Submitted:
28 May 2024
Posted:
29 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
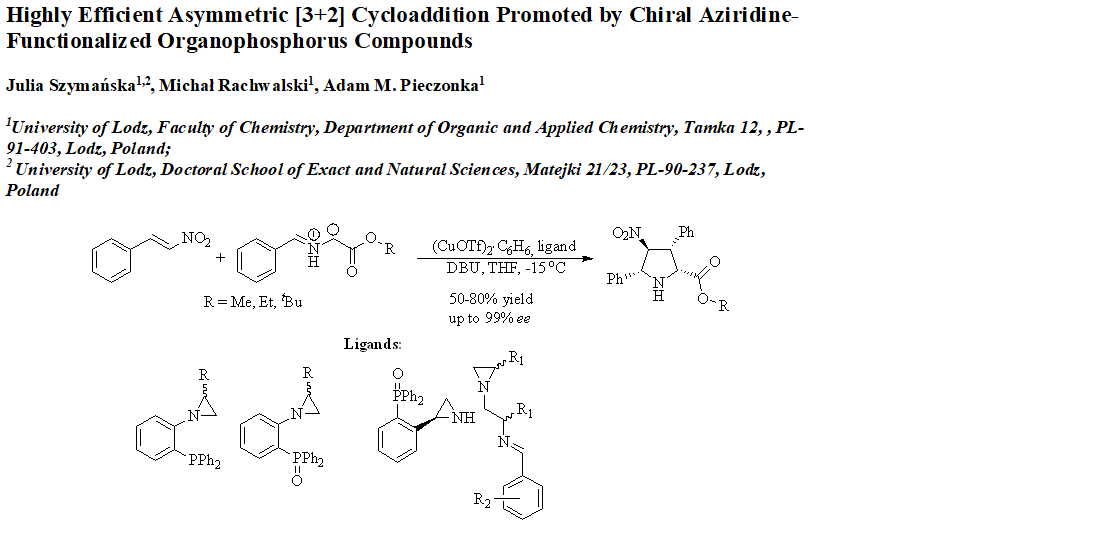
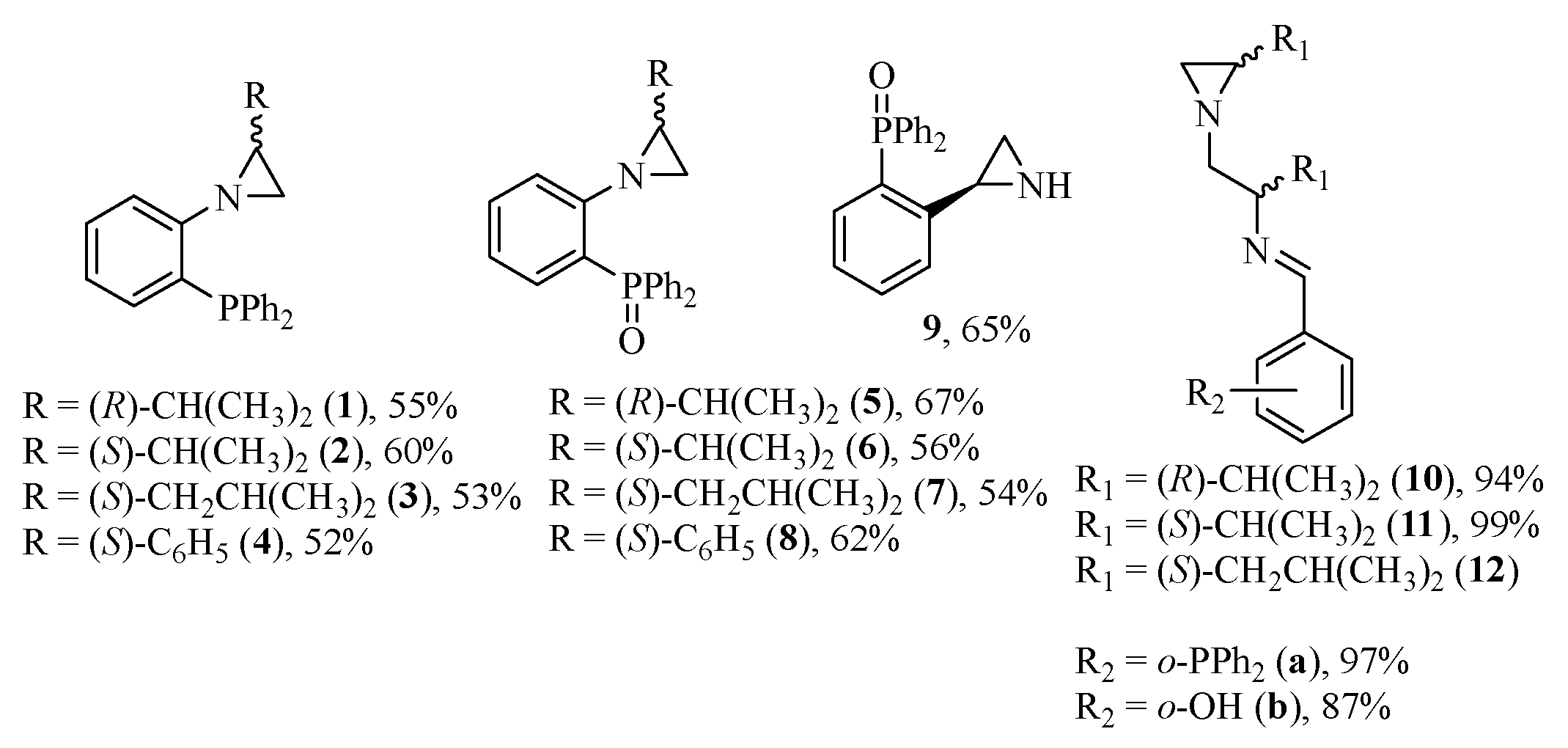
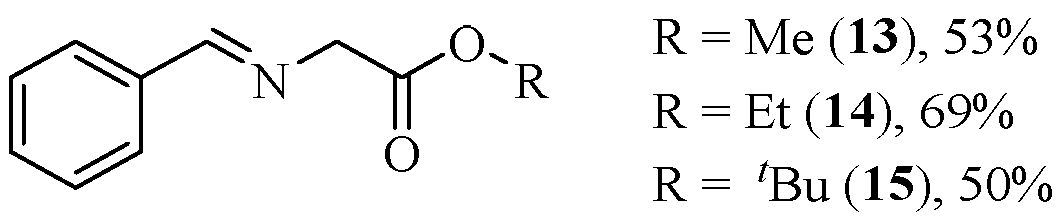
2.1. Synthesis of the Chiral Catalysts and the Starting Materials
2.2. Asymmetric [3+2]-Cycloaddition Reactions
4. Materials and Methods
4.1. General Information
4.2. Asymmetric [3+2]-Cycloaddition Reaction Catalyzed by Aziridine Derivatives 1-12 – General Procedure
Characterization of Compounds 16a-c, 17a-c, and 18b-c
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xiang, S.-H.; Tan, B. Advances in organocatalysis over the last 10 years. Nat. Commun. 2020, 11, 3786. [Google Scholar] [CrossRef] [PubMed]
- Mancheño, O. G.; Waser, M. Recent Developments and Trends in Asymmetric Organocatalysis. Eur. J. Org. Chem. 