Submitted:
16 May 2024
Posted:
17 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Chemical Composition of ELSR
2.2. Antioxidant Activity Assays
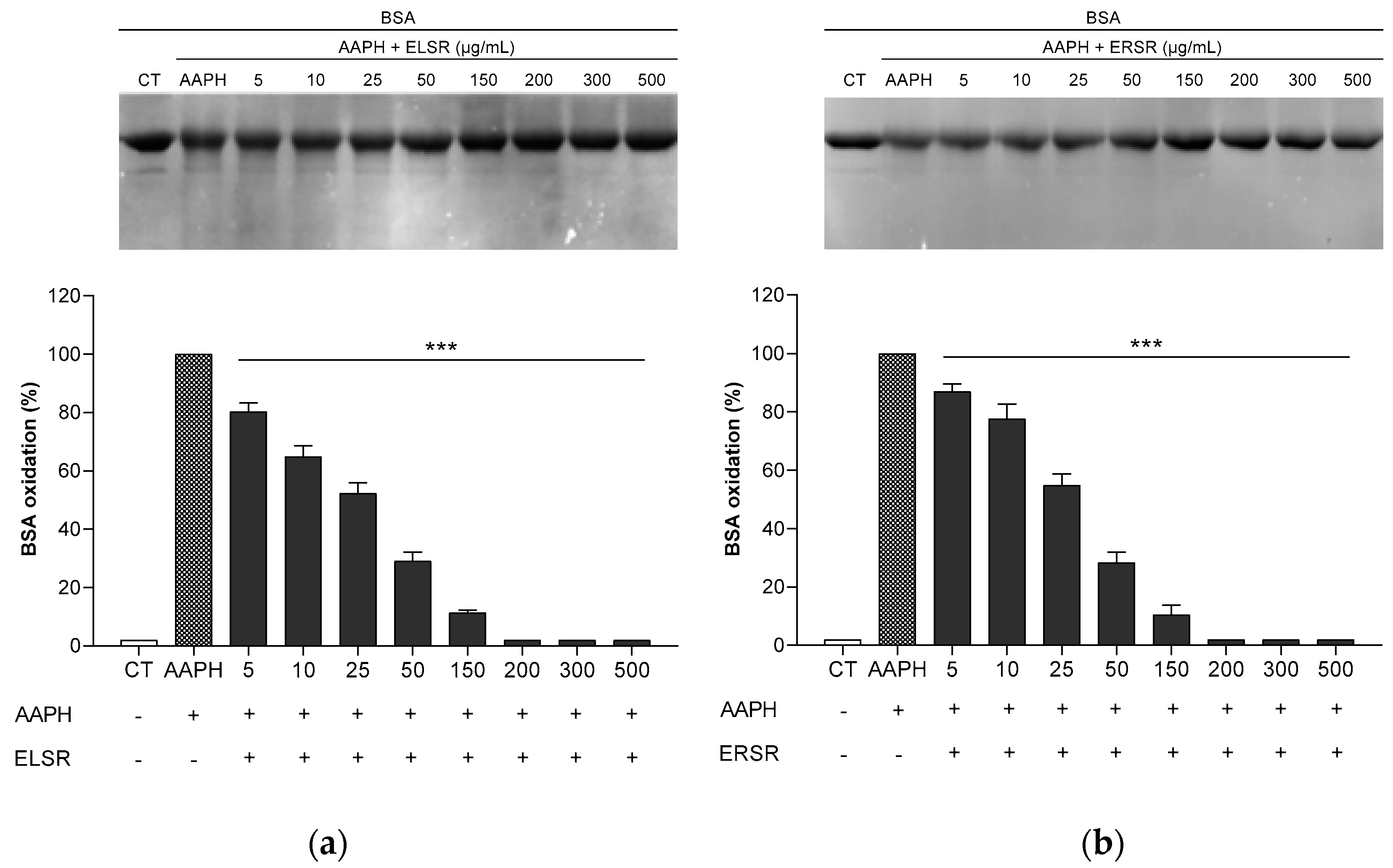
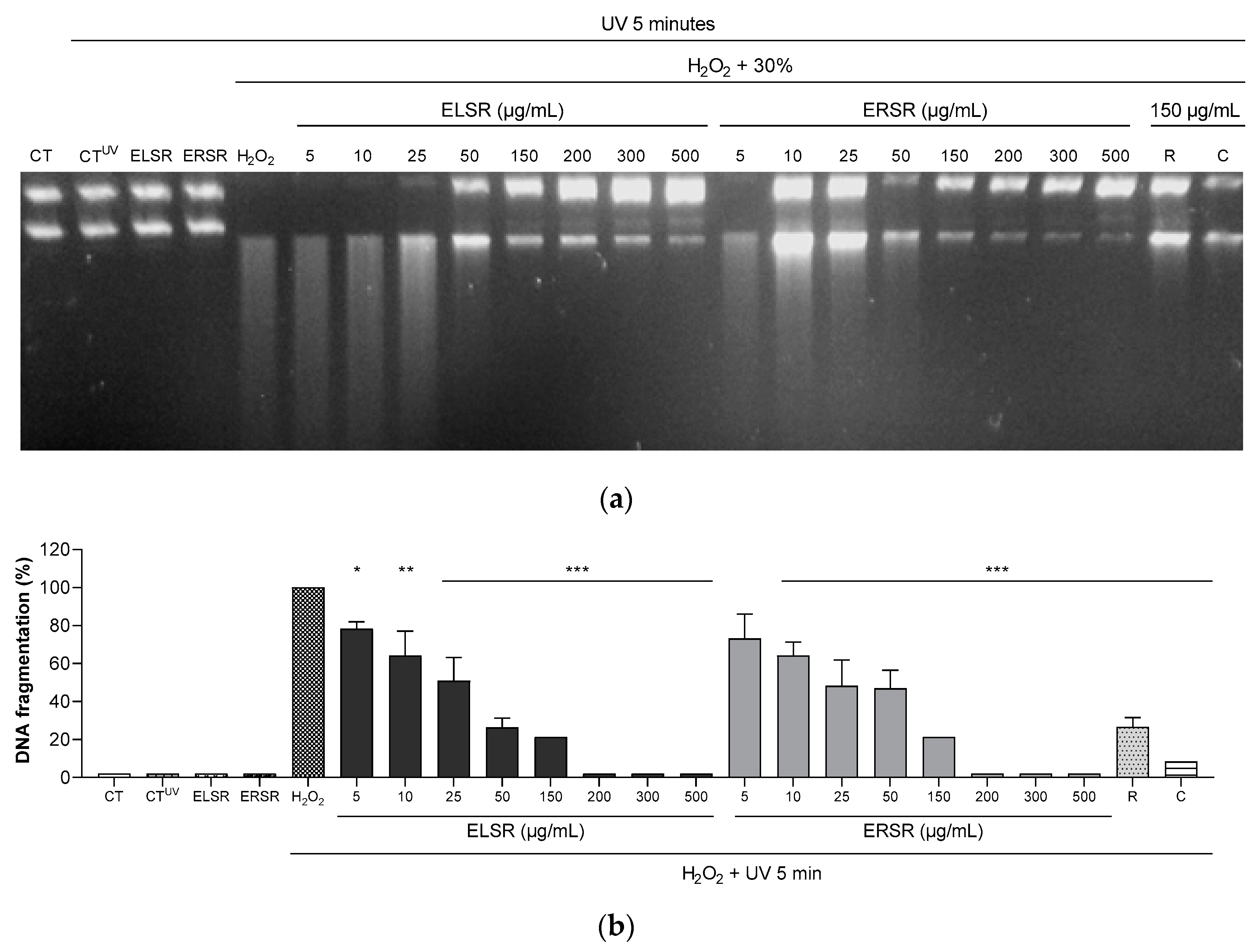
2.3. Protection of ELSR and ERSR against Oxidative Damage in Macromolecules
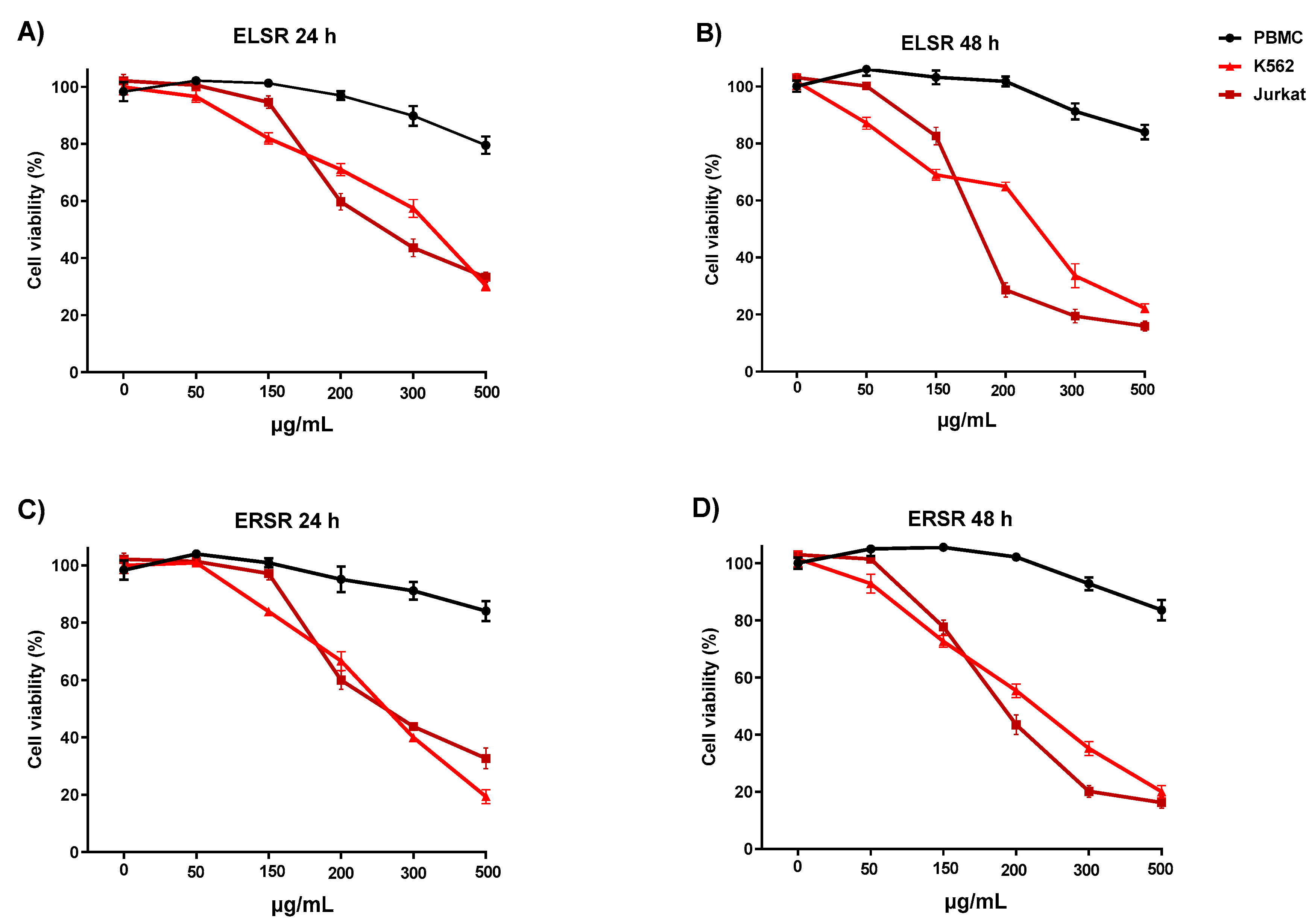
2.4. Cytotoxicity against Leukemic Strains
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection and Preparation of Plant Material
Phytochemical Analyses
4.3. Antioxidant Activity Assays
4.3.1. Direct ABTS•+ Radical Scavenging Assay
4.3.2. Direct DPPH• Radical Scavenging Assay
4.4. Protection against Oxidative Damage to the Macromolecules by ELSRs and ERSRs
4.4.1. AAPH-Induced Oxidation of Proteins
4.4.2. DNA Fragmentation induced by Hydrogen Peroxide (H2O2)
4.5. Cell-Based Assays
4.5.1. Cell Culture
4.5.2. Isolation of Mononuclear Cells from Human Peripheral Blood
4.5.3. Isolation of Mononuclear Cells from Human Peripheral Blood
4.6. Statistical Analyzes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nogueira, V.; Hay, N. Molecular Pathways: Reactive Oxygen Species Homeostasis in Cancer Cells and Implications for Cancer Therapy. Clin Cancer Res 2013, 19, 4309. