Submitted:
12 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
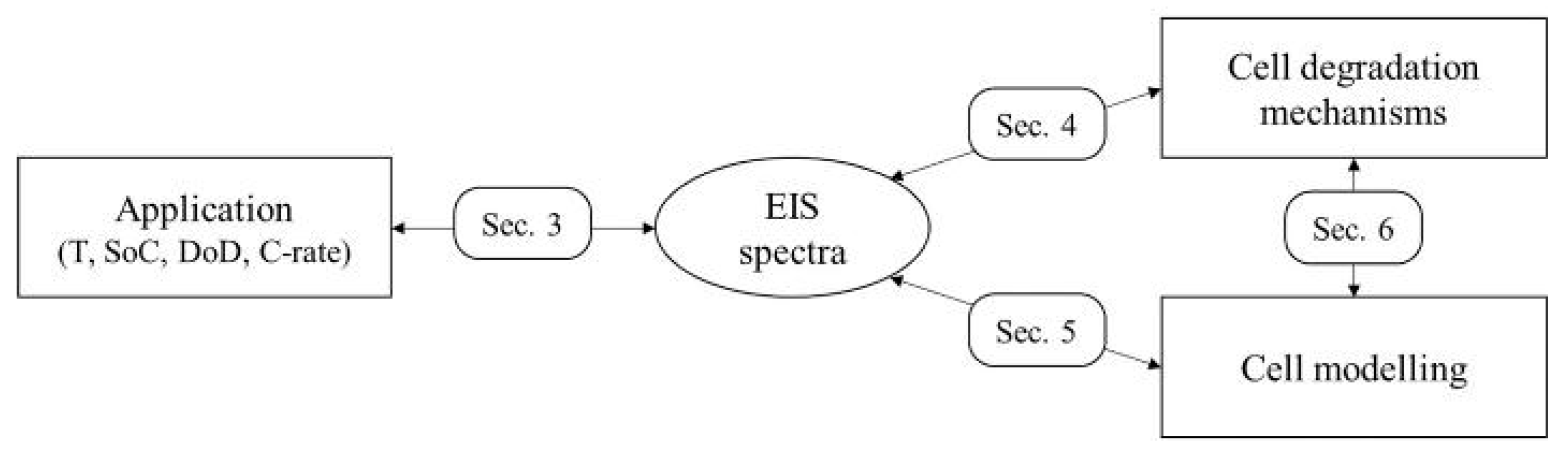
2. Methodology and Outline
3. How the Application Impacts on the EIS Spectra
4. How EIS Spectra Are Linked to Degradation
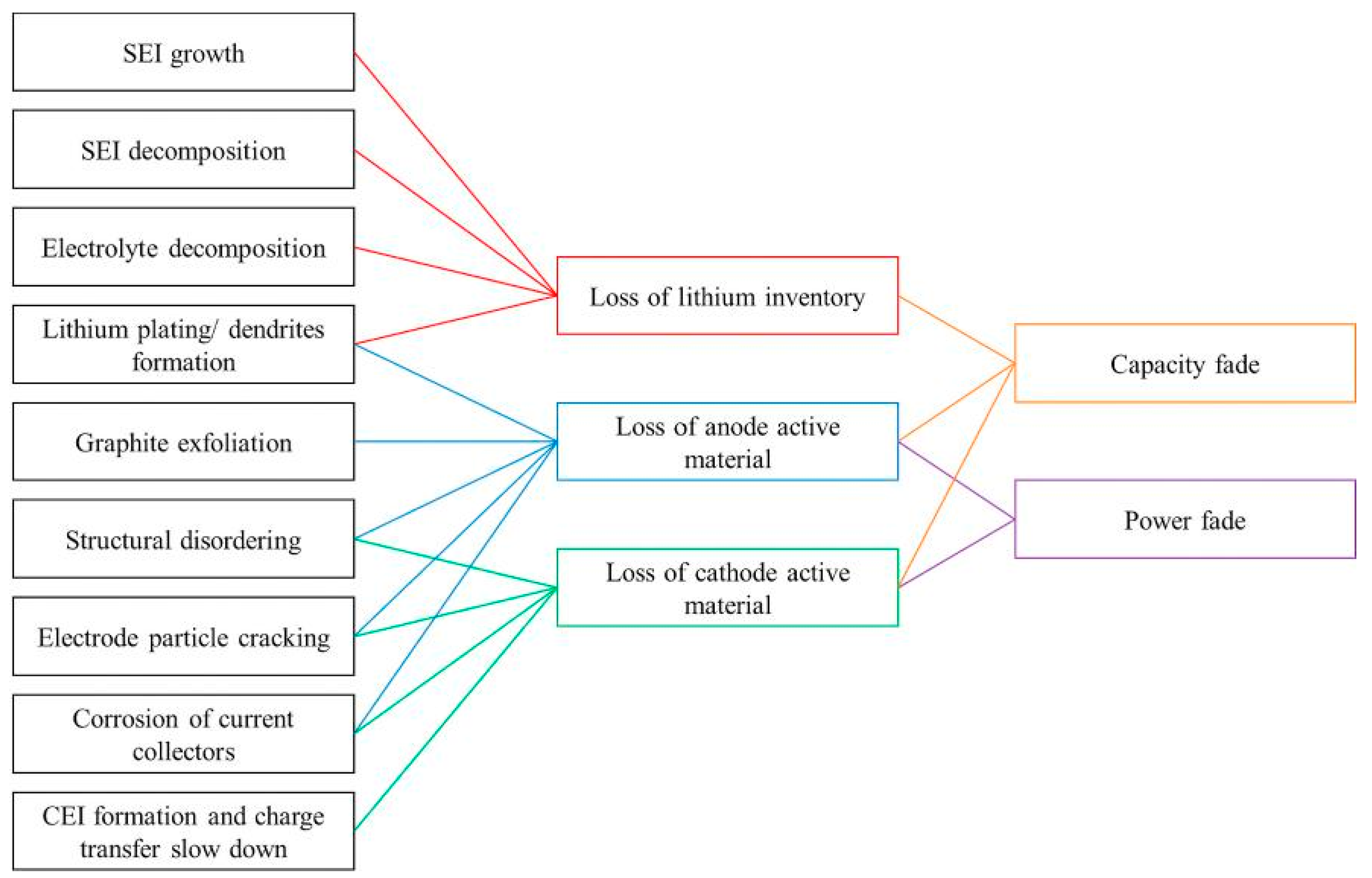
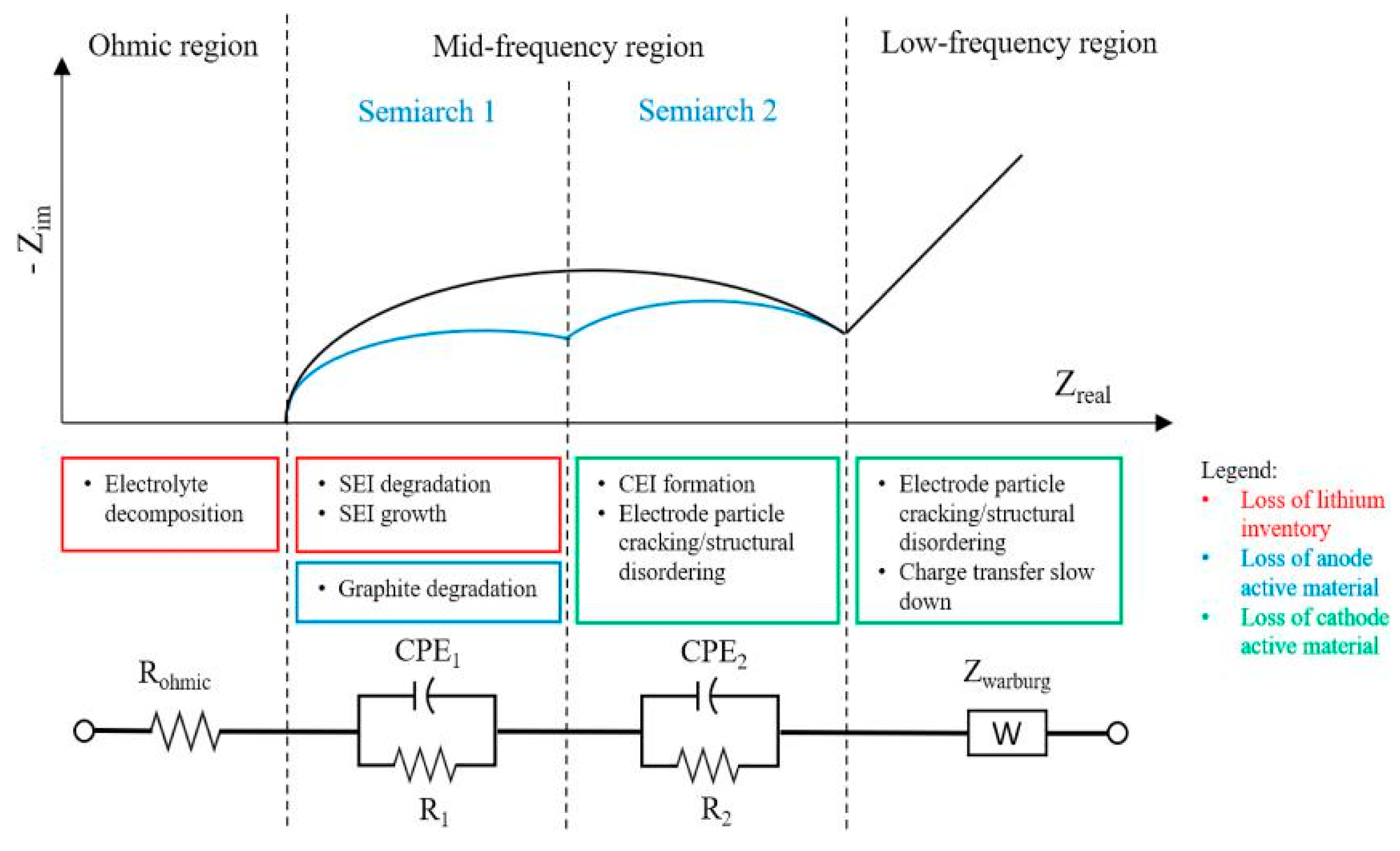
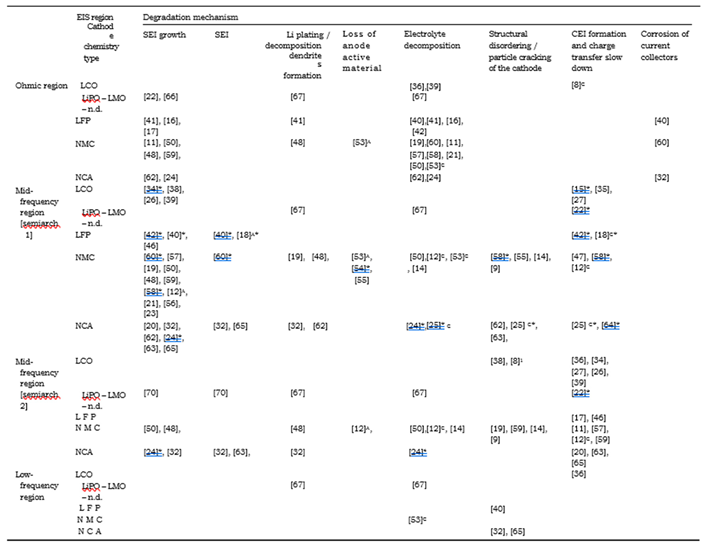
- Loss of Lithium inventory: lithium ions are consumed by processes such as parasitic reactions, decomposition reactions (SET layer decomposition, electrolyte decomposition), SET layer growth, lithium plating and they are no longer available for cycling between anode and cathode. So that, the available capacity of the cell decreases leading to capacity fade.
- Loss of anode active material: active material of the anode is no longer available due to structural disordering, particle cracking, graphite exfoliation, loss of electrical contact or blocking of the active sites by passivation surface layers. Consequently, the effects can be accounted both in capacity and power fade.
- Loss of cathode active material: this mode is similar to the previous one but it concerns the cathode. Active material is no longer available due to structural disordering, particle cracking, loss of electrical contact or Cathode Electrolyte Tnterface (CET) formation. This degradation mode leads both to capacity and power fades.
5. How EIS Spectra Are Modelled trough ECMs
6. Critical Assessment
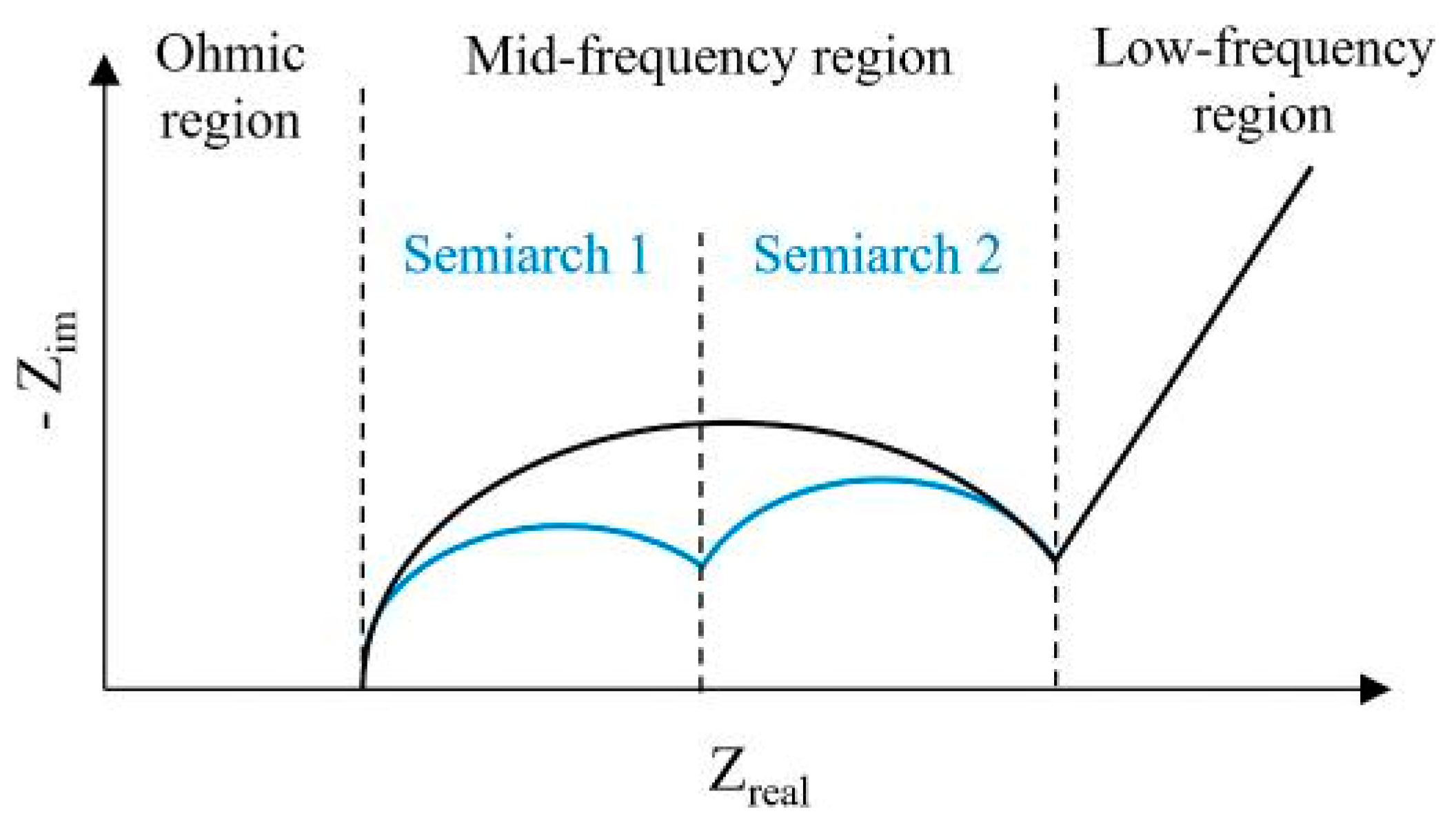
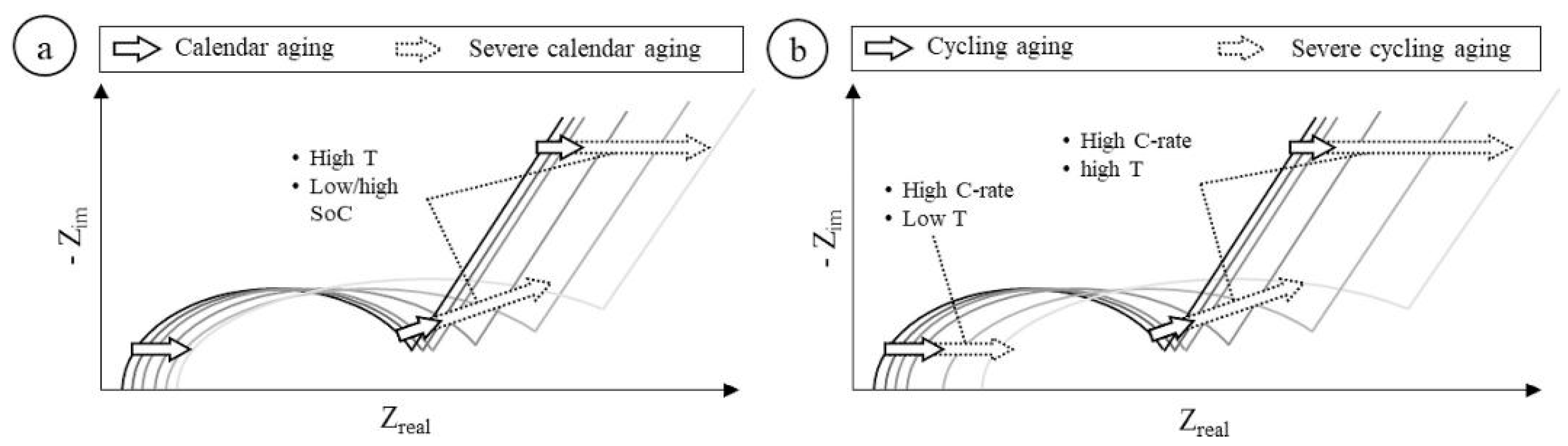
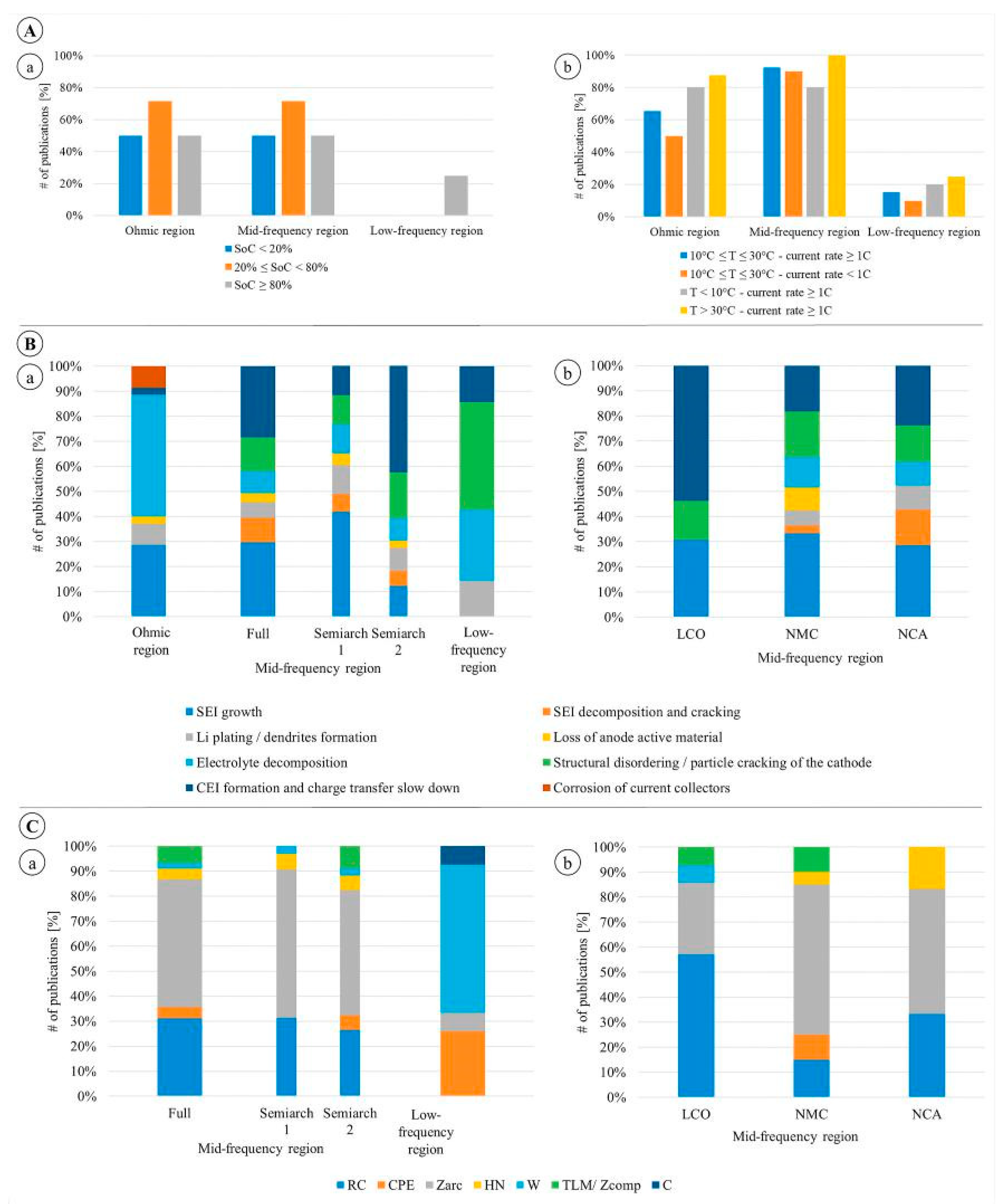
- For medium temperature (10 ◦C < T < 25 ◦C) and high current rate (equal or larger than 1C) experiments, the EIS curve is mainly impacted in the Mid-frequency region, specifically in the 2nd semiarch.
- For medium temperature and low current rate (lower than 1C) experiments, the behaviour is similar, impacting mainly Mid-frequency region. However, the EIS variations are smoother than in the previous case and are concentrated in the area of 1st semiarch.
- For low temperature (T < 10 ◦C) and high current rate experiments, the rise of ohmic resistance is stronger and the impact on EIS is equivalent both in the Ohmic and Mid-frequency regions (mainly in the 1st semiarch).
- For high temperature (T > 25 ◦C) and high current rate experiments, the impact is relevant in the ohmic region but mostly concentrated in the Mid-frequency region.
