Submitted:
29 April 2024
Posted:
29 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. AGE-Apt Inhibited Oxidative Stress, RAGE Gene Expression, Inflammation, and Apoptotic Cell Death in the Testes of Diabetic Mice
2.2. AGE-Apt Attenuated Seminiferous Tubular Dilation and Sperm Abnormalities in Diabetic Mice
2.3. TNF-α Impaired Sperm Motility and Viability
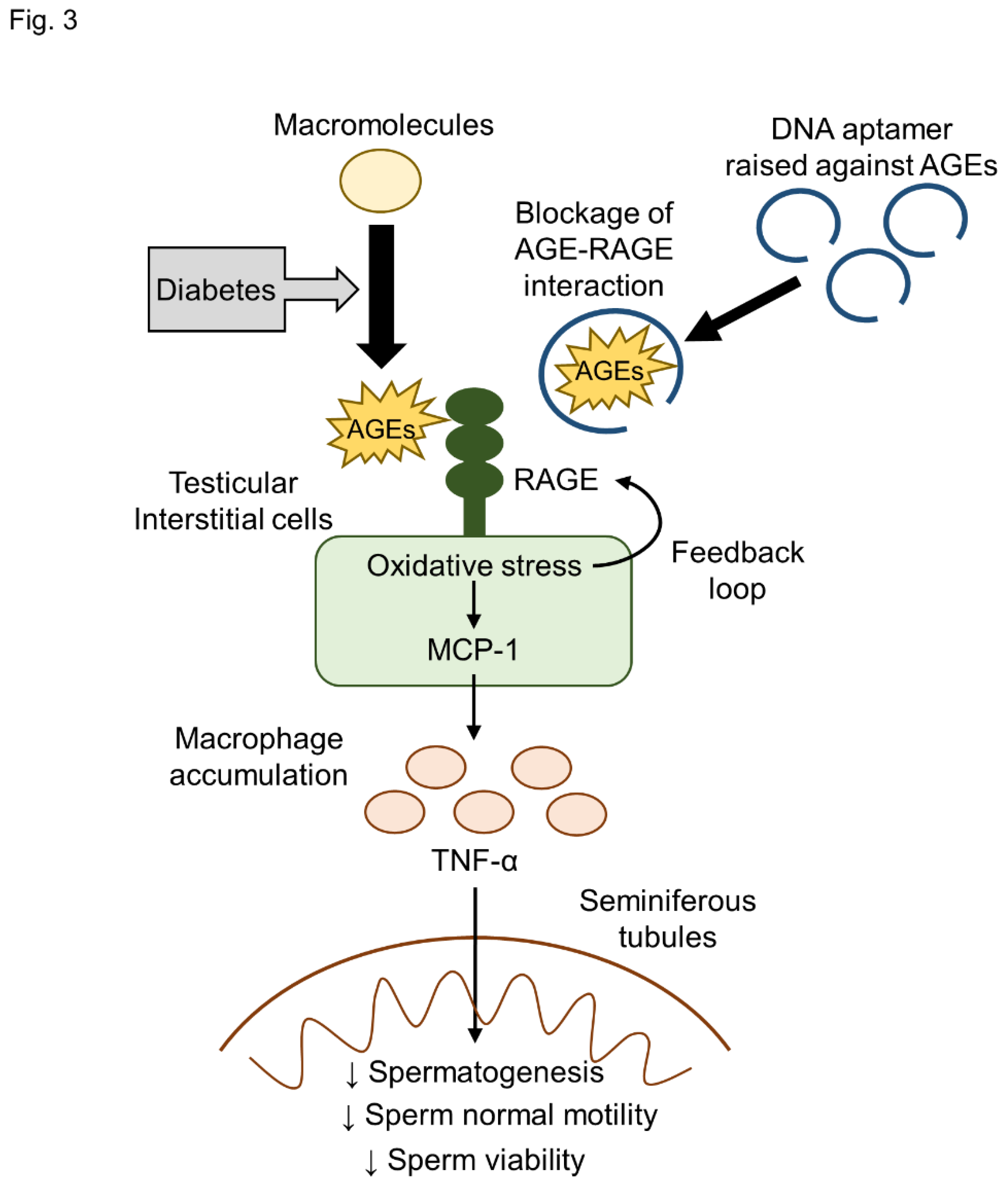
3. Discussion
4. Materials and Methods
4.1. Preparation of DNA Aptamers
4.2. Animal Study
4.3. Measurement of Biochemical Parameters, Blood Pressure, and Heart Rates
4.4. Immunofluorescence Staining
4.5. Real-Time RT-PCR
4.6. Histological Assessment of Testis
4.7. Sperm Collection
4.8. Sperm Analysis
4.9. Ex Vivo Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum Reprod Sci 2015, 8, 191–196. [Google Scholar] [CrossRef]
- Punab, M.; Poolamets, O.; Paju, P.; Vihljajev, V.; Pomm, K.; Ladva, R.; Korrovits, P.; Laan, M. Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum Reprod 2017, 32, 18–31. [Google Scholar] [CrossRef]
- Fallara, G.; Cazzaniga, W.; Boeri, L.; Capogrosso, P.; Candela, L.; Pozzi, E.; Belladelli, F.; Schifano, N.; Ventimiglia, E.; Abbate, C.; et al. Male factor infertility trends throughout the last 10 years: Report from a tertiary-referral academic andrology centre. Andrology 2021, 9, 610–617. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V. R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M. E.; Isaacs, D.; Johnson, E.L.; et al. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Prezioso, G.; Scattoni, R.; Chiarelli, F. Autonomic neuropathy in diabetes mellitus. Front Endocrinol (Lausanne) 2014, 5, 205. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.I.; Bellastella, G.; Esposito, K. Diabetes and sexual dysfunction: current perspectives. Diabetes Metab Syndr Obes 2014, 7, 95–105. [Google Scholar]
- Bener, A.; Al-Ansari, A.A.; Zirie, M.; Al-Hamaq, A. O. Is male fertility associated with type 2 diabetes mellitus? Int Urol Nephrol 2009, 41, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Paasch, U.; Heidenreich, F.; Pursche, T.; Kuhlisch, E.; Kettner, K.; Grunewald, S.; Kratzsch, J.; Dittmar, G.; Glander, H.J.; Hoflack, B.; et al. Identification of increased amounts of eppin protein complex components in sperm cells of diabetic and obese individuals by difference gel electrophoresis. Mol Cell Proteomics 2011, 10, M110.007187. [Google Scholar] [CrossRef] [PubMed]
