Submitted:
29 May 2024
Posted:
29 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Analysis of Microbial Communities
2.3. Preparation of Ethyl Acetate (EtOAc) Fraction
2.4. Cell Cultures
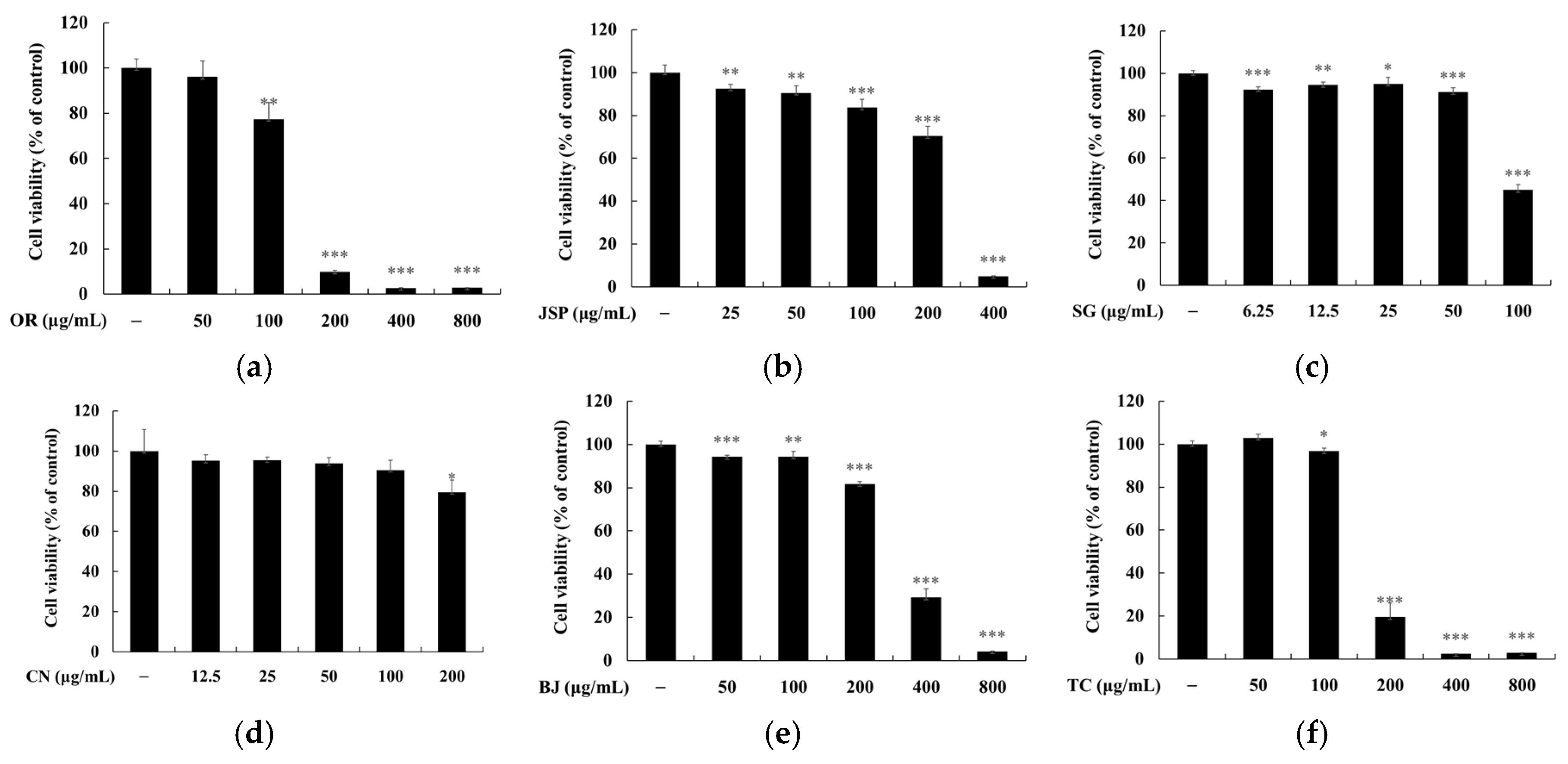
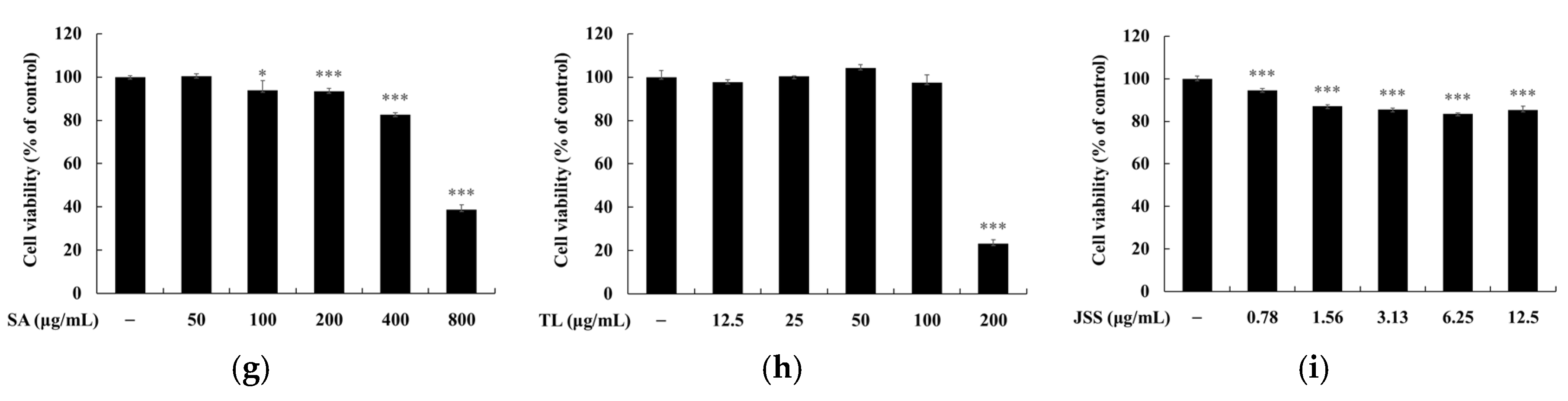
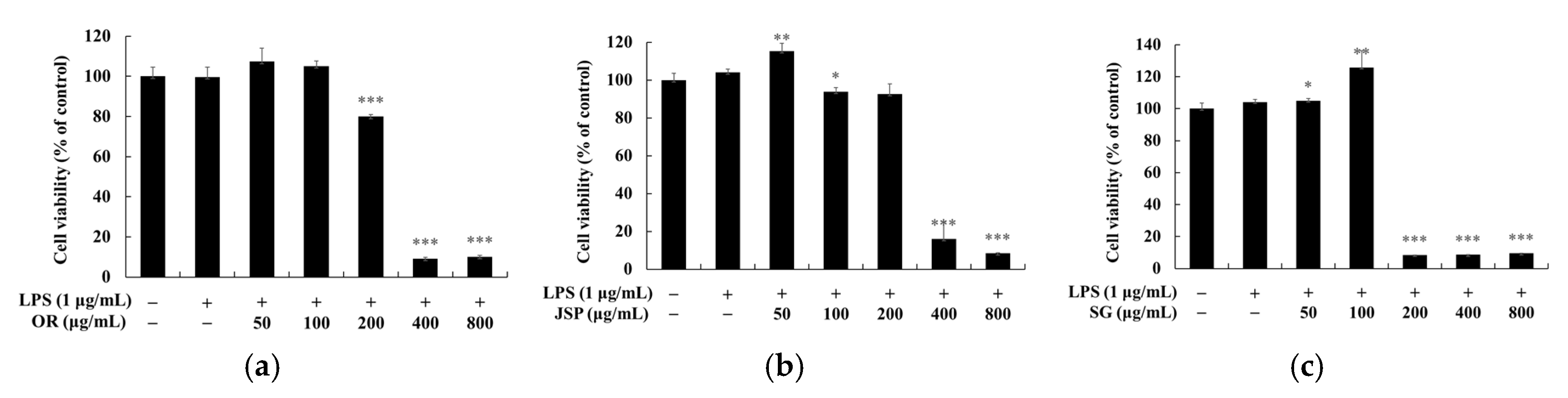
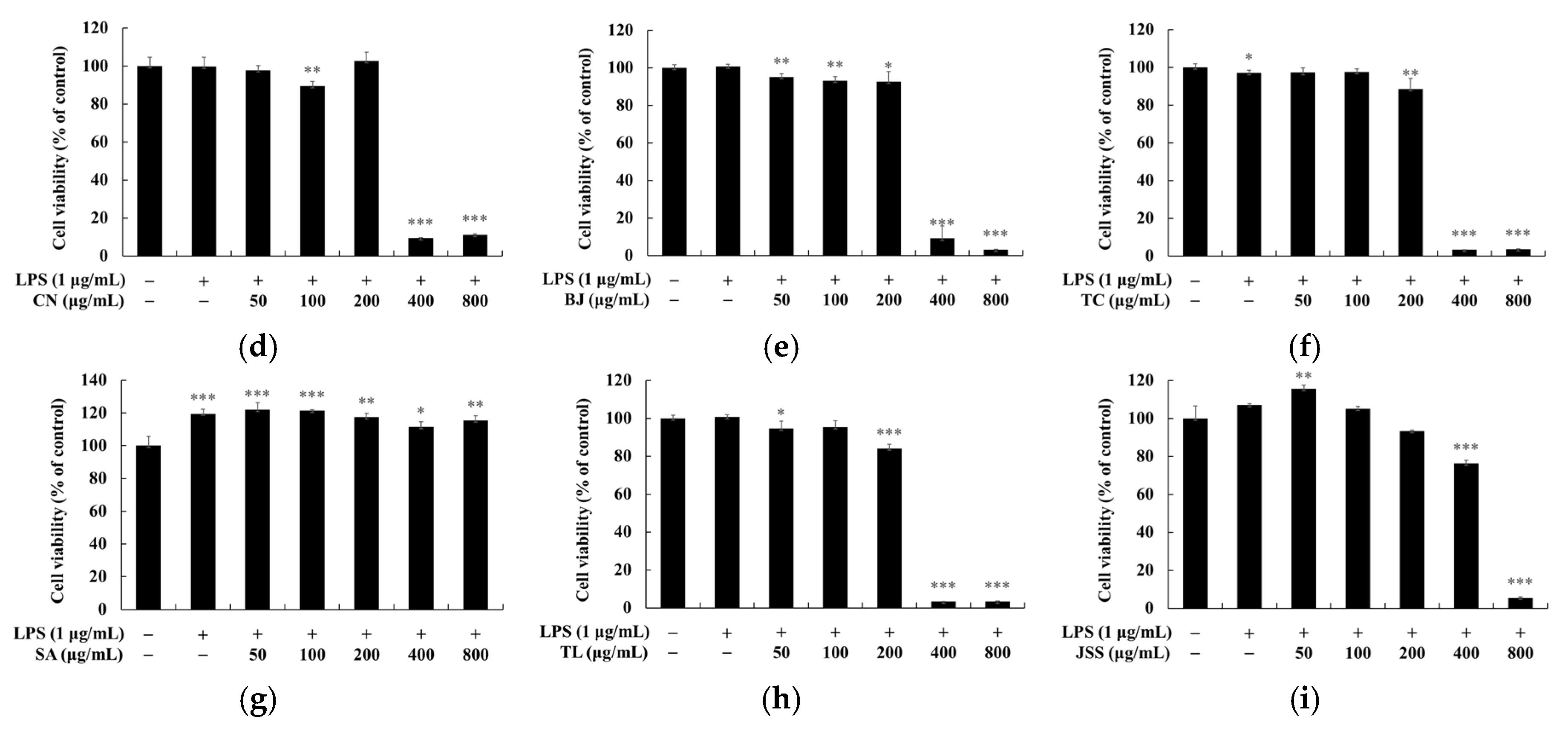
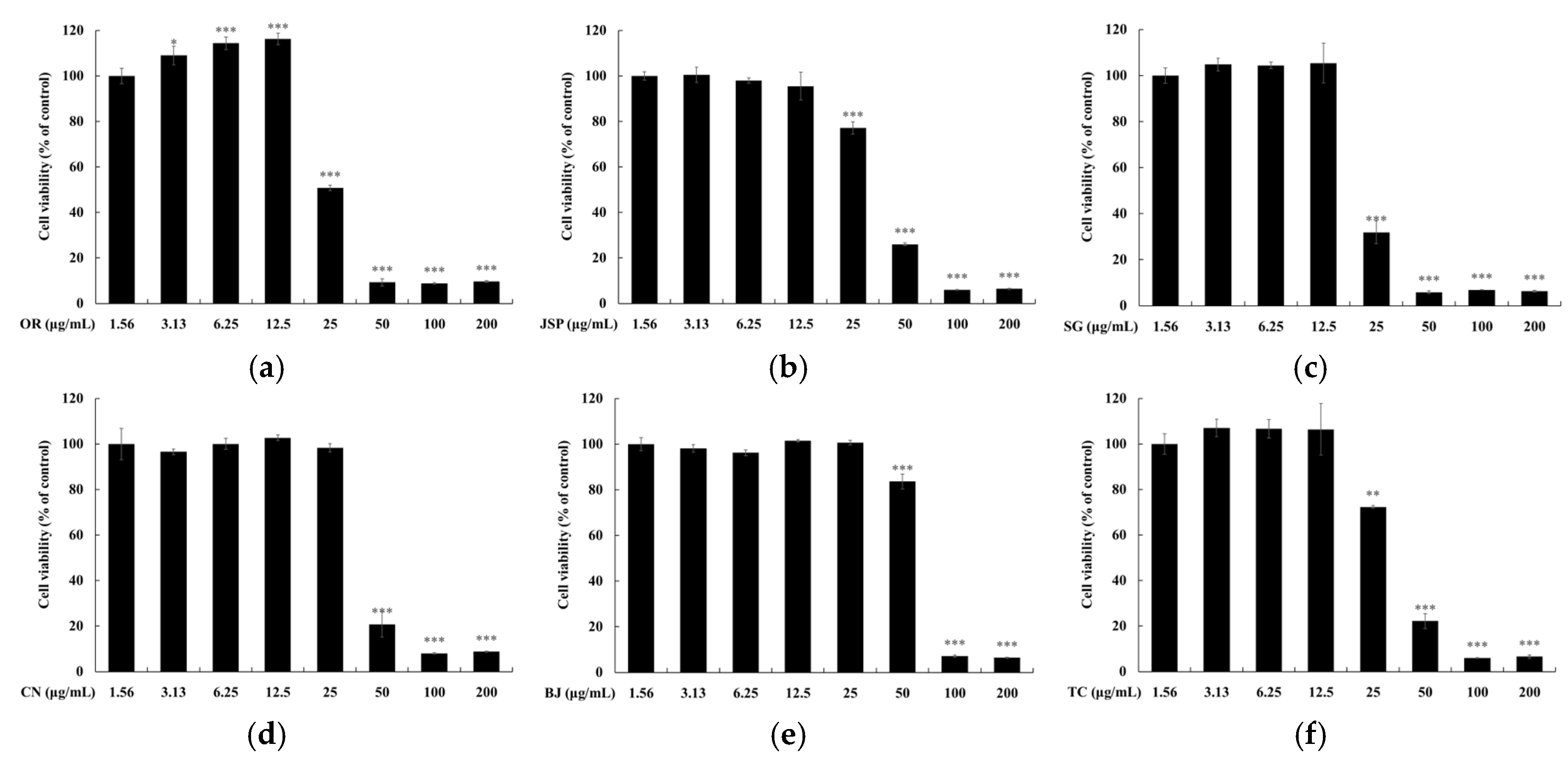
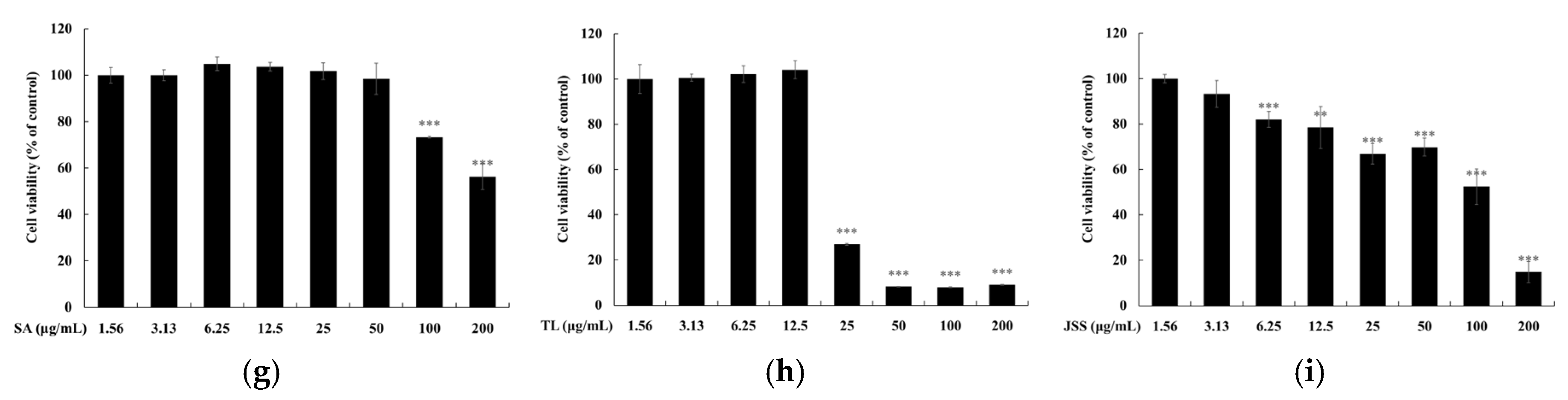
2.5. Measurement of Cell Viability
2.6. Measurement of Nitric Oxide Production
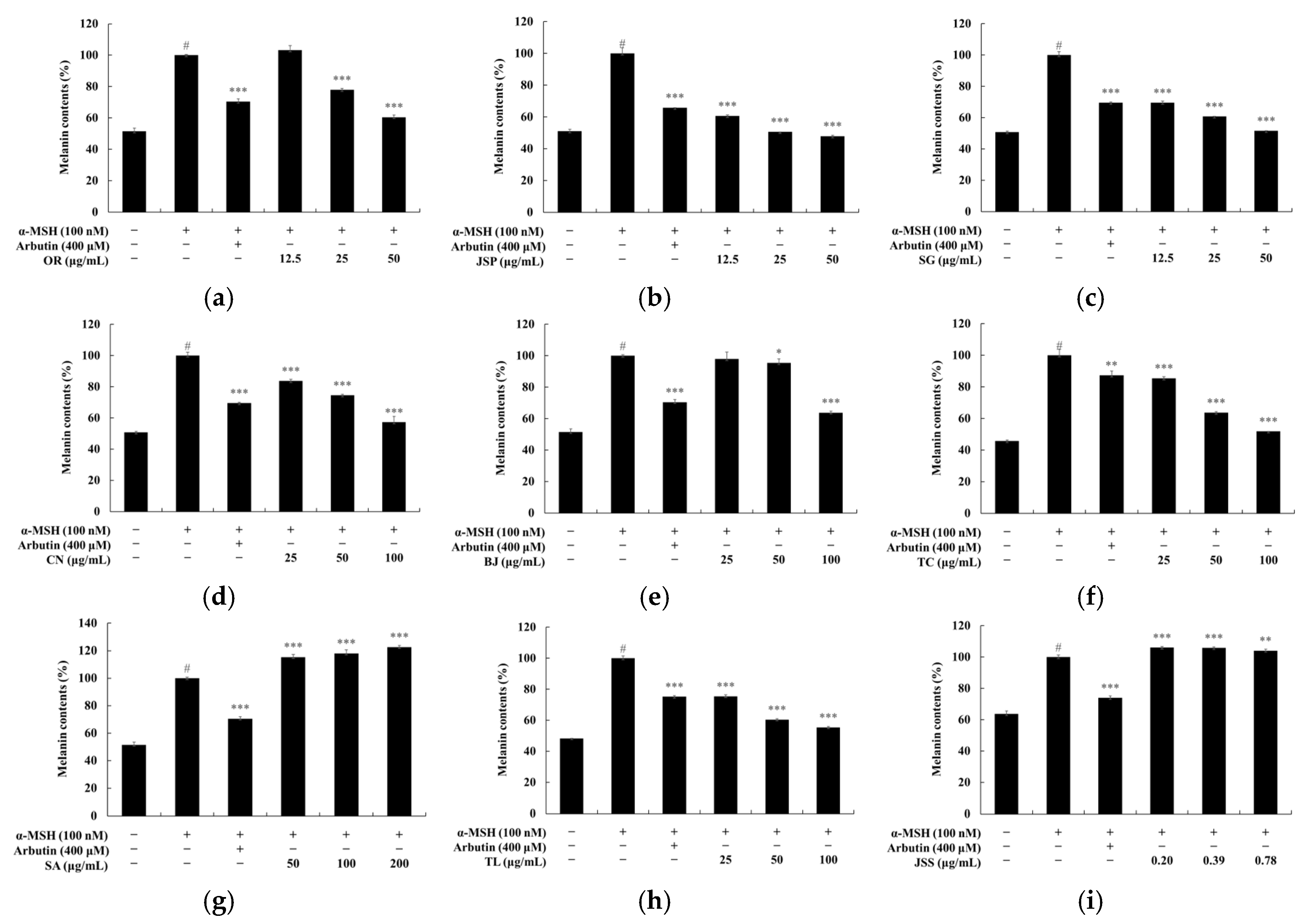
2.7. Measuring Melanin Contents
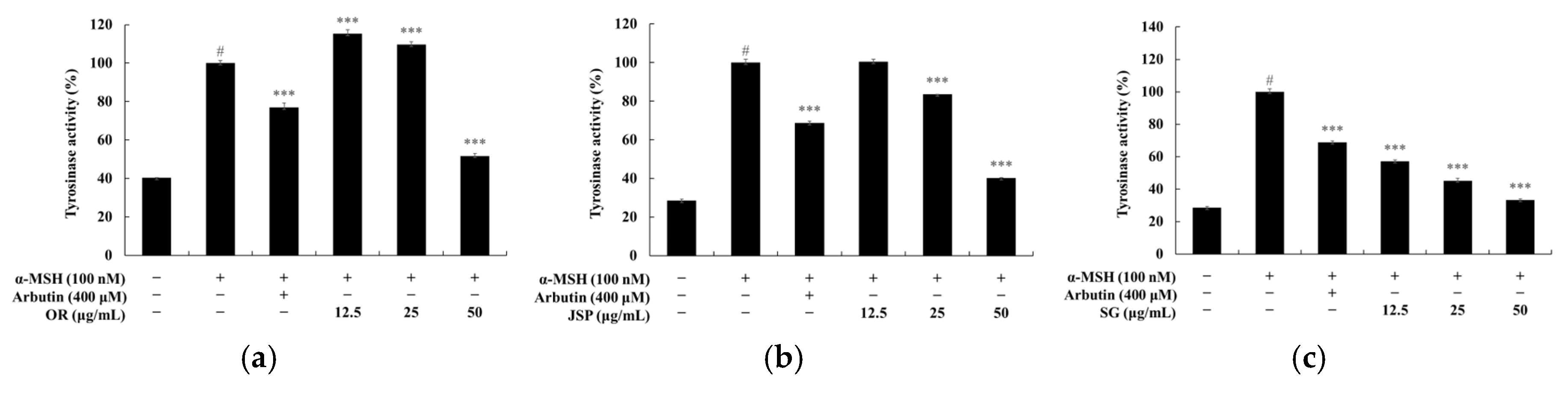
2.8. Measuring Intracellular Tyrosinase Activity
2.9. Human Skin Irritation Test
2.10. Statistical Analyses
3. Results and Discussion
3.1. Microbial Community Analysis
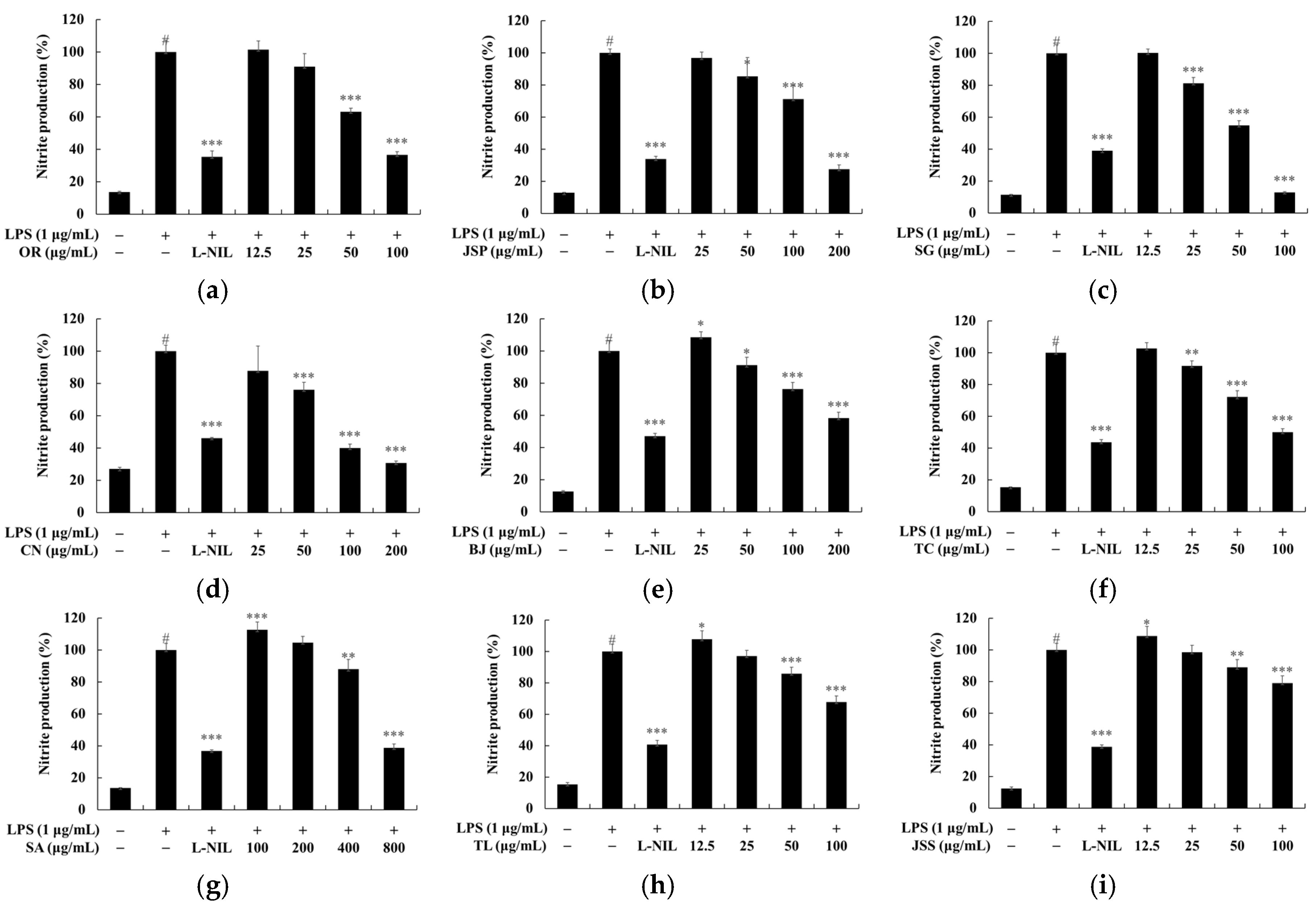
3.2. Nitric Oxide (NO) Inhibitory Effect of Jeju Fermented Foods
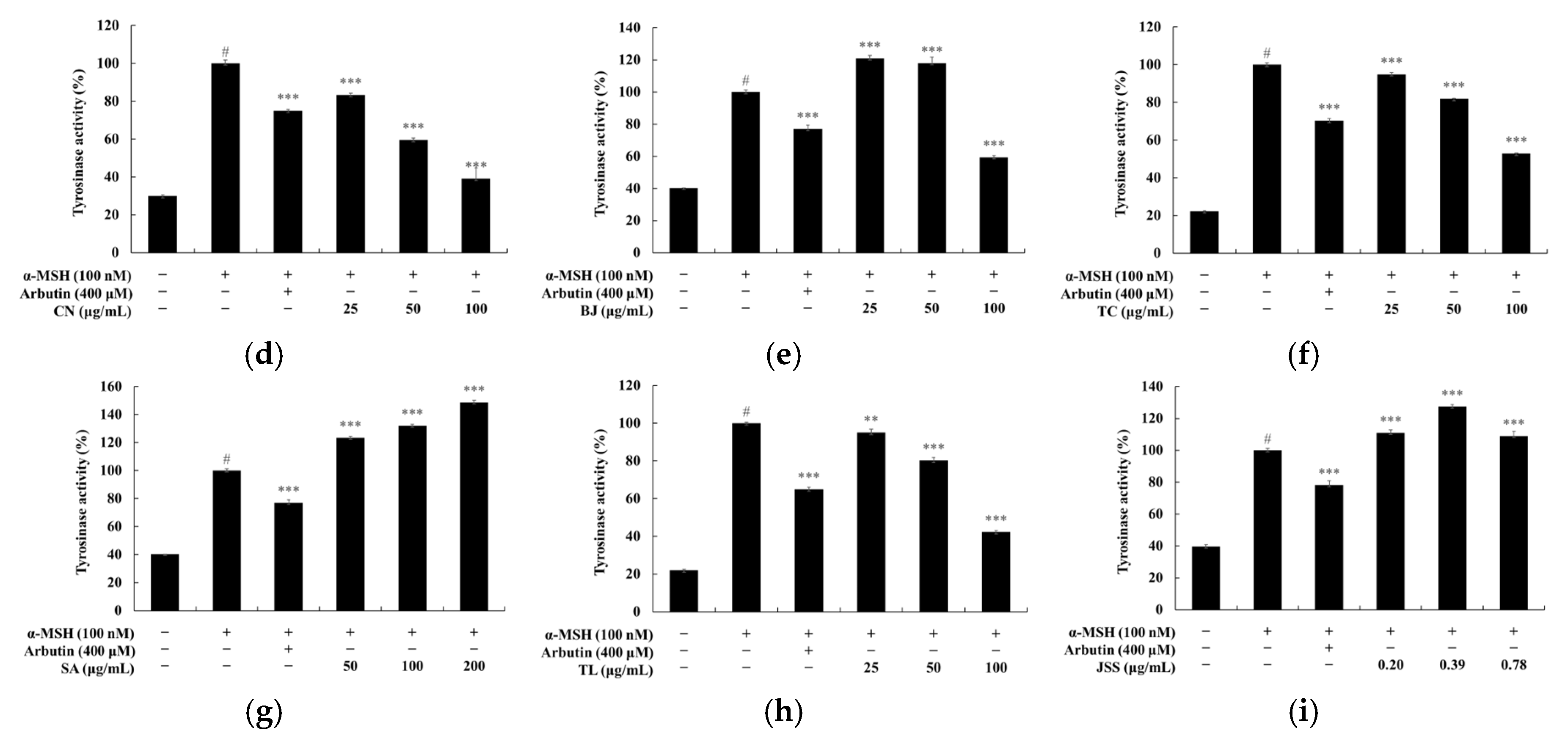
3.3. Melanin and Tyrosinase Inhibitory Effects of Jeju Fermented Foods
3.4. Skin Safety of Jeju Fermented Foods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byun, K.H.; Kang, E.J.; Kim, K.H. Environment Management for Sustainability of Hallasan National Park in Jeju Island, Korea. AMR 2014, 905, 334–338. [Google Scholar] [CrossRef]
- Lee, J. Research of the Food Culture Comparison between the Tamra & Mongolia. Trans Humanit. 2012, 5, 211–243. [Google Scholar] [CrossRef]