- Loss of lithium inventory processes. SEI layer growth and decom-position are mentioned in 40% of the studies, while electrolyte decomposition and lithium plating are mentioned in 14% of the cases.
- Loss of cathode active material. The degradation is attributed to structural disordering/particle cracking in 14% of the studies and CEI formation in 28% of the studies. Minor influence is given to loss of anode active material mentioned in less than 5% of the cases.
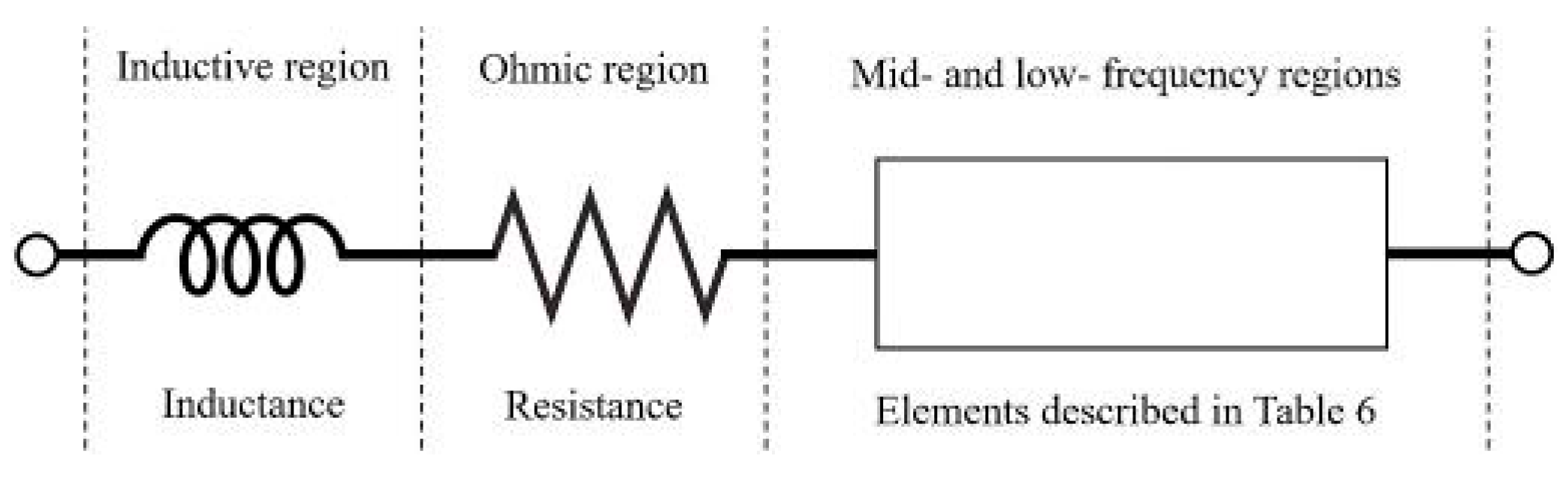
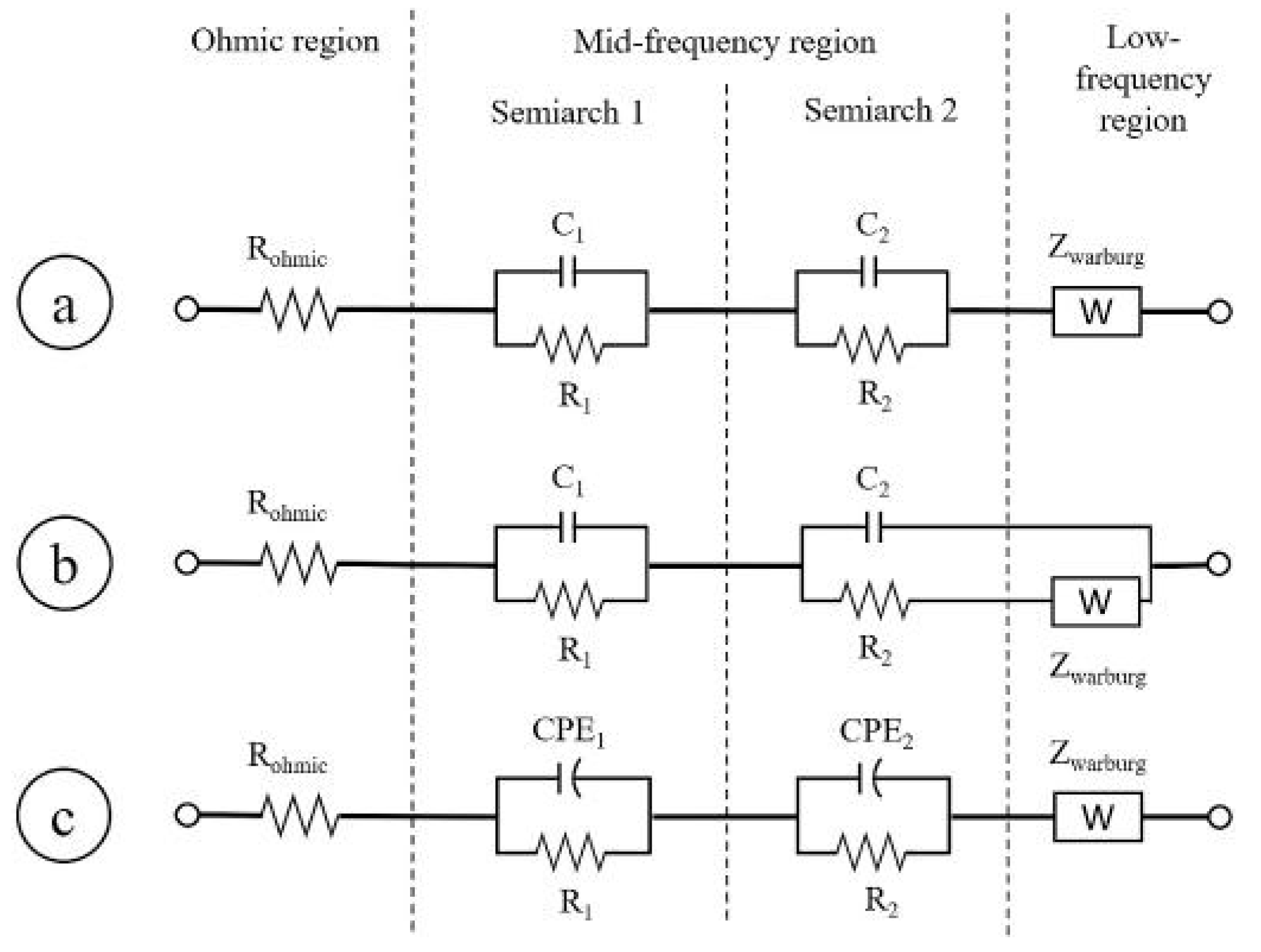
- RG element: it is the simplest element that can be used in this region, describing a perfect semicircle; it has been chosen in 31% of the cases under review.
- Zarc element: it has the advantage to reproduce the depression of the semicircles and it has been chosen in 51% of the cases. Tts disadvantage is the absence of Laplace transformation that make its use in time domain models problematic [90].
- HN element has been used in 6% of the cases, allowing to a similar description of depressed semicircle as the Zarc element.
- GPE has been used in 6% of the cases to specifically describe the diffusion processes that are usually attributed to Low-frequency region.
- Warburg element is applied in 59% of the cases. As described in Section 5, this is a more complex solution to describe the diffusive processes, with different structure depending on the boundary condition chosen for the specific problem [83]. This element has been placed on the resistive branch of RG and Zarc, such as in [10,20,32]. More complex configurations have been exploited in [35,38,52].
7. Conclusions
References
- European Commission, Communication from the Commission to the European parliament, the European council, the council, the European economic and social committee and the committee of the regions: the European Green Deal, Accessed: Oct. 15, 2020. [Online]. Available. https://ec.europa.eu/info/sites/info/files/eur opean-green-deal-communication_en.pdf, Dec. 11, 2019.
- Kabir, M.M.; Demirocak, D.E. Degradation mechanisms in Li-ion batteries: a state-of-the-art review: degradation mechanisms in Li-ion batteries: a state-of-the-art review, Int. J. Energy Res. 41 (14) (Nov. 2017) 1963–1986. [CrossRef]