- Rama, R.G.A; Jaya, P.G.; Murali, K.K.; Madan, K.; Siva, N.T.; Ravi, K.C.H. Noninsulin-dependent diabetes mellitus: effects on sperm morphological and functional characteristics, nuclear DNA integrity and outcome of assisted reproductive technique. Andrologia 2012, 44, 490–498. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La, V.S.; Mongioì, L.M.; Alamo, A.; Calogero, A.E. Diabetes Mellitus and Infertility: Different Pathophysiological Effects in Type 1 and Type 2 on Sperm Function. Front Endocrinol (Lausanne) 2018, 9, 268. [Google Scholar] [CrossRef]
- Tavares, R.S.; Escada-Rebelo, S.; Silva, A. F.; Sousa, M. I.; Ramalho-Santos, J.; Amaral, S. Antidiabetic therapies and male reproductive function: where do we stand? Reproduction 2018, 155, R13–R37. [Google Scholar] [CrossRef] [PubMed]
- Reynaert, N.L.; Gopal, P.; Rutten, E.P.A.; Wouters, E.F.M.; Schalkwijk, C.G. Advanced glycation end products and their receptor in age-related, non-communicable chronic inflammatory diseases; Overview of clinical evidence and potential contributions to disease. Int J Biochem Cell Biol 2016, 81, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab 2018, 28, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.I. Role of Advanced Glycation Endproduct (AGE)-Receptor for Advanced Glycation Endproduct (RAGE) Axis in Cardiovascular Disease and Its Therapeutic Intervention. Circ J. 2019, 83, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Stirban, A.; Heinemann, L. Skin autofluorescence - a non-invasive measurement for assessing cardiovascular risk and risk of diabetes. Eur Endocrinol. 2014, 10, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab J Urol 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Azenabor, A.; Ekun, A.O.; Akinloye, O. Impact of Inflammation on Male Reproductive Tract. J Reprod Infertil 2015, 16, 123–129. [Google Scholar] [PubMed]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, Y.; Yamagishi, S.; Nakamura, K.; Matsui, T.; Takeuchi, M.; Noguchi, M.; Inoue, H. In vitro selection of DNA aptamers that block toxic effects of AGE on cultured retinal pericytes. Microvasc Res 2007, 74, 65–69. [Google Scholar] [CrossRef]
- Higashimoto, Y.; Matsui, T.; Nishino, Y.; Taira, J.; Inoue, H.; Takeuchi, M.; Yamagishi, S. Blockade by phosphorothioate aptamers of advanced glycation end products-induced damage in cultured pericytes and endothelial cells. Microvasc Res 2013, 90, 64–70. [Google Scholar] [CrossRef]
- Kaida, Y.; Fukami, K.; Matsui, T.; Higashimoto, Y.; Nishino, Y.; Obara, N.; Nakayama, Y.; Ando, R.; Toyonaga, M.; Ueda, S.; et al. DNA aptamer raised against AGEs blocks the progression of experimental diabetic nephropathy. Diabetes 2013, 62, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Ojima, A.; Matsui, T.; Maeda, S.; Takeuchi, M.; Inoue, H.; Higashimoto, Y.; Yamagishi, S. DNA aptamer raised against advanced glycation end products inhibits melanoma growth in nude mice. Lab Invest 2014, 94, 422–429. [Google Scholar] [CrossRef]
