Submitted:
23 April 2024
Posted:
24 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
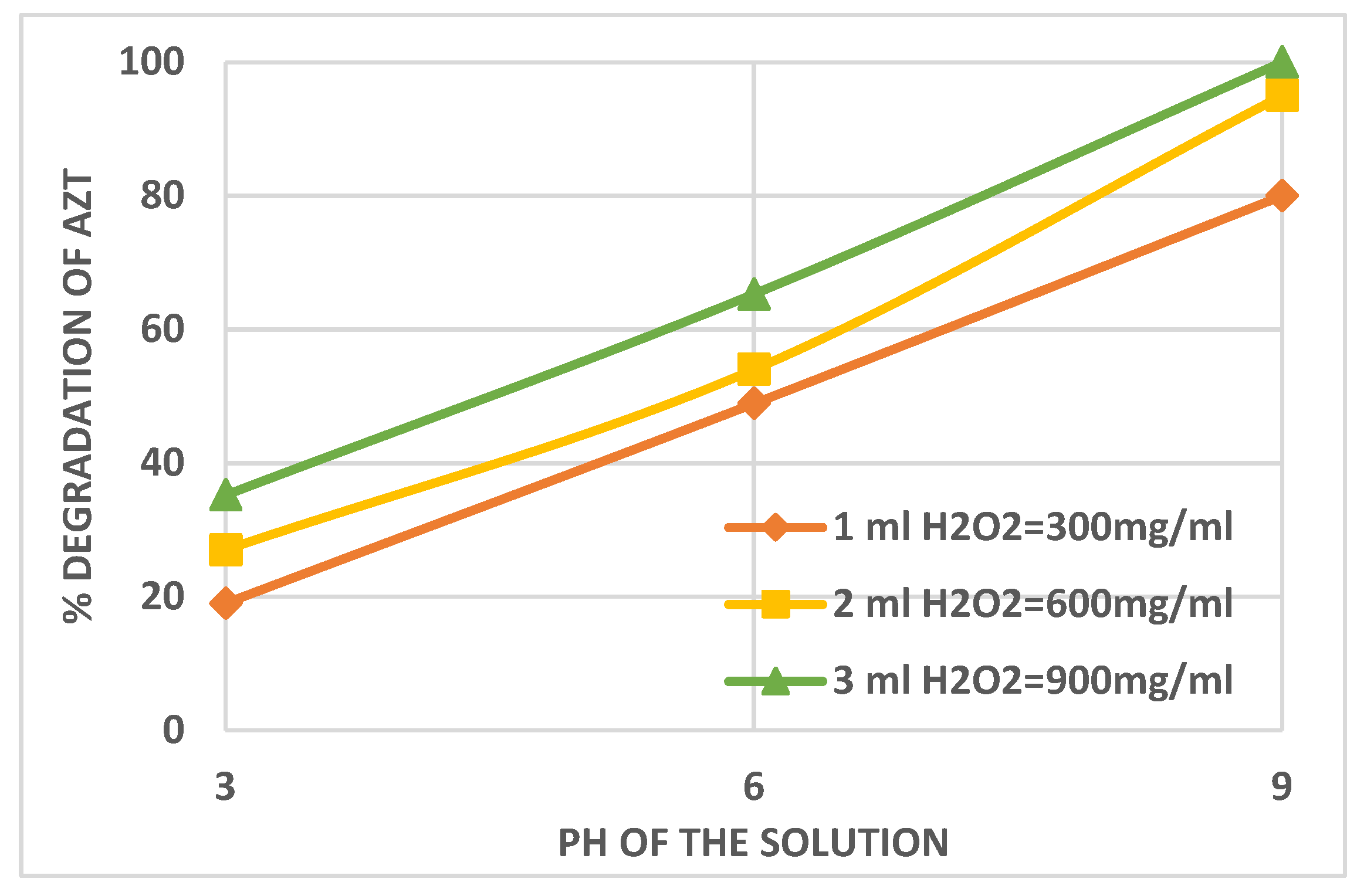
3.1. Effect of Solution pH on the AZT Degradation Efficiency
3.1. Effect of Amount/Concentration of H2O2 in the Reacting Solution
3.2. Effect of Initial AZT Concentration on the AZT Degradation
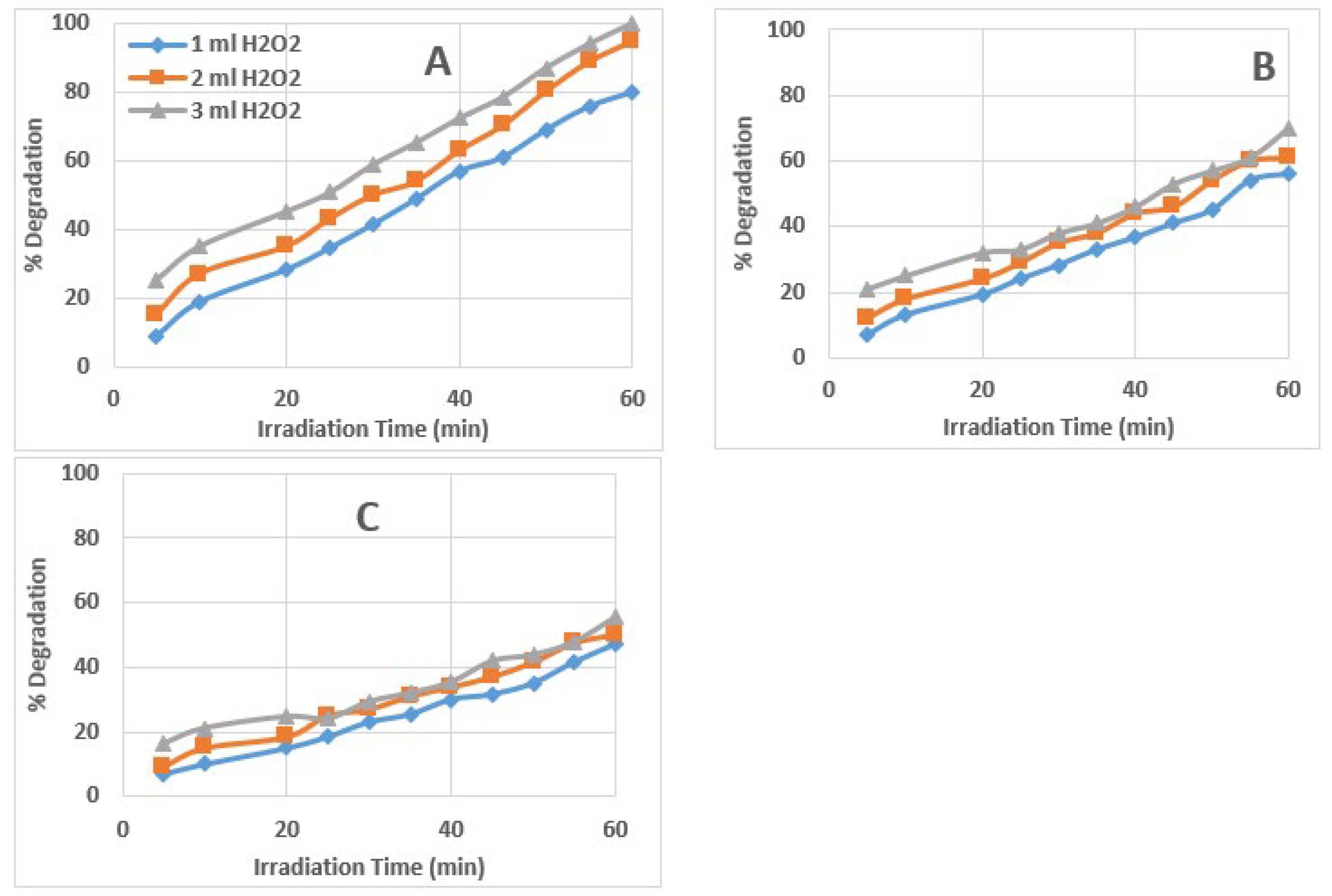
3.3. Kinetics of AZT Photodegradation
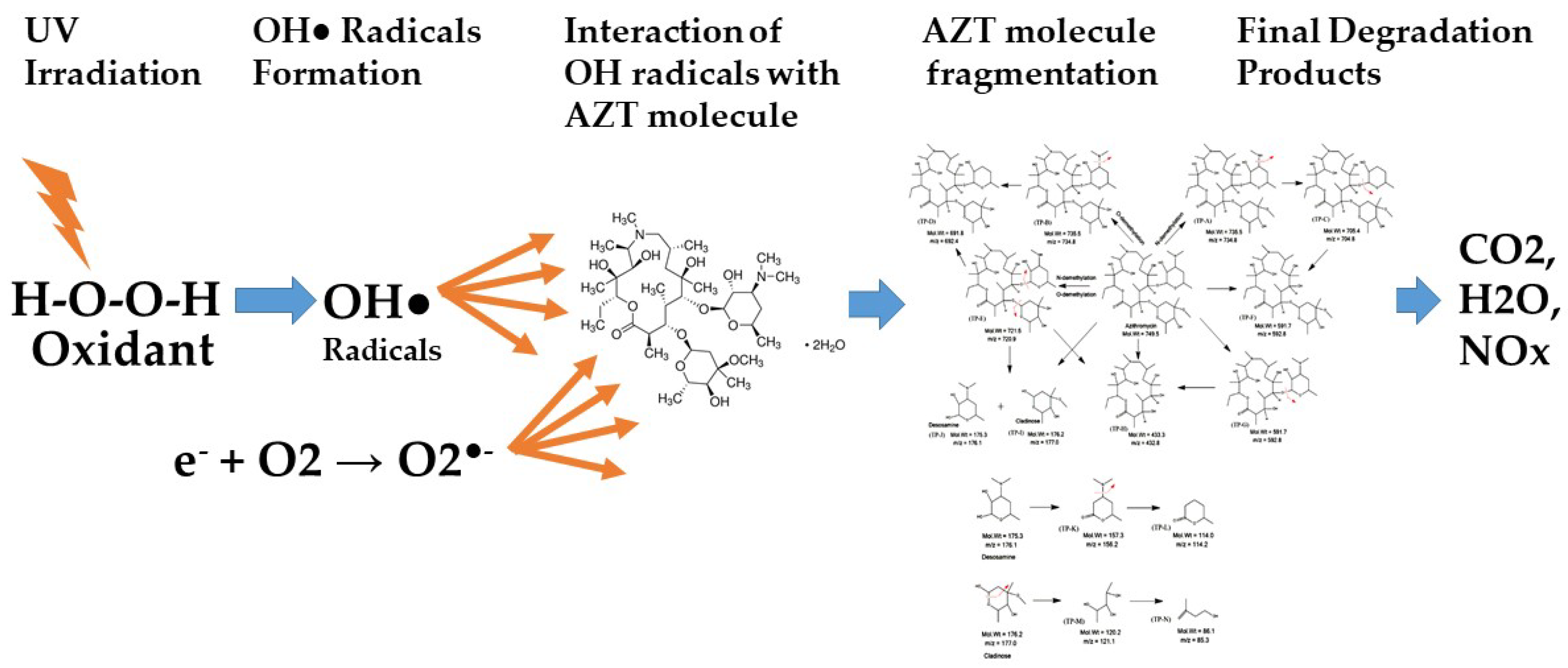
3.4. Photodegradation Mechanism
3.5. Comparison of this Work with the Literature
Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Competing or Conflicting Interest
Consent to Publish
Ethical Approval
References
- Javed, M.; Iqbal, S.; Qamar, M.A.; Shariq, M.; Ahmed, I.A.; BaQais, A.; Alzahrani, H.; Ali, S.K.; Masmali, N.A.; Althagafi, T.M.; Khan, M.S.; Fabrication of Effective Co-SnO2/SGCN Photocatalysts for the Removal of Organic Pollutants and Pathogen Inactivation. Crystals 2023, 13(2), 163; [CrossRef]
- Thilagavathi, T.; Venugopal, D.; Thangaraju, D.; Marnadu, R.; Palanivel, B.; Imran, M.; Shkir, M.; Ubaidullah, M.; AlFaify, S.; A facile co-precipitation synthesis of novel WO3/NiWO4 nanocomposite with improved photocatalytic activity, Materials Science in Semiconductor Processing, 2021, 133, 105970. [CrossRef]
- Qamar, Muhammad Azam; Sammia Shahid, Mohsin Javed, Mohammad Shariq, Mohammed M. Fadhali, Osama Madkhali, Syed Kashif Ali, Imam Saheb Syed, Majed Yusef Awaji, Mohd. Shakir Khan, et al. "Accelerated Decoloration of Organic Dyes from Wastewater Using Ternary Metal/g-C3N4/ZnO Nanocomposites: An Investigation of Impact of g-C3N4 Concentration and Ni and Mn Doping. Catalysts 2022, 12(11) 1388. [CrossRef]
- Helmy, Elsayed T.; Usama A. Soliman, A. M. Elbasiony, Ba-Son Nguyen. CuCe-Ferrite/TiO2 Nanocomposite as an Efficient Magnetically Separable Photocatalyst for Dye Pollutants Decolorization, Topics in Catalysis 2023, 66, 53–63. [CrossRef]
