Submitted:
10 April 2024
Posted:
11 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Material and Methods
Results
Discussion
Conclussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- M. E. Dilenge, A. Majnemer, and M. I. Shevell, “Long-term developmental outcome of asphyxiated term neonates.,” J Child Neurol, vol. 16, no. 11, pp. 781–92, Nov. 2001. [CrossRef]
- B. Fleiss and P. Gressens, “Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy?,” Lancet Neurol, vol. 11, no. 6, pp. 556–66, Jun. 2012. [CrossRef]
- W. A. Pulsinelli, M. Jacewicz, D. E. Levy, C. K. Petito, and F. Plum, “Ischemic brain injury and the therapeutic window.,” Ann N Y Acad Sci, vol. 835, pp. 187–93, Dec. 1997. [CrossRef]
- S. Perrone et al., “Whole body hypothermia and oxidative stress in babies with hypoxic-ischemic brain injury.,” Pediatr Neurol, vol. 43, no. 4, pp. 236–40, Oct. 2010. [CrossRef]
- E. Ramos et al., “Ischemic brain injury: New insights on the protective role of melatonin.,” Free Radic Biol Med, vol. 104, pp. 32–53, Mar. 2017. [CrossRef]
- J. Nair and V. H. S. Kumar, “Current and Emerging Therapies in the Management of Hypoxic Ischemic Encephalopathy in Neonates.,” Children (Basel), vol. 5, no. 7, Jul. 2018. [CrossRef]
- K. Martinello, A. R. Hart, S. Yap, S. Mitra, and N. J. Robertson, “Management and investigation of neonatal encephalopathy: 2017 update.,” Arch Dis Child Fetal Neonatal Ed, vol. 102, no. 4, pp. F346–F358, Jul. 2017. [CrossRef]
- R. Reiter, L. Tang, J. J. Garcia, and A. Muñoz-Hoyos, “Pharmacological actions of melatonin in oxygen radical pathophysiology.,” Life Sci, vol. 60, no. 25, pp. 2255–71, 1997. [CrossRef]
- A. Muñoz-Hoyos et al., “Melatonin’s role as an anticonvulsant and neuronal protector: experimental and clinical evidence.,” J Child Neurol, vol. 13, no. 10, pp. 501–9, Oct. 1998. [CrossRef]
- A. Jerez-Calero. et al., “Hypothermia Plus Melatonin in Asphyctic Newborns: A Randomized-Controlled Pilot Study.,” Pediatr Crit Care Med, vol. 21, no. 7, pp. 647–655, Jul. 2020. [CrossRef]
- D. Acuña-Castroviejo et al., “Melatonin, mitochondria, and cellular bioenergetics.,” J Pineal Res, vol. 30, no. 2, pp. 65–74, Mar. 2001. [CrossRef]
- M. Chahbouni et al., “Melatonin Treatment Reduces Oxidative Damage and Normalizes Plasma Pro-Inflammatory Cytokines in Patients Suffering from Charcot-Marie-Tooth Neuropathy: A Pilot Study in Three Children.,” Molecules, vol. 22, no. 10, Oct. 2017. [CrossRef]
- L. Capuron and A. H. Miller, “Immune system to brain signaling: neuropsychopharmacological implications.,” Pharmacol Ther, vol. 130, no. 2, pp. 226–38, May 2011. [CrossRef]
- A. Foster-Barber, B. Dickens, and D. M. Ferriero, “Human perinatal asphyxia: correlation of neonatal cytokines with MRI and outcome.,” Dev Neurosci, vol. 23, no. 3, pp. 213–8, 2001. [CrossRef]
- H. Hagberg et al., “Enhanced expression of interleukin (IL)-1 and IL-6 messenger RNA and bioactive protein after hypoxia-ischemia in neonatal rats.,” Pediatr Res, vol. 40, no. 4, pp. 603–9, Oct. 1996. [CrossRef]
- J. Middeldorp and E. M. Hol, “GFAP in health and disease.,” Prog Neurobiol, vol. 93, no. 3, pp. 421–43, Mar. 2011. [CrossRef]
- L. Papa et al., “Evaluation of Glial and Neuronal Blood Biomarkers Compared With Clinical Decision Rules in Assessing the Need for Computed Tomography in Patients With Mild Traumatic Brain Injury.,” JAMA Netw Open, vol. 5, no. 3, p. e221302, Mar. 2022. [CrossRef]
- L. F. Chalak, P. J. Sánchez, B. Adams-Huet, A. R. Laptook, R. J. Heyne, and C. R. Rosenfeld, “Biomarkers for Severity of Neonatal Hypoxic-Ischemic Encephalopathy and Outcomes in Newborns Receiving Hypothermia Therapy,” J Pediatr, vol. 164, no. 3, pp. 468-474.e1, Mar. 2014. [CrossRef]
- J. Espino et al., “Melatonin is able to delay endoplasmic reticulum stress-induced apoptosis in leukocytes from elderly humans.,” Age (Dordr), vol. 33, no. 4, pp. 497–507, Dec. 2011. [CrossRef]
- L. C. Wickramasinghe et al., “Granulocyte Colony-Stimulating Factor is a Determinant of Severe Bronchopulmonary Dysplasia and Coincident Retinopathy.,” Am J Pathol, vol. 193, no. 12, pp. 2001–2016, Dec. 2023. [CrossRef]
- R. B. Scheinert, A. Asokan, A. Rani, A. Kumar, T. C. Foster, and B. K. Ormerod, “Some hormone, cytokine and chemokine levels that change across lifespan vary by cognitive status in male Fischer 344 rats.,” Brain Behav Immun, vol. 49, pp. 216–32, Oct. 2015. [CrossRef]
- H. O. Dikmen, M. Hemmerich, A. Lewen, J.-O. Hollnagel, B. Chausse, and O. Kann, “GM-CSF induces noninflammatory proliferation of microglia and disturbs electrical neuronal network rhythms in situ.,” J Neuroinflammation, vol. 17, no. 1, p. 235, Aug. 2020. [CrossRef]
- H. Go et al., “Serum cytokine profiling in neonates with hypoxic ischemic encephalopathy.,” J Neonatal Perinatal Med, vol. 14, no. 2, pp. 177–182, 2021. [CrossRef]
- S. D. Paredes et al., “Melatonin Counteracts at a Transcriptional Level the Inflammatory and Apoptotic Response Secondary to Ischemic Brain Injury Induced by Middle Cerebral Artery Blockade in Aging Rats.,” Biores Open Access, vol. 4, no. 1, pp. 407–16, 2015. [CrossRef]
- F. Dehghan et al., “Does the administration of melatonin during post-traumatic brain injury affect cytokine levels?,” Inflammopharmacology, vol. 26, no. 4, pp. 1017–1023, Aug. 2018. [CrossRef]
- M. A. El-Missiry, S. Shabana, S. J. Ghazala, A. I. Othman, and M. E. Amer, “Melatonin exerts a neuroprotective effect against γ-radiation-induced brain injury in the rat through the modulation of neurotransmitters, inflammatory cytokines, oxidative stress, and apoptosis.,” Environ Sci Pollut Res Int, vol. 28, no. 24, pp. 31108–31121, Jun. 2021. [CrossRef]
- L. -L. Zhou, W. Wei, J.-F. Si, and D.-P. Yuan, “Regulatory effect of melatonin on cytokine disturbances in the pristane-induced lupus mice.,” Mediators Inflamm, vol. 2010, 2010. [CrossRef]
- A. Babaee et al., “Melatonin treatment reduces astrogliosis and apoptosis in rats with traumatic brain injury.,” Iran J Basic Med Sci, vol. 18, no. 9, pp. 867–72, Sep. 2015.
