Submitted:
11 April 2024
Posted:
12 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Mild Cognitive Impairment Due to Alzheimer's Disease
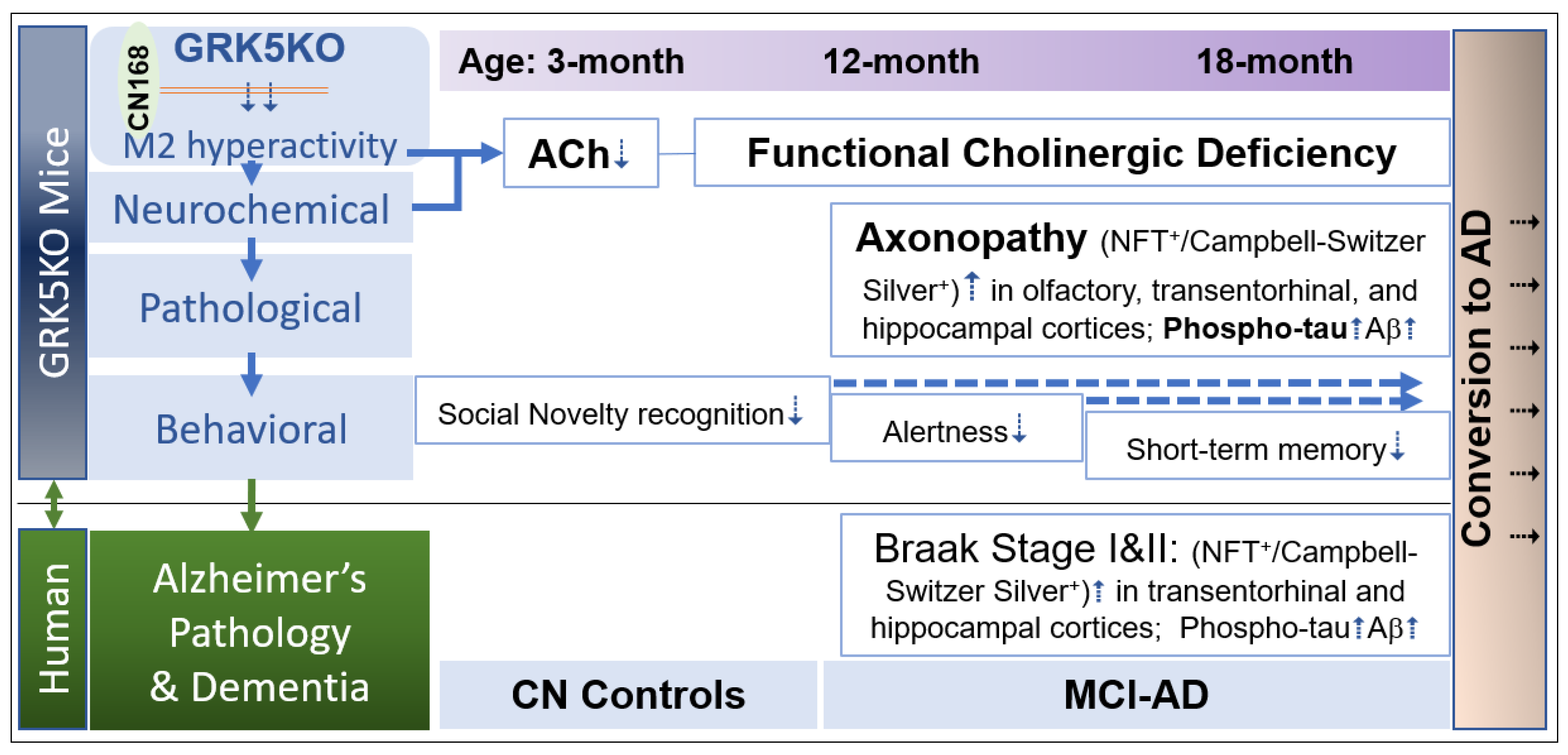
3. GRK5KO Mice—A Model of GRK5 Deficiency Recapitulates Characteristics of MCI-AD
3.1. GRK5 and Its Relevance to AD
3.2. Aged GRK5KO Mice Recaptured MCI-AD Characteristics
3.3. GRK5 Deficiency Renders Selective Cholinergic Neuronal Vulnerability-a Potential Mechanism and Therapeutical Implication
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 2023 Alzheimer's disease facts and figures. Alzheimers Dement 2023, 19, 1598–1695. [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer's disease. Nat Rev Dis Primers 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- McDade, E.; Bateman, R.J. Stop Alzheimer's before it starts. Nature 2017, 547, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Janoutova, J.; Sery, O.; Hosak, L.; Janout, V. Is Mild Cognitive Impairment a Precursor of Alzheimer's Disease? Short Review. Cent Eur J Public Health 2015, 23, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Foidl, B.M.; Humpel, C. Can mouse models mimic sporadic Alzheimer's disease? Neural regeneration research 2020, 15, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Bertens, D.; Vos, S.; Kehoe, P.; Wolf, H.; Nobili, F.; Mendonca, A.; van Rossum, I.; Hort, J.; Molinuevo, J.L.; Heneka, M.; et al. Use of mild cognitive impairment and prodromal AD/MCI due to AD in clinical care: a European survey. Alzheimers Res Ther 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Westbury, J.L.; Peterson, G.M. Rethinking psychotropics in nursing homes. The Medical journal of Australia 2013, 199, 98. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef]
- Blazhenets, G.; Frings, L.; Ma, Y.; Sorensen, A.; Eidelberg, D.; Wiltfang, J.; Meyer, P.T.; Alzheimer's Disease Neuroimaging, I. Validation of the Alzheimer Disease Dementia Conversion-Related Pattern as an ATN Biomarker of Neurodegeneration. Neurology 2021, 96, e1358–e1368. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006, 112, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Biel, D.; Brendel, M.; Rubinski, A.; Buerger, K.; Janowitz, D.; Dichgans, M.; Franzmeier, N.; Alzheimer's Disease Neuroimaging, I. Tau-PET and in vivo Braak-staging as prognostic markers of future cognitive decline in cognitively normal to demented individuals. Alzheimers Res Ther 2021, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Therriault, J.; Pascoal, T.A.; Lussier, F.Z.; Tissot, C.; Chamoun, M.; Bezgin, G.; Servaes, S.; Benedet, A.L.; Ashton, N.J.; Karikari, T.K.; et al. Biomarker modeling of Alzheimer’s disease using PET-based Braak staging. Nature Aging 2022, 2, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Morgenroth, I.; Michaud, P.; Gasparini, F.; Avrameas, A. Central and Peripheral Inflammation in Mild Cognitive Impairment in the Context of Alzheimer's Disease. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, J.A.; Freedman, N.J.; Lefkowitz, R.J. G protein-coupled receptor kinases. Annu Rev Biochem 1998, 67, 653–692. [Google Scholar] [CrossRef]
- Suo, W.Z.; Li, L. Dysfunction of G protein-coupled receptor kinases in Alzheimer's disease. TheScientificWorldJournal 2010, 10, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- The Nobel Prize in Chemistry 2012. NobelPrize.org, 2012.