- Ghaffar, Sadia; Azhar Abbas, Muhammad Naeem-Ul-Hassan, Nasir Assad, Muhammad Sher, Sami Ullah, Hassan A Alhazmi, Asim Najmi, Khalid Zoghebi, Mohammed Al Bratty, Ali Hanbashi, Hafiz A Makeen, Hatem M A Amin. Improved Photocatalytic and Antioxidant Activity of Olive Fruit Extract-Mediated ZnO Nanoparticles, Antioxidants (Basel). 2023 Jun 1;12(6):1201. [CrossRef]
- Fallatah, A.M.; Alahmari, S.D.; Farid, H.M.T. Facile Synthesis of the MOF Derived ZnMn2O4 Nanorods for Dyes Degradation in Water. J Mater Sci: Mater Electron 2023, 34, 1630. [CrossRef]
- Dilawar, Sundas; Karma Albalawi, Afaq Ullah Khan, Kamran Tahir, Magdi E.A. Zaki, Ebraheem Abdu Musad Saleh, Zainab M. Almarhoon, Talal M. Althagafi, Adel A. El-Zahhar, E. El-Bialy. Rapid photodegradation of toxic organic compounds and photoinhibition of bacteria in the presence of novel hydrothermally synthesized Ag/Mn–ZnO nanomaterial, Environmental Research, 231, Part 1, 2023, 116093. [CrossRef]
- Bessy T.C.; Sarojini V.; Chadlia El Manna, Marwah Bakri, Sasi Florence S., Johnson J., Bindhu M. R. Optical, Structural, Morphological, Antibacterial, and Photodegradation Characteristics of BaxMg0.8−xFe2O4 (x = 0.2, 0.4, and 0.6) Nanocrystalline Powders Synthesized by Combustion Method. physica status solidi (a) –applications and materials science (pssa), 2022. [CrossRef]
- Husain, A.; Khan, H.A.A.; Nadeem, A.K.; Ahmed, S.; Mehtab, M.S.; Vambol, S.; Vambol, V.; Changani, F.; Islam, S.; Pharmaceuticals of emerging concern in hospital wastewater: removal of Ibuprofen and Ofloxacin drugs using M BBR method. International Journal of Environmental Analytical Chemistry, 2023, 103(1), 140-154. [CrossRef]
- Ling Y, Hai L, Biqing L, Biaojun Z, Yixiao W, Heping H, Deyou Y, Shaobin H (2023) Efficient photocatalytic ozonation of azithromycin by three-dimensional g-C3N4 nanosheet loaded magnetic Fe-MCM-48 under simulated solar light, Appl Catal B: Environmental, 324, 122208. [CrossRef]
