Submitted:
11 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
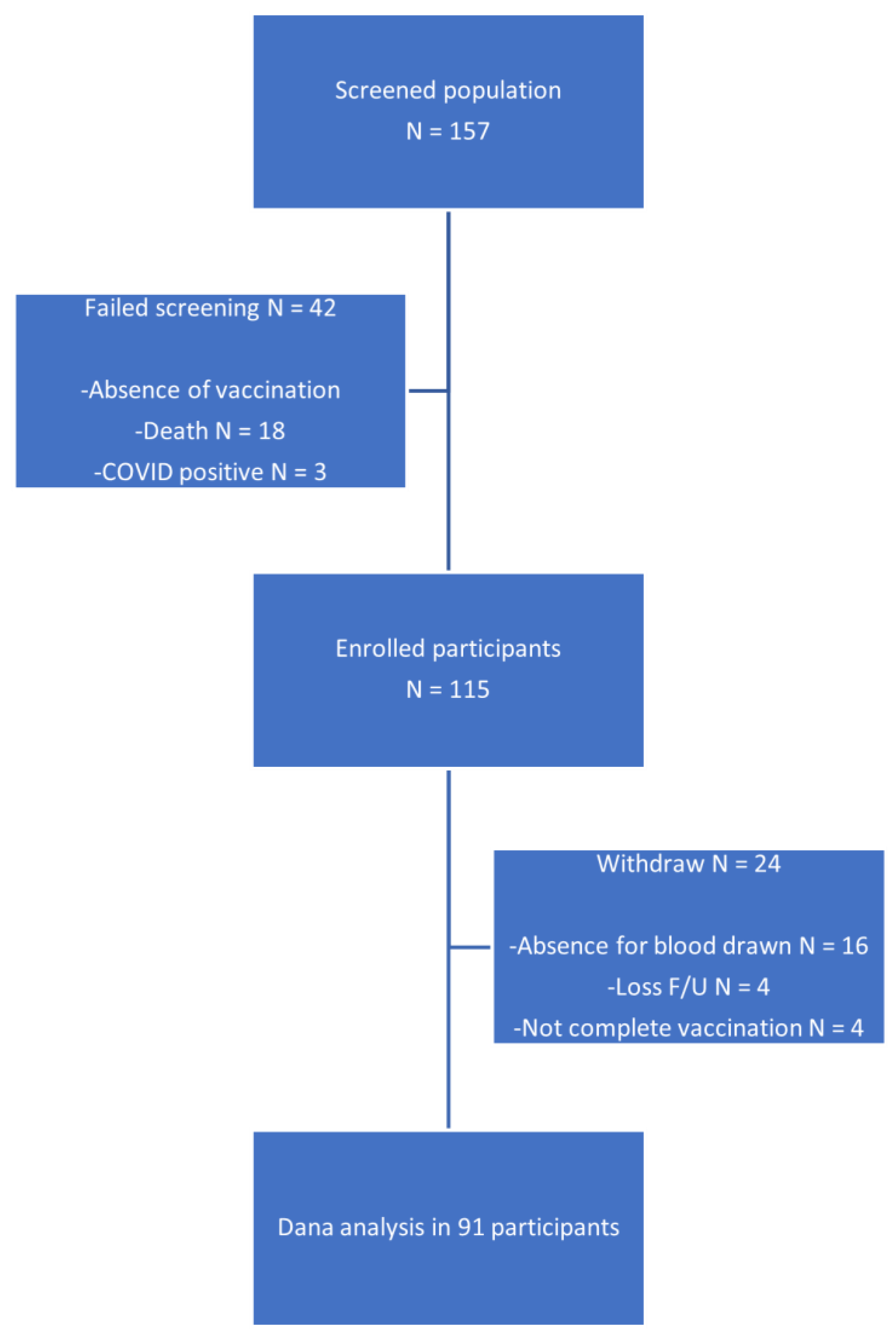
2.1. Study Design and Participants
2.2. Procedure and Materials
2.2.1. Blood and Data Collection
2.2.2. Neutralizing Antibody Assay
2.3. Outcome
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Neutralizing Antibody
3.2.1. Seroconversion Rate
3.2.2. Percent Inhibition
3.3. Factors Associated with Seroconversion
| Factors | Wild Type | Omicron Variant | ||||
|---|---|---|---|---|---|---|
| Seroconversion n (%) | OR (95% CI) |
p-Value | Seroconversion n (%) | OR (95% CI) |
p-Value | |
| Age | ||||||
| <65 yrs | 47 (81.03%) (n=58) |
1.709 (0.63-4.57) | 0.286 | 36 (62.07%) (n=58) |
1.227(0.52-2.88) | 0.638 |
| ≥65 yrs | 25 (71.43%) (n=35) |
ref | - | 20 (57.14%) (n=35) |
ref | - |
| Smoking(yes) | 16 (84.21%) (n=19) |
1.684 (0.44-6.44) | 0.447 | 12 (63.16%) (n=19) |
1.142 (0.40-3.23) | 0.801 |
| BMI | ||||||
| <18.5 (underweight) | 9 (100%) (n=9) |
7.945 (0.425-148.193) | 0.165 (vs bmi≥23) |
8 (88.89%) (n=9) |
3.737 (0.587-23.781) | 0.163 (vs bmi≥23) |
| 5.231 (0.278-98.283) | 0.269 (vs bmi 18.5-22.9) |
4.510 (0.719-28.253) | 0.108 (vs bmi 18.5-22.9) |
|||
| 18.5-22.9 (normal) | 34 (79.07%) (n=43) |
1.518 (0.561-4.106) | 0.410 (vs bmi≥23) |
24 (55.81%) (n=43) |
0.828 (0.345-1.987) | 0.674 (vs bmi≥23) |
| ≥23.0 (overweight) | 27 (71.05%) (n=38) |
ref | - | 23 (60.53%) (n=38) |
ref | - |
| Primary cancer, n (%) | ||||||
| GI | 25 (78.12%) (n=32) |
1.041 (0.372-2.912) | 0.938 | 19 (59.38%) (n=32) |
0.923 (0.386-2.205) | 0.857 |
| HBP | 13 (81.25%) (n=16) |
1.300 (0.333-5.072) | 0.706 | 10 (62.50%) (n=16) |
1.099 (0.362-3.332) | 0.867 |
| Breast | 18/26, 69.23% (n=26) |
0.531 (0.190-1.488) | 0.229 | 14 (53.85%) (n=26) |
0.678 (0.271-1.693) | 0.406 |
| Prostate | 3 (60.00%) (n=5) |
0.407 (0.063-2.614) | 0.344 | 1 (20.00%) (n=5) |
0.147 (0.015-1.374) | 0.093 |
| Others | 13/ (92.86%) (n=14) |
4.406 (0.541-35.850) | 0.166 | 13 (92.86%) (n=14) |
10.340 (1.289-82.925) | 0.028 |
| Metastasis | 36 (81.82%) (n=44) |
1.720 (0.634-4.666) | 0.286 | 28 (63.64%) (n=44) |
1.187 (0.509-2.768) | 0.691 |
| Active cancer treatment | 51 (71.83%) (n=71) |
0.134 (0.016-1.070) | 0.058 | 41 (57.75%) (n=71) |
0.455 (0.149-1.391) | 0.167 |
| Chemo vs non-chemo | 31 (75.61%) (n=41) |
0.874 (0.328-2.432) | 0.788 | 24 (58.54%) (n=41) |
0.794 (0.340-1.854) | 0.594 |
| Targeted vs non-targeted | 11 (57.89%) (n=19) |
0.302 (0.101-0.901) | 0.032 | 10 (52.63%) (n=19) |
0.628 (0.226-1.743) | 0.372 |
| Hormonal vs non-hormonal | 8 (57.14%) (n=14) |
0.322 (0.097-1.070) | 0.064 | 5 (35.71%) (n=14) |
0.283 (0.086-0.931) | 0.038 |
| No active cancer treatment | 19/20, 95.00% (n=20) |
ref | - | 15/20, 75.00% (n=20) |
ref | - |