- Tian, H.; Qin, P.; Li, K.; Zhao, Z. A review of the state of health for lithium-ion batteries: research status and suggestions, J. Clean. Prod. 261 (Jul. 2020) 120813. https://doi.org/10.1016/j.jclepro.2020.120813. [0] Y. Hu, S. Yurkovich, Y. Guezennec, B.J. Yurkovich, Electro-thermal battery model identification for automotive applications, J. Power Sources 196 (Jan. 2011), https://doi.org/10.1016/J.JPOWSOUR.2010.06.037. [1] M. Berecibar, I. Gandiaga, I. Villarreal, N. Omar, J. Van Mierlo, P. Van den Bossche, Critical review of state of health estimation methods of Li-ion batteries for real applications, Renew. Sustain. Energy Rev. 56 (Apr. 2016) 572–587, https://doi. org/10.1016/j.rser.2015.11.042. [2] P. Weicker, A Systems Approach to Lithium-Ion Battery Management, Artech House, 2013. [3] L. Ungurean, G. Caˆrstoiu, M.V. Micea, V. Groza, Battery state of health estimation: a structured review of models, methods and commercial devices: battery State of Health Estimation: a Structured Review, Int. J. Energy Res. 41 (2) (Feb. 2017) 151–181, https://doi.org/10.1002/er.3598.
- Zhou, J.; Notten, P.H.L. Studies on the degradation of Li-ion batteries by the use of microreference electrodes, J. Power Sources 177 (2) (Mar. 2008) 553–560. [CrossRef]
- Li, Y.; Bettge, M.; Polzin, B.; Zhu, Y.; Balasubramanian, M.; Abraham, D.P. ‘Understanding long-term cycling performance of Li1.2Ni0.15Mn0.55Co0.1O2–graphite lithium-ion cells’, J. Electrochem. Soc. 160 (5) (Feb. 2013) A3006. [CrossRef]
- Li, J.; Zhang, J.; Zhang, X.; Yang, C.; Xu, N.; Xia, B. Study of the storage performance of a Li-ion cell at elevated temperature, Electrochim. Acta 55 (3) (Jan. 2010) 927–934. [CrossRef]
- Stiaszny, B.; Ziegler, J.C.; Krauß, E.E.; Schmidt, J.P.; Ivers-Tiffe, E. ‘Electrochemical characterization and post-mortem analysis of aged LiMn2O4–Li(Ni0.5Mn0.3Co0.2) O2/graphite lithium ion batteries. Part I: cycle aging’, J. Power Sources 251 (Apr. 2014) 439–450. [CrossRef]
- Li, X.; et al. Degradation mechanisms of high capacity 18650 cells containing Si-graphite anode and nickel-rich NMC cathode, Electrochim. Acta 297 (Feb. 2019) 1109–1120. [CrossRef]
- Wu, M.-S.; Chiang, P.-C.J.; Lin, J.-C. Electrochemical investigations on advanced lithium-ion batteries by three-electrode measurements, J. Electrochem. Soc. 152 (1) (Nov. 2004) A47. [CrossRef]