- I. Labunets, A. Rodnichenko, S. Savosko, and T. Pivneva, “Reaction of different cell types of the brain on neurotoxin cuprizone and hormone melatonin treatment in young and aging mice.,” Front Cell Neurosci, vol. 17, p. 113 1130, 2023. [Google Scholar] [CrossRef]
- G. Baydas, M. Ozer, A. Yasar, S. T. Koz, and M. Tuzcu, “Melatonin prevents oxidative stress and inhibits reactive gliosis induced by hyperhomocysteinemia in rats.,” Biochemistry (Mosc), vol. 71 Suppl 1, pp. S91-5, 2006. [CrossRef]
- A. Babaee, S. H. E. Vaghefi, S. Dehghani Soltani, M. Asadi Shekaari, N. Shahrokhi, and M. Basiri, “Hippocampal Astrocyte Response to Melatonin Following Neural Damage Induction in Rats.,” Basic Clin Neurosci, vol. 12, no. 2, pp. 177–186, 2021. [CrossRef]
- A. Albazal, A.-A. Delshad, and M. Roghani, “Melatonin reverses cognitive deficits in streptozotocin-induced type 1 diabetes in the rat through attenuation of oxidative stress and inflammation.,” J Chem Neuroanat, vol. 112, p. 101902, Mar. 2021. [Google Scholar] [CrossRef]
- et al. A. Garcia-Alix, et al. “Development, Reliability, and Testing of a New Rating Scale for Neonatal Encephalopathy.,” J Pediatr, vol. 235, pp. 83-91.e7, Aug. 2021. [Google Scholar] [CrossRef]
- P. S. Shah, “Hypothermia: a systematic review and meta-analysis of clinical trials.,” Semin Fetal Neonatal Med, vol. 15, no. 5, pp. 238–46, Oct. 2010. [CrossRef]
- A. D. Edwards et al., “Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data.,” BMJ, vol. 340, p. c363, Feb. 2010. [CrossRef]
- S. E. Jacobs, M. Berg, R. Hunt, W. O. Tarnow-Mordi, T. E. Inder, and P. G. Davis, “Cooling for newborns with hypoxic ischaemic encephalopathy.,” Cochrane Database Syst Rev, vol. 2013, no. 1, p. CD003311, Jan. 2013. [CrossRef]
- E. Shepherd et al., “Neonatal interventions for preventing cerebral palsy: an overview of Cochrane Systematic Reviews.,” Cochrane Database Syst Rev, vol. 6, no. 6, p. CD012409, Jun. 2018. [CrossRef]
- C. A. Albers and A. J. Grieve, “Test Review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development– Third Edition. San Antonio, TX: Harcourt Assessment,” J Psychoeduc Assess, vol. 25, no. 2, pp. 180–190, Jun. 2007. [CrossRef]
- R. Palisano, P. Rosenbaum, S. Walter, D. Russell, E. Wood, and B. Galuppi, “Development and reliability of a system to classify gross motor function in children with cerebral palsy.,” Dev Med Child Neurol, vol. 39, no. 4, pp. 214–23, Apr. 1997. [CrossRef]
- M. Voorman, A. J. Dallmeijer, D. L. Knol, G. J. Lankhorst, and J. G. Becher, “Prospective longitudinal study of gross motor function in children with cerebral palsy.,” Arch Phys Med Rehabil, vol. 88, no. 7, pp. 871–6, Jul. 2007. [CrossRef]
- Tardieu, G. , Le dossier clinique de l’infirmité motrice cérébrale. Méthodes d’Évaluation et Applications Thérapeutiques., Third. Paris, 1984.
- E. Bonilla et al., “Melatonin prolongs survival of immunodepressed mice infected with the Venezuelan equine encephalomyelitis virus.,” Trans R Soc Trop Med Hyg, vol. 95, no. 2, pp. 207–10, 2001. [CrossRef]
- S. Victor, E. Rocha-Ferreira, A. Rahim, H. Hagberg, and D. Edwards, “New possibilities for neuroprotection in neonatal hypoxic-ischemic encephalopathy.,” Eur J Pediatr, vol. 181, no. 3, pp. 875–887, Mar. 2022. [CrossRef]
- E. Gitto et al., “Melatonin reduces oxidative stress in surgical neonates.,” J Pediatr Surg, vol. 39, no. 2, pp. 184–9; discussion 184-9, Feb. 2004. [CrossRef]
- E. Gitto et al., “Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: beneficial effects of melatonin.,” Am J Perinatol, vol. 21, no. 4, pp. 209–16, May 2004. [CrossRef]
- M. Korf, L. D. McCullough, and V. Caretti, “A narrative review on treatment strategies for neonatal hypoxic ischemic encephalopathy.,” Transl Pediatr, vol. 12, no. 8, pp. 1552–1571, Aug. 2023. [CrossRef]
- S. Carloni et al., “Melatonin Pharmacokinetics Following Oral Administration in Preterm Neonates.,” Molecules, vol. 22, no. 12, Dec. 2017. [CrossRef]
- N. M. Merchant et al., “Pharmacokinetics of melatonin in preterm infants.,” Br J Clin Pharmacol, vol. 76, no. 5, pp. 725–33, Nov. 2013. [CrossRef]
- M. S. El Farargy and N. A. Soliman, “A randomized controlled trial on the use of magnesium sulfate and melatonin in neonatal hypoxic ischemic encephalopathy.,” J Neonatal Perinatal Med, vol. 12, no. 4, pp. 379–384, 2019. [CrossRef]
- H. Aly et al., “Melatonin use for neuroprotection in perinatal asphyxia: a randomized controlled pilot study.,” J Perinatol, vol. 35, no. 3, pp. 186–91, Mar. 2015. [CrossRef]
- Q. M. Ahmad, A. L. Chishti, and N. Waseem, “Role of melatonin in management of hypoxic ischaemic encephalopathy in newborns: A randomized control trial.,” J Pak Med Assoc, vol. 68, no. 8, pp. 1233–1237, Aug. 2018.
