Submitted:
28 May 2024
Posted:
29 May 2024
You are already at the latest version
Abstract
Keywords:
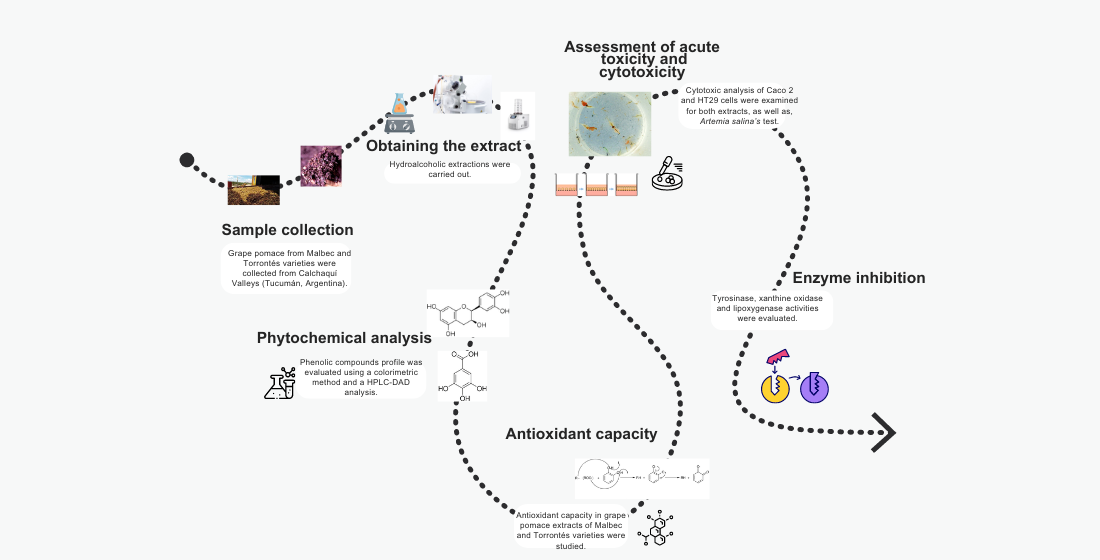
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Material Collection
2.3. Preparation of Extracts
2.4. Phytochemical Analysis
2.4.1. Quantification of Different Phenolic Groups
2.4.2. Identification of Phenolic Compounds by HPLC-DAD Analysis
2.5. Antioxidant Capacity Assays
2.5.1. Phosphomolybdenum Reducing Capacity
2.5.2. Metal Chelating Capacity
2.5.3. ABTS Cation Radical Scavenging Capacity
2.5.4. Nitric Oxide Scavenging Capacity
2.5.5. Iron Reducing Power
2.5.6. Copper Reducing Power
2.5.7. Superoxide Anion Radical Scavenging Assay
2.5.8. Hypochlorous Acid Scavenging Assay
2.5.9. Saccharomyces cerevisiae Survival Assay
2.6. Totoxicity Trials
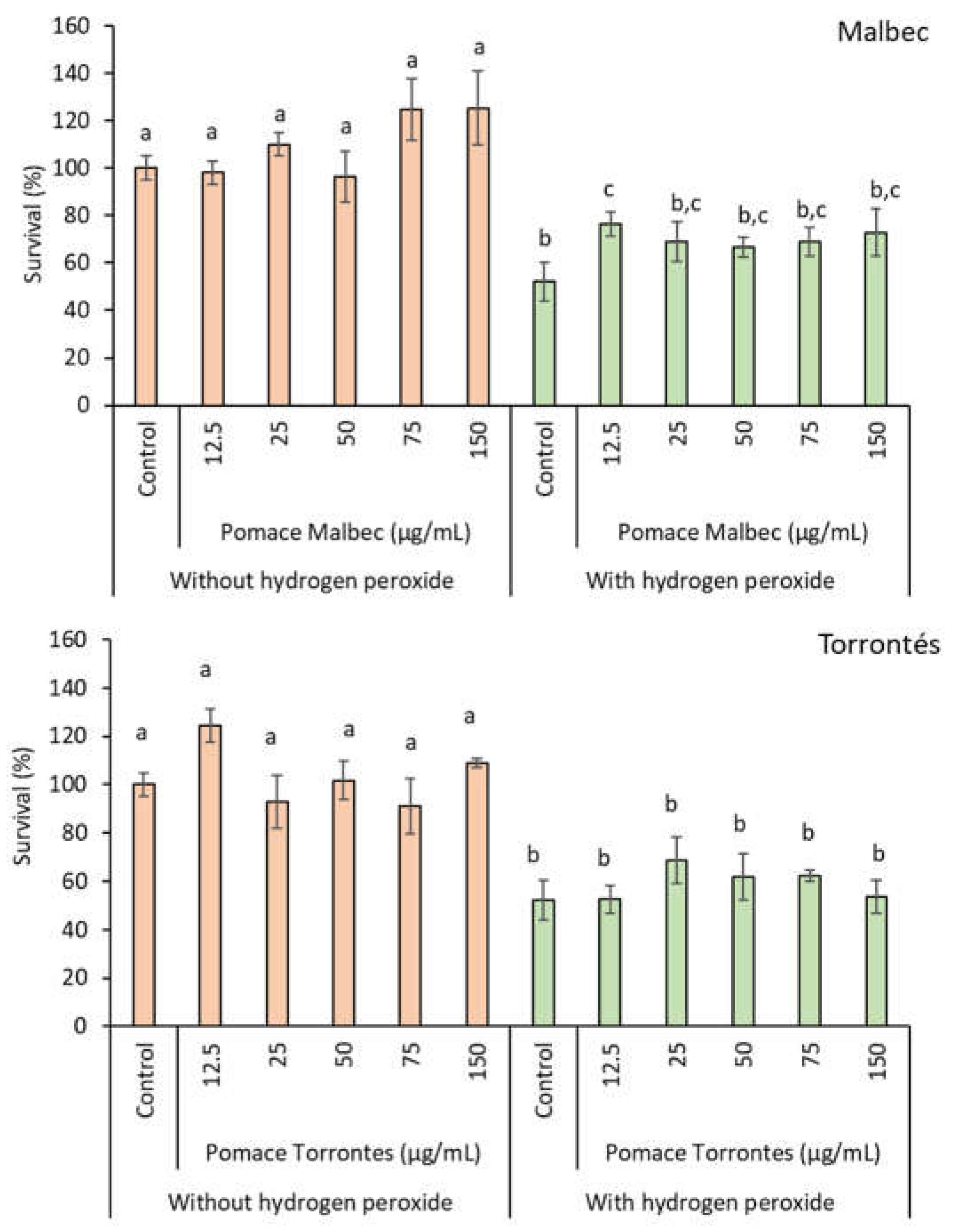
2.6.1. Artemia Salina Test
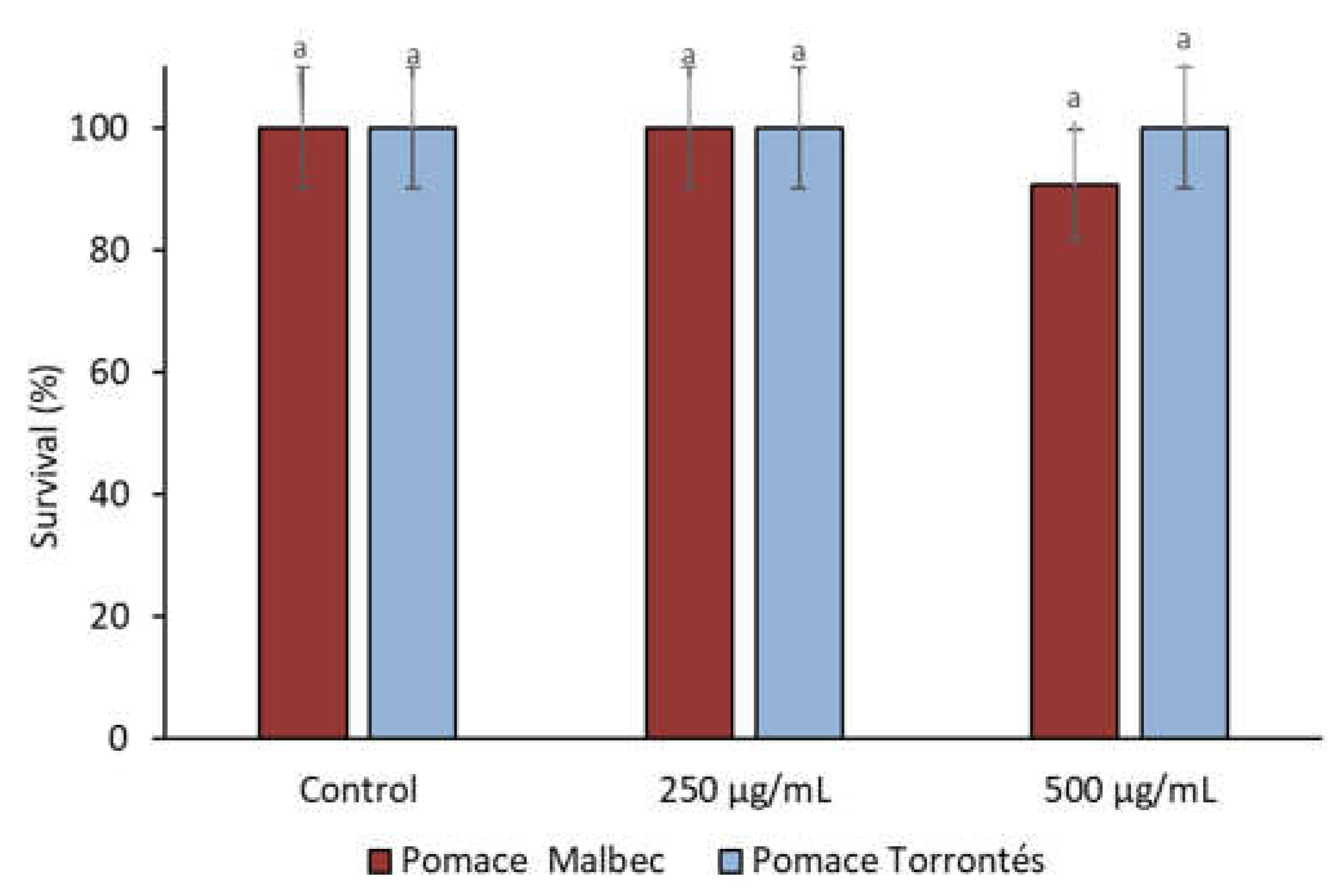
2.6.2. Hemolysis
2.6.3. Cell Viability Assay
2.7. Enzyme Inhibitions
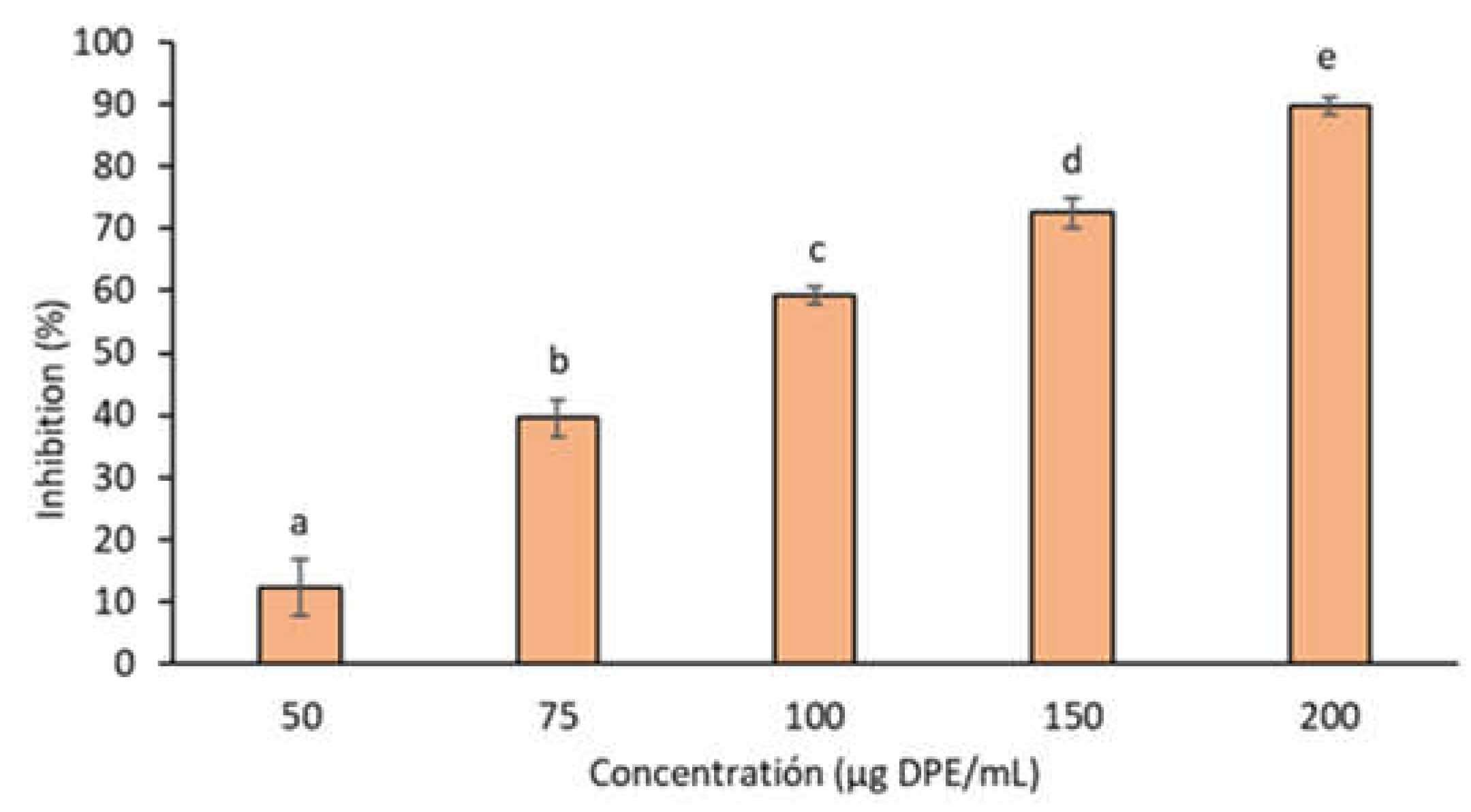
2.7.1. Tyrosinase
2.7.2. Xanthine Oxidase
2.7.3. Lipoxygenase
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Biological Activity
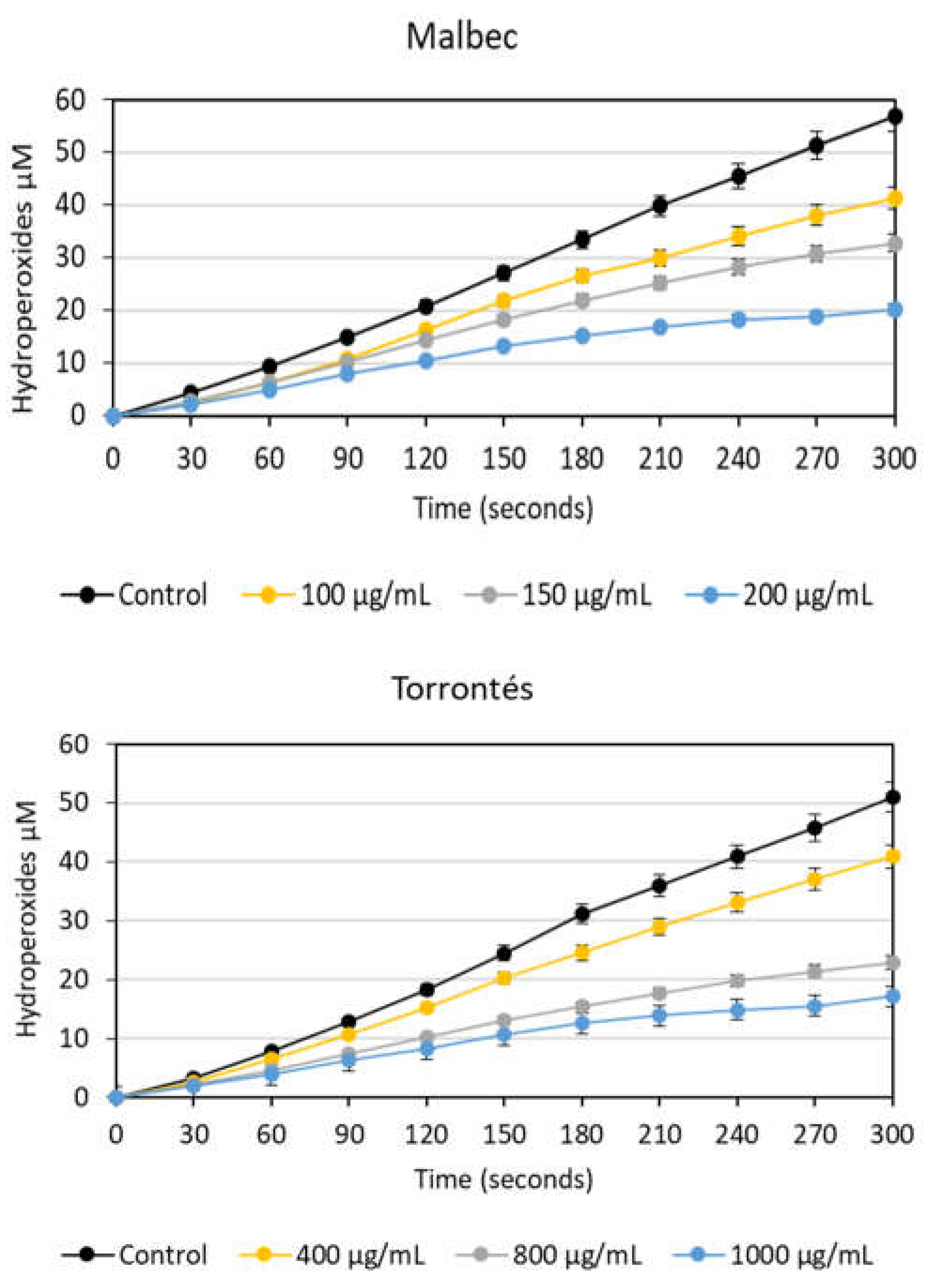
3.2.1. Antioxidant
3.2.2. Cytotoxicity
3.2.3. Toxicity
3.2.4. Enzyme Inhibition
4. Conclusion
