2. Results and Discussion
To evaluate this synthesis of dialkyl thioethers hypothesis, we screened the reaction conditions using 4-(chloromethyl)biphenyl (
1a), EtOCS
2K (
2a), and dimethylformamide (DMF) as a model reaction. Gratifyingly, sulfidation proceeds at a reaction temperature of 150 °C to afford the dialkyl thioether
3a in 77% yield (
Table 1, entry 1). The screening of various solvents reveals that the solvent is critical in the sulfidation reaction (
Table 1, entries 1–5), and a trace amount of the thioether
3a is obtained when non-polar solvent o-xylene is used (
Table 1, entry 2). The optimal results are obtained when the reaction is conducted in DMSO at 150 °C (
Table 1, entry 3), whereas unsatisfactory results are obtained using dimethylacetamide (DMAc) or N-methyl-2-pyrrolidone (NMP) as the solvent (
Table 1, entries 4–5). The yield of the thioether
3a does not change significantly when the amount of DMSO is decreased (
Table 1, entry 6). Notably, the yield decreases significantly when the dosage of
2a is decreased (
Table 1, entry 7). Further studies indicate that decreasing the reaction temperature does not decrease the yield of sulfidation (
Table 1, entries 8 and 9). However, when the reaction temperature is further decreased to 80 °C, the yield of the sulfidation product
3a decreases slightly (89%;
Table 1, entry 10). Subsequently, we investigated the reaction time, and thus, the yield of sulfidation is unaffected when the reaction time is shortened to 1 h, but shortening the time further to 0.5 h affords significant decrease in yield (
Table 1, entries 11–13). Based on these results, the optimized reaction conditions are
1a (0.5 mmol) and
2a (1.0 mmol) in 1.0 mL DMSO at 100 °C for 1 h (
Table 1, entry 12).
With the optimized conditions for use in synthesising dialkyl thioethers established, the alkyl halides applicable in the sulfuration reaction were investigated (
Scheme 2). Firstly, various substituted benzyl chlorides are compatible under the optimized conditions. Aromatic rings with electron-donating and electron-withdrawing substituents are compatible under the standard conditions. Electron-donating groups, such as –Me, –
tBu, –TMS, –OMe, –OCH
2Ph, –OCF
3, –SCF
3, –SPh, –CH
2OH, and –BPin
2 (Me = methyl,
tBu =
tert-butyl, TMS = trimethylsilyl, Ph = phenyl, Pin = pinacol), are successfully sulfated to produce dialkyl thioethers in good yields (
Scheme 2,
3a–
3k). Hindered 2-(chloromethyl)-1,3,5-trimethylbenzene, in particular, successfully undergoes the reaction, affording ethyl(2,4,6-trimethylbenzyl)sulfane
3d in 75% yield. Remarkably, the –BPin
2 group remains intact on the aromatic ring in 91% yield, and is very useful in transition-metal-catalyzed cross-coupling reactions (
Scheme 2,
3k). A crucial feature of this reaction is its tolerance of various halides, including –F, –Cl, –Br, –I, and –CF
3, with no dehalogenated by-products observed (
Scheme 2,
3l–3p). Additionally, benzyl chlorides substituted with strong electron-withdrawing groups, such as sulfone and amide, successfully undergo the reaction, furnishing thioethers
3q and
3r in yields of 91% and 93%, respectively. Moreover, fused-ring and heterocyclic-substituted alkyl halides, such as naphthalene (
3s–
3t), anthracene (
3u), thiophene (
3v), benzothiophene (
3w), quinolone (
3x), quinazoline (
3y), pyrazole (
3z), and tetrazole (
3aa), can undergo the reaction to produce the desired products in moderate-to-good yields. Next, 1,4-bis(chloromethyl)benzene, 1-(chloromethyl)adamantane, and (chloromethylene)dibenzene successfully undergo the reaction, indicating that the sulfuration reaction is characterized by a good functional group tolerance (
Scheme 2,
3ab–3ad). Finally, the use of aromatic xanthates as substituents was investigated. Gratifyingly, benzyl and phenyl substituent xanthates were well tolerated under the optimized conditions, affording the corresponding thioethers in good yields (
Scheme 2,
3ae-
3af).
To evaluate this synthesis of aryl alkyl thioethers hypothesis, we screened the reaction conditions using 3-iodopyridine (
1ae), EtOCS
2K (
2a), additive, and DMF as a model reaction. Initially, sulfidation proceeds at a reaction temperature of 150 °C for 24 h to afford the 3-(ethylthio)pyridine
4a in 37% yield (
Table 2, entry 1). The screening of various reaction time reveals that the reaction time is critical in the sulfidation reaction, and excellent results is obtained when the sulfidation reaction was carry out in 36 h (
Table 2, entries 1–3). Notably, the yield decreases significantly when the dosage of EtOCS
2K (
2a) or I
2 is decreased (
Table 2, entries 4-5). With EtOCS
2K (
2a) as sulphur source, the examination of different additive showed that NH
4I and HI was inefficient (
Table 2, entries 6-7). Further optimum solvents showed that DMF was the best choice; the other solvents—DMSO, NMP and DMAc—all decreased the yield of
4a (
Table 2, entries 8–10). Furthermore, decreasing the reaction temperature led to a decrease in yield (
Table 2, entry 11). Without the use of an iodine reagent, only a trace of the sulfidation reaction product was obtained; mostly the starting material was recovered (
Table 2, entry 12). Based on these results, the optimized reaction conditions are halopyridine (0.5 mmol) and EtOCS
2K (1.2 mmol) and I
2 (1.5 mmol) in 3.0 mL DMF at 150 °C for 36 h (
Table 1, entry 3).
The iodopyridine reactions with various substituted potassium xanthates also proceed with smooth conversions under the optimized conditions, furnishing the corresponding thioethers in moderate-to-good yields. Notably, substituted potassium xanthates with ethyl (4a), n-propyl (4b), n-butyl (4c), and n-pentyl groups (4d) are tolerated well under mild reaction conditions. When sulfuration is conducted using 3-iodoquinoline and 4-iodoisoquinoline, the thioether products 4e–4f are obtained in yields of 94% and 85%, respectively. 2-Fluoropyridines bearing various functional groups are completely converted in the presence of 2a to furnish the corresponding sulfides in good yields. 2-Fluoropyridines, substituted with both electron-donating and electron-withdrawing groups, react with 2a to generate the corresponding sulfuration products 4g–4o in good yields. The reaction tolerates various substituents, including –Me, –Ph, –NH2, –OH, –I, –OCNMe2, and –CN groups, and whether the substituent is at the 3-, 4-, 5-, or 6-position of the pyridine ring does not affect the yield of the reaction. When 2-fluoro-3-iodopyridine is used as the starting material, the F atom at the 2-position of the pyridine ring exhibits a higher reactivity, and the reaction affords the 2-(ethylthio)-3-iodopyridine product (4p) in 90% yield. Remarkably, when 3,5-dibromopyridine is used, the disulfuration product 4r is obtained in 40% yield. The activities of the halogen atoms depend more on their positions when 2-chloro-5-iodopyrimidine is used as the starting material, affording product 4s in 92% yield.
Scheme 3.
Substrate scope of the sulfuration reaction a,b. a Reaction conditions: pyridyl halide 1 (0.5 mmol), ROCS2K 2 (1.2 mmol), and I2 (1.5 mmol) in DMF (3.0 mL) at 150 °C for 36 h in a sealed tube in an air atmosphere. b Isolated yields.
Scheme 3.
Substrate scope of the sulfuration reaction a,b. a Reaction conditions: pyridyl halide 1 (0.5 mmol), ROCS2K 2 (1.2 mmol), and I2 (1.5 mmol) in DMF (3.0 mL) at 150 °C for 36 h in a sealed tube in an air atmosphere. b Isolated yields.
