Submitted:
21 May 2024
Posted:
23 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Total Flavonoid and Polyphenol Content
2.2. Antioxidant Activity
2.3. LC/MS Analysis
2.4. GC/MS Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Reagents
4.2. Total Phenolic Content
4.3. Total Flavonoid Content
4.4. Phenol Carboxylic Acid Content
4.5. DPPH Antioxidant Assay
4.6. CUPRAC Antioxidant Assay
4.7. Liquid Chromatography/Mass Spectrometry (LC/MS) Analysis
4.8. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, R.; Lu, Y.; Hao, H.; Zhang, M.D.; Xuan, J.; Zhang, Y.Q. Research progress on chemical constituents and pharmacological activities of iridoid glycosides in Lonicera japonica. Zhongguo Zhong Yao Za Zhi 2021, 46(11), 2746–2752. [Google Scholar] [CrossRef] [PubMed]
- Filipiak-Szok, A.B.; Kurzawa, M.; Szłyk, E. Simultaneous Determination of Isoquinoline Alkaloids in Medicinal Asiatic Plants by Ultrasound-Assisted Extraction and High-Performance Liquid Chromatography – Mass Spectrometry with Principal Component Analysis. Analytical Letters 2018, 51(16), 2577–2587. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ji, W.; Liu, S.; Fan, J.; Lu, H.; Wang, X. Metabolomics Analysis of Different Tissues of Lonicera japonica Thunb. Based on Liquid Chromatography with Mass Spectrometry. Metabolites 2023, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.F.; Hsiao, P.C.; Kuo, T.C.; Chiang, S.T.; Chen, S.L.; Chiou, S.J.; Ling, X.H.; Liang, M.T.; Cheng, W.Y.; Houng, J.Y. Antioxidant and anti-inflammatory activities of Lonicera japonica Thunb. var. sempervillosa Hayata flower bud extracts prepared by water, ethanol and supercritical fluid extraction techniques. Industrial Crops and Products 2016, 89, 543. [Google Scholar] [CrossRef]
