1. Introduction
Prostate cancer (PCa) is the most frequent malignancy in men, with 1.4 million new cases diagnosed each year, and 90% of them have a confined tumour [
1].
Radiotherapy (RT) is a well-established curative treatment option. Combining androgen deprivation treatment (ADT) with RT has been shown to improve overall survival (OS) among patients with intermediate- and high-risk PCa [
2,
3,
4]. Several studies have demonstrated that dose-escalated external beam radiation treatment (EBRT) has better biochemical control than standard-dose radiation [
5,
6], and dose-escalation with brachytherapy boost may be even a better option [
7,
8,
9]. As more data supports the use of SBRT for localized PCa, several investigators attempted to raise RT dosages to the whole prostate, demonstrating that 45 Gy and 47.5 Gy were safe. However, increasing the whole-prostate dose to 50 Gy resulted in higher levels of severe late toxicity [
10,
11].
Recurrences after EBRT are typically seen in the dominant intraprostatic nodule (DIN), which is the largest nodule with the most aggressive biological behaviour, and studies of patterns-of-failure following standard 78 Gy EBRT have revealed that the DIN was the primary site of tumour recurrence in more than 90% of patients [
12,
13]. However, there is limited amount of evidence on the patterns-of-recurrence after SBRT and particularly patterns of intraprostatic recurrence after dose escalated SBRT to the DIN.
We previously reported a prospective phase I/II study, in which we used SBRT to irradiate the whole prostate gland with tumoricidal doses of 36.25 Gy in five fractions while simultaneously increasing the RT dose to the DINs up to 50 Gy. To achieve this, the protocol mandated the use of a rectal balloon spacer to maximize rectal protection and fiducial markers. In that study we implemented cutting edge imaging techniques, such as multiparametric magnetic resonance imaging (mpMRI), which has been shown to improve the sensitivity and specificity in detecting and characterizing high-risk PCa foci [
14] as well as
68Ga-labelled prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (
68Ga-PSMA-PET/CT) in order to determine the site of recurrence and compared it to the original site of tumour development.
In this reanalysis of our phase I/II study, we looked at the spatial pattern association between primary and recurrent tumour sites after prostate SBRT with dose escalation to the DIN. Importantly, 97% of our patients had intermediate or high-risk PCa and only one of them received ADT, allowing us to assess the pattern-of-recurrence of RT administered in the absence of ADT.
2. Materials and Methods
This study constitutes a phase I/II dose escalation trial, sanctioned by the Ethics Committee of the Canton Vaud (ClinicalTrials.gov ID: NCT02254746). Written informed consent was obtained from all participants. Between October 2014 and April 2017, we enrolled eligible patients diagnosed with previously untreated prostate cancer (PCa), classified as low-, intermediate-, or high-risk according to the D'Amico risk classification [
15]. Patients included had stage T2 to T3 adenocarcinoma of the prostate, with no nodal (N0) or distant metastasis (M0). In case of PSA ≥ 20μg/L, and/or T3 tumor and/or Gleason Score ≥ 8 the patient must have undergone a bone scan and a CT scan of chest-abdomen and pelvis.
All patients were required to have at least one visible nodule on mpMRI. Serum PSA levels had to be < 50 μg/L, and the International Prostate Symptom Score (IPSS) had to be ≤15 (alpha blockers permitted). Exclusion criteria included pre-SBRT prostate volume on MRI exceeding 70 cm3 or tumors located within 3 mm of the urethra on mpMRI assessment.
All patients were counselled in the prostate cancer tumor board in presence of a medical oncologist (DB), an urologist (TT, MV), a radiation oncologist (FH, JB). ADT was offered to all patients in this context.
Concomitant or adjuvant ADT was allowed, but neoadjuvant ADT was an exclusion criterion.
The primary endpoint of the phase I study was to assess acute (up to 90 days after the first RT fraction) urinary and rectal toxicity. This subsequent analysis provides data on long term toxicity as well as pattern of recurrence
2.1. Radiotherapy Planning and Delivery
A biodegradable spacer (BioProtect Balloon™ Implant system, BioProtect Ltd., Tzur Igal, Israel) was perineally implanted between the prostate and the rectum under transrectal ultrasound guidance, with the patient under sedative anaesthesia. During the same procedure, four gold anchor fiducial markers (Gold Anchor, Naslund AB, Sweden) were inserted into the prostate, ensuring at least 2 cm of spacing between them to meet the fiducial spacing threshold of at least 1 cm on orthogonal imaging for precise rotational corrections. To satisfy collinearity criteria, all angles formed by at least three fiducials had to exceed 15°. Planning MRI and planning computed tomography (CT) scans were conducted 1 to 7 days following fiducial and balloon insertion. To prevent anatomical changes in the rectum that could affect image fusion, the planning MRI was immediately followed by a planning CT scan. To ensure accurate fusion, both planning MRI and planning CT scans were performed with a uniform slice thickness of 1 mm. [
16].
Following insertion of rectal spacer/fiducial markers, planning T2-weighted MRI image sets were rigidly fused to planning CT images (fiducials-based registration), omitting the use of a catheter for urethral visualization. This method was employed to ensure contouring accuracy and appropriate visualization of the DIN and organs at risk (OARs). To minimize prostate motion during planning scans and treatment, several precautions were implemented. Patients were advised to adhere to a low-fiber diet starting 5 days before planning scans until treatment completion to reduce intestinal gas. A moderate laxative was administered 48 hours prior to planned MRI and CT scans. Enemas were performed if necessary 1 hour before planning scans and before each treatment session to minimize rectal volume. Patients were encouraged to drink 200 mL of water 1 hour before scans, following complete voiding. Anatomical contours of the prostate, DIN, seminal vesicles, and OARs were delineated by lead investigators (XX and XX), then reviewed by a panel of board-certified radiation oncologists. Identification of the DIN and urethra as the region-of-interest on MRI was conducted by a radiologist (XX). The planned target volume was generated by uniformly expanding the prostate by 3 mm (PTVp). The DIN was contoured as the gross tumor volume (GTV) and expanded by 3 mm to create a PTVDIN (no clinical target volume was used around the GTV). The prescribed dose to the PTVp was 36.25 Gy in 5 fractions (7.25 Gy per fraction) at the 80% isodose line. The prescribed dose to the PTVDIN was 45, 47.5, and 50 Gy in 5 fractions, corresponding to the 80% isodose line; hence, the maximum dose point corresponded to 56.25, 59.38, and 62.5 Gy, respectively. To allow gradients for DIN boosting and to maximize PTVDIN doses, dose heterogeneity had no limits. At least 95% of the volume of interest (PTVDIN and PTVp) needed to be covered by >95% of the prescription dose. A treatment fractionation schedule mandated a minimum of 2 days and a maximum of 6 days between fractions, with no more than 2 fractions per week. The overall treatment duration was capped at 26 days.
