Submitted:
17 May 2024
Posted:
21 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Bioactive Compounds
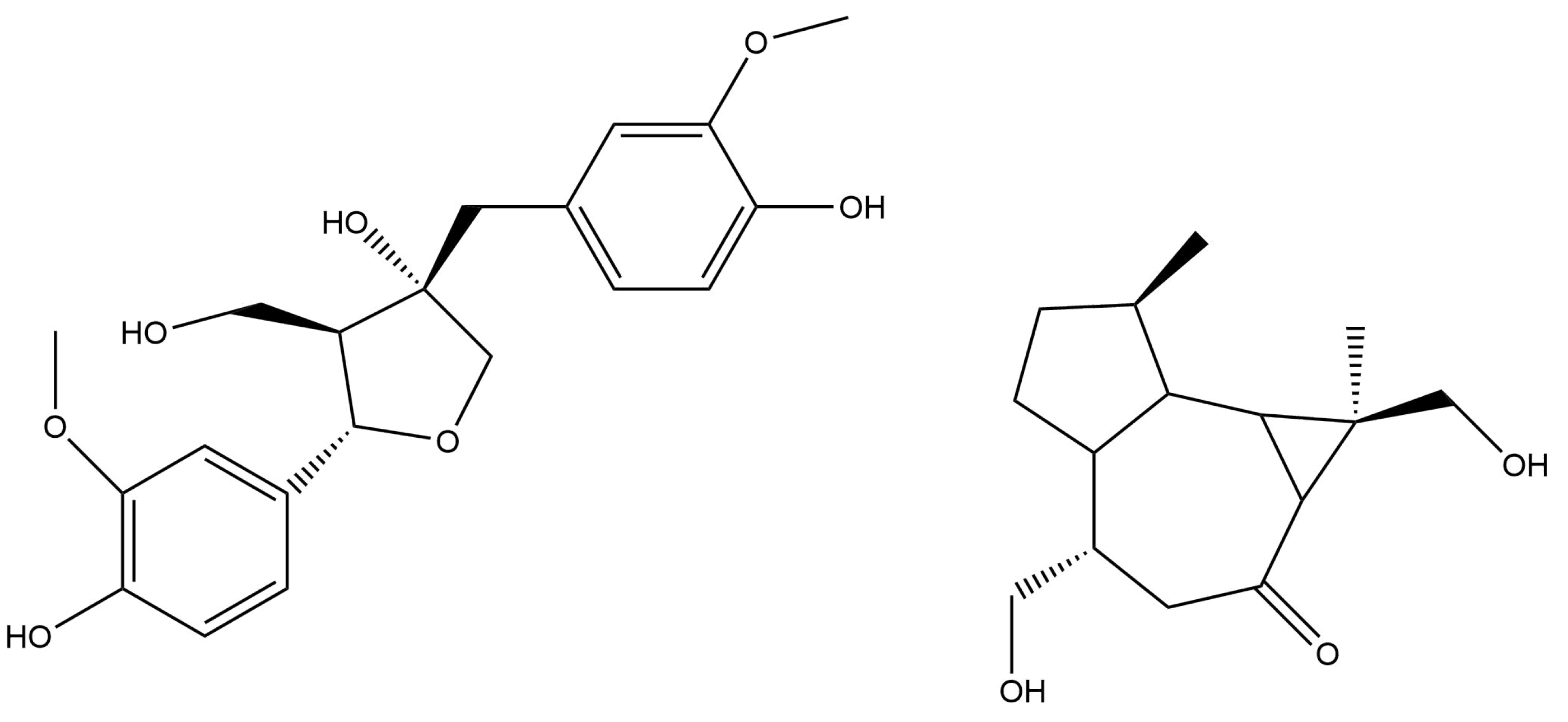
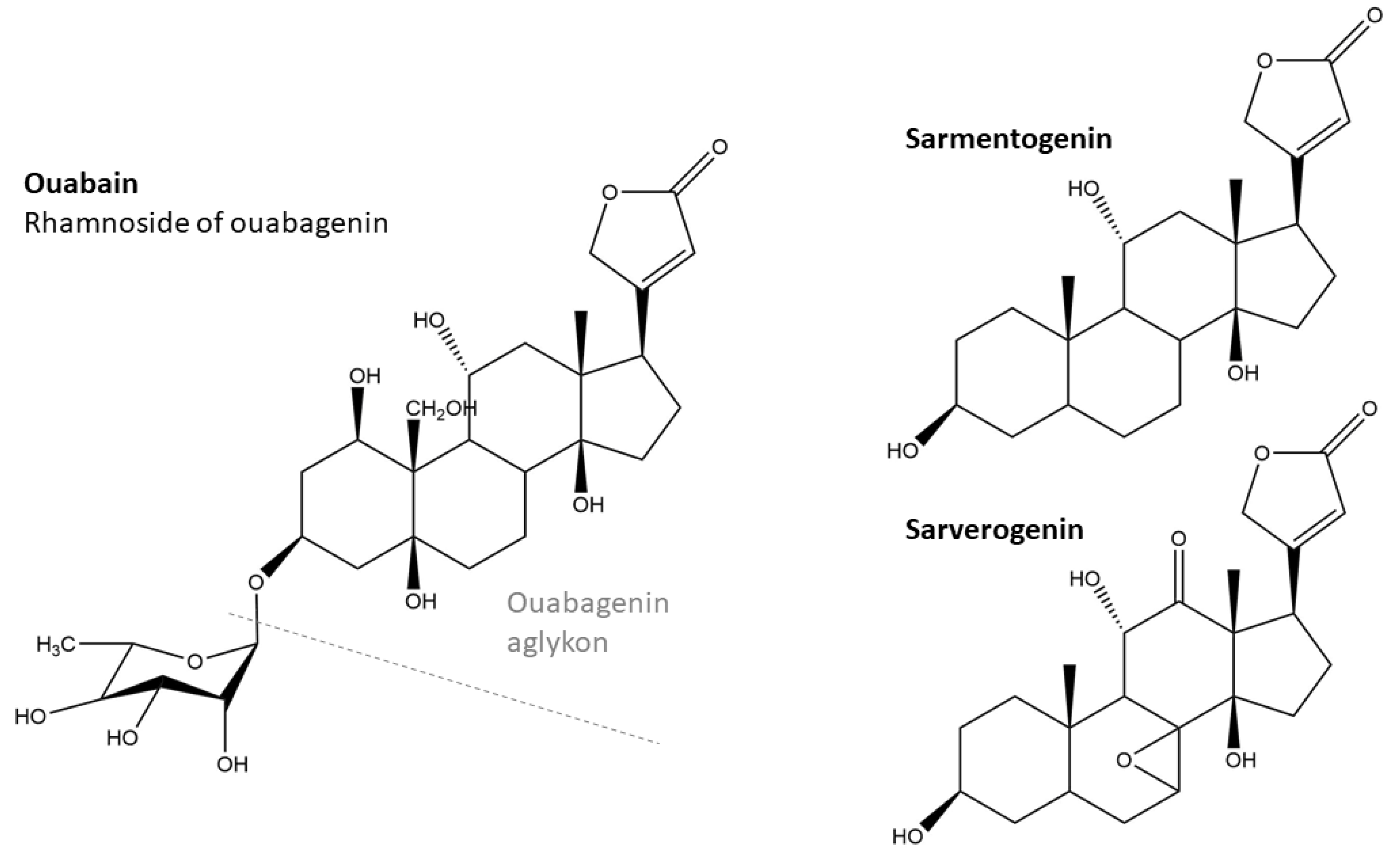
2.1. Cardiac Glycosides and Their Activity
- ouabain group: S. gardeniiflorus, S. gratus, S. thollonii
- sarmentogenin/sarverogenin group: S. welwitschii, S. amboensis, S. gerrardii, S. congoensis, S. petersianus, S. courmontii, S. sarmentosus
- strophanthidin/strophanthidol/periplogenin group: S. arnoldianus, S. hispidus, S. mirabilis, S. barteri, S. hypoleucos, S. mortehanii, S. eminii, S. kombe, S. nicholsonii, S. gracilis, S. ledienii, S. preussii
- divaricoside/caudoside group: S. caudatus, S. divaricatus, S. wightianus