| Comorbidity | ||||||
| DM | 11 (64.71%) (n=17) |
0.458 (0.145-1.439) | 0.181 | 10 (58.82%) (n=17) |
0.900 (0.308-2.630) | 0.848 |
| HT | 24 (68.57%) (n=35) |
0.464 (0.172-1.246) | 0.128 | 18 (51.43%) (n=35) |
0.529 (0.223-1.253) | 0.148 |
| Other comorbid | 30 (73.175%) (n=41) |
0.665 (0.250-1.767) | 0.414 | 26 (63.41%) (n=41) |
1.213 (0.520-2.825) | 0.654 |
| Vaccine type | ||||||
| mRNA+mRNA | 40 (93.02%) (n=43) |
14.763 (4.062-53.651) | <0.001(vs N+N) | 32 (74.42%) (n=43) |
6.990 (2.545-19.193) | <0.001 (vs N+N) |
| non-mRNA+mRNA | 19 (100.00%) (n=19) |
49.758 (2.765-895.383) | 0.008(vs N+N) | 16 (84.21%) (n=19) |
11.661 (2.940-46.249) | <0.001 (vs N+N) |
| 3.370 (0.165-68.515) | 0.429(vs M+M) | 1.668 (0.438-6.339) | 0.453 (vs M+M) |
|||
| nonRNA+nonRNA | 14 (43.755%) (n=32) |
ref | - | 9 (28.12%) (n=32) |
ref | - |
| Leucopenia | 11 (73.33%) (n=15) |
0.651 (0.167-2.526) | 0.535 | 10 (66.67%) (n=15) |
1.357 (0.400-4.603) | 0.624 |
| Neutropenia | 2 (40.00%) (n=5) |
0.141 (0.020-0.962) | 0.046 | 2 (40.00%) (n=5) |
0.388 (0.060-2.519) | 0.332 |
| Lymphopenia | 14 (70.00%) (n=20) |
0.466 (0.122-1.635) | 0.234 | 12 (60.00%) (n=20) |
0.923 (0.310-2.745) | 0.886 |
| Factors | Seroconversion, n (%) | Adjusted OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Neutropenia | 2 (40.00%) (n=5) |
0.237 (0.026-2.125) | 0.199 | |
| Diabetes Mellitus | 11 (64.71%) (n=17) |
0.153 (0.023-1.022) | 0.053 | |
| Vaccine type | ||||
| mRNA+mRNA | 40 (93.02%) (n=43) |
14.424 (1.995-104.242) | 0.008 | |
| Non-mRNA + mRNA | 19 (100%) (n=19) |
25.866 (1.399-478.06) | 0.029 | |
| Factors | Seroconversion, n (%) | Adjusted OR (95% CI) |
p-Value |
|---|---|---|---|
| Cancer type | |||
| Prostate | 1 (20.00%) (n=5) |
0.118 (0.010-1.343) | 0.085 |
| Other cancer | 13 (92.86%) (n=14) |
8.268 (0.813-84.082) | 0.074 |
| Vaccine type | |||
| mRNA+mRNA | 32 (74.42%) (n=43) |
8.900 (2.939-26.949) | <0.001 |
| Non-mRNA + mRNA | 16 (84.21%) (n=19) |
17.380 (3.653-82.669) | <0.001 |
3.4. Safety
| All Participants (n = 91) | Non-Chemotherapy (n = 50) | Chemotherapy (n = 41) | ||||
|---|---|---|---|---|---|---|
| Grade 1 n (%) |
Grade 2 n (%) |
Grade 1 n (%) |
Grade 2 n (%) |
Grade 1 n (%) |
Grade 2 n (%) |
|
| Any | 31 (34.0%) | 18 (19.7%) | 22 (44.0%) | 8 (16.0%) | 9 (21.9%) | 10 (24.3%) |
| Pain at injection site | 14 (15.3%) | 10 (10.9%) | 11 (22.0%) | 3 (6.0%) | 3 (7.3%) | 7 (17.0%) |
| Fever | 8 (8.7%) | 5 (5.4%) | 5 (10.0%) | 2 (4.0%) | 3 (7.3%) | 3 (7.3%) |
| Fatigue | 4 (4.3%) | 3 (3.2%) | 4 (8.0%) | 3 (6.0%) | 0 | 0 |
| Malaise | 3 (3.2%) | 0 | 1 (2.0%) | 0 | 2 (4.8%) | 0 |
| Diarrhea | 1 (1.0%) | 1 (1.0%) | 1 (2.0%) | 0 | 1 (2.4%) | 1 (2.4%) |