2023, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nagib, D. A. Asymmetric Catalysis in Radical Chemistry. Chem. Rev. 2022, 122, 15989–15992. [Google Scholar] [CrossRef] [PubMed]
- Das, K. K.; Kumar, P.; Ghorai, D.; Mondal, B.; Panda, S. Organoboron Compounds Towards Asymmetric Pericyclic Reaction; Exploitation to Bioactive Molecule Synthesis. Asian J. Org. Chem. 2022, 11, 1–15. [Google Scholar] [CrossRef]
- Held, F. E.; Tsogoeva, S. B. Asymmetric cycloaddition reactions catalyzed by bifunctional thiourea and squaramide organocatalysts: recent advances. Catal. Sci. Technol. 2016, 6, 645–667. [Google Scholar] [CrossRef]
- Li, J.-Y.; Kim, H. Y.; Oh, K. Brucine Diol-Catalyzed Asymmetric Synthesis of endo-Pyrrolidines: The Mechanistic Dichotomy of Imino Esters. Org. Lett. 2015, 17, 1288–1291. [Google Scholar] [CrossRef] [PubMed]
- Adrio, J.; Carretero, J. C. Stereochemical diversity in pyrrolidine synthesis by catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides. Chem. Commun. 2019, 55, 11979–11991. [Google Scholar] [CrossRef] [PubMed]
- Jeelan Basha, N.; Basavarajaiah, S. M.; Shyamsunder, K. Therapeutic potential of pyrrole and pyrrolidine analogs: an update. Mol. Divers. 2022, 26, 2915–2937. [Google Scholar] [CrossRef] [PubMed]
- Abou-Khalil, B. Levetiracetam in the treatment of epilepsy. Neuropsychiatr. Dis. Treat. 2008, 3, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Leśniak, S.; Rachwalski, M.; Pieczonka, A. M. Optically pure aziridinyl ligands as useful catalysts in the stereocontrolled synthesis. Curr. Org. Chem. 2014, 18, 3045–3065. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Asymmetric Friedel-Crafts alkylation of indoles catalyzed by chiral aziridine-phosphines. Catalysts 2020, 10, 971. [Google Scholar] [CrossRef]