Author Contributions
Funding
Data Availability Statement
Ethics approval
Consent to participate
Consent for publication
Acknowledgments
Competing interests
References
- Alimenti, C.; Lianza, M.; Antognoni, F.; Giusti, L.; Bistoni, O.; Liotta, L.; Angeloni, C.; Lupidi, G.; Beghelli, D. Characterization and biological activities of in vitro digested olive pomace polyphenols evaluated on ex vivo human immune blood cells. Molecules 2023, 28, 2122. [CrossRef]
- Chedea, V.S.; Macovei, Ștefan O.; Bocșan, I.C.; Măgureanu, D.C.; Levai, A.M.; Buzoianu, A.D.; Pop, R.M. Grape pomace polyphenols as a source of compounds for management of oxidative stress and inflammation—A possible alternative for non-steroidal anti-inflammatory drugs? Molecules 2022, 27, 6826. [CrossRef]
- Gueboudji, Z.; Kadi, K.; Mahmoudi, M.; Hannachi, H.; Nagaz, K.; Addad, D.; Yahya, L.B.; Lachehib, B.; Hessini, K. Maceration and liquid–liquid extractions of phenolic compounds and antioxidants from algerian olive oil mill wastewater. Environ. Sci. Pollut. Res. 2023, 30, 3432–3439. [CrossRef]
- Abouelenein, D.; Mustafa, A.M.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Vittori, S. Phenolic and nutritional profiles, and antioxidant activity of grape pomaces and seeds from Lacrima Di Morro d’Alba and Verdicchio Varieties. Food Biosci. 2023, 53, 102808. [CrossRef]
