program for post-covid-19 patients: A feasibility study. J. Clin. Med. 2021, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kortianou, E.A.; Tsimouris, D.; Mavronasou, A.; Lekkas, S.; Kazatzis, N.; Apostolara, Z.E.; Isakoglou, M.; Dimakou, G.; Barmparessou, Z.; Tsikrika, S.; et al. Application of a home-based exercise program combined with tele-rehabilitation in previously hospitalized patients with COVID-19: A feasibility, single-cohort interventional study. Pneumon 2022, 35, 1–10. [Google Scholar] [CrossRef]
- Theodoros, D.; Russell, T. Telerehabilitation: current perspectives. Stud. Health Technol. Inform. 2008, 131, 191–209. [Google Scholar]
- Baroni, M.P.; Jacob, M.F.A.; Rios, W.R.; Fandim, J.V.; Fernandes, L.G.; Chaves, P.I.; Fioratti, I.; Saragiotto, B.T. The state of the art in telerehabilitation for musculoskeletal conditions. Arch. Physiother. 2023, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zampolini, M.; Oral, A.; Barotsis, N.; Aguiar Branco, C.; Burger, H.; Capodaglio, P.; Dincer, F.; Giustini, A.; Hu, X.; Irgens, I.; et al. Evidence-based position paper on