- Kohout, T.A.; Lefkowitz, R.J. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol 2003, 63, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Penela, P.; Ribas, C.; Mayor, F., Jr. Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal 2003, 15, 973–981. [Google Scholar] [CrossRef]
- Hendrickx, J.O.; van Gastel, J.; Leysen, H.; Santos-Otte, P.; Premont, R.T.; Martin, B.; Maudsley, S. GRK5 - A Functional Bridge Between Cardiovascular and Neurodegenerative Disorders. Frontiers in pharmacology 2018, 9, 1484. [Google Scholar] [CrossRef]
- Premont, R.T.; Gainetdinov, R.R. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol 2007, 69, 511–534. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, E.V.; Tesmer, J.J.; Mushegian, A.; Gurevich, V.V. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther 2012, 133, 40–69. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gan, W.; Lu, L.; Dong, X.; Han, X.; Hu, C.; Yang, Z.; Sun, L.; Bao, W.; Li, P.; et al. A genome-wide association study identifies GRK5 and RASGRP1 as type 2 diabetes loci in Chinese Hans. Diabetes 2013, 62, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.I.; Gao, E.; Shang, X.; Premont, R.T.; Koch, W.J. Determining the absolute requirement of G protein-coupled receptor kinase 5 for pathological cardiac hypertrophy: short communication. Circ Res 2012, 111, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.M.; Cohn, H.I.; Pesant, S.; Eckhart, A.D. GPCR signalling in hypertension: role of GRKs. Clinical science 2008, 115, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Arawaka, S.; Wada, M.; Goto, S.; Karube, H.; Sakamoto, M.; Ren, C.H.; Koyama, S.; Nagasawa, H.; Kimura, H.; Kawanami, T.; et al. The role of G-protein-coupled receptor kinase 5 in pathogenesis of sporadic Parkinson's disease. J Neurosci 2006, 26, 9227–9238. [Google Scholar] [CrossRef] [PubMed]
- Bychkov, E.R.; Gurevich, V.V.; Joyce, J.N.; Benovic, J.L.; Gurevich, E.V. Arrestins and two receptor kinases are upregulated in Parkinson's disease with dementia. Neurobiol Aging 2008, 29, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Suo, Z.; Wu, M.; Citron, B.A.; Wong, G.T.; Festoff, B.W. Abnormality of G-protein-coupled receptor kinases at prodromal and early stages of Alzheimer's disease: an association with early beta-amyloid accumulation. J Neurosci 2004, 24, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Suo, Z.; Cox, A.A.; Bartelli, N.; Rasul, I.; Festoff, B.W.; Premont, R.T.; Arendash, G.W. GRK5 deficiency leads to early Alzheimer-like pathology and working memory impairment. Neurobiol Aging 2007, 28, 1873–1888. [Google Scholar] [CrossRef]
- Ma, S.; Gladyshev, V.N. Molecular signatures of longevity: Insights from cross-species comparative studies. Seminars in cell & developmental biology 2017, 70, 190–203. [Google Scholar] [CrossRef]
- Diez, J.J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol 2003, 148, 293–300. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Shen, M.; Ma, L. GRK5 deficiency decreases diet-induced obesity and adipogenesis. Biochem Biophys Res Commun 2012, 421, 312–317. [Google Scholar] [CrossRef]
- Xia, Z.; Yang, T.; Wang, Z.; Dong, J.; Liang, C. GRK5 intronic (CA)n polymorphisms associated with type 2 diabetes in Chinese Hainan Island. PloS one 2014, 9, e90597. [Google Scholar] [CrossRef]
- Wang, L.; Shen, M.; Wang, F.; Ma, L. GRK5 ablation contributes to insulin resistance. Biochem Biophys Res Commun 2012, 429, 99–104. [Google Scholar] [CrossRef]
- Islam, K.N.; Bae, J.W.; Gao, E.; Koch, W.J. Regulation of nuclear factor kappaB (NF-kappaB) in the nucleus of cardiomyocytes by G protein-coupled receptor kinase 5 (GRK5). J Biol Chem 2013, 288, 35683–35689. [Google Scholar] [CrossRef] [PubMed]
