Submitted:
31 May 2024
Posted:
04 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Safety, Codes, and Standards
2.1. Safety Considerations about Gaseous Hydrogen
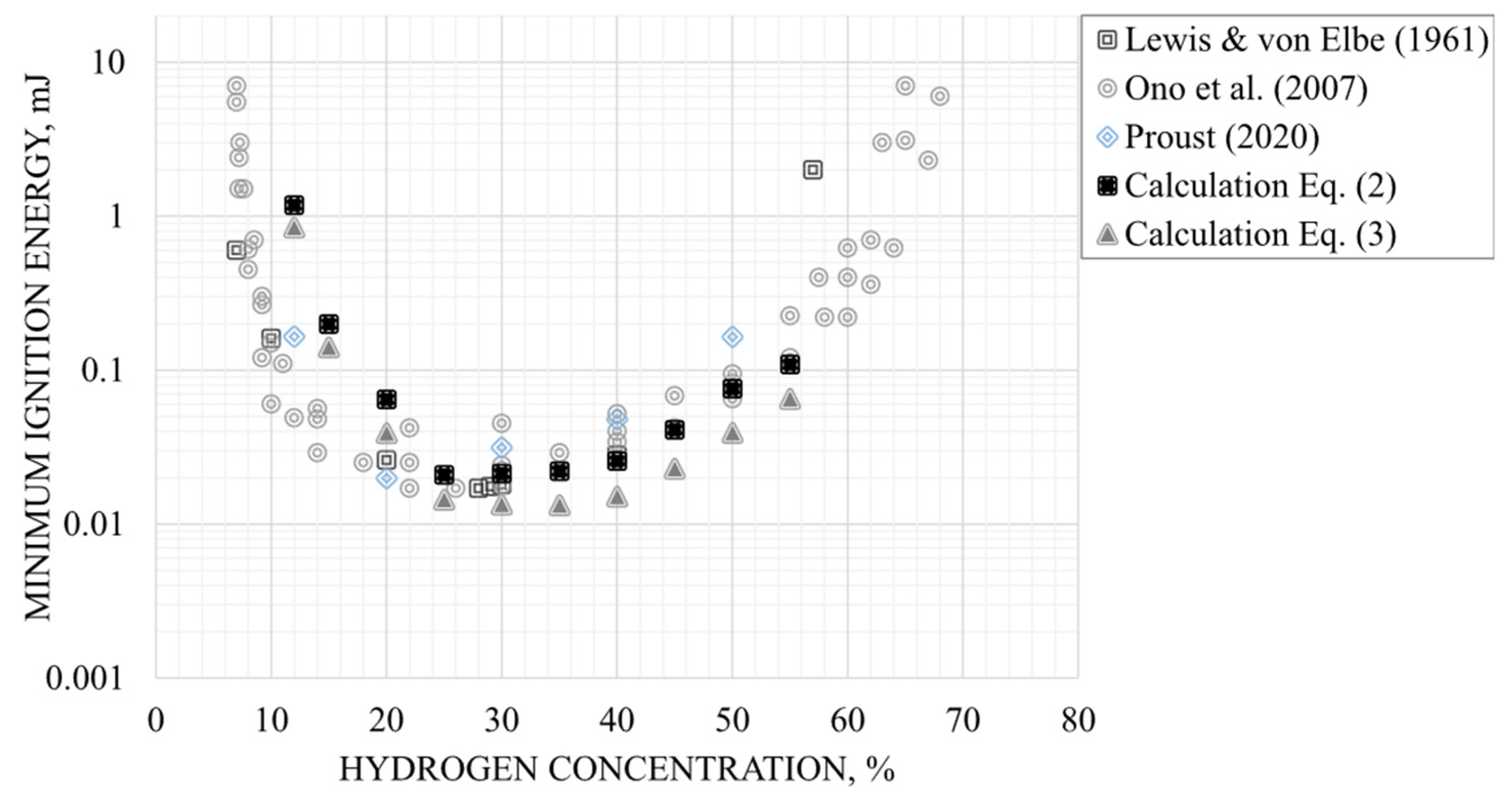
- Hydrogen ignites very easily when it is close to stoichiometric conditions where a minimum ignition energy (MIE) in air is observed at only 17 µJ [21] (Figure 2) – for comparison, an electrostatic discharge just felt by a person is around 1000 µJ – and the MIE even decreases in pure oxygen to 1.2 µJ [22]. This characteristic is reflected by the gas sub-group IIC for equipment used in potentially explosive atmospheres (ATEX) according to the EU legislation Directive 2014/34/EU [23];
- The burning velocity of hydrogen in air at stoichiometric ambient conditions is 2.37 m/s [24] and can even increase up to 3.5 m/s at a concentration of 40.1% [25]. Related to its fast chemical kinetics and high diffusivity, this burning velocity is higher than that of other hydrocarbon fuel – air mixtures and results in a greater chance for a transition of the combustion mode from deflagration to detonation;
- Due to its small-sized molecule, hydrogen leaks are more likely to occur than with other inflammable gases. Furthermore, due to its low viscosity, the volume flow rate is then nearly 3 times higher compared for instance with methane [26];
- At ambient conditions, hydrogen is about 8 times lighter than natural gas and 14 times lighter than air. While released in an open environment, it will typically rise and disperse rapidly. This is a safety advantage in an outside environment for instance with a subsonic or vertically orientated leak, but it has to be carefully taken into account in a confined space by a pertinent leak detection system and a ventilation assuring sufficient air dilution at the leak source;