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S. Y.; Alwasel, S. H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Archives of Toxicology 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Irwin, M. E.; Rivera-Del Valle, N.; Chandra, J. Redox Control of Leukemia: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid Redox Signal 2013, 18, 1349. [Google Scholar] [CrossRef]
- Xie, W.; Ma, W.; Liu, P.; Zhou, F. Overview of thioredoxin system and targeted therapies for acute leukemia. Mitochondrion 2019, 47, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Badana, A. K.; Murali Mohan, G.; Shailender, G.; Malla, R. R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark Insights 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O. M.; Akinloye, O. A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Pisoschi, A. M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Thyagarajan, A.; Sahu, R. P. Potential Contributions of Antioxidants to Cancer Therapy: Immunomodulation and Radiosensitization. Integr Cancer Ther 2018, 17, 210. [Google Scholar] [CrossRef]
- León-González, A. J.; Auger, C.; Schini-Kerth, V. B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem Pharmacol 2015, 98, 371–380. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Az-Zahra, F.; Wongso, H.; Setyawati, L. U.; Novitasari, D.; Ikram, E. H. K. Molecular Mechanism of Natural Food Antioxidants to Regulate ROS in Treating Cancer: A Review. Antioxidants 2024, 13. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Qureshi, A.; Hall, G. Leukaemias: a review. Paediatr Child Health 2017, 27, 489–494. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Ma, S. Racial Differences in Four Leukemia Subtypes: Comprehensive Descriptive Epidemiology. Sci Rep 2018, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Mwirigi, A.; Dillon, R.; Raj, K. Acute leukaemia. Medicine 2017, 45, 280–286. [Google Scholar] [CrossRef]
- O’Shea, J. J.; Schwartz, D. M.; Villarino, A. V.; Gadina, M.; McInnes, I. B.; Laurence, A. The JAK-STAT Pathway: Impact on Human Disease and Therapeutic Intervention. Annu Rev Med 2015, 66, 311. [Google Scholar] [CrossRef] [PubMed]
- Gioia, L.; Siddique, A.; Head, S. R.; Salomon, D. R.; Su, A. I. A genome-wide survey of mutations in the Jurkat cell line. BMC Genomics 2018, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rose-Inman, H.; Kuehl, D. Acute Leukemia. Hematol Oncol Clin North Am 2017, 31, 1011–1028. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Estimated number of new cases in 2020, worldwide, both sexes, all ages. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=countries&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group&5B&5D=0&ages_group%5B%5D=17&group_cancer=1&in (accessed on 10 June 2022).