- Jung, W.Y.; Jung, J.Y.; Lee, H.J.; Jeon, C.O. Functional Characterization of Bacterial Communities Responsible for Fermentation of Doenjang: A Traditional Korean Fermented Soybean Paste. Front. Microbiol. 2016, 31, 7:827. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jung, J.Y.; Jeon, C.O. Bacterial community dynamics and metabolite changes in myeolchi-aekjeot, a Korean traditional fermented fish sauce, during fermentation. Int. J. Food Microbiol. 2015, 203, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, E.J. Bacterial communities of traditional salted and fermented seafoods from Jeju Island of Korea using 16S rRNA gene clone library analysis. J. Food Sci. 2014, 79, M927–934. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, M.C.; Kwon, S.W.; Kim, S.J.; Park, I.C.; Ka, J.O.; Weon, H.Y. Analyses of bacterial communities in meju, a Korean traditional fermented soybean bricks, by cultivation-based and pyrosequencing methods. J. Microbiol. 2011, 49, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Lee, H.J.; Chun, B.H.; Jeon, C.O. Effects of Temperature on Bacterial Communities and Metabolites during Fermentation of Myeolchi-Aekjeot, a Traditional Korean Fermented Anchovy Sauce. PLoS One 2016, 11, e0151351. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kimura, B.; Fujii, T. Strictly Anaerobic Halophiles Isolated from Canned Swedish Fermented Herrings (Surströmming). Int. J. Food Microbiol. 