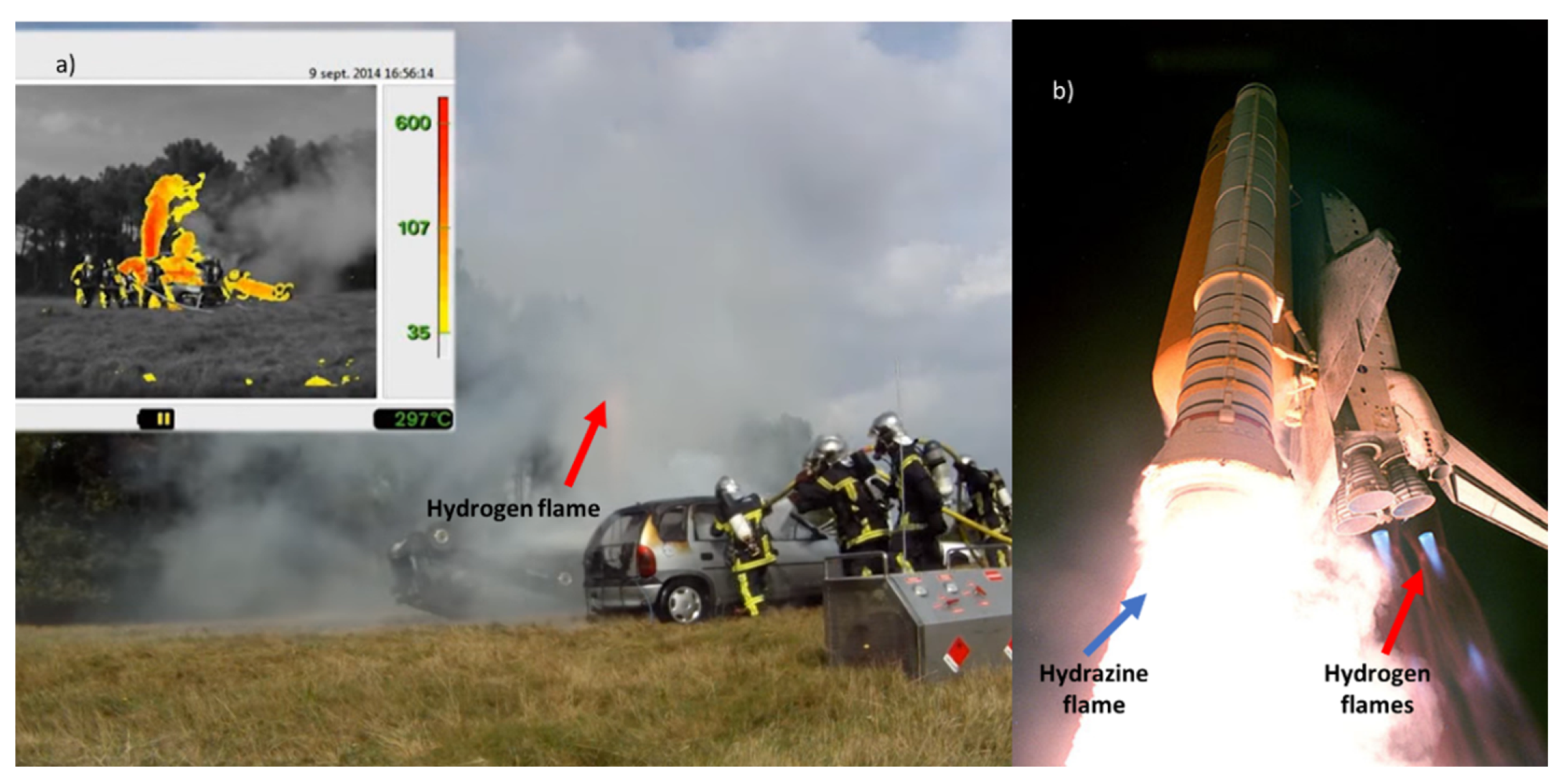
- A jet fire of hydrogen generates a pale blue flame almost invisible in daylight (Figure 3a). However, its visibility can be increased if particles are entrained in the flame (e.g. dust) and hydrogen flames are also visible at night, as shown in Figure 3b. In air, a premixed stoichiometric blend can lead to a flame temperature of 2403 K [24];
- The dominant energy emission occurs in the mid-infrared, where the peak irradiance is more than 1000 times greater than the peak measured in the ultraviolet. Thermal radiation is therefore very limited and unconventional UV detection is required [29];
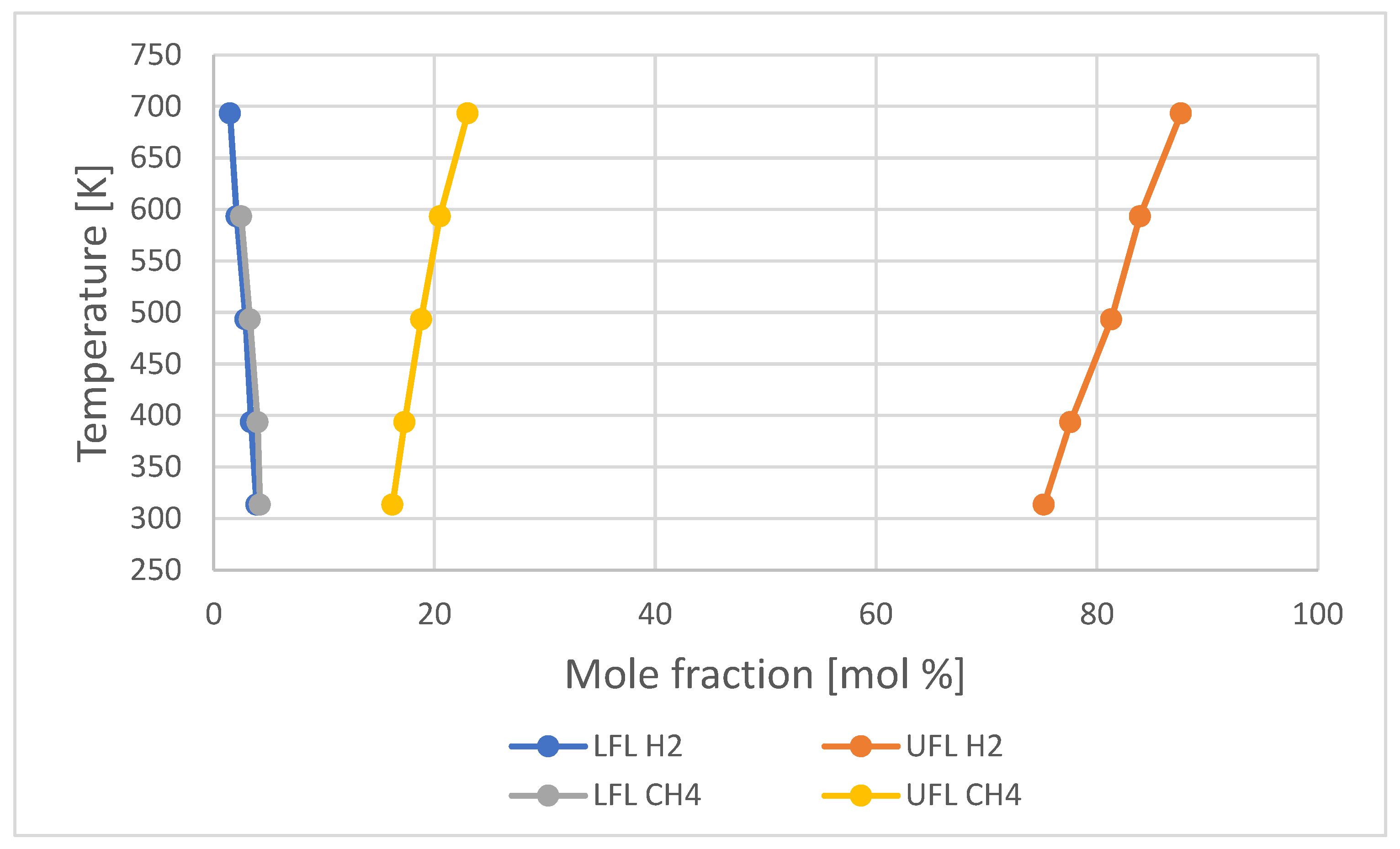
- According to different experiences with hydrogen at concentrations below 10% vol. in air [30], the explosion resembles to a flash fire with nearly no pressure rise. At higher concentrations, deflagration is observed where the flame speed is subsonic, and the maximum pressure peak is reached at 8 bar in air and 10 bar in pure oxygen [31]. At supersonic flame speeds, detonation occurs and can be observed at ranges in excess of 12,5% vol. of hydrogen where explosion pressures 15 to 20 times greater than the initial pressure can occur at the detonation front depending on concentration and turbulence conditions in the environment. The most violent reaction occurs when hydrogen is near to its stoichiometric value of 29,5% vol. in air. Table 1 summarises the main differences between deflagration and detonation;
- Various embrittlement mechanisms such as hydrogen-induced cracking or blistering are also possible with hydrogen depending both on the metal, alloy or composite selection and on the process parameters (temperature and pressure typically), so that the choice of the material constitutes a point of vigilance when designing containers or pipelines (Section 3).