Dose-volume histogram goals and RT planning details were previously published (XX). Briefly, dose-volume histogram goals for the rectum included maximum dose to 0.1 cm3 < 41 Gy and V25 < 20 (volume receiving 25 Gy < 20 cm3). Bladder dose-volume histogram limits required < 0.1 cm3 to receive < 45 Gy, and the bladder median dose was capped at 20 Gy. Urethra dose was restricted to < 1 cm3 of urethra receiving > 39 Gy, with < 0.1 cm3 not to exceed 41 Gy.
Patients were preferably treated with CyberKnife; however, Tomotherapy (Accuray Inc, Sunnyvale, CA) or VMAT (Elekta Synergy Stockholm, Sweden) were permitted in cases of CyberKnife unavailability. Treatment utilized 6 MV energy, with orthogonal x-ray imaging for image guidance based on fiducial markers position. In CyberKnife-treated patients, x-ray image shifts from planning CT scans were monitored in real time during each fraction. Tomotherapy or VMAT sessions were interrupted every 10 minutes to allow for re-scanning and verification of prostate and fiducial marker positions.
To enhance patient comfort, all patients were treated supine with a knee cushion.
2.2. Treatment Schema
The whole prostate received a radiation dose of 36.25 Gy in 5 fractions at 7.25 Gy each. Dose escalation to the DIN followed a traditional 3 + 3 design for the phase I part of the study [
17]. Dose limiting toxicities (DLTs) were defined as grade 3 or higher gastrointestinal (GI) or genito-urinary (GU) toxicity occurring from the first fraction of RT up to 90 days after completing treatment, assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 4). Patients received an initial DIN dose of 9 Gy per fraction up to 45 Gy. If no DLT occurred in the first cohort, an additional 3 patients were enrolled at the next dose level (47.5 Gy in 5 fractions of 9.5 Gy). Dose escalation continued until DLT was observed or until the maximum tolerated dose (MTD, 50 Gy in 5 fractions of 10 Gy) was reached without a DLT. If a DLT occurred in a cohort, an additional 3 patients were enrolled at that level. If 2 or more patients experienced a DLT, a lower dose level was explored to determine the MTD. Patients could be enrolled simultaneously or sequentially within a cohort. However, dose escalation was not allowed until the last patient in a cohort completed a minimum 90-day follow-up without a DLT. Following completion of the phase I trial, an interim analysis was conducted, and the trial was reviewed by an Independent Data Safety Monitoring Board (IDSMB) to determine its continuation to phase II. Patients treated at the MTD or with a DLT in phase I were included in the phase II analysis.
2.3. Phase I/II Design
The phase II part of the study used a Simon optimal two-stage design [
18] to calculate sample size, using equal or more than grade 2 acute GU and GI toxicity, occurring during treatment and up to 90 days after completion of SBRT as endpoint. We consider a grade 2 or higher toxicity rate of 10% as acceptable for promising treatment, whereas a rate of 70% or higher is deemed unacceptable. We have set a 5% probability of accepting the treatment as acceptable if the true toxicity-free rate is 70% or lower (alpha), and a 20% probability of rejecting the treatment if the true toxicity-free rate is 90% or higher (beta). An interim safety analysis by the IDSMB was conducted after treating the first six patients at the recommended phase II dose (RP2D). If two or more patients out of six would develop grade 2 or higher GU or GI toxicity, the trial would be halted; otherwise, an additional 21 patients would be enrolled in the second stage. After treating 27 patients in phase II, the treatment would be considered promising for further study if five or fewer patients developed grade 2 or higher GU or GI toxicity within the initial three months of SBRT. All adverse events were graded according to NCI-CTCAE, version 4. Secondary endpoints included late toxicity (occurring >90 days from first fraction), PSA kinetics, and patient-reported outcomes. Baseline and post-treatment assessments at 1, 3, and 6 months included European Organization for Research and Treatment of Cancer QoL Form PR25 (prostate module) and IPSS scores. QoL evaluations were optional after 6 months but collected when feasible. Health-related QoL outcomes were assessed using the European Organization for Research and Treatment of Cancer guidelines [
19] scored from zero to 100. A 10-points difference or more was deemed clinically significant [
20].
Primary endpoints results (safety) and secondary endpoints including biochemical control and quality of life were recently published elsewhere (XX).
2.4. Determination of Recurrences
Patients were followed up by having PSA measurements, and a physical examination performed every 3 months for the first 2 years, and every 6 months thereafter. The nadir +2 μg/mL failure definition was used for biochemical control [
21]. Upon confirmation of biochemical recurrence patients were staged using
68Ga-PSMA-PET/CT and prostate mpMRI which were evaluated by dedicated experienced nuclear medicine physicians (XX, XX, XX) and radiologists (XX) in order to determine the exact site of failure. Any focal 68Ga-PSMA-11 uptake above location-specific background levels was considered PSMA-positive. This is the definition according to the largest prospective analysis of PSMA-11 in over 2000 patients [
22]. We determine the distance between the DIN and the urethra/rectum and its associated grade 2 or more toxicity. Results were considered significative with a
p value ≤0,05 using Welch’s correction. Analyses were done in Prism 7 (GraphPad Software, Inc, La Jolla, CA).
The 68Ga-PSMA-PET/CT image set was fused with the initial planning CT, allowing recurrence locations to be delineated in order to determine the delivered doses to the local recurrent area. Additionally, to corroborate the location of the recurrence mpMRI was deformably fused with the 68Ga-PSMA-PET/CT images and the planning CT.