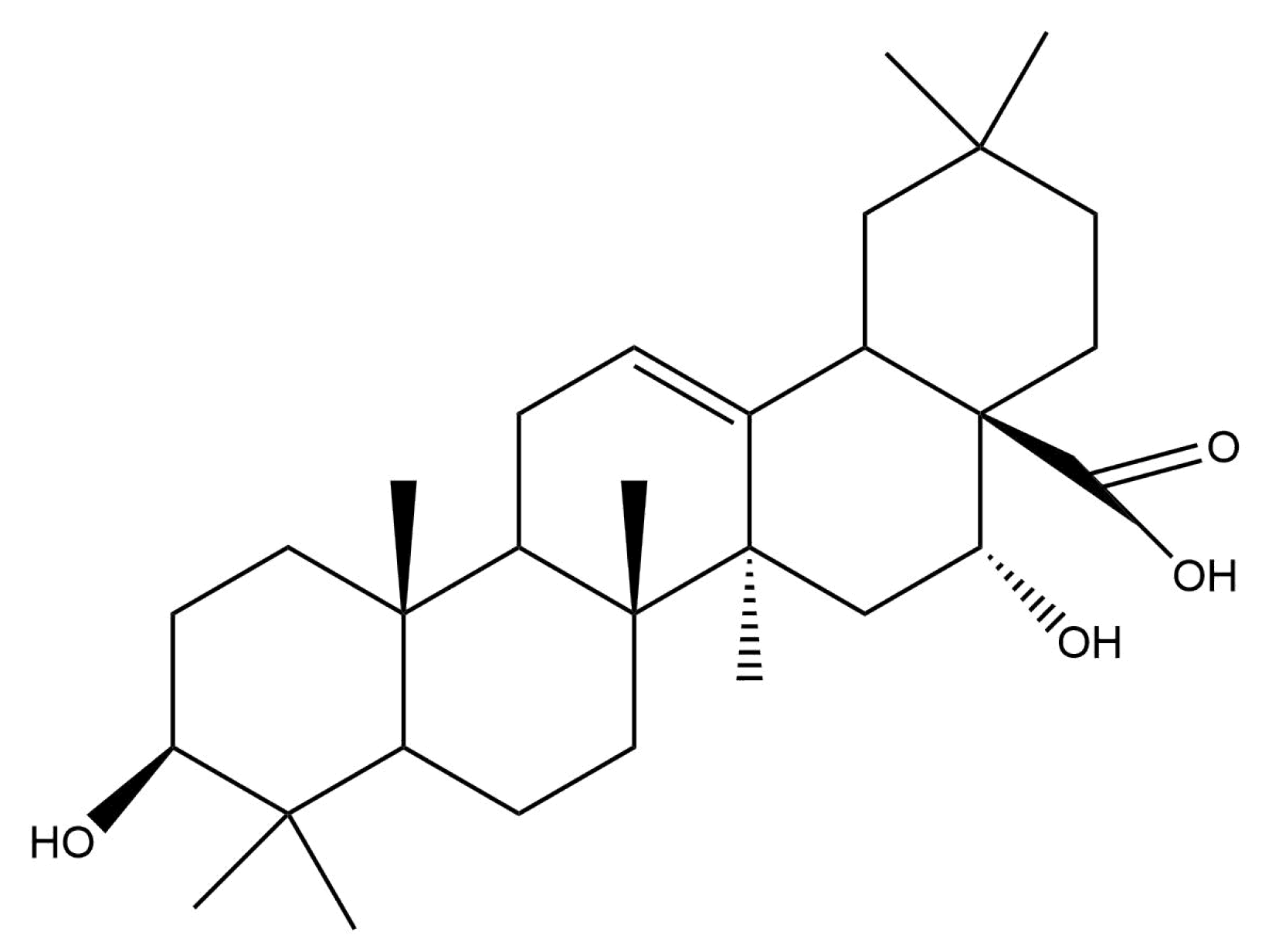
2.2. Triterpene Glycosides and Other Substances
3. Bioactivity
3.1. Toxicity and Mutagenicity
3.2. Anti-inflammatory, Antibacterial and Antioxidant Activity
3.3. Hypoglycemic Effects
3.4. Anti-Nociceptive Effects
3.5. Anti-Venomenous Activity
3.6. Anti-Phytoviral, Anti-Herpetic, Anti-Trypanasomal, Anti-Protozoal, Anti-Malarial and Hydroxynitrile Lyase Activities
4. Methods
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- H.J. Beentje. A monograph on Strophanthus DC. (Apocynaceae) 1982.
- Włodarczyk, M.; Gleńsk, M. An in-depth look into a well-known herbal drug: Fingerprinting, isolation, identification, and content estimation of saponins in different Strophanthus seeds. Planta medica 2022, 88, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Vickery, M. Plant poisons: their occurrence, biochemistry and physiological properties. Sci. Prog. 2010, 93, 181–221. [Google Scholar] [CrossRef] [PubMed]
- Makgobole, M.U.; Mpofana, N.; Ajao, A.A. Medicinal Plants for Dermatological Diseases: Ethnopharmacological Significance of Botanicals from West Africa in Skin Care. Cosmetics 2023, 10, 167. [Google Scholar] [CrossRef]
- Toxicological Evaluation of Polyherbal Medicines used for the Treatment of Tuberculosis in Eastern Cape, South Africa (accessed on 30 April 2024). (accessed on 30 April 2024).