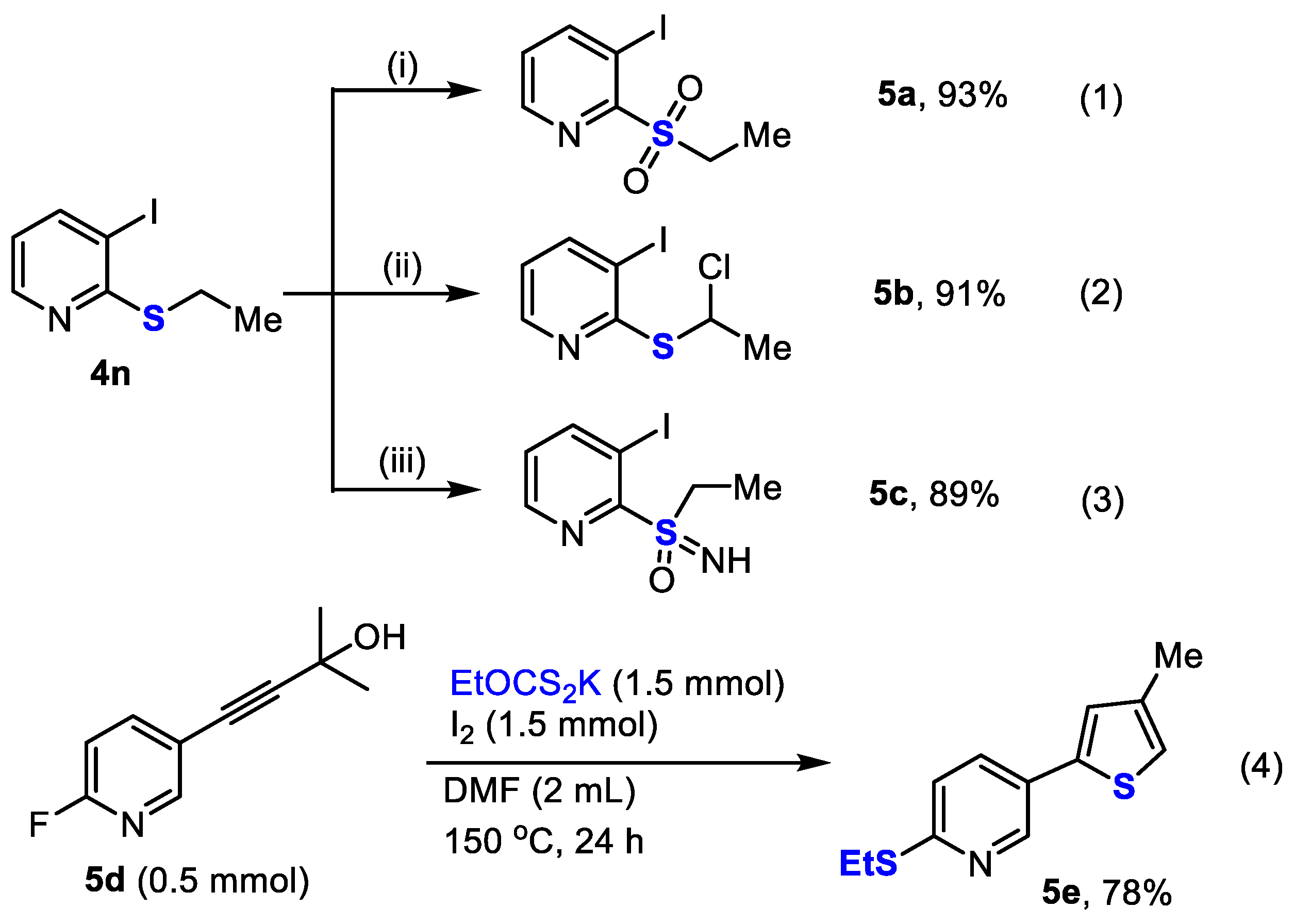
To explore the synthetic applicability of the sulfuration reaction, the newly formed thioethers were utilized in various synthetic transformations (
Scheme 5). First, 2-(ethylsulfonyl)-3-iodopyridine (
5a) may be generated in 93% yield
via m-chloroperoxybenzoic acid (
m-CPBA) oxidation (Eq. 1). Furthermore,
4n may be smoothly converted
via NCS-promoted chlorination to 2-((1-chloroethyl)thio)-3-iodopyridine (
5b) in 91% yield (Eq. 2) [
42]. Remarkably, the thioether
4n reacts successfully with (diacetoxyiodo)benzene (PIDA) and (NH
4)
2CO
3 to produce sulfoximine
5c, which has gained considerable attention owing to its unique structure and applications in medicinal chemistry, in 89% yield (Eq. 3) [
43]. Finally, in the presence of I
2 and
2a, a novel, efficient protocol affords the substituted thiophene
5e in 78% yield
via the sulfidation and sulfur cyclization of 4-(6-fluoropyridin-3-yl)-2-methylbut-3-yn-2-ol
5d with
2a (Eq. 4) [
44].
To enhance our understanding of the reaction mechanism, we designed control experiments, as shown in
Scheme 6. First, the reaction of phenylmethanethiol with
2a furnishes thioether
3ag in trace amounts and 1,2-dibenzyldisulfane
5f in 87% yield, suggesting that thiol alone cannot undergo the sulfation reaction with
2a (
Scheme 6a). When benzyl chloride and
p-tolylmethanethiol are mixed as substrates, the results of gas chromatography–mass spectrometry reveal that the thioether products
3ag and
3b are obtained in yields of 36% and 33%, respectively. This suggests that xanthate
5g generated
via the nucleophilic substitution of benzyl chloride with
2a may be the reaction intermediate (
Scheme 6b–d). However, when directly using
S-benzyl
O-ethyl carbonodithioate
5g to complete the reaction in the absence of
2a, the expected thioether
3ae is not produced, and a small amount of the 1,2-dibenzyldisulfane
5f is generated instead (
Scheme 6c). Unexpectedly, when
5g and
nBuOCS
2K (
nBu =
n-butyl) are used concurrently, sulfation proceeds smoothly to produce a mixture of thioethers
3ag and
3ah, indicating that ROCS
2K is indispensable in the reaction (
Scheme 6d).