- Sharma, M; Deepanshi R, Vinod K, Indu J, Tejraj MA, Gunda M, Ravi K, Kashyap KD (2023) Photocatalytic degradation of four emerging antibiotic contaminants and toxicity assessment in wastewater: A comprehensive study, Environmental Research, 231, Part 2, 116132. [CrossRef]
- Shajahan, S.; Abu Haija, M.; Effective removal of azithromycin by novel g-C3N4/CdS/CuFe2O4 nanocomposite under visible light irradiation, Chemosphere, 2023, 337, 139372. [CrossRef]
- Mehrdoost A, Reza J.Y, Mohammad K.M, Azadeh H, Ali A.B.; Adsorption removal and photocatalytic degradation of azithromycin from aqueous solution using PAC/Fe/Ag/Zn nanocomposite. Environ Sci Pollut Res 2022, 29, 33514–33527. [CrossRef]
- Mohammed, H,T,; Alasedi, K.K.; Ruyid, R.; ZnO/Co3O4 Nanocomposites: Novel Preparation, Characterization, and Their Performance toward Removal of Antibiotics from Wastewater. J. Nanostruct 2022, 12(3), 503-509. [CrossRef]
- Shukla, Shraddha, Himanshu Pandey, Prashansha Singh, Anish Kumar Tiwari, Vikas Baranwal, Jai Singh & Avinash C. Pandey. Time and Concentration Dependent; UV Light–Mediated Photocatalytic Degradation of Major Antibiotic Consortium Using ZnO. Braz J Phys. 2022, 52, 183. [CrossRef]
- Kumar, A.; Anamika, R.; Changsheng, G.; Gaurav, S.; Khadijah, M.S.M.K.; Fatimah, M.A.; Mu, N.; Mika, S.; Pooja, D.; Florian, J.S.; Acceleration of photo-reduction and oxidation capabilities of Bi4O5I2/SPION@calcium alginate by metallic Ag: Wide spectral removal of nitrate and azithromycin, Chemical Engineering Journal, 2021, 423, 130173. [CrossRef]
- Ospino-Atehortúa, B.A.; Zúñiga-Benítez, H.; Peñuela, G.A.; Potential Application of Persulfate and Simulated Sunlight Radiation on Azithromycin Removal. Environ. Eng. Res., 2021, 26, 200189. [CrossRef]
- Tenzin, T,; Yashas S.R.; Anilkumar, K.M.; UV–LED driven photodegradation of organic dye and antibiotic using strontium titanate nanostructures. J Mater Sci Mater Electron, 2021, 32, 21093–21105. [CrossRef]