Physical and Rehabilitation Medicine (PRM) professional practice on telerehabilitation. The European PRM position (UEMS PRM Section). Eur. J. Phys. Rehabil. Med. 2024, 60, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Rogante, M.; Grigioni, M.; Cordella, D.; Giacomozzi, C. Ten years of telerehabilitation: A literature overview of technologies and clinical applications. NeuroRehabilitation 2010, 27, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Rogante, M.; Kairy, D.; Giacomozzi, C.; Grigioni, M. A quality assessment of systematic reviews on telerehabilitation: what does the evidence tell us? Ann. Ist. Super. Sanita 2015, 51, 11–18. [Google Scholar] [CrossRef]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the State-of-the-Art and Areas of Application. JMIR Rehabil. Assist. Technol. 2017, 4, e7. [Google Scholar] [CrossRef]
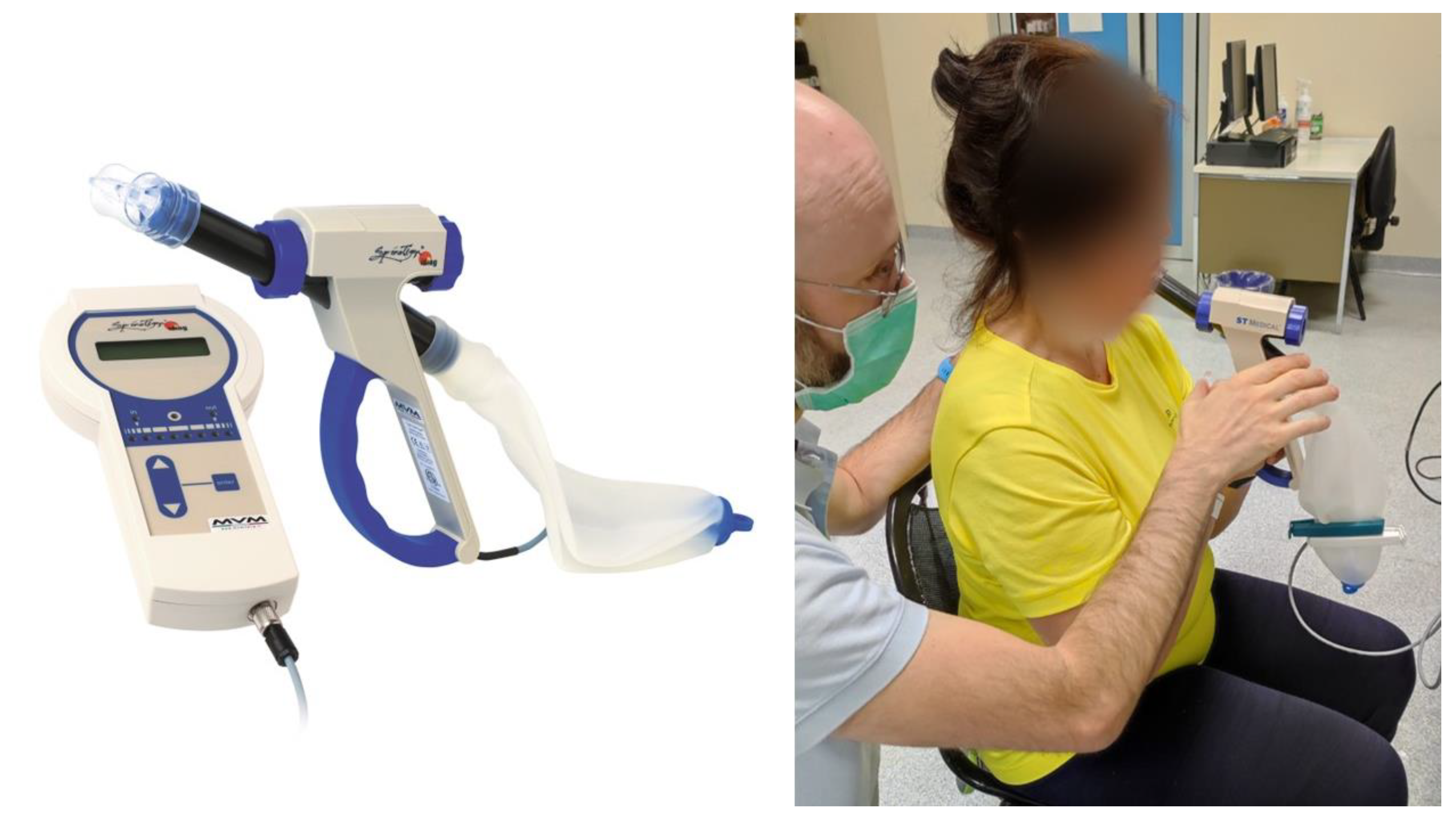
| Participants (n= 23) | |
|---|---|
| M/F | 6/17 |
| Age (years) | 55.91 ± 7.57 |
| Height (cm) | 164.58 ± 6.20 |
| Weight (kg) | 98.11 ± 17.82 |
| BMI (kg/m2) | 36.38 ± 7.06 |
| Sessions | |||
|---|---|---|---|