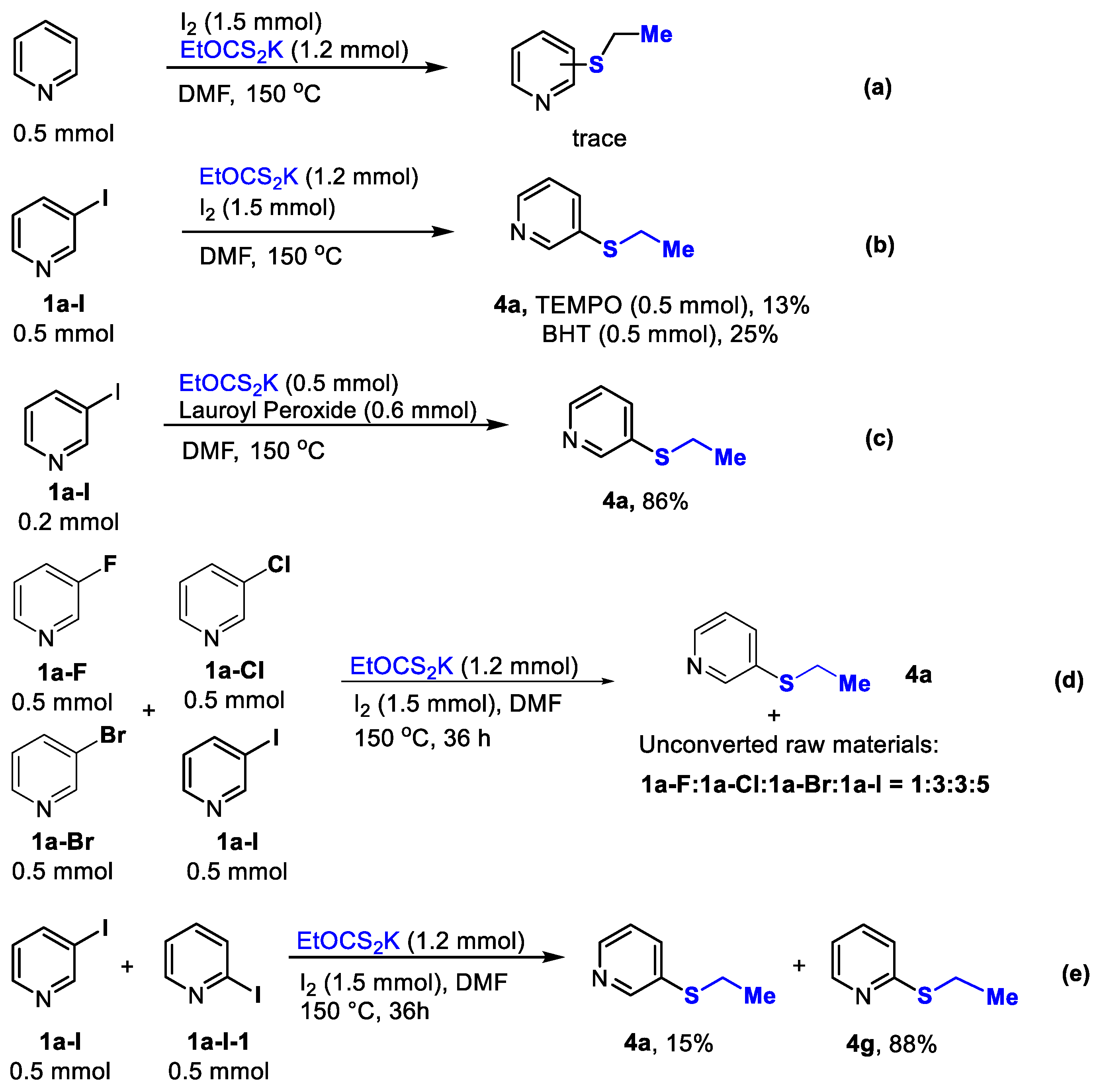
The reaction mechanism of the synthesis of pyridine thioether was then explored. First, pyridine molecules without halogen substituents do not undergo sulfation under the standard reaction conditions, indicating the necessity of halogen substituents or suitable leaving groups (
Scheme 7a). When 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) or butylated hydroxytoluene (BHT) is added as a radical scavenger, the sulfuration of 3-iodopyridine is heavily inhibited (
Scheme 7b), and thus, the sulfuration reaction may proceed
via a radical pathway. When radical initiator lauroyl peroxide was used instead of I
2, we were pleased to find that the sulfation reaction could still be completed and give
4a in 86% yields (
Scheme 7c). When using 3-fluoropyridine, 3-chloropyridine, 3-bromopyridine, and 3-iodopyridine mixed with
2a (1.2 mmol), the reactivities of the halogens follow the order F > Cl ≈ Br > I (
Scheme 7d) [
45]. When 3-iodopyridine and 2-iodopyridine are involved in the reaction, the halogen at the 2-position of pyridine exhibits a higher reactivity (
Scheme 7e).
Based on these results, a plausible mechanism for the sulfuration reaction is proposed, as shown in
Scheme 8. Initially, I
2 liberates an iodine radical (I•), which activates
2a to afford EtOCSS•, with the concomitant release of electrons [
46,
47]. The addition of the EtOCSS• radical to 3-iodopyridine then produces radical cation
A [48,49], which then releases iodine radicals to generate the intermediate xanthate
C. Subsequently, xanthate
C undergoes a hydrolysis reaction to produce pyridine-3-thiolate
D. Moreover, xanthate
C releases
O-ethyl ethoxycarbothioylsulfanylmethanethioate
E, which decomposes to generate ethyl(thioxomethylidene)oxonium
F and xanthate anions. Finally, the nucleophilic substitution reaction of the pyridine-3-thiolate
D with oxonium
F furnishes 3-(ethylthio)pyridine
4a and releases COS. Alternative, the intermediate xanthate
C may be formed through a further single-electron oxidation of intermediate
A by DMSO or O
2 to afford the intermediate xanthate carbocation
B and then releases iodine positive ions [
50,
51].
As shown in
Scheme 8b, the sulfidation reaction of benzyl halides with
2a proceeds
via a similar process. The difference is that benzyl halides reacts more easily with
2a via nucleophilic substitution to afford a similar intermediate, i.e., xanthate
G, without free radical process. The reaction under a nucleophilic attack of EtOCS
2K on the thiocarbonyl group form xanthate intermediate
G and subsequent undergoes an intramolecular elimination reaction formed intermediate thiol anion
I and
O-ethyl ethoxycarbothioylsulfanylmethanethioate
E. Finally, the nucleophilic substitution reaction of the thiol anion
I with oxonium
F furnishes dialkyl thioether
3 and releases COS.
General Procedures for the Preparation of Compounds 3a–3af.
A mixture of 4-(chloromethyl)-1,1'-biphenyl 1a (101 mg, 0.5 mmol), EtOCS2K (160 mg, 1.0 mmol) and DMSO (1 mL) was added successively in a 15 mL Schlenk tube. The Schlenk tube was then immersed in an oil bath at 100 ℃ in a sealed tube in an air atmosphere stirring for 1 h. After cooling down to room temperature, the solution was filtered through a small amount of silica gel. Then the residue was concentrated in vacuo and the crude was purified by flash chromatography with n-hexane/ethyl acetate (50/1, v/v).
([1,1'-biphenyl]-4-ylmethyl)(ethyl)sulfane (3a)
Yellow liquid (106 mg, 93% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.63 – 7.55 (m, 4H), 7.49 – 7.39 (m, 4H), 7.39 – 7.33 (m, 1H), 3.79 (s, 2H), 2.51 (d, J = 7.4 Hz, 2H), 1.29 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 140.8, 139.8, 137.7, 129.2 (2C), 128.7 (2C), 127.2, 127.2 (2C), 127.0 (2C), 35.5, 25.2, 14.4; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C15H17S+, 229.1045; found, 229.1042.
Ethyl(4-methylbenzyl)sulfane (3b)
Yellow liquid (76 mg, 92% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.25 – 7.19 (m, 2H), 7.15 – 7.10 (m, 2H), 3.87 (s, 2H), 2.48 (q, J = 7.3 Hz, 2H), 2.34 (s, 3H), 1.24 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 137.1, 134.4, 129.2 (2C), 129.1 (2C), 43.5, 32.4, 21.1, 14.3; HRMS (ESI-TOF) (m/z): [M+K]+ calcd for C10H14KS+, 205.0448; found, 205.0445.
(4-(tert-butyl)benzyl)(ethyl)sulfane (3c)
Yellow liquid (99 mg, 95% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.37 – 7.32 (m, 2H), 7.28 – 7.23 (m, 2H), 3.71 (s, 2H), 2.47 (q, J = 7.4 Hz, 2H), 1.33 (s, 9H), 1.26 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 149.7, 135.5, 128.4 (2C), 125.3 (2C), 35.4, 34.4, 31.3, 25.3, 14.3; HRMS (ESI-TOF) (m/z): [M+K]+ calcd for C13H20KS+, 247.0917; found, 247.0914.