- Packiriswamy, N.; Lee, T.; Raghavendra, P.B.; Durairaj, H.; Wang, H.; Parameswaran, N. G-protein-coupled receptor kinase-5 mediates inflammation but does not regulate cellular infiltration or bacterial load in a polymicrobial sepsis model in mice. Journal of innate immunity 2013, 5, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Suo, W.Z. GRK5 Deficiency Causes Mild Cognitive Impairment due to Alzheimer's Disease. J Alzheimers Dis 2022, 85, 1399–1410. [Google Scholar] [CrossRef]
- Wu, J.H.; Goswami, R.; Cai, X.; Exum, S.T.; Huang, X.; Zhang, L.; Brian, L.; Premont, R.T.; Peppel, K.; Freedman, N.J. Regulation of the platelet-derived growth factor receptor-beta by G protein-coupled receptor kinase-5 in vascular smooth muscle cells involves the phosphatase Shp2. J Biol Chem 2006, 281, 37758–37772. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ding, L.; Xu, J.; Williams, R.S.; Chegini, N. Leiomyoma and myometrial gene expression profiles and their responses to gonadotropin-releasing hormone analog therapy. Endocrinology 2005, 146, 1074–1096. [Google Scholar] [CrossRef]
- Nagayama, Y.; Tanaka, K.; Namba, H.; Yamashita, S.; Niwa, M. Expression and regulation of G protein-coupled receptor kinase 5 and beta-arrestin-1 in rat thyroid FRTL5 cells. Thyroid 1996, 6, 627–631. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, J.; Zhang, X.; Yue, W.; Ma, L. Acute and chronic morphine treatments and morphine withdrawal differentially regulate GRK2 and GRK5 gene expression in rat brain. Neuropharmacology 2002, 43, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Malik, A.B. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med 2003, 9, 315–321. [Google Scholar] [CrossRef]
- Pitcher, J.A.; Fredericks, Z.L.; Stone, W.C.; Premont, R.T.; Stoffel, R.H.; Koch, W.J.; Lefkowitz, R.J. Phosphatidylinositol 4,5-bisphosphate (PIP2)-enhanced G protein-coupled receptor kinase (GRK) activity. Location, structure, and regulation of the PIP2 binding site distinguishes the GRK subfamilies. J Biol Chem 1996, 271, 24907–24913. [Google Scholar] [CrossRef] [PubMed]
- Pronin, A.N.; Satpaev, D.K.; Slepak, V.Z.; Benovic, J.L. Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. J Biol Chem 1997, 272, 18273–18280. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.L.; De La Cruz, E.M.; Pollard, T.D.; Lefkowitz, R.J.; Pitcher, J.A. Regulation of G protein-coupled receptor kinase 5 (GRK5) by actin. J Biol Chem 1998, 273, 20653–20657. [Google Scholar] [CrossRef] [PubMed]
- Sallese, M.; Iacovelli, L.; Cumashi, A.; Capobianco, L.; Cuomo, L.; De Blasi, A. Regulation of G protein-coupled receptor kinase subtypes by calcium sensor proteins. Biochim Biophys Acta 2000, 1498, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Suo, W.Z. Accelerating Alzheimer’s pathogenesis by GRK5 deficiency via cholinergic dysfunction. Adv Alzheimer’s Dis 2013, 2, 148–160. [Google Scholar] [CrossRef]
- Kunapuli, P.; Benovic, J.L. Cloning and expression of GRK5: a member of the G protein-coupled receptor kinase family. Proc Natl Acad Sci U S A 1993, 90, 5588–5592. [Google Scholar] [CrossRef] [PubMed]