- Lin, H.W.; Lee, Y.J.; Yang, D.J.; Hsieh, M.C.; Chen, C.C.; Hsu, W.L.; Chang, Y.Y.; Liu, C.W. Anti-inflammatory effects of Flos Lonicerae Japonicae Water Extract are regulated by the STAT/NF-κB pathway and HO-1 expression in Virus-infected RAW264. 7 cells. International Journal of Medical Sciences 2021, 18(11), 2285–2293. [Google Scholar] [CrossRef]
- Lee, J.; Park, G.; Chang, Y.H. Nutraceuticals and antioxidant properties of Lonicera japonica Thunb. as affected by heating time. International Journal of Food Properties 2019, 22, 630. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Fu, C.; Song, Y.; Fu, Q. Lonicerae japonicae flos and Lonicerae flos: a systematic review of ethnopharmacology, phytochemistry and pharmacology. Phytochemistry Reviews 2020, 19(1), 1–61. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Ren, G.; Wu, J.; Cao, K.; Li, M.; Liu, Y.; Liu, Y.; Hu, X.; Song, M.; Wu, R. Extraction, purification, structural characterization, and antioxidant activity of a novel polysaccharide from Lonicera japonica Thunb. Frontiers in Nutrition 2022, 9, 1035760. [Google Scholar] [CrossRef]
- Tang, X.; Liu, X.; Zhong, J.; Fang, R. Potential application of Lonicera japonica extracts in animal production: from the perspective of intestinal health. Frontiers in Microbiology 2021, 12, 1. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Bai, X.; Liu, P.; Yang, Y.; Huang, J.; Zhou, L.; Min, X. Fractionation and antioxidant activities of the water-soluble polysaccharides from Lonicera japonica Thunb. International Journal of Biological Macromolecules 2020, 151, 1058. [Google Scholar] [CrossRef]