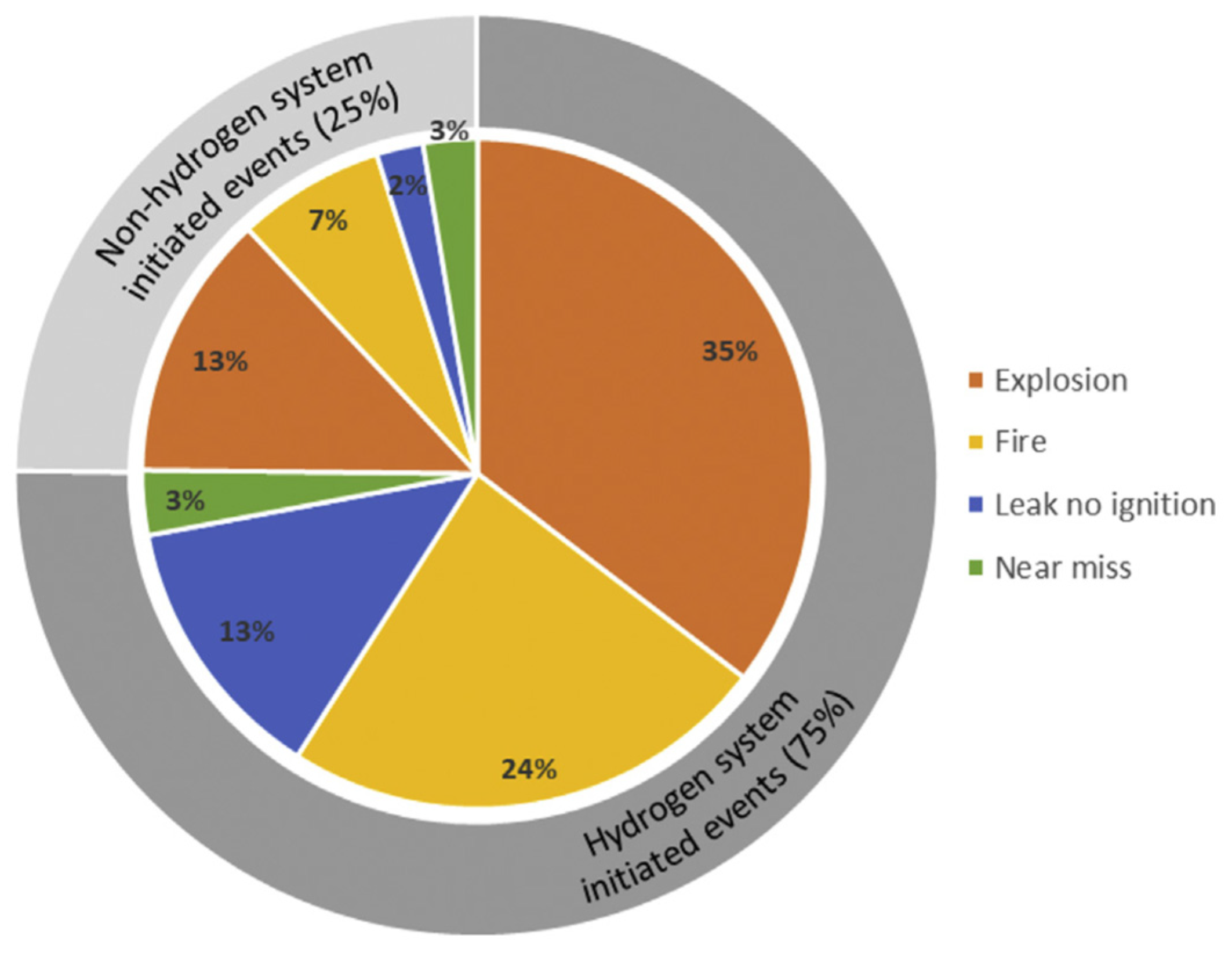
2.2. Lessons Learnt with Hydrogen
3. Gaseous Transport and Buffer Storage
- In many circumstances, hydrogen gas can weaken metallic materials, notably high-strength steels containing ferritic phases;
- The volumetric density of hydrogen gas being much lower than that of natural gas, the pressure has to be increased in order to deliver the same amount of energy whereas, thanks to the higher compressibility factor of hydrogen, its