- Premont, R.T.; Koch, W.J.; Inglese, J.; Lefkowitz, R.J. Identification, purification, and characterization of GRK5, a member of the family of G protein-coupled receptor kinases. J Biol Chem 1994, 269, 6832–6841. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Yin, M.; Cai, Y.; Liu, S.; Wang, Y.; Zhang, X.; Cao, H.; Chen, T.; Huang, P.; et al. The influence of two functional genetic variants of GRK5 on tau phosphorylation and their association with Alzheimer's disease risk. Oncotarget 2017, 8, 72714–72726. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Bohn, L.M.; Walker, J.K.; Laporte, S.A.; Macrae, A.D.; Caron, M.G.; Lefkowitz, R.J.; Premont, R.T. Muscarinic supersensitivity and impaired receptor desensitization in G protein-coupled receptor kinase 5-deficient mice. Neuron 1999, 24, 1029–1036. [Google Scholar] [CrossRef]
- Jaber, M.; Koch, W.J.; Rockman, H.; Smith, B.; Bond, R.A.; Sulik, K.K.; Ross, J., Jr.; Lefkowitz, R.J.; Caron, M.G.; Giros, B. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci U S A 1996, 93, 12974–12979. [Google Scholar] [CrossRef] [PubMed]
- Peppel, K.; Boekhoff, I.; McDonald, P.; Breer, H.; Caron, M.G.; Lefkowitz, R.J. G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J Biol Chem 1997, 272, 25425–25428. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Bohn, L.M.; Sotnikova, T.D.; Cyr, M.; Laakso, A.; Macrae, A.D.; Torres, G.E.; Kim, K.M.; Lefkowitz, R.J.; Caron, M.G.; et al. Dopaminergic supersensitivity in g protein-coupled receptor kinase 6-deficient mice. Neuron 2003, 38, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rasul, I.; Sun, Y.; Wu, G.; Li, L.; Premont, R.T.; Suo, W.Z. GRK5 deficiency leads to reduced hippocampal acetylcholine level via impaired presynaptic M2/M4 autoreceptor desensitization. J Biol Chem 2009, 284, 19564–19571. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu Rev Nutr 2016, 36, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Penela, P.; Murga, C.; Ribas, C.; Tutor, A.S.; Peregrin, S.; Mayor, F., Jr. Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc Res 2006, 69, 46–56. [Google Scholar] [CrossRef]
- Rockman, H.A.; Choi, D.J.; Rahman, N.U.; Akhter, S.A.; Lefkowitz, R.J.; Koch, W.J. Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proc Natl Acad Sci U S A 1996, 93, 9954–9959. [Google Scholar] [CrossRef]
- Niu, B.; Liu, P.; Shen, M.; Liu, C.; Wang, L.; Wang, F.; Ma, L. GRK5 Regulates Social Behavior Via Suppression of mTORC1 Signaling in Medial Prefrontal Cortex. Cereb Cortex 2018, 28, 421–432. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol Biol 2019, 1916, 105–111. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature protocols 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Alamed, J.; Wilcock, D.M.; Diamond, D.M.; Gordon, M.N.; Morgan, D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nature protocols 2006, 1, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Webber, K.M.; Casadesus, G.; Perry, G.; Atwood, C.S.; Bowen, R.; Smith, M.A. Gender differences in Alzheimer disease: the role of luteinizing hormone in disease pathogenesis. Alzheimer Dis Assoc Disord 2005, 19, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Placanica, L.; Zhu, L.; Li, Y.M. Gender- and age-dependent gamma-secretase activity in mouse brain and its implication in sporadic Alzheimer disease. PloS one 2009, 4, e5088. [Google Scholar] [CrossRef] [PubMed]
- O'Neal, M.A. Women and the risk of Alzheimer's disease. Front Glob Womens Health 2023, 4, 1324522. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.S.; Jiang, Q.W.; Chen, S.D. Sex difference in biological change and mechanism of Alzheimer's disease: From macro- to micro-landscape. Ageing Res Rev 2023, 87, 101918. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rasul, I.; Liu, J.; Zhao, B.; Tang, R.; Premont, R.T.; Suo, W.Z. Augmented axonal defects and synaptic degenerative changes in female GRK5 deficient mice. Brain Res Bull 2009, 78, 145–151. [Google Scholar] [CrossRef]