- Boakye-Yiadom, M.; Kumadoh, D.; Adase, E.; Woode, E. Medicinal Plants with Prospective Benefits in the Management of Peptic Ulcer Diseases in Ghana. BioMed Research International 2021, 2021, 5574041. [Google Scholar] [CrossRef] [PubMed]
- Traore, M.S.; Diane, S.; Diallo, M.S.T.; Balde, E.S.; Balde, M.A.; Camara, A.; Diallo, A.; Keita, A.; Cos, P.; Maes, L.; et al. In vitro antiprotozoal and cytotoxic activity of ethnopharmacologically selected guinean plants. Planta medica 2014, 80, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Esther, O. Faboro; Wisut Wichitnithad; Olatomide A. Fadare; David A. Akinpelu; Craig A. Obafemi. Antibacterial and antioxidant activities and phytochemical screening of aqueous methanol extracts of eight Nigerian medicinal and aromatic Plants. Journal of Pharmacy Research 2016, 10, 523–532. [Google Scholar]
- John Kenneth, M.; Amina, I.; Yakubu, J. Co-extract mixture from Strophanthus hispidus (roots) and Aframomum meleguta (seeds) show phytochemical synergy in its anti-inflammatory activity. Arch Pharm Pharma Sci 2019, 3, 89–100. [Google Scholar] [CrossRef]
- Taylor & Francis. A Time-Trend Hypoglycemic Study of Ethanol and Chloroform Extracts of Strophanthus hispidus. Available online: https://www.tandfonline.com/doi/full/10.1080/10496470902787386 (accessed on 30 April 2024).
- Shukurova Shoxina Tuyg’unovna. CHEMICAL COMPOSITION OF MEDICINAL PLANTS AND CLASSIFICATION. EJMMP 2023, 3, 33–35. [Google Scholar]
- Alamgir, A.N.M. Medicinal, Non-medicinal, Biopesticides, Color- and Dye-Yielding Plants; Secondary Metabolites and Drug Principles; Significance of Medicinal Plants; Use of Medicinal Plants in the Systems of Traditional and Complementary and Alternative Medicines (CAMs). Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1 2017, 73, 61–104. [Google Scholar] [CrossRef]
- HATCHER, R.A. TINCTURE OF STROPHANTHUS AND STROPHANTHIN. JAMA 1909, LII, 5. [Google Scholar] [CrossRef]
- Narayana Verlag GmbH. Strophanthin, Eberhard J. Wormer, Comeback eines Herzmittels - Narayana Verlag. Available online: https://www.narayana-verlag.de/Strophanthin-Eberhard-J-Wormer/b20490 (accessed on 29 April 2024).
- Norton, S.A. Useful plants of dermatology. III. Corticosteroids, Strophanthus, and Dioscorea. Journal of the American Academy of Dermatology 1998, 38, 256–259. [Google Scholar] [CrossRef]
- YOUNGKEN, H.W.; SIMONIAN, V.H. A study of the seeds of Strophanthus sarmentosus and some related species of Strophanthus. Journal of the American Pharmaceutical Association. American Pharmaceutical Association 1950, 39, 615–620. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, B.-E. A review of commercially important African medicinal plants. Journal of ethnopharmacology 2015, 176, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, H.; Zürcher, R.F.; Reichstein, T. Sarverogenin, vermutliche Struktur. Glykoside und Aglykone, 314. Mitteilung. Helvetica Chimica Acta 1969, 52, 616–621. [Google Scholar] [CrossRef]
- Schmelzer, G.H.; Gurib-Fakim, A.; Arroo, R.; Lemmens, R.H.M.J. Medicinal plants 1; Fondation PROTA: Wageningen, 2008; ISBN 9789057822049. [Google Scholar]
- SpringerLink. Cardiac Glycosides 1785–1985. Available online: https://link.springer.com/book/10.1007/978-3-662-11292-2 (accessed on 30 April 2024).
- Orhan, I.E.; Gokbulut, A.; Senol, F.S. Adonis sp., Convallaria sp., Strophanthus sp., Thevetia sp., and Leonurus sp. - Cardiotonic Plants with Known Traditional Use and a Few Preclinical and Clinical Studies. Current pharmaceutical design 2017, 23, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Strobach, H.; Wirth, K.E.; Rojsathaporn, K. Absorption, metabolism and elimination of strophanthus glycosides in man. Naunyn-Schmiedeberg's Arch. Pharmacol. 1986, 334, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.H. The sodium pump and hypertension: a physiological role for the cardiac glycoside binding site of the Na,K-ATPase. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 15723–15724. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Okada, M.; Kato, S.; Shinoda, Y.; Shioda, N.; Fukunaga, K.; Ui-Tei, K.; Ueda, M. Ouabagenin is a naturally occurring LXR ligand without causing hepatic steatosis as a side effect. Sci Rep 2018, 8, 2305. [Google Scholar] [CrossRef]
- Euw, J.; Gürtler, J.; Lardon, A.; Mohr, K.; Reber, F.; Richter, R.; Schindler, O.; Reichstein, T. Die Glykoside von Strophanthus sarmentosus P. DC. 8. Mitteilung. Untersuchung von Einzelpflanzen der „Sarmentogenin-produzierenden Variante b”︁ Strophanthus sarmentosus var. senegambiae (A. DC.) Monachino. Glykoside und Aglykone, 183. Mitteilung. Helvetica Chimica Acta 1957, 40, 2079–2109. [CrossRef]
- Wiley Online Library. Sarmutosid und Musarosid. Glykoside der Samen von Strophanthus sarmentosus A.P.DC. 4. Mitteilung. Glykoside und Aglykone. 113. Mitteilung. (accessed on 30 April 2024).