- Maeda, S.; Matsui, T.; Ojima, A.; Suematsu, M.; Kaseda, K.; Higashimoto, Y.; Yamakawa, R.; Yamagishi, S. DNA Aptamer Raised against Advanced Glycation End Products Prevents Abnormalities in Electroretinograms of Experimental Diabetic Retinopathy. Ophthalmic Res 2015, 54, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Ojima, A.; Matsui, T.; Nakamura, N.; Higashimoto, Y.; Ueda, S.; Fukami, K.; Okuda, S.; Yamagishi, S. DNA aptamer raised against advanced glycation end products (AGEs) improves glycemic control and decreases adipocyte size in fructose-fed rats by suppressing AGE-RAGE axis. Horm Metab Res 2015, 47, 253–258. [Google Scholar] [CrossRef] [PubMed]
- George, B.T.; Jhancy, M.; Dube, R.; Kar, S.S.; Annamma, L.M. The Molecular Basis of Male Infertility in Obesity: A Literature Review. Int J Mol Sci 2023, 25, 179. [Google Scholar] [CrossRef]
- Mounzih, K.; Lu, R.; Chehab, F.F. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology 1997, 138, 1190–1193. [Google Scholar] [CrossRef]
- Ewart-Toland, A.; Mounzih, K.; Qiu, J.; Chehab, F.F. Effect of the genetic background on the reproduction of leptin-deficient obese mice. Endocrinology 1999, 140, 732–738. [Google Scholar] [CrossRef] [PubMed]
- de Luca, C.; Kowalski, T.J.; Zhang, Y.; Elmquist, J.K.; Lee, C.; Kilimann, M.W.; Ludwig, T.; Liu, S.M.; Chua, S.C. Jr. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 2005, 115, 3484–3493. [Google Scholar] [CrossRef]
- Moroki, T.; Yoshikawa, Y.; Yoshizawa, K.; Tsubura, A.; Yasui, H. Morphological characterization of systemic changes in KK-Ay mice as an animal model of type 2 diabetes. In Vivo 2013, 27, 465–472. [Google Scholar]
- Okazaki, M.; Saito, Y.; Udaka, Y.; Maruyama, M.; Murakami, H.; Ota, S.; Kikuchi, T.; Oguchi, K. Diabetic nephropathy in KK and KK-Ay mice. Exp Anim 2002, 51, 191–196. [Google Scholar] [CrossRef]
- Yamagishi, S.; Nakamura, N.; Suematsu, M.; Kaseda, K.; Matsui, T. Advanced Glycation End Products: A Molecular Target for Vascular Complications in Diabetes. Mol Med 2015, 21, S32–40. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Thallas, V.; Thomas, M.C.; Founds, H.W.; Burns, W.C.; Jerums, G.; Cooper, M.E. The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J 2003, 17, 1762–1764. [Google Scholar] [CrossRef]