Ethyl(2,4,6-trimethylbenzyl)sulfane (3d)
Yellow liquid (73 mg, 75% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 6.85 (s, 2H), 3.78 (s, 2H), 2.61 (q, J = 7.4 Hz, 2H), 2.40 (s, 6H), 2.27 (s, 3H), 1.33 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 136.8 (2C), 136.3, 131.4, 129.0 (2C), 30.5, 26.8, 20.9, 19.6 (2C), 14.8; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C12H19S+, 195.1202; found, 195.1207.
Ethyl(3-methoxybenzyl)sulfane (3e)
Yellow liquid (73 mg, 80% yield); Rf = 0.4 (Hexane/EtOAc = 10:1); 1H NMR (400 MHz, CDCl3) δ 7.22 (t, J = 7.8 Hz, 1H), 6.94 – 6.85 (m, 2H), 6.82 – 6.76 (m, 1H), 3.81 (s, 3H), 3.70 (s, 2H), 2.45 (q, J = 7.4 Hz, 2H), 1.24 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 159.7, 140.2, 129.4, 121.2, 114.3, 112.4, 55.2, 35.9, 25.3, 14.3; HRMS (ESI-TOF) (m/z): [M+K]+ calcd for C10H14KOS+, 221.0397; found, 221.0395.
(4-(benzyloxy)benzyl)(ethyl)sulfane (3f)
Yellow solid (120 mg, 93% yield), MP: 61-62 oC; Rf = 0.4 (Hexane/EtOAc = 20:1); 1H NMR (400 MHz, CDCl3) δ 7.46 – 7.37 (m, 4H), 7.36 – 7.30 (m, 1H), 7.26 – 7.21 (m, 2H), 6.95 – 6.90 (m, 2H), 5.06 (s, 2H), 3.69 (s, 2H), 2.44 (q, J = 7.2 Hz, 2H), 1.24 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.7, 137.0, 130.9, 129.8 (2C), 128.6 (2C), 127.9, 127.4 (2C), 114.8 (2C), 79.7 – 74.5 (m), 70.0, 35.2, 25.1, 14.4; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C16H19OS+, 259.1151; found, 259.1149.
Ethyl(4-(trifluoromethoxy)benzyl)sulfane (3g)
Yellow liquid (111 mg, 94% yield); Rf = 0.5 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.34 (d, J = 8.3 Hz, 2H), 7.15 (d, J = 8.2 Hz, 2H), 3.71 (s, 2H), 2.44 (q, J = 7.4 Hz, 2H), 1.23 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 148.1 (q, J = 1.8 Hz), 137.4, 130.1 (2C), 121.0 (2C), 120.5 (q, J = 204.2 Hz), 35.1, 25.3, 14.3; HRMS (ESI-TOF) (m/z): [M+Na]+ calcd for C10H11F3NaOS+, 259.0375; found, 259.0371.
Ethyl(4-((trifluoromethyl)thio)benzyl)sulfane (3h)
Yellow liquid (166 mg, 92% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.63 – 7.56 (m, 2H), 7.40 – 7.34 (m, 2H), 3.73 (s, 2H), 2.44 (q, J = 7.4 Hz, 2H), 1.23 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 142.0, 136.4 (2C), 129.9 (2C), 129.5 (q, J = 306.1 Hz), 122.6 (q, J = 2.3 Hz), 35.4, 25.4, 14.3; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C10H12F3S2+, 253.0327; found, 253.0320.
Ethyl(4-(phenylthio)benzyl)sulfane (3i)
Yellow liquid (121 mg, 93% yield); Rf = 0.5 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.37 – 7.21 (m, 9H), 3.70 (s, 2H), 2.45 (q, J = 7.4 Hz, 2H), 1.24 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 137.7, 135.8, 134.0, 131.2 (4C), 130.8 (4C), 129.6 (4C), 129.1 (4C), 126.9, 35.4, 25.3, 14.3; HRMS (ESI-TOF) (m/z): [M+Na]+ calcd for C15H16NaS2+, 283.0586; found, 283.0593.
(4-((ethylthio)methyl)phenyl)methanol (3j)
Yellow liquid (82 mg, 90% yield); Rf = 0.5 (Hexane/EtOAc = 2:1); 1H NMR (400 MHz, CDCl3) δ 7.33 – 7.27 (m, 4H), 4.65 (s, 2H), 3.71 (s, 2H), 2.42 (q, J = 7.3 Hz, 2H), 1.91 (s, 1H), 1.22 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 139.5, 138.0, 128.9 (2C), 127.1 (2C), 65.0, 35.5, 25.2, 14.3; HRMS (ESI-TOF) (m/z): [M+K]+ calcd for C10H14KOS+, 221.0397; found, 221.0395.
2-(4-((ethylthio)methyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3k)
Yellow liquid (127 mg, 91% yield); Rf = 0.5 (Hexane/EtOAc = 20:1); 1H NMR (400 MHz, CDCl3) δ 7.78 – 7.73 (m, 2H), 7.36 – 7.30 (m, 2H), 3.72 (s, 2H), 2.41 (q, J = 7.4 Hz, 2H), 1.34 (s, 12H), 1.21 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 141.9, 141.8, 134.9 (2C), 128.2 (2C), 83.7 (2C), 35.9, 25.1, 24.8 (4C), 14.3; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C15H24BO2S+, 278.1621; found, 278.1624.
Ethyl(4-fluorobenzyl)sulfane (3l)
Yellow liquid (81 mg, 95% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.31 – 7.25 (m, 2H), 7.03 – 6.95 (m, 2H), 3.69 (s, 2H), 2.43 (q, J = 7.3 Hz, 2H), 1.23 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 161.8 (d, J = 245.2 Hz), 134.3 (d, J = 3.0 Hz), 130.3 (d, J = 8.0 Hz, 2C), 115.3 (d, J = 21.4 Hz, 2C), 35.1, 25.2, 14.3; HRMS (ESI-TOF) (m/z): [M+Na]+ calcd for C9H11FNaS+, 193.0458; found, 193.0466.
(4-chlorobenzyl)(ethyl)sulfane (3m)
Yellow liquid (84 mg, 90% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.30 – 7.26 (m, 2H), 7.24 (d, J = 8.7 Hz, 2H), 3.68 (s, 2H), 2.42 (q, J = 7.4 Hz, 2H), 1.22 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 137.1, 132.6, 130.1 (2C), 128.6 (2C), 35.2, 25.2, 14.3; HRMS (ESI-TOF) (m/z): [M+Na]+ calcd for C9H11ClNaS+, 209.0162; found, 209.0152.
(4-bromobenzyl)(ethyl)sulfane (3n)
Yellow liquid (106 mg, 92% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.47 – 7.39 (m, 2H), 7.22 – 7.16 (m, 2H), 3.66 (s, 2H), 2.42 (q, J = 7.4 Hz, 2H), 1.22 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 137.7, 131.5 (2C), 130.5 (2C), 120.6, 35.2, 25.2, 14.3; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C9H12BrS+, 230.9838; found, 230.9828.
Ethyl(4-Iodobenzyl)sulfane (3o)
Yellow liquid (132 mg, 95% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.67 – 7.58 (m, 2H), 7.10 – 7.03 (m, 2H), 3.65 (s, 2H), 2.41 (q, J = 7.4 Hz, 2H), 1.22 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 138.3, 137.5 (2C), 130.8 (2C), 92.1, 35.3, 25.2, 14.3; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C9H12IS+, 278.9699; found, 278.9693.