- Wujkowska, Z.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Phosphinoyl-aziridines as a new class of chiral catalysts for enanti-oselective Michael addition. Tetrahedron 2019, 75, 230–235. [Google Scholar] [CrossRef]
- Buchcic-Szychowska, A.; Adamczyk, J.; Marciniak, L.; Pieczonka, A. M.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Efficient asymmetric Simmons-Smith cyclopropanation and diethylzinc addition to aldehydes promoted by enantiomeric aziridine-phosphines. Catalysts (b) Pieczonka, A. M.; Marciniak, L.; Rachwalski, M.; Leśniak, S. Enantiodivergent aldol condensation in the presence of aziridine/acid/water systems. Symmetry 2020, 12, 930. https://doi.org/10.3390/sym12060930. 2021, 11, 968. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Adamczyk, J.; Pieczonka, A. M.; Rachwalski, M. Enantioselecive Mannich Reaction Promoted by Chiral Phosphinoyl-Aziridines. Catalysts 2019, 9, 837. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, G-Y. ; Zhou, Y-G.; Li, Y-X. Hydrogen Bond Directed Reversal of Enantioselectivity. J. Am. Chem. Soc. 2007, 129, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.-F.; Song, T.; Xu, Z.; Xia, C.-G.; Huang, W.-S.; Xu, L.-W. Aromatic Amide-Derived Non-Biaryl Atropisomers as Highly Efficient Ligands in Silver-Catalyzed Asymmetric Cycloaddition Reactions. Angew. Chem. Int. Ed. (b) Kumar, A. Gupta, G.; Srivastava, S. Diversity oriented synthesis of pyrroli-dines via natural carbohydrate solid acid catalyst. J. Comb. Chem. 2010, 12, 458. https://doi.org/10.1021/cc100007a. 2015, 54, 5255–5259. [Google Scholar] [CrossRef] [PubMed]
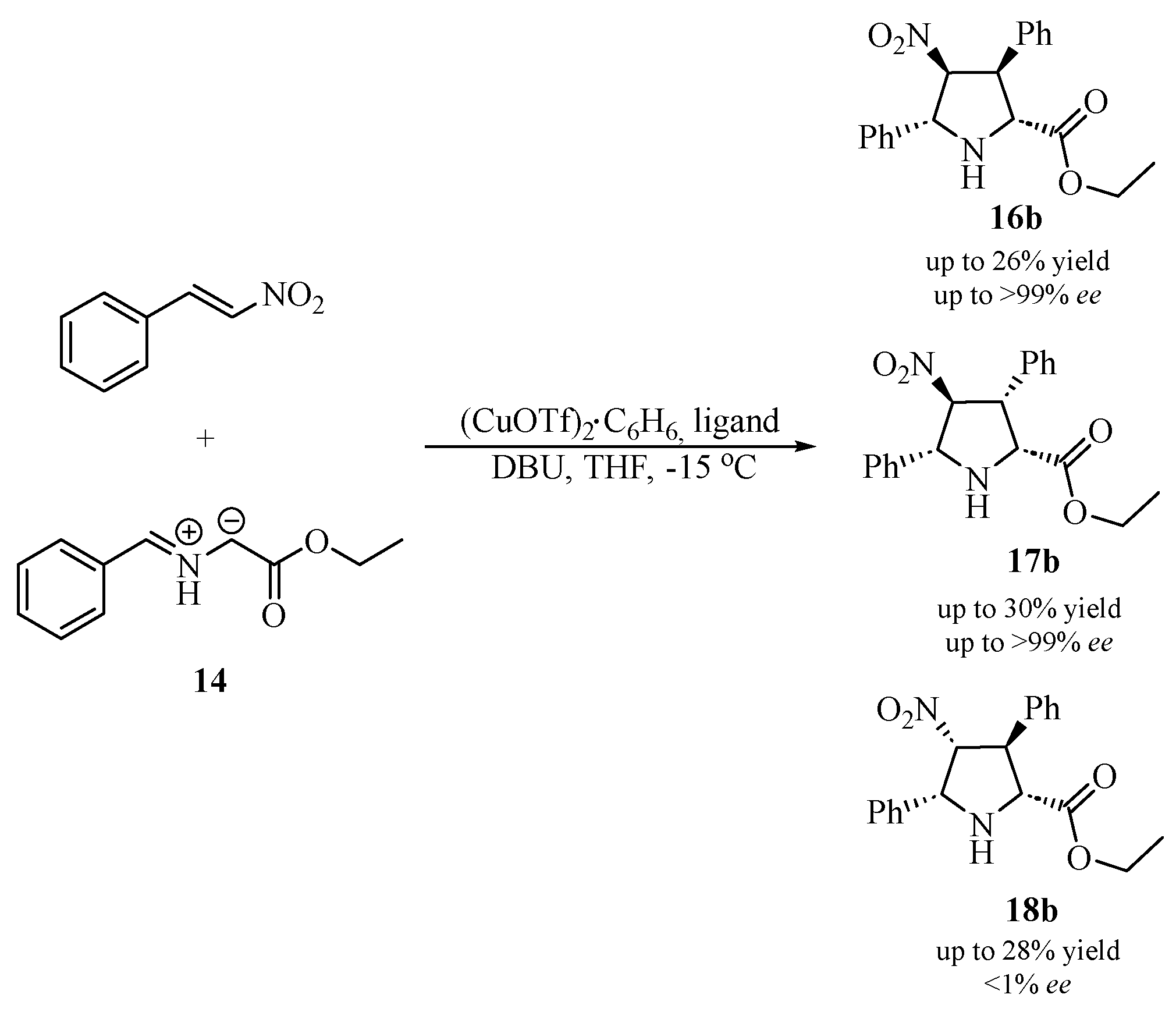
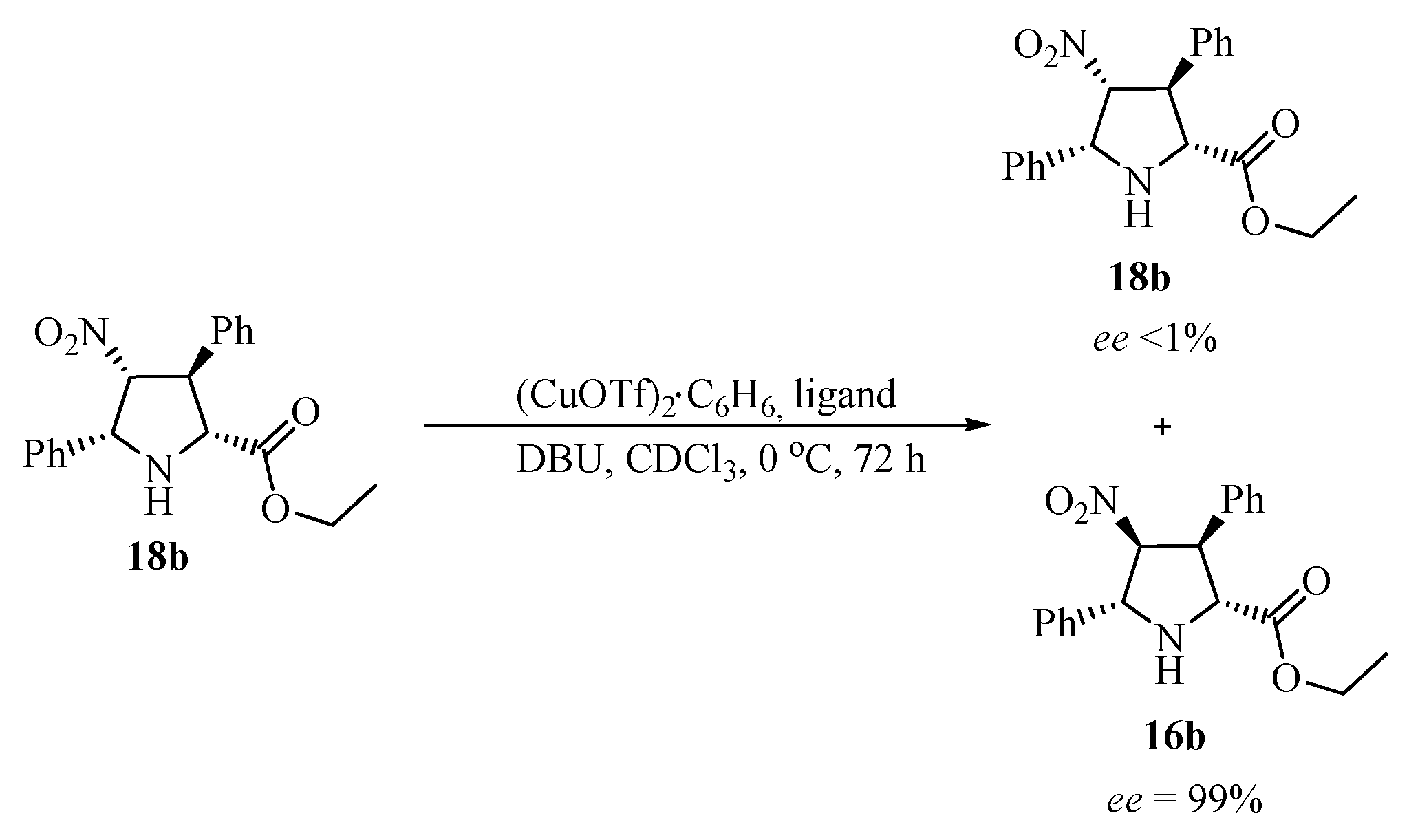