- Li, C.; Jin, H.; Hou, Z.; Guo, Y.; Study on degradation of azithromycin antibiotics by molybdenum sulfide graphene oxide composites under visible light. IOP Conf. Ser. Mater. Sci. Eng. 2020, 774, 012019. [CrossRef]
- Rueda-Márquez, J.J.; Palacios-Villarreal, C.; Manzano, M.; Blanco, E.; Ramírezdel, S.M.; Levchuk, I.; Photocatalytic degradation of pharmaceutically active compounds (PhACs) in urban wastewater treatment plants effluents under controlled and natural solar irradiation using immobilized TiO2. Sol Energy 2020, 208:480–492. [CrossRef]
- Sayadi, MH,; Sobhani S, Shekari H.; Photocatalytic degradation of azithromycin using GO@Fe3O4/ZnO/SnO2 nanocomposites. J. Clean. Prod. 2019, 232:127–136. [CrossRef]
- Čizmić M, Ljubas D, Rožman M, Ašperger D, Ćurković L, Babić S (2019) Photocatalytic Degradation of Azithromycin by Nanostructured TiO2 Film: Kinetics, Degradation Products, and Toxicity. Materials. 2019; 12(6):873. [CrossRef]
- Naraginti, S.; Yu, Y.Y.; Fang, Z.; Yong, Y.C.; Visible light degradation of macrolide antibiotic azithromycin by novel ZrO2/Ag@TiO2 nanorod composite: transformation pathways and toxicity evaluation. Proc. Saf. Environ. Prot. 2019, 125, 39–49. [CrossRef]
- Biancullo F, Nuno FF. Moreira, Ana R. Ribeiro, Célia M. Manaia, Joaquim L. Faria, Olga C. Nunes, Sérgio M. Castro-Silva, Adrián M.T. Silva. () Heterogeneous photocatalysis using UVA-LEDs for the removal of antibiotics and antibiotic-resistant bacteria from urban wastewater treatment plant effluents, Chem Eng J 2019, 367, 304-313. [CrossRef]
- Luo, X.; Hao, T.; Yue, L.; Hong, G.; Lu, Y.; Azithromycin wastewater treatment with La doping titanium dioxide /active carbon composites, International Conference on Sensors, Measurement and Intelligent Materials (ICSMIM 2015), Atlantis Press: Amsterdam, The Netherlands, 2016, 861–870.