- M. Zaigham, F. Lundberg, R. Hayes, J. Undén, and P. Olofsson, “Umbilical cord blood concentrations of ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) and glial fibrillary acidic protein (GFAP) in neonates developing hypoxic-ischemic encephalopathy.,” J Matern Fetal Neonatal Med, vol. 29, no. 11, pp. 1822–8, 2016. [CrossRef]
- D. Chen et al., “Interleukin 13 promotes long-term recovery after ischemic stroke by inhibiting the activation of STAT3.,” J Neuroinflammation, vol. 19, no. 1, p. 112, May 2022. [CrossRef]
- J. Van Broeckhoven et al., “Macrophage-based delivery of interleukin-13 improves functional and histopathological outcomes following spinal cord injury.,” J Neuroinflammation, vol. 19, no. 1, p. 102, Apr. 2022. [CrossRef]
- Y. Yan et al., “Interleukin-13 Affects the Recovery Processes in a Mouse Model of Hemorrhagic Stroke with Bilateral Tibial Fracture.,” Mol Neurobiol, vol. 59, no. 5, pp. 3040–3051, May 2022. [CrossRef]
- G. Chen et al., “Inhibiting ER Stress Weakens Neuronal Pyroptosis in a Mouse Acute Hemorrhagic Stroke Model.,” Mol Neurobiol, vol. 57, no. 12, pp. 5324–5335, Dec. 2020. [CrossRef]
- A. N. Massaro et al., “Plasma Biomarkers of Brain Injury in Neonatal Hypoxic-Ischemic Encephalopathy.,” J Pediatr, vol. 194, pp. 67-75.e1, Mar. 2018. [CrossRef]
- N. M. Simon et al., “A detailed examination of cytokine abnormalities in Major Depressive Disorder.,” Eur Neuropsychopharmacol, vol. 18, no. 3, pp. 230–3, Mar. 2008. [CrossRef]
- C. Galera et al., “Cord Serum Cytokines at Birth and Children’s Anxiety-Depression Trajectories From 3 to 8 Years: The EDEN Mother-Child Cohort.,” Biol Psychiatry, vol. 89, no. 6, pp. 541–549, Mar. 2021. [CrossRef]
- H. Yu et al., “Serum concentrations of cytokines in infants with retinopathy of prematurity.,” APMIS, vol. 122, no. 9, pp. 818–23, Sep. 2014. [CrossRef]
- D. D. Jenkins et al., “Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy.,” J Cereb Blood Flow Metab, vol. 32, no. 10, pp. 1888–96, Oct. 2012. [CrossRef]