| Ligand No. | Ligand | Yield [%] | Ratio 16b/17b/18b | Ee [%] 16b | Ee [%] 17b | Ee [%] 18b |
|---|---|---|---|---|---|---|
| (R)-1 | 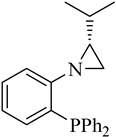 |
52 | 1.0/4.5/2.2 | 67 | 63 | 1 |
| (S)-2 | 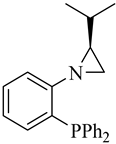 |
41 | 1.4/7.6/1.0 | 74 | 98 | 8 |
| (S)-3 | 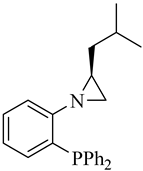 |
62 | 1.0/3.1/3.6 | 95 | 95 | 5 |
| (S)-4 | 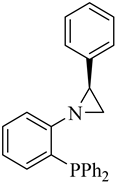 |
57 | 1.0/3.2/4.1 | 67 | 97 | 6 |
| (R)-5 | 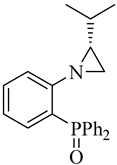 |
51 | 1.0/1.5/1.3 | 78 | >99 | 2 |
| (S)-6 | 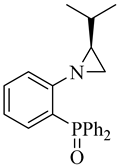 |
59 | 1.1/1.2/1.0 | 95 | 99 | 6 |
| (S)-7 | 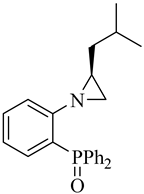 |
54 | 1.0/2.4/1.6 | 94 | 99 | 4 |
| (S)-8 |  |
54 | 1.0/2.3/1.9 | 21 | 88 | 2 |
| (S)-9 | 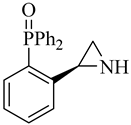 |
45 | 1.0/2.8/3.9 | 26 | 96 | 4 |