pressure drop in a pipeline over a long distance is significantly lower than that of natural gas.
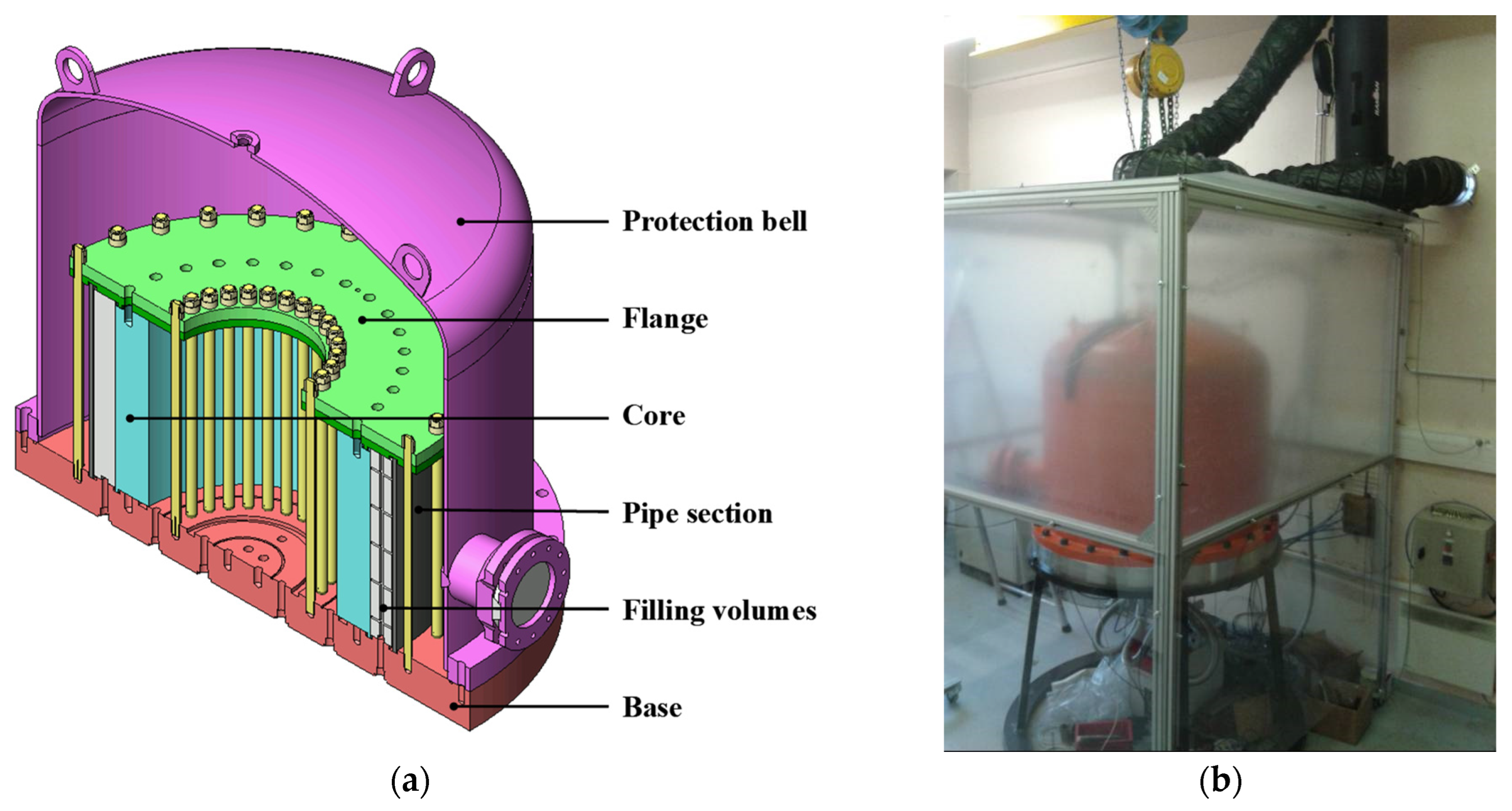
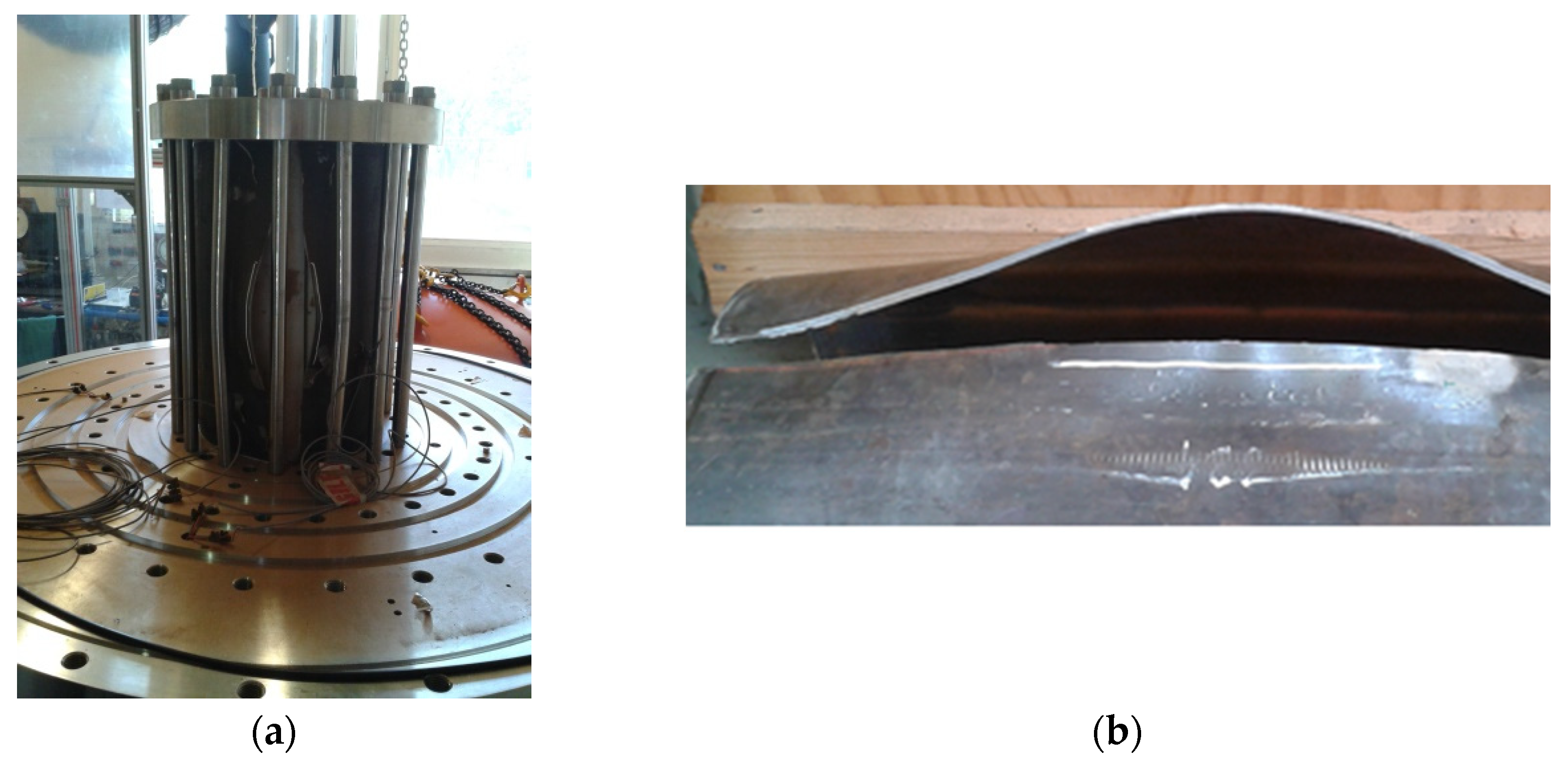
- Validation phase: the main objective was to demonstrate the capacity of the implemented bench to test a selection of representative pipe sections under cyclic loading at a low frequency (40 s per cycle typically). The possibility to test under monotonic loading was also to be demonstrated.