The recurrent tumour volume was delineated in the planning CT.
The prostate was divided into 12 sections (left/right, anterior/posterior, and base/mid-gland/apex), and the seminal vesicles were divided into 2 sections (left/right) [
23]. The primary DIN and recurrent tumour were judged separately as present or absent in each section. We computed the overlap rate between the primary DIN and the recurrent tumour. The overlap rate was defined as the number of sections which overlapped between the primary DIN and recurrent tumour divided by the total number of sections of primary DIN. A recurrent tumour was judged to be in the “same location” if there was an overlap equal to or greater than 75%. A “partial overlap” was defined as an overlap rate between 25 and 74%. A recurrence was considered to be in a “different location” from the DIN if the overlap rate was 24% or less.
3. Results
3.1. Pattern of Recurrence
All patients received SBRT to the prostate using 36.25 Gy in 5 fractions of 7.25 Gy prescribed at the 80% isodose line with dose escalation to the DIN up to 50 Gy in 5 fractions of 10 Gy. Despite ADT being offered to the entire patient population, only one patient accepted to be treated with concomitant ADT.
Table 1 shows the most important patient’s and tumour characteristics. 23 patients underwent a scintigraphy at baseline according to their risk group to confirm the absence of metastasis. Among them, 12 underwent an additional CT scan. Additionally, 2 patients benefited from a
68Ga-PSMA-PET/CT. At a median follow-up of 82 months (range: 68-130 months), PSA failure rate was 39.4% (N=13). PSA rebounds were found in three patients, two of whom later recurred. In all 13 biochemically recurred patients,
68Ga-PSMA-PET/CT and/or mpMRIs revealed local or systemic recurrences. Out of the 13 patients that recurred, 69% (N=9) had high risk (HR) disease according to the D’Amico risk classification [
15] and 30% (N=4) had intermediate risk (IR) disease. Notably, 30% (N=4) of the patients with recurrent disease harboured a T3a/b disease.
Intraprostatic recurrences occurred in 7 patients (21%) and the median time from diagnosis to local recurrence was 33 months (range: 17-76 months). We computed 100% overlap in 2 patients, a partial overlap in two others, and no overlap in the remaining 3. Recurrent tumours received a median Dmean of 42.57 Gy (range 8.67-60.59 Gy) and D98% of 36.09 Gy (range: 2.88-55 Gy). Interestingly, the seminal vesicles were affected in all recurrent tumours except one. Figure 1 describes the location of the DIN in each individual patient as well as the site of recurrence with their respective overlap rate and Dmean/D98% doses administered.
Figure 1.
legend: Tumour lesions in the different prostate areas in the patients with local recurrences. Tables show the Dmean and D98% received in each DIN region. Overlapping recurrences were calculated using the formula: number of sections (primary DIN) / number of sections overlapping (between primary DIN and recurrence).
Figure 1.
legend: Tumour lesions in the different prostate areas in the patients with local recurrences. Tables show the Dmean and D98% received in each DIN region. Overlapping recurrences were calculated using the formula: number of sections (primary DIN) / number of sections overlapping (between primary DIN and recurrence).
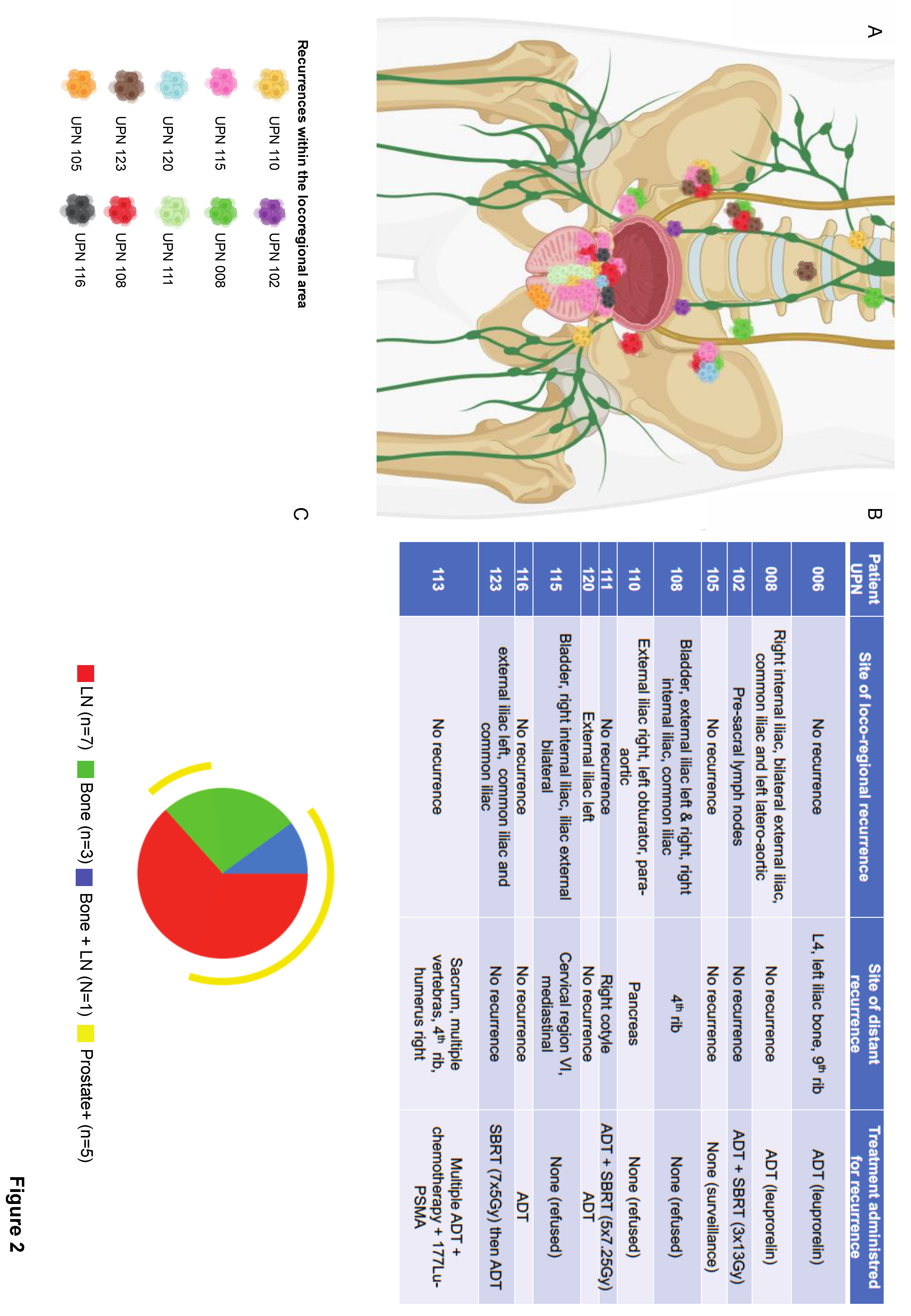
Figure 2.