- Buchberger, I.; et al. Aging analysis of graphite/LiNi1/3Mn1/3Co1/3O2 cells using XRD, PGAA, and AC impedance, J. Electrochem. Soc. 162 (14) (Oct. 2015) A2737. [CrossRef]
- Li, J.; Murphy, E.; Winnick, J.; Kohl, P.A. ‘Studies on the cycle life of commercial lithium ion batteries during rapid charge–discharge cycling’, J. Power Sources 102 (1) (Dec. 2001) 294–301. [CrossRef]
- Sarasketa-Zabala, E.; Aguesse, F.; Villarreal, I.; Rodriguez-Martinez, L.M.; Lo, C.M.; Kubiak, P. Understanding lithium inventory loss and sudden performance fade in cylindrical cells during cycling with deep-discharge steps, J. Phys. Chem. C 119 (2) (Jan. 2015) 896–906. [CrossRef]
- Wong, D.N.; Wetz, D.A.; Mansour, A.M.; Heinzel, J.M. The influence of high C rate pulsed discharge on lithium-ion battery cell degradation, in: 2015 IEEE Pulsed Power Conference (PPC), May 2015, pp. 1–6. [CrossRef]
- Kassem, M.; Delacourt, C. Postmortem analysis of calendar-aged graphite/LiFePO4 cells, J. Power Sources 235 (Aug. 2013) 159–171. [CrossRef]
- Jalkanen, K.; Karppinen, J.; Skogstro, L.; Laurila, T.; Nisula, M.; Vuorilehto, K. Cycle aging of commercial NMC/graphite pouch cells at different temperatures, Appl. Energy 154 (Sep. 2015) 160–172. [CrossRef]
- Zhang, Y.; Wang, C.-Y. Cycle-life characterization of automotive lithium-ion batteries with LiNiO2 cathode, J. Electrochem. Soc. 156 (7) (May 2009) A527. [CrossRef]
- Maheshwari, A.; Heck, M.; Santarelli, M. Cycle aging studies of lithium nickel manganese cobalt oxide-based batteries using electrochemical impedance spectroscopy, Electrochim. Acta 273 (May 2018) 335–348. [CrossRef]
- Yoshida, T.; et al. Degradation mechanism and life prediction of lithium-ion batteries, J. Electrochem. Soc. 153 (3) (Feb. 2006) A576. [CrossRef]
- Joshi, T.; Eom, K.; Yushin, G.; Fuller, T.F. Effects of dissolved transition metals on the electrochemical performance and SEI growth in lithium-ion batteries, J. Electrochem. Soc. 161 (12) (Sep. 2014) A1915. [CrossRef]
- Zhang, X. A novel quantitative electrochemical aging model considering side reactions for lithium-ion batteries, Electrochim. Acta 343 (May 2020) 136070. [CrossRef]
- Abraham, D.P.; Reynolds, E.M.; Sammann, E.; Jansen, A.N.; Dees, D.W. Aging characteristics of high-power lithium-ion cells with LiNi0.8Co0.15Al0.05O2 and Li4/3Ti5/3O4 electrodes, Electrochim. Acta 51 (3) (Oct. 2005) 502–510. https:// doi.org/10.1016/j.electacta.2005.05.008.