- Lee, J.; Park, G.; Chang, Y.H. Nutraceuticals and antioxidant properties of Lonicera japonica Thunb. as affected by heating time. International Journal of Food Properties 2019, 22(1), 630–645. [Google Scholar] [CrossRef]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Scientific Reports 2019, 9, 16021. [Google Scholar] [CrossRef] [PubMed]
- Rezende, W.P.; Borges, L.L.; Santos, D.L.; Nilda, M.A.; Alves, D.L.; Paula, J.R. Effect of Environmental Factors on Phenolic Compounds in Leaves of Syzygium jambos (L.) Alston (Myrtaceae). Modern Chemistry Applications 2015, 3(2), 1–6. [Google Scholar] [CrossRef]
- Kausar, A.; Zahra, N.; Zahra, H.; Hafeez, M.B.; Zafer, S.; Shahzadi, A.; Raza, A.; Djalovic, I.; Prasad, P.V.V. Alleviation of drought stress through foliar application of thiamine in two varieties of pea (Pisum sativum L.). Plant Signaling & Behavior 2023, 18(1). [Google Scholar] [CrossRef] [PubMed]
- Idris, Z.H.C.; Abidin, A.A.Z.; Subki, A.; Yusof, Z.N.B. The effect of oxidative stress towards the expression of thiamine biosynthesis genes (THIC and THI1/THI4) in oil palm (Elaeis guineensis). Tropical Life Sciences Research 2018, 29(1), 71–85. [Google Scholar] [CrossRef] [PubMed]
- Misra, D.; Dutta, W.; Jha, G.; Ray, P. Interactions and regulatory functions of phenolics in soil-plant-climate nexus. Agronomy 2023, 13, 280. [Google Scholar] [CrossRef]
- Albergaria, E.T.; Oliveira, A.F.M.; Albuquerque, U.P. The effect of water deficit stress on the composition of phenolic compounds in medicinal plants. South African Journal of Botany 2020, 131, 12. [Google Scholar] [CrossRef]