2000, 54, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Yoon, S.S.; Breidt, F., Jr.; Fleming, H.P.; Klaenhammer, T.R. Characterization of Six Leuconostoc fallax Bacteriophages Isolated from an Industrial Sauerkraut Fermentation. Appl. Environ. Microbiol. 2002, 68, 5452–5458. [Google Scholar] [CrossRef]
- Rizzo, G. Soy-Based Tempeh as a Functional Food: Evidence for Human Health and Future Perspective. Front. Biosci. (Elite Ed). 2024, 16, 3. [Google Scholar] [CrossRef]
- Feng, C.H. The Tale of Sushi: History and Regulations. Compr. Rev. Food Sci. Food Saf. 2012, 11, 205–220. [Google Scholar]
- Valentino, V.; Magliulo, R.; Farsi, D.; Cotter, P.D.; O’Sullivan, O.; Ercolini, D.; De Filippis, F. Fermented foods, their microbiome and its potential in boosting human health. Microb. Biotechnol. 2024, 17, e14428. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kang, X.; Wang, X.; Chen, X.; Nie, X.; Xiang, L.; Liu, D.; Zhao, Z. Potential Correlation between Microbial Diversity and Volatile Flavor Substances in a Novel Chinese-Style Sausage during Storage. Foods. 2023, 12, 3190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, Y.; Xu, Q.; Yu, Y.; Zheng, G.; Wang, Y.; Zhang, Q.; Xu, X.; Zhang, N.; Chu, J.; Zhang, Y.; Sun, Y.; Zhao, Q.; Zhang, Y.; Qu, Q.; Zhong, J. Microbial Community Succession and Its Correlation with Quality Characteristics during Gray Sufu Fermentation. Foods. 