3.5. Clinical Correlation of Seroconversion and COVID Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Chenchula, S.; Vidyasagar, K.; Pathan, S.; Sharma, S.; Chavan, M.R.; Bhagavathula, A.S.; Padmavathi, R.; Manjula, M.; Chhabra, M.; Gupta, R.; et al. Global prevalence and effect of comorbidities and smoking status on severity and mortality of COVID-19 in association with age and gender: a systematic review, meta-analysis and meta-regression. Sci Rep 2023, 13, 6415. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Sheng, Y.; Huang, C.; Jin, Y.; Xiong, N.; Jiang, K.; Lu, H.; Liu, J.; Yang, J.; Dong, Y.; et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol 2020, 21, 904–913. [Google Scholar] [CrossRef]
- Salunke, A.A.; Nandy, K.; Pathak, S.K.; Shah, J.; Kamani, M.; Kottakota, V.; Thivari, P.; Pandey, A.; Patel, K.; Rathod, P.; et al. Impact of COVID -19 in cancer patients on severity of disease and fatal outcomes: A systematic review and meta-analysis. Diabetes Metab Syndr 2020, 14, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, S.; Yoshida, T.; Kojima, Y.; Yagishita, S.; Nakahama, H.; Okinaka, K.; Matsushita, H.; Shiotsuka, M.; Kobayashi, O.; Iwata, S.; et al. Difference in SARS-CoV-2 Antibody Status Between Patients With Cancer and Health Care Workers During the COVID-19 Pandemic in Japan. JAMA Oncol 2021, 7, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, A.; Eliakim-Raz, N.; Stemmer, A.; Levy-Barda, A.; Yust-Katz, S.; Zer, A.; Benouaich-Amiel, A.; Ben-Zvi, H.; Moskovits, N.; Brenner, B.; et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol 2021, 7, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Amatu, A.; Pani, A.; Patelli, G.; Gagliardi, O.M.; Loparco, M.; Piscazzi, D.; Cassingena, A.; Tosi, F.; Ghezzi, S.; Campisi, D.; et al. Impaired seroconversion after SARS-CoV-2 mRNA vaccines in patients with solid tumours receiving anticancer treatment. Eur J Cancer 2022, 163, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Limpawittayakul, P.; Sungkasubun, P.; Chaiwiriyawong, W.; Supavavej, A.; Weerasubpong, B.; Siripaibun, J.; Phanthunane, C.; Lamlertthon, W.; Ungtrakul, T.; Tawinprai, K.; et al. Immunogenicity evaluation of ChAdox1 nCov-19 (AZD1222) vaccine in solid cancer patients in Chulabhorn Hospital. Hum Vaccin Immunother 2022, 18, 2104058. [Google Scholar] [CrossRef] [PubMed]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Byrne, F.; Cerrone, M.; Schmitt, A.M.; Joharatnam-Hogan, N.; Shum, B.; et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nat Cancer 2021, 2, 1305–1320. [Google Scholar] [CrossRef]
- Naranbhai, V.; St Denis, K.J.; Lam, E.C.; Ofoman, O.; Garcia-Beltran, W.F.; Mairena, C.B.; Bhan, A.K.; Gainor, J.F.; Balazs, A.B.; Iafrate, A.J. Neutralization breadth of SARS-CoV-2 viral variants following primary series and booster SARS-CoV-2 vaccines in patients with cancer. Cancer Cell 2022, 40, 103–108. [Google Scholar] [CrossRef]