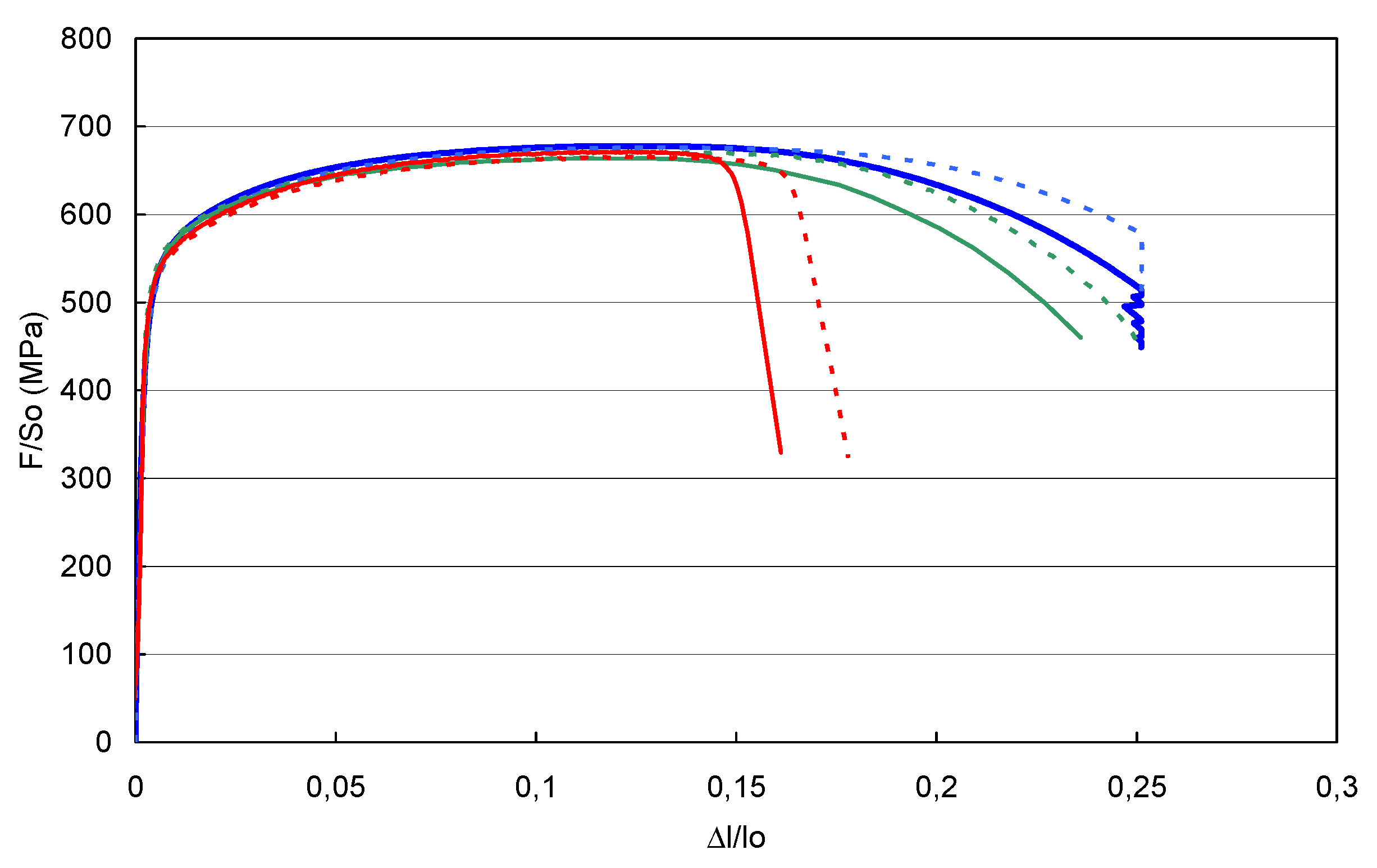
- Operational phase: a series of bursting tests under monotonic loading has been implemented on pre-notched pipe sections. The pressurised medium was pure hydrogen, which corresponds to a ‘worst-case’ scenario, for the effect to be as more significant as possible. In each case, a reference test has been carried out under nitrogen pressure for comparison with the results obtained under hydrogen. SEM observations initially aimed to determine the fragile or brittle nature of the fractures.
3.1. Validation Phase
- To validate defect failure assessment models by comparing their numerical results with the experimental ones obtained on the test bench;
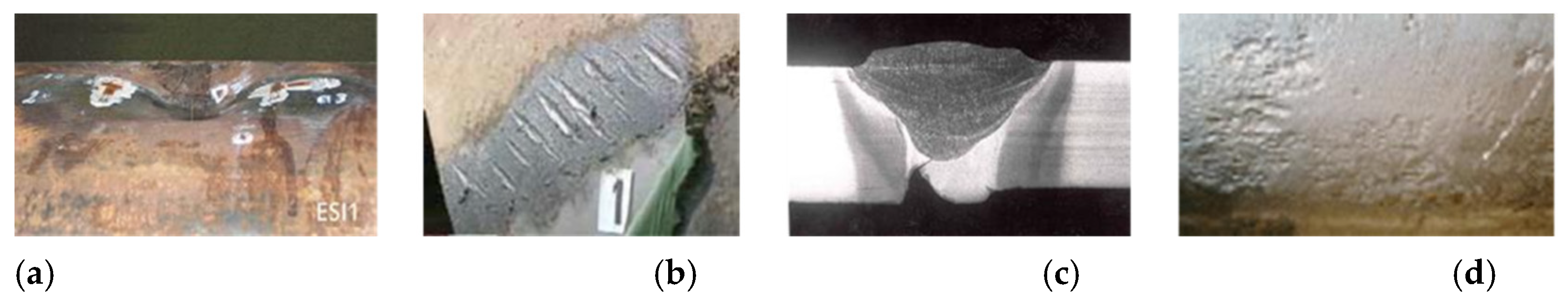
- To study the stability or instability of internal as well as external defects on hydrogen pipelines, considering the fact that, in highly populated areas such as Western Europe, the most frequent incidents on natural-gas pipelines are due to mechanical damage in relation to third-party aggressions [42]. Internal corrosion-type defects are in the minority, or even nonexistent, due to the non-corrosive qualities of the transported gas, but there may be occurrences of internal defects at the girth welds (Figure 5c);
- To validate the transferability of the fracture-mechanics results obtained on laboratory specimens to a real structure;
- To possibly serve as an instrumented hydrogen reservoir to be coupled for instance with an electrolyser, as in the case of a renewable-energy-related buffer storage.
- presence of idealised or realistic defects identical to those identified by the industrial partner (Figure 5);
- same gas/defect interactions as for a distribution pipeline;
- bi-axial stress state in the pipeline section.
3.2. Experimental Phase
4. Safe Mass Storage in the Solid State
- Physical or chemical operations as simple as possible for long-time operations;
- Kinetics fast enough to anticipate production and delivery that are both mostly intermittent;
- Safe modes of management.