- J. E. Orrock et al., “Association of brain injury and neonatal cytokine response during therapeutic hypothermia in newborns with hypoxic-ischemic encephalopathy.,” Pediatr Res, vol. 79, no. 5, pp. 742–7, May 2016. [CrossRef]
- S. E. Juul et al., “Association of High-Dose Erythropoietin With Circulating Biomarkers and Neurodevelopmental Outcomes Among Neonates With Hypoxic Ischemic Encephalopathy: A Secondary Analysis of the HEAL Randomized Clinical Trial.,” JAMA Netw Open, vol. 6, no. 7, p. e2322131, Jul. 2023. [CrossRef]
- Z. Zareen et al., “Cytokine dysregulation persists in childhood post Neonatal Encephalopathy.,” BMC Neurol, vol. 20, no. 1, p. 115, Mar. 2020. [CrossRef]
- J. D. Prasad et al., “Anti-Inflammatory Therapies for Treatment of Inflammation-Related Preterm Brain Injury,” Int J Mol Sci, vol. 22, no. 8, p. 4008, Apr. 2021. [CrossRef]
- K. Sävman, M. K. Sävman, M. Blennow, H. Hagberg, E. Tarkowski, M. Thoresen, and A. Whitelaw, “Cytokine response in cerebrospinal fluid from preterm infants with posthaemorrhagic ventricular dilatation.,” Acta Paediatr, vol. 91, no. 12, pp. 1357–63, 2002. [CrossRef]
- A. Leviton et al., “The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study.,” J Pediatr, vol. 158, no. 6, pp. 897-903.e1–5, Jun. 2011. [CrossRef]
- J. H. Gilmore, L. Fredrik Jarskog, S. Vadlamudi, and J. M. Lauder, “Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development.,” Neuropsychopharmacology, vol. 29, no. 7, pp. 1221–9, Jul. 2004. [CrossRef]
- et al. N. Kuzumaki, et al. “Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice.,” Synapse, vol. 64, no. 9, pp. 721–8, Sep. 2010. [Google Scholar] [CrossRef]
- T. M. O’Shea et al., “Elevated blood levels of inflammation-related proteins are associated with an attention problem at age 24 mo in extremely preterm infants.,” Pediatr Res, vol. 75, no. 6, pp. 781–7, Jun. 2014. [CrossRef]
- R. S. Procianoy and R. C. Silveira, “Association between high cytokine levels with white matter injury in preterm infants with sepsis.,” Pediatr Crit Care Med, vol. 13, no. 2, pp. 183–7, Mar. 2012. [CrossRef]