- De Giffoni De Carvalho, J. T.; Da Silva Baldivia, D.; Leite, D. F.; De Araújo, L. C. A.; De Toledo Espindola, P. P.; Antunes, K. A.; Rocha, P. S.; De Picoli Souza, K.; Dos Santos, E. L. Medicinal Plants from Brazilian Cerrado: Antioxidant and Anticancer Potential and Protection against Chemotherapy Toxicity. Oxid Med Cell Longev 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Castro, D. T. H.; Campos, J. F.; Damião, M. J.; Torquato, H. F. V.; Paredes-Gamero, E. J.; Carollo, C. A.; Rodrigues, E. G.; de Picoli Souza, K.; dos Santos, E. L. Ethanolic Extract of Senna velutina Roots: Chemical Composition, In Vitro and In Vivo Antitumor Effects, and B16F10-Nex2 Melanoma Cell Death Mechanisms. Oxid Med Cell Longev 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Castro, D. T. H.; Leite, D. F.; da Silva Baldivia, D.; dos Santos, H. F.; Balogun, S. O.; da Silva, D. B.; Carollo, C. A.; de Picoli Souza, K.; dos Santos, E. L. Structural Characterization and Anticancer Activity of a New Anthraquinone from Senna velutina (Fabaceae). Pharmaceuticals 2023, 16, 951. [Google Scholar] [CrossRef]
- Oladeji, O. S.; Adelowo, F. E.; Oluyori, A. P. The genus Senna (Fabaceae): A review on its traditional uses, botany, phytochemistry, pharmacology and toxicology. South African Journal of Botany 2021, 138, 1–32. [Google Scholar] [CrossRef]
- Baldivia, D. da S.; Leite, D. F.; de Castro, D. T. H.; Campos, J. F.; Dos Santos, U. P.; Paredes-Gamero, E. J.; Carollo, C. A.; Silva, D. B.; Souza, K. de P.; Dos Santos, E. L. Evaluation of In Vitro Antioxidant and Anticancer Properties of the Aqueous Extract from the Stem Bark of Stryphnodendron adstringens. Int J Mol Sci 2018, 19. [Google Scholar]
- Campos, J. F.; De Castro, D. T. H.; Damiaõ, M. J.; Vieira Torquato, H. F.; Paredes-Gamero, E. J.; Carollo, C. A.; Estevinho, L. M.; De Picoli Souza, K.; Santos, E. L. Dos The Chemical Profile of Senna velutina Leaves and Their Antioxidant and Cytotoxic Effects. Oxid Med Cell Longev 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Campos, J. F.; Espindola, P. P. de T.; Torquato, H. F. V.; Vital, W. D.; Justo, G. Z.; Silva, D. B.; Carollo, C. A.; de Picoli Souza, K.; Paredes-Gamero, E. J.; dos Santos, E. L. Leaf and Root Extracts from Campomanesia adamantium (Myrtaceae) Promote Apoptotic Death of Leukemic Cells via Activation of Intracellular Calcium and Caspase-3. Front Pharmacol 2017, 8. [Google Scholar]
- Casagrande, J. C.; Macorini, L. F. B.; Antunes, K. A.; Dos Santos, U. P.; Campos, J. F.; Dias-Júnior, N. M.; Sangalli, A.; Cardoso, C. A. L.; Do Carmo Vieira, M.; Rabelo, L. A.; et al. Antioxidant and cytotoxic activity of hydroethanolic extract from Jacaranda decurrens leaves. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Yang, C. S.; Wang, H. Cancer Preventive Activities of Tea Catechins. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Farias, D. F.; Cavalheiro, M. G.; Viana, M. P.; Queiroz, V. A.; Rocha-Bezerra, L. C. B.; Vasconcelos, I. M.; Morais, S. M.; Carvalho, A. F. U. Water extracts of Brazilian leguminous seeds as rich sources of larvicidal compounds against Aedes aegypti L. An Acad Bras Cienc 2010, 82, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, L. M.; De Paula-Souza, J.; Andrade, A.; Brandão, M. G. L. Plants from the Brazilian Traditional Medicine: species from the books of the Polish physician Piotr Czerniewicz (Pedro Luiz Napoleão Chernoviz, 1812–1881). Revista Brasileira de Farmacognosia 2017, 27, 388–400. [Google Scholar] [CrossRef]