- Del Valle, J.C.; Buide, M.L.; Whittall, J.B.; Valladares, F.; Narbona, E. UV radiation increases phenolic compound protection but decreases reproduction in Silene littorea. PLoS One 2020, 15(6), e0231611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Sousa Araújo, T.A.; de Almeida e Castro, V.T.N.; da Silva Solon, L.G.; da Silva, G.A.; das Graças Almeida, M.; da Costa, J.G.M.; de Amorim, E.L.C.; Albuquerque, U.P. Does rainfall affect the antioxidant capacity and production of phenolic compounds of an important medicinal species? Industrial Crops and Products 2015, 76, 550. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.S.; Galal, F.H.; Seufi, A.M. Effect of extreme temperature changes on phenolic, flavonoid contents and antioxidant activity of tomato seedlings (Solanum lycopersicum L. ). PeerJ 2021, 9, e11193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chowdhary, V.; Alooparampil, S.; Pandya, R.V.; Tank, J.G. 2022. Physiological Function of Phenolic Compounds in Plant Defense System [Internet]. In: Phenolic Compounds - Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications. IntechOpen. Available from:. https://doi.org/10.5772/intechopen.101131. [CrossRef]
- Liu, Z.; Fadiji, T.; Yang, J.; Li, Z. 2023. Impact of mechanical stimulation on the life cycle of horticultural plant. Horticultural Plant Journal 2023, xx, 1–14. [Google Scholar] [CrossRef]
- Guo, A.L.; Chen, L.M.; Wang, Y.M.; Liu, X.Q.; Zhang, Q.W.; Gao, H.M.; Wang, Z.M.; Wei, X.; Wang, Z.Z. 2014. Influence of sulfur fumigation on the chemical constituents and antioxidant activity of buds of Lonicera japonica. Molecules 2014, 19(10), 16640. [Google Scholar] [CrossRef]
- Kong, D.; Li, Y.; Bai, M.; Deng, Y.; Liang, G.; Wu, H. 2017. A comparative study of the dynamic accumulation of polyphenol components and the changes in their antioxidant activities in diploid and tetraploid Lonicera japonica. Plant Physiology and Biochemistry 2017, 112, 87. [Google Scholar] [CrossRef]