2023, 12, 2767. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, A.; Cheng, L.; Chen, Q.; Li, J.; Xu, Y.; Huo, D. Deep Shotgun metagenomic and 16S rRNA analysis revealed the microbial diversity of lactic acid bacteria in traditional fermented foods of eastern Hainan, China. Food Funct. 2022, 13, 12938–12952. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; Alekseeva, A.Y.; Miraji, K.F.; Phiri, S.; Linnemann, A.R.; Schoustra, S.E. Environmental Selection Shapes Bacterial Community Composition in Traditionally Fermented Maize-Based Foods from Benin, Tanzania and Zambia. Microorganisms. 2022, 10, 1354. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Fanning, S.; Proos, S.; Jordan, K.; Srikumar, S. A Review on the Applications of Next Generation Sequencing Technologies as Applied to Food-Related Microbiome Studies. Front. Microbiol. 2017, 8, 1829. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, I.; Fulci, V.; Palone, F.; Stronati, L.; Cucchiara, S.; Carissimi, C. Quantitative Assessment of Shotgun Metagenomics and 16S rDNA Amplicon Sequencing in the Study of Human Gut Microbiome. OMICS. 2018, 22, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.B.; Hyun, C.G. Anti-Inflammatory Effects and Their Correlation with Microbial Community of Shindari, a Traditional Jeju Beverage. Fermentation 2020, 6, 87. [Google Scholar] [CrossRef]