- Ferrer-Gallego, R.; Silva, P. The wine industry by-products: Applications for food industry and health benefits. Antioxidants 2022, 11, 2025. [CrossRef]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-rich extracts obtained from winemaking waste streams as natural ingredients with cosmeceutical potential. Antioxidants 2019, 8, 355. [CrossRef]
- Wani, T.A.; Majid, D.; Dar, B.N.; Makroo, H.A.; Allai, F.M. Utilization of novel techniques in extraction of polyphenols from grape pomace and their therapeutic potential: A review. J. Food Meas. Charact. 2023, 17, 5412–5425. [CrossRef]
- Szabó, É.; Marosvölgyi, T.; Szilágyi, G.; Kőrösi, L.; Schmidt, J.; Csepregi, K.; Márk, L.; Bóna, Á. Correlations between total antioxidant capacity, polyphenol and fatty acid content of native grape seed and pomace of four different grape varieties in Hungary. Antioxidants 2021, 10, 1101. [CrossRef]
- Hoss, I.; Rajha, H.N.; El Khoury, R.; Youssef, S.; Manca, M.L.; Manconi, M.; Louka, N.; Maroun, R.G. Valorization of wine-making by-products’ extracts in cosmetics. Cosmetics 2021, 8, 109. [CrossRef]
- Zeng, H.-J.; Li, Q.-Y.; Ma, J.; Yang, R.; Qu, L.-B. A Comparative study on the effects of resveratrol and oxyresveratrol against tyrosinase activity and their inhibitory mechanism. spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2021, 251, 119405. [CrossRef]