Ethyl(4-(trifluoromethyl)benzyl)sulfane (3p)
Yellow liquid (103 mg, 94% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J = 8.0 Hz, 2H), 7.43 (d, J = 8.0 Hz, 2H), 3.75 (s, 2H), 2.43 (q, J = 7.4 Hz, 2H), 1.24 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 142.9, 129.1 (2C), 129.1 (q, J = 31.7 Hz), 125.4 (q, J = 3.8 Hz, 2C), 124.2 (q, J = 269.9 Hz), 35.4, 25.3, 14.2; HRMS (ESI-TOF) (m/z): [M+Na]+ calcd for C10H11F3NaOS+, 259.0375; found, 259.0371.
Ethyl(4-(methylsulfonyl)benzyl)sulfane (3q)
Yellow liquid (105 mg, 91% yield); Rf = 0.4 (Hexane/EtOAc = 1:1); 1H NMR (400 MHz, CDCl3) δ 7.91 – 7.82 (m, 2H), 7.54 – 7.46 (m, 2H), 3.75 (s, 2H), 3.03 (s, 3H), 2.42 (q, J = 7.4 Hz, 2H), 1.21 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 145.3, 138.9, 129.6 (2C), 127.5 (2C), 44.4, 35.4, 25.4, 14.2; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C10H15O2S2+, 231.0508; found, 231.0503.
4-((ethylthio)methyl)-N-phenylbenzamide (3r)
White solid (126 mg, 93% yield), MP: 118-120 oC; Rf = 0.4 (Hexane/EtOAc = 3:1); 1H NMR (400 MHz, CDCl3) δ 8.08 (s, 1H), 7.83 – 7.76 (m, 2H), 7.66 – 7.61 (m, 2H), 7.40 – 7.30 (m, 4H), 7.16 – 7.10 (m, 1H), 3.74 (s, 2H), 2.50 – 2.36 (m, 2H), 1.23 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 165.6, 142.8, 137.9, 133.5, 129.1 (2C), 129.0 (2C), 127.3 (2C), 124.5, 120.3 (2C), 35.5, 25.3, 14.3; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C16H18NOS+, 272.1104; found, 272.1098.
Ethyl(naphthalen-1-ylmethyl)sulfane (3s)
Yellow liquid (94 mg, 93% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 8.16 (d, J = 8.3 Hz, 1H), 7.89 – 7.84 (m, 1H), 7.81 – 7.73 (m, 1H), 7.59 – 7.53 (m, 1H), 7.52 – 7.47 (m, 1H), 7.44 – 7.35 (m, 2H), 4.19 (s, 2H), 2.51 (q, J = 7.3 Hz, 2H), 1.28 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 134.1, 133.9, 131.4, 128.8, 128.0, 126.9, 126.1, 125.8, 125.1, 124.1, 33.7, 26.0, 14.4; HRMS (ESI-TOF) (m/z): [M]+ calcd for C13H14S+, 202.0811; found, 202.0816.
Ethyl(naphthalen-2-ylmethyl)sulfane (3t)
Yellow liquid (96 mg, 95% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.87 – 7.79 (m, 3H), 7.72 (s, 1H), 7.55 – 7.44 (m, 3H), 3.90 (s, 2H), 2.46 (q, J = 7.4 Hz, 2H), 1.26 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 135.9, 133.2, 132.5, 128.3, 127.6, 127.6, 127.1, 126.1, 125.6, 36.1, 25.1, 14.3; HRMS (ESI-TOF) (m/z): [M]+ calcd for C13H14S+, 202.0811; found, 202.0818.
(Anthracen-9-ylmethyl)(ethyl)sulfane (3u)
Yellow solid (53 mg, 42% yield), MP: 68-70 oC; Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 8.39 (s, 1H), 8.35 (d, J = 8.9 Hz, 2H), 8.01 (d, J = 8.4 Hz, 2H), 7.61 – 7.53 (m, 2H), 7.52 – 7.44 (m, 2H), 4.75 (s, 2H), 2.70 (q, J = 7.4 Hz, 2H), 1.37 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 131.5 (2C), 129.9 (2C), 129.5, 129.2 (2C), 127.2, 126.0 (2C), 125.0 (2C), 124.2 (2C), 28.7, 27.1, 14.8; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C17H17S+, 253.1045; found, 253.1045.
2-chloro-5-((ethylthio)methyl)thiophene (3v)
Yellow liquid (89 mg, 93% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 6.73 – 6.66 (m, 2H), 3.82 (s, 2H), 2.51 (q, J = 7.4 Hz, 2H), 1.24 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 141.4, 128.8, 125.5, 125.0, 30.4, 25.4, 14.2; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C7H10ClS2+, 192.9907; found, 192.9902.
5-chloro-3-((ethylthio)methyl)benzo[b]thiophene (3w)
Yellow liquid (111 mg, 92% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.88 – 7.84 (m, 1H), 7.74 (d, J = 8.6 Hz, 1H), 7.35 – 7.29 (m, 2H), 3.92 (s, 3H), 2.48 (q, J = 7.4 Hz, 2H), 1.26 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 139.1, 138.7, 131.9, 130.4, 125.4, 124.9, 123.8, 121.9, 28.9, 25.7, 14.2; HRMS (ESI-TOF) (m/z): [M+Na]+ calcd for C11H11ClNaS2+, 264.9883; found, 264.9884.
8-((ethylthio)methyl)quinoline (3x)
Yellow liquid (92 mg, 91% yield); Rf = 0.4 (Hexane/EtOAc = 10:1); 1H NMR (400 MHz, CDCl3) δ 8.97 – 8.93 (m, 1H), 8.16 – 8.09 (m, 1H), 7.71 (d, J = 7.7 Hz, 2H), 7.52 – 7.45 (m, 1H), 7.42 – 7.37 (m, 1H), 4.44 (s, 2H), 2.56 (q, J = 7.4 Hz, 2H), 1.28 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 149.6, 146.3, 137.3, 136.3, 129.3, 128.5, 127.0, 126.1, 121.1, 31.1, 26.1, 14.5; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C12H14NS+, 204.0842; found, 204.0843.
2-((ethylthio)methyl)-4-methylquinazoline (3y)
Yellow solid (95 mg, 87% yield), MP: 52-54 oC; Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 8.03 – 7.98 (m, 1H), 7.95 – 7.89 (m, 1H), 7.82 – 7.76 (m, 1H), 7.56 – 7.50 (m, 1H), 4.01 (s, 2H), 2.89 (s, 3H), 2.62 (q, J = 7.4 Hz, 2H), 1.24 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 168.8, 163.6, 149.6, 133.5, 128.5, 126.9, 124.8, 122.5, 39.1, 25.9, 21.7, 14.4; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C12H15N2S+, 219.0950; found, 219.0942.
1-(4-((ethylthio)methyl)phenyl)-1H-pyrazole (3z)
Yellow liquid (93 mg, 85% yield); Rf = 0.5 (Hexane/EtOAc = 10:1); 1H NMR (400 MHz, CDCl3) δ 7.95 – 7.88 (m, 1H), 7.74 – 7.70 (m, 1H), 7.64 (d, J = 8.3 Hz, 2H), 7.45 – 7.37 (m, 2H), 3.75 (s, 2H), 2.45 (q, J = 7.3, 6.8 Hz, 2H), 1.24 (t, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 141.0, 139.0, 136.9, 129.8 (2C), 126.7, 119.3 (2C), 107.6, 35.3, 25.2, 14.4; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C12H15N2S+, 219.0950; found, 219.0946.