- Jeong, D.W.; Kim, H.R.; Jung, G.; Han, S.; Kim, C.T.; Lee, J.H. Bacterial Community Migration in the Ripening of Doenjang, a Traditional Korean Fermented Soybean Food. J. Microbiol. Biotechnol. 2014, 24, 648–660. [Google Scholar] [CrossRef]
- Jang, M.; Jeong, D.W.; Lee, J.H. Identification of the Predominant Bacillus, Enterococcus, and Staphylococcus Species in Meju, a Spontaneously Fermented Soybean Prod. Microbiol. Biotechnol. Lett. 2019, 47, 359–363. [Google Scholar] [CrossRef]
- Hussein, W.E.; Abdelhamid, A.G.; Rocha-Mendoza, D.; García-Cano, I.; Yousef, A.E. Assessment of Safety and Probiotic Traits of Enterococcus durans OSY-EGY, Isolated From Egyptian Artisanal Cheese, Using Comparative Genomics and Phenotypic Analyses. Front. Microbiol. 2020, 11, 608314. [Google Scholar] [CrossRef] [PubMed]
- Carasi, .;, Racedo, S.M.; Jacquot, C.; Elie, A.M.; Serradell, M.L.; Urdaci, M.C. Enterococcus durans EP1 a Promising Anti-inflammatory Probiotic Able to Stimulate sIgA and to Increase Faecalibacterium prausnitzii Abundance. Front. Immunol. 2017, 8, 88. [CrossRef] [PubMed]
- Facchin, B.M.; Dos Reis, G.O.; Vieira, G.N.; Mohr, E.T.B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: a systematic review and meta-analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.K.; Hyun, C.G. 4-Hydroxy-7-Methoxycoumarin Inhibits Inflammation in LPS-activated RAW264.7 Macrophages by Suppressing NF-κB and MAPK Activation. Molecules 2020, 25, 4424. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Negrete, E.V.; Morales-González, Á.; Madrigal-Santillán, E.O.; Sánchez-Reyes, K.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Valadez-Vega, C.; Chamorro-Cevallos, G.; Garcia-Melo, L.F.; Morales-González, J.A. Phytochemicals and Their Usefulness in the Maintenance of Health. Plants (Basel). 2024, 13, 523. [Google Scholar] [CrossRef] [PubMed]
- Kamalian, A.; Sohrabi Asl, M.; Dolatshahi, M.; Afshari, K.; Shamshiri, S.; Momeni Roudsari, N.; Momtaz, S.; Rahimi, R.; Abdollahi, M.; Abdolghaffari, A.H. Interventions of natural and synthetic agents in inflammatory bowel disease, modulation of nitric oxide pathways. World J. Gastroenterol. 2020, 26, 3365–3400. [Google Scholar] [CrossRef] [PubMed]
- Malaspina, P.; Catellani, E.; Burlando, B.; Brignole, D.; Cornara, L.; Bazzicalupo, M.; Candiani, S.; Obino, V.; De Feo, V.; Caputo, L.; Giordani, P. Depigmenting potential of lichen extracts evaluated by in vitro and in vivo tests. PeerJ. 2020, 8, e9150. [Google Scholar] [CrossRef]
- Goelzer Neto, C.F.; do Nascimento, P.; da Silveir,a V.C.; de Mattos, A.B.N.; Bertol, C.D. Natural sources of melanogenic inhibitors: A systematic review. Int. J. Cosmet. Sci. 2022, 44, 143-153. [CrossRef]