- Rodrigues, V. E. G.; Carvalho, D. A. de Levantamento etnobotânico de plantas medicinais no domínio do Cerrado na região do Alto Rio Grande - Minas Gerais. Ciência e agrotecnologia 2001, 25, 102–123. [Google Scholar]
- Cunha, L. F.; Costa, C. M.; Barroso, P. R.; Kato, K. C.; Oliveira, F. De; Victor, C.; Filho, M.; Fernanda, C.; Grael, F.; Gregório, L. E.; et al. Pharmacognosy Phytochemical screening and biological assays of ethanolic leaf extract of Senna rugosa widely used in the popular medicine although not yet adequately investigated as to its phytoconstituents and Abstract Resumo Senna rugosa ( Fabaceae ) é. Rodriguésia 2020, 7, 2–15. [Google Scholar]
- Silva, J. G. A.; Silva, A. A.; Coutinho, I. D.; Pessoa, C. O.; Cavalheiro, A. J.; Silva, M. G. V. Chemical Profile and Cytotoxic Activity of Leaf Extracts from Senna spp. from Northeast of Brazil. J Braz Chem Soc 2016, 27, 1872–1880. [Google Scholar]
- Maia, I. R. D. O.; Trevisan, M. T. S.; Silva, M. G. D. V.; Breuer, A.; Owen, R. W. Characterization and quantitation of polyphenolic compounds in senna macranthera var pudibunda from the Northeast of Brazil. Nat Prod Commun 2019, 14. [Google Scholar] [CrossRef]
- Wang, S.; Feng, K.; Han, L.; Fang, X.; Zhang, Y.; Yu, H.; Pang, X. Glycosidic compounds from Cassia obtusifolia seeds and their inhibitory effects on OATs, OCTs and OATPs. Phytochem Lett 2019, 32, 105–109. [Google Scholar] [CrossRef]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular targets of epigallocatechin—gallate (EGCG): A special focus on signal transduction and cancer. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Almatrood, S. A.; Almatroudi, A.; Khan, A. A.; Alhumaydh, F. A.; Alsahl, M. A.; Rahmani, A. H. Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C. E.; Farzaei, M. H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers (Basel) 2020, 12, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L. F.; Costa, C. M.; Barroso, P. R.; Kato, K. C.; Oliveira, F. De; Victor, C.; Filho, M.; Fernanda, C.; Grael, F.; Gregório, L. E.; et al. Pharmacognosy Phytochemical screening and biological assays of ethanolic leaf extract of Senna rugosa widely used in the popular medicine although not yet adequately investigated as to its phytoconstituents and Abstract Resumo Senna rugosa ( Fabaceae ) é. Rodriguésia 2020, 7, 2–15. [Google Scholar]