- Soceanu, A.; Dobrinas, S.; Sirbu, A.; Manea, N.; Popescu, V. Economic Aspects of Waste Recovery in the Wine Industry. A Multidisciplinary Approach. Sci. Total Environ. 2021, 759, 143543. [CrossRef]
- Viola, C.M.; Torres-Carro, R.; Cartagena, E.; Isla, M.I.; Alberto, M.R.; Arena, M.E. Effect of wine wastes extracts on the viability and biofilm formation of pseudomonas aeruginosa and staphylococcus aureus strains. Evid. Based Complement. Alternat. Med. 2018, 2018, 1–9. [CrossRef]
- Viola, C.M.; Cartagena, E.; Arena, M.E. Desechos de vinificación como inhibidores de biopelículas de staphylococcus aureus. Nereis Interdiscip. Ibero-Am. J. Methods Model. Simul. 2021, 135–146. [CrossRef]
- Salazar, P.B.; Fanzone, M.; Zabala, B.A.; Rodriguez Vaquero, M.J.; Cilli, E.; Barroso, P.A.; Minahk, C.; Acuña, L. A Byproduct from the valles calchaquíes vineyards (Argentina) rich in phenolic compounds: A tool against endemic leishmania dissemination. Environ. Sci. Pollut. Res. 2023, 30, 97377–97385. [CrossRef]
- Instituto nacional de vitivinicultura. 2018 Regiones vitivinícolas argentinas noroeste. https://www.argentina.gob.ar/sites/default/files/region_noroeste_18.pdf.