- He, M.; Singh, P.; Cheng, S.; Zhang, Q.; Peng, W.; Ding, X.; Li, L.; Liu, J.; Premont, R.T.; Morgan, D.; et al. GRK5 Deficiency Leads to Selective Basal Forebrain Cholinergic Neuronal Vulnerability. Scientific reports 2016, 6, 26116. [Google Scholar] [CrossRef] [PubMed]
- Pepeu, G. Mild cognitive impairment: animal models. Dialogues Clin Neurosci 2004, 6, 369–377. [Google Scholar] [CrossRef]
- Frolkis, V.V.; Tanin, S.A.; Gorban, Y.N. Age-related changes in axonal transport. Exp Gerontol 1997, 32, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Vagnoni, A.; Bullock, S.L. A cAMP/PKA/Kinesin-1 Axis Promotes the Axonal Transport of Mitochondria in Aging Drosophila Neurons. Curr Biol 2018, 28, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Carlyle, B.C.; Nairn, A.C.; Wang, M.; Yang, Y.; Jin, L.E.; Simen, A.A.; Ramos, B.P.; Bordner, K.A.; Craft, G.E.; Davies, P.; et al. cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. Proc Natl Acad Sci U S A 2014, 111, 5036–5041. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Li, L.; He, S.; Liu, J.; Sun, Y.; He, M.; Grasing, K.; Premont, R.T.; Suo, W.Z. GRK5 deficiency accelerates beta-amyloid accumulation in Tg2576 mice via impaired cholinergic activity. J Biol Chem 2010, 285, 41541–41548. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, J.; Suo, W.Z. GRK5 deficiency exaggerates inflammatory changes in TgAPPsw mice. J Neuroinflammation 2008, 5, 24. [Google Scholar] [CrossRef]
- Sattler, M.; Liang, H.; Nettesheim, D.; Meadows, R.P.; Harlan, J.E.; Eberstadt, M.; Yoon, H.S.; Shuker, S.B.; Chang, B.S.; Minn, A.J.; et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 1997, 275, 983–986. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Bonni, A.; Brunet, A.; West, A.E.; Datta, S.R.; Takasu, M.A.; Greenberg, M.E. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 1999, 286, 1358–1362. [Google Scholar] [CrossRef]
- Fiscus, R.R.; Yuen, J.P.; Chan, S.L.; Kwong, J.H.; Chew, S.B. Nitric oxide and cyclic GMP as pro- and anti-apoptotic agents. J Card Surg 2002, 17, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Eliseev, R.A.; Vanwinkle, B.; Rosier, R.N.; Gunter, T.E. Diazoxide-mediated preconditioning against apoptosis involves activation of cAMP-response element-binding protein (CREB) and NFkappaB. J Biol Chem 2004, 279, 46748–46754. [Google Scholar] [CrossRef]
- Follin-Arbelet, V.; Torgersen, M.L.; Naderi, E.H.; Misund, K.; Sundan, A.; Blomhoff, H.K. Death of multiple myeloma cells induced by cAMP-signaling involves downregulation of Mcl-1 via the JAK/STAT pathway. Cancer letters 2013, 335, 323–331. [Google Scholar] [CrossRef]
- Zhang, W.; Basile, A.S.; Gomeza, J.; Volpicelli, L.A.; Levey, A.I.; Wess, J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J Neurosci 2002, 22, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Decossas, M.; Doudnikoff, E.; Bloch, B.; Bernard, V. Aging and subcellular localization of m2 muscarinic autoreceptor in basalocortical neurons in vivo. Neurobiol Aging 2005, 26, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Parnas, H.; Slutsky, I.; Rashkovan, G.; Silman, I.; Wess, J.; Parnas, I. Depolarization initiates phasic acetylcholine release by relief of a tonic block imposed by presynaptic M2 muscarinic receptors. Journal of neurophysiology 2005, 93, 3257–3269. [Google Scholar] [CrossRef] [PubMed]