- Chen, X.; Photodegradation of Azithromycin in Aqueous System under VIS/UV Irradiation, International Journal of Scientific Advances, 2022, 3, Issue: 5. [CrossRef]
- Cano, P.A.; Marcela, J-B.; Henry, Z-B.; Yudy, AL.; Gustavo, A.P.; Use of simulated sunlight radiation and hydrogen peroxide in azithromycin removal from aqueous solutions: Optimization and mineralization analysis, Emerging Contaminants, 2020, 6, 53-61. [CrossRef]
- Voigt, M.; Indra, B.; Anna, N-H.; Martin, J.; Elimination of macrolides in water bodies using photochemical oxidation, AIMS Environ Sci 2018, 5(5), 372-388. [CrossRef]
- Tong L, Peter E, Sandra P, Yanxin W, Damià B () Photodegradation of azithromycin in various aqueous systems under simulated and natural solar radiation: Kinetics and identification of photoproducts, Chemosphere, 2011, 83(3), 340-348. [CrossRef]
- Ghari, Tayebeh; Kobarfard, Farzad; Mortazavi, Seyed. Development of a Simple RP-HPLC-UV Method for Determination of Azithromycin in Bulk and Pharmaceutical Dosage forms as an Alternative to the USP Method, Iranian journal of pharmaceutical research, 2013, 12, 57-63.
- 31. Mathon, Baptiste; Martial Ferreol, Marina Coquery, Jean-Marc Choubert, Jean-Marc Chovelon, Cécile Miège, Direct photodegradation of 36 organic micropollutants under simulated solar radiation: Comparison with free-water surface constructed wetland and influence of chemical structure, Journal of Hazardous Materials, 2021, 407, 124801. [CrossRef]
- Jaramillo-Baquero, M.; Zúñiga-Benítez, H.; Peñuela, G.A.; Use of photo-Fenton for macrolide antibiotic azithromycin removal. Acta Period Technol 2020, 29–37. [CrossRef]