- Instituto nacional de vitivinicultura. 2021. Informe variedad Torrontés. https://www.argentina.gob.ar/sites/default/files/2018/10/01-Torrontés_2020.pdf.
- Torres-Carro, R.; Ana, Y.; Rojas-Márquez, J.D.; Stempin, C.C.; Zampini, I.C.; Isla, M.I.; Alberto, M.R. Argentinean puna plants with in vitro antioxidant and anti-inflammatory activities as a potential nutraceutical. J. Food Sci. 2019, 84, 3352–3363. [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant activity and polyphenols characterization of four monovarietal grape pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10(9), 1406. [CrossRef]
- Singh, P.K.; Singh, J.; Medhi, T.; Kumar, A. Phytochemical screening, quantification, FT-IR analysis, and in silico characterization of potential bioactive compounds identified in hr-lc/ms analysis of the polyherbal formulation from Northeast India. ACS Omega 2022, 7, 33067–33078. [CrossRef]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A. G.; Marcazzan, G. L.; Bogdanov, S. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem. Anal. 2004, 15(4), 235–240. [CrossRef]
- Ben Hmida, R.; Frikha, N.; Bouguerra Neji, S.; Kit, G.; Medina, F.; Bouaziz, M. Synthesis of high added value compounds through catalytic oxidation of 2-phenylethanol: A kinetic study. Int. J. Chem. Kinet. 2020, 52, 124–133. [CrossRef]
- Ricco, R.; Agudelo, I.J.; Wagner, M.L. Métodos empleados en el análisis de los polifenoles en un laboratorio de baja complejidad. Lilloa 2015, 52, 161–174.
- Carullo, G.; Spizzirri, U.G.; Loizzo, M.R.; Leporini, M.; Sicari, V.; Aiello, F.; Restuccia, D. Valorization of red grape (vitis vinifera cv. sangiovese) pomace as functional food ingredient. Ital. J. Food Sci. 2020, 32, 367–385. [CrossRef]
- Bouabid, K.; Lamchouri, F.; Toufik, H.; Faouzi, M.E.A. Phytochemical investigation, in vitro and in vivo antioxidant properties of aqueous and organic extracts of toxic plant: Atractylis gummifera L. J. Ethnopharmacol. 2020, 253, 112640. [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. Ind. Crops Prod. 2017, 104, 210–220. [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [CrossRef]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. In vivo antioxidant activity of grape, pomace and wine from three red varieties grown in argentina: Its relationship to phenolic profile. J. Funct. Foods 2016, 20, 332–345. [CrossRef]
- Quy, T.; Xuan, T. Xanthine Oxidase Inhibitory potential, antioxidant and antibacterial activities of Cordyceps militaris (l.) link fruiting Body. Medicines 2019, 6, 20. [CrossRef]
- Muñoz-Bernal, Ó.A.; Vazquez-Flores, A.A.; De La Rosa, L.A.; Rodrigo-García, J.; Martínez-Ruiz, N.R.; Alvarez-Parrilla, E. Enriched red wine: Phenolic profile, sensory evaluation and in vitro bioaccessibility of phenolic compounds. Foods 2023, 12, 1194. [CrossRef]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of polyphenols and evaluation of antioxidant capacity in grape pomace of the Cv. Malbec. Food Chem. 2015, 178, 172–178. [CrossRef]
- Guaita, M.; Motta, S.; Messina, S.; Casini, F.; Bosso, A. Polyphenolic profile and antioxidant activity of green extracts from grape pomace skins and seeds of italian cultivars. Foods 2023, 12, 3880. [CrossRef]
- Ribeiro, L.F.; Ribani, R.H.; Stafussa, A.P.; Makara, C.N.; Branco, I.G.; Maciel, G.M.; Haminiuk, C.W.I. Exploratory analysis of bioactive compounds and antioxidant potential of grape (Vitis vinifera) pomace. Acta Sci. Technol. 2022, 44, e56934. [CrossRef]
- Alvarez-Casas, M.; Pájaro, M.; Lores, M.; Garcia-Jares, C. Characterization of grape marcs from native and foreign white varieties grown in northwestern spain by their polyphenolic composition and antioxidant activity. Eur. Food Res. Technol. 2016, 242, 655–665. [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [CrossRef]
- Lisov, N.; Čakar, U.; Milenković, D.; Čebela, M.; Vuković, G.; Despotović, S.; Petrović, A. The influence of Cabernet Sauvignon ripeness, healthy state and maceration time on wine and fermented pomace phenolic profile. Fermentation 2023, 9(7), 695.