- Knittel, D.N.; Huber, U.; Stintzing, F.C.; Kammerer, D.R. Effect of extraction, microbial fermentation and storage on the cardenolide profile of Strophanthus kombé Oliv. seed preparations. Journal of pharmaceutical and biomedical analysis 2016, 129, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-F.; Abe, F.; Yamauchi, T.; Taki, M. Cardenolide glycosides of Strophanthus divaricatus. Phytochemistry 1987, 26, 2351–2355. [Google Scholar] [CrossRef]
- Makarevich, I.F.; Kovalev, S.V. Cardiac glycosides from Strophanthus kombe. Chem Nat Compd 2006, 42, 189–193. [Google Scholar] [CrossRef]
- Knittel, D.N.; Lorenz, P.; Huber, U.; Stintzing, F.C.; Kammerer, D.R. Characterization of the cardiac glycoside and lipid profiles of Strophanthus kombé Oliv. seeds. Z. Naturforsch. C J. Biosci. 2016, 71, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Grosa, G.; Allegrone, G.; Del Grosso, E. LC-ESI-MS/MS characterization of strophanthin-K. Journal of pharmaceutical and biomedical analysis 2005, 38, 79–86. [Google Scholar] [CrossRef]
- Beentje, H.; Cooke, D. 401. STROPHANTHUS SARMENTOSUS: Apocynaceae. Curtis's Botanical Magazine 2000, 17, 202–207. [Google Scholar] [CrossRef]
- Fechtig, B.; v. Euw, J.; Schindler, O.; Reichstein, T. Die Struktur der Sarmentoside. Glykoside von Strophanthus sarmentosus P. DC. 11. Mitteilung. Glykoside und Aglykone, 219. Mitteilung. Helvetica Chimica Acta 1960, 43, 1570–1584. [Google Scholar] [CrossRef]
- Owonubi, M.O.; Iwalewa, E.O.; Shok, M. CARDIO-ACTIVITY OF SARMENTOSIDE-A FROM STROPHANTHUS SARMENTOSUS SEEDS. njnpm 1997, 1, 16–18. [Google Scholar] [CrossRef]
- Maribeth R., Laurente; Mafel, C. Ysrael. THE CARDIOACTIVE SCREENING OF THE EXTRACT FROM THE BARK OF STROPHANTHUS CUMINGII A.DC. (APOCYNACEAE) USING ISOLATED FROG HEART. International Journal of Pharmacy 2015, 5, 1048–1050. [Google Scholar]
- Ojo, O.O.; Emoghwa, A.R. Methanol extracts of Strophanthus hispidus exhibit anti-apoptotic effects via alteration of cytochrome c and caspase 3 levels in rats with myocardial infarction. Chem. Pap. 2020, 74, 521–528. [Google Scholar] [CrossRef]
- Gundamaraju, R.; Vemuri, R.C.; Singla, R.K.; Manikam, R.; Rao, A.R.; Sekaran, S.D. Strophanthus hispidus attenuates the Ischemia-Reperfusion induced myocardial Infarction and reduces mean arterial pressure in renal artery occlusion. Pharmacognosy magazine 2014, 10, S557–S562. [Google Scholar] [CrossRef] [PubMed]
- Karkare, S.; Adou, E.; Cao, S.; Brodie, P.; Miller, J.S.; Andrianjafy, N.M.; Razafitsalama, J.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. Cytotoxic cardenolide glycosides of Roupellina (Strophanthus) boivinii from the Madagascar rainforest. Journal of Natural Products 2007, 70, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Rubin, B.; Redaelli, M.; Radu, C.; Barollo, S.; Cicala, M.V.; Salvà, M.; Mian, C.; Mucignat-Caretta, C.; Simioni, P.; et al. The antiproliferative effects of ouabain and everolimus on adrenocortical tumor cells. Endocrine journal 2014, 61, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Chen, Y.; Lu, Y.; Wang, Y.; Ding, L.; Jiang, M. Cardenolides from the Apocynaceae family and their anticancer activity. Fitoterapia 2016, 112, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ade Zuhrotun. Anticancer Screening of Selected Apocynaceae, Simaroubaceae and Magnoliaceae of Indonesian Plants using Mechanism-Based Yeast Bioassay. International Journal of Pharmaceutical Sciences Review and Research 2015, 35, 90–94. [Google Scholar]