- Suo, Z. Muscarinic m2 receptor blockade to delay or prevent onset of progressive memory decline. USPTO 2023, US11801241B11801242. [Google Scholar]
- Bartus, R.T.; Dean, R.L., 3rd; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Martorana, A.; Esposito, Z.; Koch, G. Beyond the cholinergic hypothesis: do current drugs work in Alzheimer's disease? CNS neuroscience & therapeutics 2010, 16, 235–245. [Google Scholar]
- Liu, P.P.; Xie, Y.; Meng, X.Y.; Kang, J.S. History and progress of hypotheses and clinical trials for Alzheimer's disease. Signal Transduct Target Ther 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Beschea Chiriac, S.I.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer's Disease Pharmacotherapy in Relation to Cholinergic System Involvement. Biomolecules 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, G.T. Cholinesterase inhibitors for the treatment of Alzheimer's disease:: getting on and staying on. Curr Ther Res Clin Exp 2003, 64, 216–235. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer's disease drug development pipeline: 2019. Alzheimers Dement (N Y) 2019, 5, 272–293. [Google Scholar] [CrossRef]
- Buccellato, F.R.; D'Anca, M.; Tartaglia, G.M.; Del Fabbro, M.; Scarpini, E.; Galimberti, D. Treatment of Alzheimer's Disease: Beyond Symptomatic Therapies. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Peng, W.; Zhang, Q.; Ding, X.; Suo, W.Z. GRK5 deficiency leads to susceptibility to intermittent hypoxia-induced cognitive impairment. Behav Brain Res 2016, 302, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, N.; O'Regan, J.; Ruthirakuhan, M.; Kiss, A.; Eryavec, G.; Williams, E.; Lanctot, K.L. A Randomized Placebo-Controlled Discontinuation Study of Cholinesterase Inhibitors in Institutionalized Patients With Moderate to Severe Alzheimer Disease. J Am Med Dir Assoc 2016, 17, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Bidzan, L.; Bidzan, M. Withdrawal syndrome after donepezil cessation in a patient with dementia. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 2012, 33, 1459–1461. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dudley, C. Discontinuation syndrome following donepezil cessation. Int J Geriatr Psychiatry 2003, 18, 282–284. [Google Scholar] [CrossRef]
- Parsons, C.; Lim, W.Y.; Loy, C.; McGuinness, B.; Passmore, P.; Ward, S.A.; Hughes, C. Withdrawal or continuation of cholinesterase inhibitors or memantine or both, in people with dementia. The Cochrane database of systematic reviews 2021, 2, CD009081. [Google Scholar] [CrossRef]
| Treatment | CN168 | ChEIs |
|---|---|---|
| Target system | Central cholinergic system | Central cholinergic system |
| Target position | Presynaptic | Postsynaptic |
| Target molecule | M2 autoreceptor | ACh |
| Direct effect | M2 inhibition | Cholinesterase inhibition |
| Acute impacts in the presence of the drug | Increased ACh release and revoked suppression of cAMP/PKA/CREB signaling | Delayed ACh degradation, prolonged ACh effects on all cholinergic receptors including M2 autoreceptor |
| Potential impacts on the cholinergic neurons | Strengthened cholinergic neuronal resilience and survival | Weakened cholinergic neuronal resilience potentially by enhancing the M2 hyperactivity |
| Impacts after the drug withdrawal | Cognitively normal | Potentially worsened symptoms |
| Disease modifying | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