- Balea, Ş.S.; Pârvu, A.E.; Pop, N.; Marín, F.Z.; Pârvu, M. Polyphenolic compounds, antioxidant, and cardioprotective effects of pomace extracts from Fetească Neagră cultivar. Oxid. Med. Cell. Longev. 2018, 2018, 1–11. [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant activity and polyphenols characterization of four monovarietal grape pomaces from salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [CrossRef]
- Onache, P.A.; Geana, E.-I.; Ciucure, C.T.; Florea, A.; Sumedrea, D.I.; Ionete, R.E.; Tița, O. Bioactive phytochemical composition of grape pomace resulted from different white and red grape Cultivars. Separations 2022, 9, 395. [CrossRef]
- Gerardi, C.; Pinto, L.; Baruzzi, F.; Giovinazzo, G. Comparison of antibacterial and antioxidant properties of red (cv. negramaro) and white (cv. fiano) skin pomace extracts. Molecules 2021, 26, 5918. [CrossRef]
- Gaafar, A.A.; Asker, M.S. The effectiveness of the functional components of grape (vitis vinifera) pomace as antioxidant, antimicrobial, and antiviral agents. 2019, 12, 625-635.
- Melo, P.S.; Massarioli, A.P.; Denny, C.; Dos Santos, L.F.; Franchin, M.; Pereira, G.E.; Vieira, T.M.F.D.S.; Rosalen, P.L.; Alencar, S.M.D. Winery By-Products: Extraction optimization, phenolic composition and cytotoxic evaluation to act as a new source of scavenging of reactive oxygen species. Food Chem. 2015, 181, 160–169. [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [CrossRef]
- Ganeshpurkar, A.; Saluja, A. The pharmacological potential of catechin. Indian j biochem biophys 2020, 57, 505-511.
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1934578X1987417. [CrossRef]
- Li, D.; Rui, Y.; Guo, S.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [CrossRef]
- Singh, S.K.; Thakur, K.; Sharma, V.; Saini, M.; Sharma, D.; Vishwas, S.; Kakoty, V.; Pal, R.S.; Chaitanya, M.V.N.L.; Babu, M.R.; et al. Exploring the multifaceted potential of chlorogenic acid: Journey from nutraceutical to nanomedicine. South Afr. J. Bot. 2023, 159, 658–677. [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New Progress in the Pharmacology of Protocatechuic Acid: A Compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current advances in naturally occurring caffeoylquinic acids: Structure, bioactivity, and synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [CrossRef]
- Li, X.; Li, K.; Xie, H.; Xie, Y.; Li, Y.; Zhao, X.; Jiang, X.; Chen, D. Antioxidant and cytoprotective effects of the di-o-caffeoylquinic acid family: The mechanism, structure–activity relationship, and conformational effect. Molecules 2018, 23, 222. [CrossRef]
- Karami, A.; Fakhri, S.; Kooshki, L.; Khan, H. Polydatin: Pharmacological mechanisms, therapeutic targets, biological activities, and health benefits. Molecules 2022, 27, 6474. [CrossRef]
- Pérez-Ortiz, J.M.; Alguacil, L.F.; Salas, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; González-Martín, C. Antiproliferative and cytotoxic effects of grape pomace and grape seed extracts on colorectal cancer cell Lines. Food Sci. Nutr. 2019, 7, 2948–2957. [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Cifuentes-Gomez, T.; Escudero-Gilete, M.L.; Heredia, F.J.; Spencer, J.P.E. Assessment of white grape pomace from winemaking as source of bioactive compounds, and its antiproliferative activity. Food Chem. 2015, 183, 78–82. [CrossRef]
- Cattivelli, A.; Conte, A.; Tagliazucchi, D. Quercetins, chlorogenic acids and their colon metabolites inhibit colon cancer cell proliferation at physiologically relevant concentrations. Int. J. Mol. Sci. 2023, 24, 12265. [CrossRef]
- Germanó, M.J.; Muñoz, M.D.; Della-Vedova, M.C.; Feresin, G.E.; Rinaldi-Tosi, M.; Enriz, R.D.; Ramirez, D.C.; Giannini, F.A. Anti-oxidant and anti-inflammatory effect of polar extracts obtained from waste product of wine making. Nat. Prod. Res. 2021, 35, 4769–4773. [CrossRef]