- Orona Návar, Carolina; Irina Levchuk, Javier Moreno-Andrés, Nancy Ornelas-Soto. Removal of pharmaceutically active compounds (PhACs) and bacteria inactivation from urban wastewater effluents by UVA-LED photocatalysis with Gd3+ doped BiVO4, Journal of Environmental Chemical Engineering 2020, 8(6), 104540. [CrossRef]
- Talaiekhozani, Amirreza; Sahar Joudaki, Farhad Banisharif, Zeinab Eskandari, Jinwoo Cho, Ghasem Moghadam, and Shahabaldin Rezania.. "Comparison of Azithromycin Removal from Water Using UV Radiation, Fe (VI) Oxidation Process and ZnO Nanoparticles" International Journal of Environmental Research and Public Health 2020, 17(5), 1758. [CrossRef]
- Fiorentino, Antonino; Paula Soriano-Molina, María Jesús Abeledo-Lameiro, Irene de la Obra, Antonio Proto, Maria Inmaculada Polo-López, José Antonio Sánchez Pérez, Luigi Rizzo, Neutral (Fe3+-NTA) and acidic (Fe2+) pH solar photo-Fenton Vs chlorination: Effective urban wastewater disinfection does not mean control of antibiotic resistance, Journal of Environmental Chemical Engineering, 2022, 10(6), 108777. [CrossRef]

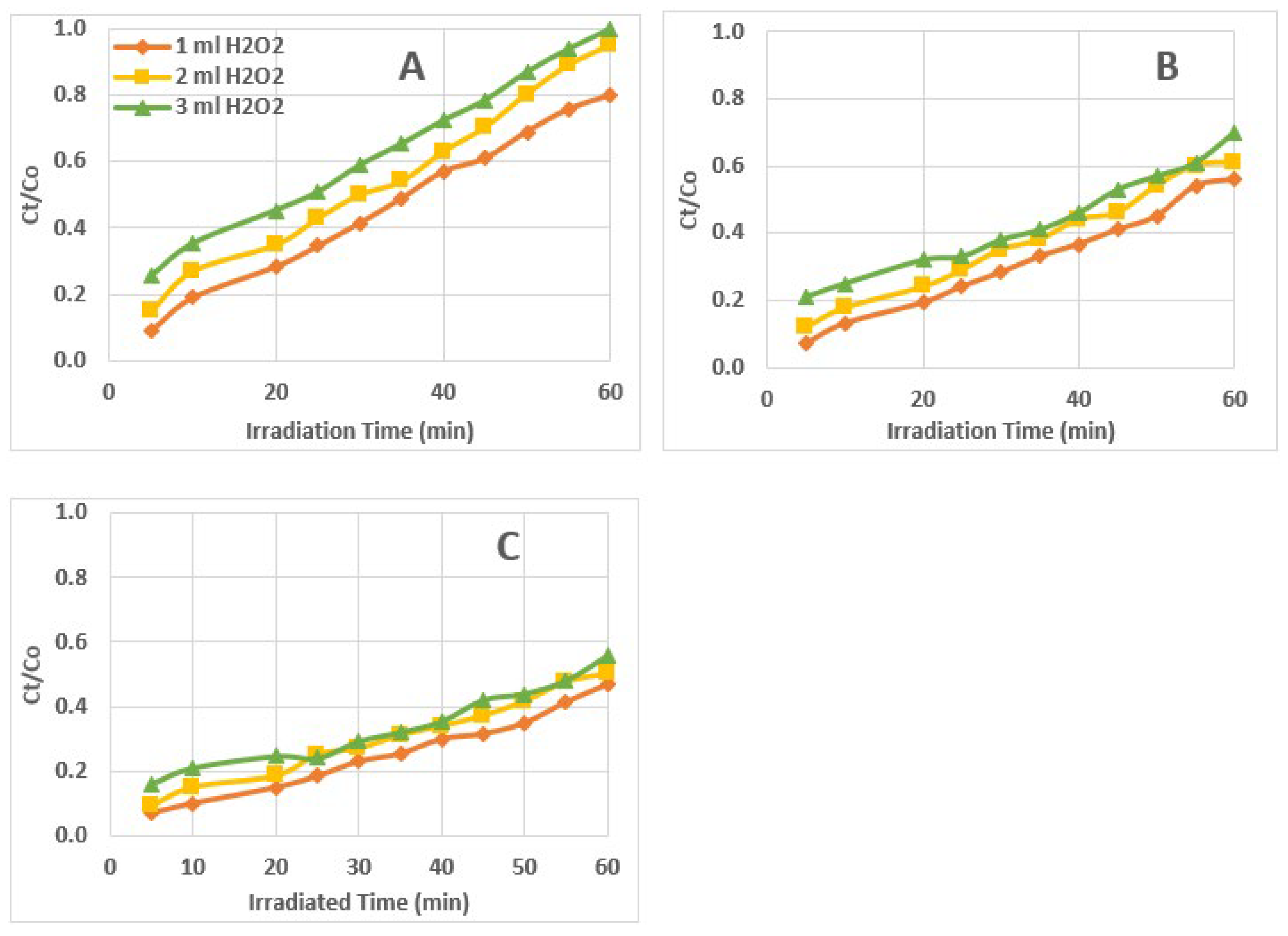
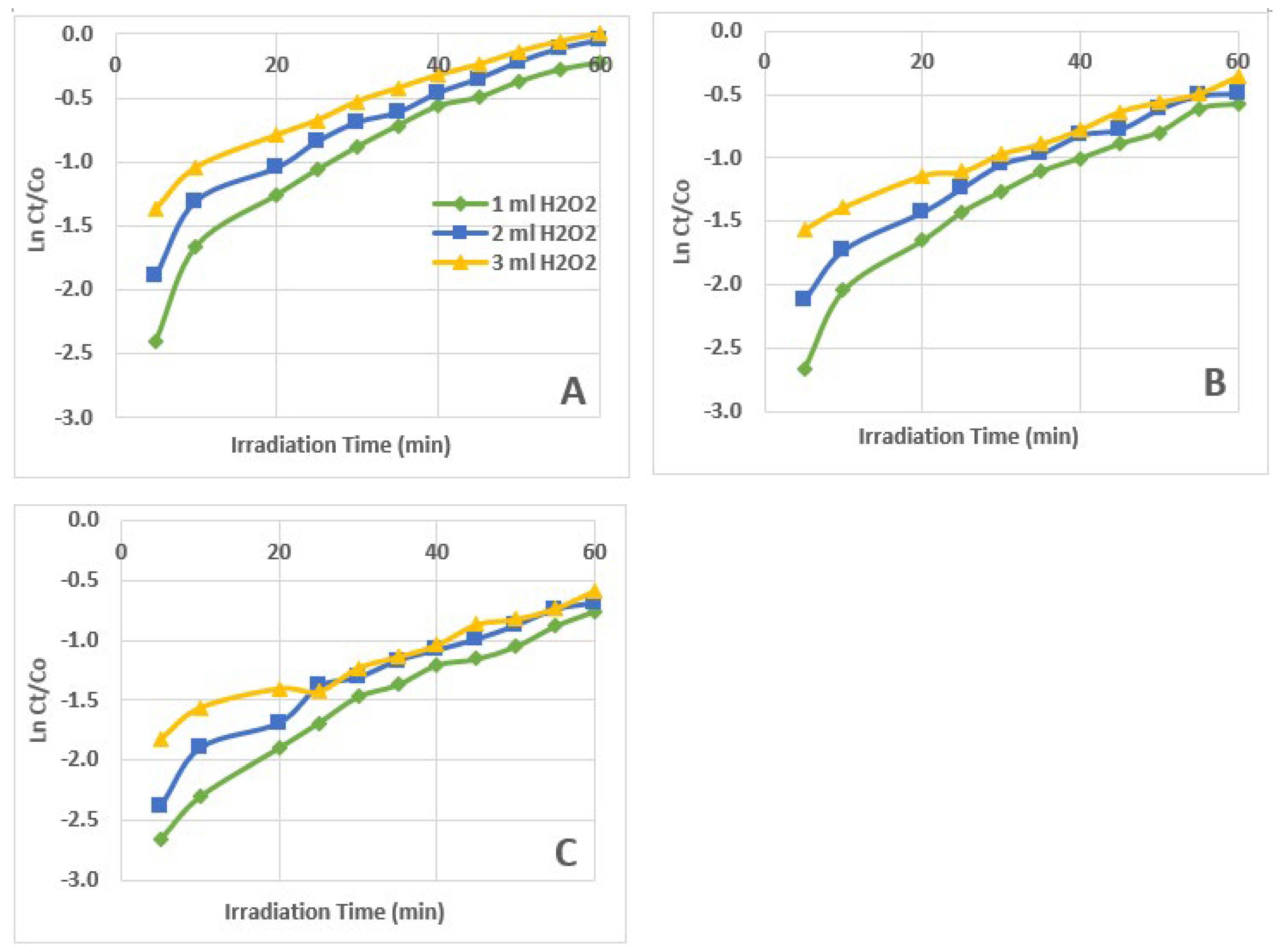
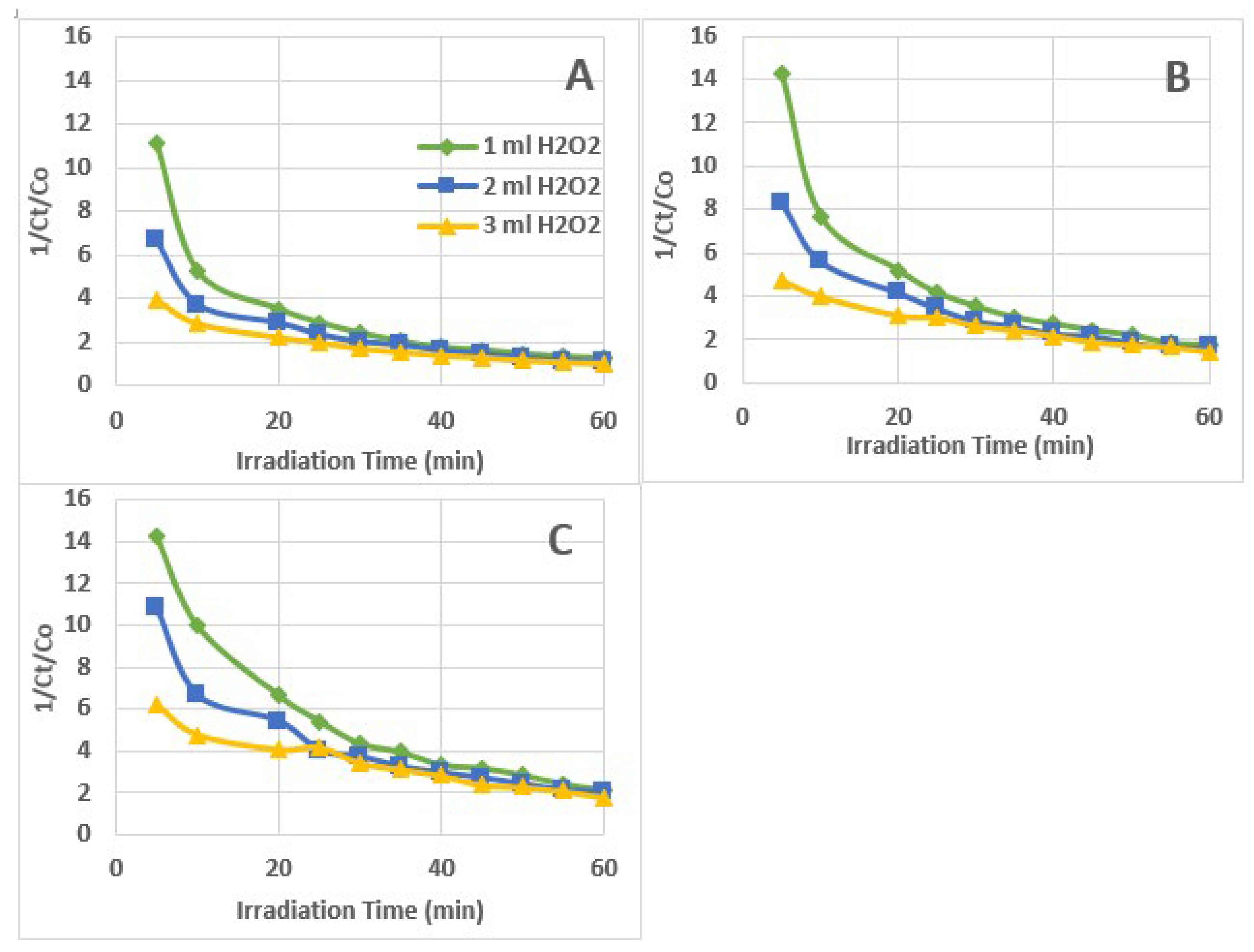
| Zero Order Kinetics (Ct/Co) vs t |
First Order Kinetics Ln (Ct/Co) vs t |
Second Order Kinetics 1/(Ct/Co) vs t |
||||
|---|---|---|---|---|---|---|
| 1 ppm AZT solution (50ml) | R2 | k, min-1 | R2 | k, min-1 | R2 | k, min-1 |
| 1 ml 30% H2O2 | 0.9968 | 0.0130 | 0.9050 | 0.0352 | 0.6569 | 0.1304 |
| 2 ml 30% H2O2 | 0.9912 | 0.0142 | 0.9418 | 0.0301 | 0.7418 | 0.0789 |