- M. D. Nist and R. H. Pickler, “An Integrative Review of Cytokine/Chemokine Predictors of Neurodevelopment in Preterm Infants.,” Biol Res Nurs, vol. 21, no. 4, pp. 366–376, Jul. 2019. [CrossRef]
- A. Leviton et al., “Systemic inflammation, intraventricular hemorrhage, and white matter injury.,” J Child Neurol, vol. 28, no. 12, pp. 1637–45, Dec. 2013. [CrossRef]
- N. J. Robertson et al., “Melatonin as an adjunct to therapeutic hypothermia in a piglet model of neonatal encephalopathy: A translational study.,” Neurobiol Dis, vol. 121, pp. 240–251, Jan. 2019. [CrossRef]
- J. Y. Lee et al., “Administration of melatonin for prevention of preterm birth and fetal brain injury associated with premature birth in a mouse model.,” Am J Reprod Immunol, vol. 82, no. 3, p. e13151, Sep. 2019. [CrossRef]
- J. Ahmed, A. K. Pullattayil S, N. J. Robertson, and K. More, “Melatonin for neuroprotection in neonatal encephalopathy: A systematic review & meta-analysis of clinical trials.,” Eur J Paediatr Neurol, vol. 31, pp. 38–45, Mar. 2021. [CrossRef]
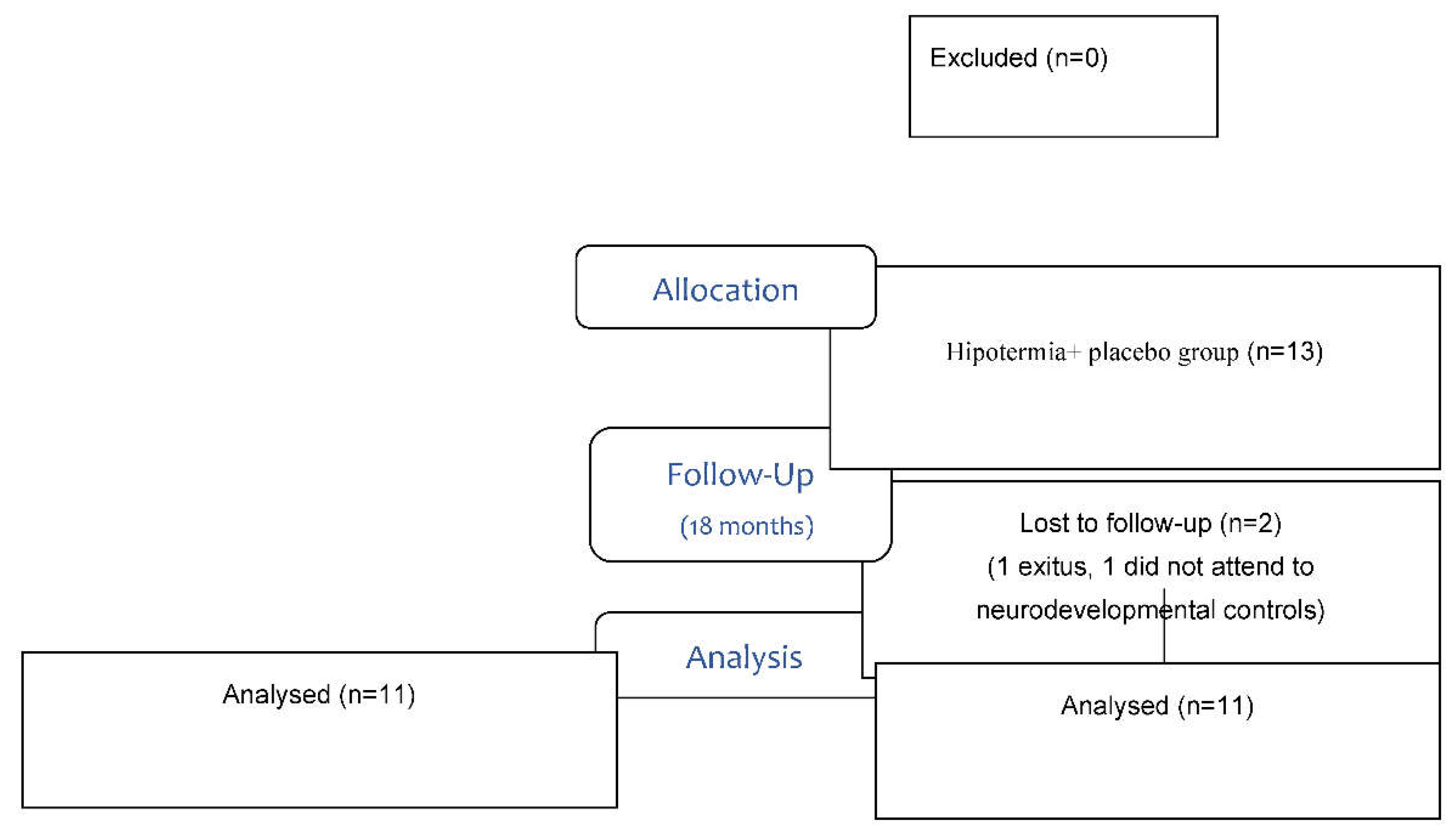
| Clinical characteristics | Placebo Group (n = 13) (Mean ± SD) | Melatonin Group (n = 12) (Mean ± SD) | p |
|---|---|---|---|
| Birth weight (grams) | 2973 +/- 660 | 3.057+/-514 | 0.86 |
| Gestational age (weeks) | 39.45+/-1.72 | 38.49+/-1.71 | 0.22 |
| Height (centimeters) | 49.96+/-2.39 | 50.73+/-2.05 | 0.56 |
| pH cord blood | 6.96+/-0.14 | 6.98+/-0.15 | 0.63 |
| Apgar score at 1 minute of life | 3.77+/-2.52 | 3.55+/-2.46 | 0.69 |
| Apgar score at 5 minutes of life | 5.42+/-2.39 | 5.91+/-1.97 | 0.78 |
| Start hypothermia therapy from birth (hours) | 4.92+/-2.56 | 3.95+/-2.49 | 0.25 |
| Sarnat-Sarnat score | 20.69+/-10.67 | 12.72+/-7.57 | 0.09 |
| Lower blood pH at NICU admittance | 7.15+/-0.16 | 7.17 +/-0.13 | 0.31 |
| Arterial Pressure (mm Hg) | 48.42 +/-15.22 | 51.7+/-11.24 | 0.41 |
| Length of stay in the NICU (days) | 11.08+/-4.25 | 11.35+/-4.37 | 0.53 |
| Plasma biomarker | n | Placebo Group | Melatonin Group | n | p |
|---|---|---|---|---|---|
| GFAP T0 | 11 | 0.096 ± 0.015 | 0.118 ± 0.122 | 10 | 0.35 |