- Ning, G.; Haran, B.; Popov, B.N. Capacity fade study of lithium-ion batteries cycled at high discharge rates, J. Power Sources 117 (1) (May 2003) 160–169. [CrossRef]
- Zhang, D.; Haran, B.S.; Durairajan, A.; White, R.E.; Podrazhansky, Y.; Popov, B.N. Studies on capacity fade of lithium-ion batteries, J. Power Sources 91 (2) (Dec. 2000) 122–129. [CrossRef]
- Pastor-Ferna, C.; Yu, T.F.; Widanage, W.D.; Marco, J. Critical review of non-invasive diagnosis techniques for quantification of degradation modes in lithium-ion batteries, Renew. Sustain. Energy Rev. 109 (Jul. 2019) 138–159. [CrossRef]
- Waag, W.; Fleischer, C.; Sauer, D. Critical review of the methods for monitoring of lithium-ion batteries in electric and hybrid vehicles, J. Power Sources 258 (Jul. 2014) 321–339. [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and its Applications, Springer, New York, NY, 2014. New York.
- Vetter, J.; et al. Ageing mechanisms in lithium-ion batteries, J. Power Sources 147 (1–2) (Sep. 2005) 269–281. [CrossRef]
- Pastor-Ferna, C.; Uddin, K.; Chouchelamane, G.H.; Widanage, W.D.; Marco, J. A comparison between electrochemical impedance spectroscopy and incremental capacity-differential voltage as Li-ion diagnostic techniques to identify and quantify the effects of degradation modes within battery management systems, J. Power Sources 360 (Aug. 2017) 301–318. [CrossRef]
- Choi, W.; Shin, H.-C.; Kim, J.M.; Choi, J.-Y.; Yoon, W.-S. Modeling and applications of electrochemical impedance spectroscopy (EIS) for lithium-ion batteries, J. Electrochem. Sci. Technol. 11 (1) (Feb. 2020) 1–13. [CrossRef]
- de Vries, H.; Nguyen, T.T.; Veld, B.O.H. Increasing the cycle life of lithium ion cells by partial state of charge cycling, Microelectron. Reliab. 55 (11) (Nov. 2015) 2247–2253. [CrossRef]
- Osaka, T.; Nakade, S.; Rajama, M.; Momma, T. Influence of capacity fading on commercial lithium-ion battery impedance, J. Power Sources 119 (121) (Jun. 2003) 929–933. [CrossRef]
- Tro, U.; Kanoun, O.; Tra, H.-R. Characterizing aging effects of lithium ion batteries by impedance spectroscopy, Electrochim. Acta 51 (8) (2005) 1664–1672. [CrossRef]
- Kato, K.; Negishi, A.; Nozaki, K.; Tsuda, I.; Takano, K. PSOC cycle testing method for lithium-ion secondary batteries, J. Power Sources 117 (1) (May 2003) 118–123. [CrossRef]
- Mukoyama, D.; Momma, T.; Nara, H.; Osaka, T. ‘Electrochemical impedance analysis on degradation of commercially available lithium ion battery during charge–discharge cycling’, Chem. Lett. 41 (4) (Apr. 2012) 444–446. [CrossRef]
- Lyu, C.; Zhang, T.; Luo, W.; Wei, G.; Ma, B.; Wang, L. SOH estimation of lithium-ion batteries based on fast time domain impedance spectroscopy, in: 2019 14th IEEE Conference on Industrial Electronics and Applications (ICIEA), Jun. 2019, pp. 2142–2147. [CrossRef]
- Schindler, S.; Danzer, M.A. A novel mechanistic modeling framework for analysis of electrode balancing and degradation modes in commercial lithium-ion cells, J. Power Sources 343 (Mar. 2017) 226–236. [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. ‘Lithium plating in a commercial lithium-ion battery – a low-temperature aging study’, J. Power Sources 275 (Feb. 2015) 799–807. [CrossRef]
- Zhang, Y.; Wang, C.-Y.; Tang, X. Cycling degradation of an automotive LiFePO4 lithium-ion battery, J. Power Sources 196 (3) (Feb. 2011) 1513–1520. [CrossRef]
- Monem, M.A.; et al. Lithium-ion batteries: evaluation study of different charging methodologies based on aging process, Appl. Energy 152 (Aug. 2015) 143–155. [CrossRef]
- Stroe, D.-I.; Swierczynski, M.; Stroe, A.-I.; Kaer, S.K.; Teodorescu, R. Lithium-ion battery power degradation modelling by electrochemical impedance spectroscopy, IET Renew. Power Gener. 11 (9) (Jul. 2017) 1136–1141. [CrossRef]
- Zavalis, T.G.; Klett, M.; Kjell, M.H.; Behm, M.; Lindstro, R.W.; Lindbergh, G. Aging in lithium-ion batteries: model and experimental investigation of harvested LiFePO4 and mesocarbon microbead graphite electrodes, Electrochim. Acta 110 (Nov. 2013) 335–348. [CrossRef]
- Wang, X.; Wei, X.; Dai, H. Estimation of state of health of lithium-ion batteries based on charge transfer resistance considering different temperature and state of charge, J. Energy Storage 21 (Feb. 2019) 618–631. [CrossRef]
- Leng, Y.; et al. Electrochemical cycle-life characterization of high energy lithium-ion cells with thick Li(Ni0.6Mn0.2Co0.2)O2 and graphite electrodes, J. Electrochem. Soc. 164 (6) (Mar. 2017) A1037. [CrossRef]
- Schuster, S.F. Nonlinear aging characteristics of lithium-ion cells under different operational conditions, J. Energy Storage 1 (Jun. 2015) 44–53. [CrossRef]
- Zhou, X.; Huang, J.; Pan, Z.; Ouyang, M. Impedance characterization of lithium-ion batteries aging under high-temperature cycling: importance of electrolyte-phase diffusion, J. Power Sources 426 (Jun. 2019) 216–222. [CrossRef]
- Westerhoff, U.; Kurbach, K.; Lienesch, F.; Kurrat, M. Analysis of lithium-ion battery models based on electrochemical impedance spectroscopy, Energy Technol. 4 (12) (Dec. 2016) 1620–1630. [CrossRef]
- Yang, Q.; Xu, J.; Li, X.; Xu, D.; Cao, B. State-of-health estimation of lithium-ion battery based on fractional impedance model and interval capacity, Int. J. Electr. Power Energy Syst. 119 (Jul. 2020) 105883. [CrossRef]
- Aurora, P.; Ramaswamy, N.; Han, T.; Adjemian, K. Electrochemical impedance spectroscopic analysis of lithium-ion battery aging mechanisms, ECS Meet. Abstr. (2013). [CrossRef]
- Klett, M.; et al. Electrode behavior RE-visited: monitoring potential windows, capacity loss, and impedance changes in Li1.03(Ni0.5Co0.2Mn0.3)0.97O2/silicon- graphite full cells, J. Electrochem. Soc. 163 (6) (Mar. 2016) A875. [CrossRef]
- Heins, T.P.; Schlüter, N.; Ernst, S.T.; Schro, U. On the interpretation of impedance spectra of large-format lithium-ion batteries and its application in aging studies, Energy Technol. 8 (2) (2020) 1900279. [CrossRef]
- Heins, T.P.; Leithoff, R.; Schlüter, N.; Schro, U.; Dro, K. Impedance spectroscopic investigation of the impact of erroneous cell assembly on the aging of lithium-ion batteries, Energy Technol. 8 (2) (2020) 1900288. [CrossRef]