| 3 ml 30% H2O2 | 0.9974 | 0.0134 | 0.9671 | 0.0235 | 0.8589 | 0.0460 |
| 3 ppm AZT solution (50ml) | R2 | k, min-1 | R2 | k, min-1 | R2 | k, min-1 |
| 1 ml 30% H2O2 | 0.9925 | 0.0089 | 0.9308 | 0.0341 | 0.7107 | 0.1730 |
| 2 ml 30% H2O2 | 0.9923 | 0.0092 | 0.9559 | 0.0282 | 0.8164 | 0.1028 |
| 3 ml 30% H2O2 | 0.9760 | 0.0085 | 0.9936 | 0.0210 | 0.9491 | 0.0563 |
| 5 ppm AZT solution (50ml) | R2 | k, min-1 | R2 | k, min-1 | R2 | k, min-1 |
| 1 ml 30% H2O2 | 0.9848 | 0.0070 | 0.9647 | 0.0325 | 0.8150 | 0.1878 |
| 2 ml 30% H2O2 | 0.9908 | 0.0073 | 0.9462 | 0.0281 | 0.7872 | 0.1295 |
| 3 ml 30% H2O2 | 0.9848 | 0.0070 | 0.9819 | 0.0210 | 0.9405 | 0.0716 |
| R2 = Correlation Coefficient, k = Rate Constant | ||||||
| No. | Catalyst | Process conditions | Degradation Efficiency | Reference |
|---|---|---|---|---|
| 1. | 10%Cu2O/TiO2 nanotubes, 1.5 g/L | Visible light irradiation, AZT 100 μg/mL, pH 7 | 100% in 1.5h | [11] |
| 2. | PAC/Fe/Ag/Zn, 0.04 g/L | UV irradiation, pH 9, AZT 10 mg/L, pseudo-first-order kinetic | 99% in 2h | [13] |
| 3. | Ag@Bi4O5I2/SPION@calcium alginate | visible light (300W Xe lamp), AZT 10 mg/L, 0.3 mg /mL | 98% in 1.5h | [16] |
| 4. | K2S2O8 5.0 to 80.0 mg/L |
Simulated solar irradiation 30 min (1.5 KW xenon lamp, 290-800nm), 50.0 mL solution, AZT 1.0 mg/L, pH 5 | 70% in 2h | [17] |
| 5. | SrTiO3, 30 mg | UV irradiation, AZT 20 mg/L, pH 12, pseudo-first-order reaction kinetics | 99% in 4h | [18] |
| 6. | (a). MoS2 (b). MoS2-GO |
Visible light irradiation, AZT 100 mg/L | (a). 75% in 3h (b). 87% in 3h |
[19] |