| GFAP T1 | 10 | 0.079 ± 0.07 | 0.036 ± 0.061 | 10 | 0.48 |
| GFAP T2 | 10 | 0.086 ± 0.095 | 0.061 ± 0.111 | 9 | 0.99 |
| GFAP T3 | 11 | 0.079 ± 0.057 | 0 ± 0.082 | 9 | 0.26 |
| GM-CSF T0 | 10 | 13.28 ± 4 | 14.77 ± 4.717 | 13 | 0.60 |
| GM-CSF T1 | 10 | 15.76 ± 11.25 | 11.77 ± 11.177 | 13 | 0.01* |
| GM-CSF T2 | 10 | 17.485 ± 8.008 | 12.275 ± 7.688 | 12 | 0.04* |
| GM-CSF T3 | 11 | 16.75 ± 6.057 | 12.78 ± 3.02 | 10 | 0.08 |
| IL1 T0 | 10 | 59.79 ± 86.88 | 32.52 ± 63.12 | 13 | 0.32 |
| IL1 T1 | 10 | 69.48 ± 108.53 | 13.28 ± 39.81 | 13 | 0.20 |
| IL1 T2 | 10 | 32.52 ± 64.455 | 36.93 ± 18.225 | 12 | 0.39 |
| IL1 T3 | 11 | 59.67 ± 32.55 | 29.9 ± 43.025 | 10 | 0.87 |
| IL2 T0 | 10 | 6.71 ± 2.46 | 6.245 ± 5.595 | 13 | 0.67 |
| IL2 T1 | 10 | 7.31 ± 5.5 | 5.27 ± 4.828 | 13 | 0.01* |
| IL2 T2 | 10 | 7.605 ± 4.608 | 5.44 ± 4.543 | 12 | 0.09 |
| IL2 T3 | 11 | 8.875 ± 3.643 | 6.09 ± 1.495 | 10 | 0.07 |
| IL7 T0 | 10 | 8.74 ± 9.61 | 11.14 ± 8.67 | 13 | 0.56 |
| IL7 T1 | 10 | 11.32 ± 14.61 | 6.635 ± 5.247 | 13 | 0.32 |
| IL7 T2 | 10 | 7.7 ± 16.438 | 7.12 ± 5.81 | 12 | 0.61 |
| IL7 T3 | 11 | 14.21 ± 11.213 | 4.95 ± 2.73 | 10 | 0.01* |
| IL13 T0 | 10 | 8.67 ± 5.47 | 8.24 ± 4.47 | 13 | 0.71 |
| IL13 T1 | 10 | 9.51 ± 7.99 | 6.015 ± 8.455 | 13 | 0.01* |
| IL13 T2 | 10 | 8.24 ± 5.037 | 7.37 ± 10.515 | 12 | 0.41 |
| IL13 T3 | 11 | 11.95 ± 3.663 | 7.81 ± 3.46 | 10 | 0.04* |
| Plasma biomarker | n | Placebo Group | n | Melatonin Group | p |
|---|---|---|---|---|---|
| GFAP decT2-T0 | 9 | -0.01 ± 0.06 | 10 | -0.01 ± 0.05 | 0.32 |
| GFAP decT3-T0 | 9 | -0.02 ± 0.06 | 11 | -0.03 ± 0.14 | 0.16 |
| GM-CSF decT2-T0 | 12 | 3.71 ± 4.78 | 9 | -2.44 ± 4 | 0.02* |
| GM-CSF decT3-T0 | 10 | 0 ± 8.55 | 9 | -2.01 ± 8.48 | 0.12 |
| IL1 decT2-T0 | 12 | 0 ± 35.83 | 9 | 15.16 ± 62.87 | 0.45 |
| IL1 decT3-T0 | 10 | -23.37 ± 96.65 | 9 | 0 ± 35.22 | 0.91 |
| IL2 decT2-T0 | 12 | 0.76 ± 1.69 | 9 | -1.38 ± 3.65 | 0.19 |
| IL2 decT3-T0 | 10 | -0.15 ± 3.37 | 9 | -1.34 ± 6.62 | 0.31 |
| IL7 decT2-T0 | 12 | 0 ± 4.24 | 9 | -0.82 ± 4.37 | 0.36 |
| IL7 decT3-T0 | 10 | -0.41 ± 12.05 | 9 | -8.22 ± 7.03 | 0.09 |