- Furukawa, K.; Tjan, L.H.; Kurahashi, Y.; Sutandhio, S.; Nishimura, M.; Arii, J.; Mori, Y. Assessment of Neutralizing Antibody Response Against SARS-CoV-2 Variants After 2 to 3 Doses of the BNT162b2 mRNA COVID-19 Vaccine. JAMA Netw Open 2022, 5, e2210780. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous Covid-19 Booster Vaccinations. N Engl J Med 2022, 386, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Pang, N.Y.; Pang, A.S.; Chow, V.T.; Wang, D.Y. Understanding neutralising antibodies against SARS-CoV-2 and their implications in clinical practice. Mil Med Res 2021, 8, 47. [Google Scholar] [CrossRef]
- Takheaw, N.; Liwsrisakun, C.; Chaiwong, W.; Laopajon, W.; Pata, S.; Inchai, J.; Duangjit, P.; Pothirat, C.; Bumroongkit, C.; Deesomchok, A.; et al. Correlation Analysis of Anti-SARS-CoV-2 RBD IgG and Neutralizing Antibody against SARS-CoV-2 Omicron Variants after Vaccination. Diagnostics (Basel) 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.; Tiu, C.; Hu, Z.; Chen, V.C.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol 2020, 38, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Santos da Silva, E.; Servais, J.Y.; Kohnen, M.; Arendt, V.; Staub, T.; The Con-Vince, C.; The CoVaLux, C.; Krüger, R.; Fagherazzi, G.; Wilmes, P.; et al. Validation of a SARS-CoV-2 Surrogate Neutralization Test Detecting Neutralizing Antibodies against the Major Variants of Concern. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Thakkar, A.; Gonzalez-Lugo, J.D.; Goradia, N.; Gali, R.; Shapiro, L.C.; Pradhan, K.; Rahman, S.; Kim, S.Y.; Ko, B.; Sica, R.A.; et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 2021, 39, 1081–1090. [Google Scholar] [CrossRef]
- Eyre, D.W.; Lumley, S.F.; Wei, J.; Cox, S.; James, T.; Justice, A.; Jesuthasan, G.; O'Donnell, D.; Howarth, A.; Hatch, S.B.; et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin Microbiol Infect 2021, 27, 1516–e1517. [Google Scholar] [CrossRef]
- Valanparambil, R.M.; Carlisle, J.; Linderman, S.L.; Akthar, A.; Millett, R.L.; Lai, L.; Chang, A.; McCook-Veal, A.A.; Switchenko, J.; Nasti, T.H.; et al. Antibody Response to COVID-19 mRNA Vaccine in Patients With Lung Cancer After Primary Immunization and Booster: Reactivity to the SARS-CoV-2 WT Virus and Omicron Variant. J Clin Oncol 2022, 40, 3808–3816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Meng, W.; Chen, L.; Ding, L.; Feng, J.; Perez, J.; Ali, A.; Sun, S.; Liu, Z.; Huang, Y.; et al. Neutralizing antibodies to SARS-CoV-2 variants of concern including Delta and Omicron in subjects receiving mRNA-1273, BNT162b2, and Ad26.COV2.S vaccines. J Med Virol 2022, 94, 5678–5690. [Google Scholar] [CrossRef]