- Yadav, P.; Parshad, B.; Manchanda, P.; Sharma, S. K. Chromones and their derivatives as radical scavengers: a remedy for cell impairment. Curr Top Med Chem 2014, 14, 2552–2575. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y. di; Jiang, Y. yan; Guo, F. xia; Chen, L. xiao; Xu, L. lu; Zhang, W.; Liu, B. The antitumor activity of naturally occurring chromones: A review. Fitoterapia 2019, 135, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Amen, Y.; Elsbaey, M.; Othman, A.; Sallam, M.; Shimizu, K. Naturally occurring chromone glycosides: Sources, bioactivities, and spectroscopic features. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, P.; Lin, H.; Tang, C.; Zhu, H.; Yang, Y. Naphthoquinones: A continuing source for discovery of therapeutic antineoplastic agents. Chem Biol Drug Des 2018, 91, 681–690. [Google Scholar] [CrossRef]
- Wellington, K. W. Understanding cancer and the anticancer activities of naphthoquinones – a review. RSC Adv 2015, 5, 20309–20338. [Google Scholar] [CrossRef]
- Klaunig, J. E. Oxidative Stress and Cancer. Curr Pharm Des 2019, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Novel aspects of oxidative stress-associated carcinogenesis. Antioxid Redox Signal 2006, 8, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C. J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J. N.; Cotter, T. G. ROS signalling in the biology of cancer. Semin Cell Dev Biol 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Abrahamse, H. Redox potential of antioxidants in cancer progression and prevention. Antioxidants 2020, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S. C.; Tyagi, A. K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural Antioxidants. Antioxidants 2019. [Google Scholar]
- Nijveldt, R. J.; Van Nood, E.; Van Hoorn, D. E. C.; Boelens, P. G.; Van Norren, K.; Van Leeuwen, P. A. M. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Panche, A. N.; Diwan, A. D.; Chandra, S. R. Flavonoids: an overview. J Nutr Sci 2016, 5. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S. M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Farha, A. K.; Gan, R. Y.; Li, H. Bin; Wu, D. T.; Atanasov, A. G.; Gul, K.; Zhang, J. R.; Yang, Q. Q.; Corke, H. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit Rev Food Sci Nutr 2022, 62, 832–859. [Google Scholar] [CrossRef]
- Lucas, D. M.; Still, P. C.; Pérez, L. B.; Grever, M. R.; Kinghorn, A. D. Potential of Plant-Derived Natural Products in the Treatment of Leukemia and Lymphoma. Curr Drug Targets 2010, 11, 812. [Google Scholar] [CrossRef]
- Arévalo, C. M.; Cruz-Rodriguez, N.; Quijano, S.; Fiorentino, S. Plant-derived extracts and metabolic modulation in leukemia: a promising approach to overcome treatment resistance. Front Mol Biosci 2023, 10, 1229760. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A. K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Mócsai, A.; Ruland, J.; Tybulewicz, V. L. J. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol 2010, 10, 387. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Gupta, R. K. Bioprotective properties of Dragon’s blood resin: in vitro evaluation of antioxidant activity and antimicrobial activity. BMC Complement Altern Med 2011, 11. [Google Scholar] [CrossRef]
- Mayo, J. C.; Tan, D. X.; Sainz, R. M.; Lopez-Burillo, S.; Reiter, R. J. Oxidative damage to catalase induced by peroxyl radicals: functional protection by melatonin and other antioxidants. Free Radic Res 2003, 37, 543–553. [Google Scholar] [CrossRef]