- Xiong, R.; Tian, J.; Mu, H.; Wang, C. A systematic model-based degradation behavior recognition and health monitoring method for lithium-ion batteries, Appl. Energy 207 (Dec. 2017) 372–383. [CrossRef]
- Stiaszny, B.; Ziegler, J.C.; Krauß, E.E.; Zhang, M.; Schmidt, J.P.; Ivers-Tiffe, E. ‘Electrochemical characterization and post-mortem analysis of aged LiMn2O4–NMC/graphite lithium ion batteries part II: calendar aging’, J. Power Sources 258 (Jul. 2014) 61–75. [CrossRef]
- Schmitt, J.; Maheshwari, A.; Heck, M.; Lux, S.; Vetter, M. Impedance change and capacity fade of lithium nickel manganese cobalt oxide-based batteries during calendar aging, J. Power Sources 353 (Jun. 2017) 183–194. [CrossRef]
- Sabet, P.S.; Warnecke, A.J.; Meier, F.; Witzenhausen, H.; Martinez-Laserna, E.; Sauer, D.U. ‘Non-invasive yet separate investigation of anode/cathode degradation of lithium-ion batteries (nickel–cobalt–manganese vs. graphite) due to accelerated aging’, J. Power Sources 449 (Feb. 2020) 227369. [CrossRef]
- Ecker, M.; et al. Development of a lifetime prediction model for lithium-ion batteries based on extended accelerated aging test data, J. Power Sources 215 (Oct. 2012) 248–257. [CrossRef]
- Waag, W.; Ka, S.; Sauer, D.U. Experimental investigation of the lithium-ion battery impedance characteristic at various conditions and aging states and its influence on the application, Appl. Energy 102 (Feb. 2013) 885–897. [CrossRef]
- Sabet, P.S.; Stahl, G.; Sauer, D.U. Non-invasive investigation of predominant processes in the impedance spectra of high energy lithium-ion batteries with Nickel-Cobalt-Aluminum cathodes, J. Power Sources 406 (Dec. 2018) 185–193. [CrossRef]
- Wong, D.; Shrestha, B.; Wetz, D.A.; Heinzel, J.M. Impact of high rate discharge on the aging of lithium nickel cobalt aluminum oxide batteries, J. Power Sources 280 (Apr. 2015) 363–372. [CrossRef]
- Jungst, R.G.; et al. Accelerated calendar and pulse life analysis of lithium-ion cells, J. Power Sources 119–121 (Jun. 2003) 870–873. [CrossRef]
- Sun, H. Quantitative analysis of degradation modes of lithium-ion battery under different operating conditions, Energies 14 (2) (Jan. 2021), 2. [CrossRef]
- Galeotti, M.; Cina, L.; Giammanco, C.; Cordiner, S.; Di Carlo, A. Performance analysis and SOH (state of health) evaluation of lithium polymer batteries through electrochemical impedance spectroscopy, Energy 89 (Sep. 2015) 678–686. [CrossRef]
- Popp, H.; Einhorn, M.; Conte, F.V. ‘Capacity Decrease vs. Impedance Increase of Lithium Batteries. A comparative study.’, p. 6.
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of lithium-ion batteries, Adv. Mater. 30 (33) (2018) 1800561. [CrossRef]
- Illig, J. ‘Physically Based Impedance Modelling of Lithium-Ion Cells’, p. 231.
- Eddahech, A.; Briat, O.; Henry, H.; Dele, J.-Y.; Woirgard, E.; Vinassa, J.-M. Ageing monitoring of lithium-ion cell during power cycling tests, Microelectron. Reliab. 51 (9) (Sep. 2011) 1968–1971. [CrossRef]
- Skoog, S.; David, S. Parameterization of linear equivalent circuit models over wide temperature and SOC spans for automotive lithium-ion cells using electrochemical impedance spectroscopy, J. Energy Storage 14 (Dec. 2017) 39–48. [CrossRef]
- Verma, P.; Maire, P.; Nova, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries, Electrochim. Acta 55 (22) (Sep. 2010) 6332–6341. [CrossRef]
- Agubra, V.; Fergus, J. Lithium ion battery anode aging mechanisms, Materials 6 (4) (Mar. 2013) 1310–1325. [CrossRef]
- Pop, V.; Bergveld, H.J.; Regtien, P.P.L.; Veld, J.H.G.O.H.; Danilov, D.; Notten, P.H.L. Battery aging and its influence on the electromotive force, J. Electrochem. Soc. 154 (8) (May 2007) A744. [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Synthesize battery degradation modes via a diagnostic and prognostic model, J. Power Sources 219 (Dec. 2012) 204–216. [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells, J. Power Sources 341 (Feb. 2017) 373–386. [CrossRef]
- Ivers-Tiffee, E.; Weber, A. Evaluation of electrochemical impedance spectra by the distribution of relaxation times, J. Ceram. Soc. Jpn. 125 (4) (2017) 193–201. [CrossRef]
- Nagasubramanian, G. Two- and three-electrode impedance studies on 18650 Li-ion cells, J. Power Sources 87 (1) (Apr. 2000) 226–229. [CrossRef]
- Macdonald, J.R. Note on the parameterization of the constant-phase admittance element, Solid State Ion. 13 (2) (May 1984) 147–149. [CrossRef]
- Abouzari, M.R.S.; Berkemeier, F.; Schmitz, G.; Wilmer, D. On the physical interpretation of constant phase elements, Solid State Ion. 180 (14) (Jun. 2009) 922–927. [CrossRef]
- Macdonald, J.R. New aspects of some small-signal ac frequency response functions, Solid State Ion. 15 (2) (Mar. 1985) 159–161. [CrossRef]
- Karan, N.K.; Pradhan, D.K.; Thomas, R.; Natesan, B.; Katiyar, R.S. ‘Solid polymer electrolytes based on polyethylene oxide and lithium trifluoro- methane sulfonate (PEO–LiCF3SO3): ionic conductivity and dielectric relaxation’, Solid State Ion. 179 (19–20) (2008) 689–696. [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, second ed., Wiley, 2005.
- Jacobsen, T.; West, K. Diffusion impedance in planar, cylindrical and spherical symmetry, Electrochim. Acta 40 (2) (Feb. 1995) 255–262. [CrossRef]
- Bisquert, J. Theory of the impedance of electron diffusion and recombination in a thin layer, J. Phys. Chem. B 106 (2) (Jan. 2002) 325–333. [CrossRef]
- Nobili, F.; Tossici, R.; Marassi, R.; Croce, F.; Scrosati, B. An AC impedance spectroscopic study of LixCoO2 at different temperatures, J. Phys. Chem. B 106 (15) (Apr. 2002) 3909–3915. [CrossRef]
- Bartoszek, J.; Liu, Y.-X.; Karczewski, J.; Wang, S.-F.; Mrozinski, A.; Jasinski, P. Distribution of relaxation times as a method of separation and identification of complex processes measured by impedance spectroscopy, in: 2017 21st European Microelectronics and Packaging Conference (EMPC) & Exhibition, Warsaw, Sep. 2017, pp. 1–5. [CrossRef]
- He, H.; Xiong, R.; Fan, J. Evaluation of lithium-ion battery equivalent circuit models for state of charge estimation by an experimental approach, Energies 4 (4) (Mar. 2011) 582–598. [CrossRef]
- Witzenhausen, D.-W.H. ‘Elektrische Batteriespeichermodelle: Modellbildung, Parameteridentifikation und Modellreduktion’, p. 276.