- Felip, E.; Pradenas, E.; Romeo, M.; Marfil, S.; Trinité, B.; Urrea, V.; Hernández, A.; Ballana, E.; Cucurull, M.; Mateu, L.; et al. Impact of chemotherapy and/or immunotherapy on neutralizing antibody response to SARS-CoV-2 mRNA-1237 vaccine in patients with solid tumors. Mol Oncol 2023, 17, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Grinshpun, A.; Rottenberg, Y.; Ben-Dov, I.Z.; Djian, E.; Wolf, D.G.; Kadouri, L. Serologic response to COVID-19 infection and/or vaccine in cancer patients on active treatment. ESMO Open 2021, 6, 100283. [Google Scholar] [CrossRef] [PubMed]
- Lyke, K.E.; Atmar, R.L.; Islas, C.D.; Posavad, C.M.; Szydlo, D.; Paul Chourdhury, R.; Deming, M.E.; Eaton, A.; Jackson, L.A.; Branche, A.R.; et al. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep Med 2022, 3, 100679. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; La, J.; Branch-Elliman, W.; Huhmann, L.B.; Han, S.S.; Parmigiani, G.; Tuck, D.P.; Brophy, M.T.; Do, N.V.; Lin, A.Y.; et al. Association of COVID-19 Vaccination With SARS-CoV-2 Infection in Patients With Cancer: A US Nationwide Veterans Affairs Study. JAMA Oncol 2022, 8, 281–286. [Google Scholar] [CrossRef]
- De Placido, P.; Pietroluongo, E.; De Angelis, C.; Tafuro, M.; Barraco, C.; Giannatiempo, R.; Buonaiuto, R.; Schettini, F.; Iervolino, A.; Vozzella, E.A.; et al. Safety and immunogenicity of the COVID-19 vaccine BNT162b2 for patients with breast and gynecological cancer on active anticancer therapy: Results of a prospective observational study. Front Oncol 2022, 12, 951026. [Google Scholar] [CrossRef]
- Lau, D.K.; Aresu, M.; Planche, T.; Tran, A.; Lazaro-Alcausi, R.; Duncan, J.; Kidd, S.; Cromarty, S.; Begum, R.; Rana, I.; et al. SARS-CoV-2 Vaccine Immunogenicity in Patients with Gastrointestinal Cancer Receiving Systemic Anti-Cancer Therapy. Oncologist 2023, 28, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Cazier, J.B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.; Curley, H.M.; Fittall, M.W.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, F.; Angelidakis, G.; Feldman, A.; Ravi, V.; Woodman, E.; Bhatti, M.; Ariza-Heredia, E.; Elhajj, P.; Spallone, A.; Jiang, Y.; Chemaly, R.F. COVID-19 in cancer patients: The impact of vaccination on outcomes early in the pandemic. Cancer Med 2023, 12, 22006–22022. [Google Scholar] [CrossRef]
- Luangdilok, S.; Wanchaijiraboon, P.; Pakvisal, N.; Susiriwatananont, T.; Zungsontiporn, N.; Sriuranpong, V.; Sainamthip, P.; Suntronwong, N.; Vichaiwattana, P.; Wanlapakorn, N.; et al. Immunogenicity after a Third COVID-19 mRNA Booster in Solid Cancer Patients Who Previously Received the Primary Heterologous CoronaVac/ChAdOx1 Vaccine. Vaccines (Basel) 2022, 10. [Google Scholar] [CrossRef]