| 7. | TiO2 P25, 227 ng/L (UV), 250 ng/L (solar) | UV irradiation 55 Wm−2, Solar 33W m−2, domestic wastewater |
52% (UV), 87% (solar) |
[20] |
| 8. | GO@Fe3O4/ZnO/SnO2, 1g/L, | UV-C Irradiation, AZT 30 mg/L, pH 3 | 90% in 2h | [21] |
| 9. | ZrO2/Ag@TiO2 | Visible light irradiation, AZT 20 mg/L (50 ml solution) | 90% in 8h | [23] |
| 10. | La-TiO2/active carbon | UV irradiation, pH 4, AZT 10 ppm | 96% in 1.5h | [25 |
| 11. | Fe (III)-oxalate | UV irradiation, AZT 10 mg/L, pH 4, | 83% in 2h | [26] |
| 12. | 35% H2O2, 482.0 ppm as oxidant | Simulated sunlight irradiation 500 W/m2, AZT 1 ppm (50 ml solution), pH 9 | 100% in 2h | [27] |
| 13. | No catalyst, only UV irradiation, | Xenon arc lamp (power 500 watts, UV power: 765 W/m2, Wavelength 300–800 nm), AZT 1.0 μg/L, pH 7, Temp. 40 °C | 90% in 7days | [31] |
| 14. | 7.5 mg/L FeSO4 + 27.5 mg/L H2O2 | Simulated solar irradiation, UV power 50mW/cm2, Wavelength 290–800 nm, AZT 3 mg/L, pH 3 | 92% in 0.5h | [32] |
| 15. | Gd3+ doped BiVO4, catalyst 2g/L | UV-LED irradiation, Wavelength 370 nm, power 4.65 mW/cm2, domestic wastewater | 63% in 3h | [33] |
| 16. | No catalyst, only UV irradiation, | UV irradiation power 163 mW/cm2, AZT 110 mg/L, pH 7. Temp. 65°C | 73% in 0.8h | [34] |
| 17. | Solar Photo-Fenton catalyst, Fe2+, 20 mg/L, H2O2 50 mg/L | Solar UV power 2.65 ± 0.68 mW/cm2, Reactor volume 2x15 L, AZT 25 ng/L, pH 7 | 24% in 3h | [35] |
| 18. | 3 ml of 30% H2O2 solution | UV irradiation intensity 500 W/m2, AZT 1.0 ppm (50 ml solution),pH 9.0, zero-order reaction kinetics | 100% in 1h | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