- Fan, Z.; Li, L.; Bai, X.; Zhang, H.; Liu, Q.; Zhang, H.; Fu, Y.; Moyo, R. 2019. Extraction optimization, antioxidant activity, and tyrosinase inhibitory capacity of polyphenols from Lonicera japonica. Food Science and Nutrition 2019, 7(5), 1786. [Google Scholar] [CrossRef] [PubMed]
- Chaowuttikul, C.; Palanuvej, C.; Ruangrungsi, N. 2020. Quantification of chlorogenic acid, rosmarinic acid, and caffeic acid contents in selected Thai medicinal plants using RP-HPLC-DAD. Brazilian Journal of Pharmaceutical Sciences 2020, 56, e17547. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Bai, M.; Haiyang, H.; Hong, W. 2019. Correlation of the temporal and spatial expression patterns of HQT with the biosynthesis and accumulation of chlorogenic acid in Lonicera japonica flowers. Horticulture Research 2019, 6, 73. [Google Scholar] [CrossRef]
- Liu, C.W.; Che, B.C.; Chen, T.M.; Chang, Y.Y. Effect of Different Extraction Methods on Major Bioactive Constituents at Different Flowering Stages of Japanese Honeysuckle (Lonicera japonica Thunb.). J Agron Agri Sci. 2020, 3, 021. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Zou, L.; Liu, X.; Chen, J.; Tan, M.; Mei, Y.; Wei, L. 2019. Comparison of Multiple Bioactive Constituents in the Flower and the Caulis of Lonicera japonica Based on UFLC-QTRAP-MS/MS Combined with Multivariate Statistical Analysis. Molecules 2019, 24(10), 1936. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. 2011. Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. Journal of Ethnopharmacology 2011, 138(1), 1–21, Epub 2011 Aug 16. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, L.; Liu, K.; Li, X.; Xie, F. 2023. Bioactive components and beneficial bioactivities of flowers, stems, leaves of Lonicera japonica Thunberg: A review. Biochemical Systematics and Ecology 2023, 106, 104570. [Google Scholar] [CrossRef]
- Xiang, T.; Xiong, Q.B.; Ketut, A.I.; Tezuka, Y.; Nagaoka, T.; Wu, L.J.; Kadota, S. 2001. Studies on the hepatocyte protective activity and the structure-activity relationships of quinic acid and caffeic acid derivatives from the flower buds of Lonicera bournei. Planta Medica 2001, 67(4), 322–325. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, B.; Bhandari, P.; Gupta, A.P.; Uniyal, S.K.; Kaul, V.K. 2005. Biflavonoids from Lonicera japonica. Phytochemistry 2005, 66(23), 2740–2744. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Dumitrescu, D.; Raducanu, M.A.; Stoicescu, I.; Badea, V. 2019. Phytochemical profile and total antioxidant capacity of Sempervivum ruthenicum Koch hydroethanolic extract. Revista de Chimie 2019, 70(1), 23–26. [Google Scholar] [CrossRef]
- Rioux, C.; Jordan, D.C.; Rattray, J.B. 1983. Colorimetric determination of catechol siderophores in microbial culture. Analytical Biochemistry 1983, 133(1), 163–169. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. 1995. Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology 1995, 28(1), 25. [Google Scholar] [CrossRef]
- Ozyürek, M.; Güngör, N.; Baki, S.; Güçlü, K.; Apak, R. 2012. Development of a silver nanoparticle-based method for the antioxidant capacity measurement of polyphenols. Anal Chem. 2012, Sep 18;84(18), 8052-9, Epub 2012 Aug 27. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.S.; Voicu, S.N.; Caruntu, S.; Nica, I.C.; Olah, N.K.; Burtescu, R.; Balta, C.; Rosu, M.; Herman, H.; Hermenean, A.; Dinischiotu, A. 2019. Antioxidant and anti-inflammatory properties of a Thuja occidentalis mother tincture for the treatment of ulcerative colitis. Antioxidants (Basel) 2019, 8(9), 416. [Google Scholar] [CrossRef] [PubMed]
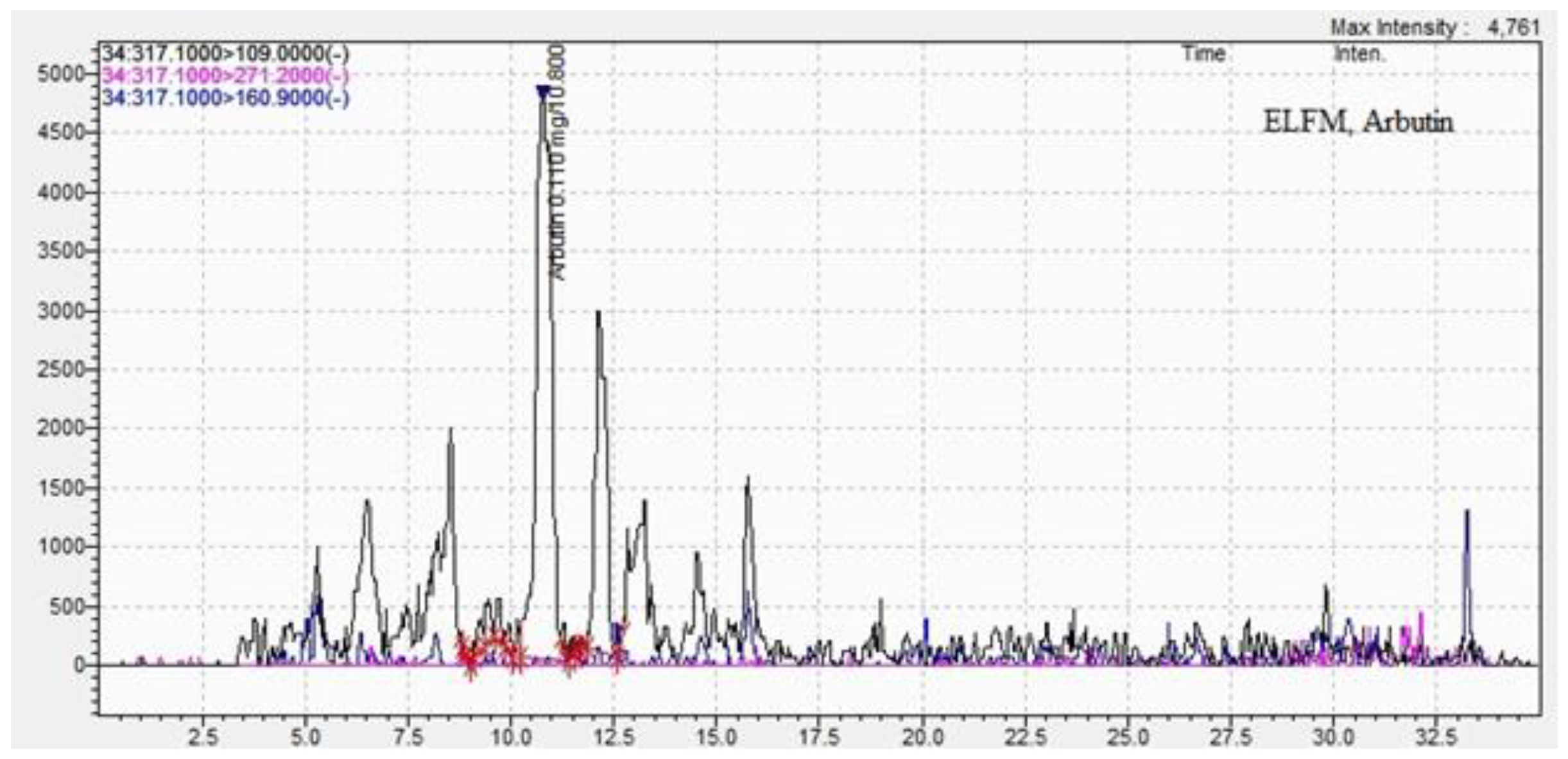
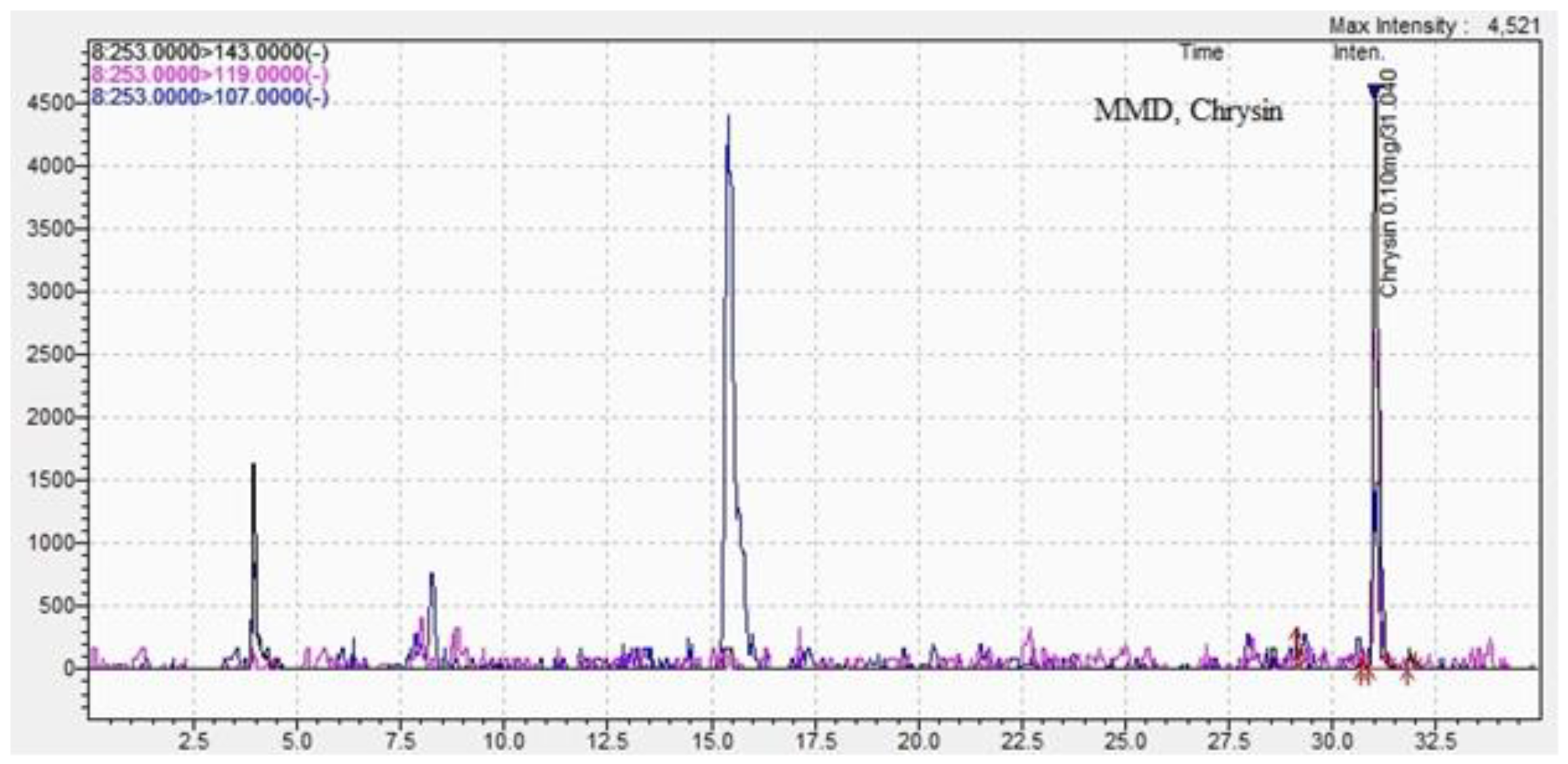
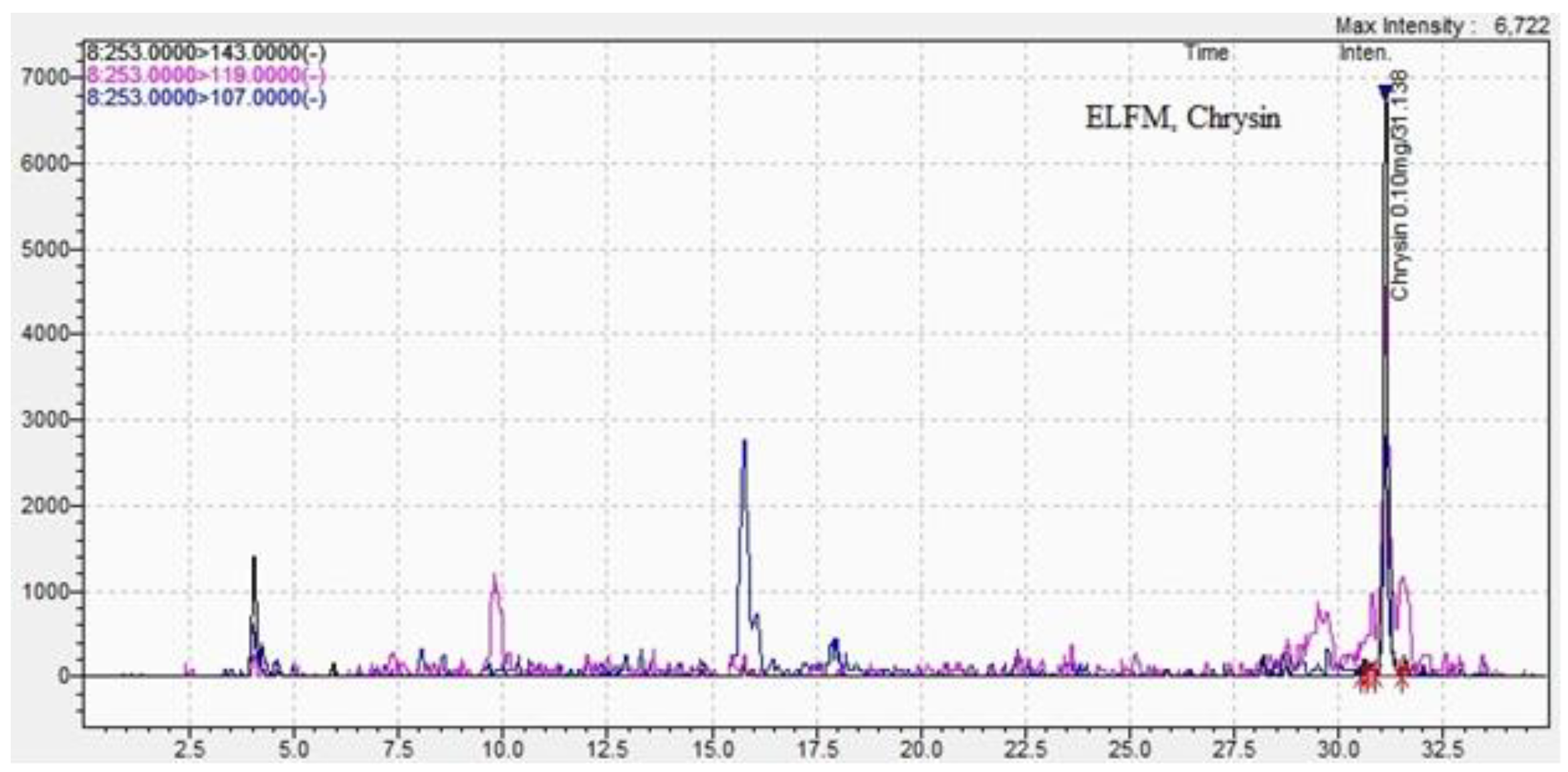
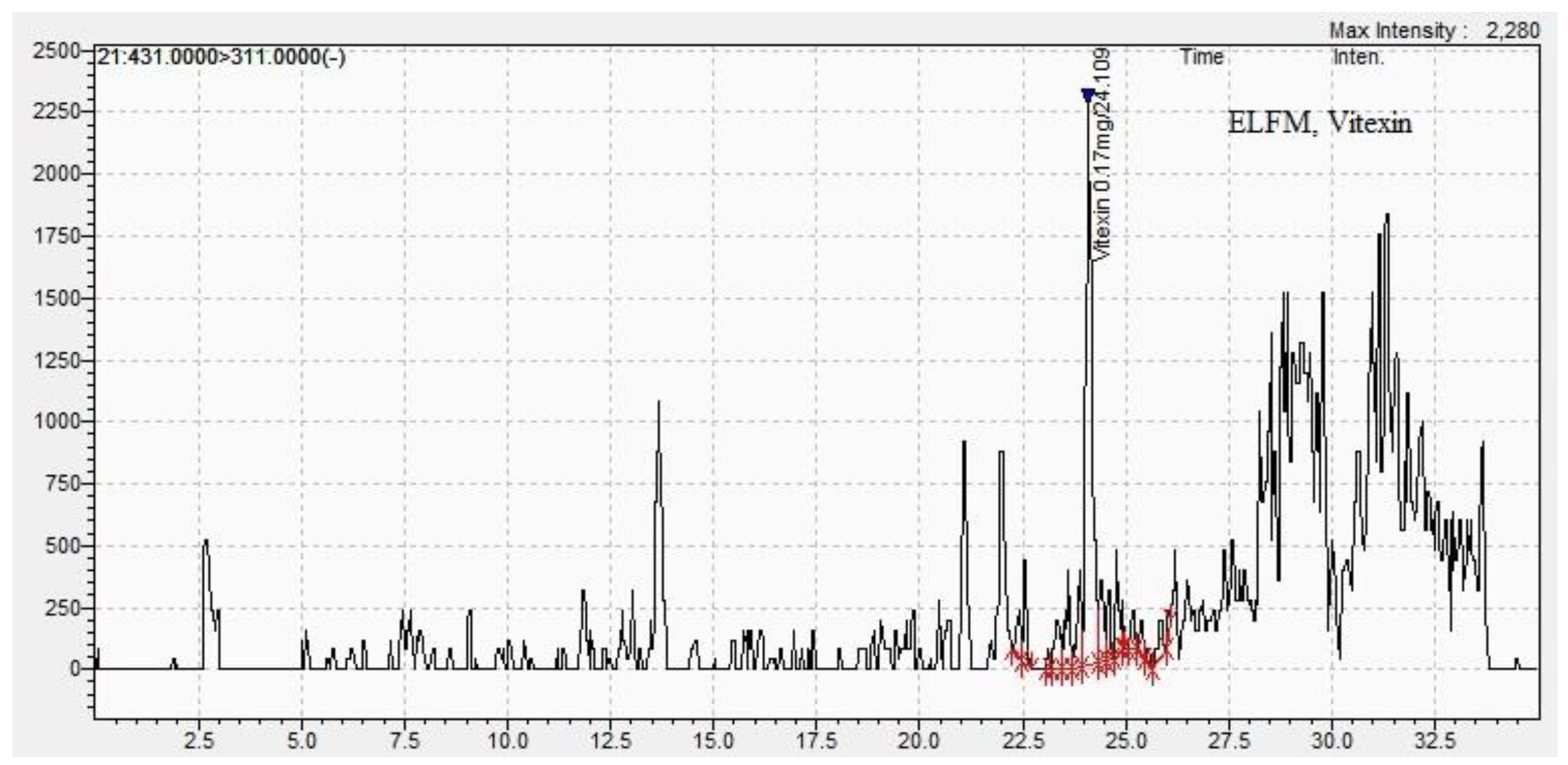
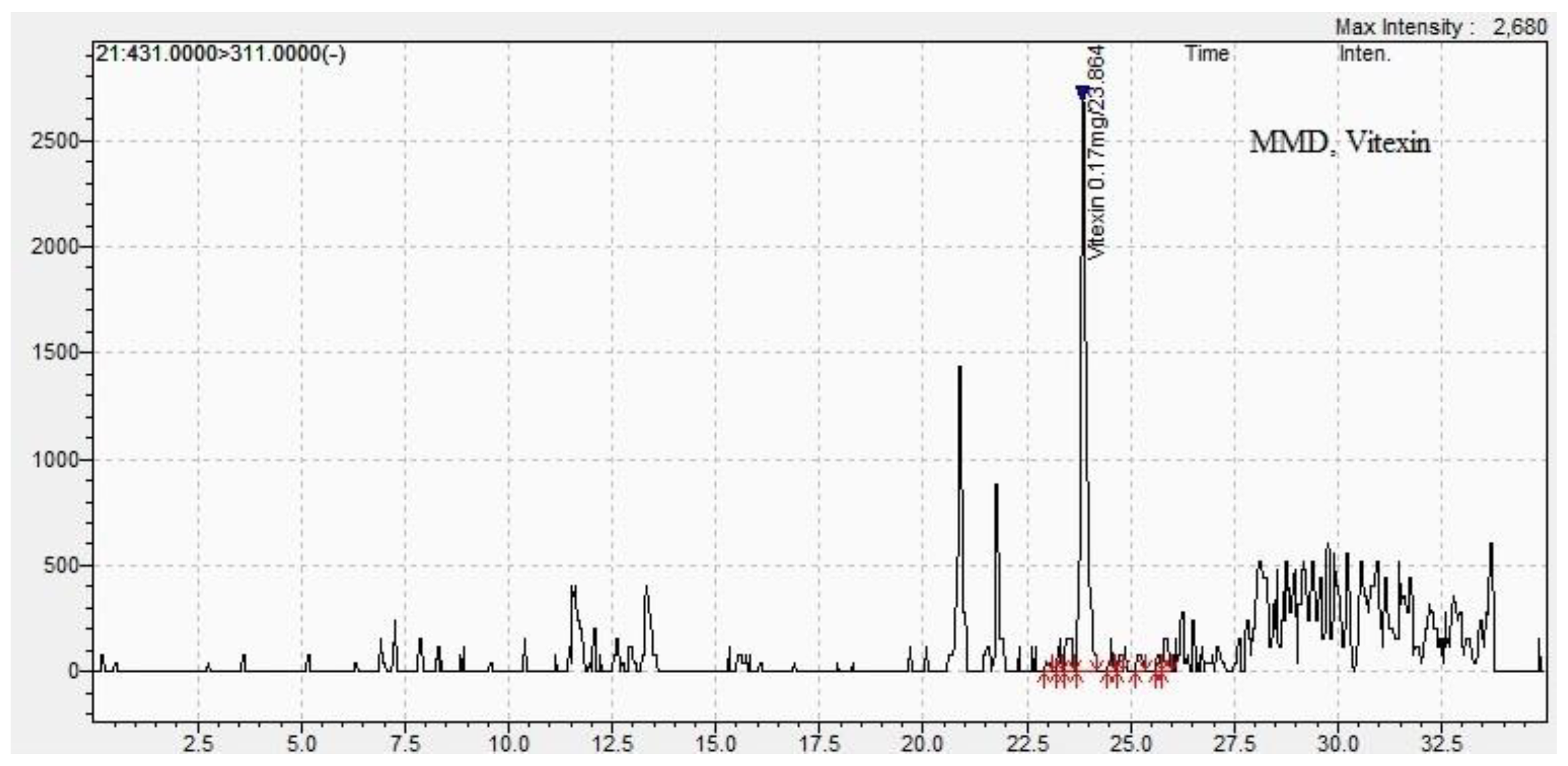
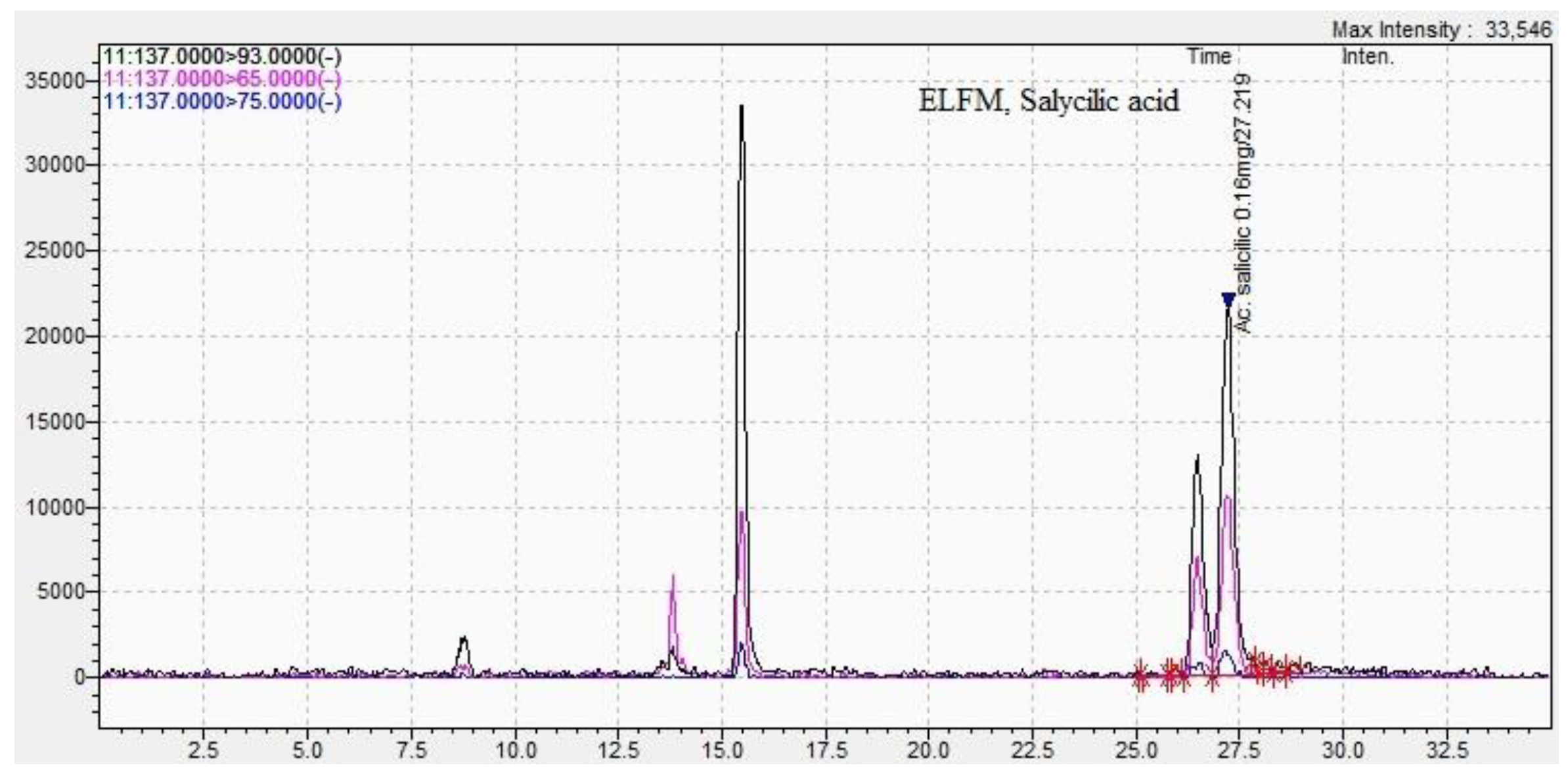
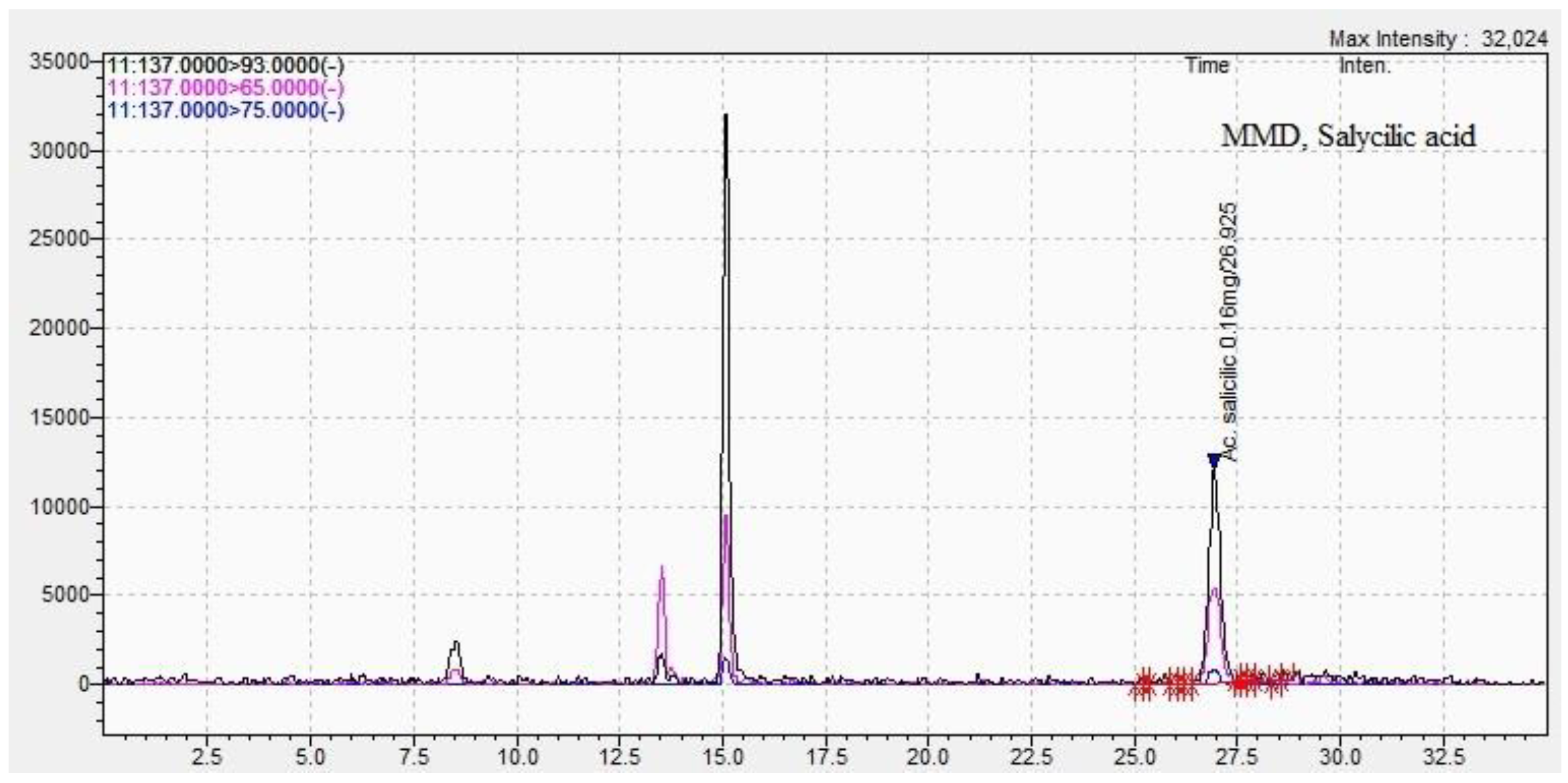
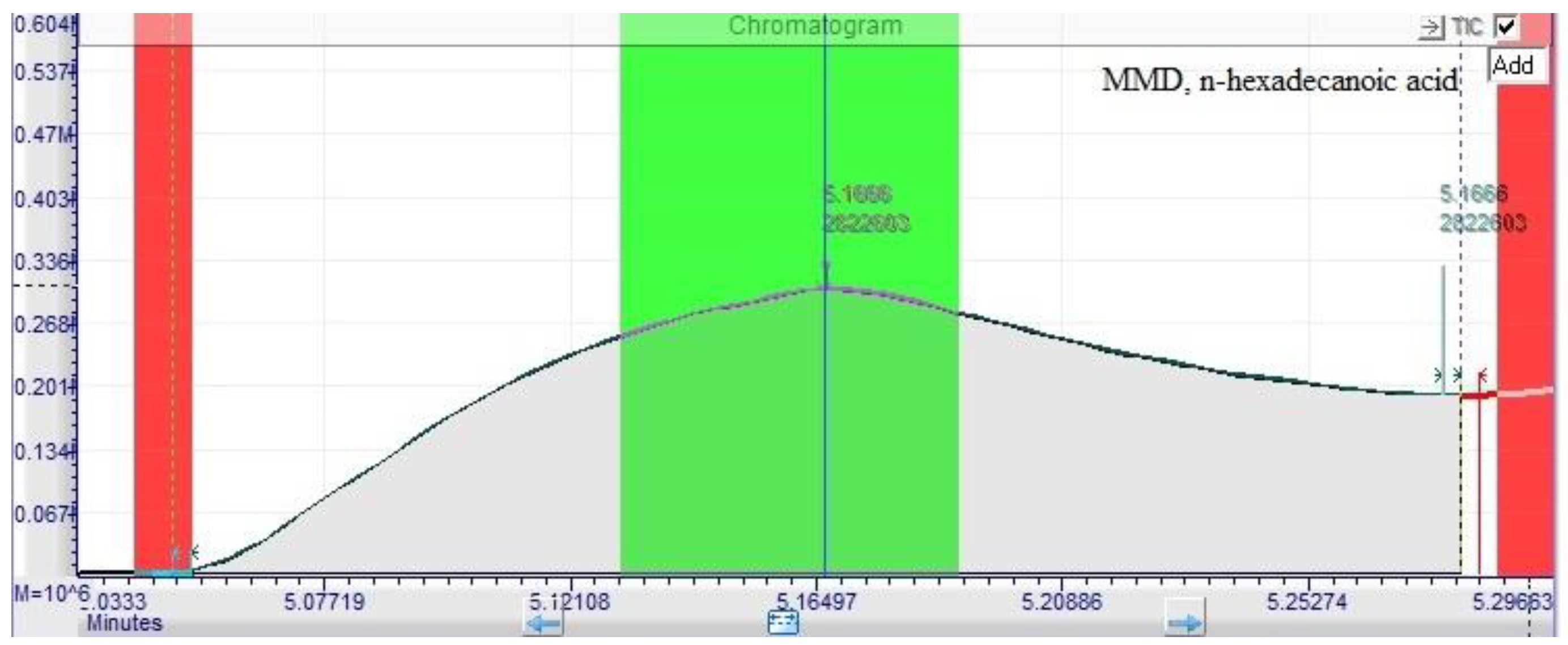

| Methods DPPH assay |
Calibration Curve Equation | R2 |
|---|---|---|
| MMD extract | y = 16.513x + 45.974 | 0.9555 |
| ELFM extract | y = 29.649x + 7.0996 | 0.9827 |
| Samples | DPPH (IC50, µg/mL) |
CUPRAC (mM TE */g dry extract) |
|---|---|---|
| MMD extract | 0.24 | 675 |
| ELFM extract | 1.45 | 637 |
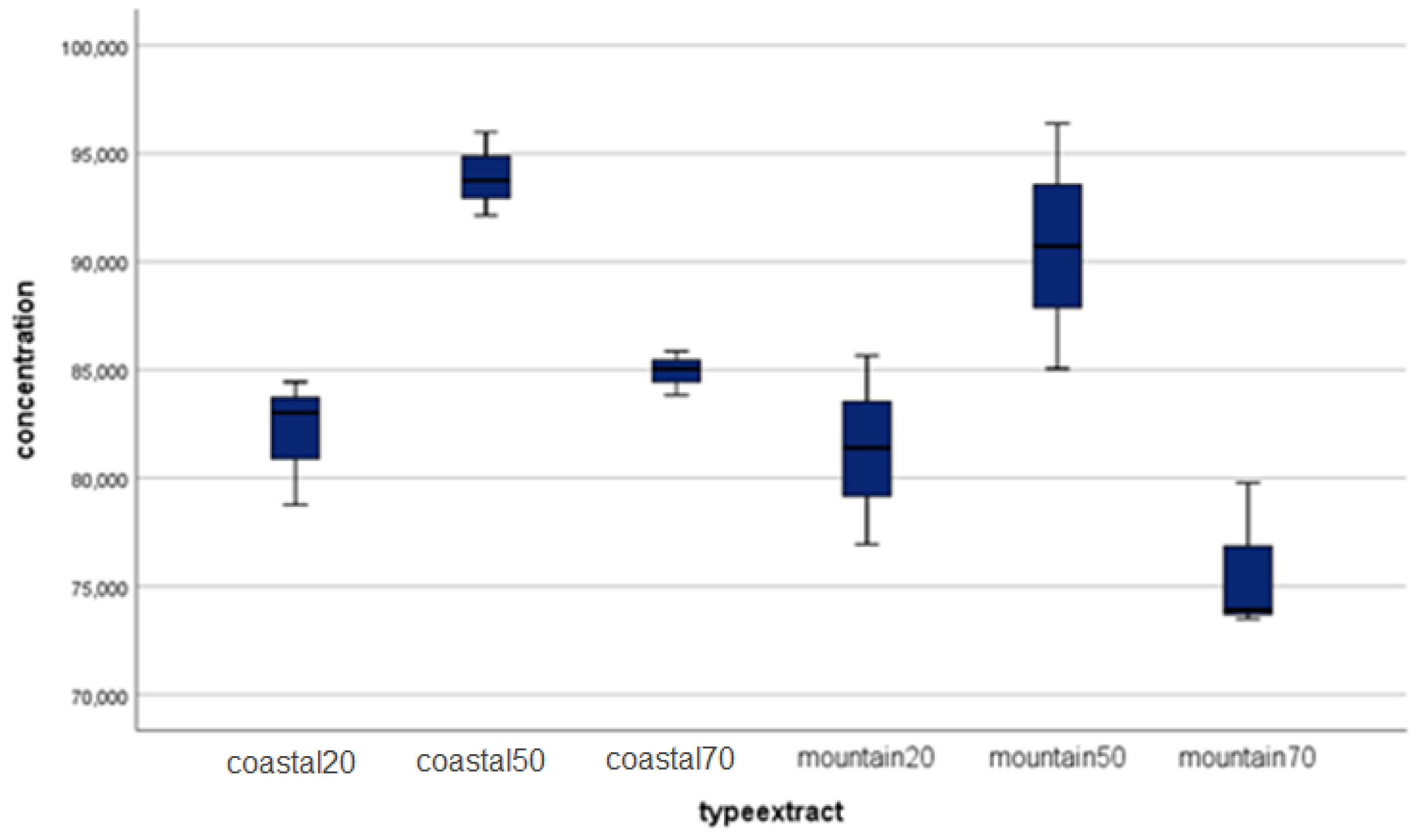
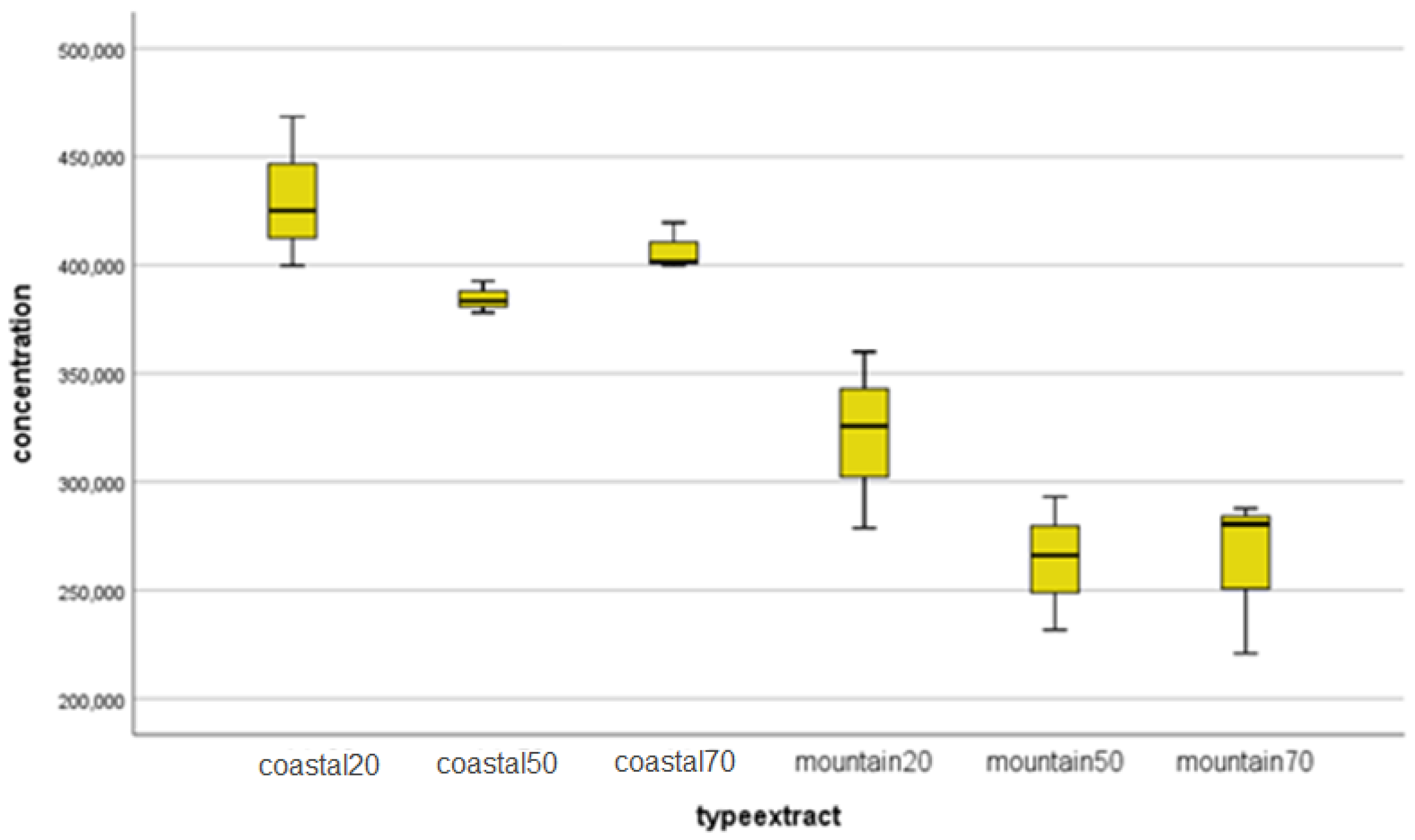
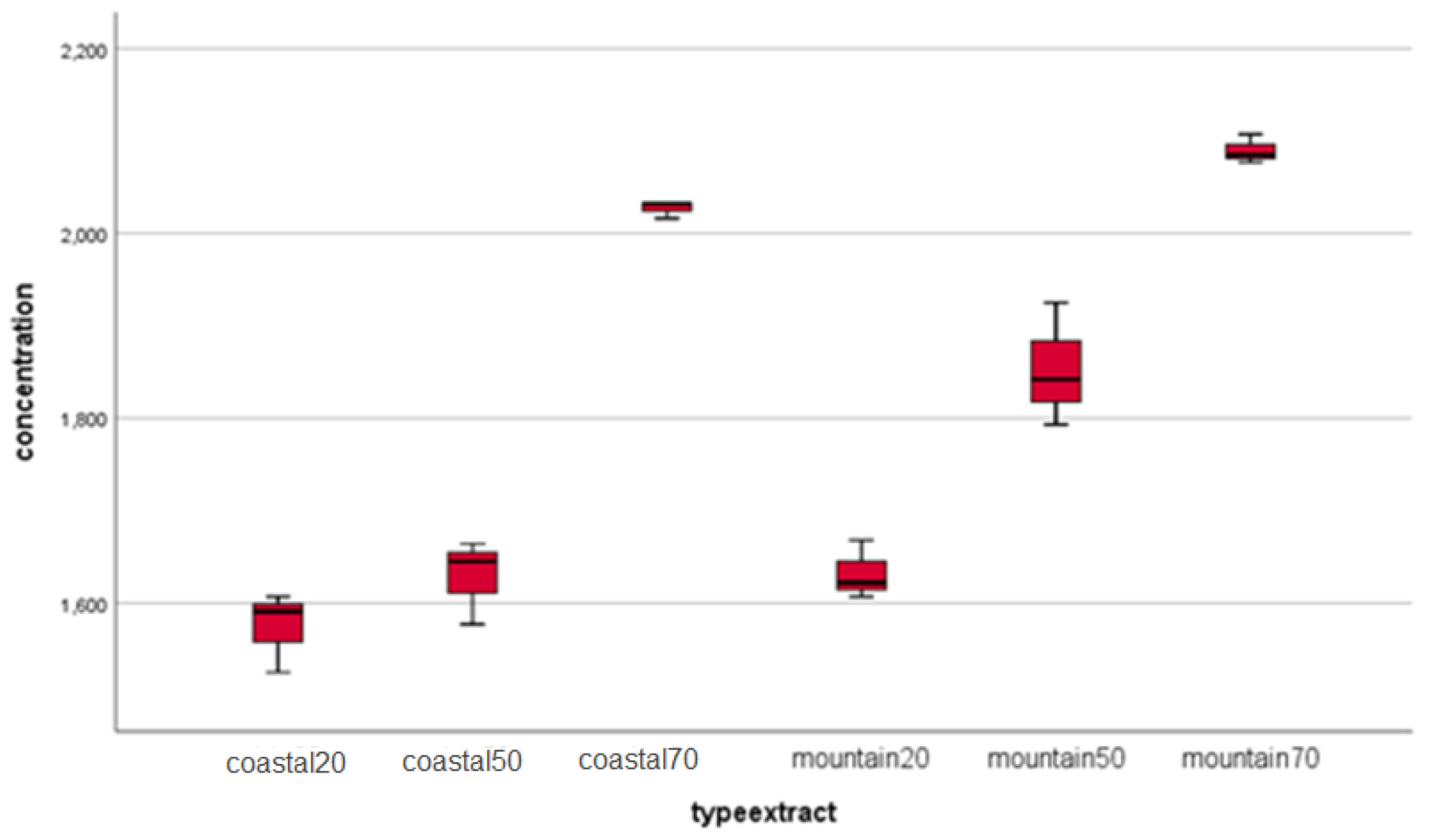
| Identified compound | Coastal region sample | Mountain region sample | ||
|---|---|---|---|---|
| Concentration Mean (mg/mL)a± SD |
Retention time Mean (min)b± SD |
Concentration Mean (mg/mL)a± SD |
Retention time Mean (min)b ± SD |
|
| Caffeic acid | 19.76 ±0.30 | 16.4±0.02 | 19.66 ± 0.30 | 16.0±0.04 |
| Chlorogenic acid | 827.79±12.40 | 13.9±0.05 | 781.41 ±11.70 | 13.7±0.01 |
| Gallic acid | 0.05±0.01 | 8.4±0.01 | 0.12 ±0.02 | 8.2±0.07 |
| Rosmarinic acid | 0.19±0.03 | 23.5±0.01 | 0.89 ±0.01 | 23.4±0.03 |
| 2-hydroxybenzoic acid | 6.05±0.09 | 27.2±0.04 | 2.98 ±0.05 | 26.9±0.03 |
| trans-p-coumaric acid | 13.31±0.20 | 20.8±0.01 | 12.26 ±0.20 | 20.5±0.01 |