- Mondaca, S.; Walbaum, B.; Le Corre, N.; Ferrés, M.; Valdés, A.; Martínez-Valdebenito, C.; Ruiz-Tagle, C.; Macanas-Pirard, P.; Ross, P.; Cisternas, B.; et al. Influence of SARS-CoV-2 mRNA Vaccine Booster among Cancer Patients on Active Treatment Previously Immunized with Inactivated versus mRNA Vaccines: A Prospective Cohort Study. Vaccines (Basel) 2023, 11. [Google Scholar] [CrossRef]
- Chi, W.Y.; Li, Y.D.; Huang, H.C.; Chan, T.E.H.; Chow, S.Y.; Su, J.H.; Ferrall, L.; Hung, C.F.; Wu, T.C. COVID-19 vaccine update: vaccine effectiveness, SARS-CoV-2 variants, boosters, adverse effects, and immune correlates of protection. J Biomed Sci 2022, 29, 82. [Google Scholar] [CrossRef] [PubMed]
- Kaku, C.I.; Champney, E.R.; Normark, J.; Garcia, M.; Johnson, C.E.; Ahlm, C.; Christ, W.; Sakharkar, M.; Ackerman, M.E.; Klingström, J.; et al. Broad anti-SARS-CoV-2 antibody immunity induced by heterologous ChAdOx1/mRNA-1273 vaccination. Science 2022, 375, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Pozzetto, B.; Legros, V.; Djebali, S.; Barateau, V.; Guibert, N.; Villard, M.; Peyrot, L.; Allatif, O.; Fassier, J.B.; Massardier-Pilonchéry, A.; et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. Nature 2021, 600, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, G.; Kabakchieva, P.; Miteva, D.; Batselova, H.; Velikova, T. Effectiveness and safety of COVID-19 vaccines in patients with diabetes as a factor for vaccine hesitancy. World J Diabetes 2022, 13, 738–751. [Google Scholar] [CrossRef]
- He, Y.F.; Ouyang, J.; Hu, X.D.; Wu, N.; Jiang, Z.G.; Bian, N.; Wang, J. Correlation between COVID-19 vaccination and diabetes mellitus: A systematic review. World J Diabetes 2023, 14, 892–918. [Google Scholar] [CrossRef]
| Characteristics | All (n = 91) |
Non-Chemotherapy (n = 50) |
Chemotherapy (n = 41) |
p-Value |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 45 (49.45%) | 19 (38.00%) | 26 (63.41%) | 0.016 |
| Female | 46 (50.55%) | 31 (62.00%) | 15 (36.59%) | |
| Age, years, mean ± SD | 60.76 ± 11.78 | 59.48 ± 12.16 | 62.48 ± 11.62 | 0.234 |
| BMI, mean ± SD | 23.44 ± 4.48 | 23.21 ± 4.29 (n = 48) | 23.50 ± 4.76 | 0.843 |
| Smoking, n (%) | 19 (20.88%) | 7 (14.00%) | 12 (29.27%) | 0.075 |
| Primary cancer, n (%) | ||||
| GI | 32 (35.16%) | 11 (22.00%) | 21 (51.22%) | 0.004 |
| HBP | 15 (16.58%) | 8 (16.00%) | 7 (17.07%) | 0.891 |
| Breast | 25 (27.47%) | 20 (40.00%) | 5 (12.20%) | 0.003 |
| Prostate | 5 (5.49%) | 2 (4.00%) | 3 (7.32%) | 0.490 |
| GU | 5 (5.49%) | 4 (8.00%) | 1 (2.44%) | 0.247 |
| others | 9 (9.89%) | 5 (10.00%) | 4 (9.76%) | 0.969 |
| Metastatic disease, n (%) | 44 (48.35%) | 17 (34.00%) | 27 (65.85%) | 0.002 |