| IL13 decT2-T0 | 12 | 0 ± 3.02 | 9 | -2.24 ± 6.03 | 0.21 |
| IL13 decT3-T0 | 10 | 0 ± 4.11 | 9 | -1.74 ± 2.32 | 0.10 |
| Plasma biomarker | Neurodevelopment test | Spearman’s correlation coefficient | p |
|---|---|---|---|
| GFAP decT2-T0 | Cog CS6 | -0.052 | 0.85 |
| L CS6 | -0.089 | 0.75 | |
| Mot CS6 | 0.057 | 0.84 | |
| Cog CS18 | 0.255 | 0.36 | |
| L CS18 | 0.326 | 0.24 | |
| Mot CS18 | 0.009 | 0.97 | |
| GMFCS6 | -0.182 | 0.52 | |
| GMFCS18 | -0.014 | 0.96 | |
| Tardieu6 | 0.134 | 0.63 | |
| Tardieu18 | 0.023 | 0.93 | |
| GFAP decT3-T0 | Cog CS6 | -0.120 | 0.65 |
| L CS6 | -0.397 | 0.11 | |
| Mot CS6 | -0.424 | 0.09 | |
| Cog CS18 | -0.043 | 0.88 | |
| L CS18 | 0.201 | 0.47 | |
| Mot CS18 | -0.133 | 0.64 | |
| GMFCS6 | 0.243 | 0.38 | |
| GMFCS18 | 0.331 | 0.23 | |
| Tardieu6 | 0.359 | 0.19 | |
| Tardieu18 | 0.290 | 0.29 | |
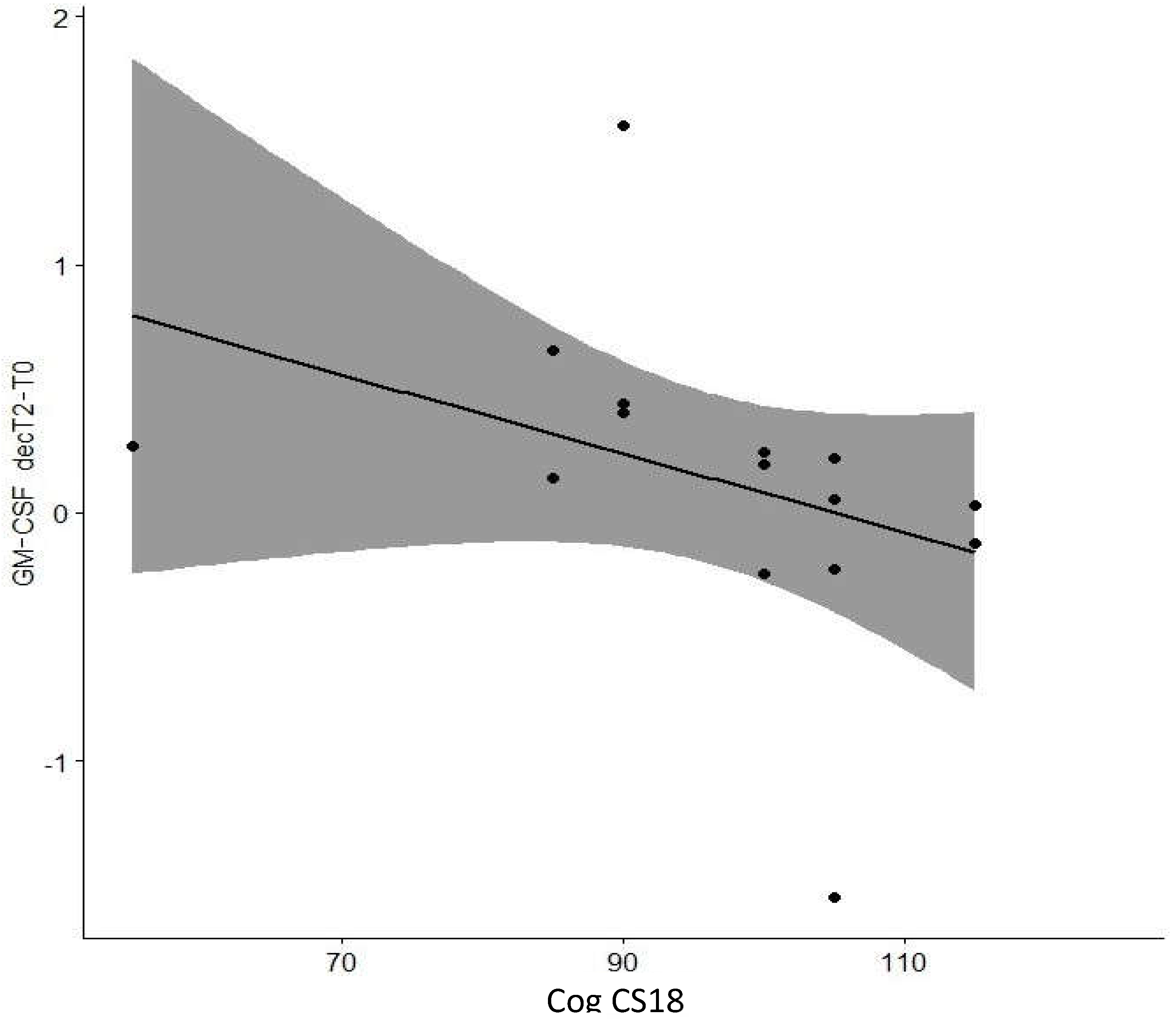
| GM-CSF decT2-T0 | Cog CS6 | -0.047 | 0.87 |
| L CS6 | -0.257 | 0.36 | |
| Mot CS6 | 0.052 | 0.85 | |
| Cog CS18 | -0.682 | 0.01* | |
| L CS18 | -0.185 | 0.51 | |
| Mot CS18 | 0.014 | 0.99 | |
| GMFCS6 | 0.187 | 0.50 | |
| GMFCS18 | 0.214 | 0.44 | |
| Tardieu6 | 0.252 | 0.36 | |
| Tardieu18 | 0.235 | 0.40 | |
| GM-CSF decT3-T0 | Cog CS6 | 0.042 | 0.89 |
| L CS6 | -0.013 | 0.96 | |
| Mot CS6 | -0.091 | 0.76 | |
| Cog CS18 | -0.189 | 0.54 | |
| L CS18 | -0.194 | 0.52 | |
| Mot CS18 | -0.150 | 0.63 | |
| GMFCS6 | 0.289 | 0.34 | |
| GMFCS18 | 0.350 | 0.24 | |
| Tardieu6 | 0.524 | 0.07 | |
| Tardieu18 | 0.510 | 0.08 | |
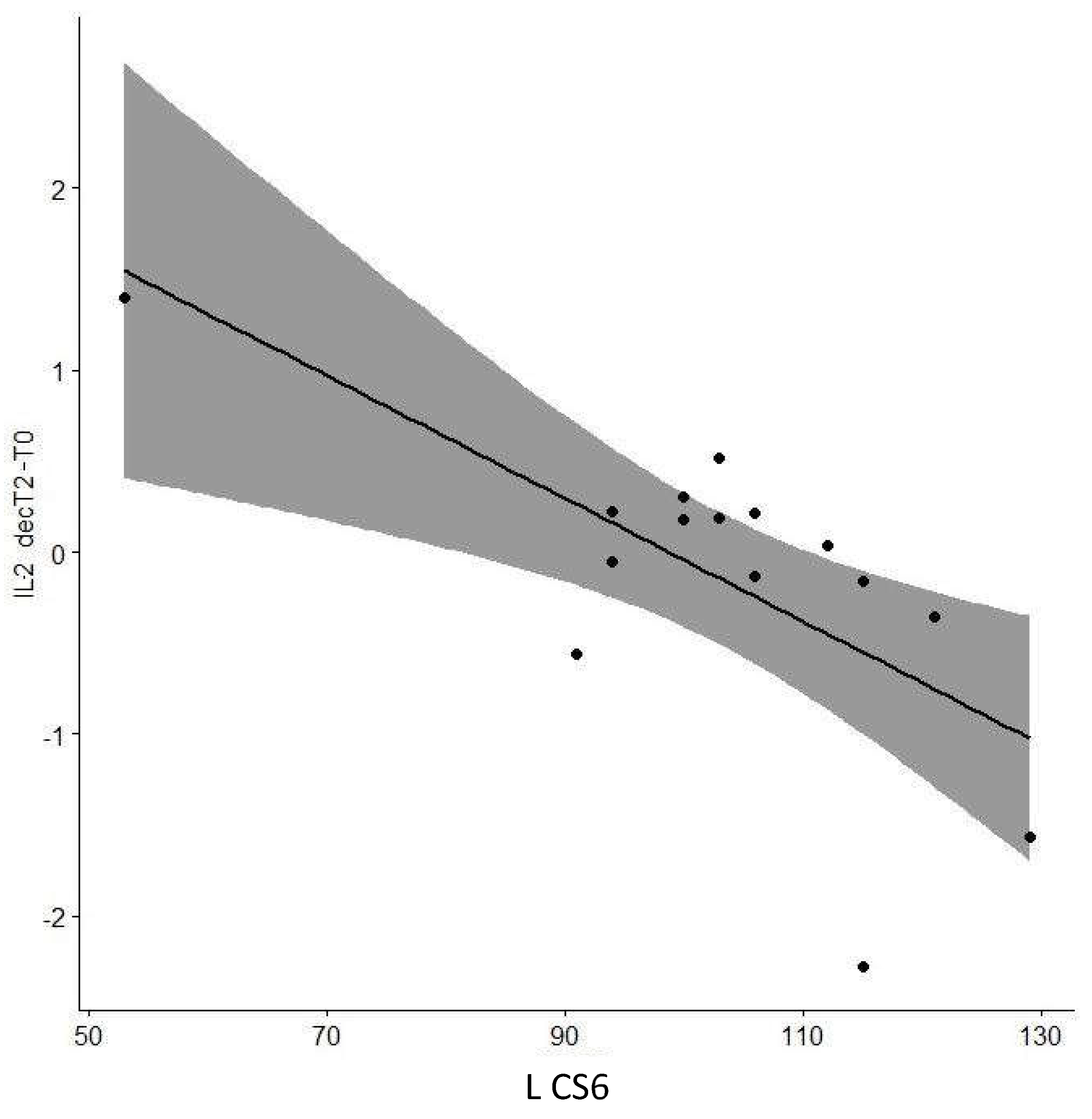
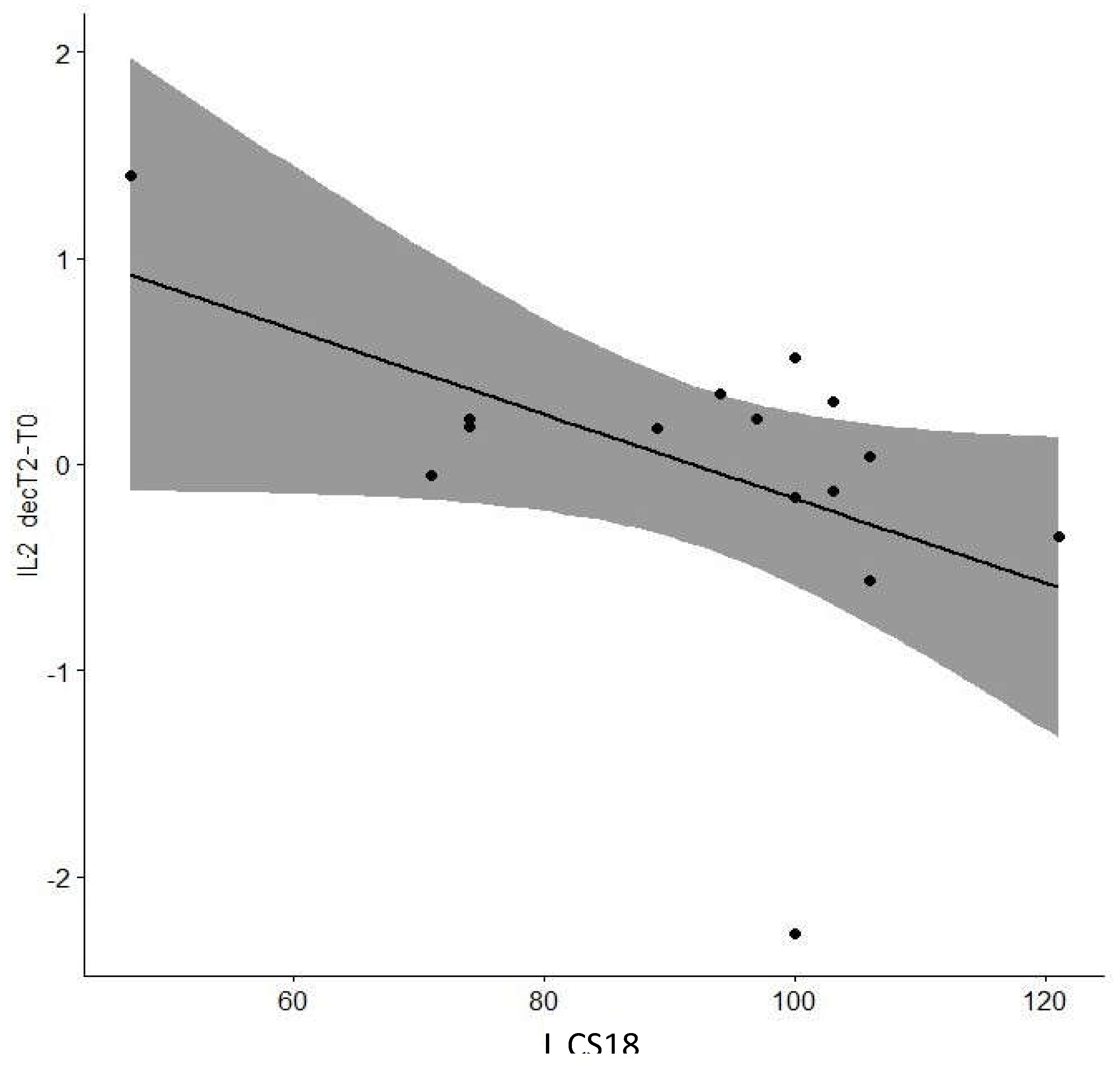
| IL13 decT2-T0 | Cog CS6 | 0.016 | 0.96 |
| L CS6 | -0.642 | 0.01* | |
| Mot CS6 | 0.003 | 0.99 | |
| Cog CS18 | -0.638 | 0.01* | |
| L CS18 | -0.593 | 0.03* | |
| Mot CS18 | -0.155 | 0.60 | |
| GMFCS6 | 0.563 | 0.09 | |
| GMFCS18 | 0.462 | 0.10 | |
| Tardieu6 | 0.400 | 0.16 | |
| Tardieu18 | 0.456 | 0.10 | |
| IL13 decT3-T0 | Cog CS6 | -0.281 | 0.33 |
| L CS6 | -0.653 | 0.01* | |
| Mot CS6 | -0.163 | 0.58 | |
| Cog CS18 | -0.287 | 0.34 | |
| L CS18 | -0.388 | 0.19 | |
| Mot CS18 | -0.496 | 0.08 | |