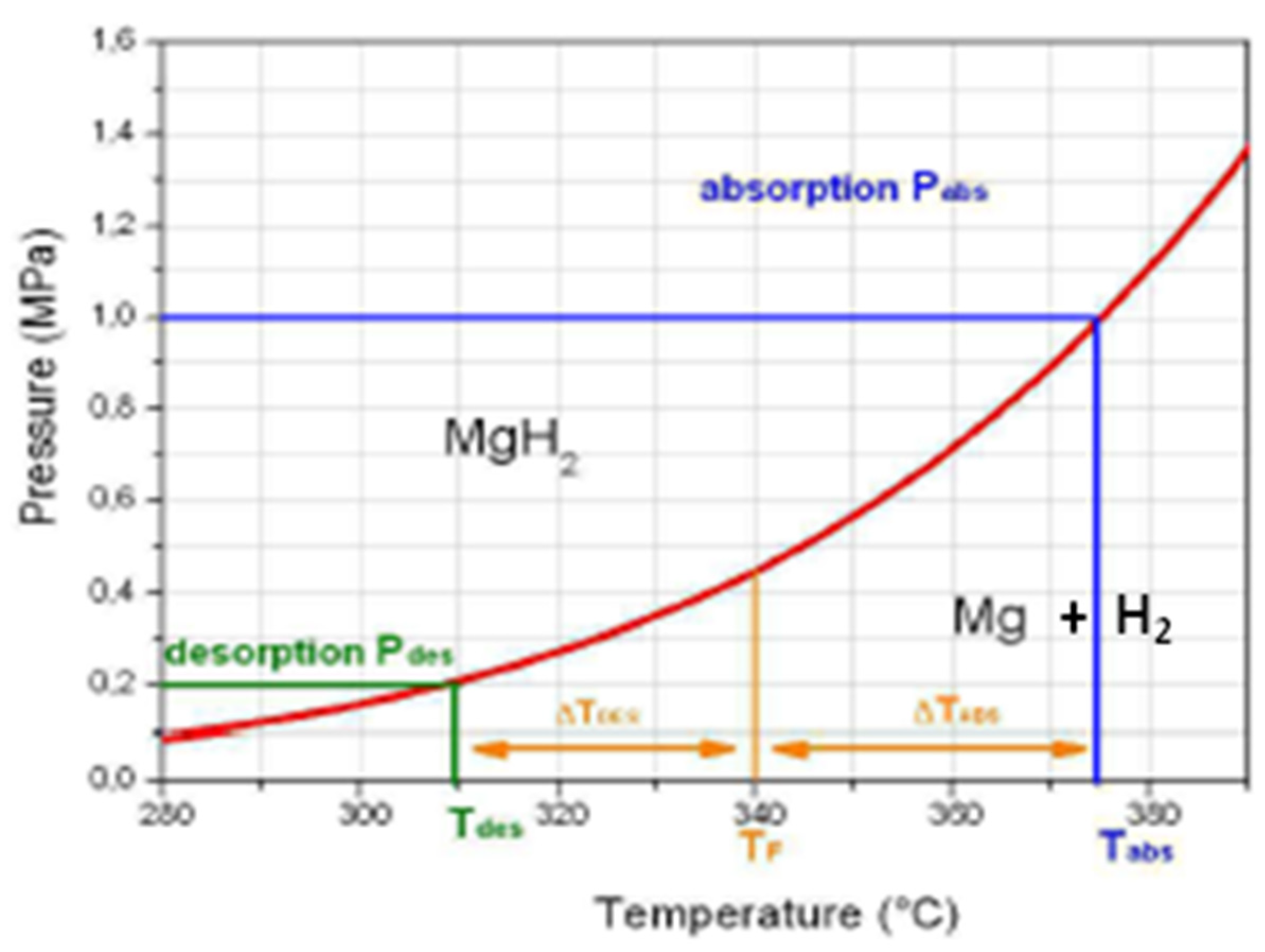
- The reaction is particularly slow, even when starting from raw powdered samples;
- Active reaction takes place at over 300 °C but, interestingly, under a few bars of hydrogen pressure.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Jia, C.; Bai, F.; Wang, W.; An, S.; Zhao, K.; Li, Z.; Li, J.; Sun, H. A comprehensive review of the promising clean energy carrier: Hydrogen production, transportation, storage, and utilization (HPTSU) technologies. Fuel 2024, 355, 129455. [Google Scholar] [CrossRef]
- Pique, S.; Weinberger, B.; de Dianous, V.; Debray, B. Comparative study of regulations, codes and standards and practices on hydrogen fuelling stations. Int. J. Hydrogen Energ. 2017, 42, 7429–7439. [Google Scholar] [CrossRef]
- Darkrim Lamari, F.; Weinberger, B.; Girodon-Boulandet, N.; Fagnon, N.; Batisse, R.; Briottet, L.; Langlois, P. High-pressure hydrogen storage for on-board applications and for coupling renewable energies to the electric grid. High Pressure Res. 2009, 29, 660–664. [Google Scholar] [CrossRef]
- Fruchart, D.; Jehan, M.; Skryabina, N.; de Rango, P. Hydrogen Solid State Storage on MgH2 Compacts for Mass Apllications. Metals 2023, 13, 992. [Google Scholar] [CrossRef]
- Guo, L.; Su, J.; Wang, Z.; Shi, J.; Guan, X.; Cao, W.; Ou, Z. Hydrogen safety: An obstacle that must be overcome on the road towards future hydrogen economy. Int. J. Hydrogen Energ. 2024, 51, 1055–1078. [Google Scholar] [CrossRef]
- Calabrese, M.; Portarapillo, M.; Di Nardo, A.; Venezia, V.; Turco, M.; Luciani, G.; Di Benedetto, A. Hydrogen Safety Challenges: A Comprehensive Review on Production, Storage, Transport, Utilization, and CFD-Based Consequence and Risk Assessment. Energies 2024, 17, 1350. [Google Scholar] [CrossRef]
- Kazemi, M.; Brennan, S.; Molkov, V. Hydrogen Safety by Design: Exclusion of Flame Blow-Out from a TPRD. Hydrogen 2024, 5, 280–292. [Google Scholar] [CrossRef]
- Di Lullo, G.; Oni, A.O.; Kumar, A. Blending blue hydrogen with natural gas for direct consumption: Examining the effect of hydrogen concentration on transportation and well-to-combustion greenhouse gas emissions. Int. J. Hydrogen Energ. 2021, 46, 19202–19216. [Google Scholar] [CrossRef]
- Di Lullo, G.; Giwa, T.; Okunlola, A.; Davis, M.; Mehedi, T.; Oni, A.O.; Kumar, A. Large-scale long-distance land-based hydrogen transportation systems: A comparative techno-economic and greenhouse gas emission assessment. Int. J. Hydrogen Energ. 2022, 47, 35293–35319. [Google Scholar] [CrossRef]
- Yang, M.; Hunger, R.; Berrettoni, S.; Sprecher, B.; Wang, B. A review of hydrogen storage and transport technologies. Clean Energy 2023, 7, 190–216. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V.; Salgansky, E.A.; Arutyunov, A.V.; Arutyunov, V.S. A Comprehensive Review on the Prospects of Using Hydrogen–Methane Blends: Challenges and Opportunities. Energies 2022, 15, 2265. [Google Scholar] [CrossRef]
- Tsiklios, C.; Hermesmann, M.; Müller, T.E. Hydrogen transport in large-scale transmission pipeline networks: Thermodynamic and environmental assessment of repurposed and new pipeline configurations. Appl. Energy 2022, 327, 120097. [Google Scholar] [CrossRef]
- Briottet, L.; Batisse, R.; de Dinechin, G.; Langlois, P.; Thiers, L. Recommendations on X80 steel for the design of hydrogen gas transmission pipelines. Int. J. Hydrogen Energ. 2012, 37, 9423–9430. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage. Int. J. Hydrogen Energ. 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Cao, Z.; Habermann, F.; Burkmann, K.; Felderhoff, M.; Mertens, F. Unstable Metal Hydrides for Possible On-Board Hydrogen Storage. Hydrogen 2024, 5, 241–279. [Google Scholar] [CrossRef]
- Lázár, M.; Mihálik, I.; Brestovič, T.; Jasminská, N.; Tóth, L.; Dobáková, R.; Duda, F.; Kmet’ová, L.; Hudák, Š. A Newly Proposed Method for Hydrogen Storage in a Metal Hydride Storage Tank Intended for Maritime and Inland Shipping. J. Mar. Sci. Eng. 2023, 11, 1643. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Sun, Y.-J.; Zhang, C.-C.; Wei, S.; Zhao, L.; Zeng, J.-L.; Cao, Z.; Zou, Y.-J.; Chu, H.-L.; Xu, F.; et al. Optimizing hydrogen ad/desorption of Mg-based hydrides for energy-storage applications. J. Mater. Sci. Technol. 2023, 141, 221–235. [Google Scholar] [CrossRef]
- Skryabina, N.; Aptukov, V.; Fruchart, D. Role of induced elastic deformations at the Mg/MgH2 transformation. J. Alloy. Metall. Systems 2024, 5, 100064. [Google Scholar] [CrossRef]
- Jehan, M.; Fruchart, D. McPhy-Energy's proposal for solid state hydrogen storage materials and systems. J. Alloys Compd. 2013, 580, 343–348. [Google Scholar] [CrossRef]
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Available online: http://data.europa.eu/eli/reg/2008/1272/2023-12-01 (also at web.archive.org).