- Weng, J.-R.; Bai, L.-Y.; Chiu, S.-J.; Chiu, C.-F.; Lin, W.-Y.; Hu, J.-L.; Shieh, T.-M. Divaricoside Exerts Antitumor Effects, in Part, by Modulating Mcl-1 in Human Oral Squamous Cell Carcinoma Cells. Computational and structural biotechnology journal 2019, 17, 151–159. [Google Scholar] [CrossRef]
- AlQathama, A.; Ezuruike, U.F.; Mazzari, A.L.D.A.; Yonbawi, A.; Chieli, E.; Prieto, J.M. Effects of Selected Nigerian Medicinal Plants on the Viability, Mobility, and Multidrug-Resistant Mechanisms in Liver, Colon, and Skin Cancer Cell Lines. Frontiers in pharmacology 2020, 11, 546439. [Google Scholar] [CrossRef]
- An, P.-P.; Cui, Y.-S.; Shi, Q.-Y.; Ren, Y.-H.; Wu, P.-Q.; Liu, Q.-F.; Liu, H.-C.; Zhou, B.; Yue, J.-M. Pregnane steroids from the twigs and leaves of Strophanthus divaricatus and their cytotoxic activities. Tetrahedron Letters 2022, 93, 153691. [Google Scholar] [CrossRef]
- Ran, H.-L.; Huang, S.-Z.; Wang, H.; Yang, L.; Gai, C.-J.; Duan, R.-J.; Dai, H.-F.; Guan, Y.-L.; Mei, W.-L. Cytotoxic steroids from the stems of Strophanthus divaricatus. Phytochemistry 2023, 210, 113668. [Google Scholar] [CrossRef]
- BROWER, L.P.; MOFFITT, C.M. Palatability dynamics of cardenolides in the monarch butterfly. Nature 1974, 249, 280–283. [Google Scholar] [CrossRef]
- Yu, H.; Li, W.; Cao, X.; Wang, X.; Zhao, Y.; Song, L.; Chen, J.; Wang, S.; Chen, B.; Xu, Y. Echinocystic acid, a natural plant extract, alleviates cerebral ischemia/reperfusion injury via inhibiting the JNK signaling pathway. European journal of pharmacology 2019, 861, 172610. [Google Scholar] [CrossRef] [PubMed]
- Esther, O. Faboro; Wisut Wichitnithad; Olatomide A. Fadare; David A. Akinpelu; Craig A. Obafemi. Antibacterial and antioxidant activities and phytochemical screening of aqueous methanol extracts of eight Nigerian medicinal and aromatic Plants. Journal of Pharmacy Research 2016, 10, 523–532. [Google Scholar]
- Taylor & Francis. A Time-Trend Hypoglycemic Study of Ethanol and Chloroform Extracts of Strophanthus hispidus. Available online: https://www.tandfonline.com/doi/full/10.1080/10496470902787386 (accessed on 7 May 2024).
- Abiola, J.L.; Aiyelaagbe, O.O. Phytochemical, Antimicrobial and Cytotoxic Activities of Strophanthus sarmentosus DC. BIOMEDNATPROCH 2022, 12, 119–126. [Google Scholar] [CrossRef]
- Karima Tani Muhammad; Abdullahi Mann; Adamu Yusuf Kabiru; Musa Bola Busari. PHYTOCHEMICAL EVALUATION AND ANTI-INFLAMMATORY ACTIVITY OF THE LEAF, STEM AND ROOT BARK EXTRACTS OF STROPHANTHUS SARMENTOSUS DC, 2015.
- Gunstone, F.D. Vegetable oils. I.—The component acids of Strophanthus sarmentosus seed oil. J Sci Food Agric 1952, 3, 185–189. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Qureshi, M.I. Glyceride studies. VIII.—The component glycerides of four Strophanthus oils containing an unsaturated hydroxy acid. J Sci Food Agric 1968, 19, 386–388. [Google Scholar] [CrossRef]
- Suhitha, S.; Devi, S.K.; Gunasekaran, K.; Pakyntein, H.C.; Bhattacharjee, A.; Velmurugan, D. Phytochemical analyses and activity of herbal medicinal plants of North- East India for anti-diabetic, anti-cancer and anti-tuberculosis and their docking studies. Current topics in medicinal chemistry 2015, 15, 21–36. [Google Scholar] [CrossRef]
- Cowan, S.; Stewart, M.; Abbiw, D.K.; Latif, Z.; Sarker, S.D.; Nash, R.J. Lignans from Strophanthus gratus. Fitoterapia 2001, 72, 80–82. [Google Scholar] [CrossRef]
- CABI Databases. The cyclitols of [leaves of] Ochrosia nakaiana, Plumeria acutifolia and Strophanthus gratus. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19710603769 (accessed on 7 May 2024).
- S. Nishibe; A. Sakushima; H. Takemura; T. Takenaka; Y. Noguchi. Cyclitols from Apocynaceae leaves, 2001.