legend: Loco-regional recurrences in individual patients. A) Anatomical location of loco-regional recurrences, each colour represents one patient (UPN, unique patient number). B) The table highlights the specific site of locoregional recurrence for each patient, as well as the site of distant metastases and the appropriate treatment received at recurrence. C) The Spice graphic was generated using Spice software. The graph illustrates the overlap of locoregional and distant metastases: bone metastases are depicted in green, lymph node metastases in red, and both bone and lymph node metastases in blue. The yellow circuit represents prostate recurrences, which did not occur in isolation but in conjunction with locoregional and/or distant metastases.
Figure 2.
legend: Loco-regional recurrences in individual patients. A) Anatomical location of loco-regional recurrences, each colour represents one patient (UPN, unique patient number). B) The table highlights the specific site of locoregional recurrence for each patient, as well as the site of distant metastases and the appropriate treatment received at recurrence. C) The Spice graphic was generated using Spice software. The graph illustrates the overlap of locoregional and distant metastases: bone metastases are depicted in green, lymph node metastases in red, and both bone and lymph node metastases in blue. The yellow circuit represents prostate recurrences, which did not occur in isolation but in conjunction with locoregional and/or distant metastases.
Notably, five of the 7 patients with intra-prostatic recurrences exhibited synchronous metastases: 3 in the lymph nodes, 1 in the bone, and 1 in both. On the other hand, six patients presented with either bone (N=2) or lymph node (N=4) metastasis in the absence of an intraprostatic recurrence (
Figure 2).
Supplementary Figure S1 details a patient’s case who recurred 28 months after treatment completion.
Upon recurrence ADT was recommended to most of the patients but refused by three of them. One patient was put under clinical and radiological follow-up after a board decision. One patient died of metastatic disease.
3.2. Safety
Toxicity appeared at a median of 25 months (range: 9-54 months), grade 2 and 3 late GU toxicity was present in 18% (N=6) and 3% (N=1) of the patients, respectively (
Table 2). Specifically, unique patient number (UPN) 113 was admitted to the hospital 4.5 years after treatment completion for urethral stenosis and a urinary infection that necessitated the placement of a temporary catheter. Grade 3 late GI toxicity appeared in two patients (6%)
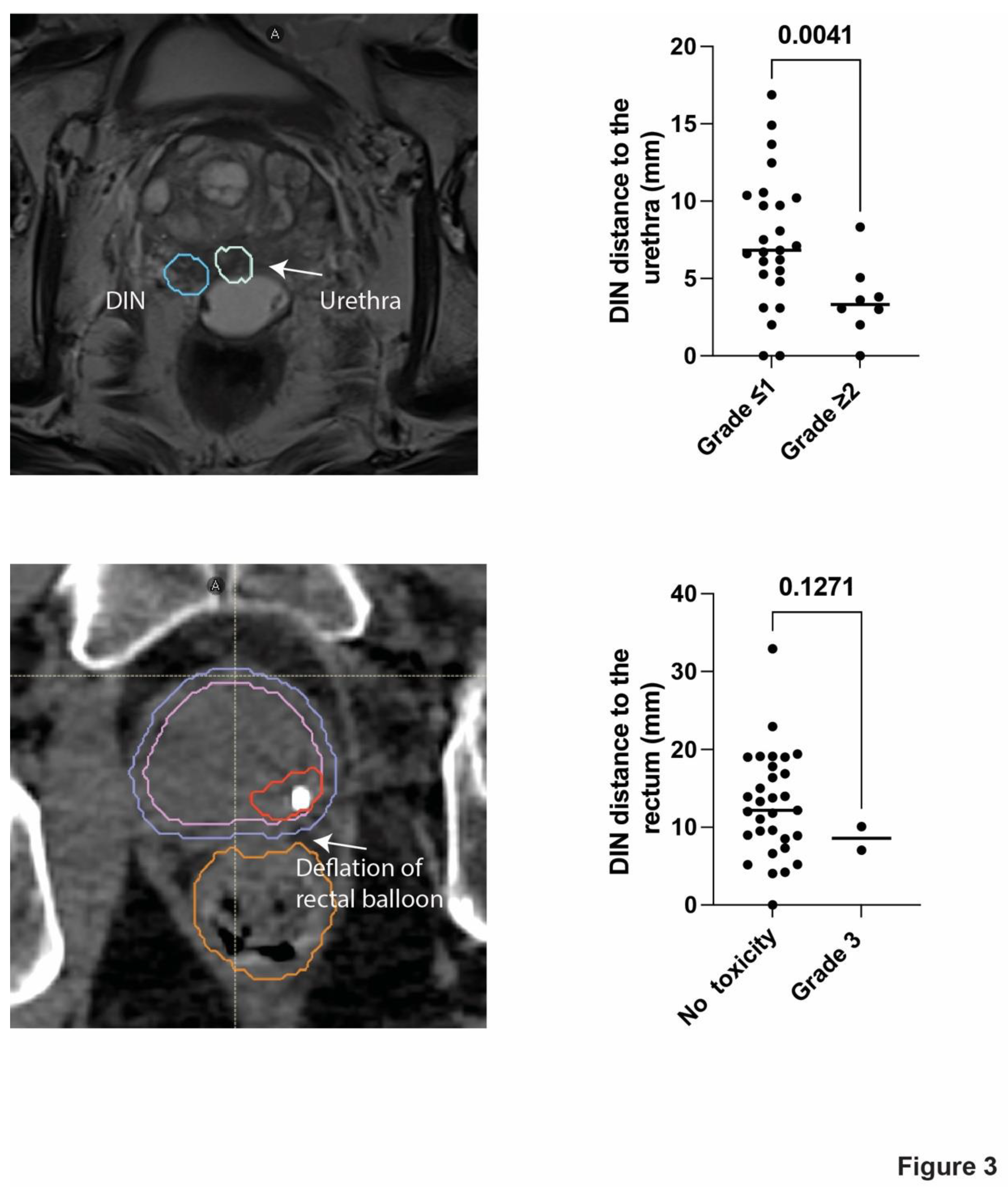
(Table 2). Specifically, UPN 111 who had undergone repeated surgical resections due to haemorrhagic diverticulosis prior to SBRT, had haemorrhagic diverticulosis 51 months after SBRT. Similarly, UPN 110 developed faecal incontinence 6 months after the end of SBRT, which was presumably caused by distal deflation of the rectal spacer (
Figure 3). There was no grade 2 late GI toxicity.