- Schroeder, V.; Holtappels, K. Explosion Characteristics of Hydrogen-Air and Hydrogen-Oxygen Mixtures at Elevated Pressures. In Proceedings of the 1st International Conference on Hydrogen Safety, Pisa, IT, 8-10 September 2005. Available online: http://conference.ing.unipi.it/ichs2005/Papers/120001.pdf (also at web.archive.org). [Google Scholar]
- Lewis, B.; von Elbe, G. Combustion, flames and explosions of gases, 2nd ed.; Academic Press: New York and London, 1961. [Google Scholar] [CrossRef]
- Kuchta, J.M. Investigation of fire and explosion accidents in the chemical, mining, and fuel-related industries. Bull., U.S. Dept. Inter. Bur. Mines 1985, 680, 1–66. [Google Scholar]
- Cirrone, D.; Makarov, D.; Proust, C.; Molkov, V. Minimum ignition energy of hydrogen-air mixtures at ambient and cryogenic temperatures. Int. J. Hydrogen Energ. 2023, 48, 16530–16544. [Google Scholar] [CrossRef]
- Directive 2014/34/EU of the European Parliament and of the Council of 26 February 2014 on the harmonisation of the laws of the Member States relating to equipment and protective systems intended for use in potentially explosive atmospheres. Available online: https://eur-lex.europa.eu/eli/dir/2014/34/oj (also at web.archive.org).
- Verfondern, K. Hydrogen fundamentals. In Hydrogen Safety for Energy Applications: Engineering Design, Risk Assessment, and Codes and Standards, 1st ed.; Kotchourko, A., Jordan, Th., Eds.; Butterworth-Heinemann, 2022; pp. 1–23. [CrossRef]
- Kroener, M.; Fritz, J.; Sattelmayer, T. Flashback limits for combustion-induced vortex breakdown in a swirl burner. J. Eng. Gas Turbines Power 2003, 125, 693–700. [Google Scholar] [CrossRef]
- Hormaza Mejia, A.; Brouwer, J.; Mac Kinnon, M. Hydrogen leaks at the same rate as natural gas in typical low-pressure gas infrastructure. Int. J. Hydrogen Energ. 2020, 45, 8810–8826. [Google Scholar] [CrossRef]
- Hydrogen vehicle burn test, Vienne Fire and Rescue Service (France). Available online: https://www.youtube.com/embed/ow47SePNz-s (accessed on 22 March 2024).
- Schefer, R.W.; Kulatilaka, W.D.; Patterson, B.D.; Settersten, T.B. Visible emission of hydrogen flames. Combust. Flame 2009, 156, 1234–1241. [Google Scholar] [CrossRef]
- Proust, C. Fire and Explosion Safety in Hydrogen Containing Processes: State of the Art and Outstanding Questions. In Proceedings of the 9th International Seminar on Fire and Explosion Hazards, Saint Petersburg, RU, 21-26 April 2019; pp. 28–40. [Google Scholar] [CrossRef]
- Kuznetsov, M.; Grune, J. Experiments on flame acceleration and DDT for stoichiometric hydrogen/air mixtures in a thin layer geometry. In Proceedings of the 7th International Conference on Hydrogen Safety, Hamburg, DE, 11-13 September 2017. Available online: https://hysafe.info/uploads/papers/2017/199.pdf (also at web.archive.org). [Google Scholar]
- Hydrogen Incident and Accident Database. https://hysafe.info/hiad-2-0-free-access-to-the-renewed-hydrogen-incident-and-accident-database/ (also available at web.archive.org).
- Hydrogen Lessons Learned Reporting Tool. https://h2tools.org/lessons (also available at web.archive.org).
- French database ARIA ‘Analyse, Recherche et Information sur les Accidents’. https://www.aria.developpement-durable.gouv.fr/?lang=en (also available at web.archive.org).
- Reports of the High-Pressure Gas Safety Institute of Japan. https://www.khk.or.jp/english/ (also available at web.archive.org).
- Campari, A.; Nakhal Akel, A.J.; Ustolin, F.; Alvaro, A.; Ledda, A.; Agnello, P.; Moretto, P.; Patriarca, R.; Paltrinieri, N. Lessons learned from HIAD 2.0: Inspection and maintenance to avoid hydrogen-induced material failures. Comput. Chem. Eng. 2023, 173, 108199. [Google Scholar] [CrossRef]
- Wen, J.X.; Marono, M.; Moretto, P.; Reinecke, E.-A.; Sathiah, P.; Studer, E.; Vyazmina, E.; Melideo, D. Statistics, lessons learned and recommendations from analysis of HIAD 2.0 database. Int. J. Hydrogen Energ. 2022, 47, 17082–17096. [Google Scholar] [CrossRef]
- Accidentologie de l’hydrogène, Synthèse ARIA. 2009. Available online: https://www.aria.developpement-durable.gouv.fr/wp-content/files_mf/1373986645SYHydrogene2008.pdf (also at web.archive.org).
- Batisse, R.; Briottet, L.; de Dinechin, G.; Wastiaux, S.; Langlois, P. Ability of X80 steel for hydrogen gas transmission pipelines. In Proceedings of the 12th International Conference on Fracture; p. 3015.
- Briottet, L.; Moro, I.; Lemoine, P. Quantifying the hydrogen embrittlement of pipe steels for safety considerations. In Proceedings of the 3rd International Conference on Hydrogen Safety, Ajaccio, FR, 16-18 September 2009; Available online: https://conference.ing.unipi.it/ichs2011/papers/186.pdf (accessed on 22 May 2024).
- Bolt, R. A Guideline: Using or Creating Incident Databases for Natural Gas Transmission Pipelines. Report of IGU Study Group 3.4, 23rd World Gas Conference, Amsterdam, NL, 1-5 June 2006.
- Sofronis, P.; Robertson, I.M.; Johnson, D.D.; Somerday, B. Hydrogen Embrittlement-Fundamentals, Modeling, and Experiment. DOE Hydrogen Pipeline Working Group Meeting, Aiken, US-SC, 23-26 September, 2007.