- Andre, D.; Meiler, M.; Steiner, K.; Walz, H.; Soczka-Guth, T.; Sauer, D.U. Characterization of high-power lithium-ion batteries by electrochemical impedance spectroscopy. II: Modelling, J. Power Sources 196 (12) (Jun. 2011) 5349–5356. [CrossRef]
- Gomez, J.; Nelson, R.; Kalu, E.E.; Weatherspoon, M.H.; Zheng, J.P. Equivalent circuit model parameters of a high-power Li-ion battery: thermal and state of charge effects, J. Power Sources 196 (10) (May 2011) 4826–4831. [CrossRef]
- Gao, Y.; Jiang, J.; Zhang, C.; Zhang, W.; Ma, Z.; Jiang, Y. Lithium-ion battery aging mechanisms and life model under different charging stresses, J. Power Sources 356 (Jul. 2017) 103–114. [CrossRef]
| Ref | Author | Available | Cathode | Testing | Invasive |
|---|---|---|---|---|---|
| online | chemistry type | type (calendar/ cycling) | Techniques | ||
| [34] | H. De Vries | 2015 | LCO | cycling | Not used |
| [10] | J. Li | 2010 | LCO | calendar | XRD, SEM, |
| half cells | |||||
| [35] | T. Osaka | 2003 | LCO | calendar, | Not used |
| cycling | |||||
| [36] | U. Tro¨ltzsch | 2005 | LCO | cycling | Not used |
| [37] | K. Kato | 2003 | LCO | cycling | Not used |
| [13] | M. -S. Wu | 2004 | LCO | cycling | Three |
| electrodes | |||||
| [38] | D. | 2012 | LCO | cycling | Not used |
| Mukoyama | |||||
| [15] | J. Li | 2001 | LCO | cycling | SEM, TEM, |
| XRD | |||||
| [27] | D. Zhang | 2000 | LCO | cycling | Half cells, |
| EPMA | |||||
| [8] | J. Zhou | 2007 | LCO | cycling | EDS, XRD, half cells, TEM |
| [26] | G. Ning | 2003 | LCO | cycling | Half cells, SEM |
| [39] | C. Lyu | 2019 | LCO | cycling | Not used |
| [67] | H. Popp | 2012 | LMO | cycling | Not used |
| [22] | T. Yoshida | 2006 | LMO | calendar | FIB, SEM, XPS |
| [66] | M. Galeotti | 2016 | LiPO | cycling | Not used |
| [40] | S. Schindler | 2017 | LFP | cycling | Not used |
| [41] | M. Petzl | 2014 | LFP | cycling | Half cells |
| [16] | E. | 2014 | LFP | cycling | SEM, EDS, |
| Sarasketa-Zabala | XRD | ||||
| [42] | Y. Zhang | 2010 | LFP | cycling | Not used |
| [17] | D. Wong | 2015 | LFP | cycling | XRD, SEM, |
| XAS | |||||
| [43] | M. A. | 2015 | LFP | cycling | Not used |
| Monem | |||||
| [44] | D.-I. Stroe | 2017 | LFP | calendar | Not used |
| [18] | M. Kassem | 2013 | LFP | calendar | FTIR, XRD, |
| SEM, half cells | |||||
| [45] | T. G. Zavalis | 2013 | LFP | calendar, | Three |
| cycling | electrodes, | ||||
| SEM | |||||
| [46] | X. Wang | 2019 | LFP | cycling | Not used |
| [11] | B. Stiaszny | 2013 | NMC | cycling | Half cells, |
| SEM, light | |||||
| microscopy | |||||
| [47] | Y. Leng | 2017 | NMC | cycling | Three |
| electrodes | |||||
| [21] | A. | 2018 | NMC | cycling | SEM, EDS |
| Maheshwari | |||||
| [48] | S. F. | 2015 | NMC | cycling | SEM |
| Schuster | |||||
| [49] | X. Zhou | 2019 | NMC | cycling | Not used |
| [50] | U. | 2016 | NMC | cycling | Not used |
| Westerhoff | |||||
| [12] | X. Li | 2019 | NMC | cycling | Half cells, |
| SEM, EDS | |||||
| [19] | K. Jalkanen | 2015 | NMC | cycling | XRD, SEM, |
| EDS | |||||
| [51] | Q. Yang | 2020 | NMC | cycling | Not used |
| [52] | P. Aurora | 2013 | NMC | cycling | Not used |
| [53] | M. Klett | 2016 | NMC | cycling | Three |
| electrodes | |||||
| [54] | T. P. Heins | 2019 | NMC | cycling | Three |
| electrodes | |||||
| [55] | T. P. Heins | 2020 | NMC | cycling | Not used |
| [56] | R. Xiong | 2017 | NMC | cycling | Not used |
| [14] | I. | 2015 | NMC | cycling | XRD, PGAA, |
| [9] | Buchberger | 2013 | NMC | cycling | Three- |
| Y. Li | electrodes | ||||
| Three electrodes, SEM, XRD, XAS, Raman Spectroscopy | |||||
| [23] | T. Joshi | 2014 | NMC | cycling | SEM, XPS |
| [57] | B. Stiaszny | 2014 | NMC | calendar | Half cells, |
| SEM, light | |||||
| microscopy | |||||
| [58] | J. Schmitt | 2017 | NMC | calendar | Not used |
| [59] | P. S. Sabet | 2020 | NMC | calendar, | Not used |
| cycling | |||||
| [60] | M. Ecker | 2012 | NMC | calendar, | Not used |
| cycling | |||||
| [61] | W. Waag | 2013 | NMC | calendar, | Not used |
| cycling | |||||
| [20] | Y. Zhang | 2009 | NCA | cycling | Three |
| electrodes | |||||
| [32] | C. P. | 2017 | NCA | cycling | Not used |
| Ferna`ndez | |||||
| [62] | P. S. Sabet | 2018 | NCA | cycling | Half cells |
| [24] | X. Zhang | 2020 | NCA | cycling | XRD, XPS, |
| SEM | |||||
| [63] | D. Wong | 2015 | NCA | cycling | Not used |
| [64] | R. G. Jungst | 2003 | NCA | calendar | Not used |
| [25] | D. P. | 2005 | NCA | calendar | Half-cells, XPS |
| Abraham | |||||
| [65] | H. Sun | 2021 | NCA | cycling | Not used |
| [70] | A. Eddahech | 2011 | n.d. | cycling | Not used |
| Ref | Cathode chemistry type | Time of storage | Temperature | SoC | Ohmic region | Mid-frequency region | Low-frequency region | ||
|---|---|---|---|---|---|---|---|---|---|
| Semiarch 1 Semiarch 2 | |||||||||
| [10] | LCO | 100 days | 55 ◦C | 0%, 50% | – | X (+) | X (+) | – | |
| [10] | LCO | 100 days | 55 ◦C | 100% | – | X (+) | X (+) | X (+) | |
| [35] | LCO | 20 days | 50 ◦C | – | – | – | X (+) | – | |
| [22] | LMO | 400 days | 40 ◦C | 50% | X (+) | X (+) | – | ||
| [44] | LFP | 12–24 months | 40 ◦C–55 ◦C | 10%, 50%, | 90% | X (+) | – | – | – |
| [18] | LFP | 240 days | 60 ◦C | 100% | – | X (+) | – | ||
| [45] | LFP | Up to EOL of cycling test | 22 ◦C | 60% | X (- ) | – | – | – | |
| [57] | NMC | 300 days | 60 ◦C | 100% | X (+) | X (+) | X (++) | – | |
| [58] | NMC | 375 days | 45 ◦C | 100% | X (+) | X (+) | – | ||
| [59] | NMC | 700 days | 35 ◦C | 100% | X (+) | X (+) | X (+) | – | |
| [60] | NMC | 420 days | 35 ◦C, 50 ◦C, 65 ◦C | 50% | X (+) | X (++) | – | ||
| [61] | NMC | 18 months | 50 ◦C–25 ◦C | – | X (+) | X (++) | – | ||
| [64] | NCA | 140 days | 55 ◦C | 100% | – | X (+) | – | ||
| [25] | NCA | 142 days | 55 ◦C | >50% | – | X (+) | X (+) | ||
| Ref | Cathode chemistry type | Number of cycles | SoH [%] | Temperature | DoD [%] | C-rate | Ohmic region |
Mid-frequency region | Low-frequency region | |
|---|---|---|---|---|---|---|---|---|---|---|
| Semiarch 1 | Semiarch 2 | |||||||||
| [34] | LCO | 890 | 80% | Tamb | 100% | C/2 d; 1C c | – | X (++) | X (+) | – |
| [35] | LCO | 20 | 78% | Tamb | 100% | 2C | – | X (-) | X (+) | – |
| [36] | LCO | 230 | 86% | 19 ◦C | 100% | C/2 d; 1C c | X (+) | – | X (+) | X (+) |
| [37] | LCO | 19,800 | 68% | 25 ◦C | 10% | 0.1/0.3C | X (+) | – | X (++) | |