In patients with late grade 2 or more GU toxicity the mean distance between the PTV-DIN and the urethra was 3.6 mm compared to 7.5 mm in patients without significant signs of toxicity (P<0.0041) (Figure 3), there was no statistically significant difference in mean prostate volume between patients with and without toxicity (64.5 ml versus 57.3 ml, P<0.29). The distance between the rectum and the DIN was not significantly shorter in patients with late grade 3 GI toxicity (Figure 3).
4. Discussion
In the first five years following treatment, 5 to 15% of PCa patients who had current hypofractionated RT will experience a biochemical recurrence, and the rate of recurrences varies according to the initial disease risk [
24]. There has been a noticeable decrease in local failures in recent studies [
25] adopting focally dose-escalated radiation schemes, although the prostate and seminal vesicles continue to be the most common first-recurrence sites after conventional RT.
In this study, which remains small and failed to provide effective ADT treatment due to patient refusal, we found a 39.4% cumulative incidence of recurrence at 6.5 years, with 69% of recurrent patients having a high-risk disease and a failure pattern of distant metastases in most patients. Among those with distant metastases, five patients (45%) also experienced a local recurrence, which was also predicted by an unfavourable cancer pathology at baseline (
Figure 1), implying that precise patient selection is necessary for the local treatment to be effective and that the delivery of ADT is important. Local recurrence was shown to originate within the initial tumour site in 2 patients, a partial overlap in other two (40 and 33% OR) making a 12% intra-DIN recurrence, and no overlap in three patients (9% extra-DIN), indicating that, although speculative, residual tumours after DIN dose escalated SBRT expanded to areas adjacent to the original site and/or recurrences occurred in an original microscopic multifocal tumour. According to the FLAME phase III trial [
26], which evaluated the benefit of focal boost to the DIN in the context of EBRT and ADT, the biochemical disease-free survival rate was significantly better in the focal-boost arm (HR 0.45; 95% CI: 0.28-0.71,
p<0.001) with only 3% of local intra-prostatic recurrences [
25] . Due to the small number of local recurrences compared to the number of distant metastases our study provides preliminary information for the rational of adding focal boost to SBRT.
This study presented significant challenges, including difficulties in treatment positioning and planning; for example, the accuracy of mpMRI and CT-planning fusion was achieved by implementing fiducial marker-guided rigid fusions and a rectal balloon spacer; on the other hand, hot spots located in close proximity to healthy organs (urethra, bladder, and rectum) had to be manipulated in order to respect dose constraints; all of these challenges may raise the possibility of target missing in those 4 patients where the disease appear in close proximity to the DIN which we cannot rule out. Recurrent tumours received a median Dmean of 42.57 Gy (range 8.67-60.59 Gy) and D98% of 36.09 Gy (range: 2.88-55 Gy). The lower range limit (8.67 and 2.88 Gy) corresponds to three patients who recur in non-irradiated seminal vesicles; two of those patients had large prostate volumes, and in order to limit sigmoid doses, irradiation of the seminal vesicles was omitted in view of the absence of infiltration at baseline.
68Ga-PSMA-PET/CT was not available at our institution at the time patients were recruited into the trial and thus we relied on mpMRI scans. It is possible that the incorporation
68Ga-PSMA-PET/CT would have helped to identify eventual millimetric disease that could have spread around the DIN. Despite these limitations, our results could serve as a baseline data to the lack of high-level evidence regarding these issues. A recently completed meta-analysis of 38 prospective series of SBRT that included over 2,900 patients with intermediate-risk disease reporting 5-year biochemical freedom from recurrence of 92.1% [
27]. These outcomes were not stratified by favourable or unfavourable intermediate risk groups; nevertheless, available evidence suggests that SBRT treated men with unfavourable intermediate-risk PCa have equal outcomes when compared to conventionally fractionated radiation. The recently published HYPO-RT-PC randomized phase III non-inferiority trial included 1,200 patients, of which 89% were intermediate risk and showed identical 5-year failure-free survival between ultra-hypofractionated and conventionally fractionated radiation [
28]. A recent article of completed prospective trials that pooled results for low and intermediate-risk patients did stratify results into favourable and unfavourable intermediate-risk groups. The 7-year biochemical failure free survival rate was 93 and 85% for favourable intermediate and unfavourable intermediate-risk groups, respectively [
24]. This excellent outcome is confirmed by another multicentric prospective trial, with 5-year rates of 100 and 93.1%, respectively, for favourable intermediate and unfavourable intermediate-risk patients [
29]. In addition, the previously discussed results from pooled trials also support low rates of distant metastatic disease (1.7 and 3.0%, respectively), with no patients dying of prostate cancer [
24]. In our own analysis, 11 patients developed metastatic disease with one of those patients having prostate cancer–specific mortality and 2 patients had intraprostatic recurrence alone. Our higher incidence of metastatic failures is probably linked to the higher proportion of high-risk patients (55% of the trial population) and the lack of ADT in the majority of them. Data supporting the use of SBRT in high-risk prostate cancer are more limited. The meta-analysis discussed previously also included 470 patients with high-risk disease, but was unable to provide a high-risk cohort estimate of biochemical freedom from recurrence given that many of the included studies did not provide estimates based on risk group [
27]. Numerous retrospective/single institution experiences have included high-risk patients from 7.4 to 65.9% of their patient cohorts, with variable total doses (32–40 Gy in 5 fractions) and ADT use [
30,
31,
32,
33,
34,
35,
36,
37]. In addition, a large National Cancer Database study did not find a difference in overall survival between SBRT and conventionally fractionated patients when propensity-matched high-risk subpopulations of Gleason score 8+ or PSA >10µg/l [