| T0 | T1 | T2 | |
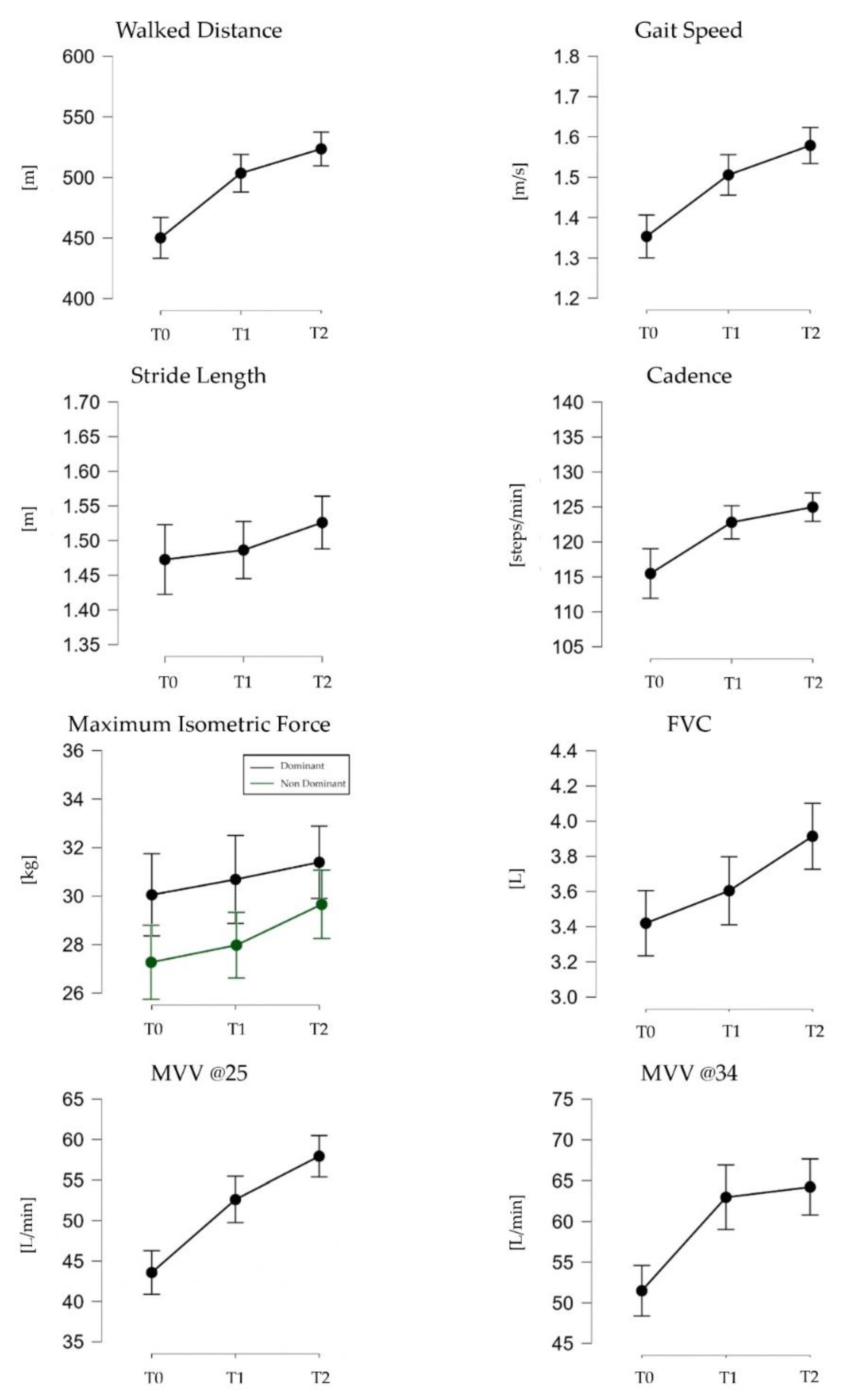
| Walked Distance (m) | 450.16 (69.24) | 503.52 (63.85) * | 523.59 (57.59) * |
| Gait speed (m/s) | 1.35 (0.22) | 1.51 (0.20) * | 1.58 (0.18) * |
| Stride Length (m) | 1.47 (0.21) | 1.49 (0.16) | 1.53 (0.16) |
| Cadence (steps/min) | 115.48 (14.59) | 122.81 (9.44) | 124.99 (8.43) * |
| Maximum Isometric Force (kg) (D) | 30.05 (7.56) | 30.69 (8.53) | 31.40 (6.81) |
| Maximum Isometric Force (kg) (ND) | 27.39 (8.05) | 28.22 (7.48) | 30.20 (7.62) |
| FVC (L) | 3.42 (0.81) | 3.60 (0.91) | 3.91 (0.88) *,+ |
| MVV @25 (L/min) | 43.57 (12.09) | 52.61 (12.38)* | 57.93 (12.02) *,+ |
| MVV @34 (L/min) | 51.49 (12.89) | 62.97 (18.54)* | 64.23 (16.09)* |
| Hear Rate (bpm) | SpO2(%) | |||
|---|---|---|---|---|
| Session | Pre-test | Post-test | Pre-test | Post-test |
| T0 | 76.39 (11.55) | 101.41 (17.14)* | 95.33 (2.45) | 91.06 (6.19)* |
| T1 | 81.40 (14.52) | 108.55 (20.46)* | 94.50 (2.36) | 93.40 (3.85) |
| T2 | 85.05 (17.41) | 112.10 (20.04)* | 94.05 (5.39) | 92.40 (4.13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