- Ran, H.-L.; Huang, S.-Z.; Wang, H.; Yang, L.; Yu, M.; Gai, C.-J.; Duan, R.-J.; Dai, H.-F.; Guan, Y.-L.; Mei, W.-L. Three new sesquiterpenoids from the stems of Strophanthus divaricatus. Phytochemistry Letters 2021, 46, 100–104. [Google Scholar] [CrossRef]
- Chen, N.-H.; Zhang, Y.-B.; Li, Z.-H.; Jiang, J.-W.; Li, G.-Q.; Wang, G.-C.; Li, Y.-L. Two New Sesquiterpenoids from the Root of Strophanthus divaricatus. chem. Lett. 2015, 44, 1119–1121. [Google Scholar] [CrossRef]
- Mulula, A.; Bouzina, A.; Kalulu, T.; Ntumba, J.; phine; Tshingamb, M.; Mambu, H.; Zaki, A. HPLC Fingerprint profile, In-vitro Cytotoxity and Anti-Herpes Simplex Virus Activity of Methanol Extract from Strophanthus hispidus DC (Stem bark). Microbes and Infectious Diseases 2022, 0, 0. [CrossRef]
- A Mulula; A D Bouzina; H B Mambu; J K Ntumba; K M Taba. HPLC Fingerprint profile and Antioxidant, Antibacterial Activities of Methanol Extract of Strophanthus hispidus DC (Stem bark). IOSR Journal of Applied Chemistry 2021, 14, 21–27. [Google Scholar] [CrossRef]
- INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES AND RESEARCH | IJPSR. PHYTOCHEMICAL SCREENING, ANTIBACTERIAL AND ANTIOXIDANT ACTIVITIES OF AQUEOUS AND ORGANICS STEM EXTRACTS OF STROPHANTHUS HISPIDUS DC. | INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES AND RESEARCH. (accessed on 8 May 2024).
- Ezuruike, U.F.; Chieli, E.; Prieto, J.M. In Vitro Modulation of Glibenclamide Transport by P-glycoprotein Inhibitory Antidiabetic African Plant Extracts. Planta medica 2019, 85, 987–996. [Google Scholar] [CrossRef] [PubMed]
- MS, T.; T, C.; S, D.; A, C.; MA, B.; MST, D.; AK, C.; AM, B. Ethnobotanical survey of the adverse and toxic effects of medicinal plants used in Guinean traditional medicine. J Pharmacogn Phytochem 2022, 11, 186–194. [Google Scholar] [CrossRef]
- Fageyinbo, M.S.; Akindele, A.J.; Agbaje, E.O. Sub-chronic toxicological evaluation of Strophanthus hispidus DC (Apocynaceae) aqueous root extract. Journal of complementary & integrative medicine 2021, 18, 753–760. [Google Scholar] [CrossRef] [PubMed]
- M. Osibemhe; G.O. Omaji; I.O. Onoagbe. Sub-chronic toxicity of extracts of strophanthus hispidus stem bark in normal rats. Pharmacologyonline 2017, 2, 140–161. [Google Scholar]
- Oppong Bekoe, E.; Agyare, C.; Boakye, Y.D.; Baiden, B.M.; Asase, A.; Sarkodie, J.; Nettey, H.; Adu, F.; Otu, P.B.; Agyarkwa, B.; et al. Ethnomedicinal survey and mutagenic studies of plants used in Accra metropolis, Ghana. Journal of ethnopharmacology 2020, 248, 112309. [Google Scholar] [CrossRef] [PubMed]
- Ofori – Baah, S.; Borquaye, L.S. Ethanolic leaf extract from Strophanthus gratus (Hook.) Franch. (Apocynaceae) exhibits anti-inflammatory and antioxidant activities. Cogent Biology 2019, 5, 1710431. [Google Scholar] [CrossRef]
- Adaramoye, O.A.; Olajuyin, A. A Comparative In Vitro Study on the Antioxidant and Anti-acetylcholinesterase Properties of Aerial Parts of Strophanthus preusii Engl & Pax. The West Indian Medical Journal 2014, 63, 408–415. [Google Scholar] [CrossRef]
- Agyare, C.; Dwobeng, A.S.; Agyepong, N.; Boakye, Y.D.; Mensah, K.B.; Ayande, P.G.; Adarkwa-Yiadom, M. Antimicrobial, Antioxidant, and Wound Healing Properties of Kigelia africana (Lam.) Beneth. and Strophanthus hispidus DC. Advances in pharmacological sciences 2013, 2013, 692613. [Google Scholar] [CrossRef]
- John Kenneth, M.; Amina, I.; Yakubu, J. Co-extract mixture from Strophanthus hispidus (roots) and Aframomum meleguta (seeds) show phytochemical synergy in its anti-inflammatory activity. Arch Pharm Pharma Sci 2019, 3, 89–100. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Cai, Y.-Z.; Xing, J.; Corke, H.; Sun, M. A Potential Antioxidant Resource: Endophytic Fungi from Medicinal Plants. Econ Bot 2007, 61, 14–30. [Google Scholar] [CrossRef]
- Elya, B.; Basah, K.; Mun'im, A.; Yuliastuti, W.; Bangun, A.; Septiana, E.K. Screening of α-glucosidase inhibitory activity from some plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. Journal of biomedicine & biotechnology 2012, 2012, 281078. [Google Scholar] [CrossRef] [PubMed]