- Batisse, R.; Cuni, A.; Wastiaux, S.; Briottet, L.; Lemoine, P.; de Dinechin, G.; Chagnot, C.; Castilan, F.; Klosek, V.; Langlois, P.; et al. Investigation of X80-steel grade for hydrogen gas transmission pipelines. In Proceedings of the 6th International Gas union Research Conference, Paris, FR, 8-10 October 2008. [Google Scholar]
- Moro, I.; Briottet, L.; Lemoine, P.; Andrieu, E.; Blanc, C.; Odemer, G. Hydrogen embrittlement susceptibility of a high strength steel X80. Mater. Sci. Eng. A 2010, 527, 7252–7260. [Google Scholar] [CrossRef]
- Pasquini, L.; Sakaki, K.; Akiba, E.; Alendorf, M.D.; Alvares, E.; Ares, J.R.; Babai, D.; Baricco, M.; Bellosta von Colbe, J.; Bereznitsky, M.; et al. Magnesium- and intermetallic alloys-based hydrides for energy storage: Modelling, synthesis and properties. Prog. Energy 2022, 4, 032007. [Google Scholar] [CrossRef]
- Edalati, K.; Akiba, E.; Botta, W.J.; Estrin, Y.; Ricardo, F.; Fruchart, D.; Grosdidier, T.; Horita, Z.; Huot, J.; Li, H.-W.; et al. Impact of Severe Plastic Deformation on Kinetics and Thermodynamics of Hydrogen Storage in Magnesium and Its Alloys. J. Mater. Sci. Technol. 2023, 146, 221–239. [Google Scholar] [CrossRef]
- Edalati, K.; Yamamoto, A.; Horita, Z.; Ishihara, T. High-pressure torsion of pure magnesium: Evolution of mechanical properties, microstructures and hydrogen storage capacity with equivalent strain. Scripta Mater. 2011, 64, 880–883. [Google Scholar] [CrossRef]
- Révész, Á.; Kánya, Z.; Verebélyi, T.; Szabó, P.J.; Zhilyaev, A.P.; Spassov, T. The effect of high-pressure torsion on the micro-structure and hydrogen absorption kinetics of ball-milled Mg70Ni30. J. Alloys Compd. 2010, 504, 83–88. [Google Scholar] [CrossRef]
- Skrypnyuk, V.M.; Rabkin, E.; Estrin, Y.; Lapovok, R. The effect of ball milling and equal channel angular pressing on the hydrogen absorption/desorption properties of Mg–4.95 wt% Zn–0.71 wt% Zr (ZK60) alloy. Acta Mater. 2004, 52, 405–414. [Google Scholar] [CrossRef]
- Skryabina, N.; Aptukov, V.; Romanov, P.; Fruchart, D.; de Rango, P.; Girard, G.; Grandini, C.; Sandim, H.; Huot, J.; Lang, J.; et al. Microstructure Optimization of Mg-Alloys by the ECAP Process Including Numerical Simulation, SPD Treatments, Characterization, and Hydrogen Sorption Properties. Molecules 2019, 24, 24010089. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.; Tousignant, M. Effet of Cold Rolling on Metal Hydrides. Mater. Trans. 2019, 60, 1571–1576. [Google Scholar] [CrossRef]
- Floriano, R.; Leiva, D.R.; Melo, G.C.; Ishikawa, T.T.; Huot, J.; Kaufman, M.; Figueroa, S.J.A.; Mendoza-Zélis, L.A.; Damonte, L.C.; Botta, W.J. Low temperature rolling of AZ91 alloy for hydrogen storage. Int. J. Hydrogen Energ. 2017, 42, 29394–29405. [Google Scholar] [CrossRef]
- Skryabina, N.; Aptukov, V.; de Rango, P.; Fruchart, D. Effect of temperature on fast forging process of Mg-Ni samples for fast formation of Mg2Ni for hydrogen storage. Int. J. Hydrogen Energ. 2020, 45, 3008–3015. [Google Scholar] [CrossRef]
- de Rango, P.; Fruchart, D.; Aptukov, V.; Skryabina, N. Fast forging: A new SPD method to synthesize Mg-based alloys for hydrogen storage. Int. J. Hydrogen Energ. 2020, 45, 7912–7916. [Google Scholar] [CrossRef]
- Chaise, A.; de Rango, P.; Marty, P.; Fruchart, D.; Miraglia, S.; Olivès, R.; Garrier, S. Enhancement of hydrogen sorption in magnesium hydride using expanded natural graphite. Int. J. Hydrogen Energ. 2009, 34, 8589–8596. [Google Scholar] [CrossRef]
- Garrier, S.; Delhomme, B.; de Rango, P.; Marty, P.; Fruchart, D.; Miraglia, S. A new MgH2 tank concept using a phase-change material to store the heat of reaction. Int. J. Hydrogen Energ. 2013, 38, 9766–9771. [Google Scholar] [CrossRef]
- de Rango, P.; Marty, P.; Fruchart, D. Hydrogen storage systems based on magnesium hydride: From laboratory tests to fuel cell integration. Appl. Phys. A 2016, 122, 126–146. [Google Scholar] [CrossRef]
- Delhomme, B.; de Rango, P.; Marty, P.; Bacia, M.; Zawilski, B.; Raufast, C.; Miraglia, S.; Fruchart, D. Large scale magnesium hydride tank coupled with an external heat source. Int. J. Hydrogen Energ. 2012, 37, 9103–9111. [Google Scholar] [CrossRef]
- Garrier, S. Conception et simulation d'un réservoir à hydrure de magnésium avec récupération de la chaleur de réaction à l'aide d'un matériau à changement de phase. Ph.D. Thesis, Université de Grenoble, Grenoble, FR, 2011. https://theses.hal.science/tel-00940452/document. [Google Scholar]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Rajicic, B.; Sedmak, A.; Wasim, M.; Perisic, J. Hydrogen embrittlement mechanisms in steels at different length scales. In Proceedings of the Communication at the 1st International Conference on Innovative Materials in Extreme Conditions, Belgrade, RS, 24–25 March 2022. [Google Scholar]


| Deflagration | Detonation | |||
|---|---|---|---|---|
| Speed of flame propagation |
Subsonic, e.g. never exceeding the level of 10 m/s for lean hydrogen-air mixtures in a smooth 10 mm-thick channel |
Supersonic, e.g. for stoichiometric hydrogen – air mixtures: 1,600 to 2,000 m/s |
||
| Mechanism of propagation |
The flame front propagates by transferring heat and mass to the unburnt hydrogen–air mixture ahead of the front |
Powerful pressure wave compressing the unburnt gas ahead of the wave up to a temperature above the auto-ignition temperature |
||
| Protection by venting | Effective explosion protection | Ineffective protection |
| Consequence | Number of accidents* | % |
|---|---|---|
| Fatal outcome·s | 25 | 12 |
| Serious injuries | 28 | 13 |
| Injuries (serious ones included) | 70 | 33 |
| Onsite damage | 183 | 86 |
| Offsite damage | 17 | 8 |
| Operating losses | 89 | 42 |
| Population evacuation | 8 | 3.8 |
| * Out of 213 accidents where consequences are known, as collected until 2010 by BARPI. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