| Comorbid, n (%) | ||||
| DM | 17 (18.68%) | 9 (18.00%) | 8 (19.51%) | 0.854 |
| HT | 34 (37.46%) | 15 (30.00%) | 19 (46.34%) | 0.109 |
| Other | 40 (43.96%) | 20 (40.00%) | 20 (48.78%) | 0.401 |
| Vaccines, n (%) | ||||
| RNA+RNA | 41 (45.05%) | 21 (42.00%) | 20 (48.78%) | 0.518 |
| nonRNA+RNA | 19 (20.88%) | 12 (24.00%) | 7 (17.07%) | 0.419 |
| nonRNA+nonRNA | 31 (34.07%) | 17 (34.00%) | 14 (34.15%) | 0.988 |
| Third vaccine booster | 21 (23.08%) | 12 (24.00%) | 9 (21.95%) | 0.817 |
| WBC | 6158 (±2461) 5985, 4400, 7280 |
6637 (±2544) 6270, 4730, 7540 |
5679 (±2317) 5040, 4160, 6350 |
0.613 0.085 |
| Neutrophil count | 3557 (±1847) 3085, 2430, 4190 |
3824 (±2034) 3560, 2520, 4190 |
3290 (±1627) 2840, 2300, 4560 |
0.227 0.231 |
| Lymphocyte count | 1853 (±1024) 1720, 1210, 2270 |
2124 (±1227) 1870, 1370, 2570 |
1582 (±689) 1490, 950, 1910 |
0.002 0.057 |
| Hematologic AE during F/U | ||||
| Leukopenia | 15 (24.19%) | 4 (12.9%) | 11 (35.48%) | 0.038 |
| Neutropenia | 5 (8.06%) | 0 | 5 (16.13%) | 0.020 |
| Lymphopenia | 20 (32.26%) | 7 (22.58%) | 13 (41.94%) | 0.103 |
| Neutralizing Antibody | Group | Baseline | Day 28 | Month 6 |
p-Value (Month 6 vs Day 28) |
|
|---|---|---|---|---|---|---|
| Percent Inhibition(wild type) | Non-chemotherapy | N | 48 | 50 | 45 | |
| Mean (95% CI) | 6.28 (4.87 – 7.69) | 62.63 (53.13 – 72.12) | 69.73 (59.95 – 79.51) | 0.170 | ||
| Chemotherapy | N | 41 | 41 | 35 | ||
| Mean (95% CI) | 5.24 (4.01 – 6.47) | 61.79 (50.79 – 72.78) | 67.31 (56.17 – 78.44) | 0.254 | ||
| p-Value (chemo vs non-chemo) | 0.907 | 0.791 | ||||
| Total | N | 89 | 91 | 80 | ||
| Mean (95% CI) | 5.80 (4.86 – 6.74) | 62.25 (55.19 – 69.30) | 68.67 (61.48 – 75.86) | 0.074 | ||
| Percent Inhibition(Omicron variant) | Non-chemotherapy | N | 48 | 50 | 45 | |
| Mean (95% CI) | 10.63 (8.72 – 12.54) | 43.87 (36.59 – 51.16) | 52.29 (42.99 – 61.60) | 0.052 | ||
| Chemotherapy | N | 41 | 41 | 35 | ||
| Mean (95% CI) | 7.26 (5.45 – 9.07) | 40.09 (32.19 – 47.99) | 42.88 (32.99 – 52.77) | 0.512 | ||
| p-Value (chemo vs non-chemo) | 0.476 | 0.180 | ||||
| Total | N | 89 | 91 | 80 | ||
| Mean (95% CI) | 9.08 (7.73 – 10.42) | 42.17 (36.90 – 47.44) | 48.18 (41.45 – 54.90) | 0.061 | ||
| Seroconversion(wild type) | Non-chemotherapy | N | 48 | 50 | 45 | |
| Mean (95% CI) | 0 (0 – 0.07) | 0.78 (0.64 – 0.88) | 0.80 (0.65 – 0.90) | 0.778 | ||
| Chemotherapy | N | 41 | 41 | 35 | ||
| Mean (95% CI) | 0 (0 – 0.09) | 0.76 (0.60 – 0.88) | 0.83 (0.66 – 0.93) | 0.236 | ||
| p-Value (chemo vs non-chemo) | 0.789 | 0.743 | ||||
| Total | N | 89 | 91 | 80 | ||
| Mean (95% CI) | 0 (0 – 0.04) | 0.77 (0.67 – 0.85) | 0.81 (0.71 – 0.89) | 0.365 | ||
| Seroconversion(Omicron variant) | Non-chemotherapy | N | 48 | 50 | 45 | |