| GMFCS6 | 0.577 | 0.04* | |
| GMFCS18 | 0.535 | 0.04* | |
| Tardieu6 | 0.678 | 0.01* | |
| Tardieu18 | 0.732 | 0.10 | |
| IL1 decT2-T0 | Cog CS6 | -0.121 | 0.68 |
| L CS6 | -0.311 | 0.28 | |
| Mot CS6 | 0.420 | 0.13 | |
| Cog CS18 | -0.579 | 0.17 | |
| L CS18 | -0.538 | 0.24 | |
| Mot CS18 | -0.040 | 0.89 | |
| GMFCS6 | 0.299 | 0.30 | |
| GMFCS18 | 0.292 | 0.31 | |
| Tardieu6 | 0.292 | 0.31 | |
| Tardieu18 | 0.299 | 0.30 | |
| IL1 decT3-T0 | Cog CS6 | -0.036 | 0.91 |
| L CS6 | -0.157 | 0.63 | |
| Mot CS6 | 0.041 | 0.90 | |
| Cog CS18 | -0.188 | 0.56 | |
| L CS18 | -0.398 | 0.20 | |
| Mot CS18 | -0.248 | 0.44 | |
| GMFCS6 | 0.261 | 0.41 | |
| GMFCS18 | 0.230 | 0.47 | |
| Tardieu6 | 0.408 | 0.19 | |
| Tardieu18 | 0.469 | 0.12 | |
| IL2 decT2-T0 | Cog CS6 | -0.249 | 0.37 |
| L CS6 | -0.578 | 0.02* | |
| Mot CS6 | -0.014 | 0.96 | |
| Cog CS18 | -0.484 | 0.07 | |
| L CS18 | -0.498 | 0.04* | |
| Mot CS18 | -0.188 | 0.50 | |
| GMFCS6 | 0.465 | 0.08 | |
| GMFCS18 | 0.500 | 0.35 | |
| Tardieu6 | 0.490 | 0.06 | |
| Tardieu18 | 0.452 | 0.09 | |
| IL2 decT3-T0 | Cog CS6 | -0.180 | 0.54 |
| L CS6 | -0.126 | 0.67 | |
| Mot CS6 | 0.055 | 0.85 | |
| Cog CS18 | -0.056 | 0.86 | |
| L CS18 | -0.227 | 0.46 | |
| Mot CS18 | 0.058 | 0.85 | |
| GMFCS6 | 0.367 | 0.22 | |
| GMFCS18 | 0.494 | 0.09 | |
| Tardieu6 | 0.584 | 0.03* | |
| Tardieu18 | 0.498 | 0.08 | |
| IL7 decT2-T0 | Cog CS6 | -0.070 | 0.82 |
| L CS6 | 0.202 | 0.50 | |
| Mot CS6 | 0.114 | 0.71 | |
| Cog CS18 | -0.403 | 0.17 | |
| L CS18 | -0.266 | 0.38 | |
| Mot CS18 | -0.010 | 0.97 | |
| GMFCS6 | 0.464 | 0.11 | |
| GMFCS18 | 0.367 | 0.22 | |
| Tardieu6 | 0.086 | 0.78 | |
| Tardieu18 | 0.052 | 0.86 | |
| IL7 decT3-T0 | Cog CS6 | -0.258 | 0.39 |
| L CS6 | -0.338 | 0.26 | |
| Mot CS6 | -0.204 | 0.50 | |
| Cog CS18 | -0.141 | 0.65 | |
| L CS18 | -0.145 | 0.64 | |
| Mot CS18 | -0.380 | 0.20 | |
| GMFCS6 | 0.553 | 0.55 | |
| GMFCS18 | 0.599 | 0.43 | |
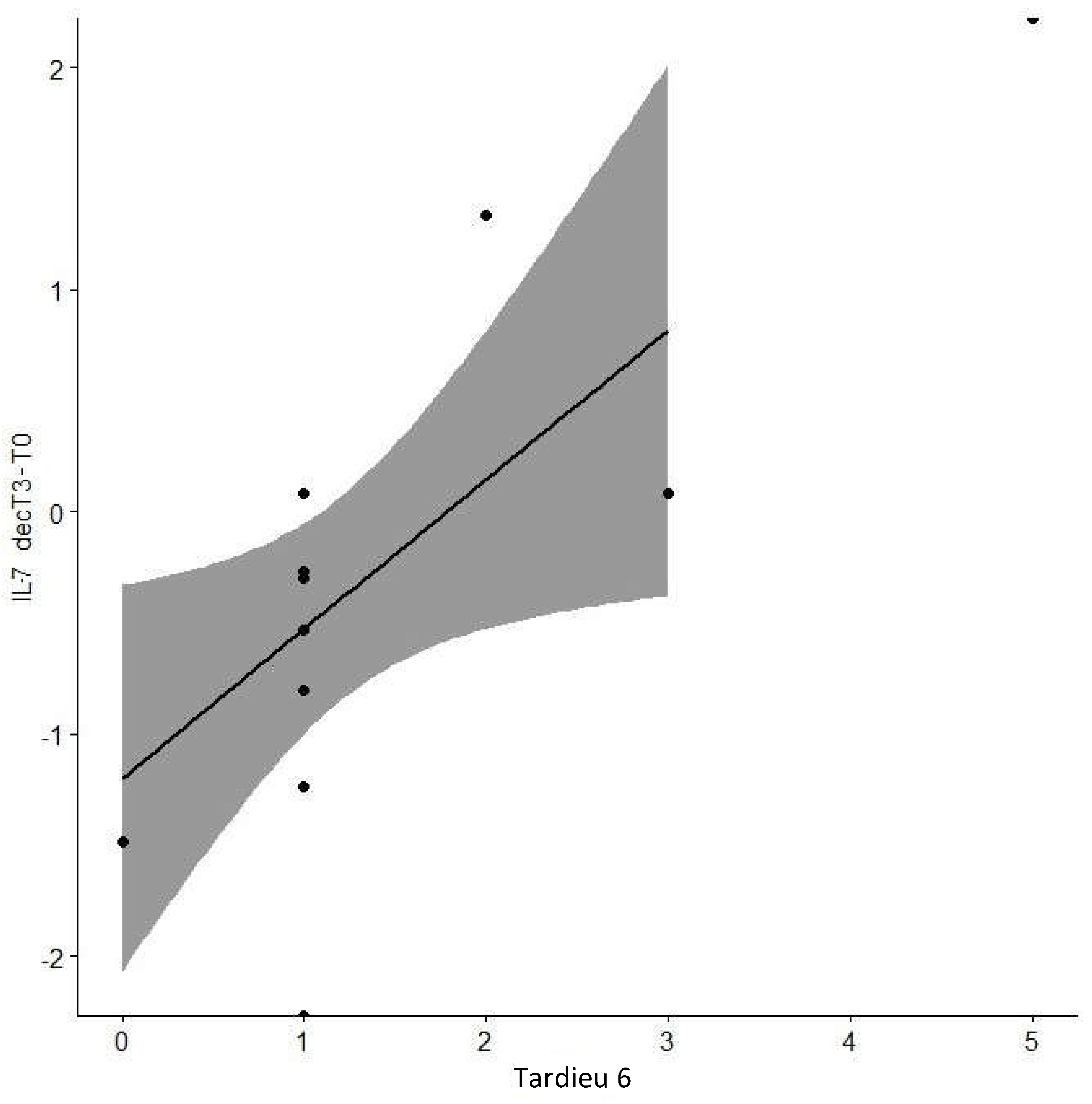
| Tardieu6 | 0.728 | 0.01* | |
| Tardieu18 | 0.711 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