38]. Despite potential selection bias, results are promising with 5-year biochemical failure free recurrence between 70 and 80% in most series, and therefore our study is aligned with those results. One of the largest series of high-risk patients (
n = 52) with long follow-up (median 60 months) was the Katz et al. study which reported 6-year biochemical failure free recurrence of 69%, comparable with the 7-year rate of 68% in high-risk patients treated on a dose-escalated conventionally fractionated trial [
36,
37]. The HYPO-RT-PC trial also included 11% of patients with high-risk disease but did not stratify their biochemical outcomes by risk group. Given the small proportion of high-risk patients, the authors concluded that there is not enough evidence to support SBRT as standard-of-care for high-risk patients contrary to their conclusion for intermediate-risk patients. In addition, this trial did not use ADT, which is standard- of-care for high-risk patients [
28]. A consortium of seven institutional phase II studies (SHARP) investigated the efficacy and toxicity of SBRT in 344 high-risk prostate cancer patients [
39]. Most trials within the consortium administered SBRT to the entire prostate, with doses ranging between 5 fractions of 7 to 5 fractions of 8 Gy. With a median follow-up of 49.5 months, 72% of patients received ADT (median duration of 9 months), and some patients underwent elective nodal radiotherapy. The 4-year biochemical recurrence-free survival was 81.7% (95% CI, 77.2%-86.5%), and the distant metastasis-free survival was 89.1% (95% CI, 85.3%-93.1%). The crude incidences of late grade ≥3 genitourinary and gastrointestinal toxicity were 2.3% and 0.9%, respectively.
Due to the rising concerns associated with clinical observations of significant late toxicity in other dose escalation trials [
10], we present data with a long term follow-up focused on treatment safety. Although late grade 3 toxicity remains low (3% GU and 6% GI) in this study, we demonstrate a significant association between the volume of irradiation and the risk of late toxicity in patients treated with dose escalated SBRT. To place the results of the present series in the appropriate context, we extracted the rates of late severe (i.e., grade
3 or more) toxic events after treatment reported in other series with long-term follow-up (Supplementary Table S1). Overall, the outcomes after SBRT compare very favourably, without evidence of unanticipated increased late toxic effects. Menkarios et al. [
40], reported 5% GU and 11% GI toxicity at approximately 2 years following IMRT and delivering 45 Gy in nine 5 Gy fractions, once weekly. In that studies grade 3 or more GI toxicities consisted of rectal bleeding with one patient experiencing haemorrhagic cystitis that require blood transfusion and a radical cysto-prostatectomy for bladder necrosis. Similarly, Hannan et al reported 6% of GU and 7% GI grade 3 or more toxicity at 5-years. Late grade 3 GU toxicity consisted of cystitis requiring uretero-ileal diversion and mainly observed in the 50 Gy dose escalation arm [
41].
Chen et al recently reviewed 249 low-intermediate and high-PCa patients treated in different series with dose escalated SBRT up to 45 Gy in five fractions. With a median follow-up of 14.9 months late GU and GI grade 3 or more toxicity occurred in 6 and 1.5% of the patients, respectively [
42].
It is possible that the relatively low incidence of severe late GI toxicity in our 50 Gy treated patients is due to the placement of a rectal balloon spacer. Folkert et al recently reported a series of 44 men treated with SBRT at 45 Gy in 5 fractions using a rectal balloon spacer and showed no incidence of grade 3 toxicity in any domain with an excellent biochemical control of 93% in intermediate risk patients at 48 months median follow-up [
43].
To better put our results into context we also compared different rates of self-reported patient’s perception in quality-of-life at baseline and at 3, 6, 9 and 12 months follow up in different series and acknowledging the differences in self-reported questionnaires used by the different trials we observed that scores gradually decreased reaching a plateau at 12 months and subsequently improving.
The mechanisms of GU and GI toxicity are many and complex. They include patient-related characteristics such as baseline urine symptoms, prostate volume, the presence of the urethra in the high dose volume area, radiation delivery processes such as bladder and rectal filling during treatment, and dosimetric parameters. While our efforts to develop an innovative strategy succeeded quite satisfactorily, the precise dosimetric restrictions to prevent GU and GI toxicity are not well understood and are a subject of active investigation.
The use of a rectal spacer prior to 50 Gy-DIN SBRT, as well as rectal and bladder/urethra limitations learned from our phase I/II trial experience, most likely contributed to lower rectal toxicity risks in our patients compared to previous publications that also attempted to deliver 50 Gy to the prostate.
5. Conclusions
Among patients with intermediate-high risk PCa undergoing focal dose-escalated SBRT without ADT, DIN recurrences were occasional (12%). When present, these recurrences were typically located at the original site or adjacent to the initial tumor. Conversely, relapses beyond the DIN (9%) and in extra-prostatic (metastatic) sites were prevalent, underscoring the significance of systemic ADT in managing this patient population.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Minna Cloître: data collection, figures and tables of the paper, supplementary material, redaction of the manuscript. Fernanda Herrera, MD, PhD: principal investigator of the study, figures and tables, corrections and redaction of the manuscript. Jean Bourhis : principal investigator. Sofian Benkhaled, MD: data collection of some of the irradiation parameters. Sarah Boughdad, MD: data collection of the SUVs of the recurrent tumors on PET-CT. Niklaus Schaefer, MD, PhD, John Prior, MD, PhD: help with their expertise in nuclear medicine. Michele Zeverino, MSc: insightful input for the pertinence of our manuscript, help in data collection for physics parameters of the radiotherapy treatment. Dominik Berthold, MD: referent medical oncologist in this study. Thomas Tawadros, MD, PhD, Paul Martel, MD, Chantal Rohner, Massimo Valerio, MD, PhD: surgical urology team, responsible for Rectal Spacer’s placement and follow-up. Leonie Heym, Frederic Duclos, Veronique Vallet, PhD: medical dosimetrists involved in patient’s treatment according to protocol.