1-cyclohexyl-5-(4-(ethylthio)butyl)-1H-tetrazole (3aa)
Yellow liquid (123 mg, 92% yield); Rf = 0.4 (Hexane/EtOAc = 2:1); 1H NMR (400 MHz, CDCl3) δ 4.21 – 4.04 (m, 1H), 2.85 (t, 2H), 2.61 – 2.48 (m, 3H), 2.09 – 1.87 (m, 8H), 1.82 – 1.66 (m, 4H), 1.48 – 1.30 (m, 3H), 1.24 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 153.5, 57.6, 32.9 (2C), 30.9, 28.6, 26.2, 25.9, 25.3 (2C), 24.8, 22.9, 14.8; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C13H25N4S+, 269.1794; found, 269.1790.
1,4-bis((ethylthio)methyl)benzene (3ab)
Yellow liquid (99 mg, 88% yield); Rf = 0.5 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.25 (s, 4H), 3.70 (s, 4H), 2.43 (q, J = 7.4 Hz, 4H), 1.23 (t, J = 7.4 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 137.2 (2C), 128.9 (4C), 35.5, 25.2, 14.4; HRMS (ESI-TOF) (m/z): [M]+ calcd for C12H18S2+, 226.0845; found, 226.0836.
(((1s,3s)-adamantan-1-yl)methyl)(ethyl)sulfane (3ac)
Yellow liquid (42 mg, 40% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 2.51 (q, J = 7.4 Hz, 2H), 2.32 (s, 2H), 1.97 (s, 3H), 1.73 – 1.66 (m, 3H), 1.65 – 1.59 (m, 3H), 1.58 – 1.54 (m, 6H), 1.24 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 47.3, 41.9 (3C), 36.9 (3C), 33.9, 28.6 (3C), 28.2, 15.0; HRMS (ESI-TOF) (m/z): [M+K]+ calcd for C13H22KS+, 249.1074; found, 249.1075.
Benzhydryl(ethyl)sulfane (3ad)
Yellow liquid (80 mg, 70% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.50 – 7.44 (m, 4H), 7.38 – 7.31 (m, 4H), 7.30 – 7.21 (m, 2H), 5.22 (s, 1H), 2.44 (q, J = 7.4 Hz, 2H), 1.25 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 141.5 (2C), 128.5 (4C), 128.2 (4C), 127.0 (2C), 53.7, 26.2, 14.2; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C15H17S+, 229.1045; found, 229.1046.
Dibenzylsulfane (
3ae)[
52]
White solid (100 mg, 93% yield), MP: 61-62 oC; Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.36 – 7.22 (m, 10H), 3.61 (s, 4H); 13C NMR (100 MHz, CDCl3) δ 138.1 (2C), 129.0 (4C), 128.5 (4C), 127.0 (2C), 35.6 (2C).
benzyl(phenyl)sulfane(
3af) [
53]
White solid (90 mg, 90% yield), MP: 39-40 oC; Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.34 – 7.22 (m, 9H), 7.21 – 7.15 (m, 1H), 4.13 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 137.4, 136.3, 129.8 (2C), 128.8 (2C), 128.8 (2C), 128.5 (2C), 127.2, 126.3, 39.0.
Benzyl(ethyl)sulfane (3ag)
Yellow liquid (27 mg, 36% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (500 MHz, CDCl3) δ 7.35 – 7.28 (m, 4H), 7.26 – 7.21 (m, 1H), 3.73 (s, 2H), 2.44 (q, J = 7.4 Hz, 2H), 1.23 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 138.6, 128.8 (2C), 128.4 (2C), 126.8, 35.8, 25.2, 14.3; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C9H13S+, 153.0732; found, 153.0735.
Benzyl(butyl)sulfane (3ah)
Yellow liquid (33 mg, 37% yield); Rf = 0.6 (Hexane/EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.35 – 7.25 (m, 4H), 7.28 – 7.19 (m, 1H), 3.71 (s, 2H), 2.49 – 2.35 (m, 2H), 1.60 – 1.49 (m, 2H), 1.38 (dt, J = 8.1, 7.0 Hz, 2H), 0.89 (t, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3) 138.7, 128.8 (2C), 128.4 (2C), 126.8, 36.2, 31.3, 31.0, 22.0, 13.7; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C11H17S+, 181.1045; found, 181.1050.
General Procedures for the Preparation of Compounds 4a–4s.
A mixture of 3-Iodine pyridine (103 mg, 0.5 mmol), EtOCS2K (192 mg, 1.2 mmol), I2 (381 mg, 1.5 mmol), and DMF (3 mL) was added successively in a 15 mL Schlenk tube. The Schlenk tube was then immersed in an oil bath at 150 ℃ in a sealed tube in an air atmosphere stirring for 36 h. After cooling down to room temperature, the solution was filtered through a small amount of silica gel. Then the residue was concentrated in vacuo and the crude was purified by flash chromatography with n-hexane/ethyl acetate (3/1, v/v).
3-(Ethylthio)pyridine (
4a) [
54]
Yellow liquid (64 mg, 92 % yield); Rf = 0.5 (Hexane/EtOAc = 3:1); 1H NMR (500 MHz, CDCl3) δ 8.56 (s, 1H), 8.41 (d, J = 4.8 Hz, 1H), 7.66 (ddd, J = 8.0, 2.4, 1.5 Hz, 1H), 7.23 (dd, J = 8.0, 4.8 Hz, 1H), 2.96 (q, J = 7.4 Hz, 2H), 1.32 (t, J = 7.4 Hz, 3H);13C NMR (125 MHz, CDCl3) δ 149.6, 146.5, 137.1, 134.0, 123.7, 27.7, 14.3.
3-(Propylthio)pyridine (
4b) [
55]
Yellow liquid (58 mg, 75%); Rf = 0.5 (Hexane/EtOAc = 3:1); 1H NMR (500 MHz, CDCl3) δ 8.56 (d, J = 1.8 Hz, 1H), 8.48 – 8.35 (m, 1H), 7.69 – 7.60 (m, 1H), 7.21 (dd, J = 7.9, 4.8 Hz, 1H), 2.96 – 2.74 (m, 2H), 1.67 (h, J = 7.3 Hz, 2H), 1.03 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 149.9, 146.7, 136.8, 134.1, 123.6, 35.7, 22.4, 13.3.
3-(Butylthio)pyridine (
4c) [
56]
Yellow liquid (62 mg, 74%); Rf = 0.5 (Hexane/EtOAc = 3:1); 1H NMR (500 MHz, CDCl3) δ 8.55 (s, 1H), 8.41 (d, J = 4.2 Hz, 1H), 7.66 (dt, J = 8.0, 1.8 Hz, 1H), 7.24 (dd, J = 7.9, 4.8 Hz, 1H), 2.97 – 2.90 (m, 2H), 1.63 (p, J = 7.4 Hz, 2H), 1.45 (dq, J = 14.6, 7.3 Hz, 2H), 0.92 (t, J = 7.3 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 149.2, 146.2, 137.0, 134.6, 123.7, 33.3, 31.1, 21.8, 13.6.
3-(Pentylthio)pyridine (4d)
Yellow liquid (67 mg, 74%); Rf = 0.5 (Hexane/EtOAc = 3:1); 1H NMR (500 MHz, CDCl3) δ 8.55 (s, 1H), 8.41 (s, 1H), 7.66 (d, J = 7.9 Hz, 1H), 7.24 – 7.21 (m, 1H), 2.92 (t, J = 7.4 Hz, 2H), 1.64 (p, J = 7.4 Hz, 2H), 1.40 (dt, J = 14.3, 6.9 Hz, 2H), 1.32 (dq, J = 14.3, 6.9 Hz, 2H), 0.89 (t, J = 7.2 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 149.4, 146.4, 136.9, 134.4, 123.6, 33.6, 30.8, 28.7, 22.2, 13.9. HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C10H16NS+, 182.0998; found, 182.0995.