- Takeuchi, M.; Bucala, R.; Suzuki, T.; Ohkubo, T.; Yamazaki, M.; Koike, T.; Kameda, Y.; Makita, Z. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J Neuropathol Exp Neurol 2000, 59, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yonekura, H.; Watanabe, T.; Sakurai, S.; Li, H.; Harashima, A.; Myint, K.M.; Osawa, M.; Takeuchi, A.; Takeuchi, M.; et al. Short-chain aldehyde-derived ligands for RAGE and their actions on endothelial cells. Diabetes Res Clin Pract 2007, 77, S30–40. [Google Scholar] [CrossRef]
- Johnson, J.; Flores, M.G.; Rosa, J.; Han, C.; Salvi, A.M.; DeMali, K.A.; Jagnow, J.R.; Sparks, A.; Haim, H. The High Content of Fructose in Human Semen Competitively Inhibits Broad and Potent Antivirals That Target High-Mannose Glycans. J Virol 2020, 94, e01749–01719. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: what is the best choice? Nutr Diabetes 2020, 10, 24. [Google Scholar] [CrossRef]
- Sotokawauchi, A.; Matsui, T.; Higashimoto, Y.; Yamagishi, S.I. Fructose causes endothelial cell damage via activation of advanced glycation end products-receptor system. Diab Vasc Dis Res 2019, 16, 556–561. [Google Scholar] [CrossRef]
- Matsui, T.; Higashimoto, Y.; Nishino, Y.; Nakamura, N.; Fukami, K.; Yamagishi, S.I. RAGE- Aptamer Blocks the Development and Progression of Experimental Diabetic Nephropathy. Diabetes 2017, 66, 1683–1695. [Google Scholar] [CrossRef]
- Mori, Y.; Ohara, M.; Terasaki, M.; Osaka, N.; Yashima, H.; Saito, T.; Otoyama-Kataoka, Y.; Omachi, T.; Higashimoto, Y.; Matsui, T.; et al. Subcutaneous Infusion of DNA-Aptamer Raised against Advanced Glycation End Products Prevents Loss of Skeletal Muscle Mass and Strength in Accelerated-Aging Mice. Biomedicines 2023, 11, 3112. [Google Scholar] [CrossRef]
- Terré, B.; Lewis, M.; Gil-Gómez, G.; Han, Z.; Lu, H.; Aguilera, M.; Prats, N.; Roy, S.; Zhao, H.; Stracker, T.H. Defects in efferent duct multiciliogenesis underlie male infertility in GEMC1-, MCIDAS- or CCNO-deficient mice. Development 2019, 146, 162628. [Google Scholar] [CrossRef]
- Creasy, D.M. Pathogenesis of male reproductive toxicity. Toxicol Pathol 2001, 29, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liu, Y.; Peng, H.; Tang, C.; Hennig, G.W.; Wang, Z.; Wang, L.; Yu, T.; Klukovich, R.; Zhang, Y.; et al. Proc Natl Acad Sci U S A 2019, 116, 3584–3593. Heuser, A.; Mecklenburg, L.; Ockert, D.; Kohler, M.; Kemkowski, J. Selective inhibition of PDE4 in Wistar rats can lead to dilatation in testis, efferent ducts, and epididymis and subsequent formation of sperm granulomas. Toxicol Pathol 2013, 41, 615–627.