Funding
Accuray Inc: Sunnyvale, CA; and BioProtect Ltd., Tzur Igal, Israel.
Institutional Review Board Statement
This phase 1/2 study was approved on the 17.02.2014 by the Ethics Committee of the Canton Vaud (ClinicalTrials.gov ID: NCT02254746) and conducted according to the guidelines of the Declaration of Helsinki.
Informed Consent Statement
All patients enrolled in this study signed informed consent.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Acknowledgments
The authors would like to thank Eloise Sychold for her help on Supplementary Tables S1 and S2.
Conflicts of Interest
F.G. Herrera reports grants from Bristol-Myers Squibb, Nanobiotix, BioProtect, San Salvatore Foundation and Prostate Cancer Foundation Challenge Award during the conduct of the study. Personal fees from Johnson & Johnson, Seagen, MSD, AstraZeneca, Roche, Eisai as well as nonfinancial support from European Organization for Research and Treatment of Cancer. All other authors report no conflicts of interest.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. [CrossRef]
- Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. The Lancet. 2009 Jan 24;373(9660):301–8. [CrossRef]
- Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010 Nov 1;11(11):1066–73.
- Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. The Lancet. 2011 Dec 17;378(9809):2104–11. [CrossRef]
- Peeters STH, Heemsbergen WD, Koper PCM, van Putten WLJ, Slot A, Dielwart MFH, et al. Dose-Response in Radiotherapy for Localized Prostate Cancer: Results of the Dutch Multicenter Randomized Phase III Trial Comparing 68 Gy of Radiotherapy With 78 Gy. J Clin Oncol. 2006 May;24(13):1990–6. [CrossRef]
- Dearnaley DP, Sydes MR, Graham JD, Aird EG, Bottomley D, Cowan RA, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007 Jun 1;8(6):475–87. [CrossRef]
- Morris WJ, Tyldesley S, Rodda S, Halperin R, Pai H, McKenzie M, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int J Radiat Oncol. 2017 Jun 1;98(2):275–85. [CrossRef]
- Spratt DE, Soni PD, McLaughlin PW, Merrick GS, Stock RG, Blasko JC, et al. American Brachytherapy Society Task Group Report: Combination of brachytherapy and external beam radiation for high-risk prostate cancer. Brachytherapy. 2017 Jan 1;16(1):1–12. [CrossRef]
- Hoskin PJ, Rojas AM, Ostler PJ, Bryant L, Lowe GJ. Randomised trial of external-beam radiotherapy alone or with high-dose-rate brachytherapy for prostate cancer: Mature 12-year results. Radiother Oncol. 2021 Jan 1;154:214–9. oi:10.1016/j.radonc.2020.09.047.
- Boike TP, Lotan Y, Cho LC, Brindle J, DeRose P, Xie XJ, et al. Phase I Dose-Escalation Study of Stereotactic Body Radiation Therapy for Low- and Intermediate-Risk Prostate Cancer. J Clin Oncol. 2011 May 20;29(15):2020–6. [CrossRef]
- Kim DWN, Cho LC, Straka C, Christie A, Lotan Y, Pistenmaa D, et al. Predictors of Rectal Tolerance Observed in a Dose-Escalated Phase 1-2 Trial of Stereotactic Body Radiation Therapy for Prostate Cancer. Int J Radiat Oncol. 2014 Jul 1;89(3):509–17. [CrossRef]
- Arrayeh E, Westphalen AC, Kurhanewicz J, Roach M, Jung AJ, Carroll PR, et al. Does Local Recurrence of Prostate Cancer After Radiation Therapy Occur at the Site of Primary Tumor? Results of a Longitudinal MRI and MRSI Study. Int J Radiat Oncol. 2012 Apr 1;82(5):e787–93. [CrossRef]
- Cellini N, Morganti AG, Mattiucci GC, Valentini V, Leone M, Luzi S, et al. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: implications for conformal therapy planning. Int J Radiat Oncol. 2002 Jul 1;53(3):595–9. [CrossRef]
- Puech P, Sufana Iancu A, Renard B, Villers A, Lemaitre L. Detecting prostate cancer with MRI — why and how. Diagn Interv Imaging. 2012 Apr 1;93(4):268–78. [CrossRef]
- D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998 Sep 16;280(11):969–74. [CrossRef]
- Collins S, Lei S, Piel N, Oermann E, Chen V, Ju A, et al. Six-Dimensional Correction of Intra-Fractional Prostate Motion with CyberKnife Stereotactic Body Radiation Therapy. Front Oncol [Internet]. 2011 [cited 2022 Mar 6];1. Available from: https://www.frontiersin.org/article/10.3389/fonc.2011.00048.