| (R,R)-10a | 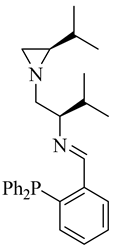 |
66 | 1.0/1.2/1.5 | 95 | 42 | 1 |
| (S,S)-11a | 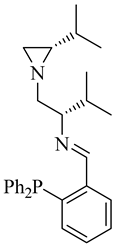 |
37 | 1.3/1.4/1.0 | >99 | 94 | 5 |
| (S,S)-12a | 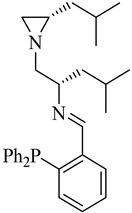 |
64 | 1.0/1.2/1.0 | 3 | 98 | 5 |
| (R,R)-10b | 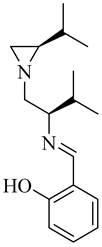 |
71 | 1.2/1.5/1.0 | 83 | 72 | 4 |
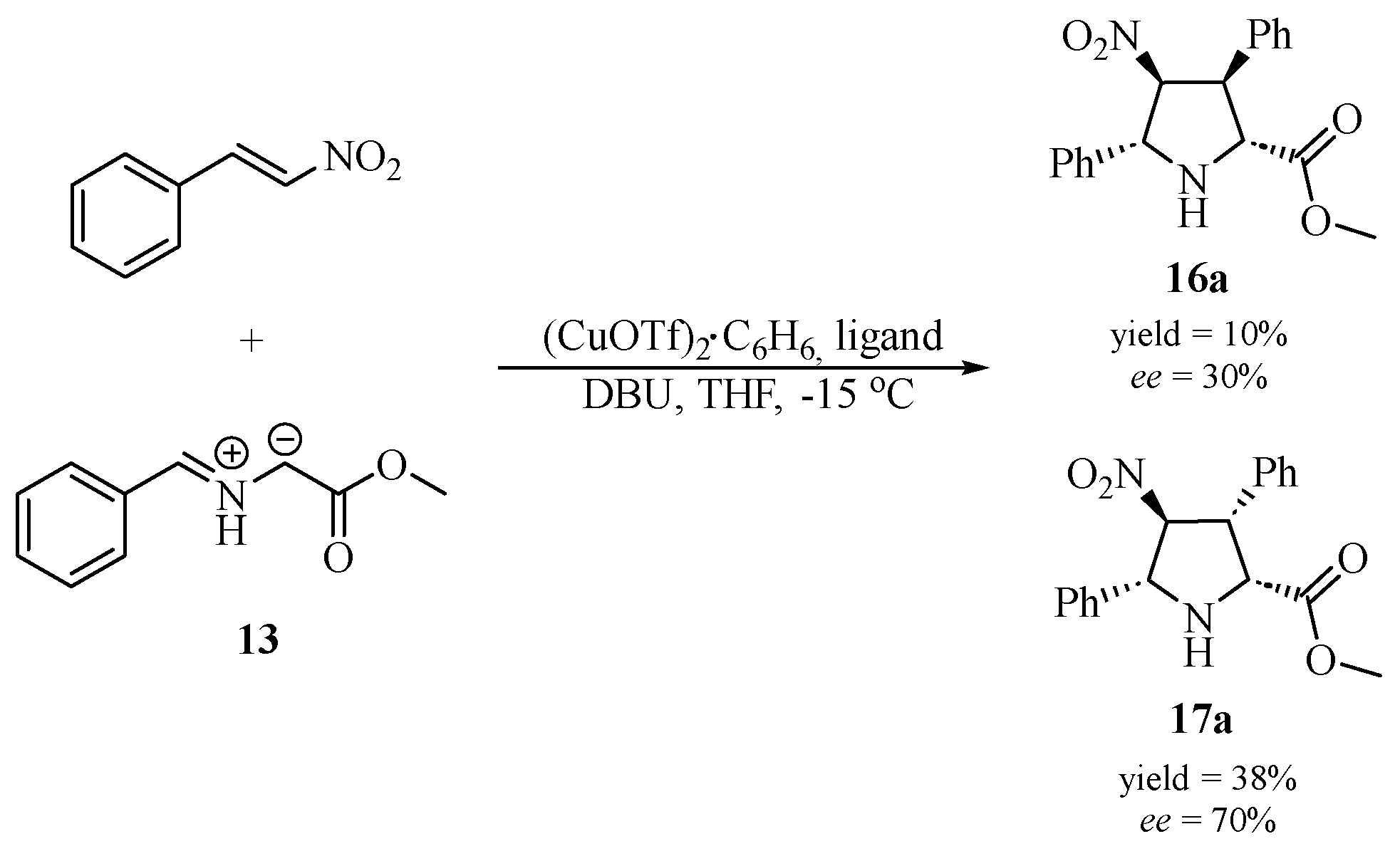
| Ligand No. | Ligand | Yield [%] | Ratio 16a/17a | Ee [%] 16a | Ee [%] 17a |
|---|---|---|---|---|---|
| (S)-6 | 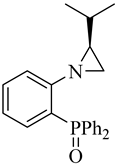 |
48 | 1.0/3.8 | 30 | 70 |
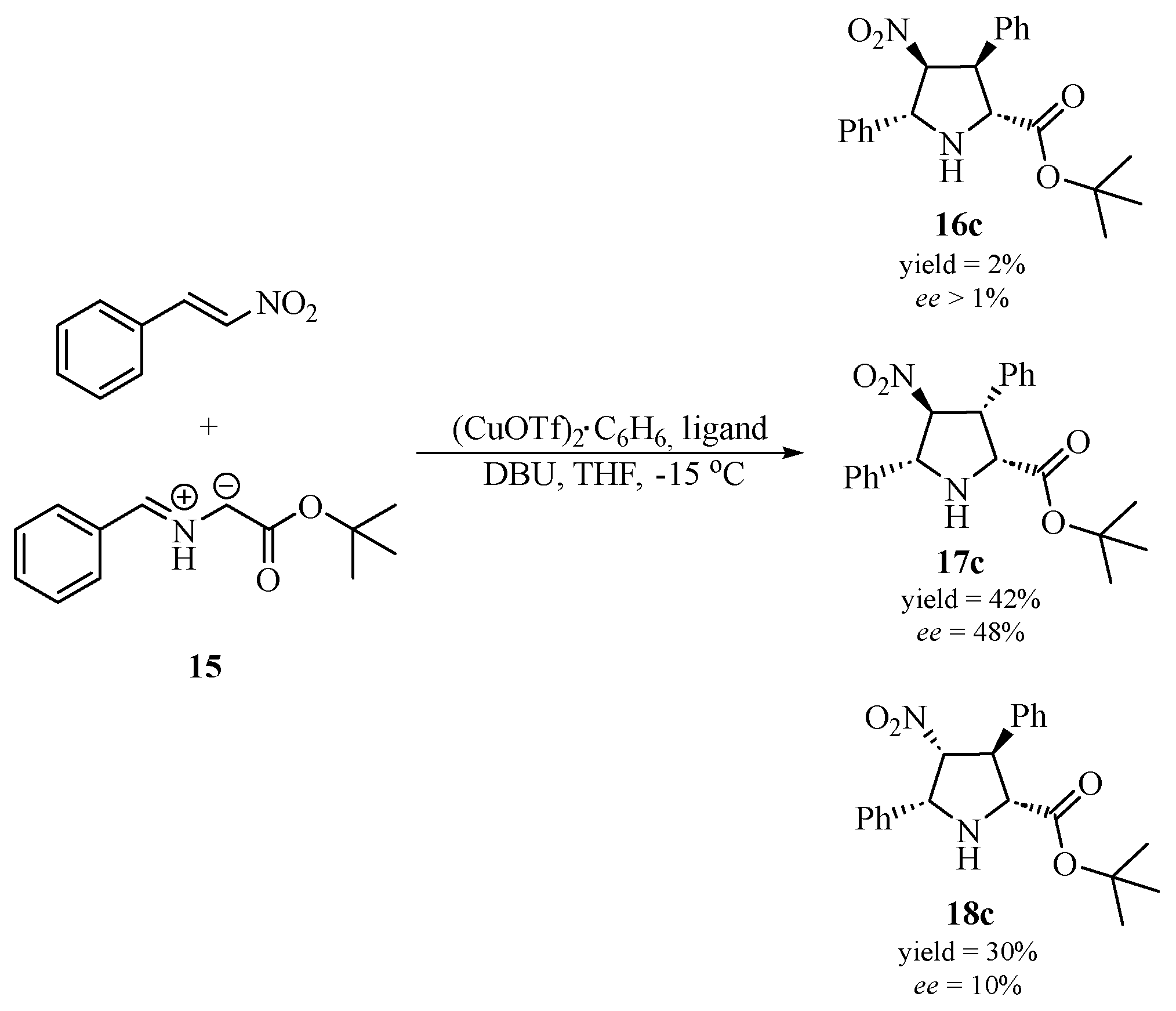
| Ligand No. | Ligand | Yield [%] | Ratio16c/17c/18c | Ee [%] 16c | Ee [%] 17c | Ee [%] 18c |
|---|---|---|---|---|---|---|
| (S)-6 | 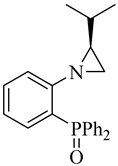 |
74 | 1.0/19.0/13.0 | >1 | 48 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