| [13] | LCO | 200 | 94% | – | 100% | 0.2C | X (+) | X (+) | X (+) | |
| [38] | LCO | 550 | <23% | Tamb | 100% | 1C | X (+) | X (+) | X (+) | |
| [15] | LCO | 286 | 70% | Tamb | 100% | 1C | X (-) | X (+) | X (+) | |
| [27] | LCO | 800 | 68% | – | 100% | 0.8C | – | X (+) | X (+) | |
| [8] | LCO | 500 | 87% | – | 100% | 0.5C | – | X (+) | X (++) | |
| [26] | LCO | 300 | 83% | Tamb | 100% | 3C | – | X (+) | X (+) | |
| [39] | LCO | 400 | – | 25 ◦C | 100% | 1C | X (+) | X (+) | appears X | |
| [39] | LCO | 360 | – | 25 ◦C | 100% | 2C | X (+) | X (+) | appears and X | – |
| (++) | ||||||||||
| [67] [66] |
LMO LiPO | 1400 315 |
67% 60% |
23 ◦C 25 ◦C | 100%100% | 2C 1C | X (+) X (+) | X (+) X (+) | appears X X (+) | X (+) – |
| [70] | n.d. | 3.8 × 105 | 81% | 25 ◦C | 100% | Pulses 1C up to | X (+) | – | appears and X | – |
| 7C | (++) | |||||||||
| [40] | LFP | 100 | 80% | -22 ◦C | 100% | 2C | X (+) | X (+) | X (+) | |
| [41] | LFP | 300 | 74% | -22 ◦C | 100% | 1C | X (+) | – | – | – |
| [16] [42] |
LFP LFP | 3200 600 |
80% 86% |
30 ◦C 45 ◦C | 100%50% | 1C US EPA UDDS |
X (+) X (+) | – | – X (++) |
– X (+) |
| [17] | LFP | 40 | 0% | – | 100% | Pulses ≤ 15C | X (+) | – | appears and X | – |
| (+) | ||||||||||
| [43] | LFP | 500 | 95% | Tamb | 100% | Pulses ≤ 1C c; | X (+) | – | X (+) | X (+) |
| 5C d | ||||||||||
| [45] | LFP | n.d. | 80% | 22 ◦C | 100% | HDC | X (+) | – | – | – |
| [46] | LFP | 800 | 80% | 45 ◦C | 100% | 2C | X (+) | X (+) | X (+) | – |
| [11] | NMC | 800 | 5% | Tamb | 100% | 1C | X (++) | X (-) | X (+) | – |
| [47] | NMC | 1500 | 75% | 25 ◦C | 100% | 1C c; 2C d | – | X (+) | X (+) | – |
| [21] | NMC | 500 | 55% | 20 ◦C | 50% | 1C c; 2C d | X (++) | X (+) | – | – |
| [48] | NMC | 977 | 50% | 35 ◦C | 100% | 1C | X (+) | X (+) | – | |
| [49] | NMC | n.d. | 71% | 45 ◦C | 100% | ≥1C | X (+) | X (+) | X (+) | X (+) |
| [50] | NMC | n.d. | 86% | Range 50 to | 100% | 1C | X (+) | X (+) | X (++) | |
| -20 ◦C | ||||||||||
| [12] | NMC | 250 | 84% | Tamb | 100% | 0.5C | – | X (+) | X (-) | – |
| [19] | NMC | 2500 | 80% | Tamb | 100% | 1C | X (+) | X (+) | X (+) | – |
| [19] | NMC | 1500 | 80% | 45 ◦C c; 65 ◦C d | 100% | 1C | X (++) | X (+) | X (++) | – |
| [51] | NMC | 550 | 82% | Tamb | 100% | C/3 | X (+) | X (+) | X (++) | – |
| [52] | NMC | 50 | 97% | – | 100% | 1C | X (+) | – | X (+) | – |
| [53] | NMC | 50 | 95% | 30 ◦C | 100% | C/3 | X (+) | X (+) | – | X (+) |
| [54] | NMC | 1000 | 95% | – | 100% | 1.5C | – | X (+) | – | |
| [55] | NMC | 450 | 85% | 20 ◦C | 100% | 1C | – | X (+) | – | – |
| [56] | NMC | 650 | 70% | 10 ◦C | 100% | 1C c; 1C, 2C, 3C d | X (+) | |||
| [14] | NMC | 300 | 60% | 25 ◦C, 60 ◦C | 100% | 1C | – | X (+) | X (+) | |
| [9] | NMC | 1500 | 43% | 30 ◦C | 100% | 0.5C | – | X (++) | X (+) | |
| [23] | NMC | 50 | 96% | Tamb | 100% | 0.5C | – | X (+) | – | |
| [59] | NMC | 3000 | 92%, 85% | 25 ◦C | 80%, | C/3 c; 1C d | X (+) | X (+) | X (+) | |
| 100% | ||||||||||
| [61] | NMC | 200, 1900 | 94%, 90% | 40 ◦C to -10 ◦C | 100% | EV profile | X (+) | X (++) | ||
| [20] | NCA | 5250 | 82% | 40 ◦C | 100% | 5C | X (+) | X (+) | X (++) | |
| [32] | NCA | 500 | 84% | 25 ◦C | 100% | C/2 c; 1C d | X (+) | X (+) | X (+) | |
| [62] | NCA | 120 | 70% | ≥ -10 ◦C | 100% | ≤3C | X (+) | X (+) | X (+) | |
| [24] | NCA | 400 | 75%, 72%, | 25 ◦C | 100% | 1C, | X (++) | X (+) | ||
| 64% | 2C, 3C | |||||||||
| [63] | NCA | 400 | 80% | Tamb | 100% | Pulses ≤ 83C | X (+) | X (+) | X (++) | – |
| [65] | NCA | 250 | – | 25 ◦C | 100% | 1C | X(++) | X(+) | X(+) | X(+) |
| [65] | NCA | 250 | – | 45 ◦C | 100% | 1C | X(+) | X(+) | X (+) | X(+) |
 |
| Reference | Cathode chemistry | Mid-frequency region [0.1 Hz < f < 1000 Hz] | Low frequency region [f < 0.1 Hz] | Voltage source | |
|---|---|---|---|---|---|
| Semiarch 1 | Semiarch 2 | ||||
| [34] | LCO | RC | C//(R – CPE) | N | |
| [10] anode half cell | LCO | Zarc | Zarc | CPE | N |
| [10] cathode half cell | LCO | Zarc | C//(R – W) | N | |
| [35] | LCO | C//(R – W) | C//(R – W)//(R – W) | N | |
| [36] | LCO | RC | Z_composite_electrode | N | |
| [37] | LCO | RC-RC-RC | C//(R – W) | [Not measured] | N |
| [38] | LCO | Zarc | CPE//(R – W) – CPE//(R – W)//(R – W) | N | |
| [15] | LCO | Zarc | Zarc | N | |
| [8] cathode half cell | LCO | RC | RC | C//W | N |
| [26] | LCO | RC – RC – RC | W – C | N | |
| [39] | LCO | C//(RC-R) | RC | [Not in the model] | N |
| [66] | LiPO | Zarc | Zarc | CPE | N |
| [70] | n.d. | Zarc | Zarc | CPE | Y |
| [43] | LFP | (R – W)//C | Y | ||
| [44] | LFP | Zarc | Zarc | N | |
| [46] | LFP | Zarc | Zarc | W | N |
| [11] anode half cell | NMC | Zarc | Zarc – Zarc | [Not in the model] | N |
| [11] cathode half cell | NMC | Zarc | TLM | [Not in the model] | N |
| [47] | NMC | Zarc | Zarc | [Not in the model] | N |
| [21] | NMC | Zarc | Zarc | CPE | N |
| [12] anode half cell | NMC | Zarc | Zarc | W | N |
| [12] cathode half cell | NMC | Zarc | Zarc – CPE//(R – CPE) | N | |
| [51] | NMC | Zarc | Zarc | N | |
| [52] | NMC | Zarc | CPE//(R – W) – CPE//(R – W) | N | |
| [54,55] | NMC | RC – RC | RC – RC | W | N |
| [56] | NMC | Zarc | Zarc | W | Y |
| [56] | NMC | RC | RC | [Not in the model] | N |
| [57] | NMC | Zarc – Zarc | TLM | [Not in the model] | N |
| [58] | NMC | Zarc | CPE | [Not in the model] | N |
| [59] | NMC | HN | HN | W | N |
| [60] | NMC | Zarc | Zarc | [Not in the model] | N |
| [61] | NMC | RC | [Not in the model] | Y | |
| [20] positive electrode | NCA | RC | C//(R – W) | N | |
| [32] | NCA | Zarc | CPE//(R – W) | Y | |
| [62] | NCA | HN | HN | W | N |
| [63] | NCA | RC | Zarc | CPE | N |
| [65] | NCA | Zarc | CPE//(R – W) | N | |
| EIS region | Ohmic region |
Mid-frequency region | Low-frequency region | |
|---|---|---|---|---|
| Degradation | Loss of | Loss of lithium inventory: 54% | Loss of | |
| mode | lithium | Loss of cathode active material: | cathode | |
| inventory: 78% | 42% | active material: | ||
| 57% | ||||
| Semiarch 1 | Semiarch 2 | |||
| Loss of Lithium | Loss of | |||
| inventory + | cathode | |||
| loss of anode | active | |||
| active material: | material: | |||
| 78% | 61% | |||
| Circuital | Resistor | Zarc element: 50% | Warburg | |
| element for | 100% | element: | ||
| ECM | 59% | |||
| Semiarch 1 | Semiarch 2 | |||
| Zarc element: | Zarc | |||
| 59% | element: | |||
| 50% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