- Angelini, P.; Flores, G.A.; Piccirilli, A.; Venanzoni, R.; Acquaviva, A.; Di Simone, S.C.; Libero, M.L.; Tirillini, B.; Zengin, G.; Chiavaroli, A.; et al. Polyphenolic composition and antimicrobial activity of extracts obtained from grape processing by-products: Between green biotechnology and nutraceutical. Process Biochem. 2022, 118, 84–91. [CrossRef]
- El-Nashar, H.A.S.; El-Din, M.I.G.; Hritcu, L.; Eldahshan, O.A. Insights on the inhibitory power of flavonoids on tyrosinase activity: A survey from 2016 to 2021. Molecules 2021, 26, 7546. [CrossRef]
- Ferri, M.; Rondini, G.; Calabretta, M.M.; Michelini, E.; Vallini, V.; Fava, F.; Roda, A.; Minnucci, G.; Tassoni, A. White grape pomace extracts, obtained by a sequential enzymatic plus ethanol-based extraction, exert antioxidant, anti-tyrosinase and anti-inflammatory activities. New Biotechnol. 2017, 39, 51–58. [CrossRef]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I.R.N.A. Potential application of grape (vitis vinifera l.) stem extracts in the cosmetic and pharmaceutical industries: Valorization of a by-product. Ind. Crops Prod. 2020, 154, 112675. [CrossRef]
- Sinha, S.; Doble, M.; Manju, S.L. 5-Lipoxygenase as a drug target: A review on trends in inhibitors structural design, sar and mechanism based approach. Bioorg. Med. Chem. 2019, 27, 3745–3759. [CrossRef]
- Vishnupriya, P.; Aparna, A.; Viswanadha, V.P. Lipoxygenase (lox) pathway: A promising target to combat cancer. Curr. Pharm. Des. 2021, 27, 3349–3369. [CrossRef]
- Mollica, A.; Scioli, G.; Della Valle, A.; Cichelli, A.; Novellino, E.; Bauer, M.; Kamysz, W.; Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Castillo-López, R.; et al. Phenolic analysis and in vitro biological activity of red wine, pomace and grape seeds oil derived from vitis vinifera L. Cv. Montepulciano d’Abruzzo. Antioxidants 2021, 10, 1704. [CrossRef]
- Bucić-Kojić, A.; Fernandes, F.; Silva, T.; Planinić, M.; Tišma, M.; Šelo, G.; Šibalić, D.; Pereira, D.M.; Andrade, P.B. Enhancement of the anti-inflammatory properties of grape pomace treated by Trametes Versicolor. Food Funct. 2020, 11, 680–688. [CrossRef]
- Dwibedi, V.; Jain, S.; Singhal, D.; Mittal, A.; Rath, S.K.; Saxena, S. Inhibitory activities of grape bioactive compounds against enzymes linked with human diseases. Appl. Microbiol. Biotechnol. 2022, 106, 1399–1417. [CrossRef]
| Phytochemical group | Malbec | Torrontés |
|---|---|---|
| mg/g DPE | mg/g DPE | |
| Total Phenolics (GAE) | 156.01 ± 3.49a | 19.91 ± 1.21b |
| Hydroxycinnamic acids (CAE) | 11.39 ± 0.32a | 0.47 ± 0.01b |
| Orthodiphenols (CAE) | 31.79 ± 0.62a | 4.71 ± 0.28b |
| Anthocyanins (C3GE) | 6.30 ± 0.49a | <LOQ |
| Non-flavonoid phenolics (GAE) | 30.49 ± 1.15a | 8.01 ± 0.94b |
| Tannins (CE) | 23.20 ± 1.30a | 17.37 ± 0.47b |
| Total flavonoids (QE) | 327.25 ± 6.30a | 43.67 ± 1.39b |
| Flavones/Flavonols (QE) | 73.56 ± 1.41a | 1.17 ± 0.07b |
| Flavanones/Dihydroflavonols (NE) | 63.65 ± 5.56a | 10.10 ± 0.85b |
| Phenolic compound | Retention time | mg/100 g DPE | mg/100 g DP | ||
|---|---|---|---|---|---|
| (min) | Malbec | Torrontés | Malbec | Torrontés | |
| Phenolic acids | |||||
| Gallic acid | 5.618 | 245.0 ± 12.0a | 89.3 ± 4.5b | 39.2 ± 1.9a | 65.2 ± 3.3b |
| Protocatechuic acid | 9.935 | 700.0 ± 35.0a | 71.2 ± 3.6b | 112.0 ± 5.6a | 51.9 ± 2.6b |
| Neochlorogenic acid | 10.219 | 6.1 ± 0.3a | 1.9 ± 0.1b | 0.9 ± 0.1a | 1.4 ± 0.1b |
| Caftaric acid | 15.436 | <LOQ | 90.6 ± 4.5 | <LOQ | 66.1 ± 3.3b |
| Chlorogenic acid | 17.869 | 6.6 ± 0.3a | 6.8 ± 0.3a | 1.1 ± 0.1a | 5.0 ± 0.2b |
| 4-O-caffeoylquinic acid | 19.897 | 130.0 ± 6.0a | 118.0 ± 6.0a | 20.8 ± 1.0a | 86.1 ± 4.4b |
| Vanillic acid | 20.748 | ND | ND | ND | ND |
| Caffeic acid | 21.224 | 6.7 ± 0.31a | 18.7 ± 0.9b | 0.9 ± 0.1a | 13.7 ± 0.7b |
| Syringic acid | 22.283 | 38.6 ± 1.9a | 27.6 ± 1.4b | 6.2 ± 0.3a | 20.2 ± 1.0b |