| Apigenin | 5.35±0.08 | 29.8±0.03 | 4.68 ±0.07 | 28.2±0.07 |
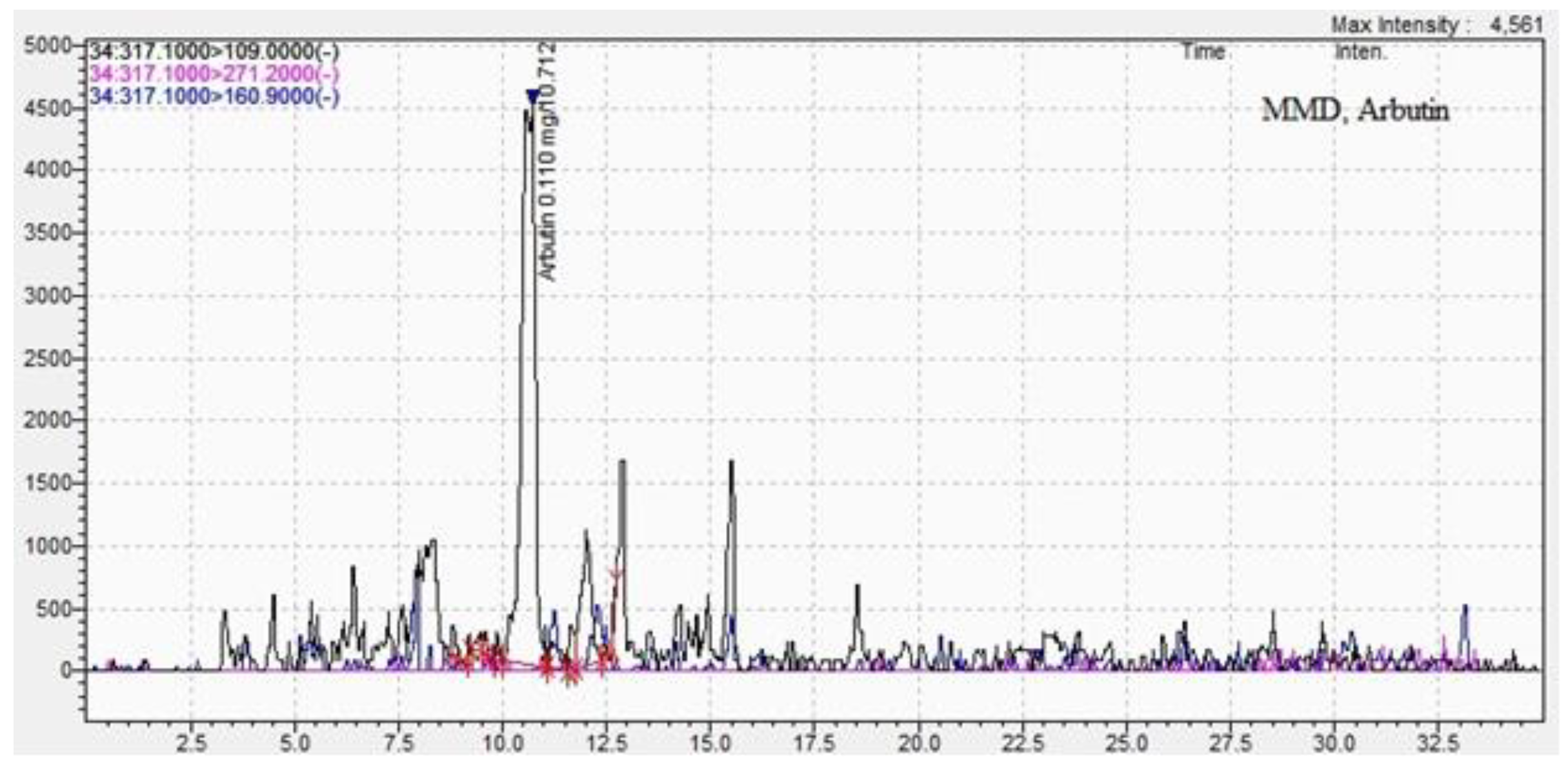
| Arbutin | 0.10±0.02 | 10.8±0.09 | 0.008 ±0.01 | 10.7±0.02 |
| Carnosol | 3.20±0.05 | 31.7±0.01 | 0.80 ±0.01 | 31.6±0.01 |
| Chrysin | 0.30±0.01 | 31.1±0.01 | 0.23 ±0.01 | 31.0±0.04 |
| Hesperetin | 0.58±0.01 | 28.7±0.02 | 0.39 ±0.01 | 28.6±0.02 |
| Hyperoside | 28.62±0.40 | 22.9±0.01 | 34.65 ±0.50 | 22.8±0.06 |
| Kaempferol | 1.31±0.02 | 28.9±0.11 | 0.95 ±0.010 | 28.8±0.04 |
| Luteolin-7-O-glucozid | 34.37±0.50 | 22.2±0.01 | 33.88 ±0.50 | 22.1±0.02 |
| Luteolin | 8.19±0.10 | 28.9±0.01 | 7.68 ±0.10 | 28.8±0.09 |
| Myricetin | 12.88±0.20 | 13.8±0.06 | 23.04 ±0.30 | 13.5±0.04 |
| Naringenin | 0.72±0.01 | 28.7±0.01 | 0.93 ±0.01 | 28.6±0.02 |
| Quercetin | 5.03±0.08 | 28.4±0.05 | 3.71 ±0.06 | 28.3±0.04 |
| Rutoside | 319.80±4.80 | 22.7±0.03 | 277.19 ±4.20 | 22.7±0.11 |
| Vitexin | 0.01±0.01 | 24.1±0.11 | 0.01 ±0.01 | 23.9±0.01 |
| Esculetin | 207.61±3.10 | 15.4±0.01 | 168.66 ± 2.50 | 15.1±0.09 |
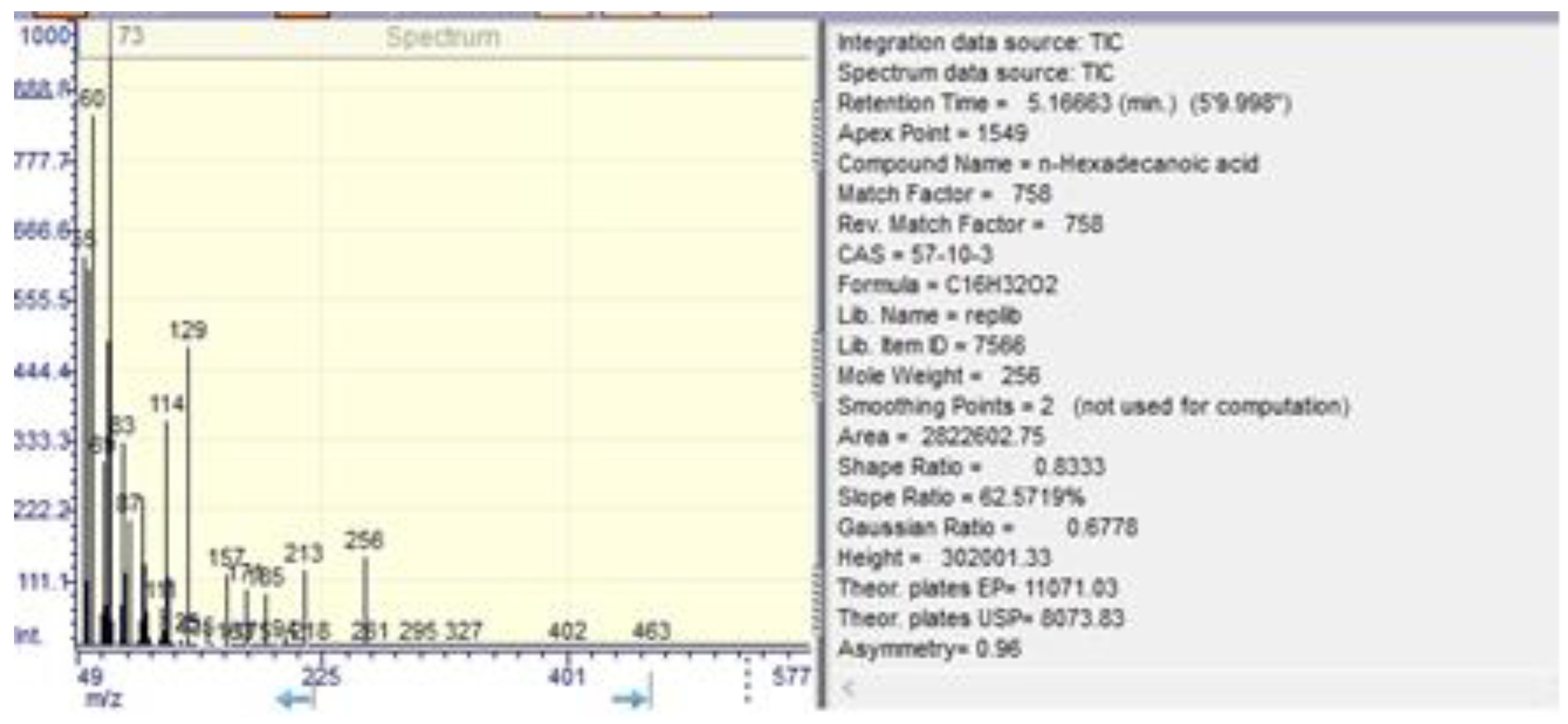
| n-hexadecanoic acid | 6.61±0.02 | 5.11±0.02 | 0.01 ±0.01 | 5.10±0.03 |
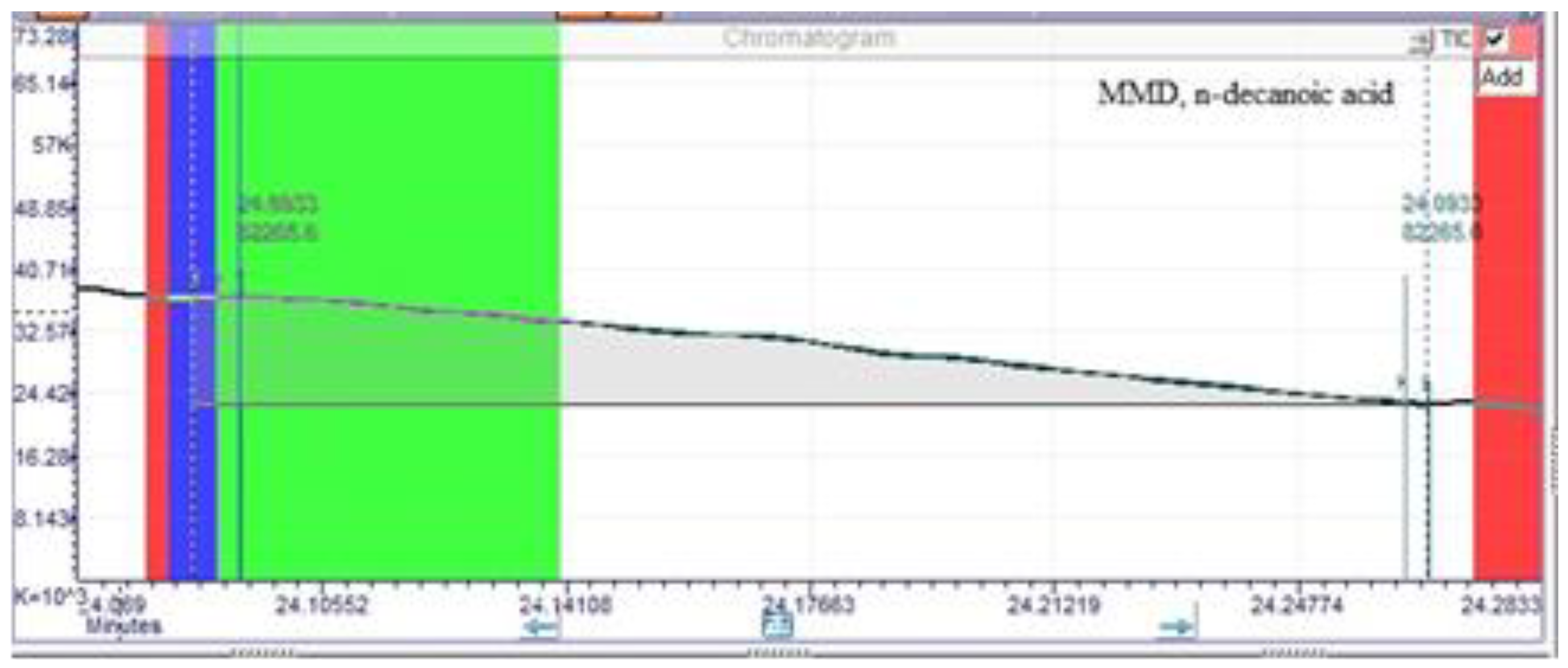
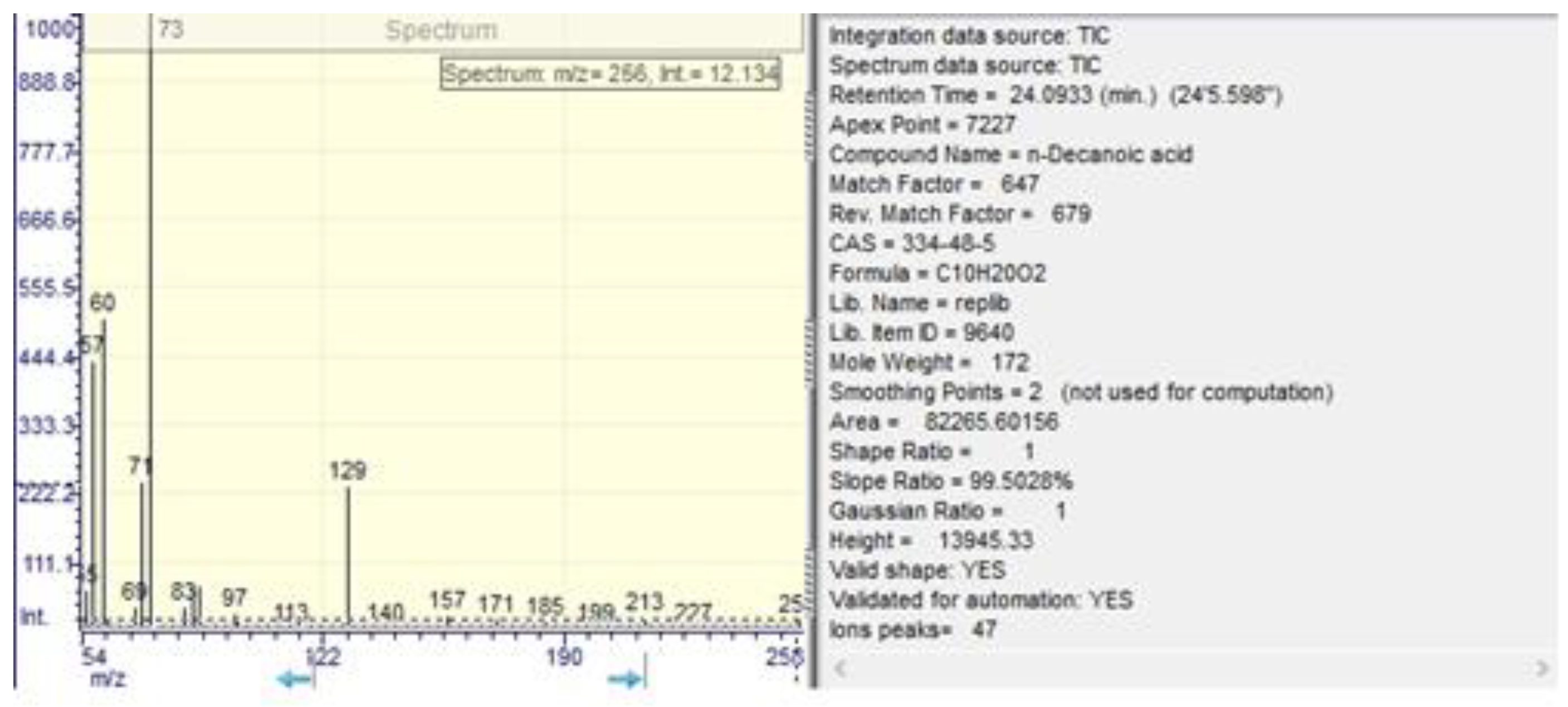
| n-decanoic acid | 5.21 ±0.01 | 24.09±0.01 | 0.01 ±0.01 | 24.06±0.01 |
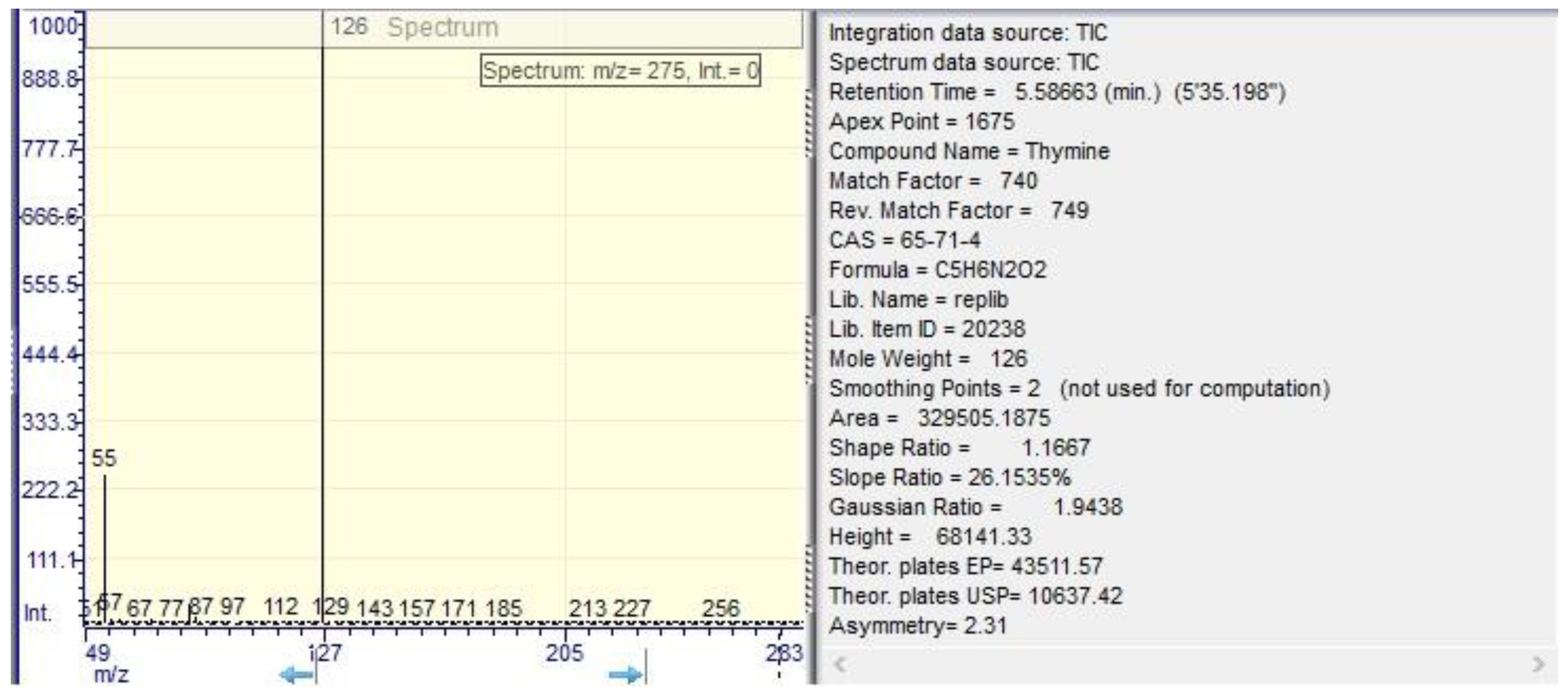
| thyamine | 1.71 ±0.01 | 5.58 ±0.02 | 0.01 ±0.01 | 5.57 ±0.01 |
| Compound | Retention time, min. | Sample area | Content by normalization, % |
|---|---|---|---|
| n-hexadecanoic acid | 5.20 | 4.254.87 | 66.00 |
| thymine | 5.60 | 329505.20 | 5.10 |
| n-decanoic acid | 24.20 | 112038.00 | 1.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