- Kumar, A.; Chattopadhyay, S. DNA damage protecting activity and antioxidant potential of pudina extract. Food Chem 2007, 100, 1377–1384. [Google Scholar] [CrossRef]
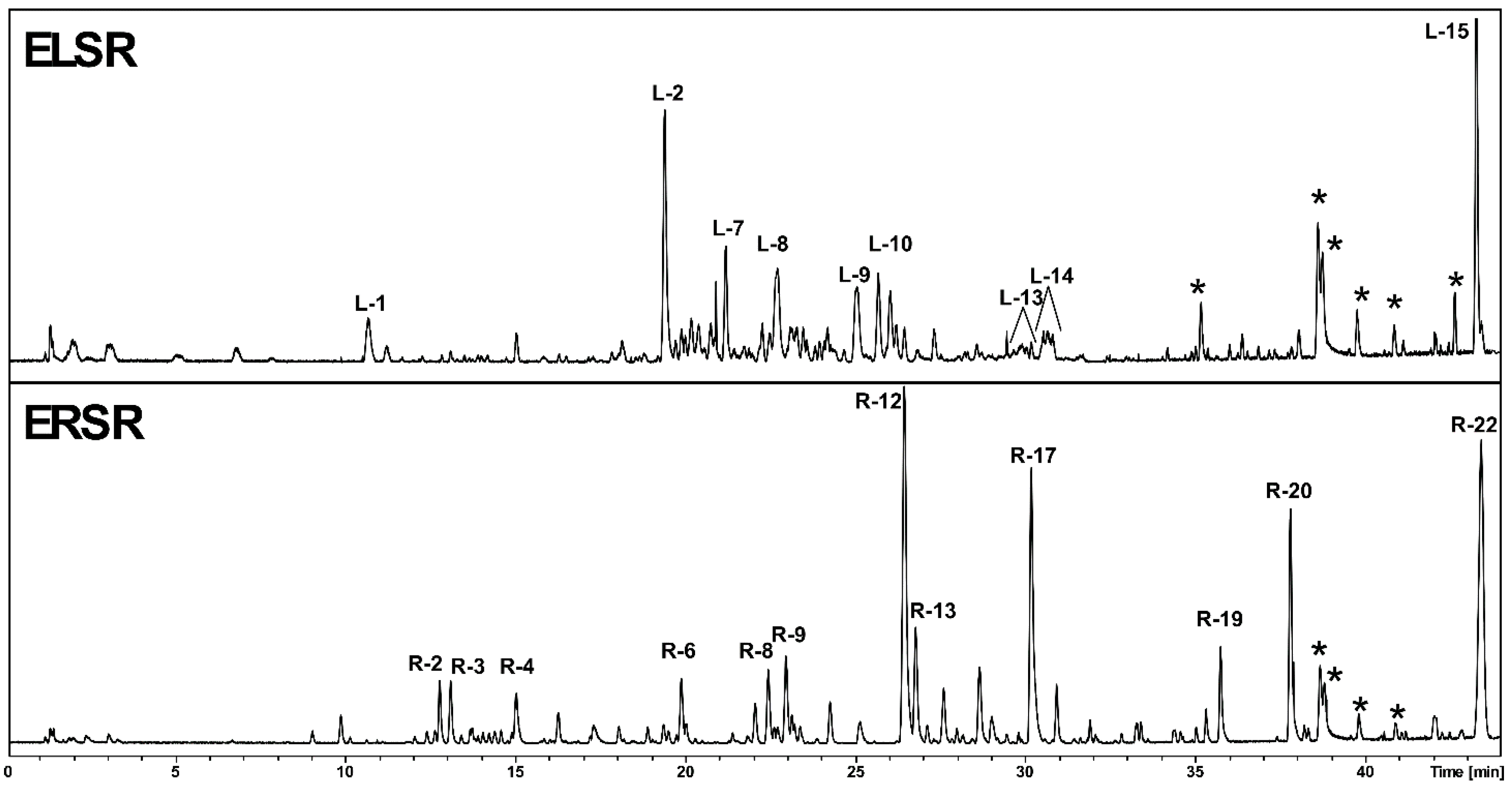
| ELSR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak | Time(min) | UV | Formula | [M+H]+ | MS/MS | Classes | Compound | |||
| L-1 | 10.6 | 280 | C15H14O6 | 291.0870 | - | Flavan-3-ol | Catechin | |||
| L-2 | 19.4 | 256/353 | C27H30O16 | 611.1599 | 465 (C21H21O12), 449 (C21H21O11), 303 (C15H11O7) | Flavonol | Rutin | |||
| L-3 | 19.8 | 313 | C15H14O5 | 275.0914 | 225 (C14H9O3), 213 (C13H9O3), 197 (C13H9O2), 185 (C12H9O2), 157 (C11H9O) | Stilbene | 3,3',5,5'-Tetrahydroxy-4-methoxystilbene | |||
| L-4 | 20.1 | 280 | C30H26O10 | 547.1580 | 393 (C22H17O7), 285 (C16H13O5), 271 (C15H11O5), 259 (C14H11O5), 241 (C14H9O4), 147 (C9H7O2) | Proanthocyanidin | Epiafzelechin-epiafzelechin derivative | |||
| L-5 | 20.3 | 280 | C30H26O10 | 547.1582 | 393 (C22H17O7), 271 (C15H11O5), 241 (C14H9O4) | Proanthocyanidin | Epiafzelechin-epiafzelechin derivative | |||
| L-6 | 20.7 | 280 | C30H26O10 | 547.1589 | 409 (C22H17O8), 269 (C16H13O4), 243 (C14H11O4), 163 (C9H7O3) | Proanthocyanidin | Epiafzelechin-epiafzelechin derivative | |||
| L-7 | 21.1 | 265/347 | C27H30O15 | 595.1651 | 449 (C21H21O11), 287 (C15H11O6) | Flavonol | Kaempferol 3-O-rutinoside | |||
| L-8 | 22.6 | 280 | C30H26O9 | 531.1644 | 393 (C22H17O7), 269 (C16H13O4) | Proanthocyanidin | Trihydroxyflavan-epiafzelechin derivative | |||
| L-9 | 24.9 | 280 | C30H26O9 | 531.1648 | 393 (C22H17O7), 269 (C16H13O4) | Proanthocyanidin | Trihydroxyflavan-epiafzelechin derivative | |||
| L-10 | 25.6 | 280 | C30H26O9 | 531.1648 | 393 (C22H17O7), 269 (C16H13O4), 243 (C14H11O4), 207 (C11H11O4) | Proanthocyanidin | Trihydroxyflavan-epiafzelechin derivative | |||
| L-11 | 25.9 | 277/345 | C15H10O6 | 287.0546 | 241 (C14H9O4), 153 (C7H5O4) | Flavone | Luteolin | |||
| L-12 | 26.3 | 280 | C30H26O9 | 531.1651 | - | Proanthocyanidin | Trihydroxyflavan-epiafzelechin derivative | |||
| L-13 | 27.2 | 279/350 | C16H12O7 | 317.0662 | - | 3-metoxyluteolin | ||||
| L-14 | 29.2-30.1 | 280 | C45H38O13 | 787.2360 | - | Proanthocyanidin | Trimeric procyanidins | |||
| L-15 | 30.2-30.9 | 280 | C45H38O12 | 771.2412 | - | Proanthocyanidin | Trimeric procyanidins | |||
| L-16 | 43.2 | 406 | C34H40O9 | 593.2750 | 533 (C32H37O7), 461 (C29H33O5) | Tetrahydroanthracene | Dimeric tetrahydroanthracene derivative | |||
| ERSR | ||||||||||
| Peak | Time (min) | UV | Formula | [M+H]+ | MS/MS | Classes | Compound | |||
| R-1 | 9.8 | 253/298 | C24H32O15 | 561.1791 | 489 (C24H25O11), 423 (C20H23O10), 345 (C18H17O7), 291 (C15H15O6) | - | unknown | |||
| R-2 | 12.7 | 254/299 | C20H26O11 | 443.1542 | 323 (C16H19O7), 293 (C15H17O6), 235 (C12H11O5), 205 (C11H9O4) | Chromone | Obtusichromoneside derivative | |||
| R-3 | 13 | 252/295 | C20H26O11 | 443.1548 | 371 (C20H19O7), 259 (C14H11O5), 235 (C12H11O5), 205 (C11H9O4) | Chromone | Obtusichromoneside derivative | |||
| R-4 | 14.9 | 320 | C14H12O5 | 261.0757 | 197 (C13H9O2), 169 (C12H9O) | Naphthoquinone | 2-Methoxystypandrone | |||