| p-Coumaric acid | 33.758 | 28.2 ± 1.4a | 36.7 ± 1.8b | 4.5 ± 0.2a | 26.8 ± 1.3b |
| trans-Ferulic acid | 37.289 | 45.7 ± 2.3a | 74.2 ± 3.7b | 7.3 ± 0.4a | 54.2 ± 2.7b |
| Sinapic acid | 37.662 | 108.0 ± 5.0a | 89.6 ± 4.5b | 17.3 ± 0.8a | 65.4 ± 3.3b |
| 3,5-di-O-caffeoylquinic acid | 50.127 | 7.2 ± 0.4a | 45.0 ± 2.2b | 1.2 ± 0.1a | 32.9 ± 1.6b |
| Ellagic acid | 55.284 | 33.4 ± 1.7 | ND | 5.3 ± 0.3 | ND |
| 4,5-di-O-caffeoylquinic acid | 56.781 | 50.7 ± 2.5a | 258.0 ± 13.0b | 8.1 ± 0.4a | 188.3 ± 9.5b |
| Cinnamic acid | 58.47 | ND | ND | ND | ND |
| Flavonoids | |||||
| (+)-Catechin | 14.143 | 618.0 ± 31.0a | 70.1 ± 3.5b | 98.9 ± 5.0a | 51.2 ± 2.6b |
| (-)-Epicatechin | 23.294 | 43.2 ± 2.2a | 17.2 ± 0.9b | 6.9 ± 0.4a | 12.6 ± 0.7b |
| Naringin | 49.847 | 31.9 ± 1.6a | 54.6 ± 2.7b | 5.1 ± 0.3a | 39.9 ± 2.0b |
| Quercetin-3-O-galactoside | 52.177 | 25.2 ± 1.3a | 43.8 ± 2.2b | 4.0 ± 0.2a | 32.0 ± 1.6b |
| Quercetin-3-O-glucopyranoside | 52.735 | ND | ND | ND | ND |
| Rutin | 53.284 | 19.1 ± 1.0a | 16.4 ± 0.8b | 3.1 ± 0.2a | 12.0 ± 0.6b |
| Phloridzin | 54.355 | 32.3 ± 1.6a | ND | 5.2 ± 0.3 | ND |
| Myricetin | 57.943 | 17.6 ± 0.9a | 18.9 ± 0.9a | 2.8 ± 0.1a | 13.8 ± 0.7b |
| Quercitrin | 59.07 | ND | ND | ND | ND |
| Kaempferol-3-O-glucoside | 59.466 | ND | 116.0 ± 6.0 | ND | 84.7 ± 4.4 |
| Kaempferol-3-O-rutinoside | 60.01 | <LOD | 66.6 ± 3.3 | <LOD | 48.6 ± 2.4 |
| Isorhamnetin-3-O-glucoside | 60.277 | ND | 95.6 ± 4.8 | ND | 69.8 ± 3.5 |
| Isorhamnetin-3-O-rutinoside | 61.568 | 24.2 ± 1.2a | 10.2 ± 0.5b | 3.9 ± 0.2a | 7.4 ± 0.4b |
| Naringenin | 68.149 | <LOD | <LOD | <LOD | <LOD |
| Quercetin | 71.031 | 18.6 ± 0.9a | 11.4 ± 0.6b | 3.0 ± 0.1a | 8.3 ± 0.4b |
| Phloretin | 72.269 | <LOQ | <LOD | <LOQ | <LOD |
| Tiliroside | 76.233 | 22.7 ± 1.1 | <LOQ | 3.6 ± 0.2 | <LOQ |
| Kaempferol | 79.854 | 3.4 ± 0.2a | 4.4 ± 0.2b | 0.6 ± 0.1a | 3.2 ± 0.2b |
| Apigenin | 81.44 | <LOD | <LOD | <LOD | <LOD |
| Chrysin | 90.832 | <LOD | <LOD | <LOD | <LOD |
| Stilbenoids and others | |||||
| trans-Polydatin | 39.182 | 18.9 ± 0.9a | 3.6 ± 0.2b | 3.0 ± 0.1a | 2.6 ± 0.1b |
| Resveratrol | 52.507 | ND | ND | ND | ND |
| trans-Epsilon viniferin | 69.158 | 6.5 ± 0.3a | 6.2 ± 0.3a | 1.0 ± 0.1a | 4.5 ± 0.2b |
| Sample | Phosphomolybdenum reducing capacity (μgAAE/mg DPE) |
Cupric reducing capacity (μgGAE/mg DPE) |
ABTS•+ scavenging IC50 (μg/mL) |
NO scavenging IC50 (μg/mL) |
Fe+3 reducing RC50 (μg/mL) |
Iron chelating CC50 (μg/mL) |
O2●- scavenging IC50 (µg/mL) |
HOCl scavenging IC50 (µg/mL) |
| Malbec | 178.57 ± 4.99a | 171,18 ± 2,2a | 7.79 ± 0.17a | 414.19 ± 5.79b | 10.22 ± 0.16a | 41.82% ± 0.48%* | 74.17 ± 4.12a,b | 6.71 ± 0.36b |
| Torrontés | 4.74 ± 0.16b | 26,12 ± 0,52b | 49.5 ± 1.46b | 15.34% ± 0.78%** | 84.62 ± 0.95b | 11.28% ± 1.05%* | 874.61 ± 15.71c | 27.40 ± 0.19c |
| Controls | ||||||||
| BHT | - | - | - | - | 11.37 ± 0.13a | - | - | - |
| Trolox | - | - | 3.74 ± 0.06a | - | - | - | - | - |
| Ascorbic acid | - | - | - | 36.13 ± 6.01a | - | - | - | - |
| EDTA | - | - | - | - | - | 13.97 ± 0.06 | - | - |
| Catechin | - | - | - | - | - | - | 99.21 ± 0.85b | 0.095 ± 0.006a |
| Gallic acid | - | - | - | - | - | - | 52.49 ± 1.58a | 0.82 ± 0.06a |
| Cell Viability (%) | ||||
| Concentration (µg DPE/mL) |
HT29-MTX cells | Caco-2 cells | ||
| Malbec | Torrontés | Malbec | Torrontés | |
| 0.1 | 132.25 ± 10.18a | 109.33 ± 19.90a | 120.67 ± 6.92a | 70.29 ± 10.03b |
| 1.0 | 133.03 ± 12.93a | 110.34 ± 9.63a | 79.08 ± 6.94b | 65.14 ± 14.27b |
| 10 | 131.85 ± 14.80a | 103.57 ± 16.69a | 82.82 ± 11.80b | 66.85 ± 7.36b |
| 100 | 122.19 ± 16.94a | 66.87 ± 13.06b | 88.48 ± 12.45b | 66.85 ± 10.52b |
| 1000 | 104.75 ± 23.03b | 33.56 ± 4.38c | 81.26 ± 12.83b | 52.51 ± 1.53c |
| Medium | 100.00 ± 8.86a | |||
| Triton X-100 | 0.00 ± 0.00 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