| Mean (95% CI) | 0 (0 – 0.07) | 0.64 (0.49 – 0.77) | 0.71 (0.56 – 0.84) | 0.316 | ||
| Chemotherapy | N | 41 | 41 | 35 | ||
| Mean (95% CI) | 0 (0 – 0.09) | 0.59 (0.42 – 0.74) | 0.60 (0.42 – 0.76) | 0.875 | ||
| p-Value (chemo vs non-chemo) | 0.597 | 0.311 | ||||
| Total | N | 89 | 91 | 80 | ||
| Mean (95% CI) | 0 (0 – 0.04) | 0.62 (0.51 – 0.72) | 0.66 (0.55 – 0.76) | 0.420 | ||
| Strain | Baseline | Day 28 | Month 6 | ||
|---|---|---|---|---|---|
| Percent Inhibition (non-chemotherapy) |
Wild type | N | 48 | 50 | 45 |
| Mean (95% CI) | 6.28 (4.87 – 7.69) | 62.63 (53.13 – 72.12) | 69.73 (59.95 – 79.51) | ||
| Omicron variant | N | 48 | 50 | 45 | |
| Mean (95% CI) | 10.63 (8.72 – 12.54) | 43.87 (36.59 – 51.16) | 52.29 (42.99 – 61.60) | ||
| Wild vs Omicron, p-Value | 0.000 | 0.000 | |||
| Percent Inhibition (chemotherapy) |
Wild type | N | 41 | 41 | 35 |
| Mean (95% CI) | 5.24 (4.01 – 6.47) | 61.79 (50.79 – 72.78) | 67.31 (56.17 – 78.44) | ||
| Omicron variant | N | 41 | 41 | 35 | |
| Mean (95% CI) | 7.26 (5.45 – 9.07) | 40.09 (32.19 – 47.99) | 42.88 (32.99 – 52.77) | ||
| Wild vs Omicron, p-Value | 0.000 | 0.000 | |||
| Seroconversion (non-chemotherapy) |
Wild type | N | 48 | 50 | 45 |
| Mean (95% CI) | 0 (0 – 0.07) | 0.78 (0.64 – 0.88) | 0.80 (0.65 – 0.90) | ||
| Omicron variant | N | 48 | 50 | 45 | |
| Mean (95% CI) | 0 (0 – 0.07) | 0.64 (0.49 – 0.77) | 0.71 (0.56 – 0.84) | ||
| Wild vs Omicron, p-Value | 0.008 | 0.103 | |||
| Seroconversion (chemotherapy |
Wild type | N | 41 | 41 | 35 |
| Mean (95% CI) | 0 (0 – 0.09) | 0.76 (0.60 – 0.88) | 0.83 (0.66 – 0.93) | ||
| Omicron variant | N | 41 | 41 | 35 | |
| Mean (95% CI) | 0 (0 – 0.09) | 0.59 (0.42 – 0.74) | 0.60 (0.42 – 0.76) | ||
| Wild vs Omicron, p-Value | 0.008 | 0.005 | |||
| Company | Vaccine Platform | |
|---|---|---|
| Non-mRNA vaccines | ||
| AZD1222, ChAdOx1 nCoV-19 | AstraZeneca | Replication-deficient chimpanzee adenoviral vector |
| CoronaVac, SinoVac | Sinovac Biotech | Whole inactivated virus |
| BBIBP-CorV, BIBP vaccine | Sinopharm | Whole inactivated virus |
| mRNA vaccine | ||
| BTN162b2, Comirnaty | Pfizer–BioNTech | nucleoside-modified mRNA |
| mRNA-1273, Spikevax | Moderna | nucleoside-modified mRNA |
| All (91) | Non-Chemotherapy (50) | Chemotherapy (41) | |
|---|---|---|---|
| mRNA + mRNA | 41 | 21 | 20 |
| Pfizer+ Pfizer | 21 | 8 | 13 |
| Moderna+ Moderna | 19 | 12 | 7 |
| Others | 1 | 1 | 0 |
| Non-mRNA+ mRNA | 19 | 12 | 7 |
| Astrazeneca+Pfizer | 18 | 11 | 7 |
| Others | 1 | 1 | 0 |
| Non-mRNA+ non-mRNA | 31 | 17 | 14 |
| Sinopharm + Sinopharm | 17 | 12 | 5 |
| Sinovac + Astrazeneca | 9 | 4 | 5 |
| Astrzeneca + Astrazeneca | 3 | 0 | 3 |
| Others | 2 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