| R-5 | 16.2 | 313 | C15H14O5 | 275.091 | 213 (C13H9O3), 197 (C13H9O2), 169 (C12H9O) | Stilbene | Tetrahydroxy-methoxy stilbene derivative | |||
| R-6 | 19.8 | 313 | C15H14O5 | 275.0914 | 225 (C14H9O3), 213 (C13H9O3), 197 (C13H9O2), 185 (C12H9O2), 157 (C11H9O) | Stilbene | 3,3',5,5'-Tetrahydroxy-4-methoxystilbene | |||
| R-7 | 22 | 280/334 | C16H14O6 | 303.0867 | 271 (C15H11O5), 243 (C14H11O4), 203 (C11H7O4) | Chromone | chromone deriative | |||
| R-8 | 22.3 | 278/326/400 | C25H28O14 | 553.1554 | 259 (C14H11O5) | Naphthopyrone | Putative norrubrofusarin gentiobioside | |||
| R-9 | 22.9 | 279/312/366 | C26H30O14 | 567.1699 | 273 (C15H13O5) | Flavanone | Putative hexahydroxy Flavanonol pentosyl-hexosyl | |||
| R-10 | 24.2 | 277/324/399 | C27H32O15 | 597.18 | 273 (C15H13O5) | Naphthopyrone | Rubrofusarin gentiobioside | |||
| R-11 | 25.1 | 276/310/369 | C15H12O8S | 353.0314 | 273 (C15H13O5), 230 (C13H10O4) | Flavanone | Putative hexahydroxy Flavanonol sulfate | |||
| R-12 | 26.4 | 277/324/395 | C26H30O14 | 567.1709 | 273 (C15H13O5) | Naphthopyrone | Cassiaside B | |||
| R-13 | 26.7 | 285/320/379 | C27H32O14 | 581.1864 | 449 (C22H25O10), 287 (C16H15O5) | Flavanone | Putative hexahydroxy-methoxy Flavanonol pentosyl-hexosyl | |||
| R-14 | 27.5 | 277/324/401 | C21H22O10 | 435.1286 | 273 (C15H13O5) | Naphthopyrone | Rubrofusarin-O-glucopyranoside | |||
| R-15 | 28.6 | 279/330/413 | C28H34O15 | 611.1963 | 449 (C22H25O10), 287 (C16H15O5) | - | unknown | |||
| R-16 | 28.9 | 285/321/377 | C16H14O8S | 367.0472 | 287 (C16H15O5) | Flavanone | Putative hexahydroxy-methoxy Flavanonol sulfate | |||
| R-17 | 30.1 | 258/278/331/411 | C27H32O14 | 581.1861 | 449 (C22H25O10), 419 (C21H23O9), 287 (C16H15O5) | - | unknown | |||
| R-18 | 30.8 | 279/330/411 | C22H24O10 | 449.1433 | 287 (C16H15O5) | - | unknown | |||
| R-19 | 35.7 | 277/325/402 | C15H12O5 | 273.0758 | 230 (C13H10O4) | Naphthopyrone | Rubrofusarin | |||
| R-20 | 37.7 | 281/337/425 | C16H15O5 | 287.0917 | 272 (C15H12O5), 254 (C15H10O4), 244 (C14H12O4), 226 (C15H10O3), 198 (C13H10O2) | - | unknown | |||
| R-21 | 43.4 | 410 | C36H44O9 | 621.308 | 561 (C34H41O7) | - | unknown | |||
| R-22 | 44.5 | 410 | C36H44O9 | 621.3077 | 561 (C34H41O7) | - | unknown | |||
| Samples | ABTS•+ | DPPH• | |||||
|---|---|---|---|---|---|---|---|
| IC50 (µg/mL) |
Maximum Activity (%) |
(µg/mL) | IC50 (µg/mL) |
Maximum Activity (%) |
(µg/mL) | ||
| AA | 1.32 + 0.04 | 99. 28 + 0.16 | 5 | 2.35 + 0.34 | 94. 88 +0.16 | 10 | |
| BHT | 6.08 + 0.77 | 98. 98 + 0.24 | 50 | 71.86 + 2.32 | 93. 16 +0.23 | 500 | |
| ELSR | 4.86 + 0.51 | 98. 15 + 0.53 | 50 | 19.98 + 1.96 | 95. 24 +0.20 | 100 | |
| ERSR | 8.33 + 0.90 | 98. 40 + 0.33 | 25 | 13.37 + 1.05 | 94. 98 +0.27 | 100 | |
| Cell line | ELSR IC50 (μg/mL) |
ERSR IC50 (μg/mL) |
||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| PBMC | ND | ND | ND | ND |
| K562 | 345.01 ± 2.53 | 242.54 ± 2,38 | 257.49 ± 2.41 | 223.00 ± 2.34 |
| Jurkat | 255.33 ± 2.40 | 171.45 ± 2,25 | 256.65 ± 2.40 | 189.30 ± 2.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