- Le Tourneau C, Lee JJ, Siu LL. Dose Escalation Methods in Phase I Cancer Clinical Trials. JNCI J Natl Cancer Inst. 2009 May 20;101(10):708–20. [CrossRef]
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989 Mar;10(1):1–10. [CrossRef]
- Fayers PM. Interpreting quality of life data: population-based reference data for the EORTC QLQ-C30. Eur J Cancer Oxf Engl 1990. 2001 Jul;37(11):1331–4. [CrossRef]
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol Off J Am Soc Clin Oncol. 1998 Jan;16(1):139–44. [CrossRef]
- Roach M, Hanks G, Thames H, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006 Jul 15;65(4):965–74. [CrossRef]
- Abghari-Gerst M, Armstrong WR, Nguyen K, Calais J, Czernin J, Lin D, et al. A comprehensive assessment of 68Ga-PSMA-11 PET in biochemically recurrent prostate cancer: Results from a prospective multi-center study in 2005 patients. J Nucl Med [Internet]. 2021 Jul 1 [cited 2024 Feb 9]; Available from: https://jnm.snmjournals.org/content/early/2021/07/29/jnumed.121.262412. [CrossRef]
- Barrier A, Ouzzane A, Villers A. Rôle de l’IRM prostatique dans le cancer de la prostate en 2016: mise au point et perspectives d’avenir. Afr J Urol. 2017 Dec 1;23(4):272–7. [CrossRef]
- Kishan AU, Dang A, Katz AJ, Mantz CA, Collins SP, Aghdam N, et al. Long-term Outcomes of Stereotactic Body Radiotherapy for Low-Risk and Intermediate-Risk Prostate Cancer. JAMA Netw Open. 2019 Feb 8;2(2):e188006. [CrossRef]
- Groen VH, Haustermans K, Pos FJ, Draulans C, Isebaert S, Monninkhof EM, et al. Patterns of Failure Following External Beam Radiotherapy With or Without an Additional Focal Boost in the Randomized Controlled FLAME Trial for Localized Prostate Cancer. Eur Urol. 2022 Sep 1;82(3):252–7. [CrossRef]
- Kerkmeijer LGW, Groen VH, Pos FJ, Haustermans K, Monninkhof EM, Smeenk RJ, et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J Clin Oncol. 2021 Mar;39(7):787–96. [CrossRef]
- Jackson WC, Silva J, Hartman HE, Dess RT, Kishan AU, Beeler WH, et al. Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis of Over 6,000 Patients Treated On Prospective Studies. Int J Radiat Oncol. 2019 Jul 15;104(4):778–89. [CrossRef]
- Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. The Lancet. 2019 Aug 3;394(10196):385–95. [CrossRef]
- Meier RM, Bloch DA, Cotrutz C, Beckman AC, Henning GT, Woodhouse SA, et al. Multicenter Trial of Stereotactic Body Radiation Therapy for Low- and Intermediate-Risk Prostate Cancer: Survival and Toxicity Endpoints. Int J Radiat Oncol. 2018 Oct 1;102(2):296–303. [CrossRef]
- Macias VA, Blanco ML, Barrera I, Garcia R. A Phase II Study of Stereotactic Body Radiation Therapy for Low-Intermediate-High-Risk Prostate Cancer Using Helical Tomotherapy: Dose-Volumetric Parameters Predicting Early Toxicity. Front Oncol [Internet]. 2014 [cited 2023 Oct 13];4. Available from: https://www.frontiersin.org/articles/10.3389/fonc.2014.00336.
- Bolzicco G, Favretto MS, Satariano N, Scremin E, Tambone C, Tasca A. A single-center study of 100 consecutive patients with localized prostate cancer treated with stereotactic body radiotherapy. BMC Urol. 2013 Oct 17;13(1):49.
- Tree AC, Ostler P, Hoskin P, Dankulchai P, Nariyangadu P, Hughes RJ, et al. Prostate Stereotactic Body Radiotherapy — First UK Experience. Clin Oncol. 2014 Dec 1;26(12):757–61. oi:10.1016/j.clon.2014.08.007.
- Fan CY, Chao HL, Huang WY, Lin CS, Chen CM, Lo CH. Stereotactic Ablative Radiotherapy with CyberKnife in the Treatment of Locally Advanced Prostate Cancer: Preliminary Results. Tumori J. 2015 Nov 1;101(6):684–91. [CrossRef]
- Kotecha R, Djemil T, Tendulkar RD, Reddy CA, Thousand RA, Vassil A, et al. Dose-Escalated Stereotactic Body Radiation Therapy for Patients With Intermediate- and High-Risk Prostate Cancer: Initial Dosimetry Analysis and Patient Outcomes. Int J Radiat Oncol. 2016 Jul 1;95(3):960–4. [CrossRef]
- King CR, Freeman D, Kaplan I, Fuller D, Bolzicco G, Collins S, et al. Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013 Nov 1;109(2):217–21. [CrossRef]
- Katz A, Kang J. Stereotactic body radiotherapy with or without external beam radiation as treatment for organ confined high-risk prostate carcinoma: a six year study. Radiat Oncol. 2014 Jan 1;9(1):1. [CrossRef]
- Spratt DE, Pei X, Yamada J, Kollmeier MA, Cox B, Zelefsky MJ. Long-term Survival and Toxicity in Patients Treated With High-Dose Intensity Modulated Radiation Therapy for Localized Prostate Cancer. Int J Radiat Oncol. 2013 Mar 1;85(3):686–92. [CrossRef]
- Ricco A, Hanlon A, Lanciano R. Propensity Score Matched Comparison of Intensity Modulated Radiation Therapy vs Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Survival Analysis from the National Cancer Database. Front Oncol [Internet]. 2017 [cited 2023 Oct 13];7. Available from: https://www.frontiersin.org/articles/10.3389/fonc.2017.00185.
- van Dams R, Jiang NY, Fuller DB, Loblaw A, Jiang T, Katz AJ, et al. Stereotactic Body Radiotherapy for High-Risk Localized Carcinoma of the Prostate (SHARP) Consortium: Analysis of 344 Prospectively Treated Patients. Int J Radiat Oncol Biol Phys. 2021 Jul 1;110(3):731–7. [CrossRef]
- Menkarios C, Vigneault É, Brochet N, Nguyen DH, Bahary JP, Jolicoeur M, et al. Toxicity report of once weekly radiation therapy for low-risk prostate adenocarcinoma: preliminary results of a phase I/II trial. Radiat Oncol. 2011 Sep 9;6(1):112. [CrossRef]
- Hannan R, Tumati V, Xie XJ, Cho LC, Kavanagh BD, Brindle J, et al. Stereotactic body radiation therapy for low and intermediate risk prostate cancer—Results from a multi-institutional clinical trial. Eur J Cancer. 2016 May 1;59:142–51. [CrossRef]
- Chen L, Gannavarapu BS, Desai NB, Folkert MR, Dohopolski M, Gao A, et al. Dose-Intensified Stereotactic Ablative Radiation for Localized Prostate Cancer. Front Oncol. 2022;12:779182. [CrossRef]
- Folkert MR, Zelefsky MJ, Hannan R, Desai NB, Lotan Y, Laine AM, et al. A Multi-Institutional Phase 2 Trial of High-Dose SAbR for Prostate Cancer Using Rectal Spacer. Int J Radiat Oncol Biol Phys. 2021 Sep 1;111(1):101–9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).