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol 2021, 101, 107598. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Inagaki, Y.; Okamoto, T.; Amano, S.; Koga, K.; Takeuchi, M.; Makita, Z. Advanced glycation end product-induced apoptosis and overexpression of vascular endothelial growth factor and monocyte chemoattractant protein-1 in human-cultured mesangial cells. J Biol Chem 2002, 277, 20309–20315. [Google Scholar] [CrossRef]
- Inagaki, Y.; Yamagishi, S.; Okamoto, T.; Takeuchi, M.; Amano, S. Pigment epithelium- derived factor prevents advanced glycation end products-induced monocyte chemoattractant protein-1 production in microvascular endothelial cells by suppressing intracellular reactive oxygen species generation. Diabetologia 2003, 46, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr 2010, 20, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yao, T.; Zhou, Z.; Zhu, J.; Zhang, S.; Hu, W.; Shen, C. Advanced Glycation End Products Enhance Macrophages Polarization into M1 Phenotype through Activating RAGE/NF-κB Pathway. Biomed Res Int 2015, 2015, 732450. [Google Scholar] [CrossRef] [PubMed]
- Perdichizzi, A.; Nicoletti, F.; La Vignera, S.; Barone, N.; D'Agata, R.; Vicari, E.; Calogero, A.E. Effects of tumour necrosis factor-alpha on human sperm motility and apoptosis. J Clin Immunol 2007, 27, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, W.; Jiang, Q.; Gong, M.; Chen, R.; Wu, H.; Han, R.; Chen, Y.; Han, D. Lipopolysaccharide-induced testicular dysfunction and epididymitis in mice: a critical role of tumor necrosis factor alpha. Biol Reprod 2019, 100, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Said, T.M.; Agarwal, A.; Falcone, T.; Sharma, R.K.; Bedaiwy, M.A.; Li, L. Infliximab may reverse the toxic effects induced by tumor necrosis factor alpha in human spermatozoa: an in vitro model. Fertil Steril 2005, 83, 1665–1673. [Google Scholar] [CrossRef]
- Jiang, X.H.; Bukhari, I.; Zheng, W.; Yin, S.; Wang, Z.; Cooke, H.J.; Shi, Q.H. Blood-testis barrier and spermatogenesis: lessons from genetically-modified mice. Asian J Androl 2014, 16, 572–580. [Google Scholar]
- Alves, M.G.; Martins, A.D.; Cavaco, J.E.; Socorro, S.; Oliveira, P.F. Diabetes, insulin- mediated glucose metabolism and Sertoli/blood-testis barrier function. Tissue Barriers 2013, 1, e23992. [Google Scholar] [CrossRef]
- Kumari, N.; Khan, A.; Shaikh, U.; Lobes, K.; Kumar, D.; Suman, F.; Bhutto, N.S.; Anees, F.; Shahid, S.; Rizwan, A. Comparison of Testosterone Levels in Patients With and Without Type 2 Diabetes. Cureus 2021, 13, e16288. [Google Scholar] [CrossRef]