- Qu, Y.; Zhan, Q.; Du, S.; Ding, Y.; Fang, B.; Du, W.; Wu, Q.; Yu, H.; Li, L.; Huang, W. Catalysis-based specific detection and inhibition of tyrosinase and their application. J. Pharm. Anal. 2020, 10, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Saghaie, L.; Pourfarzam, M.; Fassihi, A.; Sartippour, B. Synthesis and tyrosinase inhibitory properties of some novel derivatives of kojic acid. Res. Pharm. Sci. 2013, 8, 233–242. [Google Scholar]
- Oh, S.Y.; Hyun, C.G. Chrysoeriol Enhances Melanogenesis in B16F10 Cells Through the Modulation of the MAPK, AKT, PKA, and Wnt/β-Catenin Signaling Pathways. Nat. Prod. Commun. 2022, 17. [Google Scholar] [CrossRef]
- Potez, M.; Trappetti, V.; Bouchet, A.; Fernandez-Palomo, C.; Güç, E.; Kilarski, W.W.; Hlushchuk, R.; Laissue, J.; Djonov, V. Characterization of a B16-F10 melanoma model locally implanted into the ear pinnae of C57BL/6 mice. PLoS One 2018, 13, e0206693. [Google Scholar] [CrossRef]
- Kim, T.; Hyun, C.G. Imperatorin Positively Regulates Melanogenesis through Signaling Pathways Involving PKA/CREB, ERK, AKT, and GSK3β/β-Catenin. Molecules. 2022, 27, 6512. [Google Scholar] [CrossRef]
- Cuamatzin-García, L.; Rodríguez-Rugarcía, P.; El-Kassis, E.G.; Galicia, G.; Meza-Jiménez, M.L.; Baños-Lara, M.D.R.; Zaragoza-Maldonado, D.S.; Pérez-Armendáriz, B. Traditional Fermented Foods and Beverages from around the World and Their Health Benefits. Microorganisms. 2022, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
| No | Test Samples | 30 min | 24 h | 48 h | Reaction Grade | |||
|---|---|---|---|---|---|---|---|---|
| 30 min | 24 h | 48 h | Mean | |||||
| 1 | BJ (100 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | BJ (200 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | JSP (100 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | JSP (200 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | SA (100 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | SA (200 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | TC (50 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | TC (100 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | TL (50 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | TL (100 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 11 | OR (50 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | OR (100 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13 | SG (50 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | SG (100 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | CN (50 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | CN (100 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 17 | JSS (50 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | JSS (100 µg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