3-(Ethylthio)quinoline (
4e) [
57]
Yellow liquid (89 mg, 94 % yield); Rf = 0.4 (Hexane/EtOAc = 5:1); 1H NMR (500 MHz, CDCl3) δ 8.84 (d, J = 2.3 Hz, 1H), 8.10 (d, J = 8.4 Hz, 1H), 8.07 (d, J = 2.3 Hz, 1H), 7.75 (dd, J = 8.2, 1.4 Hz, 1H), 7.68 (ddd, J = 8.4, 6.9, 1.4 Hz, 1H), 7.56 (ddd, J = 8.2, 6.9, 1.2 Hz, 1H), 3.06 (q, J = 7.4 Hz, 2H), 1.37 (t, J = 7.4 Hz, 3H);13C NMR (125 MHz, CDCl3) δ 151.6, 146.2, 134.8, 130.6, 129.3, 129.0, 128.2, 127.2, 126.9, 27.9, 14.3.
4-(Ethylthio)isoquinoline (
4f) [
57]
Yellow liquid (80 mg, 85 % yield); Rf = 0.4 (Hexane/EtOAc = 5:1); 1H NMR (500 MHz, CDCl3) δ 9.12 (s, 1H), 8.55 (s, 1H), 8.31 (d, J = 8.4 Hz, 1H), 7.98 (d, J = 8.1 Hz, 1H), 7.77 (ddd, J = 8.3, 6.8, 1.3 Hz, 1H), 7.64 (ddd, J = 8.1, 6.8, 1.1 Hz, 1H), 3.01 (q, J = 7.3 Hz, 2H), 1.32 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 151.0, 143.7, 135.9, 130.9, 128.7, 128.4, 128.2, 127.67, 124.1, 28.3, 14.5.
2-(Ethylthio)pyridine (
4g) [
58]
Yellow liquid (63 mg, 90% yield); Rf = 0.4 (Hexane/EtOAc = 5:1); 1H NMR (500 MHz, CDCl3) δ 8.41 (ddd, J = 5.0, 1.9, 1.0 Hz, 1H), 7.50 – 7.41 (m, 1H), 7.15 (dt, J = 8.1, 1.1 Hz, 1H), 6.95 (ddd, J = 7.3, 4.9, 1.1 Hz, 1H), 3.16 (q, J = 7.4 Hz, 2H), 1.36 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 159.3, 149.3, 135.8, 122.1, 119.2, 24.4, 14.5.
2-(Ethylthio)-3-methylpyridine (4h)
Yellow liquid (71 mg, 93% yield); Rf = 0.4 (Hexane/EtOAc = 5:1); 1H NMR (500 MHz, CDCl3) δ 8.29 (dd, J = 4.9, 1.7 Hz, 1H), 7.30 (ddd, J = 7.4, 1.8, 0.9 Hz, 1H), 6.90 (dd, J = 7.4, 4.9 Hz, 1H), 3.22 (q, J = 7.4 Hz, 2H), 2.24 (s, 3H), 1.38 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 158.2, 146.4, 136.2, 130.9, 118.8, 24.0, 18.6, 14.6. HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C8H12NS+, 154.0685; found, 154.0685.
4-2-(Ethylthio)-4-methylpyridine (4i)
Yellow liquid (69 mg, 90% yield); Rf = 0.4 (Hexane/EtOAc = 5:1); 1H NMR (500 MHz, CDCl3) δ 8.28 (d, J = 5.1 Hz, 1H), 6.99 (s, 1H), 6.79 (dd, J = 5.2, 1.5 Hz, 1H), 3.15 (q, J = 7.4 Hz, 2H), 2.26 (s, 3H), 1.36 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 158.9, 148.9, 147.2, 122.7, 120.7, 24.4, 20.8, 14.6. HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C8H12NS+, 154.0685; found, 154.0685.
6-(Ethylthio)pyridin-3-amine (
4j) [
59]
Brown liquid (72 mg, 94% yield); Rf = 0.4 (Hexane/EtOAc = 3:1); 1H NMR (500 MHz, CDCl3) δ 8.02 (d, J = 2.9 Hz, 1H), 7.04 (dd, J = 8.4, 0.7 Hz, 1H), 6.91 (dd, J = 8.4, 2.9 Hz, 1H), 3.51 (s, 2H), 3.07 (q, J = 7.3 Hz, 2H), 1.31 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 146.5, 139.9, 136.9, 123.9, 123.4, 25.9, 14.7.
2-(Ethylthio)pyridin-3-amine (
4k) [
60]
Brown liquid (67 mg, 87% yield); Rf = 0.4 (Hexane/EtOAc = 3:1); 1H NMR (500 MHz, CDCl3) δ 7.97 (dd, J = 4.2, 2.0 Hz, 1H), 6.93 – 6.83 (m, 2H), 3.23 (q, J = 7.4 Hz, 2H), 1.35 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 143.0, 140.9, 139.4, 120.6, 120.4, 25.2, 14.9.
6-(Ethylthio)pyridin-3-ol (4l)
Pale-yellow solid (73 mg, 94% yield); Rf = 0.4 (Hexane/EtOAc = 5:1); 1H NMR (500 MHz, CDCl3) δ 8.12 (dd, J = 2.8, 0.8 Hz, 1H), 7.22 (dd, J = 8.6, 2.8 Hz, 1H), 7.18 (dd, J = 8.7, 0.7 Hz, 1H), 3.02 (q, J = 7.3 Hz, 2H), 1.29 (t, J = 7.3 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 152.0, 148.1, 137.0, 125.8, 125.1, 26.9, 14.5. HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C7H10NOS+, 156.0478; found, 156.0476.
6-(Ethylthio)-4-methylpyridin-3-amine (4m)
Red liquid (80 mg, 95% yield); Rf = 0.5 (Hexane/EtOAc = 2:1); 1H NMR (500 MHz, CDCl3) δ 7.93 (s, 1H), 6.95 (t, J = 0.7 Hz, 1H), 3.07 (q, J = 7.4 Hz, 2H), 2.13 (d, J = 0.8 Hz, 3H), 1.31 (t, J = 7.3 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 146.7, 138.8, 136.5, 132.2, 124.9, 25.8, 16.8, 14.8. HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C8H13N2S+, 169.0794; found, 169.0792.
2-(Ethylthio)-3-phenylpyridine (4n)
Yellow liquid (91 mg, 85% yield); Rf = 0.5 (Hexane/EtOAc = 10:1); 1H NMR (500 MHz, CDCl3) δ 8.46 (dd, J = 4.9, 1.7 Hz, 1H), 7.50 – 7.42 (m, 5H), 7.41 (dd, J = 7.4, 1.8 Hz, 1H), 7.06 (dd, J = 7.5, 4.9 Hz, 1H), 3.19 (q, J = 7.3 Hz, 2H), 1.35 (t, J = 7.4 Hz, 3H);13C NMR (125 MHz, CDCl3) δ 157.5, 147.9, 138.2, 136.3, 136.0, 129.1, 128.3, 128.0, 118.8, 24.6, 14.3. HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C13H14NS+, 216.0841; found, 216.0839.
6-(Ethylthio)-N,N-dimethylpicolinamide (4o)
Yellow liquid (92 mg, 88% yield); Rf = 0.4 (Hexane/EtOAc = 2:1); 1H NMR (500 MHz, CDCl3) δ 7.54 (t, J = 7.8 Hz, 1H), 7.29 (d, J = 7.5 Hz, 1H), 7.16 (d, J = 8.1 Hz, 1H), 3.15 (q, J = 7.4 Hz, 2H), 3.10 (d, J = 17.2 Hz, 6H), 1.34 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 168.5, 158.1, 154.0, 136.6, 122.7, 119.1, 39.0, 35.8, 24.2, 14.6. HRMS (ESI-TOF) (m/z): [M+Na]+ calcd for C10H14N2NaOS+, 233.0719; found, 233.0715.