- Taofik O., Sunmonu; Regina, N. Ugbaja; Obasijuade Ogunlesi; Daniel Olafimihan; Tolulope Shodeinde; Mubaraq A. Toriola; Dorcas I. Akinloye; Elizabeth A. Balogun. Effects of Strophanthus hispidus DC. (Apocynaceae) aqueous root extract on antioxidant status in Streptozotocin-induced diabetic rats. biokem 2015, 27, 89–97. [Google Scholar]
- Fageyinbo, M.S.; Akindele, A.J.; Adenekan, S.O.; Agbaje, E.O. Evaluation of in-vitro and in-vivo antidiabetic, antilipidemic and antioxidant potentials of aqueous root extract of Strophanthus hispidus DC (Apocynaceae). Journal of complementary & integrative medicine 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Ojiako, O.; Igwe, C. A Time-Trend Hypoglycemic Study of Ethanol and Chloroform Extracts of Strophanthus hispidus. J. of Herbs, Spices & Medicinal Plants 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Osibemhe, M.; Ibrahim, M.; Onoagbe, I.O. Anti-diabetic potential assessment of aqueous and ethanol extracts of arrow poison (Strophanthus hispidus) plant stem bark in Wistar male rats. jasem 2019, 23, 603. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Oyedemi, B.O.; Ijeh, I.I.; Ohanyerem, P.E.; Coopoosamy, R.M.; Aiyegoro, O.A. Alpha-Amylase Inhibition and Antioxidative Capacity of Some Antidiabetic Plants Used by the Traditional Healers in Southeastern Nigeria. TheScientificWorldJournal 2017, 2017, 3592491. [Google Scholar] [CrossRef] [PubMed]
- INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES AND RESEARCH | IJPSR. ANTI-NOCICEPTIVE ACTIVITY AND POSSIBLE MECHANISMS OF ACTION OF AQUEOUS ROOT EXTRACT OF STROPHANTHUS HISPIDUS DC (APOCYNACEAE) | INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES AND RESEARCH. Available online: https://ijpsr.com/bft-article/anti-nociceptive-activity-and-possible-mechanisms-of-action-of-aqueous-root-extract-of-strophanthus-hispidus-dc-apocynaceae/ (accessed on 8 May 2024).
- Ishola, I.O.; Awodele, O.; Oreagba, I.A.; Murtala, A.A.; Chijioke, M.C. Antinociceptive, anti-inflammatory and antiulcerogenic activities of ethanol root extract of Strophanthus hispidus DC (Apocynaceae). Journal of basic and clinical physiology and pharmacology 2013, 24, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Molander, M.; Nielsen, L.; Søgaard, S.; Staerk, D.; Rønsted, N.; Diallo, D.; Chifundera, K.Z.; van Staden, J.; Jäger, A.K. Hyaluronidase, phospholipase A2 and protease inhibitory activity of plants used in traditional treatment of snakebite-induced tissue necrosis in Mali, DR Congo and South Africa. Journal of ethnopharmacology 2014, 157, 171–180. [Google Scholar] [CrossRef]
- Houghton, P.J.; Skari, K.P. The effect on blood clotting of some west African plants used against snakebite. Journal of ethnopharmacology 1994, 44, 99–108. [Google Scholar] [CrossRef]
- Yazdi, S.E.; Mulabisana, J.; Prinsloo, G.; Cloete, M.; Kritzinger, Q. Plants containing cardiac glycosides showing antiphytoviral activity against Potato virus Y (PVYNTN) on tobacco plants. Journal of Plant Protection Research 2023, 58, 397–403. [Google Scholar] [CrossRef]
- Innocent, E.; Moshi, M.J.; Masimba, P.J.; Mbwambo, Z.H.; Kapingu, M.C.; Kamuhabwa, A. Screening of traditionally used plants for in vivo antimalarial activity in mice. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 163–167. [Google Scholar] [CrossRef] [PubMed]
- ONOTU, C.S; ABEDO, J.A; ANCHUA, R.G; SHETTIMA, F.A. T; ABDULMALIK U; SAMBO F. Invivo antitrypanosomal activity of methanolic stem extract of strophanthus sarmentosus dc on wistar white mice infected with trypanosomal brucei brucei spp (federe strain). Advances in Life Science and Technology 2014, 16, 10–16. [Google Scholar]
- Kassim, M.A.; Sooklal, S.A.; Archer, R.; Rumbold, K. Screening for hydroxynitrile lyase activity in non-commercialised plants. South African Journal of Botany 2014, 93, 9–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).