- Dhindsa, S.; Prabhakar, S.; Sethi, M.; Bandyopadhyay, A.; Chaudhuri, A.; Dandona, P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2004, 89, 5462–5468. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br J Pharmacol 2020, 177, 3617–3624. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed; National Academies Press (US), Washington (DC), USA, 2011; pp. 1–220.
- Mori, Y.; Terasaki, M.; Hiromura, M.; Saito, T.; Kushima, H.; Koshibu, M.; Osaka, N.; Ohara, M.; Fukui, T.; Ohtaki, H.; Tsutomu, H.; Yamagishi, S.I. Luseogliflozin attenuates neointimal hyperplasia after wire injury in high-fat diet-fed mice via inhibition of perivascular adipose tissue remodeling. Cardiovasc Diabetol 2019, 18, 143. [Google Scholar] [CrossRef]
- Montoto, L.G.; Arregui, L.; Sánchez, N.M.; Gomendio, M.; Roldan, E.R. Postnatal testicular development in mouse species with different levels of sperm competition. Reproduction. 2012, 143, 333–346. [Google Scholar] [CrossRef]
- Zhang, E.; Xu, F.; Liang, H.; Yan, J.; Xu, H.; Li, Z.; Wen, X.; Weng, J. GLP-1 Receptor Agonist Exenatide Attenuates the Detrimental Effects of Obesity on Inflammatory Profile in Testis and Sperm Quality in Mice. Am J Reprod Immunol 2015, 74, 457–466. [Google Scholar] [CrossRef]
- World Health Organization. WHO laboratory manual for the examination and processing of human semen, 6th edition. WHO Press, Geneva, Switzerland, 2016, pp. 1–276.
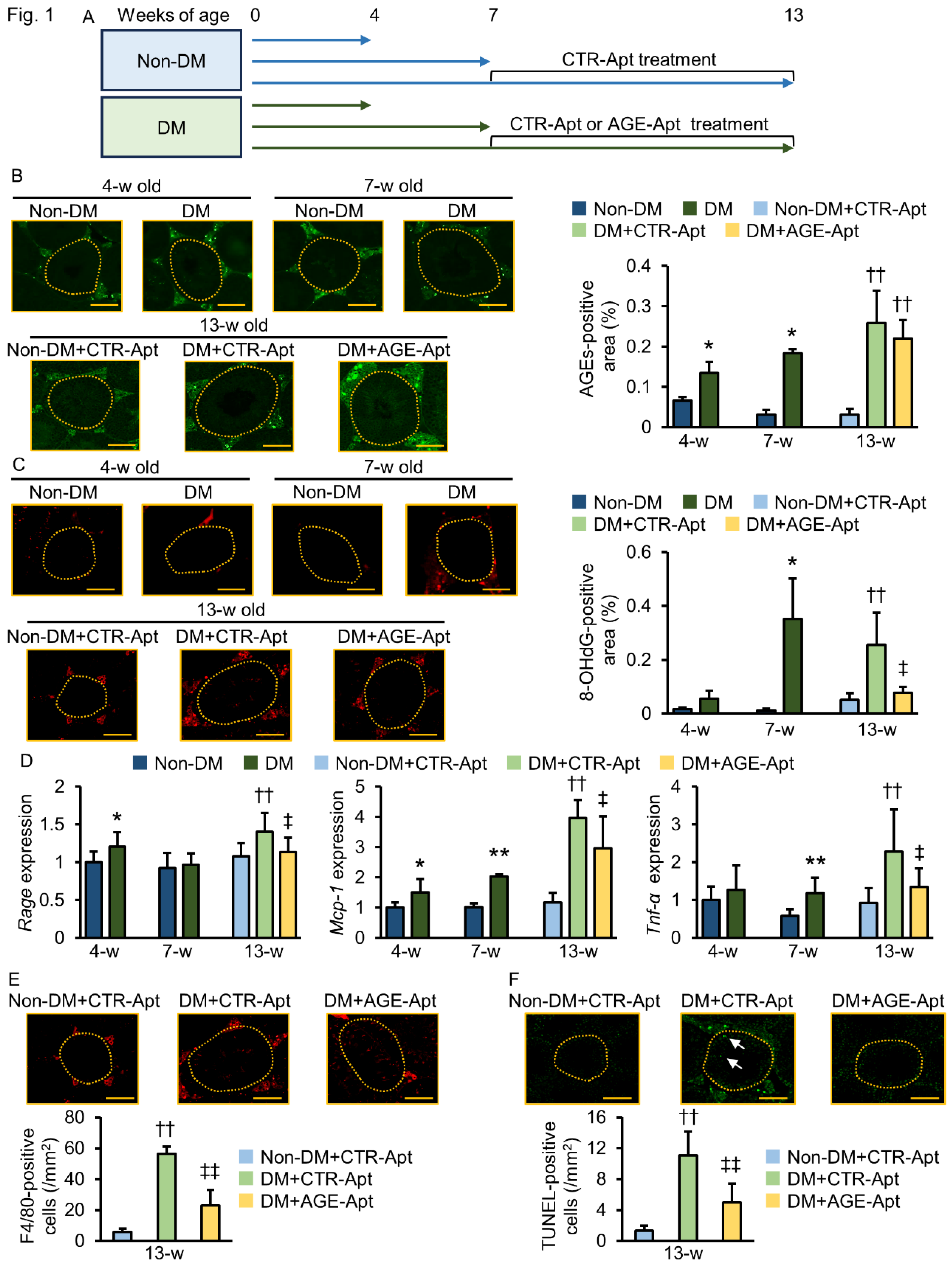

| 4 Weeks Old | 7 Weeks Old | 13 Weeks Old | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-DM | DM | Non-DM | DM | Non-DM +CTR-Apt |
DM +CTR-Apt |
DM +AGE-Apt |
|||
| Number | 7 | 6 | 7 | 8 | 8 | 8 | 8 | ||
| Food intake (g/day) | N.A. | N.A. | N.A. | N.A. | 5.0 ± 0.5 | 7.3 ± 0.6† | 7.1 ± 0.4† | ||
| Body weight (g) | 18.4 ± 1.1 | 22.5 ± 1.6* | 21.4 ± 1.0 | 35.8 ± 2.6* | 27.0 ± 1.2 | 44.0 ± 2.2† | 42.4 ± 1.5† | ||
| Testis weight (mg) | 132 ± 18 | 111 ± 8 | 190 ± 9 | 206 ± 21 | 213 ± 7 | 205 ± 25 | 218 ± 13 | ||
| HbA1c (%) | <4.0 | <4.0 | 4.7 ± 0.3 | 7.0 ± 0.9* | 4.8 ± 0.2 | 10.2 ± 1.0† | 10.4 ± 1.0† | ||
| Plasma glucose (mg/dL) |
160 ± 11 | 201 ± 16* | 142 ± 14 | 207 ± 38* | 155 ± 9 | 190 ± 29† | 174 ± 22 | ||
| Plasma insulin (ng/mL) |
N.A. | N.A. | N.A. | N.A. | 0.24 ± 0.06 | 2.44 ± 1.77† | 1.93 ± 1.17† | ||
| Plasma total cholesterol (mg/dL) |
N.A. | N.A. | N.A. | N.A. | 38 ± 3 | 68 ± 5† | 62 ± 11† | ||
| Plasma triglycerides (mg/dL) |
N.A. | N.A. | N.A. | N.A. | 39 ± 9 | 115 ± 19† | 105 ± 36† | ||
| Plasma testosterone (ng/mL) |
N.A. | N.A. | N.A. | N.A. | 1.6 ± 1.1 | 35.6 ± 26.0† | 26.6 ± 11.3† | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