2-(Ethylthio)-3-iodopyridine (4p)
Brown liquid (119 mg, 90% yield); Rf = 0.4 (Hexane/EtOAc = 5:1); 1H NMR (500 MHz, CDCl3) δ 8.39 (dd, J = 4.7, 1.6 Hz, 1H), 7.90 (dd, J = 7.7, 1.6 Hz, 1H), 6.70 (dd, J = 7.7, 4.7 Hz, 1H), 3.13 (q, J = 7.4 Hz, 2H), 1.37 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 161.8, 148.1, 145.7, 119.9, 93.7, 26.9, 14.0. HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C7H9INS+, 265.9500; found, 265.9504.
6-(Ethylthio)picolinonitrile (4q)
Brown solid (71 mg, 87% yield); MP: 50-52 oC, Rf = 0.4 (Hexane/EtOAc = 3:1); 1H NMR (500 MHz, CDCl3) δ 8.64 (d, J = 2.5 Hz), 7.63 (dd, J = 8.4, 2.2 Hz), 7.21 (dd, J = 8.4, 0.9 Hz), 3.19 (q, J = 7.4 Hz, 2H), 1.37 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 165.6, 152.1, 137.6, 121.7, 117.1, 104.3, 24.5, 14.2. HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C8H9N2S+, 165.0481; found, 165.0481.
3,5-Bis(ethylthio)pyridine (4r)
Brown liquid (40 mg, 40% yield); Rf = 0.4 (Hexane/EtOAc = 5:1); 1H NMR (500 MHz, CDCl3) δ 8.32 (s, 2H), 7.55 (s, 1H), 2.95 (q, J = 7.4 Hz, 4H), 1.31 (t, J = 7.4 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 146.5 (2C), 136.6 (2C), 134.0, 27.6 (2C), 14.2 (2C). HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C9H14NS2+, 200.0562; found, 200.0559.
2-(Ethylthio)-5-iodopyrimidine (4s)
Brown solid (122 mg, 92% yield); MP: 64-65 oC, Rf = 0.5 (Hexane/EtOAc = 3:1); 1H NMR (500 MHz, CDCl3) δ 8.64 (s, 2H), 3.09 (q, J = 7.4 Hz, 2H), 1.36 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.1, 162.2 (2C), 86.2, 25.4, 14.2. HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C6H8IN2S+, 266.9447; found, 266.9450.
4-(Ethylsulfonyl)aniline (5a)
Yellow liquid (138 mg, 93% yield); Rf = 0.5 (Hexane/EtOAc = 3:1); 1H NMR (500 MHz, CDCl3) δ 8.58 (dd, J = 4.6, 1.5 Hz, 1H), 8.40 (dd, J = 8.0, 1.5 Hz, 1H), 7.20 (dd, J = 8.0, 4.5 Hz, 1H), 3.67 (q, J = 7.4 Hz, 2H), 1.46 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 157.3, 150.9, 147.2, 127.3, 86.1, 46.0, 7.2. HRMS (ESI-TOF) (m/z): [M]+ calcd for C7H8INO2S+, 296.9553; found, 296.9551.
2-((1-Chloroethyl)thio)-3-iodopyridine (5b)
Yellow liquid (136 mg, 91% yield); Rf = 0.5 (Hexane/EtOAc = 5:1); 1H NMR (500 MHz, CDCl3) δ 8.50 (dt, J = 4.7, 1.2 Hz, 1H), 7.98 (dt, J = 7.7, 1.2 Hz, 1H), 6.82 (ddd, J = 7.8, 4.7, 0.8 Hz, 1H), 6.19 (q, J = 6.9 Hz, 1H), 2.01 (d, J = 6.9 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 158.8, 148.4, 146.2, 121.1, 93.2, 61.9, 25.7. HRMS (ESI-TOF) (m/z): [M+Na]+ calcd for C7H7ClINNaS+, 321.8925; found, 321.8917.
Ethyl(imino)(3-iodopyridin-2-yl)-λ6-sulfanone (5c)
Yellow liquid (132 mg, 89% yield); Rf = 0.5 (Hexane/EtOAc = 3:1); 1H NMR (500 MHz, CDCl3) δ 8.51 (dd, J = 4.6, 1.5 Hz, 1H), 8.32 (dd, J = 7.9, 1.5 Hz, 1H), 7.11 (dd, J = 7.9, 4.6 Hz, 1H), 3.71 (ddt, J = 70.8, 14.2, 7.2 Hz, 2H), 1.43 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 159.3, 150.4, 147.1, 126.4, 85.1, 46.5, 7.7. HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C7H10IN2OS+, 296.9553; found, 296.9551.
2-(Ethylthio)-5-(4-methylthiophen-2-yl)pyridine (5e)
Yellow liquid (92 mg, 78% yield); Rf = 0.4 (Hexane/EtOAc = 5:1); 1H NMR (500 MHz, CDCl3) δ 8.88 (s, 1H), 8.58 (d, J = 4.5 Hz, 1H), 8.06 (dt, J = 7.9, 2.0 Hz, 1H), 7.39 (dd, J = 7.9, 4.9 Hz, 1H), 7.08 (d, J = 1.2 Hz, 1H), 2.52 (q, J = 7.4 Hz, 3H), 2.36 (d, J = 1.0 Hz, 2H), 1.01 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 149.3, 147.9, 142.2, 141.9, 137.4, 131.1, 128.9, 123.3, 120.7, 29.9, 16.0, 14.5. HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C12H14NS2+, 236.0557; found, 236.0562.
Benzyl(ethyl)sulfane (3ae)
Yellow liquid (27 mg, 36% yield); Rf = 0.6 (Hexane /EtOAc = 50:1); 1H NMR (500 MHz, CDCl3) δ 7.35 – 7.28 (m, 4H), 7.26 – 7.21 (m, 1H), 3.73 (s, 2H), 2.44 (q, J = 7.4 Hz, 2H), 1.23 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 138.6, 128.8 (2C), 128.4 (2C), 126.8, 35.8, 25.2, 14.3; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C9H13S+, 153.0732; found, 153.0735.
Benzyl(butyl)sulfane (3af)
Yellow liquid (33 mg, 37% yield); Rf = 0.6 (Hexane /EtOAc = 50:1); 1H NMR (400 MHz, CDCl3) δ 7.35 – 7.25 (m, 4H), 7.28 – 7.19 (m, 1H), 3.71 (s, 2H), 2.49 – 2.35 (m, 2H), 1.60 – 1.49 (m, 2H), 1.38 (dt, J = 8.1, 7.0 Hz, 2H), 0.89 (t, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3) 138.7, 128.8 (2C), 128.4 (2C), 126.8, 36.2, 31.3, 31.0, 22.0, 13.7; HRMS (ESI-TOF) (m/z): [M+H]+ calcd for C11H17S+, 181.1045; found, 181.1050.
1,2-dibenzyldisulfane (
5f) [
61]
White solid (107 mg, 87% yield), MP: 71-72 oC; Rf = 0.5 (Hexane/EtOAc = 20:1); 1H NMR (400 MHz, CDCl3) δ 7.36 – 7.29 (m, 5H), 7.29 – 7.23 (m, 5H), 3.62 (s, 4H); 13C NMR (100 MHz, CDCl3) δ 137.4 (2C), 129.4 (4C), 128.5 (4C), 127.4 (2C), 43.4 (2C).